Abstract
Aims/Introduction
We investigated the effect of renal impairment on cognitive function during a 3‐year follow up in elderly type 2 diabetic patients, and an association with microinflammation.
Materials and Methods
Four cognitive function tests – Mini‐Mental State Examination (MMSE), word recall, Digit Symbol Substitution (DSS) and Stroop Color Word – were carried out in 67 patients. Renal impairment was defined as the presence of albuminuria and a decline in estimated glomerular filtration (eGFR) <60 mL/min/1.73 m2. Inflammatory markers, such as highly sensitive C‐reactive protein (hs‐CRP), tumor necrotizing factor‐α (TNF‐α), interleukin (IL)‐1β and IL‐6, were measured at baseline.
Results
At baseline, cognitive decline was found in patients with renal impairment. The DSS test was independently associated with eGFR decline, whereas MMSE tended to be associated with albuminuria after adjusting for confounding factors. Regarding changes in cognitive function and renal impairment, changes in urinary albumin to creatinine ratios were strongly and independently associated with changes in word recall scores. In patients with persistent eGFR decline, there was a tendency toward a greater decrease in MMSE and DSS scores, whereas in those with newly detected albuminuria, there was a tendency toward a greater decrease in word recall scores. Increased baseline levels of hs‐CRP, TNF‐α and IL‐6 were associated with renal impairment and cognitive function, especially DSS tests, respectively. However, the increased levels were not independent predictors for cognitive decline.
Conclusions
The present study showed a reciprocal relationship between cognitive decline and renal impairment, especially progression of albuminuria. Thus, monitoring treatment using renal biomarkers will be important for preserving both renal and cognitive function.
Keywords: Cognitive impairment, Microinflammation, Renal impairment
Introduction
The complication of renal impairment in diabetic patients might not only bring about serious conditions, such as end‐stage renal failure, but it also causes the onset of cardiovascular events. It is well recognized that impaired cognitive function is likely to be found in diabetic patients as compared with non‐diabetic subjects, as previously reported by the Rotterdam study1 and Hisayama study2. An association between renal impairment and cognitive dysfunction has been focused on recently3, although many mechanisms have been considered for cognitive impairment in diabetic patients6. The combined effects of renal impairment and cognitive dysfunction in diabetic patients could synergistically impair their quality of life and cause mortality. Therefore, it is becoming more important than ever to elucidate the mechanisms of an association between renal and cognitive impairment, and prevent the progression of both.
The brain and kidneys are thought to be associated through a variety of mechanisms, and one of them might involve hemodynamic similarities in vascular beds and similarities in the histopathological appearance of small vessels in both organs7. Also, it is likely that hyperglycemia and concomitant hypertension in diabetic patients impair microvessels through endothelial dysfunction as a result of microinflammation and oxidative stress, leading to a decreased glomerular filtration rate (GFR) or albuminuria in the kidney4, and similarly, they might affect the incidence and progression of cerebral small vessel disease (SVD) in the brain10, which could lead to cognitive impairment12.
In a 3‐year longitudinal observation, we observed that the presence or progression of SVD seen on magnetic resonance imaging was associated with a decline in cognitive function in elderly diabetic patients14, and, recently, we reported that renal impairment was associated with cognitive dysfunction independently of SVD in a cross‐sectional study15. However, we did not assess how changes in renal impairment affect various aspects of cognitive function in the previous study.
It has also been reported that chronic microinflammation was involved in the pathogenesis of diabetic nephropathy16, as well as chronic kidney disease in non‐diabetic patients19 or cognitive impairment in diabetic patients21 and the general population23. However, few studies have considered whether microinflammation is associated with both renal and cognitive impairment. Therefore, in the present 3‐year observation, first, we investigated how the presence of and changes in renal impairment were associated with cognitive decline during follow up; and, second, whether microinflammation was a common risk factor for both types of impairment.
Materials and Methods
A total of 79 outpatients with type 2 diabetes aged more than 65 years, who were living independently at home without apparent dementia, had the ability to understand this study and freely consent to taking part were recruited for it. A total of 10 patients dropped out during the 3‐year follow up. Eight of them were institutionalized or moved, and the remaining two patients died due to lung cancer and cerebral bleeding. In addition, two patients with severe cognitive impairment (Mini‐Mental State Examination [MMSE] score <19 points) were excluded at baseline. Therefore, the study was carried out on 67 patients (mean 74.6 ± 5.5, range 65–89 years) whose known duration of diabetes was 17.0 ± 8.2 years and education years were 9.34 ± 2.25 years. Microvascular complications, such as renal insufficiency (serum creatinine ≥ 2.0 mg/dL), severe retinopathy, history of stroke, transient cerebral ischemia and atrial fibrillation, were also exclusion criteria.
Cognitive Function Tests
We selected the following standardized psychological tests for measurement of each function as reported previously14. MMSE was used to assess orientation, registration, attention, calculation, language and recall with a score range from 0 to 3026. We carried out word recall, a subtest of the Alzheimer's Disease Assessment Scale with a score ranging from 0 to 10, to assess verbal memory27. Complex psychomotor skills were evaluated by the Digit Symbol Substitution (DSS) test, a subtest of the Wechsler Adult Intelligence Scale‐Revised with a score ranging from 0 to 9328. We used the modified Stroop Color Word (Stroop) test to assess attention/executive function, in which the seconds to completion are recorded, and the difference between the time required to read the word card and that required to read the dot card is calculated. A wider time difference generally means lower cognitive performance29. All neuropsychological tests were carried out twice in 2006 and 2009 by well‐trained psychological testers.
We calculated z‐scores (individual test score minus mean test score divided by the standard deviation [SD]) for the neuropsychological tests at baseline and the end of follow up using the mean and SD of the whole population. Cognitive decline was calculated by subtracting the z‐scores for word recall (Δword recall), DSS test (ΔDSS) and Stroop test (ΔStroop) at baseline from the z‐scores at end of follow up. Change in MMSE score (ΔMMSE) was calculated by subtracting baseline scores from the scores at the end of follow up using raw test scores.
Definition of Renal Impairment
Each patient's estimated glomerular filtration rate (eGFR) was calculated from the following three‐variable Japanese equation: GFR (mL/min/1.73 m2) = 194 × serum creatinine−1.094 × age−0.287 × 0.739 (if female)30. Decline in eGFR was defined as <60 mL/min/1.73 m2. Albuminuria was defined as urinary albumin to creatinine ratios (ACR), which were calculated from two measurements of albumin (mg) and creatinine (g) in spot urine, as follows: normoalbuminuria, <30 mg/g creatinine; microalbuminuria, 30–299 mg/g creatinine; and macroalbuminuria, ≥300 mg/g creatinine. Changes in ACR (Δlog ACR) and eGFR (ΔeGFR) were calculated by subtracting baseline from end of follow up after log transformation for ACR.
Evaluation of Patients
Fasting blood samples were separated and analyzed. Total cholesterol, high‐density lipoprotein (HDL) cholesterol, triglycerides, low‐density lipoprotein (LDL) cholesterol, and fasting blood glucose were measured using an autoanalyzer and routine enzymatic techniques. Hemoglobin A1c (HbA1c) was measured using high‐performance liquid chromatography (HLC‐723G7; Tosoh, Tokyo, Japan), and HbA1c (%) was estimated as a National Glycohemoglobin Standardization Program (NGSP) value (%), calculated by the formula HbA1c (%) = HbA1c (Japan Diabetes Society [JDS]) (%) × 1.02 + 0.2531. Plasma insulin was measured by chemiluminescence enzyme immunoassay. Inflammatory markers were assayed using the quantitative sandwich enzyme technique of the enzyme‐linked immunosorbent assay QuantiGlo kit (R&D System, Minneapolis, MN, USA) for tumor necrotizing factor‐α (TNF‐α) and interleukin‐6 (IL‐6), and Quantikine kit (R&D System) for interleukin‐1β (IL‐1β). For levels of TNF‐α and IL‐1β below the detection limits of the methods used to determine them, the respective detection limits of 0.55 pg/mL (n = 2) and 0.125 pg/mL (n = 16) were used as measurement values. High‐sensitivity C‐reactive protein (hs‐CRP) was assayed using a monoclonal antibody coated onto polystyrene particles and fixed‐timed kinetic nephelometric measurements (BN II; Dade Behring, Marberg, Germany).
Hypertension was defined as prior diagnosis, current treatment with an antihypertensive agent or blood pressure >140/90 mmHg when enrolled in the study and during the follow‐up period. Dyslipidemia was defined as current treatment with a lipid‐lowering drug or LDL cholesterol ≥140 mg/dL and/or HDL cholesterol <40 mg/dL and/or triglycerides ≥150 mg/dL according to Treatment Guide for Diabetes32.
The present study was approved by the Ethics Committee of Chubu Rosai Hospital. After informed consent was obtained from each of the participants, the study was carried out in accordance with the principles of the Declaration of Helsinki.
Statistical Analysis
Results were expressed as mean ± SD. Statistical analysis was carried out using the t‐test, for comparisons between two groups. Comparisons for factors not forming normal distributions were carried out after log transformation. The chi square test was also carried out, for independence. Comparisons among the groups were carried out using analysis of variance (anova) followed by Bonferroni's post‐hoc test. Repeated‐measures anova was used to examine the interaction between changes in cognitive function and each group. We calculated Pearson's correlation coefficients and partial correlation coefficients adjusted by variables. We carried out multiple linear regression or multiple logistic regression where appropriate, and variables with P < 0.1 in univariate analysis were entered for multiple regression analysis. Also, if two variables had collinearity, one of them was not entered. Statistical analyses were carried out using spss version 21 software (SPSS, Chicago, IL, USA), and P < 0.05 was considered to be statistically significant.
Results
Clinical and Biochemical Characteristics for Presence or Absence of Albuminuria and eGFR Decline at Baseline in Elderly Diabetic Patients
At baseline, 18 patients (27%) were being treated by diet only, 37 (55%) were using oral glucose‐lowering medications and 12 (18%) were using insulin. These patients were moderately well‐controlled during the 3 years with an average HbA1c of 7.0 ± 0.7%. The average blood pressure during follow up was 139.2 ± 12.5/75.4 ± 7.2 mmHg, and there were 50 patients with hypertension, of whom 43 (86%) were receiving renin–angiotensin system blockers during follow up. No patients experienced a severe hypoglycemic episode requiring admission, and no patients had a stroke during the follow‐up period.
In patients with albuminuria at baseline, higher frequency of RAS blocker use, higher levels of triglycerides, serum creatinine and fasting blood glucose, and lower levels of HDL‐C were observed; whereas patients with eGFR decline were older, had lower levels of HDL‐C and diastolic blood pressure, and higher levels of serum creatinine (Table 1).
Table 1. Clinical and biochemical characteristics for presence and absence of albuminuria and estimated glomerular filtration rate decline at baseline.
| Total | Albuminuria | eGFR (mL/min/m2) | |||
|---|---|---|---|---|---|
| (−) | (+) | eGFR ≥60 | eGFR <60 | ||
| n (male/female) | 67 (28/39) | 47 (17/30) | 20 (11/9) | 47 (19/28) | 20 (9/11) |
| Age (years) | 74.6 ± 5.5 | 73.9 ± 5.5 | 76.1 ± 5.3 | 73.4 ± 4.8 | 77.3 ± 6.2** |
| Duration of diabetes (years) | 17.0 ± 8.2 | 16.6 ± 8.4 | 18.2 ± 7.8 | 15.8 ± 7.5 | 19.9 ± 9.1# |
| Body mass index (kg/m2) | 22.9 ± 3.3 | 22.6 ± 3.5 | 23.5 ± 2.9 | 22.7 ± 3.3 | 23.2 ± 3.5 |
| Smoking (non/current) | 54/13 | 40/7 | 14/6 | 36/11 | 18/2 |
| Serum creatinine (mg/dL) | 0.79 ± 0.23 | 0.74 ± 0.20 | 0.92 ± 0.24*** | 0.70 ± 0.12 | 1.02 ± 0.25 |
| Systolic BP (mmHg) | 139.2 ± 17.9 | 137.9 ± 19.0 | 142.5 ± 14.8 | 137.8 ± 17.2 | 142.7 ± 19.3 |
| Diastolic BP (mmHg) | 77.2 ± 7.9 | 77.5 ± 7.7 | 76.5 ± 8.5 | 78.5 ± 7.8 | 74.0 ± 7.3* |
| RAS blockers use (+/−) | 40/27 (59.7%) | 24/23 (51.1%) | 16/4 (80.0%)* | 25/22 (53.2%) | 15/5 (75.0%)# |
| T‐cholesterol (mg/dL) | 206.2 ± 29.6 | 207.1 ± 29.0 | 204.0 ± 31.5 | 203.4 ± 30.2 | 212.8 ± 27.6 |
| HDL cholesterol (mg/dL) | 53.5 ± 15.3 | 57.1 ± 15.6 | 45.1 ± 10.9*** | 56.1 ± 15.8 | 47.5 ± 12.4* |
| Triglycerides (mg/dL) | 127.0 ± 88.2 | 107.3 ± 55.4 | 173.3 ± 127.9*** | 115.3 ± 60.0 | 154.5 ± 131.0 |
| LDL cholesterol (mg/dL) | 124.3 ± 24.0 | 125.4 ± 25.4 | 121.8 ± 20.6 | 122.4 ± 25.7 | 129.0 ± 19.4 |
| Statin use (+/−) | 29/38 (43.3%) | 18/29 (38.3%) | 11/9 (55.0%) | 19/28 (40.4%) | 10/10 (50.0%) |
| Fasting blood glucose (mg/dL) | 137.2 ± 30.0 | 132.0 ± 29.0 | 149.7 ± 29.3* | 136.5 ± 30.3 | 139.1 ± 30.0 |
| Fasting insulin (μU/mL) | 7.64 ± 5.40 | 6.69 ± 3.62 | 9.88 ± 7.88# | 7.59 ± 6.00 | 7.75 ± 3.75 |
| HbA1c (%) | 7.08 ± 0.83 | 6.97 ± 0.78 | 7.34 ± 0.91 | 7.09 ± 0.81 | 7.07 ± 0.90 |
| hs‐CRP (mg/L) | 0.85 ± 1.26 | 0.51 ± 0.58 | 1.64 ± 1.93* | 0.48 ± 0.56 | 1.73 ± 1.89 |
| TNF‐α (pg/mL) | 2.47 ± 1.02 | 2.21 ± 0.79 | 3.07 ± 1.25*** | 2.13 ± 0.68 | 3.27 ± 1.22 |
| IL‐1β (pg/mL) | 0.34 ± 0.23 | 0.31 ± 0.20 | 0.42 ± 0.29# | 0.34 ± 0.26 | 0.33 ± 0.18 |
| IL‐6 (pg/mL) | 1.60 ± 1.56 | 1.16 ± 0.95 | 2.61 ± 2.17**** | 1.24 ± 0.85 | 2.44 ± 2.36* |
| MMSE | 26.1 ± 2.6 | 26.7 ± 2.4 | 24.7 ± 2.7*** | 26.6 ± 2.5 | 25.0 ± 2.7* |
| Word recall (z‐score) | 0.12 ± 0.94 | 0.20 ± 1.01 | −0.05 ± 0.74 | 0.28 ± 0.93 | −0.25 ± 0.86* |
| DSS test (z‐score) | 0. 08 ± 0.92 | 0.25 ± 0.88 | −0.34 ± 0.91* | 0.34 ± 0.90 | −0.57 ± 0.60**** |
| Stroop test (z‐score) | 0. 03 ± 0.92 | −0.24 ± 0.77 | 0.47 ± 1.14*** | −0.13 ± 0.76 | 0.21 ± 1.21 |
Values are expressed as mean ± standard deviation. #P < 0.1, *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001 different from albuminuria (–) or estimated glomerular filtration rate (eGFR) ≥60. Statistical analyses were carried out using the unpaired t‐test for comparison of two means, and the chi square test for independence. Triglycerides, fasting insulin, high‐sensitivity C‐reactive protein (hs‐CRP), tumor necrosis factor (TNF)‐α, interleukin (IL)‐1β, and IL‐6 are log transformed and comparison of the two means is carried out. BP, blood pressure; DSS, Digit Symbol Substitution; HbA1c, glycated hemoglobin; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; RAS, renin–angiotensin system; T‐cholesterol, total cholesterol.
Regarding an association between renal impairment and cognitive functions at baseline, significant differences in MMSE, DSS test and Stroop test scores were found at baseline in patients with albuminuria as compared with normoalbuminuria, and decreased scores for MMSE, word recall and DSS tests were found in patients with a decline in eGFR.
Individual associations of albuminuria and eGFR decline with each cognitive function were examined in multiple logistic regression analysis with age, duration of diabetes, HDL cholesterol, RAS use, log triglycerides, log fasting insulin and fasting blood glucose at baseline as covariables. Only DSS test scores at baseline were independently associated with eGFR decline (P = 0.015), while there was a tendency toward an association between MMSE at baseline and albuminuria (P = 0.065) after adjustment for the aforementioned covariables.
Association Between Changes in Cognitive Function Scores and Changes in Renal Impairment During Follow‐Up
After 3 years, cognitive function scores except those for the Stroop test declined (MMSE 26.1 ± 2.6 at baseline vs 24.0 ± 3.9 at 3 years of follow up, P < 0.001; word recall 6.86 ± 1.51 vs 6.46 ± 1.69, P = 0.017; DSS test 33.4 ± 10.2 vs 31.7 ± 12.0, P = 0.075; Stroop test 20.7 ± 13.6 vs 21.5 ± 15.7, P = 0.743).
Factors associated with changes in cognitive function scores during follow up are shown in Table 2. Multivariate analysis showed that independent factors were MMSE scores at baseline for ΔMMSE, Δlog ACR for Δword recall, age and fasting blood glucose at baseline for ΔDSS test; and Stroop test scores at baseline, average diastolic blood pressure during follow up, and HbA1c at baseline for ΔStroop test. Regarding associations between changes in renal impairment and cognitive decline, only Δlog ACR was strongly and independently associated with Δword recall (Figure 1).
Table 2. Factors associated with changes in cognitive function scores during follow up.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Correlation coefficient | P‐value | Standardized β coefficient (SE) | P‐value | |
| ΔMMSE | r2 = 0.306, P = 0.001 | |||
| Age | −0.309 | 0.011 | −0.209 (0.078) | 0.092 |
| DBP at baseline | 0.261 | 0.033 | 0.176 (0.052) | 0.127 |
| eGFR at baseline | 0.260 | 0.034 | 0.130 (0.029) | 0.306 |
| Log IL‐6 at baseline | −0.254 | 0.040 | −0.139 (1.406) | 0.200 |
| MMSE at baseline | −0.235 | 0.056 | −0.367 (0.153) | 0.002 |
| HDL‐C at baseline | 0.205 | 0.096 | 0.145 (0.026) | 0.209 |
| ΔWord recall (z‐score) | r2 = 0.297, P < 0.001 | |||
| ΔLog ACR | −0.476 | 0.000 | −0.427 (0.208) | 0.000 |
| Word recall at baseline | −0.292 | 0.016 | −0.204 (0.102) | 0.084 |
| Education years | −0.207 | 0.093 | 0.110 (0.392) | 0.323 |
| Log TG at baseline | 0.230 | 0.061 | −0.045 (0.042) | 0.698 |
| ΔDSS test (z‐score) | r2 = 0.229, P = 0.009 | |||
| Age | −0.305 | 0.014 | −0.261 (0.013) | 0.041 |
| DBP at baseline | 0.296 | 0.016 | 0.242 (0.009) | 0.051 |
| Average DBP during follow up | 0.244 | 0.051 | Not applied | |
| FBG at baseline | −0.214 | 0.084 | −0.272 (0.002) | 0.026 |
| Education years | 0.207 | 0.093 | −0.137 (0.030) | 0.253 |
| Log IL‐6 at baseline | −0.212 | 0.093 | −0.053 (0.236) | 0.682 |
| ΔStroop test (z‐score) | r2 = 0.628, P < 0.001 | |||
| Stroop score at baseline | −0.570 | 0.000 | −0.632 (0.097) | 0.000 |
| Average DBP during follow up | 0.402 | 0.001 | 0.252 (0.019) | 0.021 |
| HbA1c at baseline | −0.318 | 0.009 | −0.175 (0.133) | 0.046 |
| Smoking | −0.250 | 0.043 | −0.128 (0.272) | 0.134 |
| Average SBP during follow up | 0.219 | 0.077 | −0.046 (0.011) | 0.658 |
| DBP at baseline | 0.208 | 0.094 | Not applied | |
No smoking was 0, smoking was 1. Δ, change; ACR, albumin to creatinine ratio; DBP, diastolic blood pressure; DSS, Digit Symbol Substitution; eGFR, estimated glomerular filtration rate; FBG, fasting blood glucose; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; IL, interleukin; MMSE, Mini‐Mental State Examination; SBP, systolic blood pressure; SE, standard error; TG, triglycerides.
Figure 1.
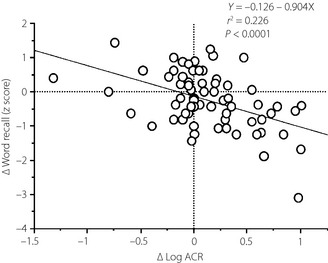
Correlation between changes in log albumin to creatinine ratios (Δlog ACR) and changes in word recall (Δword recall) during follow up. ΔLog ACR was calculated by subtracting baseline from the end of follow up after log transformation for ACR. ΔWord recall was calculated by subtracting the z‐scores for word recall at baseline from the z‐scores at the end of follow up.
At baseline, 13 patients had microalbuminuria and seven patients had macroalbuminuria. During follow up, five patients regressed to normoalbuminuria from microalbuminuria and eight patients progressed to microalbuminuria. After 3 years, 23 patients had albuminuria and in two of them, microalbuminuria had developed into macroalbuminuria. The eGFR had improved in two patients and deteriorated in seven patients.
We divided patients into three groups: no renal impairment, newly detected renal impairment and persistent renal impairment during follow‐up, and patients with improved renal impairment were assigned to the no renal impairment group. MMSE and DSS test scores showed a tendency to deteriorate to a greater extent in patients with persistent decline in eGFR than in those with new decline or without decline in eGFR. In contrast, decreases in word recall scores tended to be greater in patients with newly detected albuminuria than in the other groups (Figure 2). However, no such interaction among groups with regard to cognitive function was observed for the Stroop test (data not shown).
Figure 2.
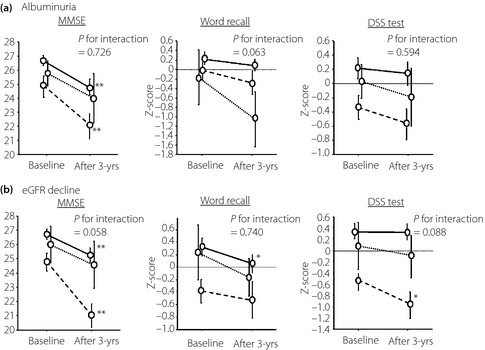
Association between changes in renal impairment and changes in Mini‐Mental State Examination (MMSE), word recall and Digit Symbol Substitution (DSS) test scores during follow up. Data are expressed as mean ± standard error at baseline and after the 3‐year (3‐yrs) follow up. Broken line: presence of albuminuria (n = 15) or estimated glomerular filtration rate (eGFR) decline <60 mL/min/1.73 m2 (n = 18); dotted line: newly detected albuminuria (n = 8) or eGFR decline (n = 7); solid line: absence of albuminuria (n = 44) or eGFR decline (n = 42). *P < 0.05, **P < 0.01 compared with baseline by paired t‐test. For MMSE, (a) albuminuria main effect of time P = 0.025, main effect of group P < 0.001, time × group interaction P = 0.726; (b) eGFR decline, main effect of time P < 0.001, main effect of group P < 0.001, time × group interaction P = 0.058. For word recall, (a) albuminuria, main effect of time P = 0.057, main effect of group P < 0.005, time × group interaction P = 0.063; (b) eGFR decline main effect of time P = 0.042, main effect of group P = 0.041, time × group interaction P = 0.740. For DSS test, (a) albuminuria main effect of time P = 0.093, main effect of group P = 0.052, time × group interaction P = 0.594; (b) eGFR decline main effect of time P < 0.001, main effect of group P = 0.038, time × group interaction P = 0.088, P‐values show the results of repeated‐measures anova.
We also investigated an association between cognitive function and the presence of albuminuria or eGFR decline, assigning participants to four groups categorized by the presence or absence of albuminuria or eGFR. Although the group with both types of renal impairment had significantly poorer cognitive scores in MMSE, DSS and Stroop tests at baseline as compared with the group without either, no significant differences in changes in individual cognitive functions during follow up among the four groups were observed (data not shown).
Association Between Levels of Inflammatory Markers at Baseline and Renal Impairment
Significantly higher levels of hs‐CRP, TNF‐α and IL‐6 were observed in patients with albuminuria and eGFR decline (Table 1). Log IL‐6, log IL‐1β, log TNF‐α and log hs‐CRP were associated with albuminuria (P = 0.004, P = 0.024, P = 0.064, P = 0.088, respectively), whereas log hs‐CRP and log TNF‐α were independently associated with eGFR decline (P = 0.014, P = 0.011, respectively) after adjusting for variables as aforementioned. In addition, levels of TNF‐α at baseline were significantly lower in patients without albuminuria (2.24 ± 0.79 pg/mL, P < 0.05) and eGFR decline (2.10 ± 0.71 pg/mL, P < 0.001) than in those with persistent albuminuria (3.11 ± 1.34 pg/mL) and eGFR decline (3.29 ± 1.28 pg/mL) during follow up, and the baseline levels in patients with newly diagnosed albuminuria (2.53 ± 1.04 pg/mL) and new eGFR decline (2.57 ± 0.54 pg/mL) were between those in patients with normal renal function and persistent renal impairment, although the differences were not significant. Hs‐CRP and IL‐6 levels also showed a similar tendency (data not shown).
In the group with both albuminuria and eGFR decline, levels of hs‐CRP, TNF‐α and IL‐6 at baseline were significantly higher (2.87 ± 2.10 mg/L, 3.84 ± 1.29 pg/mL, 3.44 ± 2.69 pg/mL, respectively) as compared with the group without either type of renal impairment (0.49 ± 0.62 mg/L, 2.08 ± 0.72 pg/mL, 1.09 ± 0.73 pg/mL, respectively).
Correlation Between Levels of Inflammatory Markers at Baseline and Cognitive Function at Baseline, After 3‐year Follow‐Up and Changes in Them
Significant correlations between inflammatory markers, such as hs‐CRP, TNF‐α and IL‐6, and cognitive function indicators, such as MMSE, DSS and Stroop tests, were mainly observed at baseline and after 3 years, but not for changes in them during follow up. However, such significant associations found at baseline and after 3 years disappeared after adjustment for the variables shown in Table 3, and tendencies toward correlation were only found between hs‐CRP and TNF‐α levels at baseline, and DSS tests at baseline, and between TNF‐α and IL‐6 levels at baseline and ΔStroop test.
Table 3. Correlation between levels of inflammatory markers at baseline and cognitive function at baseline, after 3‐year follow up, and the change in it.
| MMSE | Word recall (z‐score) | DSS test (z‐score) | Stroop test (z‐score) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Base | After | Δ | Base | After | Δ | Base | After | Δ | Base | After | Δ | |
| Log hs‐CRP |
−0.272* (−0.198) |
−0.278* (−0.071) |
−0.103 (−0.036) |
−0.226# (−0.150) |
−0.131 (−0.014) |
0.091 (0.146) |
−0.376*** (−0.239#) |
−0.365*** (−0.211) |
−0.075 (0.000) |
0.311* (0.131) |
0.267* (0.122) |
0.000 (0.041) |
| Log TNF‐α |
−0.249* (−0.009) |
−0.372*** (−0.052) |
−0.221# (−0.032) |
−0.249* (−0.023) |
−0.327** (−0.064) |
−0.135 (−0.149) |
−0.489**** (−0.240#) |
−0.439**** (−0.067) |
−0.035 (0.202) |
0.200 (−0.122) |
0.260* (−0.113) |
0.074 (0.247#) |
| Log IL‐1β |
−0.258* (−0.196) |
−0.165 (−0.055) |
−0.007 (0.127) |
−0.121 (−0.018) |
−0.126 (−0.071) |
−0.022 (−0.022) |
−0.164 (−0.015) |
−0.162 (0.007) |
−0.036 (0.062) |
0.141 (0.139) |
−0.059 (−0.102) |
−0.149 (−0.109) |
| Log IL‐6 |
−0.246* (−0.038) |
−0.400*** (−0.194) |
−0.254* (−0.141) |
−0.185 (−0.029 |
−0.136 (−0.130) |
0.036 (−0.041) |
−0.370*** (−0.199) |
−0.431**** (−0.166) |
−0.212# (−0.025) |
0.252* (0.017) |
0.321** (0.226) |
0.087 (0.244#) |
Values are correlation coefficients and values in parenthesis are partial correlation coefficient adjusted by age, sex, years of education, duration of diabetes, statin and renin–angiotensin system use, systolic blood pressure, diastolic blood pressure, glycated hemoglobin (HbA1c), current smoking, the presence of dyslipidemia, log ACR and estimated glomerular filtration rate. #P < 0.1, *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001. DSS, Digit Symbol Substitution; hs‐CRP, high‐sensitivity C‐reactive protein; IL, interleukin; MMSE, Mini‐Mental State Examination; TNF‐α, tumor necrosis factor‐α.
Discussion
Cross‐sectional and longitudinal studies on an association between renal and cognitive impairment have recently been carried out in diabetic patients4, as well as the general population3. Although it is widely accepted that chronic kidney disease is a potent risk factor for cognitive impairment, the details remain controversial36. In many studies, the estimation of cognitive function was carried out using MMSE, a simple, global test for screening. In the present study, a decline in MMSE was also partially associated with renal impairment. Regarding specific cognitive domains, it is generally agreed that frontal lobe functions, such as psychomotor speed, executive function and attention, are impaired in patients with renal impairment4, and impairment of memory has also been reported4. Our recent15 and the present study cross‐sectionally observed an association between renal impairment and DSS and Stroop tests, which reflect frontal lobe function.
Regarding an association between types of renal impairment and cognitive impairment, albuminuria and eGFR decline, respectively, have been reported to be associated with cognitive dysfunction. A recent meta‐analysis by Etgen et al.36 showed that significant cognitive decline was observed in subjects with eGFR decline in both cross‐sectional and longitudinal studies, although they mentioned that there was insufficient data available for a separate analysis on albuminuria. However, it has also been reported that albuminuria is more associated with cognitive decline than eGFR decline, and that albuminuria was associated with decreased verbal memory performance and impaired processing speed and executive function4. In the present study, although impaired cognitive functions, except word recall, were significantly related to albuminuria at baseline, significance disappeared after adjusting for confounding factors, and there was only a tendency toward an association for MMSE. In contrast, a significant decline in DSS tests was observed in patients with eGFR decline at baseline, even after adjustment. In addition, poorer scores for MMSE, DSS, and Stroop tests were found in patients with both albuminuria and eGFR decline as compared with those without either. In the Reasons for Geographic and Racial Differences in Stroke (REGARD) study, albuminuria and low eGFR were complementary, but not additive, even though they were independently associated with incident cognitive impairment5. Which type of renal impairment is more associated with cognitive dysfunction remains controversial, because it is likely that many factors, such as the selected subjects and their age38, selected cognitive domains, and classification bias regarding renal impairment will affect the results. In this regard, further studies will be required for clarification.
Regarding the progression of renal impairment and cognitive decline, the large‐scale ONTARGET/TRANSCEND studies35 reported that the presence and degree of albuminuria, and the progression to albuminuria were associated with worsened cognitive function. In addition, in the recent subanalysis of the ACCORD trial, it was reported that persistent albuminuria and progressive albuminuria were associated with a decline in cognitive function in relatively young individuals with diabetes and unimpaired eGFR39. Another longitudinal study also reported that cognitive function was declining in a subject group with renal impairment during the follow‐up period, though there was no association between renal and cognitive impairments at baseline13. In the present study, a decline in verbal memory was strongly associated with the degree of change in albuminuria rather than status of albuminuria at baseline, suggesting that progressive changes in albuminuria could accelerate cognitive decline. Accordingly, supreme treatment for preventing the incidence and progression of albuminuria at the early stage will be required in order to avoid not only end‐stage renal disease, but also dementia.
In contrast, an association between changes in eGFR and cognitive function was not found in the present study, and only a tendency toward decreasing scores in MMSE and DSS tests was seen in patients with persistent eGFR decline. However, patients with persistent eGFR decline were older as compared with patients without it. As we previously reported that cognitive decline was more developed in older patients in the same subject group14, it is likely that age had a strong impact on declining cognitive function.
Many studies have suggested that cerebral SVD is related to renal impairment10 and cognitive dysfunction12. Our previous results14 also were in line with the findings of these studies. As the brain and kidney might be considered as end organs having similar low‐resistance vascular beds and endothelial structures, which are affected by inflammation and oxidative stress, impaired endothelial function in the brain could lead to SVD or neurodegeneration through damage to the blood–brain barrier41, whereas endothelial dysfunction in the kidney might contribute to impaired glomerular filtration and secondary protein leakage4. Therefore, the findings of the recent studies39 and the present study suggest that endothelial dysfunction is a common factor for renal and cognitive impairment, as albuminuria is a measure for endothelial pathology.
Microinflammation is also thought to be one of the potent factors for such impairment, and inflammatory markers have been reported to be associated with cerebral SVD42, cognitive impairment21 and renal disease16. The rise in hs‐CRP, TNF‐α and IL‐6 levels observed in patients with renal impairment in the present study was consistent with previous research results. Similarly, levels of inflammatory markers at baseline were associated with MMSE and DSS tests at baseline and after 3 years, whereas they were not associated with changes during follow up. Also, IL‐6 levels at baseline were correlated with changes in MMSE and DSS tests, in accordance with the findings of previous studies22. However, such significant correlations disappeared after adjusting for confounders, including renal markers. Thus, it still remains unclear whether inflammatory markers are an independent predictor for cognitive decline, and further large‐scale studies are expected to clarify this.
There were several limitations to the present study. It was a small‐scale hospital‐based study, and cognitive impairment as a result of vascular dementia was indistinguishable from that as a result of Alzheimer's disease, and the effect of this cannot be ignored.
In conclusion, the present study showed a reciprocal relationship between cognitive decline and renal impairment, especially the progression of albuminuria. Thus, early and effective treatment that prevents the development of renal impairment will be important for preserving both renal and cognitive function. Monitoring treatment using renal biomarkers is expected to maintain a good quality of life for elderly diabetic patients.
Acknowledgements
This research was supported by research funds to promote the hospital function from the Japan Labor Health and Welfare Organization. We thank Mr Takahito Kaji for his statistical advice. The authors have no conflicts of interest to declare.
J Diabetes Invest 2014; 5: 597–605
References
- 1.Ott A, Stolk RP, van Harskamp F, et al Diabetes mellitus and the risk of dementia. The Rotterdam Study. Neurology 1999; 53: 1937–1942 [DOI] [PubMed] [Google Scholar]
- 2.Ohara T, Doi Y, Ninomiya T, et al Glucose tolerance status and risk of dementia in the community. The Hisayama Study. Neurology 2011; 77: 1126–1134 [DOI] [PubMed] [Google Scholar]
- 3.Kurella Tamura M, Wadley V, Yaffe K, et al Kidney function and cognitive impairment in US adults: The REGARDS (Reasons for Geographic and Racial Differences in Stroke) Study. Am J Kidney Dis 2008; 52: 227–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray AM, Barzilay JI, Lovato JF, et al Biomarkers of renal function and cognitive impairment in patients with diabetes. Diabetes Care 2011; 34: 1827–1832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurella Tamura M, Muntner P, Wadley V, et al Albuminuria, kidney function, and the incidence of cognitive impairment among adults in the United States. Am J Kidney Dis 2011; 58: 756–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawamura T, Umemura T, Hotta N. Cognitive impairment in diabetic patients: can diabetic control prevent cognitive decline? J Diabetes Invest 2012; 3: 413–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson CS, Hakim AM. Living beyond our physiological means. Small vessel disease of the brain is an expression of a systemic failure in arteriolar function: a unifying hypothesis. Stroke 2009; 40: e322–e330 [DOI] [PubMed] [Google Scholar]
- 8.Murray AM. The brain and the kidney connection. A model of accelerated vascular cognitive impairment. Neurology 2009; 73: 916–917 [DOI] [PubMed] [Google Scholar]
- 9.Mogi M, Horiuchi M. Clinical interaction between brain and kidney in small vessel disease. Cardiol Res Pract 2011; 2011: 306189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wada M, Nagasawa H, Iseki C, et al Cerebral small vessel disease and chronic kidney disease (CKD): results of a cross‐sectional study in community‐based Japanese elderly. J Neurol Sci 2008; 272: 36–42 [DOI] [PubMed] [Google Scholar]
- 11.Bouchi R, Babazono T, Yoshida N, et al Relationship between chronic kidney disease and silent cerebral infarction in patients with Type 2 diabetes. Diabet Med 2010; 27: 538–543 [DOI] [PubMed] [Google Scholar]
- 12.Weiner DE, Bartolomei K, Scott T, et al Albuminuria, cognitive functioning, and white matter hyperintensities in Homebound elders. Am J Kidney Dis 2009; 53: 438–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Bresser J, Reijmer YD, van den Berg E, et al Microvascular deteminants of cognitive decline and brain volume change in elderly patients with type 2 diabetes. Dement Geriatr Cogn Disord 2010; 30: 381–386 [DOI] [PubMed] [Google Scholar]
- 14.Imamine R, Kawamura T, Umemura T, et al Does cerebral small vessel disease predict future decline of cognitive function in well controlled elderly patients with type 2 diabetes? Diabetes Res Clin Pract 2011; 94: 91–99 [DOI] [PubMed] [Google Scholar]
- 15.Umemura T, Kawamura T, Umegaki H, et al Association of chronic kidney disease and cerebral small‐vessel disease with cognitive impairment in elderly patients with type 2 diabetes. Dement Geriatr Cogn Dis Extra 2013; 3: 212–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Navarro‐González JF, Mora‐Fernández C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol 2008; 19: 433–442 [DOI] [PubMed] [Google Scholar]
- 17.Sahakyan K, Klein BEF, Lee KE, et al Inflammatory and endothelial dysfunction markers and proteinuria with type 1 diabetes mellitus. Eur J Endocrinol 2010; 162: 1101–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shikata K, Makino H. Microinflammation in the pathogenesis of diabetic nephropathy. J Diabetes Invest 2013; 4: 142–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Upadhyay A, Larson MG, Guo C‐Y, et al Inflammation, kidney function and albuminuria in the Framingham offspring cohort. Nephrol Dial Transplant 2011; 26: 920–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shankar A, Sun L, Klein BEK, et al Markers of inflammation predict the long‐term risk of developing chronic kidney disease: a population‐based cohort study. Kidney Int 2011; 80: 1231–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki M, Umegaki H, Ieda S, et al Factors associated with cognitive impairment in elderly patients with diabetes mellitus. J Am Geriatr Soc 2006; 54: 558–559 [DOI] [PubMed] [Google Scholar]
- 22.Marioni RE, Strachan MW, Reynolds RM, et al Association between raised inflammatory markers and cognitive decline in elderly people with type 2 diabetes. The Edinburgh Type 2 Diabetes Study. Diabetes 2010; 59: 710–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan ZS, Beiser AS, Vasan RS, et al Inflammatory markers and the risk of Alzheimer disease. The Framingham Study. Neurology 2007; 68: 1902–1908 [DOI] [PubMed] [Google Scholar]
- 24.Hoshi T, Yamagami H, Furukado S, et al Serum inflammatory proteins and frontal lobe dysfunction in patients with cardiovascular risk factors. Eur J Neurol 2010; 17: 1134–1140 [DOI] [PubMed] [Google Scholar]
- 25.Wersching H, Duning T, Lohmann H, et al Serum C‐reactive protein is linked to cerebral microstructural integrity and cognitive function. Neurology 2010; 74: 1022–1029 [DOI] [PubMed] [Google Scholar]
- 26.Tombaugh TN, McIntyre NJ. The mini‐mental state examination. A comprehensive review. J Am Geriatr Soc 1992; 40: 922–935 [DOI] [PubMed] [Google Scholar]
- 27.Mohs RC, Rosen WG, Davis KL. The Alzheimer's disease assessment scale: an instrument for assessing treatment efficacy. Psychopharmacol Bull 1983; 19: 448–450 [PubMed] [Google Scholar]
- 28.Shinagawa F, Kobayashi S, Fujita K, et al Japanese Manual for the Wechsler Adult Intelligence Scale‐Revised. 1990; Nihon‐bunka‐kagaku‐sya. Tokyo; 1990: 115–8 (Japanese).
- 29.Stoop JR. Studies of interference in serial verbal reactions. J Exp Psychol 1935; 18: 643–662 [Google Scholar]
- 30.Matsuo S, Imai E, Horio M, et al Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992 [DOI] [PubMed] [Google Scholar]
- 31.Kashiwagi A, Kasuga M, Araki E, et al International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Invest 2012; 3: 39–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Japan Diabetes Society . Treatment Guide for Diabetes. BUNKODO. Co., Ltd., Tokyo, Japan, 2007; 21–23 [Google Scholar]
- 33.Bruce DG, Davis WA, Casey GP, et al Predictors of cognitive decline in older individuals with diabetes. Diabetes Care 2008; 31: 2103–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacobson AM, Ryan CM, Cleary PA, et al Biomedical risk factors for decreased cognitive functioning in type 1 diabetes: an 18 year follow‐up of diabetes control and complications trial (DCCT) cohort. Diabetologia 2011; 54: 245–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barzilay JI, Gao P, O'Donnell M, et al Albuminuria and decline in cognitive function. The ONTARGET/TRANSCEND Studies. Arch Intern Med 2011; 171: 142–150 [DOI] [PubMed] [Google Scholar]
- 36.Etgen T, Chonchol M, Förstl H, et al Chronic kidney disease and cognitive impairment: a systematic review and meta‐analysis. Am J Nephrol 2012; 35: 474–482 [DOI] [PubMed] [Google Scholar]
- 37.Barzilay JI, Fitzpatrick AL, Luchsinger J, et al Albuminuria and dementia in the elderly: a community study. Am J Kidney Dis 2008; 52: 216–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joosten H, Izaks GJ, Slates JPJ, et al Association of cognitive function with albuminuria and eGFR in the general population. Clin J Am Soc Nephrol 2011; 6: 1400–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barzilay J, Lovato JF, Murray AM, et al Albuminuria and cognitive decline in people with diabetes and normal renal function. Clin J Am Soc Nephrol 2013; 8: 1907–2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jokinen H, Gouw AA, Madureria S, et al Incident lacunes influence cognitive decline. The LADIS study. Neurology 2011; 76: 1872–1878 [DOI] [PubMed] [Google Scholar]
- 41.Wardlaw JM, Sandercock PAG, Dennis MS, et al Is breakdown of the blood–brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke 2003; 34: 806–812 [DOI] [PubMed] [Google Scholar]
- 42.Hoshi T, Kitagawa K, Yamagami H, et al Relations of serum high‐sensitivity C‐reactive protein and interleukin‐6 levels with silent brain infarction. Stroke 2005; 36: 768–772 [DOI] [PubMed] [Google Scholar]
- 43.Kawamura T, Umemura T, Kanai A, et al Soluble adhesion molecules and C‐reactive protein in the progression of silent cerebral infarction in patients with type 2 diabetes mellitus. Metabolism 2006; 55: 461–466 [DOI] [PubMed] [Google Scholar]
- 44.Umemura T, Kawamura T, Umegaki H, et al Endothelial and inflammatory markers in relation to progression of ischaemic cerebral small‐vessel disease and cognitive impairment: a 6‐year longitudinal study in patients with type 2 diabetes mellitus. J Neurol Neurosurg Psychiatry 2011; 82: 1186–1194 [DOI] [PubMed] [Google Scholar]


