Abstract
BACKGROUND:
Pulmonary aspiration is an important recognized cause of ARDS. Better characterization of patients who aspirate may allow identification of potential risks for aspiration that could be used in future studies to mitigate the occurrence of aspiration and its devastating complications.
METHODS:
We conducted a secondary analysis of the Lung Injury Prediction Score cohort to better characterize patients with aspiration, including their potential risk factors and related outcomes.
RESULTS:
Of the 5,584 subjects at risk for ARDS and who required hospitalization, 212 (3.8%) presented with aspiration. Subjects who aspirated were likely to be male (66% vs 56%, P < .007), slightly older (59 years vs 57 years), white (73% vs 61%, P = .0004), admitted from a nursing home (15% vs 5.9%, P < .0001), have a history of alcohol abuse (21% vs 8%, P < .0001), and have lower Glasgow Coma Scale (median, 13 vs 15; P < .0001). Aspiration subjects were sicker (higher APACHE [Acute Physiology and Chronic Health Evaluation] II score), required more mechanical ventilation (54% vs 32%, P < .0001), developed more moderate to severe ARDS (12% vs 3.8%, P < .0001), and were twofold more likely to die in-hospital, even after adjustment for severity of illness (OR = 2.1; 95% CI, 1.2-3.6). Neither obesity nor gastroesophageal reflux was associated with aspiration.
CONCLUSIONS:
Aspiration was more common in men with alcohol abuse history and a lower Glasgow Coma Scale who were admitted from a nursing home. It is independently associated with a significant increase in the risk for ARDS as well as morbidity and mortality. Findings from this study may facilitate the design of future clinical studies of aspiration-induced lung injury.
The Lung Injury Prediction Score (LIPS) study identified aspiration as among the leading risk factors for ARDS.1‐7 However, most of our knowledge on aspiration and its associated risks and outcomes is based on observational studies where aspiration is determined retrospectively or concurrent to the clinical consequence (eg, ARDS).8,9 We systematically and prospectively characterized patients who aspirated and required hospitalization from the LIPS cohort, with the goal of identifying the burden of illness, consequences of aspiration, and risk factors associated with aspiration. Findings from this study are meant to be descriptive and hypothesis-generating to support the design of future clinical trials on aspiration, with long-term goals to identify potential therapeutic targets to mitigate aspiration-induced lung injury and its other consequences.
Materials and Methods
This is an a priori planned secondary analysis of the LIPS17 cohort to characterize patients who aspirated and required hospitalization. The LIPS1 study developed and validated the LIPS to facilitate clinical trials aimed at preventing ARDS. Because only deidentified clinical data were collected and aggregated, a formal informed consent was waived per each center’s institutional review board.7 This secondary analysis was approved under Mayo Clinic institutional review board #08-008726. Subjects were enrolled prospectively at 19, and retrospectively at three, centers over 6 months (starting March 2009), and were followed to hospital discharge or death.
Every patient admitted with a predisposing condition (e-Appendix 1 (93.2KB, pdf) ) for ARDS,7 including those with aspiration, was identified.7 We aimed to further characterize this subgroup in-depth, including an analysis of risks for aspiration, its associations, and outcomes specific to aspiration that were not reported in the primary study. Aspiration was defined as “witnessed or suggestive history of inhalation of food or regurgitated gastric contents”10 and was determined along with all baseline covariates from review of medical documentation and clinically obtained tests, within 6 h of hospital. Principal outcomes included acute lung injury (Pao2/Fio2 < 300) and ARDS (Pao2/Fio2 < 200) per the prevailing definition11 and mortality. Given the interval update in the definition,12 henceforth, ARDS will refer to Pao2/Fio2 < 300, and moderate to severe ARDS to Pao2/Fio2 < 200. Covariates and outcomes were predefined and systematically captured: demographics, ARDS “risk modifiers” (e-Appendix 1 (93.2KB, pdf) ), medications, APACHE (Acute Physiology and Chronic Health Evaluation ) II at admission, LIPS,7 ventilator settings, length of hospital and ICU stay, and costs of hospitalization. Comparisons were done between patients who aspirated (“aspirators”) vs those who did not aspirate (“nonaspirators”), as well as between those who aspirated (with or without pneumonia) vs those who met Centers for Disease Control and Prevention (CDC) criteria for pneumonia13 (without aspiration).
This is primarily a descriptive report, and all analytic statistics should be considered exploratory. Descriptive statistics are summarized as medians and interquartile ranges (IQRs), and exploratory statistical hypothesis testing was performed using nonparametric assumptions: Mann-Whitney or Fisher exact tests. ORs with 95% CI are reported as appropriate. Bivariate analyses distinguishing aspirators from nonaspirators were the primary hypothesis-generating aim of the report. Particular hypotheses of interest included whether aspirators would have more gastroesophageal reflux disease (GERD) and be on agents that suppress consciousness (eg, benzodiazepines, opiates, alcohol). Multivariate logistic regression models were used to identify independent predictors of aspiration from baseline covariates (e-Appendix 1 (93.2KB, pdf) ). We also analyzed whether medications that affect acid reflux, the cough reflex, and host response to aspiration would influence outcomes (invasive ventilation, ARDS, death). Statistical analyses were completed using JMP10 (SAS Institute Inc).
Results
The LIPS1 cohort enrolled 5,584 subjects and has been previously described.7 The mean age was 56 years, and 57% were men, with 5.1% overall mortality. ARDS developed in 377 (6.8%).7
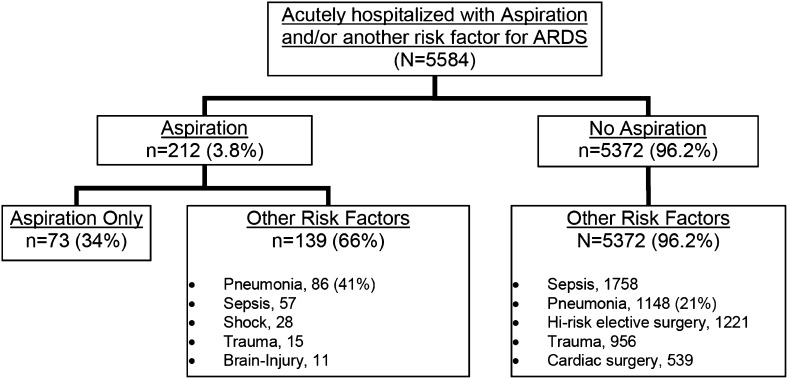
Aspiration was identified in 212 patients (3.8%) at admission, 41% of whom also met CDC criteria for pneumonia (Fig 1). Aspirators were more likely to be male, older, white, and admitted from nursing home, compared with nonaspirators (Table 1). There was no difference in BMI or smoking status, but aspirators had more excessive alcohol use than nonaspirators. Aspiration was also associated with both higher APACHE II score and base risk for ARDS (modified LIPS) at hospital admission. The Glasgow Coma Scale (GCS) was slightly lower among aspirators (Table 1).
Figure 1 –
Lung Injury Prediction Score 1 cohort.
TABLE 1 ] .
Baseline Characteristics
| Characteristics | Any Aspiration (n = 212) | No Aspiration (n = 5,372) | P Value |
| Age, median (IQR), y | 59 (43-77) | 57 (43-70) | .05 |
| Male sex, % | 66 | 56 | .0072 |
| Hispanic, % | 8.3 | 10 | .53 |
| White, % | 73 | 61 | .0004 |
| Black, % | 13 | 21 | .004 |
| BMI, median (IQR), kg/m2 | 26 (22-30) | 27 (23-31) | .066 |
| Nursing home | 15 | 5.9 | < .0001 |
| Smoking, % | .58 | ||
| Never | 51 | 50 | |
| Former | 21 | 24 | |
| Current | 28 | 26 | |
| Excessive alcohol | 21 | 8.0 | < .0001 |
| APACHE II score, median (IQR) | 13 (8-19) | 8.0 (5-13) | < .0001 |
| LIPS, median (IQR) | 5.5 (4-7.5) | 2.5 (1.5-4) | < .0001 |
| Modified LIPSa | 3.5 (2-5.5) | 2.5 (1.5-4) | < .0001 |
| GCS | 13 (7-15) | 15 (15-15) | < .0001 |
APACHE = Acute Physiology and Chronic Health Evaluation; GCS = Glasgow Coma Scale; IQR = interquartile range; LIPS = Lung Injury Prediction Score.
LIPS without the numerical contribution from aspiration.
Among the predefined ARDS risk modifiers, prior chest radiation was the only preexisting condition that was more common among aspirators, while active immunosuppression was less common (Table 2). Despite more aspirators taking proton pump inhibitors (PPIs), there was no difference in the frequency of clinically documented GERD. The prehospital use of opiates and benzodiazepines were no more common among aspirators than nonaspirators, but antipsychotics were more common in univariate though not adjusted analyses (Table 3).
TABLE 2 ] .
Predefined Predisposing Conditions for ARDS and ARDS Risk Modifiers
| Conditions | Any Aspiration (n = 212) | No Aspiration (n = 5,372) | P Value |
| Predisposing conditions for ARDS, % | |||
| Pneumonia | 41 | 21 | < .0001 |
| Pancreatitis | 0.9 | 6 | .0005 |
| Sepsis | 27 | 33 | .085 |
| Shock | 13 | 7 | .0016 |
| Lung contusion | 2 | 3 | .56 |
| Smoke inhalation | 0.9 | 0.5 | .27 |
| Near drowning | 0 | 0.1 | 1.0 |
| Long-bone fractures | 0.5 | 6 | < .0001 |
| Brain injury | 5 | 9 | .063 |
| Acute abdomen | 0.9 | 5 | .0014 |
| High-risk trauma | 7 | 18 | < .0001 |
| Emergency surgery | 3 | 6 | .10 |
| ARDS risk modifiers, % | |||
| GERD | 13 | 13 | .75 |
| DM | 20 | 23 | .36 |
| Cirrhosis | 4 | 2 | .053 |
| Chronic hemodialysis | 4 | 4 | 1.0 |
| CHF (NYHA IV) | 2 | 3 | .56 |
| COPD | 11 | 11 | .91 |
| Asthma | 6 | 8 | .43 |
| ILD | 2 | 0.9 | .12 |
| Immunosuppression | 3 | 9 | .0019 |
| Lymphoma | 0.5 | 1.6 | .26 |
| Leukemia | 0.5 | 1 | .73 |
| Metastatic solid cancer | 8 | 5 | .11 |
| Chest radiation | 3 | 1 | .018 |
| Sleep apnea | 4 | 5 | .74 |
CHF = congestive heart failure; DM = diabetes mellitus; GERD = gastroesophageal reflux disease; ILD = interstitial lung disease; NYHA = New York Heart Association.
TABLE 3 ] .
Prehospital Medication Use in Aspirators vs Nonaspirators
| Medication Use | Any Aspiration (n = 212) | No Aspiration (n = 5,372) | P Value |
| Prehospital medications, % (No. = 5,584) | |||
| Proton pump inhibitor | 29 | 23 | .045 |
| H2 antagonist | 4 | 5 | .64 |
| ACE inhibitor | 19 | 20 | .73 |
| ARB | 5 | 6 | .76 |
| Inhaled β-agonist | 12 | 14 | .61 |
| Inhaled steroids | 6 | 9 | .18 |
| Systemic steroids | 6 | 8 | .20 |
| Statin | 21 | 25 | .20 |
| Aspirin | 25 | 27 | .64 |
| Amiodarone | 2 | 0.8 | .11 |
| Oral hypoglycemic | 10 | 11 | .82 |
| Insulin | 9 | 10 | .73 |
| Other medications, % (No. = 4,609) | |||
| Benzodiazepines | 11 | 12 | .91 |
| Opiates | 18 | 20 | .53 |
| Antipsychotics | 9 | 4 | .0090 |
| Metoclopramide | 3 | 1 | .13 |
| Macrolide | 0 | 1 | .18 |
| ACE inhibitor | 19 | 20 | .73 |
| β-Blocker | 26 | 26 | .99 |
Prehospital medications were obtained systematically on all enrolled patients per protocol. Other medications was a manually extracted list of other medications submitted by centers. ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; H2 = histamine-2.
Independent predictors significantly associated with aspiration in the multivariate logistic regression model included male sex (OR = 1.5), white (OR = 2.0), admission from a nursing home (OR = 2.9), excessive alcohol use (OR = 2.8), and prior history of chest radiation (OR = 3.7). GCS (OR = 0.77), admission for sepsis (OR = 0.57), and high-risk trauma (OR = 0.13) were inversely associated with likelihood of being diagnosed with aspiration.
Aspirators vs Nonaspirators
Despite higher severity of illness scores, subjects who aspirated were no more likely to be admitted to the ICU than patients with other predisposing risks for ARDS (55% vs 59%, P = .29). However, among the 3,274 subjects who eventually required ICU admission, those with clinically defined aspiration at the time of admission had longer ICU (median, 4 days vs 2 days; P < .0001) and hospital length of stay (LOS) (median, 8 days vs 6 days; P < .0001). Additionally, aspirators required significantly more noninvasive (17% vs 9.6%, P = .0012) and invasive ventilatory support (54% vs 32%, P < .0001) (Table 4).
TABLE 4 ] .
Outcomes Between Those Who Aspirated vs Nonaspirators
| Outcomes | Any Aspiration (n = 212) | No Aspiration (n = 5,372) | P Value |
| LOS, median (IQR), d | |||
| Hospital | 8 (4-14) | 6 (4-10) | < .0001 |
| ICU (No. = 3,262) | 4 (2-6.5) | 2 (1-5) | < .0001 |
| Ventilatory support, % | |||
| Noninvasive | 17 | 9.6 | .0012 |
| Invasive | 54 | 32 | < .0001 |
| ARDS, % | 17 | 6.4 | < .0001 |
| Mild | 4.3 | 2.6 | .18 |
| Moderate to severea | 12 | 3.8 | < .0001 |
| Mortality, % | |||
| Hospital death | 15 | 4.8 | < .0001 |
| ICU death | 9.9 | 3.5 | < .001 |
| Costs, median, $ | |||
| Hospital (No. = 1,313) | 44,915 | 27,300 | .021 |
| ICU (No. = 765) | 44,960 | 18,360 | .025 |
LOS = length of stay. See Table 1 legend for expansion of other abbreviations.
Moderate to severe = Pao2/Fio2 < 200.
For the primary outcomes, ARDS occurred more frequently among subjects who aspirated compared with those who did not (17% vs 6.4%; OR = 2.9; 95% CI, 2.0-4.2), and ARDS was more likely to be of at least moderate severity (12% vs 3.8%; OR = 3.6; 95% CI, 2.3-5.5) (Table 4). Mortality was threefold higher among aspirators than nonaspirators (15% vs 4.8%; OR = 3.4; 95% CI, 2.3-5.1), including ICU deaths (9.9% vs 3.5%; OR = 3.1; 95% CI, 1.0-4.9). After adjusting for age, sex, APACHE II score, and baseline risk for developing ARDS (modified LIPS score), aspiration remained a significant risk for the development of moderate to severe ARDS (OR = 1.8; 95% CI, 1.1-2.9) and death (OR = 1.8; 95% CI, 1.1-2.8), though not statistically significant for mild ARDS (OR = 1.5; 95% CI, 1.0-2.3). Although blacks were less likely to aspirate than other races, they were more likely to develop moderate to severe ARDS (26% vs 10%, P = .03) if they did aspirate. Also, among those who aspirated, an increased weight (P = .012) though not BMI (P = .15) was associated with developing ARDS.
Of the 212 subjects who aspirated, approximately two-thirds (n = 139) had another of the predefined predisposing conditions for ARDS, most commonly pneumonia, sepsis, and shock (Fig 1). We explored whether there would be any differences between those who just aspirated without any other predisposing condition (n = 73) vs those who aspirated and had another predisposing condition for ARDS (n = 139). In unadjusted comparisons, those who just aspirated were younger (median age, 52 years vs 65 years; P = .015), less sick (median APACHE II score of 11 vs 15, P = .0006), at a lower risk for ARDS (median modified LIPS 2 vs 3, P < .0001), and had a diagnosis of GERD more frequently (21% vs 9.4%, P = .032). ARDS was much less common in those who aspirated without any other predisposing condition for ARDS (8.2% vs 21%, P = .020), though no statistically significant differences in death (8.2% vs 18%, P = .066) or the need for invasive ventilation (52% vs 55%, P = .77) was noted. When adjusted in a multivariate logistic regression model for age, sex, APACHE II score, and the modified LIPS, with or without the significant covariates identified in the univariate analyses, there were no significant differences in the risk for ARDS, death, nor invasive ventilation, between those who aspirated with, from those without, any other predisposing condition for ARDS.
Aspiration vs Pneumonia Alone
In unadjusted analyses comparing patients admitted with aspiration (with or without other predisposing conditions for ARDS) vs pneumonia alone, ARDS was still more common (17% vs 7.9%; OR = 2.3; 95% CI, 1.5-3.5) and was more moderate to severe in severity (12% vs 4.7%; OR = 2.8; 95% CI, 1.7-4.6) among aspirators. Overall mortality was also higher among aspirators compared with those with pneumonia alone (15% vs 5.2%; OR = 3.1; 95% CI, 2.0-4.9), as was the ICU mortality (9.9% vs 2.8%; OR = 3.8; 95% CI, 2.2-6.8). After adjusting for age, sex, modified LIPS, and APACHE II score, mortality remained significantly higher among aspirators, doubling the risk over those admitted with pneumonia alone (OR = 2.1; 95% CI, 1.2-3.6); the risks for ARDS (OR = 1.0; 95% CI, 0.6-1.6) or moderate to severe ARDS (OR = 1.3; 95% CI, 0.7-2.2) were no longer significant.
Prehospital Medications
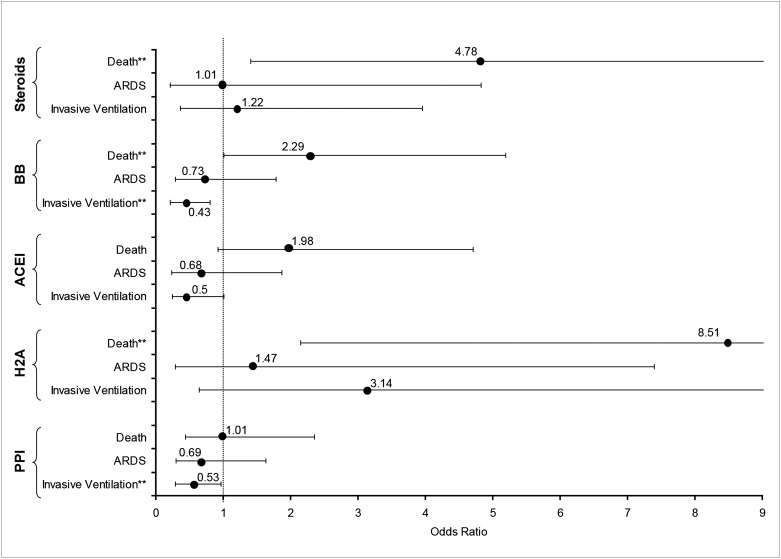
Among the 212 subjects who aspirated, we explored whether the prehospital use of specific medications that affect GERD (PPI), the cough reflex (angiotensin-converting enzyme [ACE] inhibitors), or our host response to aspiration (corticosteroids, β-blockers) might influence the risk for invasive ventilation, ARDS, and death. Figure 2 summarizes the unadjusted risk of these outcomes from the use of PPI, histamine-2 antagonists, β-blockers, ACE inhibitors, and systemic steroids. In univariate analyses, both PPI and β-blockers appear to be protective against the need for invasive ventilation, though not significantly for ARDS. Steroids had no significant effect on respiratory outcomes, and increased the risk of death. After adjusting for age, sex, LIPS, and APACHE, PPI was no longer protective against the need for invasive ventilation (OR = 0.52; 95% CI, 0.25-1.1), while β-blockers remained protective (OR = 0.45; 95% CI, 0.20-0.97). Neither of these had significant effects on ARDS or death in the adjusted analyses.
Figure 2 –
Effect of prehospital medications on need for invasive ventilation, ARDS, and death among patients who aspirated. ORs are shown with their 95% CIs. **Statistically significant. ACEI = angiotensin-converting enzyme inhibitor; BB = β-blocker; H2A = H2 antagonist; PPI = proton pump inhibitor.
Hospital and ICU Costs
Among the four US centers that collected cost data (n = 1,313), aspiration was associated with higher absolute hospital and ICU costs when compared with nonaspirators (Table 4). When comparing to the more analogous group of patients with pneumonia (without aspiration), the absolute hospital cost associated with aspiration was more than fivefold higher (median, $44,915 vs $8,559; P = .0009). However, when adjusted for age, sex, modified LIPS, and APACHE, there was no significant difference in the adjusted costs.
Discussion
To our knowledge, this study represents the largest and most complete characterization of a cohort of patients who aspirate and require hospitalization. Not unexpectedly, aspiration was associated with older age, admission from a nursing home, excessive alcohol use, a lower GCS, and higher severity of illness scores. Somewhat unexpectedly, neither obesity nor a history of GERD was a significant risk factor for all aspirators, while prior chest radiation was more common in those who aspirated. We also identified potential racial differences, with whites being more likely to present with aspiration, though once aspiration occurred, blacks were more likely to progress to ARDS. Regarding medications that may mitigate aspiration-induced lung injury, we found that β-blockers may be protective against the need for invasive mechanical ventilation, though not mortality. Although the subset of patients who aspirate and have another predisposing condition for ARDS are older and sicker, there were no significant differences in outcomes between those who aspirated with or without other predisposing condition for ARDS. Finally, our study indicated that aspiration in unadjusted analyses was associated with higher absolute hospital costs, longer hospital LOS, and almost double the risk for moderate-to-severe ARDS and death.
Significant limitations are inherent in this type of observational report and the findings from the analytic statistics must be considered cautiously. The temporal relationship of the baseline variables to the aspiration event or the subsequent outcomes cannot be firmly established in an observational design. Patients are also influenced by helpful interventions and potentially harmful “hits” (eg, transfusion, excessive fluid administration, iatrogenic infections) during the course of hospitalization (ie, health-care systems factors) that further displace the precise effect of aspiration to its outcomes. Specifically, we did not capture all interventions nor medications administered during the hospital course that may have affected patient outcomes. To correct for this, we attempted to adjust for this with global markers of severity of illness and the subject’s predilection for lung injury. Furthermore, this is possibly the only study in which aspiration was comprehensively and systematically identified at the time of hospital admission, and then followed for outcomes. Although we captured acute neurologic emergencies and a consciousness scale, another important limitation was the lack of capture of chronic neurologic conditions that may impair swallowing such as Parkinson disease, prior strokes, and advanced dementia. These may have confounded our findings with respect to patients admitted from nursing homes.
Another limitation is in the definition of aspiration used. Aspiration is clinically defined, clinician dependent, and often a disease of exclusion. Without a confident biomarker or validated clinical criteria, misclassification bias is likely present. In particular, our findings are confined to “gross” or macroaspiration, and not microaspiration. Although there is renewed interest in biomarkers to more objectively diagnose and quantify aspiration (eg, pepsin),14‐16 none has been validated, and the clinical impact of unwitnessed aspiration or microaspiration events remains uncertain.17 Despite the difficulties in the diagnosis, the findings of this study suggest that the simple definition for aspiration, used in this and prior ARDS epidemiologic studies,7,8 is highly clinically relevant.
Similarly, the distinction between aspiration and pneumonia is also challenging.10 Bacteria can be isolated following aspiration, but most suggest that infection is a secondary consequence, resulting from a reduction in bacterial clearance following aspiration.18 As bronchoscopy and or tracheal aspirates are inappropriate for most community-acquired pneumonia, the CDC clinical criteria13 was used, independent of their diagnosis of aspiration. Despite the difficulties in distinguishing uncomplicated aspiration from infectious pneumonia complicating aspiration from aspiration-induced lung injury, we were able to demonstrate the relative validity of these simple clinical definitions given the significantly worsened prognosis among aspirators, along with a longer LOS and higher hospital costs, compared with patients with pneumonia alone. Despite these and other inherent weaknesses of an observational study, the large multicentered sample, the complete and systematic characterization starting from the “exposure” of aspiration at the time of admission, and the utilization of clinically simple definitions are the major strengths of this study. A randomized controlled trial of aspiration is not possible.
Although some findings were consistent with prior observations, several are noteworthy. The median GCS among aspirators in this cohort was 13, significantly higher than the dogmatic 8 threshold where elective endotracheal intubation is often considered to prevent aspiration. However, the effect of a single unit increase in GCS is not trivial, resulting in a 23% decrease in the odds of aspiration. Based on this and others,19 there is unlikely to be a single threshold in which the risk of aspiration can be confidently assessed by GCS alone. Another notable negative finding was the lack of relationship between aspiration and clinically diagnosed GERD. GERD is a prerequisite for “gastropulmonary” aspiration,20 but additional “hits,” such as infection, the quantity and nature of the aspirate material, and the host immune response, combine to determine the actual consequence of aspiration.17 While reducing acidity may protect against respiratory complications, PPI did not appear to significantly benefit patients who aspirated. It may be because nonacidic gastric contents are critical and synergistic with acid in the development of lung injury.21‐23 Hence, approaching aspiration as a problem of acid-induced lung injury and treating with acid suppression alone may be inadequate, while potentiating the risk of pneumonia.24 Once aspiration occurs, ACE inhibitors, by increasing the cough reflex,25‐27 may potentially protect against pneumonia28; however, no protective effect was observed in our cohort. Although ACE inhibitors may protect against microaspiration, it is unlikely that a “more sensitive” cough reflex will protect against gross aspiration, as identified and defined in this study. Host response is also likely critical in determining whether clinical pathology develops.17,29,30 As with prior reports, steroids proved possibly harmful,16 but β-blockade appeared to reduce the respiratory sequelae of aspiration in this study. β-blockade has been suggested to be helpful in other conditions with significant systemic inflammatory response31,32; and in one animal model of aspiration, β-blockers protected against pneumonia, bacteremia, and death.33 Thus, host response could potentially be targeted to lessen the clinical consequences of aspiration. In the subgroup within aspirators who did not have any other comorbid predisposing condition for ARDS, it was notable that they were younger, had more GERD, and were less ill as compared with other aspirators with additional risks for lung injury. This confirms, not unsurprisingly, the heterogeneity and complexity of aspirators, and may imply that aspiration could be potentially be viewed as two broad subgroups: one in which aspiration is a “complication” of other acute illnesses, chronic comorbidities, aging, and health-care associated factors, and another in which aspiration is a primary event potentiated by issues directly related to the pathway from the stomach to the airways, such as GERD and swallow function. Distinguishing such phenotypes among aspirators in the future may theoretically be helpful toward systematic investigations trying to identify potential interventions. Finally, it was interesting to note that chest radiation appeared to be independently associated with the risk for aspiration, although we are unable to tease out whether this was an effect of acute radiation injury (eg, to the esophagus, lungs, airways, vagus nerve, mucositis) or whether this is also a potential concern for patients with more distant chest radiation from chronic radiation damage to these same structures. Further investigations on these associations would be of interest.
Conclusions
In summary, aspiration is a heterogeneous10,16,22,34 disorder with a variety of manifestations that depend on the nature of the aspirate material, the quantity aspirated, host defenses against aspiration, and the host immune response. To impact on aspiration, the traditional view of aspiration as solely an acid-induced lung injury must be discarded, and each of these steps in the evolution to complications must be better explored. Future targets or interventions may be behavioral at the patient level, process-related at the health-care systems level, and potentially pharmacologic or surgical. We provide here a comprehensive and systematic characterization of gross aspiration and its clinical consequences that should prove informative to both clinicians and researchers. Future studies should further elaborate on characterizing different aspiration syndromes, the continued development of reliable biomarkers, and clinical trials to prevent and mitigate the devastating consequences of aspiration.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: A. L. is the guarantor of the content of this article, including data and analysis. A. L., E. F., P. K. P., K. R., O. D., A. A., O. G., and R. R. B. contributed to conception and design of the study; A.L., E. F., P. K. P., K. R., O. D., A. A., O. G., and R. R. B. contributed to collection, analysis, and interpretation of data; A. L., E. F., P. K. P., K. R., and R. R. B. contributed to generation of figures; A. L., E. F., and R. R. B. contributed to drafting of the article; A. L., E. F., P. K. P., K. R., O. D., A. A., O. G., and R. R. B. contributed to critical revision of the article; and A. L., E. F., P. K. P., K. R., O. D., A. A., O. G., and R. R. B. approved the final version of this article, including the authorship list.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The United States Critical Illness and Injury Trials Group contributed to conception and design of the study, collection of data, and critical revision of the article.
Additional information: The e-Appendix can be found in the Supplemental Materials section of the online article.
ABBREVIATIONS
- ACE
angiotensin-converting enzyme
- APACHE
Acute Physiology and Chronic Health Evaluation
- CDC
Centers for Disease Control and Prevention
- GCS
Glasgow Coma Scale
- GERD
gastroesophageal reflux disease
- IQR
interquartile range
- LIPS
Lung Injury Prediction Score
- LOS
length of stay
- PPI
proton pump inhibitor
Footnotes
Portions of this analysis were presented in abstract form at the 40th Critical Care Congress, January 15-19, 2011, San Diego, CA.
FUNDING/SUPPORT: This work was supported by the National Institutes of Health [HL78743-01A1, KL2 RR024151], the Mayo Clinic Critical Care Research Committee, Mayo Clinic Foundation, and the National Center for Advancing Translational Sciences (NCATS) [CTSA UL1-TR000135].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Pepe PE, Potkin RT, Reus DH, Hudson LD, Carrico CJ. Clinical predictors of the adult respiratory distress syndrome. Am J Surg. 1982;144(1):124-130 [DOI] [PubMed] [Google Scholar]
- 2.Fowler AA, Hamman RF, Good JT, et al. Adult respiratory distress syndrome: risk with common predispositions. Ann Intern Med. 1983;98(5 Pt 1):593-597 [DOI] [PubMed] [Google Scholar]
- 3.Doyle RL, Szaflarski N, Modin GW, Wiener-Kronish JP, Matthay MA. Identification of patients with acute lung injury. Predictors of mortality. Am J Respir Crit Care Med. 1995;152(6 Pt 1):1818-1824 [DOI] [PubMed] [Google Scholar]
- 4.Zilberberg MD, Epstein SK. Acute lung injury in the medical ICU: comorbid conditions, age, etiology, and hospital outcome. Am J Respir Crit Care Med. 1998;157(4 Pt 1):1159-1164 [DOI] [PubMed] [Google Scholar]
- 5.Eisner MD, Thompson T, Hudson LD, et al. ; Acute Respiratory Distress Syndrome Network. Efficacy of low tidal volume ventilation in patients with different clinical risk factors for acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164(2):231-236 [DOI] [PubMed] [Google Scholar]
- 6.Spragg RG, Bernard GR, Checkley W, et al. Beyond mortality: future clinical research in acute lung injury. Am J Respir Crit Care Med. 2010;181(10):1121-1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gajic O, Dabbagh O, Park PK, et al. ; US Critical Illness and Injury Trials Group: Lung Injury Prevention Study Investigators (USCIITG-LIPS). Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. Am J Respir Crit Care Med. 2011;183(4):462-470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685-1693 [DOI] [PubMed] [Google Scholar]
- 9.Teramoto S, Fukuchi Y, Sasaki H, Sato K, Sekizawa K, Matsuse T; Japanese Study Group on Aspiration Pulmonary Disease. High incidence of aspiration pneumonia in community- and hospital-acquired pneumonia in hospitalized patients: a multicenter, prospective study in Japan. J Am Geriatr Soc. 2008;56(3):577-579 [DOI] [PubMed] [Google Scholar]
- 10.Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665-671 [DOI] [PubMed] [Google Scholar]
- 11.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 pt 1):818-824 [DOI] [PubMed] [Google Scholar]
- 12.Ferguson ND, Fan E, Camporota L, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38(10):1573-1582 [DOI] [PubMed] [Google Scholar]
- 13.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309-332 [DOI] [PubMed] [Google Scholar]
- 14.Bohman JK, Kor DJ, Kashyap R, et al. Airway pepsin levels in otherwise healthy surgical patients receiving general anesthesia with endotracheal intubation. Chest. 2013;143(5):1407-1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trinick R, Johnston N, Dalzell AM, McNamara PS. Reflux aspiration in children with neurodisability—a significant problem, but can we measure it? J Pediatr Surg. 2012;47(2):291-298 [DOI] [PubMed] [Google Scholar]
- 16.Raghavendran K, Nemzek J, Napolitano LM, Knight PR. Aspiration-induced lung injury. Crit Care Med. 2011;39(4):818-826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during the perioperative period. Anesthesiology. 1993;78(1):56-62 [DOI] [PubMed] [Google Scholar]
- 18.Rotta AT, Shiley KT, Davidson BA, Helinski JD, Russo TA, Knight PR. Gastric acid and particulate aspiration injury inhibits pulmonary bacterial clearance. Crit Care Med. 2004;32(3):747-754 [DOI] [PubMed] [Google Scholar]
- 19.Manno EM, Rabinstein AA, Wijdicks EF, et al. A prospective trial of elective extubation in brain injured patients meeting extubation criteria for ventilatory support: a feasibility study. Crit Care. 2008;12(6):R138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bice T, Li G, Malinchoc M, Lee AS, Gajic O. Incidence and risk factors of recurrent acute lung injury. Crit Care Med. 2011;39(5):1069-1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knight PR, Rutter T, Tait AR, Coleman E, Johnson K. Pathogenesis of gastric particulate lung injury: a comparison and interaction with acidic pneumonitis. Anesth Analg. 1993;77(4):754-760 [DOI] [PubMed] [Google Scholar]
- 22.Raghavendran K, Davidson BA, Mullan BA, et al. Acid and particulate-induced aspiration lung injury in mice: importance of MCP-1. Am J Physiol Lung Cell Mol Physiol. 2005;289(1):L134-L143 [DOI] [PubMed] [Google Scholar]
- 23.Knight PR, Davidson BA, Nader ND, et al. Progressive, severe lung injury secondary to the interaction of insults in gastric aspiration. Exp Lung Res. 2004;30(7):535-557 [DOI] [PubMed] [Google Scholar]
- 24.Eom CS, Jeon CY, Lim JW, Cho EG, Park SM, Lee KS. Use of acid-suppressive drugs and risk of pneumonia: a systematic review and meta-analysis. CMAJ. 2011;183(3):310-319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fox AJ, Lalloo UG, Belvisi MG, Bernareggi M, Chung KF, Barnes PJ. Bradykinin-evoked sensitization of airway sensory nerves: a mechanism for ACE-inhibitor cough. Nat Med. 1996;2(7):814-817 [DOI] [PubMed] [Google Scholar]
- 26.Ebihara T, Sekizawa K, Ohrui T, Nakazawa H, Sasaki H. Angiotensin-converting enzyme inhibitor and danazol increase sensitivity of cough reflex in female guinea pigs. Am J Respir Crit Care Med. 1996;153(2):812-819 [DOI] [PubMed] [Google Scholar]
- 27.Yeo WW, Chadwick IG, Kraskiewicz M, Jackson PR, Ramsay LE. Resolution of ACE inhibitor cough: changes in subjective cough and responses to inhaled capsaicin, intradermal bradykinin and substance-P. Br J Clin Pharmacol. 1995;40(5):423-429 [PMC free article] [PubMed] [Google Scholar]
- 28.Ohkubo T, Chapman N, Neal B, Woodward M, Omae T, Chalmers J; Perindopril Protection Against Recurrent Stroke Sutdy Collaborative Group. Effects of an angiotensin-converting enzyme inhibitor-based regimen on pneumonia risk. Am J Respir Crit Care Med. 2004;169(9):1041-1045 [DOI] [PubMed] [Google Scholar]
- 29.Kikuchi R, Watabe N, Konno T, Mishina N, Sekizawa K, Sasaki H. High incidence of silent aspiration in elderly patients with community-acquired pneumonia. Am J Respir Crit Care Med. 1994;150(1):251-253 [DOI] [PubMed] [Google Scholar]
- 30.Neubert H, Gale J, Muirhead D. Online high-flow peptide immunoaffinity enrichment and nanoflow LC-MS/MS: assay development for total salivary pepsin/pepsinogen. Clin Chem. 2010;56(9):1413-1423 [DOI] [PubMed] [Google Scholar]
- 31.Patel MB, McKenna JW, Alvarez JM, et al. Decreasing adrenergic or sympathetic hyperactivity after severe traumatic brain injury using propranolol and clonidine (DASH After TBI Study): study protocol for a randomized controlled trial. Trials. 2012;13:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pereira CT, Jeschke MG, Herndon DN. Beta-blockade in burns. Novartis Found Symp. 2007;280:238-248 [DOI] [PubMed] [Google Scholar]
- 33.Prass K, Braun JS, Dirnagl U, Meisel C, Meisel A. Stroke propagates bacterial aspiration to pneumonia in a model of cerebral ischemia. Stroke. 2006;37(10):2607-2612 [DOI] [PubMed] [Google Scholar]
- 34.Marik PE. Pulmonary aspiration syndromes. Curr Opin Pulm Med. 2011;17(3):148-154 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement