Abstract
Advances in critical care practice have led to a substantial decline in the incidence of ARDS over the past several years. Low tidal volume ventilation, timely resuscitation and antimicrobial administration, restrictive transfusion practices, and primary prevention of aspiration and nosocomial pneumonia have likely contributed to this reduction. Despite decades of research, there is no proven pharmacologic treatment of ARDS, and mortality from ARDS remains high. Consequently, recent initiatives have broadened the scope of lung injury research to include targeted prevention of ARDS. Prediction scores have been developed to identify patients at risk for ARDS, and clinical trials testing aspirin and inhaled budesonide/formoterol for ARDS prevention are ongoing. Future trials aimed at preventing ARDS face several key challenges. ARDS has not been validated as an end point for pivotal clinical trials, and caution is needed when testing toxic therapies that may prevent ARDS yet potentially increase mortality.
Significant advances in the care of critically ill patients have decreased the incidence of ARDS over the past 20 years.1,2 Among key contributing measures are lung-protective ventilation,3-5 timely resuscitation and antimicrobial administration,6,7 restrictive transfusion strategies,8,9 and ventilator care bundles.10
Despite these measures, mortality among patients with ARDS remains high, up to 45% for severe disease when patients with important comorbidities are included.11 Current initiatives have broadened the scope of lung injury research to include primary prevention of ARDS12,13 and prevention of morbidity among ARDS survivors.14,15 Prediction scores have been derived to identify patients at risk for ARDS, and two interventional trials are ongoing that target primary prevention. To facilitate these efforts, the National Institutes of Health has formed the Prevention and Early Treatment of Acute Lung Injury (PETAL) Network to conduct multicenter clinical trials aimed at further decreasing the disease burden of ARDS.
Clinical trials targeting the prevention of ARDS face several key design challenges. Perhaps the greatest challenge is to identify at-risk patients in whom ARDS is likely to develop and who would benefit in a meaningful way if ARDS is indeed prevented. The selection of appropriate study end points, adequate statistical power for these end points, and efficient trial design are additional challenges. The following review directly addresses these practical challenges for studies of ARDS prevention and discusses potential therapies for this novel indication, including those already under investigation in early stage trials.
Gains Through Improved Critical Care Delivery
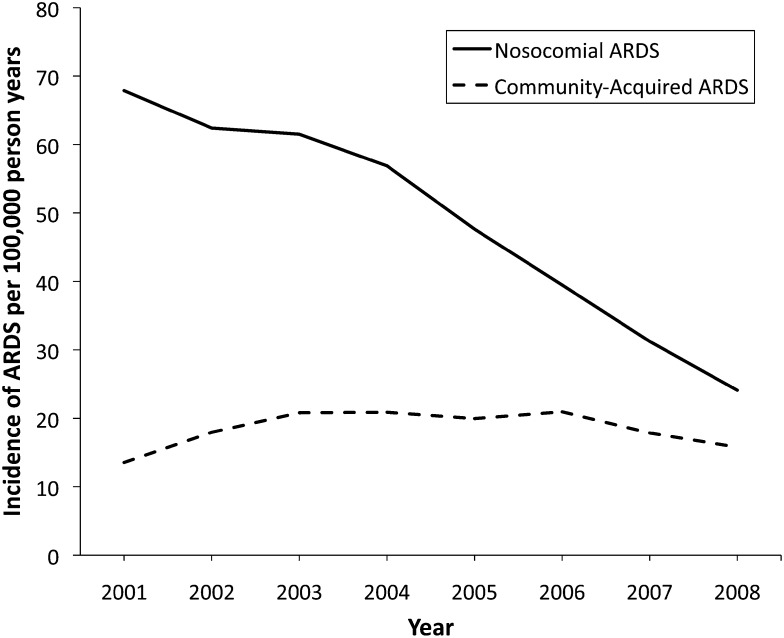
The incidence of ARDS has declined steadily over the past decade. A pivotal study by Li et al1 found that this decline is primarily due to a decrease in nosocomial ARDS (Fig 1). In contrast, the incidence of community-acquired ARDS, defined as meeting diagnostic criteria within 6 h of hospital admission, has not changed. A decrease in the use of routine chest radiographs16 and arterial blood gas analyses17 may have led to underdiagnosis of nosocomial ARDS over time, partially accounting for this trend. Similarly, adoption of restrictive fluid management strategies may have reduced the incidence of hydrostatic pulmonary edema misdiagnosed as ARDS.18,19 Several major advances in critical care practice likely also contributed to this trend.
Figure 1 –
Trends in incidence of community-acquired and nosocomial ARDS from 2001 to 2008 in Olmsted County, Minnesota. Incidence of community-acquired ARDS, defined as fulfilling all diagnostic criteria within 6 h of admission, did not change significantly over time (P = .887). Incidence of nosocomial ARDS, defined as first fulfilling all diagnostic criteria> 48 h after admission, decreased significantly over time (P < .001). Adapted with permission from Li et al.1
Low Tidal Volume Ventilation
Since the landmark National Heart, Lung, and Blood Institute (NHLBI) ARDS Network trial of lung-protective ventilation,3 tidal volumes in clinical practice have decreased over time, even for patients without ARDS.1 High tidal volumes cause direct biomechanical injury to the lung20 and induce a systemic inflammatory response that contributes to pulmonary and extrapulmonary organ injury.21,22 High tidal volumes alone precipitate lung injury in animal models.23 Observational studies have repeatedly identified high tidal volume as an independent risk factor for ARDS in patients without lung injury at baseline.24,25
Mounting evidence suggests that low tidal volumes also prevent lung injury and morbidity in at-risk patients without ARDS. A randomized trial of lung-protective ventilation for critically ill patients without ARDS demonstrated a reduction in ARDS incidence among those randomized to 6 mL/kg predicted body weight (PBW) compared with 10 mL/kg PBW.26 Similarly, a multicenter trial found that intraoperative low tidal volume ventilation during major abdominal surgery reduced pulmonary and extrapulmonary complications and shortened hospital stay.5 A meta-analysis of 20 studies involving 2,822 patients without ARDS at enrollment found reduced mortality, hospital length of stay, and incidence of ARDS with low vs traditional tidal volumes (mean, 6.45 mL/kg PBW vs 10.60 mL/kg PBW).4 Together, these findings suggest that low tidal volumes prevent ARDS in at-risk patients. Still, potential benefits must be balanced by the potential risks of increased sedation requirements and patient-ventilator dyssynchrony.27 How best to weigh these potential risks and benefits remains to be determined.
Timely Resuscitation and Antimicrobials
Timely resuscitation and antimicrobial administration for sepsis, a major risk factor for ARDS, also play important roles in ARDS prevention. Early antiviral therapy was associated with decreased ARDS incidence and improved mortality during the 2009 influenza A(H1N1) pandemic.28,29 Delays in antimicrobial administration and resuscitation have been identified as independent risk factors for ARDS.30 A landmark trial by Rivers et al6 found that early goal-directed therapy, compared with then-standard therapy, decreased the need for mechanical ventilation, reversed shock more rapidly, and improved mortality. Prompt fluid resuscitation and appropriate antimicrobial administration7,31 attenuate the systemic inflammatory response of sepsis that predisposes to lung injury and ARDS.
Transfusion and Donor Strategies
Evolving transfusion practices have also contributed to the declining incidence of ARDS. Erythrocyte, platelet, and plasma transfusions are independent risk factors for ARDS.32-34 A multicenter randomized trial demonstrated that a conservative erythrocyte transfusion strategy using a transfusion threshold hemoglobin of 7 g/dL in most patients reduced pulmonary complications and mortality compared with a higher threshold of 10 g/dL.8 Recognition that plasma and platelets carry a higher risk of transfusion-related acute lung injury has led to greater caution in their use.33 Additionally, donor-related factors have been targeted directly to reduce lung injury. In many regions, multiparous female donors are prohibited from donating, whereas in other regions, parity is used to trigger HLA antibody testing to screen for high-risk donor exclusion.9,35,36
Prevention of Aspiration and Nosocomial Pneumonia
Measures to prevent aspiration and nosocomial pneumonia also may have contributed to the declining incidence of ARDS.1 Aspiration of gastric contents is an important and largely preventable cause of ARDS.37 In a randomized trial of body positioning during mechanical ventilation, nosocomial pneumonia occurred four times more often in patients assigned to supine positioning than in those assigned to semirecumbent (head of bed 45°) positioning.38 Use of care bundles to prevent ventilator-associated pneumonia that incorporate body positioning, antiseptic oral care, and daily evaluation for readiness to liberate from mechanical ventilation have been associated with a lower rate of ventilator-associated pneumonia in a broad range of ICUs,10 almost certainly also contributing to a decline in nosocomial ARDS.
The Checklist for Lung Injury Prevention
In a single-center study, implementation of protocol-guided tidal volume and transfusion management was associated with a significantly lower incidence of ARDS (OR, 0.21; 95% CI, 0.10-0.40).32 Similarly, protocol-driven resuscitation for severe sepsis and septic shock has been shown to prevent respiratory failure requiring mechanical ventilation.6 Together, these and other best practices in critical care have been bundled into the Checklist for Lung Injury Prevention (CLIP) to standardize care in clinical trials of ARDS prevention (Table 1).39 The utility of adopting CLIP as a patient-level checklist may vary depending on an institution’s local practices, but the measures in CLIP should be viewed as the current best practice for ARDS prevention.
TABLE 1 ] .
The Checklist for Lung Injury Prevention (CLIP)
| CLIP Element | Best Practice |
| Lung-protective mechanical ventilation | Tidal volume between 6 and 8 mL/kg predicted body weight and plateau pressure < 30 cm H2O |
| PEEP ≥ 5 cm H2O | |
| Minimize Fio2, targeting Sao2 to 88%-92% after early shock | |
| Aspiration precautions | Rapid-sequence intubation performed or supervised by experienced provider |
| Elevated head of bed | |
| Antiseptic oral care | |
| Gastric acid neutralization absent enteral nutrition | |
| Early reassessment of noninvasive ventilation | Reevaluate work of breathing and clinical status 30 min after initiating noninvasive ventilation to prevent delay in intubation if necessary |
| Adequate empirical antimicrobial treatment and source control | According to suspected site of infection, health-care exposure, and immune status |
| Optimal fluid management | Early fluid resuscitation in septic shock |
| Simplified ARDS Network FACTT protocol after early shock | |
| Restrictive transfusion | Hemoglobin target ≥ 7 g/dL absent active bleeding or ischemia |
| Avoid platelet and plasma transfusions absent active bleeding | |
| Appropriate communication during transfer of patients at risk | Structured handoff to ICU providers of at-risk patients who require ICU admission, such as through SBAR |
Adapted with permission from Kor et al.39 CLIP = Checklist for Lung Injury Prevention; FACTT = Fluid and Catheter Treatment Trial; PEEP = positive end-expiratory pressure; Sao2 = arterial oxygen saturation; SBAR = situation, background, assessment, recommendation.
Early Recognition of the At-Risk Patient
ARDS does not develop in most patients with discernible risk factors for lung injury.12,40 Investigating new therapies targeting ARDS prevention necessitates reliable early identification of patients at risk for lung injury. Moreover, minimizing unnecessary exposure to harm from candidate therapies requires reliable risk stratification to include only patients likely to benefit from the intervention.
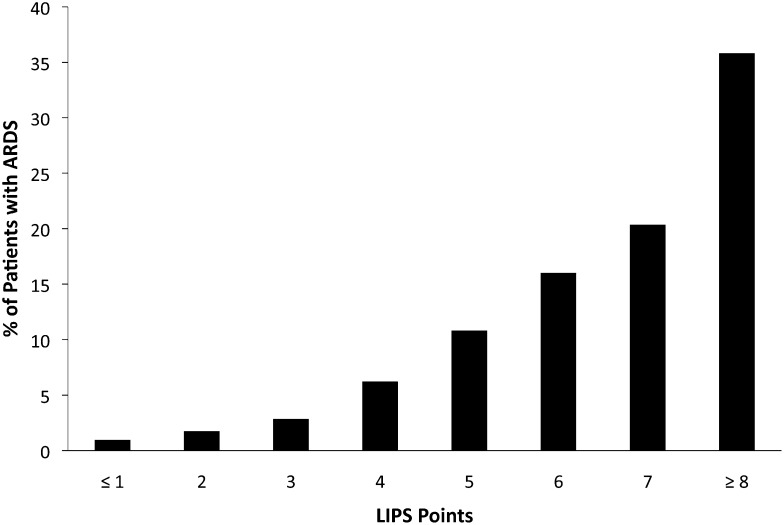
To that end, Gajic et al12 derived the Lung Injury Prediction Score (LIPS) to stratify patients at risk for acute lung injury (Fig 2, Table 2). LIPS was validated in a 22-hospital observational study of 5,584 patients with at least one risk factor for ARDS.12 The score is calculated from 22 variables considering predisposing conditions (shock, aspiration, sepsis, pneumonia, high-risk surgery, and trauma), physiologic data (oxygenation, respiratory rate, and acid-base status), and risk modifiers (alcohol use, diabetes, obesity, serum albumin level, and chemotherapy). A LIPS totaling ≥ 4 points has a sensitivity of 0.69, specificity of 0.78, positive predictive value of 0.18, and negative predictive value of 0.97.
Figure 2 –
Frequency of ARDS development according to LIPS points. Reproduced with permission from Gajic et al.12 LIPS = Lung Injury Prediction Score.
TABLE 2 ] .
Calculation of the Lung Injury Prediction Score (LIPS)
| Characteristic | Points Awarded |
| Predisposing conditions | |
| Shock | 2 |
| Aspiration | 2 |
| Sepsis | 1 |
| Pneumonia | 1.5 |
| High-risk surgerya | |
| Orthopedic spine | 1 |
| Acute abdomen | 2 |
| Cardiac | 2.5 |
| Aortic vascular | 3.5 |
| High-risk trauma | |
| Traumatic brain injury | 2 |
| Smoke inhalation | 2 |
| Near drowning | 2 |
| Lung contusion | 1.5 |
| Multiple fractures | 1.5 |
| Risk modifiers | |
| Alcohol abuse | 1 |
| Obesity (BMI > 30 kg/m2) | 1 |
| Hypoalbuminemia (albumin < 3.5 g/dL) | 1 |
| Chemotherapy | 1 |
| Fio2 > 0.35 (> 4 L/min) | 2 |
| Tachypnea (respirations > 30/min) | 1.5 |
| Spo2 < 95% | 1 |
| Acidosis (pH < 7.35) | 1.5 |
| Diabetes mellitus with sepsis | −1 |
Adapted with permission from Gajic et al.12 Spo2 = oxygen saturation as measured by pulse oximetry.
Add 1.5 points if emergency surgery.
The number of variables required to calculate LIPS may limit feasibility in clinical practice without automated support. A secondary analysis of the LIPS cohort found oxygen saturation by pulse oximetry (Spo2)/Fio2 recorded within 6 h of hospitalization to be an independent predictor of risk for ARDS.41 An alternative three-component Early Acute Lung Injury score emphasizing clinical utility was derived by Levitt et al13 in a single-center study of patients at risk for ARDS. This simpler score considers only supplemental oxygen requirement (one point for 2-6 L/min, two points for > 6 L/min), respiratory rate (one point for ≥ 30/min), and immunosuppression (one point if present), proposing a threshold of ≥ 2 points for optimal performance. Although the Early Acute Lung Injury score performed comparably to LIPS in its derivation cohort, multicenter validation is needed.
Notably absent from both clinical practice and research are reliable biomarkers to prospectively identify patients at risk for ARDS. Biomarkers of alveolar epithelial injury (surfactant protein D, club cell secretory protein-16, receptor for advanced glycation end products), vascular endothelial injury (angiopoietin-2, von Willebrand factor), inflammation (IL-6, IL-8), and coagulation (protein C, thrombomodulin, plasminogen activator inhibitor-1) all correlate with morbidity and mortality in patients with ARDS.42,43 Plasma biomarker panels may distinguish ARDS from severe sepsis or trauma without ARDS.44,45 Their utility in identifying at-risk patients without ARDS remains to be determined.
More recently, angiopoietin-2 used together with LIPS was shown to predict subsequent development of ARDS better than either alone (area under the curve, 0.74 vs 0.84 for either measure alone vs together, respectively).46 Genomic studies also have identified several candidate genes associated with an increased risk for ARDS, although phenotypic heterogeneity has limited their use in clinical research thus far.47,48 These preliminary results are promising, but no biomarkers have yet found their way into prospective clinical trials to guide the identification of patients vulnerable to ARDS who might benefit from therapies aimed at prevention.
As clinical prediction rules evolve with or without biomarkers, timing between initial presentation and ARDS onset must be considered.49 The pathogenesis, progression, response to therapy, and outcome of established ARDS may differ for various predisposing conditions.50 In the LIPS cohort, ARDS developed a median of 2 days after hospitalization. However, onset in 25% of all patients with ARDS occurred within the first 24 h of admission and in another 25% ≥ 4 days after admission.12 Other studies have found that ARDS occurs in more than one-half of patients within the first day of hospitalization.40,51 Using electronic medical records with better integration of automated surveillance may facilitate early recognition of at-risk patients within this narrow window for prevention and early treatment.52
The variation in timing of ARDS onset may reflect various underlying pathophysiologic processes.40 Accordingly, in at-risk patients, the early acute lung injury phenotype may respond differently to therapies than slower or only partially activated pathophysiologic processes. Moreover, certain therapies may be effective only at particular stages of pathogenesis. Distinguishing among these phenotypes and understanding better the underlying biology will have important implications for planning new clinical investigations and interpreting results of ongoing trials.53
Potential Therapies
Two pioneering studies published almost 30 years ago examined the role for IV corticosteroids to prevent ARDS in high-risk patients and found no effect on ARDS development or overall mortality.54,55 Shortly thereafter, ketoconazole and dipyridamole were tested in pilot studies for ARDS prevention without success.56,57 Pharmacologic attempts at ARDS prevention since have been largely dormant. More recently, several potential therapies have been proposed for ARDS prevention.53 Early phase multicenter randomized trials of aspirin, inhaled corticosteroids, and aerosolized β2-agonists are ongoing. Keratinocyte growth factor (KGF) is being studied for the treatment of established ARDS and also has been proposed for ARDS prevention.
Aspirin
Platelets play an increasingly recognized role in ARDS pathogenesis through their effects on immune function, inflammation, and vascular permeability.58 Direct alveolar injury and systemic inflammation trigger a release of P-selectin, thromboxane A2, and other molecules that promote platelet aggregation and platelet-endothelial cell adhesion.59 In turn, activated platelets induce endothelial intercellular adhesion molecule 1 expression and neutrophil recruitment to the lung, promoting a proinflammatory cascade that leads to increased vascular permeability and contributes to the development of ARDS.60
Several preclinical and epidemiologic studies have suggested a role for aspirin in preventing ARDS.34,60,61 In a murine model of acid aspiration-induced lung injury, aspirin improved oxygenation and decreased lung neutrophil recruitment and edema.60 Similarly, in a murine model of transfusion-related acute lung injury, aspirin decreased thromboxane B2 levels, platelet sequestration in the lungs, development of lung injury, and mortality.34 Aspirin also promotes 15-epi-lipoxin A4 production, which overrides myeloperoxidase-mediated suppression of neutrophil apoptosis and enhances resolution of acute lung injury.62
Supporting translation of these findings, a single-center observational study of 161 at-risk patients found that prehospitalization antiplatelet therapy was independently associated with a decreased risk for ARDS (OR, 0.34; 95% CI, 0.13-0.88).61 However, a secondary analysis of the multicenter cohort used to derive LIPS did not find a significant association between prehospitalization aspirin use and ARDS among at-risk patients (OR, 0.84; 95% CI, 0.50-1.38).63 Differences in processes of care, study populations, and analytical techniques may have contributed to these divergent findings.
To address this discrepancy, the LIPS investigators are conducting an NHLBI-funded multicenter, double-blind, placebo-controlled, phase 2 trial of aspirin for ARDS prevention among at-risk patients (LIPS-A: Lung Injury Prevention Study With Aspirin [LIPS-A]).64 Patients are randomized to aspirin 325 mg on the first day, followed by 81 mg for the next 6 days, or to placebo for 7 days. Important cointerventions are standardized through the use of CLIP. The primary outcome is the development of ARDS within 7 days of hospitalization. Hospitalized adult patients at high risk for ARDS as determined with a LIPS ≥ 4 are enrolled within 12 h of hospital presentation. Key exclusion criteria are antiplatelet therapy in the previous 7 days, ARDS prior to randomization, active or high risk for bleeding, and severe chronic liver disease. Study enrollment is to be completed in late 2014 with results expected in 2015.
Inhaled Corticosteroids
Inflammation plays a central role in the pathogenesis of ARDS and associated extrapulmonary organ failure.21,22 Multiple preclinical studies have suggested that inhaled corticosteroids may prevent ARDS through inhibition of the proinjurious inflammatory response. In a murine model of ARDS induced by intratracheal lipopolysaccharide, pretreatment with inhaled budesonide limited pulmonary edema formation and neutrophil infiltration and reduced BAL levels of tumor necrosis factor-α, IL-1β, and IL-6 compared with untreated animals.65 In a porcine model of lung injury induced by chlorine gas inhalation, budesonide given concurrently with chlorine gas attenuated impairment in lung compliance and oxygenation compared with inhaled saline control.66 Importantly, concurrent treatment with budesonide was also superior to administration 60 min after the exposure, suggesting inhibition of the initial inflammatory cascade may be more effective than therapy after the proinjury pathways are activated.
Similar findings have been observed in animal models of ARDS using an extrapulmonary trigger. Porcine models of bacteremia-induced ARDS demonstrated diminished pulmonary granulocyte accumulation and attenuated impairment in oxygenation and lung compliance with inhaled beclomethasone given within 30 to 60 min of the initial insult.67,68 Although limited clinical data exist, prehospitalization use of inhaled corticosteroids was associated with a decreased risk for ARDS in a secondary analysis of the LIPS cohort (OR, 0.39; 95% CI, 0.14-0.93).69 Two early clinical trials of IV methylprednisolone found it ineffective for ARDS prevention in critically ill patients at high risk for lung injury.54,55 However, considerable advances in critical care since their completion > 2 decades ago limits interpretation of these findings in current practice. The role for systemic steroids in the treatment of ARDS remains unclear.70,71
Aerosolized β2-Agonists
Alveolar fluid clearance is impaired in most patients with ARDS, and the degree of impairment is associated with increased mortality.72 Pulmonary β2-adrenergic receptor stimulation promotes alveolar fluid clearance through cyclic adenosine monophosphate-mediated upregulation and activation of sodium and chloride transport proteins.73 A murine model of acid aspiration-induced lung injury found that clinically relevant concentrations of β2-agonists stimulate maximal cyclic adenosine monophosphate-dependent alveolar fluid clearance and decrease pulmonary edema.74 Aerosolized albuterol achieves therapeutic levels in the pulmonary edema fluid of patients with ARDS receiving mechanical ventilation.75 Moreover, prophylactic salmeterol inhalation has been shown to prevent high-altitude pulmonary edema in humans, demonstrating its effectiveness for primary prevention of at least one form of pulmonary edema.76
However, in two multicenter randomized trials, selective β2-agonists for the treatment of established ARDS failed to produce a clinical benefit and appeared to actually worsen outcomes.77,78 Whether these findings translate to ARDS prevention is unknown. Intact alveolar epithelium is required for alveolar fluid clearance.72 Thus, β2-agonists may be effective in augmenting fluid clearance only in the setting of intact alveolar epithelium prior to ARDS onset. Still, a recent multicenter trial of perioperative salmeterol inhalation did not find salmeterol to be effective at preventing lung injury following esophagectomy (OR, 1.25; 95% CI, 0.71-2.22).79 Suboptimal dosing or timing of salmeterol administration may have contributed to these negative results, highlighting the need for further investigation.
The LIPS investigators have begun a multicenter, double-blind, placebo-controlled, phase 2 trial of an aerosolized β2-agonist/corticosteroid combination for ARDS prevention among at-risk patients (LIPS-B: Lung Injury Prevention Study With Budesonide and Beta Agonist [Formoterol]).80 Patients are randomized to receive doses of combined aerosolized budesonide/formoterol 0.5 mg/20 μg or placebo every 12 h for 5 days. As in LIPS-A, CLIP is being used to standardize cointerventions. The primary outcome is change in oxygen saturation as measured by pulse oximetry/Fio2 from baseline to study day 5. Inclusion criteria are similar to LIPS-A. Key exclusion criteria include inhaled β-agonist or corticosteroid therapy on admission or within the previous 7 days, new-onset arrhythmia, chronic atrial fibrillation with a current rate > 110 beats/min, and presentation suggestive of acute coronary ischemia. Study enrollment is to be completed in early 2015 with results available later that year.
Keratinocyte Growth Factor
KGF is an epithelial cell-specific paracrine mediator of cell repair expressed primarily by mesenchymal cells.81 In the lung, KGF expression increases in response to injury, likely stimulated in part by proinflammatory cytokines, including IL-1β, IL-6, and tumor necrosis factor-α.82 Potential mechanisms by which KGF may be lung-protective are type 2 pneumocyte proliferation, increased surfactant production, enhanced alveolar fluid clearance, and promotion of DNA repair after oxidant injury.81
Several preclinical ARDS models have found exogenous KGF that is given prior to the pulmonary insult prevents lung injury.83,84 In a rat model, intrabronchial KGF pretreatment 72 h before acid aspiration reduced histologic lung injury and mortality.83 Pretreatment only 24 h before acid aspiration did not prevent injury or improve outcomes. In a bacterial pneumonia model, intratracheal KGF pretreatment augmented alveolar fluid clearance and decreased bacterial translocation into the circulation.84 A healthy human lipopolysaccharide model of lung injury found that IV KGF pretreatment increased BAL concentrations of surfactant protein D and matrix metalloproteinase 9, markers of type 2 pneumocyte proliferation and epithelial repair, respectively.85 Indeed, several of the promising effects of mesenchymal stem cells, under investigation as a novel treatment of established ARDS, may be attributed to their secretion of KGF.86
A British group recently completed enrollment for a multicenter, double-blind, placebo-controlled, phase 2 trial testing whether IV KGF improves the oxygenation index in patients with ARDS.87 Results from this trial are pending.
Other Potential Therapies
A number of other therapies have been proposed for the prevention of ARDS among at-risk patients and have been reviewed.53,88 A three-center trial of preoperative vitamin D3 supplementation for the prevention of postesophagectomy ARDS is ongoing and highlights the unique potential for preventive therapies targeting patients undergoing high-risk elective surgery.89 Other candidate therapies at various stages of preclinical or clinical investigation are statins, aerosolized hypertonic saline, metformin, curcumin, aerosolized heparin, aerosolized and IV N-acetylcysteine, ascorbic acid, inhibitors of the renin-angiotensin system, protease inhibitors, prone positioning, and noninvasive positive pressure ventilation.
Key Challenges in Study End Points and Trial Design
Epidemiologic studies and early phase clinical trials focusing on mechanism of action play crucial roles in shaping future investigation into ARDS prevention. However, these and later-phase clinical trials evaluating patient-centered outcomes face important challenges to feasibility unique to their focus on prevention. Selection of study end points, the clinical population to be studied, and flexible trial design all must be considered carefully in the planning of prevention trials.
Traditional Study End Points and Patient Selection
Traditionally, clinical trials of ARDS have focused on mortality as the primary outcome. However, powering ARDS prevention trials for mortality may not be feasible. To illustrate this challenge, consider the multicenter cohort study used to derive the LIPS model.12 In this study, 22 hospitals enrolled a total of 5,584 at-risk patients. The overall mortality rate was 5.1%, and the overall incidence of ARDS was 6.8%. The proportion of all at-risk patients in whom ARDS developed and who subsequently died was 1.6%. Thus, a treatment so effective that it successfully prevented one-half of all ARDS-related deaths among at-risk patients would decrease overall mortality from 5.1% to 4.3%. A sample size of > 20,000 patients would be required to detect this difference with 80% power.
Ventilator-free days often is a primary or coprimary outcome in ARDS treatment trials. This outcome combines mortality with time to successful liberation from mechanical ventilation among survivors. Ventilator-free days may allow for smaller sample sizes if the treatment reduces both duration of mechanical ventilation and mortality.90 However, ventilator-free days is not clearly a patient-centered outcome, treats death and prolonged mechanical ventilation as equal, and may fail to detect differences between groups even when a significant mortality difference exists.91,92
The incidence of ARDS at first glance seems an obvious choice for a primary outcome of ARDS prevention trials. However, it is unproven that ARDS prevention itself is a meaningful or appropriate surrogate end point for mortality. For example, a randomized trial of low tidal volumes for primary prevention of ARDS demonstrated a decreased incidence of ARDS but no mortality difference.26 It is also conceivable that a drug that effectively prevents ARDS could actually increase overall mortality. In β-Agonist Lung Injury Trial-2 (BALTI-2), the IV β2-agonist salbutamol reduced lung water in established ARDS but increased mortality by 11%.78 By aerosol, salmeterol effectively prevents high-altitude pulmonary edema76 but not postesophagectomy ARDS.79 It is entirely possible that IV salbutamol effectively prevents ARDS but, as seen in BALTI-2, increases overall mortality.
The very risk factors that place patients at higher risk for ARDS also increase the risk of death from other causes.12 For this same reason, restricting study eligibility to a subset of patients at higher risk for ARDS would not improve feasibility. To address this issue, a prevention trial could target enrollment to the subset of patients at high risk for ARDS who also are at low risk for death if ARDS does not develop. This particular subset of at-risk patients has not yet been described. More work is needed to identify the optimal population subsets for prevention trials, especially when testing agents with potentially serious side effects.
Alternative Study End Points
Given these limitations of conventional outcomes, alternative primary end points should be considered in ARDS prevention trials and require further exploration (Table 3). The ideal primary end point would be a patient-centered surrogate for mortality, quality of life, and/or cost of care that can be measured reasonably within the practical and financial constraints of a clinical trial. For example, incidence of acute respiratory failure requiring positive pressure ventilation might serve as a more relevant outcome than the occurrence of ARDS per se. However, noninvasive and invasive ventilation would need to be considered separately given their dissimilar impact on patient experience and level of care.
TABLE 3 ] .
Benefits and Limitations of Possible End Points for ARDS Prevention Trials
| Candidate End Point | Benefits | Limitations |
| Mortality | Patient centered | Requires identifying a subset of patients at high risk for ARDS who also are at low risk for death if ARDS does not develop |
| Gold standard for ARDS treatment trials | Much larger sample size required for adequate power compared with trials of established ARDS | |
| Ventilator-free days | Reduces sample size needed for adequate power | Not clearly patient centered |
| Common primary or secondary outcome for ARDS treatment trials | Large sample size still may be required for adequate power | |
| Risk of bias in unblinded trials unless weaning from mechanical ventilation is fully protocolized | ||
| ARDS incidence | Intuitive | Not clearly patient centered |
| Variability in interpretation of chest radiographs | ||
| Risk of therapy preventing ARDS and yet paradoxically increasing mortality | ||
| Acute respiratory failure requiring positive pressure ventilation (distinguishing noninvasive and invasive) | Patient centered | Risk of bias in threshold applied for needing positive pressure ventilation |
| Associated with cost of care and resource utilization | Early, short-term invasive ventilation may be beneficial in some cases where prolonged noninvasive ventilation may be harmful | |
| Large sample size still may be required | ||
| Hospital-free days | Patient centered | Subject to variability in service availability and insurance coverage that influence access to long-term care |
| Associated with cost of care and resource utilization | ||
| Functional and neurocognitive testing | Patient centered | No accepted method for addressing nonsurvivors |
| Associated with cost of care and resource utilization |
Alternatively, hospital-free days to day 90 could be calculated in the same manner as ventilator-free days93 and would offer greater patient relevance while maintaining the benefits to statistical power.90 Hospital-free days is particularly relevant in the era of chronic critical illness and prolonged multistage hospitalization spanning multiple institutions that frequently characterizes recovery from critical illness.94 However, this more patient-centered end point would be subject to variability in service availability and insurance coverage that influence access to long-term acute care and home services.95
Impaired functional status and neurocognitive and physical performance are increasingly recognized important effects of critical illnesses, including ARDS.14,96 These measures have yet to be used as end points in clinical trials of ARDS treatment or prevention. However, they have been advanced as key outcomes in the postcardiac arrest literature for > 10 years,97 and several methodologies could be adopted for ARDS prevention trials without the need to develop new instruments.92 Further experience with these measures in patients with and at risk for ARDS will be required to produce reliable effect estimates for powering clinical trials.
Going forward, standardization of end points for clinical trials would facilitate interpretability, comparison of interventions across studies, and patient-level meta-analyses.92 Even when similar end points are used, definitions often vary.98 Expert consensus to standardize data collected and select essential study end points to be used across major clinical trials of ARDS prevention is needed.92,98 In addition, the burden of developing ARDS (or not) from the patient’s perspective needs to be captured to gain a full measure of the effect of future prevention efforts.
Trial Design for Process-of-Care Interventions
There are several instances where alternatives to the conventional randomized controlled trial may be appropriate for ARDS prevention studies. Certain process-of-care interventions may not be amenable to study through a patient-level randomized trial due to cross-contamination of the control group. In such cases, a cluster randomized trial assigning interventions at the hospital level should be considered. Under very limited circumstances, hospital-level interventions may not require individual informed consent because some process-of-care interventions (eg, transfusion thresholds and aspiration precautions in CLIP) could be integrated into usual care as quality improvement initiatives.
Conclusions
Advances in multiple aspects of critical care delivery have contributed to a substantial decline in the incidence of ARDS over the past several years. In the coming years, additional improvements in prevention and management of predisposing conditions, including shock, aspiration, sepsis, pancreatitis, pneumonia, trauma, and intraoperative and perioperative care, will contribute further to preventing ARDS. LIPS, a validated prediction score for lung injury, has enabled ongoing phase 2 clinical trials of aspirin and inhaled budesonide/formoterol for ARDS prevention. Additional NHLBI initiatives, including the PETAL Network, have the potential to further reduce the incidence of ARDS while simultaneously improving mortality and quality of life for patients at risk.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
ABBREVIATIONS
- CLIP
Checklist for Lung Injury Prevention
- KGF
keratinocyte growth factor
- LIPS
Lung Injury Prediction Score
- LIPS-A
Lung Injury Prevention Study With Aspirin
- NHLBI
National Heart, Lung, and Blood Institute
- PBW
predicted body weight
Footnotes
FUNDING/SUPPORT: This work was funded in part by National Institutes of Health [Grants 5-R01-HL60710-9, 1-P01-HL108801, and 5-T32-HL007633].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Li G, Malinchoc M, Cartin-Ceba R, et al. Eight-year trend of acute respiratory distress syndrome: a population-based study in Olmsted County, Minnesota. Am J Respir Crit Care Med. 2011;183(1):59-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ciesla DJ, Moore EE, Johnson JL, et al. Decreased progression of postinjury lung dysfunction to the acute respiratory distress syndrome and multiple organ failure. Surgery. 2006;140(4):640-647 [DOI] [PubMed] [Google Scholar]
- 3.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301-1308 [DOI] [PubMed] [Google Scholar]
- 4.Serpa Neto A, Cardoso SO, Manetta JA, et al. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. JAMA. 2012;308(16):1651-1659 [DOI] [PubMed] [Google Scholar]
- 5.Futier E, Constantin JM, Paugam-Burtz C, et al. ; IMPROVE Study Group. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med. 2013;369(5):428-437 [DOI] [PubMed] [Google Scholar]
- 6.Rivers E, Nguyen B, Havstad S, et al. ; Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368-1377 [DOI] [PubMed] [Google Scholar]
- 7.Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589-1596 [DOI] [PubMed] [Google Scholar]
- 8.Hébert PC, Wells G, Blajchman MA, et al. ; Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med. 1999;340(6):409-417 [DOI] [PubMed] [Google Scholar]
- 9.Gajic O, Rana R, Winters JL, et al. Transfusion-related acute lung injury in the critically ill: prospective nested case-control study. Am J Respir Crit Care Med. 2007;176(9):886-891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenthal VD, Rodrigues C, Álvarez-Moreno C, et al. ; INICC members. Effectiveness of a multidimensional approach for prevention of ventilator-associated pneumonia in adult intensive care units from 14 developing countries of four continents: findings of the International Nosocomial Infection Control Consortium. Crit Care Med. 2012;40(12):3121-3128 [DOI] [PubMed] [Google Scholar]
- 11.Ranieri VM, Rubenfeld GD, Thompson BT, et al. ; ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526-2533 [DOI] [PubMed] [Google Scholar]
- 12.Gajic O, Dabbagh O, Park PK, et al. ; US Critical Illness and Injury Trials Group: Lung Injury Prevention Study Investigators (USCIITG-LIPS). Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. Am J Respir Crit Care Med. 2011;183(4):462-470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levitt JE, Calfee CS, Goldstein BA, Vojnik R, Matthay MA. Early acute lung injury: criteria for identifying lung injury prior to the need for positive pressure ventilation. Crit Care Med. 2013;41(8):1929-1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herridge MS, Tansey CM, Matté A, et al. ; Canadian Critical Care Trials Group. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293-1304 [DOI] [PubMed] [Google Scholar]
- 15.Fan E, Dowdy DW, Colantuoni E, et al. Physical complications in acute lung injury survivors: a two-year longitudinal prospective study. Crit Care Med. 2014;42(4):849-859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hejblum G, Chalumeau-Lemoine L, Ioos V, et al. Comparison of routine and on-demand prescription of chest radiographs in mechanically ventilated adults: a multicentre, cluster-randomised, two-period crossover study. Lancet. 2009;374(9702):1687-1693 [DOI] [PubMed] [Google Scholar]
- 17.Pilon CS, Leathley M, London R, et al. Practice guideline for arterial blood gas measurement in the intensive care unit decreases numbers and increases appropriateness of tests. Crit Care Med. 1997;25(8):1308-1313 [DOI] [PubMed] [Google Scholar]
- 18.Wiedemann HP, Wheeler AP, Bernard GR, et al. ; National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564-2575 [DOI] [PubMed] [Google Scholar]
- 19.Mekontso Dessap A, Katsahian S, Roche-Campo F, et al. Ventilator-associated pneumonia during weaning from mechanical ventilation: role of fluid management. Chest. 2014;146(1):58-65 [DOI] [PubMed] [Google Scholar]
- 20.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013;369(22):2126-2136 [DOI] [PubMed] [Google Scholar]
- 21.Ranieri VM, Suter PM, Tortorella C, et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1999;282(1):54-61 [DOI] [PubMed] [Google Scholar]
- 22.Imai Y, Parodo J, Kajikawa O, et al. Injurious mechanical ventilation and end-organ epithelial cell apoptosis and organ dysfunction in an experimental model of acute respiratory distress syndrome. JAMA. 2003;289(16):2104-2112 [DOI] [PubMed] [Google Scholar]
- 23.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295(3):L379-L399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gajic O, Dara SI, Mendez JL, et al. Ventilator-associated lung injury in patients without acute lung injury at the onset of mechanical ventilation. Crit Care Med. 2004;32(9):1817-1824 [DOI] [PubMed] [Google Scholar]
- 25.Gajic O, Frutos-Vivar F, Esteban A, Hubmayr RD, Anzueto A. Ventilator settings as a risk factor for acute respiratory distress syndrome in mechanically ventilated patients. Intensive Care Med. 2005;31(7):922-926 [DOI] [PubMed] [Google Scholar]
- 26.Determann RM, Royakkers A, Wolthuis EK, et al. Ventilation with lower tidal volumes as compared with conventional tidal volumes for patients without acute lung injury: a preventive randomized controlled trial. Crit Care. 2010;14(1):R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferguson ND. Low tidal volumes for all? JAMA. 2012;308(16):1689-1690 [DOI] [PubMed] [Google Scholar]
- 28.Bramley AM, Dasgupta S, Skarbinski J, et al. ; 2009 Pandemic Influenza A (H1N1) Virus Hospitalizations Investigation Team. Intensive care unit patients with 2009 pandemic influenza A (H1N1pdm09) virus infection - United States, 2009. Influenza Other Respi Viruses. 2012;6(6):e134-e142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muthuri SG, Venkatesan S, Myles PR, et al. ; PRIDE Consortium Investigators. Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: a meta-analysis of individual participant data. Lancet Respir Med. 2014;2(5):395-404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iscimen R, Cartin-Ceba R, Yilmaz M, et al. Risk factors for the development of acute lung injury in patients with septic shock: an observational cohort study. Crit Care Med. 2008;36(5):1518-1522 [DOI] [PubMed] [Google Scholar]
- 31.Puskarich MA, Trzeciak S, Shapiro NI, et al. ; Emergency Medicine Shock Research Network (EMSHOCKNET). Association between timing of antibiotic administration and mortality from septic shock in patients treated with a quantitative resuscitation protocol. Crit Care Med. 2011;39(9):2066-2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yilmaz M, Keegan MT, Iscimen R, et al. Toward the prevention of acute lung injury: protocol-guided limitation of large tidal volume ventilation and inappropriate transfusion. Crit Care Med. 2007;35(7):1660-1666 [DOI] [PubMed] [Google Scholar]
- 33.Khan H, Belsher J, Yilmaz M, et al. Fresh-frozen plasma and platelet transfusions are associated with development of acute lung injury in critically ill medical patients. Chest. 2007;131(5):1308-1314 [DOI] [PubMed] [Google Scholar]
- 34.Looney MR, Nguyen JX, Hu Y, Van Ziffle JA, Lowell CA, Matthay MA. Platelet depletion and aspirin treatment protect mice in a two-event model of transfusion-related acute lung injury. J Clin Invest. 2009;119(11):3450-3461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gajic O, Yilmaz M, Iscimen R, et al. Transfusion from male-only versus female donors in critically ill recipients of high plasma volume components. Crit Care Med. 2007;35(7):1645-1648 [DOI] [PubMed] [Google Scholar]
- 36.Kleinman S, Grossman B, Kopko P. A national survey of transfusion-related acute lung injury risk reduction policies for platelets and plasma in the United States. Transfusion. 2010;50(6):1312-1321 [DOI] [PubMed] [Google Scholar]
- 37.Ahmed AH, Litell JM, Malinchoc M, et al. The role of potentially preventable hospital exposures in the development of acute respiratory distress syndrome: a population-based study. Crit Care Med. 2014;42(1):31-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drakulovic MB, Torres A, Bauer TT, Nicolas JM, Nogué S, Ferrer M. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial. Lancet. 1999;354(9193):1851-1858 [DOI] [PubMed] [Google Scholar]
- 39.Kor DJ, Talmor DS, Banner-Goodspeed VM, et al. ; US Critical Illness and Injury Trials Group: Lung Injury Prevention with Aspirin Study Group (USCIITG: LIPS-A). Lung Injury Prevention with Aspirin (LIPS-A): a protocol for a multicentre randomised clinical trial in medical patients at high risk of acute lung injury. BMJ Open. 2012;2(5):e001606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferguson ND, Frutos-Vivar F, Esteban A, et al. Clinical risk conditions for acute lung injury in the intensive care unit and hospital ward: a prospective observational study. Crit Care. 2007;11(5):R96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Festic E, Bansal V, Kor DJ, Gajic O, US Critical Illness and Injury Trials Group: Lung Injury Prevention Study Investigators (USCIITG-LIPS). SpO2/FiO2 ratio on hospital admission is an indicator of early acute respiratory distress syndrome development among patients at risk [published online ahead of print December 20, 2013]. J Intensive Care Med. doi:10.1177/08850666136516411 [DOI] [PubMed] [Google Scholar]
- 42.Agrawal A, Zhuo H, Brady S, et al. Pathogenetic and predictive value of biomarkers in patients with ALI and lower severity of illness: results from two clinical trials. Am J Physiol Lung Cell Mol Physiol. 2012;303(8):L634-L639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terpstra ML, Aman J, van Nieuw Amerongen GP, Groeneveld ABJ. Plasma biomarkers for acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care Med. 2014;42(3):691-700 [DOI] [PubMed] [Google Scholar]
- 44.Fremont RD, Koyama T, Calfee CS, et al. Acute lung injury in patients with traumatic injuries: utility of a panel of biomarkers for diagnosis and pathogenesis. J Trauma. 2010;68(5):1121-1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ware LB, Koyama T, Zhao Z, et al. Biomarkers of lung epithelial injury and inflammation distinguish severe sepsis patients with acute respiratory distress syndrome. Crit Care. 2013;17(5):R253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agrawal A, Matthay MA, Kangelaris KN, et al. Plasma angiopoietin-2 predicts the onset of acute lung injury in critically ill patients. Am J Respir Crit Care Med. 2013;187(7):736-742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao L, Barnes KC. Recent advances in genetic predisposition to clinical acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2009;296(5):L713-L725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tejera P, Meyer NJ, Chen F, et al. Distinct and replicable genetic risk factors for acute respiratory distress syndrome of pulmonary or extrapulmonary origin. J Med Genet. 2012;49(11):671-680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151(2):293-301 [DOI] [PubMed] [Google Scholar]
- 50.Gong MN, Thompson BT, Williams P, Pothier L, Boyce PD, Christiani DC. Clinical predictors of and mortality in acute respiratory distress syndrome: potential role of red cell transfusion. Crit Care Med. 2005;33(6):1191-1198 [DOI] [PubMed] [Google Scholar]
- 51.Levitt JE, Bedi H, Calfee CS, Gould MK, Matthay MA. Identification of early acute lung injury at initial evaluation in an acute care setting prior to the onset of respiratory failure. Chest. 2009;135(4):936-943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pickering BW, Gajic O, Ahmed A, Herasevich V, Keegan MT. Data utilization for medical decision making at the time of patient admission to ICU. Crit Care Med. 2013;41(6):1502-1510 [DOI] [PubMed] [Google Scholar]
- 53.Levitt JE, Matthay MA. Clinical review: early treatment of acute lung injury - paradigm shift toward prevention and treatment prior to respiratory failure. Crit Care. 2012;16(3):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weigelt JA, Norcross JF, Borman KR, Snyder WH., III Early steroid therapy for respiratory failure. Arch Surg. 1985;120(5):536-540 [DOI] [PubMed] [Google Scholar]
- 55.Luce JM, Montgomery AB, Marks JD, Turner J, Metz CA, Murray JF. Ineffectiveness of high-dose methylprednisolone in preventing parenchymal lung injury and improving mortality in patients with septic shock. Am Rev Respir Dis. 1988;138(1):62-68 [DOI] [PubMed] [Google Scholar]
- 56.Sinuff T, Cook DJ, Peterson JC, Fuller HD. Development, implementation, and evaluation of a ketoconazole practice guideline for ARDS prophylaxis. J Crit Care. 1999;14(1):1-6 [DOI] [PubMed] [Google Scholar]
- 57.Vincent JL, Brimioulle S, Berre J, Kahn RJ. Prevention of the adult respiratory distress syndrome with dipyridamole. Crit Care Med. 1985;13(10):783-785 [DOI] [PubMed] [Google Scholar]
- 58.Zarbock A, Ley K. The role of platelets in acute lung injury (ALI). Front Biosci (Landmark Ed). 2009;14:150-158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kiefmann R, Heckel K, Schenkat S, Dörger M, Węsierska-Gadek J, Goetz AE. Platelet-endothelial cell interaction in pulmonary micro-circulation: the role of PARS. Thromb Haemost. 2004;91(4):761-770 [DOI] [PubMed] [Google Scholar]
- 60.Zarbock A, Singbartl K, Ley K. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J Clin Invest. 2006;116(12):3211-3219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Erlich JM, Talmor DS, Cartin-Ceba R, Gajic O, Kor DJ. Prehospitalization antiplatelet therapy is associated with a reduced incidence of acute lung injury: a population-based cohort study. Chest. 2011;139(2):289-295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.El Kebir D, József L, Pan W, et al. 15-epi-lipoxin A4 inhibits myeloperoxidase signaling and enhances resolution of acute lung injury. Am J Respir Crit Care Med. 2009;180(4):311-319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kor DJ, Erlich J, Gong MN, et al. ; US Critical Illness and Injury Trials Group: Lung Injury Prevention Study Investigators (USCIITG-LIPS). Association of prehospitalization aspirin therapy and acute lung injury: results of a multicenter international observational study of at-risk patients. Crit Care Med. 2011;39(11):2393-2400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gajic O, Gong MN, Talmor D. LIPS-A: lung injury prevention study with aspirin. NCT01504867. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT01504867. Bethesda, MD: National Institutes of Health; 2012. Updated March 17, 2014.
- 65.Jansson AH, Eriksson C, Wang X. Effects of budesonide and N-acetylcysteine on acute lung hyperinflation, inflammation and injury in rats. Vascul Pharmacol. 2005;43(2):101-111 [DOI] [PubMed] [Google Scholar]
- 66.Wang J, Zhang L, Walther SM. Inhaled budesonide in experimental chlorine gas lung injury: influence of time interval between injury and treatment. Intensive Care Med. 2002;28(3):352-357 [DOI] [PubMed] [Google Scholar]
- 67.Walther S, Jansson I, Berg S, Lennquist S. Pulmonary granulocyte accumulation is reduced by nebulized corticosteroid in septic pigs. Acta Anaesthesiol Scand. 1992;36(7):651-655 [DOI] [PubMed] [Google Scholar]
- 68.Walther S, Jansson I, Berg S, Olsson Rex L, Lennquist S. Corticosteroid by aerosol in septic pigs—effects on pulmonary function and oxygen transport. Intensive Care Med. 1993;19(3):155-160 [DOI] [PubMed] [Google Scholar]
- 69.Ortiz-Diaz E, Li G, Kor DJ, et al. Preadmission use of inhaled corticosteroids is associated with a reduced risk of direct acute lung injury/acute respiratory distress syndrome. Chest. 2011;140(4_MeetingAbstracts):912A [Google Scholar]
- 70.Tang BMP, Craig JC, Eslick GD, Seppelt I, McLean AS. Use of corticosteroids in acute lung injury and acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care Med. 2009;37(5):1594-1603 [DOI] [PubMed] [Google Scholar]
- 71.Peter JV, John P, Graham PL, Moran JL, George IA, Bersten A. Corticosteroids in the prevention and treatment of acute respiratory distress syndrome (ARDS) in adults: meta-analysis. BMJ. 2008;336(7651):1006-1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163(6):1376-1383 [DOI] [PubMed] [Google Scholar]
- 73.Folkesson HG, Matthay MA. Alveolar epithelial ion and fluid transport: recent progress. Am J Respir Cell Mol Biol. 2006;35(1):10-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McAuley DF, Frank JA, Fang X, Matthay MA. Clinically relevant concentrations of β2-adrenergic agonists stimulate maximal cyclic adenosine monophosphate-dependent airspace fluid clearance and decrease pulmonary edema in experimental acid-induced lung injury. Crit Care Med. 2004;32(7):1470-1476 [DOI] [PubMed] [Google Scholar]
- 75.Atabai K, Ware LB, Snider ME, et al. Aerosolized β(2)-adrenergic agonists achieve therapeutic levels in the pulmonary edema fluid of ventilated patients with acute respiratory failure. Intensive Care Med. 2002;28(6):705-711 [DOI] [PubMed] [Google Scholar]
- 76.Sartori C, Allemann Y, Duplain H, et al. Salmeterol for the prevention of high-altitude pulmonary edema. N Engl J Med. 2002;346(21):1631-1636 [DOI] [PubMed] [Google Scholar]
- 77.Matthay MA, Brower RG, Carson S, et al. ; National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Randomized, placebo-controlled clinical trial of an aerosolized β2-agonist for treatment of acute lung injury. Am J Respir Crit Care Med. 2011;184(5):561-568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gao Smith F, Perkins GD, Gates S, et al. ; BALTI-2 Study Investigators. Effect of intravenous β-2 agonist treatment on clinical outcomes in acute respiratory distress syndrome (BALTI-2): a multicentre, randomised controlled trial. Lancet. 2012;379(9812):229-235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Perkins GD, Gates S, Park D, et al. ; BALTI-Prevention Collaborators. The beta agonist lung injury trial prevention: a randomized controlled trial. Am J Respir Crit Care Med. 2014;189(6):674-683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Festic E, Levitt JE. LIPS-B: lung injury prevention study with budesonide and beta agonist (formoterol). NCT01783821. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT01783821. Bethesda, MD: National Institutes of Health; 2013. Updated December 13, 2013.
- 81.Ware LB, Matthay MA. Keratinocyte and hepatocyte growth factors in the lung: roles in lung development, inflammation, and repair. Am J Physiol Lung Cell Mol Physiol. 2002;282(5):L924-L940 [DOI] [PubMed] [Google Scholar]
- 82.Brauchle M, Angermeyer K, Hübner G, Werner S. Large induction of keratinocyte growth factor expression by serum growth factors and pro-inflammatory cytokines in cultured fibroblasts. Oncogene. 1994;9(11):3199-3204 [PubMed] [Google Scholar]
- 83.Yano T, Deterding RR, Simonet WS, Shannon JM, Mason RJ. Keratinocyte growth factor reduces lung damage due to acid instillation in rats. Am J Respir Cell Mol Biol. 1996;15(4):433-442 [DOI] [PubMed] [Google Scholar]
- 84.Viget NB, Guery BP, Ader F, et al. Keratinocyte growth factor protects against Pseudomonas aeruginosa-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2000;279(6):L1199-L1209 [DOI] [PubMed] [Google Scholar]
- 85.Shyamsundar M, McAuley DF, Ingram RJ, et al. Keratinocyte growth-factor promotes epithelial survival and resolution in a human model of lung injury [published online ahead of print April 9, 2014]. Am J Respir Crit Care Med.doi:10.1164/rccm.201310-1892OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee JW, Krasnodembskaya A, McKenna DH, Song Y, Abbott J, Matthay MA. Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am J Respir Crit Care Med. 2013;187(7):751-760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cross LJM, O’Kane CM, McDowell C, Elborn JJ, Matthay MA, McAuley DF. Keratinocyte growth factor in acute lung injury to reduce pulmonary dysfunction—a randomised placebo-controlled trial (KARE): study protocol. Trials. 2013;14:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Albert RK. The role of ventilation-induced surfactant dysfunction and atelectasis in causing acute respiratory distress syndrome. Am J Respir Crit Care Med. 2012;185(7):702-708 [DOI] [PubMed] [Google Scholar]
- 89.Thickett D. Vitamin D replacement to prevent acute lung injury following oesophagectomy (VINDALOO). ISRCTN27673620. Controlled-Trials.com http://www.controlled-trials.com/ISRCTN27673620. Birmingham, England: University of Birmingham; 2011. Updated December 21, 2011.
- 90.Schoenfeld DA, Bernard GR; ARDS Network. Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med. 2002;30(8):1772-1777 [DOI] [PubMed] [Google Scholar]
- 91.Willson DF, Thomas NJ, Markovitz BP, et al. ; Pediatric Acute Lung Injury and Sepsis Investigators. Effect of exogenous surfactant (calfactant) in pediatric acute lung injury: a randomized controlled trial. JAMA. 2005;293(4):470-476 [DOI] [PubMed] [Google Scholar]
- 92.Spragg RG, Bernard GR, Checkley W, et al. Beyond mortality: future clinical research in acute lung injury. Am J Respir Crit Care Med. 2010;181(10):1121-1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Young P, Hodgson C, Dulhunty J, et al. ; ANZICS Clinical Trials Group. End points for phase II trials in intensive care: recommendations from the Australian and New Zealand Clinical Trials Group consensus panel meeting. Crit Care Resusc. 2012;14(3):211-215 [PubMed] [Google Scholar]
- 94.Kahn JM, Benson NM, Appleby D, Carson SS, Iwashyna TJ. Long-term acute care hospital utilization after critical illness. JAMA. 2010;303(22):2253-2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lane-Fall MB, Iwashyna TJ, Cooke CR, Benson NM, Kahn JM. Insurance and racial differences in long-term acute care utilization after critical illness. Crit Care Med. 2012;40(4):1143-1149 [DOI] [PubMed] [Google Scholar]
- 96.Hopkins RO, Weaver LK, Collingridge D, Parkinson RB, Chan KJ, Orme JF., Jr Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005;171(4):340-347 [DOI] [PubMed] [Google Scholar]
- 97.Becker LB, Aufderheide TP, Geocadin RG, et al. ; American Heart Association Emergency Cardiovascular Care Committee; Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Primary outcomes for resuscitation science studies: a consensus statement from the American Heart Association. Circulation. 2011;124(19):2158-2177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Blackwood B, Clarke M, McAuley DF, McGuigan PJ, Marshall JC, Rose L. How outcomes are defined in clinical trials of mechanically ventilated adults and children. Am J Respir Crit Care Med. 2014;189(8):886-893 [DOI] [PubMed] [Google Scholar]