Abstract
BACKGROUND:
The underlying mechanisms of idiopathic pulmonary fibrosis (IPF) are unknown. This progressive disease has high mortality rates, and current models for prediction of mortality have limited value in identifying which patients will progress. We previously showed that the glycoprotein fibulin-1 is involved in enhanced proliferation and wound repair by mesenchymal cells and, thus, may contribute to lung fibrosis in IPF.
METHODS:
Serum, lung tissue, and lung function values were obtained from four independent locations (Sydney, NSW, and Perth, WA, Australia; San Francisco, CA; and Modena, Italy). Patients with IPF were followed for a minimum of 1 year and progression was defined as a significant decline in lung function or death. Primary parenchymal lung fibroblasts of 15 patients with and without IPF were cultured under nonstimulatory conditions. Fibulin-1 levels in serum, and secreted or deposited by fibroblasts, were measured by western blot and in lung tissue by immunohistochemistry.
RESULTS:
Serum fibulin-1 levels were increased in patients with IPF compared with subjects without lung disease (P = .006). Furthermore, tissue fibulin-1 levels were increased in patients with IPF (P = .02) and correlated negatively with lung function (r = −0.9, P < .05). Primary parenchymal fibroblasts from patients with IPF produced more fibulin-1 than those from subjects without IPF (P < .05). Finally, serum fibulin-1 levels at first blood draw predicted disease progression in IPF within 1 year (area under the curve , 0.71; 95% CI, 0.57-0.86; P = .012).
CONCLUSIONS:
Fibulin-1 is a novel potential biomarker for disease progression in IPF and raises the possibility that it could be used as a target for the development of new treatments.
Idiopathic pulmonary fibrosis (IPF) is the most common of the idiopathic interstitial lung diseases (ILDs) and has the highest mortality.1 Forty-four percent of all patients with IPF are expected to die within 5 years, compared with 33% of patients with ILD-associated connective tissue disease and 2% of patients with pulmonary sarcoidosis, two other ILDs.1
Treatments for IPF have been largely unsuccessful,2,3 mainly because the underlying mechanisms of IPF are unresolved and there is no biomarker available to pinpoint individuals with the risk of accelerated disease progression. Biomarkers in IPF reflecting states of active fibrogenesis in the lung are needed to indicate those patients who will rapidly decline, so as to prioritize them for intensive management care and lung transplantation.
It is likely that common pathways driving fibrogenesis exist in many diseases with a fibrotic component.4 The composition of the extracellular matrix (ECM) is an important and unifying factor in the pathophysiology of fibrosis, including lung fibrosis.5 A number of ECM proteins also circulate in the blood. However, the utility of bloodborne ECM proteins as serum biomarkers in IPF has not been well investigated.6,7 This reflects our lack of knowledge as to which components of the ECM contribute particularly to the disease pathology of pulmonary fibrogenesis and progression of the disease.
The glycoprotein fibulin-1, which is found in both the ECM and the blood, is necessary for embryonic morphogenesis8 and is essential for alveolar septa formation.9 It is widely accepted in the literature that there are similarities between the development of fibrosis and embryonic morphogenesis,10 concepts not limited to the lung.11
Fibulin-1 is produced by lung fibroblasts12 and has been shown to play a role in the pathophysiology of patients with asthma,13 a disease characterized in part by airway fibrosis.14 We reasoned that dysregulated fibulin-1 expression may be involved in lung diseases with more extensive fibrosis as mechanisms of fibrogenesis are likely to be shared.15
This study explored the role of fibulin-1 in disease pathogenesis and progression of IPF. To this aim, we examined serum and tissue fibulin-1 levels in patients with IPF and related it to lung function. We measured fibulin-1 production by lung fibroblasts derived from patients with IPF and from subjects without lung fibrosis. Lastly, we investigated whether serum fibulin-1 could serve as a biomarker and assessed its prognostic utility in several cohorts of patients with IPF.
Materials and Methods
This study contained three components. First we measured serum and lung tissue levels of fibulin-1 in patients with IPF and from individuals without lung disease. Next, we used fibroblast cultures to assess fibulin-1 production. Finally, we explored the utility of serum fibulin-1 as a biomarker of disease progression.
Study Approvals
This study was approved by the ethics committees of each of the six institutions involved in three countries; informed, written consent was obtained from all participants or their next of kin. Detailed ethics information can be found in e-Appendix 1 (2.8MB, pdf) .
Fibulin-1 Levels in Serum
Serum was collected from three independent populations in Sydney, NSW, Australia; San Francisco, CA; and Modena, Italy, and included 72 patients with IPF and 17 individuals without lung disease (Table 1). A diagnosis of IPF was determined by multidisciplinary review as recommended by current guidelines.16 Detailed characteristics of the three cohorts of patients with IPF are found in e-Table 1 (2.8MB, pdf) . Characteristics of 79 additional patients with ILD are found in e-Table 2 (2.8MB, pdf) . Diagnoses included sarcoidosis (n = 12), hypersensitivity pneumonitis (n = 32), and “other” ILDs defined as connective tissue disease-related ILD (n = 26), nonspecific interstitial pneumonia (n = 4), lymphangioleiomyomatosis (n = 4), and drug-induced ILD (n = 1). Fibulin-1 levels were measured using densitometric analysis of western blots compared with a placental fibulin-1 positive control.
TABLE 1 ] .
Characteristics of Patients With IPF Used for Serum Analysis
| Characteristics | All IPF (N = 72) | Stable (n = 21) | Progressed (n = 27) | P Value |
| Mean (SD) | ||||
| Age, y | 68 (9) | 65 (11) | 70 (9) | nsa |
| FEV1, % predicted | 79 (20) | 79 (19) | 76 (20) | nsa |
| FVC, % predicted | 74 (20) | 78 (18) | 68 (21) | nsa |
| Dlco, % predicted | 41 (16) | 48 (18) | 33 (14) | .012a |
| CPI, units | 52 (13) | 44 (14) | 58 (10) | .002a |
| TLC, % predicted | 67 (13) | 75 (12) | 63 (12) | .027a |
| Serum fibulin-1, units | 2.04 (1.05) | 1.59 (0.83) | 2.34 (1.18) | .013a |
| No. (%) | ||||
| Male sex | 41 (57) | 10 (48) | 15 (56) | nsb |
| History of smoking | 45 (63) | 14 (74) | 19 (70) | nsb |
There were a total of 72 patients with IPF and of those 48 had follow-up information available. Continuous data of patients with IPF that either remained stable or progressed in the year following blood draw were compared by the Kruskal-Wallis test, and categorical data were compared with the Pearson χ2 test. CPI = composite physiologic index; Dlco = diffusing capacity of the lung for carbon monoxide; IPF = idiopathic pulmonary fibrosis; ns = nonsignificant; TLC = total lung capacity.
P value for Kruskal-Wallis test.
P value for Pearson χ2 test.
Fibulin-1 Levels in Tissue
Lung parenchymal tissue was collected from four independent cohorts: Sydney, NSW, and Perth, WA, Australia; San Francisco, CA; and Modena, Italy. Lung function was measured within 30 days of tissue sampling. Characteristics of patients used for analysis of formalin-fixed lung tissue are found in Table 2, while those for patients used for whole-lung lysate analysis were not available. Fibulin-1 levels were measured by quantification of immunohistochemical staining (e-Fig 1 (2.8MB, pdf) ) or western blot, respectively. Measurement of total collagen by quantification of Masson trichrome staining was used as a positive control for fibrosis in tissue sections.
TABLE 2 ] .
Characteristics of Patients Used for Lung Tissue Analysis
| Characteristics | Nondiseased Control (n = 5) | IPF (n = 20) | P Value |
| Mean (SD) | |||
| Age, y | 38 (14) | 59 (8) | .03a |
| FEV1, % predicted | n/a | 77 (21) | n/aa |
| FVC, % predicted | n/a | 74 (20) | n/aa |
| Dlco, % predicted | n/a | 42 (20) | n/aa |
| CPI, units | n/a | 52 (16) | n/aa |
| TLC, % predicted | n/a | 81 (18) | n/aa |
| Count (%) | |||
| Male | 4 (80) | 14 (70) | .24b |
Continuous data were compared by unpaired t tests, and categorical data by Fisher exact test. n/a = not applicable. See Table 1 legend for expansion of other abbreviations.
P value for unpaired t test.
P value for Fisher exact test.
Fibulin-1 Levels in Parenchymal Fibroblasts
Human distal parenchymal fibroblasts were isolated from lung tissue obtained from patients with IPF (n = 8) or pathologist-identified macroscopically nondiseased tissue from age-matched and sex-matched donors (n = 7) undergoing resection for either thoracotomy or transplantation. Demographic information for people from whom fibroblasts were derived is found in e-Table 3 (2.8MB, pdf) . Lung function measurements and smoking history were not available. Fibulin-1 levels were measured by western blot.
Serum Fibulin-1 as a Biomarker of Disease Progression
Patients with IPF were followed up for a minimum of 1 year (365 ± 1 day) after blood draw. A progression event was defined as any of the following occurring within the first 1-year follow-up period: ≥ 10% relative fall in % predicted FVC, ≥ 15% relative fall in % predicted diffusing capacity of the lung for carbon monoxide (Dlco), or death, as previously published.17
Statistics
Statistical analysis was performed using SPSS (Version 21) (IBM). The coefficient of repeatability and intraclass correlation coefficient were calculated.18 Distributions of serum, parenchyma, and fibroblast levels of fibulin-1, and lung function parameters were tested for normality. Receiver-operator characteristic (ROC) curves were used to model the utility of serum fibulin-1 as a marker of disease progression. Cox regression analysis was used to model the impact of serum fibulin-1 levels on predicting progression. Kaplan-Meier curves were used to model time-to-progression event. Correlations between fibulin-1 and lung function parameters in patients with IPF were performed using Pearson product-moment tests. Between-group differences were assessed by unpaired t, Kruskal-Wallis, Fisher exact, χ2, and log-rank tests, or analysis of covariance where appropriate. A P value ≤ .05 was considered significant. Additional methods can be found in e-Appendix 1 (2.8MB, pdf) .
Results
Serum Fibulin-1 Levels Are Increased in Patients With IPF
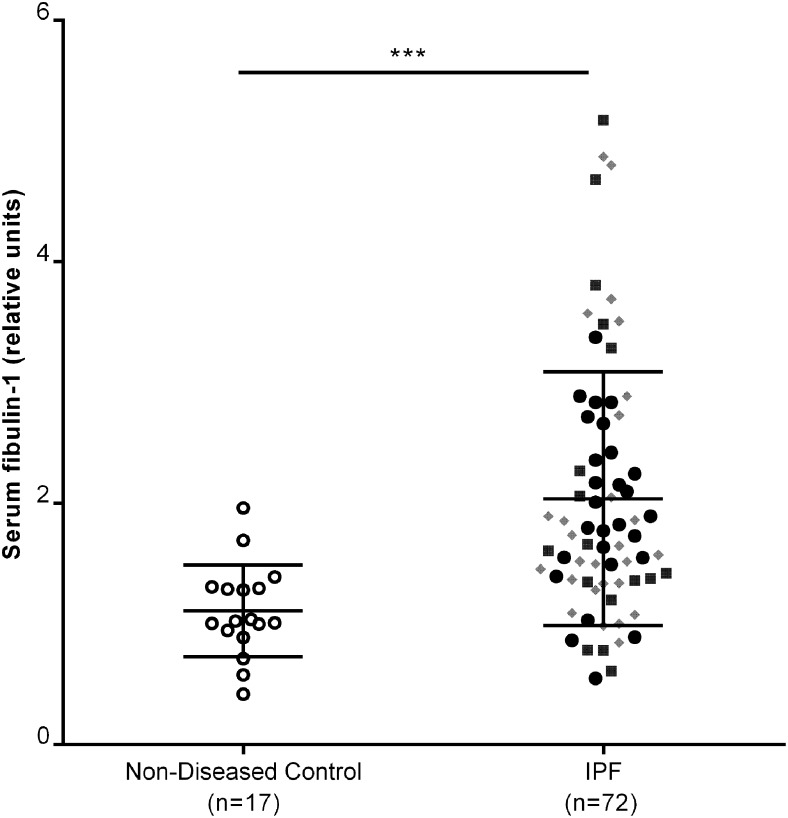
Serum fibulin-1 levels were significantly higher in patients with IPF compared with subjects without lung disease after adjustments for potential confounding variables: age, sex, and smoking history (Fig 1, e-Table 4 (2.8MB, pdf) ). The serum fibulin-1 levels were comparable in the three independent populations of patients with IPF (e-Table 1 (2.8MB, pdf) ). Serum fibulin-1 levels were also increased in patients with IPF compared with patients with sarcoidosis, and patients with other ILDs, but there was no difference compared with patients with hypersensitivity pneumonitis (e-Fig 2 (2.8MB, pdf) , e-Table 4 (2.8MB, pdf) ). Serum fibulin-1 levels did not correlate with lung function variables in patients with only IPF (e-Table 5 (2.8MB, pdf) ) but did correlate with lung function when all ILDs were included in the analysis (e-Fig 3 (2.8MB, pdf) , e-Table 6 (2.8MB, pdf) ).
Figure 1 –
Serum fibulin-1 levels are increased in patients with IPF compared with nondiseased subjects. Serum fibulin-1 levels in patients with IPF, measured by western blot analysis of equal volumes of serum, were normalized against a standard serum sample loaded onto every gel. Densitometric values were transformed to natural log values before analysis. Data were adjusted for age, sex, and smoking history. Analysis of covariance, Sydney cohort n = 27; ●, San Francisco cohort, n = 17; ■, Modena cohort, n = 28; gray diamond, posttest Tukey; ***P = .006, median ± 25th and 75th percentiles. IPF = idiopathic pulmonary fibrosis.
Tissue Fibulin-1 Levels Are Higher in Patients With IPF
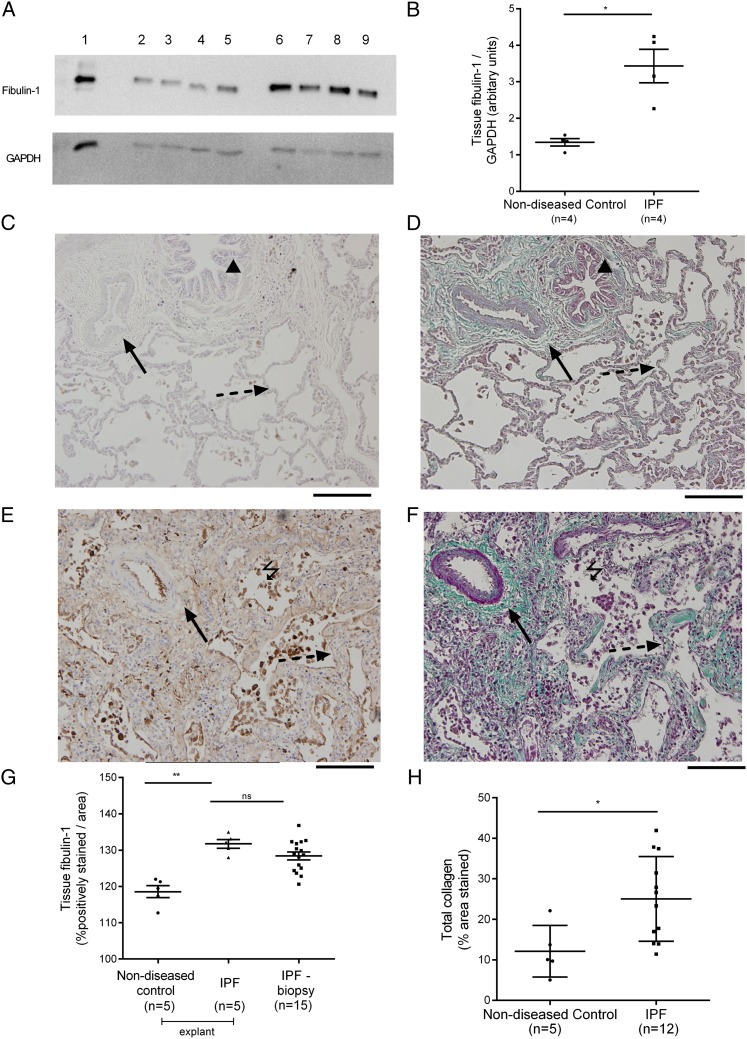
The level of fibulin-1 in whole-lung lysates from patients with IPF (mean, 3.43 units; SD, 0.91) was 2.6-fold higher than in subjects without lung disease (mean, 1.34; SD, 0.2; P = .02) (Fig 2A). Representative images of fibulin-1 and Masson trichrome-stained tissue from patients with IPF and subjects without lung disease are shown in Figure 2B. The level of fibulin-1 in formalin-fixed tissue sections was significantly higher in patients with IPF (mean, 129.2 units; SD, 4.32) compared with subjects without lung disease (mean, 118.6; SD, 3.73; P < .001) (Fig 2C). There was no significant difference in fibulin-1 levels in explanted lung tissue (P = .24) (Fig 2C). More collagen (percentage area stained with Masson trichrome) was measured in tissue from patients with IPF (n = 12; mean, 25.0% area; SD, 10.5) than in tissue from subjects without lung disease (n = 5; mean, 12.1; SD, 6.4; P = .02) (Fig 2D).
Figure 2 –
Tissue fibulin-1 and collagen levels in lung parenchyma from IPF and nondiseased subjects. A, Levels of fibulin-1 in lysates of parenchyma from patients with IPF (n = 4) and nondiseased subjects (n = 4) were analyzed by western blot and densitometric values were normalized to GAPDH detected on the same blots. A representative blot is shown. Lane 1, Cell lysate from fibulin-1 transfected HT1080 fibrosarcoma cells. Total protein (1.5 μg) of whole-lung lysates from nondiseased subjects (lanes 2-5) and patients with IPF (lanes 6-9) were loaded. B, The normalized densitometric values were compared by unpaired t test; *P < .05. C-F, Immunostaining of fibulin-1, detected using a chemical chromophore DAB (brown) and Masson trichrome staining for detection of collagen (green) in formalin-fixed tissue sections from patients with IPF and nondiseased subjects was analyzed by computerized image analysis. Twenty images were taken at random from two to six sections per patient (fibulin-1 IPF: n = 5 explanted lungs, n = 15 surgical biopsies, nondiseased subjects n = 5; collagen IPF: n = 12 surgical biopsies, nondiseased subjects n = 5). Representative images in nondiseased subjects (C, fibulin-1; D, collagen) and patients with IPF (E, fibulin-1; F, collagen). In images, black triangle indicates airway, black solid arrow indicates blood vessel, black dashed arrow indicates alveolar septa. Fibulin-1 staining is distributed throughout the lung parenchyma but may be more concentrated in cells within the alveolar space (black lightning bolt). G-H, Averaged staining levels for (G) fibulin-1 and (H) collagen were compared using analysis of variance (**P = .005, fibulin-1) and unpaired t test (*P < .05, collagen). Values are expressed in all graphs as mean ± SD. Magnification, ×20; black scale bar, 200 μm. DAB = diaminobenzidine; GAPDH = glyceraldehyde 3-phosphate dehydrogenase. See Figure 1 legend for expansion of other abbreviation.
Tissue Fibulin-1 Correlates With Lung Function in Patients With IPF
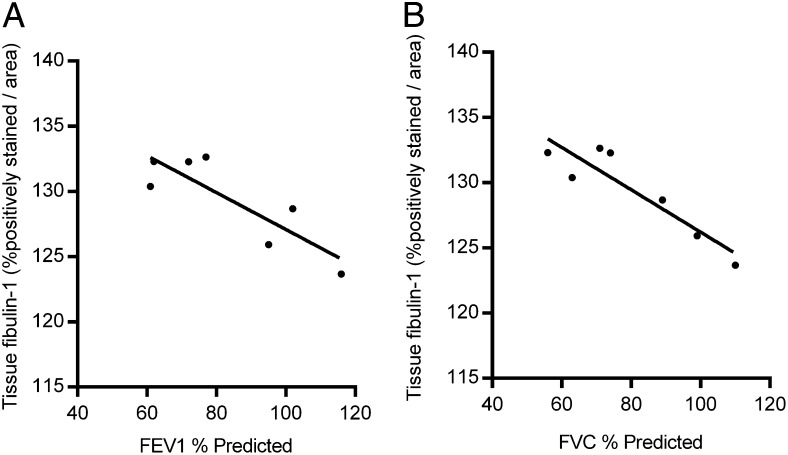
Higher tissue fibulin-1 levels correlated significantly with lower % predicted FEV1 and FVC measurements (FEV1: r = −0.86, P = .014; FVC: r = −0.92, P = .004) (Fig 3). Tissue fibulin-1 levels did not significantly correlate with age, Dlco, composite physiologic index (CPI), or % predicted total lung capacity (TLC) (data not shown).
Figure 3 –
Lung tissue fibulin-1 levels in patients with IPF inversely correlate with lung function measurements. A-B, Averaged tissue fibulin-1 levels for each patient with IPF (n = 7) were compared with their (A) FEV1 and (B) FVC measurements. Each solid circle represents the average lung tissue fibulin-1 level of two to six individual biopsies per patient. Pearson product-moment coefficients: FEV1: r = −0.86, P = .014; FVC: r = 0.92, P = .004. See Figure 1 legend for expansion of abbreviation.
Fibroblasts From Patients With IPF Produce More Fibulin-1
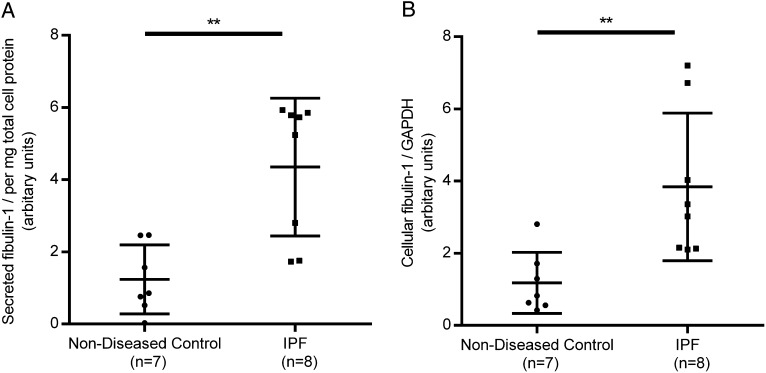
To investigate a potential source of the increase in fibulin-1, we compared the secreted and cell-associated levels of fibulin-1 in primary parenchymal fibroblasts from patients with IPF with age-matched and sex-matched subjects without lung disease under nonstimulated cell-culture conditions in fibroblasts at low passage number (at most five passages). Fibroblasts derived from patients with IPF produced significantly more secreted fibulin-1 than fibroblasts from subjects without IPF (P = .003) (Fig 4A). Fibroblasts from patients with IPF produced more cell-associated fibulin-1 than fibroblasts derived from subjects without IPF (P = .007) (Fig 4B).
Figure 4 –
Parenchymal fibroblasts from patients with IPF produce more fibulin-1 than fibroblasts from patients without respiratory disease. Primary parenchymal fibroblasts from patients with IPF (n = 8) and age-matched and sex-matched patients without respiratory disease (n = 7) were grown for 72 h in 5% fetal bovine serum (FBS), quiesced in 0.1% FBS for 24 h and maintained in fresh 0.1% FBS for a further 72 h. Supernatants and cell lysates were collected and analyzed by western blot. A-B, Densitometric values were normalized to total protein (supernatants, A) or GAPDH detected on the same blots (cell lysates, B). Unpaired t test. **P < .01. Values are expressed as median and interquartile range. See Figure 1 and 2 legends for expansion of abbreviations.
Serum Fibulin-1 Predicts Progression in IPF
Of the 72 patients with IPF, 48 patients were available for 1-year follow-up. In the year following blood draw, 27 patients had a progression event and 21 patients remained stable. Patients who later progressed had poorer Dlco, CPI, and TLC at the time of blood draw than those who did not progress (Table 1). There was no difference in FEV1, FVC, age, sex, or history of smoking between the two groups.
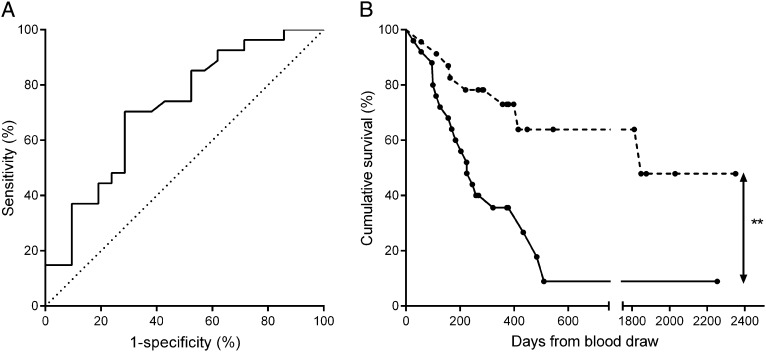
Serum fibulin-1 levels were 1.5 times higher in patients with IPF who experienced a progression event (mean 2.34 units, SD 1.18) compared with patients who remained stable (mean 1.59 units, SD 0.83; P = .013) (Table 1). The area under the ROC curve was 0.71 (95% CI, 0.57-0.86; P = .012) (Fig 5A). From this curve, thresholds for fibulin-1 levels were generated and sensitivity and specificity at each fibulin-1 level were estimated. Three thresholds (1.1, 1.6, and 2.9) were chosen for their differing specificity and sensitivity profiles and subjected to further analysis. At a threshold of 1.6 units, serum fibulin-1 identified patients with IPF who progressed with 70% sensitivity and 71% specificity. At thresholds of 1.1 and 2.9 units, the sensitivities and specificities were 93% and 33%, and 30% and 90%, respectively (e-Table 7 (2.8MB, pdf) ). Kaplan-Meier curves showed that patients with IPF with a serum fibulin-1 level > 1.6 units had a shorter progression-free survival time than those with ≤ 1.6 units (P = .003) (Fig 5B, Table 3). The progression-free survival rates of patients with IPF with high or low serum fibulin-1 levels were also significantly different if thresholds of 1.1 units or 2.9 units were used to stratify the groups (1.1 units, P = .016; 2.9 units, P = .019) (Table 3). An increase in 1 unit of serum fibulin-1 level in patients with IPF carried a significant hazard ratio for the likelihood of disease progression of 1.69 (95% CI, 1.2-2.3; P = .001).
Figure 5 –
Serum fibulin-1 levels predict disease progression in patients with IPF. Patients with IPF were followed up for at least 1 year (365 ± 1 d) following blood draw. A, Fibulin-1 levels in patients who progressed (n = 27) were compared with the levels in patients who did not progress (n = 21) and a receiver operating characteristic (ROC) curve was generated. The area under the curve was 0.71 (95% CI, 0.57-0.86; P = .012). B, Kaplan-Meier survival curves showing time to progression event were generated. Patients who had a serum fibulin-1 of ≤ 1.6 units (n = 23, dashed line) were compared with patients who had a level of > 1.6 units (n = 25, solid line). Circles on each line represent time censoring. Mantel-Cox log rank, **P = .003. See Figure 1 legend for expansion of abbreviation.
TABLE 3 ] .
Progression-Free Survival Time Comparison in Patients With IPF
| Threshold and Fibulin-1 Level | Total No. | No. of Progressed | Average Time to Progression Event (d) | ||||
| Estimate | SE | 95% CI | P Value | ||||
| Lower | Upper | ||||||
| 1.1 units | .016 | ||||||
| Low | 10 | 2 | 1,772 | 360 | 1,066 | 2,478 | |
| High | 38 | 25 | 704 | 167 | 377 | 1,031 | |
| 1.6 units | .003 | ||||||
| Low | 23 | 8 | 1,509 | 241 | 1,036 | 1,982 | |
| High | 25 | 19 | 433 | 156 | 128 | 738 | |
| 2.9 units | .019 | ||||||
| Low | 39 | 20 | 1,056 | 197 | 670 | 1,441 | |
| High | 9 | 7 | 215 | 35 | 146 | 283 | |
Patients with IPF who presented with a high level of serum fibulin-1 had a shorter progression-free survival time compared with those who had a low level of serum fibulin-1. The progression-free survival time of patients who presented with a high level of fibulin-1 (greater than the threshold of 1.1, 1.6 or 2.9) and low level of fibulin-1 (less than or equal to the threshold of 1.1, 1.6 or 2.9) were compared using the Mantel-Cox log-rank test. See Table 1 legend for expansion of abbreviation.
Univariate logistic regression showed that age, history of smoking, or FVC did not predict disease progression in our study population. When the variables serum fibulin-1, age, history of smoking, FVC, and Dlco were analyzed simultaneously in the multivariate model, the independent contribution of serum fibulin-1 increased to 2.11 (95% CI, 1.3-3.5; P = .004) (e-Table 8 (2.8MB, pdf) ). Dlco did not predict disease progression in the multivariate model.
A patient with IPF with a level of serum fibulin-1 > 1.6 units was five times more likely to progress compared with a patient with a level of serum fibulin-1 ≤ 1.6 units (hazard ratio, 5.2; 95% CI, 2.0-13.3; P = .001).
Discussion
To our knowledge, this is the first study reporting the significance of fibulin-1 in the severity and disease progression of IPF. The increased levels of fibulin-1 found in the serum, lung tissue, and primary fibroblast cultures of patients with IPF indicate fibulin-1 may contribute to this disease and importantly, a high serum fibulin-1 level may serve as a biomarker for disease progression. Moreover, the findings of this study were derived from four separate patient cohorts from three different countries, which is important, as IPF is a variable and global disease.
We have previously observed raised fibulin-1 levels in the serum and BAL fluid of patients with asthma,13 a disease in which fibrosis of the airways is associated with disease progression.14 Across diseases in which fibrosis occurs there are similar mechanisms contributing to fibrogenesis.15 Hence, it was logical to explore whether fibulin-1 would also be important in a disease predominantly characterized by fibrosis, that is, IPF. Alterations in fibulin-1 levels have been observed in a number of diseases. Plasma levels of fibulin-1 were identified as a potential marker for kidney malfunction,19 and increased levels of fibulin-1 were detected in sera from patients with preeclampsia.20 It is likely that the feature common to these diseases and the lung disease we studied is active fibrosis.
If fibulin-1 were to be biologically important during active fibrosis, then levels of fibulin-1 would likely be related to measurements of disease severity. While much of the focus in the literature has centered on the importance of collagen to fibrosis,21 it is possible that smaller ECM-connecting proteins,22 like fibulin-1 which binds to elastic fibers,12 can also act to alter the mechanical properties of the lung.23 In this study, as the levels of tissue fibulin-1 increased, the lung function of the patients decreased. Given that fibulin-1 is a known modulator of the ECM during the progression of some diseases,24 this finding is indicative of the potential importance of fibulin-1 as a target during fibrogenesis.
Primary parenchymal fibroblasts derived from patients with IPF produced more secreted and cell-associated fibulin-1 than fibroblasts derived from patients without IPF. The contribution of particular ECM molecules to the development of fibrogenesis has not been extensively investigated in IPF, as research has largely focused on either chemokines or circulating progenitors of myofibroblasts as targets to dampen the “profibrotic environment” that drives fibrosis.25 Furthermore, there is evidence that the stiffness of the underlying matrix alters fibroblast behavior.26 It is possible that excessive production of secreted fibulin-1 by the resident fibroblasts of patients with IPF contributes to the increased fibulin-1 level found in the blood. In addition, the increased production of fibulin-1 likely reflects in the activated fibrogenic state of fibroblasts derived from patients with IPF.
A number of serum biomarkers of disease severity and progression in IPF have been identified and reviewed.6,7 These include the MUC5B gene polymorphism,27 mucin-1 (KL-6), surfactant proteins SP-A and SP-D, matrix metalloproteinases 1 and 7, chemokines CCL18 and CXCL8, calgranulin B (S100A12), intracellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion protein 1 (VCAM-1), and periostin.28 While it is likely that numerous factors will contribute to the development of fibrosis, the strength of our focus on fibulin-1 is the consistent finding in multiple patient cohorts and the multiple forms, soluble and tissue incorporated, of the protein that were increased. In addition, lung tissue fibulin-1 levels relate to physiologic measurements of lung function in patients with IPF.
An important finding from this study for the future management of IPF is the observation that serum fibulin-1 levels have predictive value for acute disease progression in patients with IPF. There was a 71% chance that there was a higher serum fibulin-1 level in a patient with IPF who progressed within 1 year compared with a patient who remained stable. In addition, patients with a high level of serum fibulin-1 had a higher likelihood of progression and a shorter progression-free survival time compared with their low fibulin-1 counterparts. Serum levels were highest in patients with IPF compared with patients with other ILDs but correlated with lung function variables in all patients with ILD, suggesting that serum fibulin-1 levels may predict disease progression in other fibrosing ILDs, such as idiopathic nonspecific pneumonia.
It is crucial to identify factors able to categorize which patients with IPF will rapidly decline compared with those patients with a more stable form of the disease. Further follow-up studies in a larger sample size are needed to confirm the potential use of fibulin-1 levels for the stratification of patients with IPF. This information will enable clinicians to optimize early referral to transplantation programs and palliative care. Fibulin-1 may be a useful tool to stratify patients for inclusion in future therapeutic trials and to guide future management decisions.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: J. J. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. J. J., B. G. O., J. L. B., J. K. B. contributed to study conception and design; J. J., T. J. C., M. K., P. J. W., L. R., and S. C. contributed to patient recruitment; T. J. C., M. K., P. J. W., L. R., S. C., and C. M. P. contributed to diagnosis ascertainment; J. J., S. U., P. J. W., S. C., C. M. P., W. S. A., R. A. O. contributed to serum, tissue, and sample handling; J. J. contributed to protein and cell culture assays, and statistical analysis; J. J. contributed to the writing of the manuscript; and S. U., T. J. C., M. K., P. J. W., L. R., S. C., C. M. P., P. M. H., W. S. A., R. A. O., B. G. O., J. L. B., and J. K. B. contributed to the revision of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Corte has received unrestricted educational grants from Boehringer Ingleheim GmbH, InterMune, and Actelion Pharmaceuticals US, Inc and has acted in an advisory role to Pfizer Inc, ActelionPharmaceuticals US, Inc, and GlaxoSmithKline. Dr Wolter’s research is funded by grants from the National Institutes of Health, University of California San Francisco, and Genentech, Inc. Dr Richeldi reports grants and personal fees from Boehringer Ingelheim GmbH, InterMune, MedImmune, LLC, Biogen Idec, sanofi-aventis US LLC, F. Hoffmann-La Roche Ltd, Takeda Pharmaceuticals USA, ImmuneWorks, and Shionogi Inc. Dr Black received grant funding from the National Health and Medical Research Council, Australia and holds a patent (pending) relating to this work. Dr Burgess received grant funding from the National Health and Medical Research Council, Australia and holds a patent (pending) relating to this work. Mss Jaff ar and Unger and Drs Keller, Cerri, Prêle, Hansbro, Argraves, R. A. Oliver, and B. G. Oliver have reported that no potential conflicts of interest existwith any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: No funding body had influence in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Other contributions: We acknowledge the collaborative efforts of the transplant team and pathologists at St Vincent’s Hospital Sydney and the surgical teams at Strathfield Private Hospital (Ramsay Health Care) and Royal Prince Alfred Hospital (Sydney Local Health District). We thank the pathologists at Royal Prince Alfred Hospital (Sydney Local Health District) and at Douglass H. Moir, Macquarie Park. For organizing serum collection, we thank Liarna Honeysett, BSc; Susanne Webster, MS; members of the Interstitial Lung Disease Clinic from the Royal Prince Alfred Hospital (Sydney Local Health District), and Elisa Persiani from the University of Modena and Reggio Emilia. For statistical advice, we thank Guy Marks, MD, PhD (University of New South Wales, The Woolcock Institute of Medical Research). We thank Dirkje Postma, MD, PhD (University of Groningen, University Medical Center of Groningen [UMCG]) for her helpful comments and advice during the preparation of the manuscript.
Additional information: The e-Appendix, e-Figures, and e-Tables can be found in the Supplemental Materials section of the online article.
ABBREVIATIONS
- CPI
composite physiologic index
- Dlco
diffusing capacity of the lung for carbon monoxide
- ECM
extracellular matrix
- ILD
interstitial lung disease
- IPF
idiopathic pulmonary fibrosis
- ROC
receiver-operator characteristic
- TLC
total lung capacity
Footnotes
Part of this article has been presented and published in abstract form at the American Thoracic Society Annual Scientific Conference, May 17-22, 2013, Philadelphia, PA (Jaffar J, Unger S, Corte T, et al. Am J Respir Crit Care Med. 2013;187[1_MeetingAbstracts]:A3382) and at the Thoracic Society of Australia and New Zealand Annual Scientific Conference, March 23-27, 2013, Darwin, Australia (Jaffar J, Tjin G, Unger S, Black JL, Oliver BG, Burgess JK. Respirology. 2013;18[suppl 2]:27).
FUNDING/SUPPORT: This work was supported by the National Health and Medical Research Council, Australia [Grant 1003263]. Ms Jaffar was supported by a Rebecca L Cooper PhD Scholarship. Dr Burgess was supported by an NHM RC Career Development Fellowship [1032695]. Dr Black was supported by a NHMRC Senior Principal Research [Fellowship 571098]. Dr B. G. Oliver was supported by an NHMRC Career Development [Fellowship 1026880]. Dr Argraves was supported by a National Institutes of Health [Grant HL095067]. Serum acquisition from San Francisco was funded by the Nina Ireland Lung Disease Program.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Demedts M, Wells AU, Antó JM, et al. Interstitial lung diseases: an epidemiological overview. Eur Respir J Suppl. 2001;32:2s-16s [PubMed] [Google Scholar]
- 2.Raghu G, Behr J, Brown KK, et al. ; ARTEMIS-IPF Investigators. Treatment of idiopathic pulmonary fibrosis with ambrisentan: a parallel, randomized trial. Ann Intern Med. 2013;158(9):641-649 [DOI] [PubMed] [Google Scholar]
- 3.Shulgina L, Cahn AP, Chilvers ER, et al. Treating idiopathic pulmonary fibrosis with the addition of co-trimoxazole: a randomised controlled trial. Thorax. 2013;68(2):155-162 [DOI] [PubMed] [Google Scholar]
- 4.Araya J, Nishimura SL. Fibrogenic reactions in lung disease. Annu Rev Pathol. 2010;5:77-98 [DOI] [PubMed] [Google Scholar]
- 5.Tschumperlin DJ, Jones JC, Senior RM. The fibrotic matrix in control: does the extracellular matrix drive progression of idiopathic pulmonary fibrosis? Am J Respir Crit Care Med. 2012;186(9):814-816 [DOI] [PubMed] [Google Scholar]
- 6.Vij R, Noth I. Peripheral blood biomarkers in idiopathic pulmonary fibrosis. Transl Res. 2012;159(4):218-227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richards TJ, Kaminski N, Baribaud F, et al. Peripheral blood proteins predict mortality in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;185(1):67-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miosge N, Götz W, Sasaki T, Chu ML, Timpl R, Herken R. The extracellular matrix proteins fibulin-1 and fibulin-2 in the early human embryo. Histochem J. 1996;28(2):109-116 [DOI] [PubMed] [Google Scholar]
- 9.Kostka G, Giltay R, Bloch W, et al. Perinatal lethality and endothelial cell abnormalities in several vessel compartments of fibulin-1-deficient mice. Mol Cell Biol. 2001;21(20):7025-7034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beers MF, Morrisey EE. The three R’s of lung health and disease: repair, remodeling, and regeneration. J Clin Invest. 2011;121(6):2065-2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carew RM, Wang B, Kantharidis P. The role of EMT in renal fibrosis. Cell Tissue Res. 2012;347(1):103-116 [DOI] [PubMed] [Google Scholar]
- 12.Roark EF, Keene DR, Haudenschild CC, Godyna S, Little CD, Argraves WS. The association of human fibulin-1 with elastic fibers: an immunohistological, ultrastructural, and RNA study. J Histochem Cytochem. 1995;43(4):401-411 [DOI] [PubMed] [Google Scholar]
- 13.Lau JY, Oliver BG, Baraket M, et al. Fibulin-1 is increased in asthma—a novel mediator of airway. remodeling? PLoS ONE. 2010;5(10):e13360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Royce SG, Cheng V, Samuel CS, Tang ML. The regulation of fibrosis in airway remodeling in asthma. Mol Cell Endocrinol. 2012;351(2):167-175 [DOI] [PubMed] [Google Scholar]
- 15.Kisseleva T, Brenner DA. Mechanisms of fibrogenesis. Exp Biol Med (Maywood). 2008;233(2):109-122 [DOI] [PubMed] [Google Scholar]
- 16.Raghu G, Collard HR, Egan JJ, et al. ; ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788-824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryerson CJ, Urbania TH, Richeldi L, et al. Prevalence and prognosis of unclassifiable interstitial lung disease. Eur Respir J. 2013;42(3):750-757 [DOI] [PubMed] [Google Scholar]
- 18.Bland JM, Altman DG. Measurement error. BMJ. 1996;313(7059):744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neiman M, Hedberg JJ, Dönnes PR, et al. Plasma profiling reveals human fibulin-1 as candidate marker for renal impairment. J Proteome Res. 2011;10(11):4925-4934 [DOI] [PubMed] [Google Scholar]
- 20.Liu C, Zhang N, Yu H, et al. Proteomic analysis of human serum for finding pathogenic factors and potential biomarkers in preeclampsia. Placenta. 2011;32(2):168-174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calabresi C, Arosio B, Galimberti L, et al. Natural aging, expression of fibrosis-related genes and collagen deposition in rat lung. Exp Gerontol. 2007;42(10):1003-1011 [DOI] [PubMed] [Google Scholar]
- 22.Bensadoun ES, Burke AK, Hogg JC, Roberts CR. Proteoglycan deposition in pulmonary fibrosis. Am J Respir Crit Care Med. 1996;154(6 pt 1):1819-1828 [DOI] [PubMed] [Google Scholar]
- 23.Le Saux O, Teeters K, Miyasato S, et al. The role of caveolin-1 in pulmonary matrix remodeling and mechanical properties. Am J Physiol Lung Cell Mol Physiol. 2008;295(6):L1007-L1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Argraves WS, Tanaka A, Smith EP, et al. Fibulin-1 and fibrinogen in human atherosclerotic lesions. Histochem Cell Biol. 2009;132(5):559-565 [DOI] [PubMed] [Google Scholar]
- 25.du Bois RM. Strategies for treating idiopathic pulmonary fibrosis. Nat Rev Drug Discov. 2010;9(2):129-140 [DOI] [PubMed] [Google Scholar]
- 26.Liu F, Mih JD, Shea BS, et al. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol. 2010;190(4):693-706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seibold MA, Wise AL, Speer MC, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med. 2011;364(16):1503-1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naik PK, Bozyk PD, Bentley JK, et al. ; COMET Investigators. Periostin promotes fibrosis and predicts progression in patients with idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2012;303(12):L1046-L1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement