Abstract
Background
Papanicolaou (Pap) triage, with high specificity, has been recommended for primary Human papillomavirus (HPV) testing but is flawed by poor sensitivity and cytologist dependence. We evaluated the potential role of microRNA (miRNA) detection in cervical exfoliated cells in HPV-positive women from a clinic-based population.
Methods
Primary HPV testing as well as Pap test were performed on all eligible women. Six miRNAs (miR-424/miR-375/miR-34a/miR-218/miR-92a/miR-93) were detected by RT-qPCR in cervical exfoliated cells. All HPV-positive women underwent colposcopy and further biopsy if indicated. Mann–Whitney U test, the receiver operating characteristic curve, logistic regression, and Pearson’s Chi-square were used to assess data. All tests of statistical significance were two-sided.
Results
A total of 1021 eligible HPV-positive women were enrolled. The expression of miR-424/miR-375/miR-34a/miR-218 in high-grade cervical intraepithelial neoplasia (CIN) and abnormal cytology was statistically significantly lower than that in low-grade CIN and normal cytology, respectively (all P < .05). Compared with the Pap test, both miR-424 and miR-375 detection achieved higher sensitivity (76.0% and 74.9% vs 63.8%, P < .05), higher negative predictive value (NPV) (85.7% and 85.4% vs 79.3%, P < .05), and comparable specificity while identifying CIN2 or worse (CIN2+). Similar results were achieved while identifying CIN3+. Multi-marker panels based on miR-424, miR-375, and miR-218 further improved the performance over any single miRNA test or Pap test.
Conclusion
Single miR-424 or miR-375 detection and miR-424/miR-375/miR-218–based multimarker panels in cervical exfoliated cells show superior performance over Pap triage for high-grade CIN identification in a clinic-based population. Detection of miRNA may provide a new triage option for HPV-positive women.
Introduction
Cervical cancer ranks as the third most common malignancy in women worldwide (1), and about 80% of worldwide incidence occurs in less developed countries (2). Persistent infection of high-risk human papillomavirus (HR-HPV) is a causal factor for the development of cervical cancer (3). The etiologic association of HR-HPV infection with the development of cervical cancer leads to the introduction of HR-HPV DNA testing for cervical cancer screening. Accumulated evidence shows that HPV testing offers a higher sensitivity but a lower specificity than cytology while identifying high-grade cervical intraepithelial neoplasia (CIN) (4,5). Supported by clinical trials (4,6–8), HPV testing has been recommended as a primary screening for cervical cancer in Europe and some less developed areas (9). However, HPV infection is a frequent event, especially in young girls, and most HPV infections are transient, with nearly 90% eliminated spontaneously in two years (10). Thus, HPV testing alone may result in increased psychological burden and unnecessary management, and a proper triage is indispensable for HPV-positive populations. The introduction of cytological screening has successfully reduced the incidence and mortality of cervical cancer, and cytology triage has become a recommended option for HPV-positive women (9). However, cytology still presents disadvantages, mainly because of its limited sensitivity, ranging from 38% to 65% for a singe round test for CIN2+ (CIN grade 2 or worse) identification (5). Furthermore, cytology triage needs well-trained cytologists, who are underrepresented in less developed countries. Therefore, it seems of considerable importance to search out new triage approaches for HPV-positive women.
MicroRNAs (miRNAs), as important regulators of gene expression, are involved in many important intracellular pathways as tumor suppressor genes or oncogenes (11). Dysregulated miRNA expression has been found in many human malignancies (12). Recently, several studies (13–16) focused on miRNAs as biomarkers for cancer diagnosis and obtained promising results. In those studies, most miRNAs were detected in serum or plasma (13), and some in other samples such as urine (14), saliva (15), and gastric juice (16). However, miRNA detection in cervical exfoliated cells has not been reported yet. miRNA dysregulation has been identified in cervical cancer. In a previous study, we identified 14 downregulated miRNAs, including miR-375/miR-424/miR-218 and 17 upregulated miRNAs, including miR-92a/miR-93 in cervical cancer and precancerous tissues, using a microarray platform. Of these, six miRNAs (including miR-375/miR-92a) were further confirmed in invasive cervical cancer (ICC) and CIN2-3 tissues by reverse transcription and quantitative polymerase chain reaction (RT-qPCR) (17). Reduced miR-34a expression was found in HPV-positive cervical tissues (18). The roles of some of these dysregulated miRNAs in cervical carcinogenesis have been confirmed; miR-375, miR-424, and miR-218 participate in cervical carcinogenesis via targeting of Sp1, Chk1, and LAMB3, respectively (19–21), and miR-34a is involved in the HPV E6-p53 pathway (22). Dysregulated expression of miRNAs implies their potential application as biomarkers for cervical cancer screening.
In this study, we chose six of these previously studied dysregulated miRNAs (miR-424/miR-375/miR-34a/miR-218/miR-92a/miR-93) as candidate biomarkers for cervical cancer screening in HPV-positive women. We performed Papanicolaou (Pap) test and miRNA detection in cervical exfoliated cell samples from HPV-positive women and evaluated the performance of miRNA detection for high-grade CIN identification.
Methods
Subject Recruitment and Sample Collection
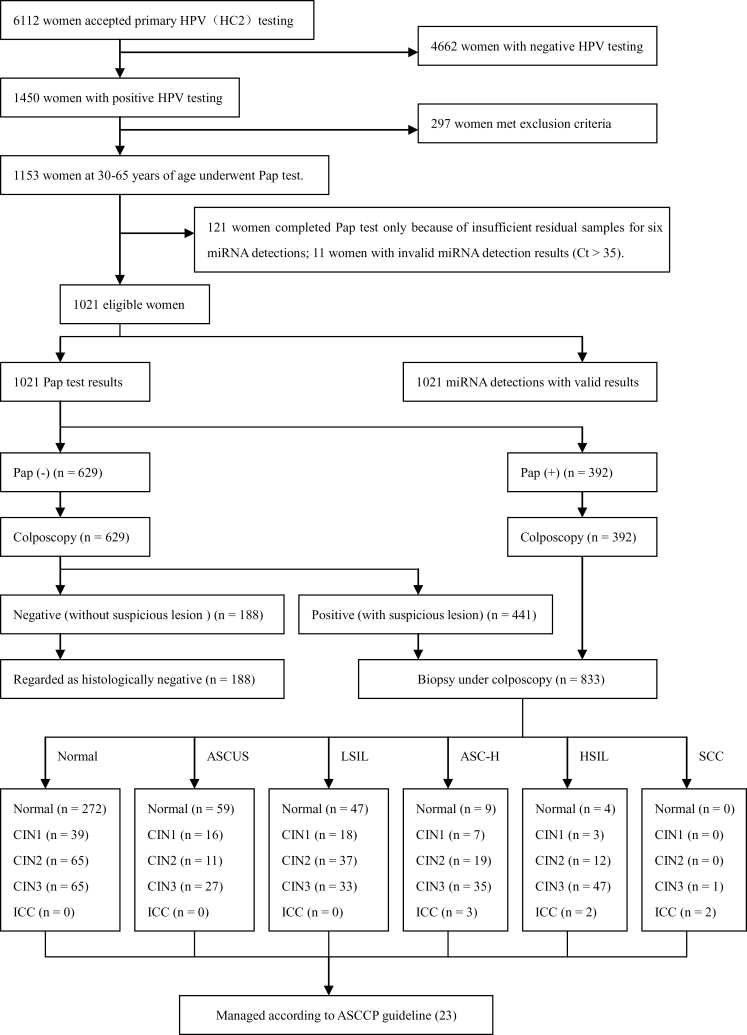
From August 2012 to May 2013, women who visited the gynecologic clinic of our hospital for various gynecologic disorders and underwent primary HPV testing were prospectively recruited. Women were excluded from the study according to the following criteria: 1) previously confirmed CIN, cervical cancer, or other malignancies, 2) previous therapeutic procedure to cervix, 3) aged younger than 30 years or older than 65 years, and 4) pregnancy. Included women with positive HPV test results were enrolled as eligible subjects. All HPV-positive eligible women also underwent Pap tests for triage. Those with normal Pap tests underwent colposcopy alone; if suspicious lesions were detected colposcopically, biopsy was performed. Those with abnormal Pap tests, that is, abnormal squamous cells of uncertain significance (ASCUS) or worse, underwent colposcopically directed multiple biopsies, including random and suspicious lesions, if present. All women with abnormal histological diagnosis were managed according to American Society for Colposcopy and Cervical Pathology (ASCCP) 2006 guidelines (23). The management procedure for all eligible women is shown in Figure 1. In line with current clinical practice, colposcopists were aware of the Pap test and HPV testing results but blinded to any miRNA detection results, and the results of miRNA detection were used for research only and not for triage clinically. MiRNA detection of each eligible woman was performed in residual cervical exfoliated cell samples after Pap test. Informed consent was obtained from all women for the collection of their residual cell samples and from HPV-positive/cytology-negative women for colposcopic inspection. The study was approved by the Ethics Committee of the Hospital and conducted in accordance with the 2008 Declaration of Helsinki.
Figure 1.
Test results and outcomes. At least 1 pg/mL HPV DNA or more were identified as positive for the Digene HC2 HPV testing. Pap (+) denotes positive Pap test result and is defined as ASCUS or worse. Pap (-) denotes negative Pap test result. ASC-H = atypical squamous cells-cannot exclude high-grade squamous intraepithelial lesion; ASCUS = abnormal squamous cells of uncertain significance; CIN = cervical intraepithelial neoplasia; HPV = human papillomavirus; HSIL = high-grade squamous intraepithelial lesion; ICC = invasive cervical cancer; LSIL = low-grade squamous intraepithelial lesion; SCC = squamous cell carcinoma.
HPV Testing and Pap Test
High-risk HPV DNA was detected by Hybrid Capture 2 assay (HC2, Digene, Gaithersburg, MD) according to the manufacturer’s instructions. At least 1 pg/mL HPV DNA or more was required to be identified as positive. The technology of liquid-based cytology (LBC) was used for the Pap test. Thin-layer LBC was processed by a ThinPrep 2000 processor (Cytyc Corporation, Marlborough, MA). The cytological diagnosis was made by cytologists of the hospital according to the Bethesda System (TBS, 2001) (24).
MiRNA Detection
The total RNA containing miRNA of each sample was extracted with Trizol Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Stem-loop real-time RT-qPCR was used for miRNA detection as previously described (17). cDNA was synthesized from 0.5 μg of total RNA in a 10 μL reaction volume with the PrimeScript RT reagent Kit (TaKaRa, Dalian, China), and the reverse transcription (RT) reaction program was as follows: 30min at 16°C, 30min at 42°C, 5min at 85°C, and end at 4°C. qPCR was performed to quantify the expression of target miRNAs using SYBR Premix Ex Taq kit (TaKaRa, Dalian, China) on an ABI 7900HT fast real-time system (Applied Biosystems, Foster City, CA). One microliter of the RT product was added in a total reaction volume of 20 μL, and the reactions were incubated in a 96-well plate at 95°C for 30 seconds, followed by 40 cycles of 95°C for 5 seconds and 60°C for 30 seconds. For normalization, U6 was used as an endogenous control. The relative quantitative method was used and the relative expression level of miRNA was calculated based on the following equation: F = 2-ΔCt,where ΔCt = Ct (miRNA) – Ct (U6). All results of miRNA detection were considered invalid when one or more cycle threshold (Ct) values of six miRNAs were more than 35 in one sample. A higher F value indicated a higher relative expression level. MiRNA detection was blind to Pap test results and histological diagnosis. Primers used in miRNA detection are shown in Supplementary Table 1 (available online).
Histological Diagnosis
Histological diagnosis was made by pathologists of the hospital according to Pathology and Genetics of Tumors of the Breast and Female genital organs (World Health Organization, 2003) (25). If there was a disagreement on histological diagnoses, a discussion was held by the pathologists until a consensus diagnosis was reached. All HPV-positive, cytology-normal, and colposcopy-negative women were regarded as histologically normal on ethical grounds. All reviewers (pathologists) were blinded to miRNA detection results.
Statistical Analysis
SPSS software (version 17.0; SPSS Inc, Chicago, IL) was used in this study. P less than .05 (two-sided) was considered statistically significant. Statistical comparisons between two groups were performed by the nonparametric Mann–Whitney U test. The receiver operating characteristic (ROC) curve and logistic regression analysis were used to assess the performance of miRNA detection in detecting high-grade CIN. The optimal cutoff values of each miRNA were determined by the maximal Youden index (26,27). Two histological cutoffs were used: CIN2+ and CIN3+. For the Pap test, ASCUS+ was used as the cutoff value. Pearson Chi-Square was used to compare performance efficiency between the Pap test and miRNA detection. The 95% confidence intervals (CIs) of proportions were calculated based on the following equation: p ± 1.96 , where n was the case number involved in the calculation of proportion.
Results
As shown in Figure 1, a total of 6112 women underwent primary HPV testing during the period, of which 1450 women presented as HPV positive, but 297 HPV-positive women were excluded by the exclusion criteria. Among the remaining 1153 HPV-positive women, 132 were further excluded, including 121 with deficient residual samples for detection of the six miRNAs and 11 with invalid miRNA detection results (Ct > 35). Among 1021 eligible women who finally completed both Pap test and miRNA detection with valid results, 833 underwent cervical biopsies under colposcopy, among whom 392 presented with abnormal Pap tests (ASCUS+) and 441 presented with normal Pap tests but positive colposcopies. The remaining 188 women with normal Pap tests and negative colposcopies did not undergo biopsies.
Table 1 shows the relative expression levels of six candidate miRNAs in 1021 HPV-positive women. The relative expression levels of miR-218, miR-34a, miR-424, and miR-375, but not miR-92a and miR-93, in CIN2+ and CIN3+ groups were statistically significantly lower than those in CIN1- (CIN1 or better) and CIN2- groups, respectively (all P < .001). Supplementary Figure 1 (available online) shows the data distributions of two representative miRNAs (miR-424/miR-375) by final histology, and their distributions were skewed. Similarly, the relative expression levels of miR-218, miR-34a, miR-424, and miR-375, but not miR-92a and miR-93, in ASCUS+ women were statistically significantly lower than those in women with normal cytology (P < .001 for all except miR-34a, where P = .004).
Table 1.
Comparison of relative expression levels of miRNAs (2-ΔCT) in cervical exfoliated cells among HPV-positive women with different grades of cervical cancer precursors and cytology outcomes*
| Variable | CIN1- (n = 662) Median (IQR) | CIN2+ (n = 359) Median (IQR) | P | CIN2- (n = 806) Median (IQR) | CIN3+ (n = 215) Median (IQR) | P | Pap (-) (n = 629) Median (IQR) | Pap (+) (n = 392) Median (IQR) | P |
|---|---|---|---|---|---|---|---|---|---|
| Age, y | 42.0 (36.8–48.0) | 42.0 (36.0–47.0) | .93 | 42.0 (37.0–48.0) | 41.0 (36.0–46.0) | .22 | 42.0 (36.5–48.0) | 42.0 (36.0–47.0) | .76 |
| miR-424 (×10–5) | 2.571 (1.500–3.938) | 0.651 (0.260–1.339) | <.001 | 2.277 (1.114–3.668) | 0.543 (0.183–1.091) | <.001 | 2.499 (1.187–3.842) | 0.893 (0.397–1.923) | <.001 |
| miR-375 (×10–3) | 1.691 (1.056–3.384) | 0.589 (0.351–0.996) | <.001 | 1.515 (0.829–3.060) | 0.565 (0.365–0.825) | <.001 | 1.503 (0.785–3.123) | 0.787 (0.430–1.866) | <.001 |
| miR-34a (×10–3) | 0.583 (0.287–1.073) | 0.438 (0.147–0.734) | <.001 | 0.556 (0.272–0.985) | 0.432 (0.110–0.816) | <.001 | 0.575 (0.260–1.039) | 0.484 (0.194–0.856) | .004 |
| miR-218 (×10–5) | 3.526 (1.838–6.132) | 1.788 (0.672–4.348) | <.001 | 3.210 (1.541–5.880) | 1.805 (0.608–4.238) | <.001 | 3.379 (1.604–6.235) | 2.117 (0.870–4.663) | <.001 |
| miR-92a (×10–3) | 3.873 (1.971–8.040) | 4.645 (2.055–8.315) | .45 | 4.018 (2.082–7.882) | 4.598 (1.819–8.772) | .80 | 4.227 (2.254–8.341) | 3.693 (1.612–8.066) | .06 |
| miR-93 (×10–3) | 0.828 (0.460–1.824) | 0.982 (0.489–1.816) | .23 | 0.865 (0.460–1.775) | 1.030 (0.506–1.915) | .21 | 0.883 (0.464–1.827) | 0.926 (0.458–1.813) | .89 |
* Pap (+) denotes positive Pap test result and is defined as abnormal squamous cells of uncertain significance or worse; Pap (-) denotes negative Pap test result. All statistical tests were non-parametric Mann–Whitney U test, and were two-sided. ΔCt = Ct of miRNA minus Ct of U6; CIN = cervical intraepithelial neoplasia; CINx- = CIN grade x or better; CINx+ = CIN grade x or worse; HPV = human papillomavirus; IQR = inter-quartile range.
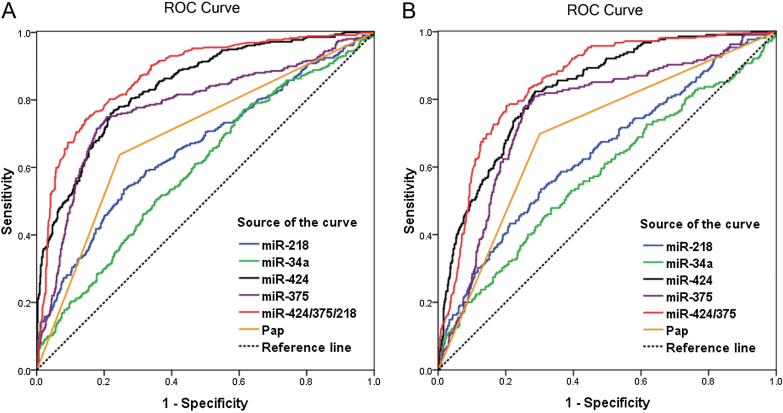
Further, we plotted ROC curves for miR-424, miR-375, miR-34a, and miR-218, as well as Pap test (Figure 2). According to the maximal Youden index, 1.353×10–5, 0.965×10–3, 0.759×10–3, and 1.928×10–5 were identified as the cutoff values of miR-424, miR-375, miR-34a, and miR-218 for high-grade CIN identification, respectively. The results of miRNA detection and Pap tests in women of different histological groups (Normal, CIN1, CIN2, CIN3, and ICC) are shown in Table 2, and the results of miRNA detection in women with different cytology outcomes are shown in Table 3. Table 4 shows the performance parameters of miRNA detection for identifying high-grade CIN. To identify CIN2+, compared with Pap test, miR-424 detection achieved greater area under curve (AUC) (0.840 vs 0.696) with statistically significantly higher sensitivity (76.0% vs 63.8%, P < .001), positive predictive value (PPV) (65.3% vs 58.4%, P = .04), NPV (85.7% vs 79.3%, P = .003), and comparable specificity (78.1% vs 75.4%, P = .24). In addition, miR-375 detection also achieved greater AUC (0.779 vs 0.696) with statistically significantly higher sensitivity (74.9% vs 63.8%, P = .001), PPV (66.3% vs 58.4%, P = .02), NPV (85.4% vs 79.3%, P = .01), and comparable specificity (79.3% vs 75.4%, P = .09). To identify CIN3+, both miR-424 and miR-375 detection also showed advantages over Pap tests with greater AUCs (0.828 and 0.760 vs 0.699, respectively) and significant higher sensitivity (82.3% vs 69.8, P = .002 and 80.9% vs 69.8%, P = 0.01, respectively), NPV (93.7% vs 89.7%, P = .01 and 93.3% vs 89.7%, P = .02, respectively), and comparable specificity and PPV (P > .05). Further, logistic regression analysis identified two multimarker panels, miR-424/375/218 and miR-424/375 for CIN2+ and CIN3+ identification, respectively. Both panels achieved greater AUCs than the Pap test and any single miRNA detection (Figure 2), with statistically significantly higher sensitivity, specificity, PPV, and NPV than the Pap test, and statistically significantly higher specificity and PPV, similar sensitivity and NPV, compared with single miR-424 or miR-375 detection (Table 4).
Figure 2.
The receiver operating characteristic (ROC) curves of miRNA detection and Pap test in identifying high-grade cervical intraepithelial neoplasia (CIN). A) miRNA detection and Pap test to identify CIN2+ (CIN grade 2 or worse). B) miRNA detection and Pap test to identify CIN3+.
Table 2.
The results of miRNA detection and Papanicolaou (Pap) test in different cervical lesions*
| Subgroup | Result | miR-424, No. (%) | miR-375, No. (%) | miR-218, No. (%) | miR-34a, No. (%) | Pap test, No.(%) |
|---|---|---|---|---|---|---|
| Normal† | + | 114 (19.7) | 112 (19.3) | 140 (24.2) | 358 (61.8) | 119 (20.6) |
| (n = 579) | - | 465 (80.3) | 467 (80.7) | 439 (75.8) | 221 (38.2) | 460 (79.4) |
| CIN1 | + | 31 (37.3) | 25 (30.1) | 31 (37.3) | 48 (57.8) | 39 (47.0) |
| (n = 83) | - | 52 (62.7) | 58 (69.9) | 52 (62.7) | 35 (42.2) | 44 (53.0) |
| CIN2 | + | 96 (66.7) | 94 (65.3) | 77 (53.5) | 117 (81.3) | 79 (54.9) |
| (n = 144) | - | 48 (33.3) | 50 (34.7) | 67 (46.5) | 27 (18.7) | 65 (45.1) |
| CIN3 | + | 172 (82.7) | 169 (81.3) | 109 (52.4) | 155 (74.5) | 143 (68.8) |
| (n = 208) | - | 36 (17.3) | 39 (18.7) | 99 (47.6) | 53 (25.5) | 65 (31.2) |
| ICC | + | 5 (71.4) | 5 (71.4) | 6 (85.7) | 4 (57.1) | 7 (100.0) |
| (n = 7) | - | 2 (28.6) | 2 (28.6) | 1 (14.3) | 3 (42.9) | 0 (0.0) |
* Cutoffs of miR-424, miR-375, miR-34a, miR-218, and Pap test were 1.353E-5, 0.965E-3, 0.759E-3, 1.928E-05, and abnormal squamous cells of uncertain significance (ASCUS) respectively; “+” (positive) is defined as the miRNA-relative expression level that is lower than cutoff and the result of Pap test that was ASCUS or worse; “-” (negative) is defined as the miRNA relative expression level that is equal to or higher than cutoff and the negative result of Pap test. CIN = cervical intraepithelial neoplasia; ICC = invasive cervical cancer;
† Including 188 normal Pap tests and colposcopy-negative women who did not undergo cervical biopsy.
Table 3.
The results of miRNA detection in women with different cytology outcomes*
| Subgroup | Result | miR-424, No. (%) | miR-375, No. (%) | miR-218, No. (%) | miR-34a, No. (%) |
|---|---|---|---|---|---|
| Normal | + | 174 (27.7) | 187 (29.7) | 179 (28.5) | 399 (63.4) |
| (n = 629) | - | 455 (72.3) | 442 (72.3) | 450 (71.5) | 230 (36.6) |
| ASCUS | + | 41 (36.3) | 46 (40.7) | 44 (38.9) | 71 (62.8) |
| (n = 113) | - | 72 (63.7) | 67 (59.3) | 69 (61.1) | 42 (37.2) |
| LSIL | + | 95 (70.4) | 71 (52.6) | 62 (45.9) | 102 (75.6) |
| (n = 135) | - | 40 (29.6) | 64 (47.4) | 73 (54.1) | 33 (24.4) |
| ASC-H | + | 57 (78.1) | 48 (65.8) | 39 (53.4) | 59 (80.8) |
| (n = 73) | - | 16 (21.9) | 25 (34.2) | 34 (46.6) | 14 (19.2) |
| HSIL and SCC | + | 51 (71.8) | 54 (76.1) | 38 (53.5) | 52 (73.2) |
| (n = 71) | - | 20 (28.2) | 17 (23.9) | 33 (46.5) | 19 (26.8) |
* “+” is defined as the positive miRNA detection result that is less than cutoff; “-” is defined as the negative miRNA detection result that is equal to or higher than cutoff; Cutoffs of miR-424, miR-375, miR-34a, and miR-218 were 1.353E-5, 0.965E-3, 0.759E-3, and 1.928E-05, respectively. ASC-H = atypical squamous cells-cannot exclude high-grade squamous intraepithelial lesion; ASCUS = abnormal squamous cells of uncertain significance; HSIL = high-grade squamous intraepithelial lesion; LSIL = low-grade squamous intraepithelial lesion; SCC = squamous cell carcinoma.
Table 4.
Comparison of performance efficiency between miRNA detection and Papanicolaou (Pap) test for identifying CIN2+ and CIN3+*
| Test | AUC (95% CI) | Sensitivity | Specificity | Positive predictive value | Negative predictive value | ||||
|---|---|---|---|---|---|---|---|---|---|
| % (95% CI) | P | % (95% CI) | P | % (95% CI) | P | % (95% CI) | P | ||
| To identify CIN2+ | |||||||||
| miR-424 | 0.840 (0.815 to 0.865) | 76.0 (71.6 to 80.5) | <.001 | 78.1 (74.9 to 81.2) | .24 | 65.3 (60.7 to 69.9) | .04 | 85.7 (82.9 to 88.5) | .003 |
| miR-375 | 0.779 (0.746 to 0.811) | 74.9 (70.4 to 79.4) | .001 | 79.3 (76.2 to 82.4) | .09 | 66.3 (61.7 to 70.9) | .02 | 85.4 (82.6 to 88.2) | .01 |
| miR-34a | 0.598 (0.561 to 0.634) | 77.2 (72.8 to 81.5) | <.001 | 38.7 (35.0 to 42.2) | <.001 | 40.6 (36.9 to 44.2) | <.001 | 75.7 (71.2 to 80.3) | .20 |
| miR-218 | 0.659 (0.623 to 0.695) | 53.5 (48.3 to 58.6) | .01 | 74.2 (70.8 to 77.5) | .61 | 52.9 (47.8 to 58.0) | .13 | 74.6 (71.3 to 77.9) | .05 |
| miR-424/375/218† | 0.874 (0.852 to 0.897) | 74.4 (69.9 to 78.9) | .002 | 85.3 (82.7 to 88.0) | <.001 | 73.4 (68.8 to 77.9) | <.001 | 86.0 (83.3 to 88.7) | .002 |
| Pap test | 0.696 (0.661 to 0.731) | 63.8 (58.8 to 68.8) | 75.4 (72.1 to 78.7) | 58.4 (53.5 to 63.3) | 79.3 (76.2 to 82.5) | ||||
| To identify CIN3+ | |||||||||
| miR-424 | 0.828 (0.799 to 0.858) | 82.3 (77.2 to 87.4) | .002 | 70.1 (66.9 to 73.3) | .96 | 42.3 (37.6 to 47.1) | .24 | 93.7 (91.8 to 95.6) | .01 |
| miR-375 | 0.760 (0.723 to 0.797) | 80.9 (75.7 to 86.2) | .01 | 71.2 (68.1 to 74.3) | .58 | 42.9 (38.0 to 47.7) | .19 | 93.3 (91.4 to 95.3) | .02 |
| miR-34a | 0.578 (0.533 to 0.623) | 74.0 (68.1 to 79.8) | .33 | 35.0 (31.7 to 38.3) | <.001 | 23.3 (20.1 to 26.4) | <.001 | 83.4 (79.5 to 87.4) | .01 |
| miR-218 | 0.641 (0.598 to 0.684) | 53.5 (46.8 to 60.2) | <.001 | 69.2 (66.0 to 72.4) | .75 | 31.7 (26.9 to 36.5) | .06 | 84.8 (82.1 to 87.5) | .01 |
| miR-424/375† | 0.853 (0.826 to 0.879) | 78.1 (72.6 to 83.7) | .048 | 79.8 (77.0 to 82.5) | <.001 | 50.8 (45.4 to 56.1) | .001 | 93.2 (91.3 to 95.1) | .022 |
| Pap test | 0.699 (0.659 to 0.739) | 69.8 (63.6 to 75.9) | 70.0 (66.8 to 73.1) | 38.3 (33.5 to 43.1) | 89.7 (87.3 to 92.0) | ||||
* AUC = area under curve; CI = confidence interval; CINx+ = cervical intraepithelial neoplasia grade x or worse.
† Multimarker panels are determined by logistic regression analysis. Cutoffs of miR-424, miR-375, miR-34a, miR-218, and Pap test were 1.353E-5, 0.965E-3, 0.759E-3, 1.928E-05, and abnormal squamous cells of uncertain significance (ASCUS) respectively. Results from Pearson Chi-square test. All statistical tests were two-sided.
Discussion
Cervical exfoliated cells as ideal samples have been widely used in cervical cancer screening, both for HPV testing and the Pap test. To our knowledge, this is the first report on miRNA detection in cervical exfoliated cells. Here, among 1021 HPV-positive women, we found that the expression of miR-424/miR-375/miR-34a/miR-218 in cervical exfoliated cells was statistically significantly lower in women with high-grade CIN and abnormal cytology (ASCUS+). Our and other previous studies have reported that the suppressed expression of the above four miRNAs plays roles in cervical cancer carcinogenesis or progression. Thus, our results here suggest that those miRNAs in cervical exfoliated cells could be utilized as candidate biomarkers in cervical cancer screening.
HPV testing offers advantages such as higher sensitivity, prolonged screening interval (4), and independence of cytologists and has been recommended as the primary cervical cancer screening for women aged 30 years or older in many countries (9). Because of a prevalent lack of well-trained cytologists, HPV primary screening is especially appropriate in less developed countries, including China. However, HPV testing also presents disadvantages, especially poor positive predictive value and therefore overreferral to colposcopy. Cytology triage is a recommended option for HPV-positive women (9). Indeed, disadvantages of HPV testing can be, to some extent, overcome by cytology triage. The results from four randomized controlled trails showed that cytology triage could avoid substantial increases in expenditures and overmanagement for women older than 30–35 years in HPV screening (4,6,8,28).
The Pap test is flawed by poor interobserver agreement, and even within a single cytology laboratory, the interpretations may differ substantially among personnel (29). The sensitivity of the Pap test is also quite poor and varies greatly among published studies. In six fair- and good-quality designed studies, the sensitivity of a single round Pap test ranged from 38% to 65% and 46% to 50% for CIN2+ and CIN3+ detection, respectively (5). Even using LBC instead of conventional Pap smear cannot improve the sensitivity or specificity for high-grade CIN detection (30). Additionally, in the situation of triage for HPV-positive women, HPV infection induces morphologic changes in cervical epithelial cells and consequently affects Pap smear interpretation by recognizing more histologically normal or low-grade CIN as cytologically abnormal (31) and therefore results in more unnecessary referral to colposcopy. In our study, we found that the specificity of Pap test triage was 75.4% and 70.0%, respectively, for CIN2+ and CIN3+ identification, similar to a recent report in which the specificity of Pap triage in HPV-positive women was 76% and 71.7%, respectively (32).
MiRNAs obtained from serum and other samples have been used as biomarkers for cancer diagnosis. In this study, we found for the first time that, compared with the Pap test, both miR-424 and miR-375 detection in cervical exfoliated cells gains greater AUC and statistically significantly higher sensitivity and NPV, without the cost of decreased specificity and PPV, while identifying high-grade CIN. Our results suggest better performance of miR-424 or miR-375 detection triage than that of the Pap test in high-grade CIN identification. The higher sensitivity and NPV of miR-424 and miR-375 detection suggest that miRNA triage possesses stronger effectiveness and longer retesting intervals, which are very appealing for triage situations. To explore the potential complementary roles among miRNA markers, logistic regression analysis was used, and two multimarker panels (miR-424/375/218 for CIN2+ and miR-424/375 for CIN3+) were identified, both of which improved the performance parameters further and were statistically significantly better than any single miRNA test or Pap test.
This study also had some limitations. Although the results of this study are promising compared with Pap triage, cytology is still one of the most specific triage methods currently available and has been recommended as a triage for HPV-positive women, based on many trials. Our findings were derived from a clinic-based population instead of a general population, and it may possess biases such as higher HPV-positive proportion in recruited women and higher abnormal cytology and histology proportion in HPV-positive women. Since test performance might be population-dependent, the thresholds (cutoffs) should be extended from a clinic-based population to a general population with caution. Additionally, we did not observe statistically significant upregulation of miR-92a or miR-93 in high-grade CIN and abnormal cytology. Despite substantial downregulation of miR-34a and miR-218 in high-grade CIN and abnormal cytology, neither gained promising performance in triage for HPV-positive women. Previous studies indicated that oncogenic HPV E6 was able to downregulate miR-34a and miR-218 and upregulate miR-93 via direct or indirect p53 degradation pathway (21,22,33,34). Thus, it is possible that the altered expression of miRNAs closely related to HPV infection, such as miR-34a, miR-218, and miR-93, has already occurred before morphologic change, and therefore the performance in triage is weakened. In addition, since both underregulated miR-424 and miR-375 produce negative readouts in identifying high-grade CIN, a standard procedure for sample collection is needed to reduce the variation of miRNA results among individuals, because of the admixture of normal cells in various proportions.
In conclusion, our findings from a clinic-based population demonstrated that detection of both miR-424 and miR-375 in primary HPV-positive women offers statistically significantly higher sensitivity and NPV than the Pap test while detecting high-grade CIN, without the cost of decreased specificity and PPV. Multimarker panels on the basis of miR-424, miR-375, and miR-218 achieved statistically significantly better performance over any single miRNA test or the Pap test. Further research in a general population in a testing set is needed to validate the findings. Our study suggests a potential application of miRNA detection in cervical exfoliated cells, especially in areas where well-trained cytologists are lacking. In addition, miRNA detection may provide an additional option for triage of HPV-positive women.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers 81172475 and 81272861) and Special Funds for Scientific Research of Zhejiang University (grant number 2012-RH-BF-03).
QF Tian, Yang Li, and FF Wang had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. X Xie, WG Lu, QF Tian, and XY Wan organized the design of the study. Yang Li, Ying Li, FF Wang, and F Ye designed the primers. QF Tian, Yang Li, Ying Li, FF Wang, YM Shen, JF Xu, and YX Chen did sample collection and miRNA detection. FF Wang, XY Wang, and XD Cheng acquired and interpreted the data. QF Tian and FF Wang did statistical analysis. QF Tian, XD Cheng, YX Chen, and XY Wang drafted the manuscript. X Xie, WG Lu, and XY Wan revised the manuscript.
We thank pathologists Bingjian Lu and Xiaoduan Chen of the Pathology Department of our hospital for their cytological and histological diagnosis for all enrolled women; statistician Professor Kun Chen of Public Health School, Zhejiang University, for his help in statistical analysis of the manuscript, all clinicians in our hospital for their help in the collection of samples, and all the grants supporting our study.
References
- 1. Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90 [DOI] [PubMed] [Google Scholar]
- 2. Arbyn M, Castellsagué X, de Sanjosé S, et al. Worldwide burden of cervical cancer in 2008. Ann Oncol. 2011;22(12):2675–2686 [DOI] [PubMed] [Google Scholar]
- 3. Schiffman M, Castle PE, Jeronimo J, et al. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890–907 [DOI] [PubMed] [Google Scholar]
- 4. Naucler P, Ryd W, Törnberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med. 2007;357(16):1589–1597 [DOI] [PubMed] [Google Scholar]
- 5. Whitlock EP, Vesco KK, Eder M, et al. Liquid-based cytology and human papillomavirus testing to screen for cervical cancer: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155(10):687–97, W214–W215 [DOI] [PubMed] [Google Scholar]
- 6. Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol. 2009;10(7):672–682 [DOI] [PubMed] [Google Scholar]
- 7. Mayrand MH, Duarte-Franco E, Rodrigues I, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357(16):1579–1588 [DOI] [PubMed] [Google Scholar]
- 8. Ronco G, Giorgi-Rossi P, Carozzi F, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11(3):249–257 [DOI] [PubMed] [Google Scholar]
- 9. Franceschi S, Denny L, Irwin KL, et al. EUROGIN 2010 roadmap on cervical cancer prevention. Int J Cancer. 2011;128(12):2765–2774 [DOI] [PubMed] [Google Scholar]
- 10. de Sanjosé S, Diaz M, Castellsagué X, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007;7(7):453–459 [DOI] [PubMed] [Google Scholar]
- 11. Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11(9):597–610 [DOI] [PubMed] [Google Scholar]
- 12. Pencheva N, Tavazoie SF. Control of metastatic progression by microRNA regulatory networks. Nat Cell Biol. 2013;15(6):546–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA. 2008;105(30):10513–10518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Puerta-Gil P, Garcia-Baquero R, Jia AY, et al. miR-143, miR-222, and miR-452 Are Useful as Tumor Stratification and Noninvasive Diagnostic Biomarkers for Bladder Cancer. Am J Pathol. 2012;180(5):1808–1815 [DOI] [PubMed] [Google Scholar]
- 15. Xie ZJ, Chen G, Zhang XC, et al. Salivary MicroRNAs as Promising Biomarkers for Detection of Esophageal Cancer. Plos One. 2013;8(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cui L, Zhang XJ, Ye GL, et al. Gastric Juice MicroRNAs as Potential Biomarkers for the Screening of Gastric Cancer. Cancer. 2013;119(9):1618–1626 [DOI] [PubMed] [Google Scholar]
- 17. Li Y, Wang FF, Xu JF, et al. Progressive miRNA expression profiles in cervical carcinogenesis and identification of HPV-related target genes for miR-29. J Pathol. 2011;224(4):484–495 [DOI] [PubMed] [Google Scholar]
- 18. Li B, Hu Y, Ye F, et al. Reduced miR-34a expression in normal cervical tissues and cervical lesions with high-risk human papillomavirus infection. Int J Gynecol Cancer. 2010;20(4):597–604 [DOI] [PubMed] [Google Scholar]
- 19. Wang FF, Li Y, Zhou JS, et al. miR-375 Is Down-Regulated in Squamous Cervical Cancer and Inhibits Cell Migration and Invasion via Targeting Transcription Factor SP1. Am J Pathol. 2011;179(5):2580–2588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xu J, Li Y, Wang F, et al. Suppressed miR-424 expression via upregulation of target gene Chk1 contributes to the progression of cervical cancer. Oncogene. 2013;32(8):976–987 [DOI] [PubMed] [Google Scholar]
- 21. Martinez I, Gardiner AS, Board KF, et al. Human papillomavirus type 16 reduces the expression of microRNA-218 in cervical carcinoma cells. Oncogene. 2008;27(18):2575–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang X, Meyers C, Guo M, et al. Upregulation of p18Ink4c expression by oncogenic HPV E6 via p53-miR-34a pathway. Int J Cancer. 2011;129(6):1362–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wright TC, Jr, Massad LS, Dunton CJ, et al. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197(4):346–355 [DOI] [PubMed] [Google Scholar]
- 24. Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002;287(16):2114–2119 [DOI] [PubMed] [Google Scholar]
- 25. Tavassoli FA, Devilee P. Pathology and genetics of tumours of the breast and female genital organs. Lyon, France: IARC Press; 2003 [Google Scholar]
- 26. Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–35 [DOI] [PubMed] [Google Scholar]
- 27. Schisterman EF, Perkins NJ, Liu A, et al. Optimal cut-point and its corresponding youden index to discriminate individuals using pooled blood samples. Epidemiology. 2005;16(1):73–81 [DOI] [PubMed] [Google Scholar]
- 28. Rijkaart DC, Berkhof J, Rozendaal L, et al. Human papillomavirus testing for the detection of high-grade cervical intraepithelial neoplasia and cancer: final results of the POBASCAM randomised controlled trial. Lancet Oncol. 2012;13(1):78–88 [DOI] [PubMed] [Google Scholar]
- 29. Condel JL, Mahood LK, Grzybicki DM, et al. Papanicolaou tests diagnosed as atypical by a cytotechnologist and downgraded to benign by a pathologist - A measure of laboratory quality. Am J Clin Pathol. 2002;117(4):534–540 [DOI] [PubMed] [Google Scholar]
- 30. Siebers AG, Klinkhamer PJ, Grefte JM, et al. Comparison of Liquid-Based Cytology With Conventional Cytology for Detection of Cervical Cancer Precursors: A Randomized Controlled Trial. JAMA. 2009;302(16):1757–1764 [DOI] [PubMed] [Google Scholar]
- 31. Wright TC, Jr, Schiffman M. Adding a test for human papillomavirus DNA to cervical cancer screening. N Engl J Med. 2003;348(6):489–490 [DOI] [PubMed] [Google Scholar]
- 32. Zappacosta R, Caraceni D, Ciccocioppo L, et al. Implementing specificity of HPV-DNA primary screening in a successful organised cervical cancer prevention programme. Gynecol Oncol. 2013;128(3):427–432 [DOI] [PubMed] [Google Scholar]
- 33. Brosh R, Shalgi R, Liran A, et al. p53-Repressed miRNAs are involved with E2F in a feed-forward loop promoting proliferation. Mol Syst Biol. 2008;4:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. He L, He X, Lim LP, et al. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447(7148):1130–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]