Abstract
BACKGROUND:
The surviving sepsis guidelines recommend early aggressive fluid resuscitation within 6 h of sepsis onset. Although rapid fluid administration may offer benefit, studies on the timing of resuscitation are lacking. We hypothesized that there is an association between quicker, adequate fluid resuscitation and patient outcome from sepsis onset time.
METHODS:
This is a retrospective cohort study of consecutive adults with severe sepsis and septic shock admitted to a quaternary care medical ICU between January 2007 and December 2009. Data were collected from a previously validated electronic medical database. Multivariate regression modeling was performed, adjusting for age, admission weight, Sequential Organ Failure Assessment score, APACHE (Acute Physiology and Chronic Health Examination) III score, and total fluid administration within the first 6 h of sepsis onset time.
RESULTS:
Of 651 patients with severe sepsis and septic shock screened, 594 had detailed fluid data. In a univariate analysis, the median amount of fluid within the first 3 h for survivors at discharge was 2,085 mL (940-4,080 mL) and for nonsurvivors, 1,600 mL (600-3,010 mL; P = .007). In comparison, during the latter 3 h, the median amount was 660 mL (290-1,485 mL) vs 800 mL (360-1,680 mL; P = .09), respectively. After adjusting for confounders, the higher proportion of total fluid received within the first 3 h was associated with decreased hospital mortality (OR, 0.34; 95% CI, 0.15-0.75; P = .008).
CONCLUSIONS:
Earlier fluid resuscitation (within the first 3 h) is associated with a greater number of survivors with severe sepsis and septic shock.
Sepsis is the leading cause of death in noncoronary ICUs, with a fatality rate of 20% to 40%,1,2 and is the 11th leading cause of death overall in the United States.3 Furthermore, the incidence of sepsis and sepsis-related deaths has increased in the past 2 decades, despite the decrease in overall in-hospital mortality.2,4 Those who survive sepsis were more likely to require long-term care than those recovering from other acute conditions.5 The estimated cost of sepsis burden in the United States was $14.6 billion in 2008 and has risen annually by 11.9%.1,5
More than 1 decade ago, the term “early-goal directed therapy” (EGDT) was introduced, a protocol for resuscitation within the first 6 h of hospitalization for patients with severe sepsis and septic shock.6 A mortality benefit was found that resulted in global educational efforts and bundled recommendations from the Surviving Sepsis Campaign to help manage severe sepsis and septic shock. Adherence to these bundled recommendations has been associated with improved outcomes, including decrease in-hospital mortality from sepsis.6‐10
Despite proven benefits, there continues to be debate on which elements of EGDT actually prevent mortality. Optimal fluid resuscitation is recognized as a critical component, and studies have addressed the crystalloid/colloid debate.11‐15 However, limited data guide fluid management in the ICU in a time-sensitive manner. Multiple studies have demonstrated harm with standardizing liberal fluid resuscitation, notably when given beyond the initial hours of EGDT.16‐19 On the contrary, anecdotal experience and a few studies show the benefit of out-of-hospital fluid resuscitation by emergency medical services, which suggest that early fluid resuscitation might be better.20,21 The present study is the first, to our knowledge, to examine the timing of fluid resuscitation in patients with severe sepsis and septic shock within the first 6 h in the ICU. The aim was to evaluate for mortality differences in patients who received adequate fluid resuscitation within the first 3 h (hours 0-3) compared with the latter 3 h (hours 3.1-6) of EGDT.
Materials and Methods
Design and Selection
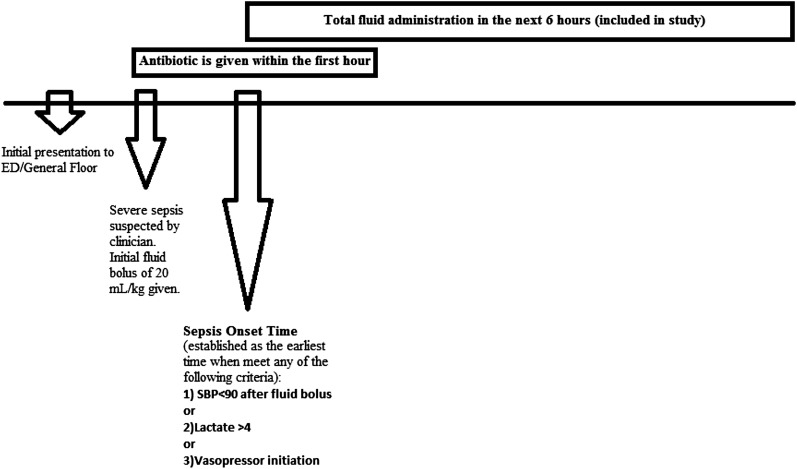
In a single-center retrospective cohort study, consecutive adults aged > 18 years were screened for severe sepsis or septic shock after admission to a medical ICU of a quaternary care academic hospital between January 2007 and December 2009. The study period was selected based on the completeness and accuracy of the available data, which took several years to collect, recheck, and validate against errors. The diagnosis of severe sepsis or septic shock was made based on the 2003 International Sepsis Definitions Consensus Conference.22 We included patients who had suspected infection and one of the following: (1) fluid-resistant hypotension of < 90 mm Hg systolic BP after an initial 20 mL/kg fluid bolus, (2) lactate level > 4 mmol/L, or (3) vasopressor initiation.22 Sepsis onset time was based on when the patient met any of these criteria (Fig 1). Two independent reviewers manually appraised the medical charts for accuracy in meeting the inclusion criteria and determining the sepsis onset time. Discrepancies were resolved by consensus. We excluded mixed shock states (ie, hypovolemia due to hemorrhage or trauma, cardiogenic, neurogenic), patients aged < 18 years, and patients placed on comfort care.
Figure 1 –
Timeline and definition of sepsis onset time. SBP = systolic BP.
The requirement for consent was waived because of the observational nature of the study. Patients were treated according to the institution’s sepsis protocol, and all were given antibiotics and fluids. The study was approved by the Mayo Clinic Institutional Review Board (IRB # 277-04).
Data Collection
The data were derived from a previously validated database, the ICU datamart, which is a real-time relational database generated from the electronic medical record.23 Baseline demographics, BMI on admission, Sequential Organ Failure Assessment (SOFA) score over the first 24 h of sepsis onset time, APACHE (Acute Physiology and Chronic Health Evaluation) III score, Charlson comorbidity index, and hemodynamic variables measured in the sixth hour of sepsis resuscitation, including central venous pressure (CVP), central venous oxygen saturation (Scvo2), and mean arterial pressure (MAP), were collected. Length of hospital stay, duration of mechanical ventilation, presence of oliguria (urine output < 0.5 mL/kg/h), and in-hospital mortality data were also collected. After the initial bolus of 20 mL/kg fluid, the total amount of fluid in milliliters was tallied from the electronic medical record and calculated for the first 3 h and then the latter 3 h for all patients in the cohort. All patients received lactated Ringer’s solution, 0.9% normal saline, or albumin for fluid resuscitation. No starches, dextrans, or gelatins were given. The reported fluid amounts were unbalanced.
Statistical Analysis
Quantitative variables are reported as median with interquartile range. Categorical variables, such as sex, vasopressor use, and presence of oliguria, are reported as the percentage of patients within the subgroup of survivors or nonsurvivors. The primary hypothesis was tested using multivariate logistic regression modeling, adjusted for age, admission weight, SOFA score, APACHE III score, and total fluid given within the first 6 h of sepsis onset time. For univariate analysis, Student t test, χ2 test, and Wilcoxon signed rank test were used as appropriate. The covariates identified in the univariate analysis were adjusted in the multivariate logistic regression model. Total amount of fluid given in the first 3 h and later 3 h had a skewed distribution, so they were log-transformed to satisfy regression assumptions. SAS/JMP, version 9.1 (SAS Institute Inc) software was used for data analysis. CIs and P values were calculated using the standard t test, with P < .05 considered statistically significant.
Results
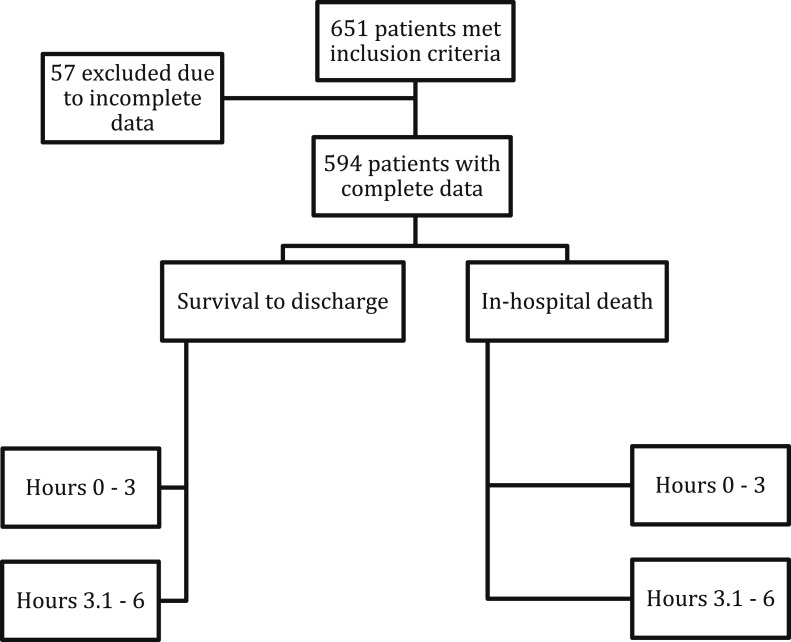
Of the 651 patients who met the inclusion criteria, 57 (8.7%) were excluded due to incomplete data (Fig 2). The median age was 70 years (range, 58-80 years) and 54% (n = 326) were men. Among the cohort, 452 patients survived to discharge and 142 died, resulting in 24% all-cause in-hospital mortality. Table 1 shows the baseline demographics. On the basis of univariate analysis, the survivors were younger, male, and had lower APACHE III scores than nonsurvivors. There was no difference in BMI, timing of antibiotic initiation relative to sepsis onset time, percentage of central venous catheter use, or hospital length of stay between survivors and nonsurvivors. Total amount of fluids given in the first 6 h, age, and APACHE III score were later adjusted in multivariate logistic regression. All patients received resuscitation that complied with the 2008 protocol, which is to give antibiotics within the first hour, start fluid resuscitation as soon as IV access can be established, measure hemodynamic variables as soon as a central venous catheter is placed, and aim for EGDT by 6 h.
Figure 2 –
Study flowchart.
TABLE 1 ] .
Baseline Characteristics of Study Participants
| Variable | Nonsurvivors (n = 142) | Survivors (n = 452) | P Value |
| Male sex | 64 (45) | 262 (58) | < .01a |
| Age, y | 74 (60 to 82) | 69 (58 to 80) | .04a |
| Weight, kg | 82 (65 to 92) | 77 (66 to 99) | .26 |
| BMI, kg/m2 | 28.1 (22.7 to 33.5) | 27.9 (23.7 to 34.4) | .37 |
| APACHE III score | 60 (46.8 to 75.5) | 56 (42 to 70) | .045a |
| Charlson comorbidity index | 5 (3 to 7) | 5 (3 to 7) | .2 |
| Time to antibiotic initiation relative to sepsis onset time, h | 0.13 (−2.2 to 1) | 0.28 (−1.9 to 1.3) | .23 |
| Fluid received in hours 0-3, mL | 1,600 (600 to 3,010) | 2,085 (940 to 4,080) | .007a |
| Fluid received in hours 3.1-6, mL | 880 (360 to 1,680) | 660 (290 to 1,485) | .09 |
| Total fluid received in 6 h, mL | 2,875 (1,390 to 47,20) | 3,150 (1,630 to 5,665) | .10 |
| Net positive fluid balance hours 0-3, mL | 1,460 (550 to 2,815) | 1,790 (705 to 3,665) | .051 |
| Net positive fluid balance hours 3.1-6, mL | 665 (250 to 1,595) | 465 (100 to 1,170) | .0032a |
Data are presented as No. (%) or median (interquartile range). APACHE = Acute Physiology and Chronic Health Evaluation.
P < .05.
In univariate analysis, the median amount of fluid within the first 3 h for survival at discharge vs nonsurvival was 2,085 mL (interquartile range, 940-4,080 mL) vs 1,600 mL (600-3,010 mL; P = .007). In comparison, during the latter 3 h, the median amount of fluid administered was 660 mL (290-1,485 mL) vs 800 mL (360-1,680; P = .09), respectively. The optimal cut point from receiver operating characteristic analysis that jointly maximizes sensitivity and specificity for numbers of survivors based on fluid administration was 1.5 h from sepsis onset time. Table 2 shows hemodynamic variables comparing the survivors and nonsurvivors according to the institutional protocol for sepsis resuscitation. There was no statistical significance between the two groups regarding CVP measured at 6 h from sepsis onset time. However, the nonsurvivors had significantly lower MAP, lower Scvo2, more vasopressor use in the initial 24 h, more oliguria, and higher SOFA scores. In other words, the nonsurvivor group at the end of 6-h resuscitation exhibited worse clinical markers than the survivor group. Although survivors and nonsurvivors did not differ in the number of hospital days, survivors had more ICU-free days and spent more of their hospitalization on a general care floor (Table 3).
TABLE 2 ] .
Clinical Hemodynamic Outcomes
| Variable | Nonsurvivors | Survivors | P Value |
| CVP in hour 6, cm H2O | 10 (5-16) (n = 88) | 10 (5-14) (n = 279) | .52 |
| MAP in hour 6, mm Hg | 64.5 (59-72) (n = 142) | 68.5 (62-77) (n = 452) | < .01a |
| Scvo2 in hour 6 | 68.5 (61-78) (n = 75) | 73 (68-78) (n = 252) | < .01a |
| Vasopressor use in first 24 h, % | 76 (n = 108) | 54 (n = 242) | < .01a |
| Oliguria in hour 6, % | 71 (n = 101) | 41 (n = 186) | < .01a |
| SOFA score day 1 | 8 (6-12) (n = 142) | 6 (4-9) (n = 452) | < .01a |
Data are presented as median (interquartile range) or % unless otherwise indicated. CVP = central venous pressure; MAP = mean arterial pressure; Scvo2 = central venous oxygen saturation; SOFA = Sequential Organ Failure Assessment.
P < .05.
TABLE 3 ] .
Secondary Outcomes
| Outcome | Nonsurvivors (n = 142) | Survivors (n = 452) | P Value |
| ICU length of stay, d | 4.6 (2.2-8.3) | 2.1 (1.2-4.2) | <.001a |
| ICU-free days | 2.8 (0-9.4) | 6.1 (3.1-11.9) | <.001a |
| Hospital length of stay, d | 9.16 (5.58-16.6) | 9.55 (5.5-17.1) | .87 |
Data are presented as median (interquartile range).
P < .05.
Furthermore, the data were incorporated into a multivariate logistic regression adjusting for total fluid administration, age, admission weight, APACHE III score on admission, and SOFA score. There was a statistically significant difference for the amount of fluid given within the first 3 h between survivors and nonsurvivors. The higher proportion of total fluid received within the first 3 h was associated with decreased hospital mortality (OR, 0.34; 95% CI, 0.15-0.75; P = .008) (Table 4).
TABLE 4 ] .
Multivariate Regression
| Variable | OR (95% CI) | P Value |
| Proportion of fluid in first 3 h | 0.34 (0.15-0.75) | .0076 |
| Total fluid in 6 h | 1.00 (1.00-1.00) | .0138 |
| Age | 1.02 (1.01-1.04) | .0050 |
| Weight | 1.00 (0.99-1.01) | .7008 |
| Admission APACHE III score | 1.00 (0.98-1.01) | .4670 |
| SOFA score on day 1 | 1.20 (1.14-1.27) | < .0001a |
Discussion
In this retrospective cohort study of fluid resuscitation in patients with severe sepsis and septic shock, survivors were likely to receive a larger amount of fluid in the first 3 h than nonsurvivors from sepsis onset time. Nonsurvivors were older with higher APACHE III scores, indicating a greater burden of chronic illness. Nonsurvivors had more oliguria, more vasopressor use, worsened hypotension, and lower SOFA scores at the end of 6-h resuscitation. After multivariate adjustment, more fluid resuscitation within the first 3 h was associated with those who survived with severe sepsis and septic shock, even when adjusted for the total amount of fluid in the first 6 h.
This study was performed before the revised guidelines were published24,25; however, to our knowledge, the study is the first to show evidence supporting the new recommendation that initial fluid bolus should be increased from 20 to 30 mL/kg.25 The importance of fluid resuscitation in the first hour has been recognized since the inception of sepsis as a definition and the knowledge of adequate tissue perfusion pressure.26,27 Oliveira et al28 found in children with septic shock who received a< 20 mL/kg dose of fluid in the first hour had a significantly higher mortality rate than those who received 40 mL/kg fluid (73% vs 33%, P < .05). Ospina-Tascon et al29 found that fluids improved sublingual microcirculation perfusion in the early (within 24 h of severe sepsis diagnosis) but not late phase (> 48 h after diagnosis) of sepsis. Furthermore, there is mounting evidence that late sepsis resuscitation results in harmful effects, such as increased mortality and worsening lung and renal function.18,30‐33
Debate continues on how best to assess fluid status during resuscitation. CVP is widely used, but studies have shown that CVP poorly predicts a sustained and significant hemodynamic response to a fluid challenge as defined by an increase in cardiac output or MAP.34,35 The present findings neither support nor contradict the use of CVP as a predictor for outcome because the goal of CVP was achieved in both survivors and nonsurvivors. This is in accordance with the recently published Protocol-Based Care for Early Septic Shock (ProCESS) trial, a randomized controlled trial in 31 EDs across the United States that assigned 1,341 patients to three groups of resuscitation methods.36 The ProCESS investigators found no difference in mortality between usual-care resuscitation, which is bedside, physician-directed care, and the two protocolized methods studied, including EGDT with central venous catheter monitoring and a standardized protocol model that used adequate peripheral venous access for resuscitation with optional central venous access if peripheral access could not be obtained. The present findings support using Scvo2, MAP, or both as surrogate hemodynamic markers for resuscitation.24 Some nonsurvivors did not reach the goal Scvo2 or MAP by the end of the sixth hour for EGDT, which was statistically significant. Oliguria was significantly more prevalent in the nonsurvivor group, further indicating that fluid resuscitation did not achieve adequate tissue perfusion and probably resulted in end-organ damage. Nonsurvivors had a larger fluid balance in hours 3.1 to 6 than survivors. Total fluid administration was not different between the two groups, which likely reflects the decreased urine output of the nonsurvivors (Table 2).
The ProCESS trial also measured hourly fluid volume administration, and the total volume of fluid given in the 6 h of initial resuscitation varied among the three groups (protocol-based standard therapy, 3.3 L; protocol-based EGDT, 2.8 L; usual care, 2.3 L; P < .0001).36 The standard therapy group received the most fluid initially compared with the usual-care and EDGT groups, and the EGDT group received fluid at the most consistent rate (P = .007). The present study had similar inclusion criteria to the ProCESS trial; however, some key differences may account for the conclusions. In the ProCESS trial, fluid volume was tracked from the time of randomization into the study, which may have been up to 2 h after the sepsis onset time or time of initial shock. By then, subjects had already received 20 to 30 mL/kg fluid before randomization. Additionally, all three arms of the ProCESS trial received more fluid in the earlier part of the 6 h, with less fluid given over time. The earlier fluid resuscitation may account for the lack of outcome differences in the trial and may have contributed to the overall low 60-day in-hospital mortality rate of 19%.
Another retrospective cohort study found that there were more nonsurvivors than survivors with a higher fluid balance after 8 days and a trend with higher fluid balance in the first 24 h from septic shock onset.37 The authors found an association between normal and slightly elevated left ventricular function in nonsurvivors, suggesting the presence of vasoplegia.37 This study correlates with the present findings in that nonsurvivors required more vasopressors and had worsened SOFA scores in the initial 24 h from sepsis onset time. It is possible that nonsurvivors exhibited multiorgan dysfunction and vasoplegia beyond EGDT and, therefore, received more fluid after initial resuscitation and throughout the following days, resulting in more total fluid after ≥ 24 h.
The major strengths in this study are the intentionally broad inclusion criteria to be sensitive in capturing the most patients with severe sepsis and septic shock and minimal loss of data due to use of ICU Datamart database, resulting in a large cohort. The 8.7% excluded because of data incompleteness was due to computer medical error. ED fluid resuscitation was included in the calculation of initial fluid bolus. Because laboratory and fluid data from the ED are integrated into the database with review of the electronic medical record, the sepsis onset time could be determined systematically and based on each patient’s clinical parameters. The secondary outcome of survivors having more ICU-free days despite a similar number of overall hospitalization days has implications for health-care costs because higher costs are incurred in the ICU.38 Further study on cost-effectiveness is warranted.
This study is limited in that the prehospital fluid resuscitation and fluid administration before progression to severe sepsis and septic shock may not be consistently reported and accounted for. Furthermore, we do not have an explanation documented in the charts for unachieved resuscitative goals in the nonsurvivor group at the end of the 6-h resuscitation. We conjecture that a predominate factor for unachieved goal resuscitation was lack of timely, adequate vascular access because there was missing CVP and Scvo2 data, measurements that require a central venous catheter. It is possible that attempts were made to achieve venous access when a diagnosis of severe sepsis or septic shock was made; however, variability in patient anatomy and operator skill may have led to lack of timely central venous access, EGDT goal measurements, and resuscitation. Alternatively, nonsurvivors had higher APACHE III and SOFA scores than survivors, indicating a greater burden of critical illness, which may not have been reversible with resuscitation and antibiotic treatment. We incorporated APACHE III and SOFA scores into the multivariate logistic regression model, but unknown confounding factors and bias may not be fully accounted for by the measurements in the retrospective analysis. We also did not stratify the fluid amounts hour by hour because this would require a much larger study to achieve significant power. The data were limited to 3 years and collected before the current guidelines were published because it took several years to collect and validate the data against errors. It is unknown whether a maximum ceiling dose of fluid exists in sepsis resuscitation such that it becomes harmful with subsequent complications, such as brisk electrolyte shifting, ischemic-reperfusion injuries, abdominal compartment syndrome, cerebral edema, or other dangerous edematous states.3,39‐42 Furthermore, this is a single-center study at an academic quaternary center, so the results may not be generalizable to community-based and other models of ICU care. Finally, the study does not prove causality or reduction in mortality, limitations inherent to a retrospective analysis, and these will require prospective validation. The observed association may have been due to an unmeasured risk factor, thereby confounding results. However, the association was present in univariate and multivariate analyses. It is interesting to note that the two study groups (survivors and nonsurvivors) stratified by the end of the 6-h time frame of EGDT, which supports the notion that early intervention is key to prevent sustained organ damage and may set the course for the rest of the hospitalization. We hope that this article is hypothesis generating for future studies on the timing of fluid resuscitation in sepsis.
Conclusions
The purpose of this study is to aid intensivists in the management of fluid resuscitation in sepsis by focusing on the timing of administration. In this multivariate logistic regression analysis adjusting for the total amount of fluid given and severity of illnesses, survivors received more fluid resuscitation within the first 3 h after diagnosis of severe sepsis and septic shock than did nonsurvivors. To our knowledge, this study is the first of its kind to examine the timing of fluid resuscitation within an EGDT time frame for critically ill adults with severe sepsis and septic shock. The findings support the recently published guidelines recommendation of increasing the initial fluid bolus to 30 mL/kg for sepsis resuscitation.
Acknowledgments
Author contributions: R. K. had full access to all of the data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analysis. S. J. L. and R. K. contributed to the research design, data collection, and writing of the manuscript; S. J. L., G. L., and R. K. contributed to the data analysis and interpretation; K. R., J. G. P., and O. G. contributed to the critical revisions important for the intellectual content of the manuscript; and S. J. L., K. R., J. G. P., O. G., G. L., and R. K. contributed to the approval of the final version of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
ABBREVIATIONS
- APACHE
Acute Physiology and Chronic Health Evaluation
- CVP
central venous pressure
- EGDT
early goal-directed therapy
- MAP
mean arterial pressure
- ProCESS
Protocol-Based Care for Early Septic Shock
- Scvo2
central venous oxygen saturation
- SOFA
Sequential Organ Failure Assessment
Footnotes
Part of this article has been presented in abstract form at the 42nd Critical Care Congress, January 19-23, 2013, San Juan, Puerto Rico.
FUNDING/SUPPORT: This publication was supported by the National Center for Advancing Translational Sciences [Grant UL1 TR000135].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303-1310 [DOI] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546-1554 [DOI] [PubMed] [Google Scholar]
- 3.Maitland K, George EC, Evans JA, et al. ; FEAST Trial Group. Exploring mechanisms of excess mortality with early fluid resuscitation: insights from the FEAST trial. BMC Med. 2013;11:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA. 2014;311(13):1308-1316 [DOI] [PubMed] [Google Scholar]
- 5.Hall MJ, Williams SN, DeFrances CJ, Golosinskiy A. Inpatient care for septicemia or sepsis: a challenge for patients and hospitals. NCHS Data Brief. 2011;(62): 1-8 [PubMed] [Google Scholar]
- 6.Gurnani PK, Patel GP, Crank CW, et al. Impact of the implementation of a sepsis protocol for the management of fluid-refractory septic shock: a single-center, before-and-after study. Clin Ther. 2010;32(7):1285-1293 [DOI] [PubMed] [Google Scholar]
- 7.Trzeciak S, Dellinger RP, Abate NL, et al. Translating research to clinical practice: a 1-year experience with implementing early goal-directed therapy for septic shock in the emergency department. Chest. 2006;129(2):225-232 [DOI] [PubMed] [Google Scholar]
- 8.Cruz AT, Perry AM, Williams EA, Graf JM, Wuestner ER, Patel B. Implementation of goal-directed therapy for children with suspected sepsis in the emergency department. Pediatrics. 2011;127(3):e758-e766 [DOI] [PubMed] [Google Scholar]
- 9.Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med. 2010;36(2):222-231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levy MM, Dellinger RP, Townsend SR, et al. ; Surviving Sepsis Campaign. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med. 2010;38(2):367-374 [DOI] [PubMed] [Google Scholar]
- 11.Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R; SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350(22):2247-2256 [DOI] [PubMed] [Google Scholar]
- 12.Myburgh JA, Finfer S, Bellomo R, et al. ; CHEST Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. 2012;367(20):1901-1911 [DOI] [PubMed] [Google Scholar]
- 13.Perner A, Haase N, Guttormsen AB, et al. ; 6S Trial Group; Scandinavian Critical Care Trials Group. Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med. 2012;367(2):124-134 [DOI] [PubMed] [Google Scholar]
- 14.Haase N, Perner A. Is hydroxyethyl starch 130/0.4 safe? Crit Care. 2012;16(2):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bayer O, Reinhart K, Kohl M, et al. Effects of fluid resuscitation with synthetic colloids or crystalloids alone on shock reversal, fluid balance, and patient outcomes in patients with severe sepsis: a prospective sequential analysis. Crit Care Med. 2012;40(9):2543-2551 [DOI] [PubMed] [Google Scholar]
- 16.Durairaj L, Schmidt GA. Fluid therapy in resuscitated sepsis: less is more. Chest. 2008;133(1):252-263 [DOI] [PubMed] [Google Scholar]
- 17.Vincent JL, Sakr Y, Sprung CL, et al. ; Sepsis Occurrence in Acutely Ill Patients Investigators. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34(2):344-353 [DOI] [PubMed] [Google Scholar]
- 18.Wiedemann HP, Wheeler AP, Bernard GR, et al. ; National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564-2575 [DOI] [PubMed] [Google Scholar]
- 19.Bihari S, Prakash S, Bersten AD. Post resuscitation fluid boluses in severe sepsis or septic shock: prevalence and efficacy (price study). Shock. 2013;40(1):28-34 [DOI] [PubMed] [Google Scholar]
- 20.Seymour CW, Cooke CR, Mikkelsen ME, et al. Out-of-hospital fluid in severe sepsis: effect on early resuscitation in the emergency department. Prehosp Emerg Care. 2010;14(2):145-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Studnek JR, Artho MR, Garner CL, Jr, Jones AE. The impact of emergency medical services on the ED care of severe sepsis. Am J Emerg Med. 2012;30(1):51-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy MM, Fink MP, Marshall JC, et al. ; SCCM/ESICM/ACCP/ATS/SIS. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250-1256 [DOI] [PubMed] [Google Scholar]
- 23.Herasevich V, Kor DJ, Li M, Pickering BW. ICU data mart: a non-IT approach. A team of clinicians, researchers and informatics personnel at the Mayo Clinic have taken a homegrown approach to building an ICU data mart. Healthc Inform. 2011;28(11):42, 44-5 [PubMed] [Google Scholar]
- 24.Dellinger RP, Levy MM, Rhodes A, et al. ; Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580-637 [DOI] [PubMed] [Google Scholar]
- 25.Dellinger RP, Levy MM, Rhodes A, et al. ; Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165-228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644-1655 [DOI] [PubMed] [Google Scholar]
- 27.Marik PE, Varon J. The hemodynamic derangements in sepsis: implications for treatment strategies. Chest. 1998;114(3):854-860 [DOI] [PubMed] [Google Scholar]
- 28.Oliveira CF, Nogueira de Sá FR, Oliveira DS, et al. Time- and fluid-sensitive resuscitation for hemodynamic support of children in septic shock: barriers to the implementation of the American College of Critical Care Medicine/Pediatric Advanced Life Support Guidelines in a pediatric intensive care unit in a developing world. Pediatr Emerg Care. 2008;24(12):810-815 [DOI] [PubMed] [Google Scholar]
- 29.Ospina-Tascon G, Neves AP, Occhipinti G, et al. Effects of fluids on microvascular perfusion in patients with severe sepsis. Intensive Care Med. 2010;36(6):949-955 [DOI] [PubMed] [Google Scholar]
- 30.Alsous F, Khamiees M, DeGirolamo A, Amoateng-Adjepong Y, Manthous CA. Negative fluid balance predicts survival in patients with septic shock: a retrospective pilot study. Chest. 2000;117(6):1749-1754 [DOI] [PubMed] [Google Scholar]
- 31.Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39(2):259-265 [DOI] [PubMed] [Google Scholar]
- 32.Maitland K, Kiguli S, Opoka RO, et al. ; FEAST Trial Group. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364(26):2483-2495 [DOI] [PubMed] [Google Scholar]
- 33.Hanson JP, Lam SW, Mohanty S, et al. Fluid resuscitation of adults with severe falciparum malaria: effects on acid-base status, renal function, and extravascular lung water. Crit Care Med. 2013;41(4):972-981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magder S, Bafaqeeh F. The clinical role of central venous pressure measurements. J Intensive Care Med. 2007;22(1):44-51 [DOI] [PubMed] [Google Scholar]
- 35.Marik PE, Baram M, Vahid B. Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest. 2008;134(1):172-178 [DOI] [PubMed] [Google Scholar]
- 36.Yealy DM, Kellum JA, Huang DT, et al. ; ProCESS Investigators. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370(18)L1683-1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Micek ST, McEvoy C, McKenzie M, Hampton N, Doherty JA, Kollef MH. Fluid balance and cardiac function in septic shock as predictors of hospital mortality. Crit Care. 2013;17(5):R246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chalupka AN, Talmor D. The economics of sepsis. Crit Care Clin. 2012;28(1):57-76 [DOI] [PubMed] [Google Scholar]
- 39.Lee JW. Fluid and electrolyte disturbances in critically ill patients. Electrolyte Blood Press. 2010;8(2):72-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beger HG, Rau B, Mayer J, Pralle U. Natural course of acute pancreatitis. World J Surg. 1997;21(2):130-135 [DOI] [PubMed] [Google Scholar]
- 41.Mahmood I, Mahmood S, Parchani A, et al. Intra-abdominal hypertension in the current era of modern trauma resuscitation. ANZ J Surg. 2014;84(3):166-171 [DOI] [PubMed] [Google Scholar]
- 42.Turner J, Nicholl J, Webber L, Cox H, Dixon S, Yates D. A randomised controlled trial of prehospital intravenous fluid replacement therapy in serious trauma. Health Technol Assess. 2000;4(31):1-57 [PubMed] [Google Scholar]