Abstract
BACKGROUND:
Approximately 65% of elderly patients with lung cancer who are admitted to the ICU will die within 6 months. Efforts to improve end-of-life care for this population must first understand the patient factors that underlie admission to the ICU.
METHODS:
We performed a retrospective cohort study examining all fee-for-service inpatient claims in the Surveillance, Epidemiology, and End Results (SEER)-Medicare registry for elderly patients (aged > 65 years) who had received a diagnosis of lung cancer between 1992 and 2005 and who were hospitalized for reasons other than resection of their lung cancer. We calculated yearly rates of ICU admission per 1,000 hospitalizations via room and board codes or International Classification of Diseases, Ninth Revision, Clinical Modification and diagnosis-related group codes for mechanical ventilation, stratified the rates by receipt of mechanical ventilation and ICU type (medical/surgical/cardiac vs intermediate), and compared these rates over time.
RESULTS:
A total of 175,756 patients with lung cancer in SEER were hospitalized for a reason other than surgical resection of their tumor during the study period, 49,373 (28%) of whom had at least one ICU stay. The rate of ICU admissions per 1,000 hospitalizations increased over the study period from 140.7 in 1992 to 201.7 in 2005 (P < .001). The majority of the increase in ICU admissions (per 1,000 hospitalizations) between 1992 and 2005 occurred among patients who were not mechanically ventilated (118.2 to 173.3, P < .001) and among those who were in intermediate ICUs (20.0 to 61.9, P < .001), but increased only moderately in medical/surgical/cardiac units (120.7 to 139.9, P < .001).
CONCLUSIONS:
ICU admission for patients with lung cancer increased over time, mostly among patients without mechanical ventilation who were largely cared for in intermediate ICUs.
Approximately one in four elderly patients with lung cancer who are admitted to the ICU will die during their hospital stay, and 65% will die within 6 months.1,2 Despite significant advancements in the treatment of lung cancer and in the care of critically ill patients over the last 15 years, these survival statistics remain unchanged.2 When confronting the end of life, the vast majority of patients with lung cancer would rather forego aggressive nonpalliative care, stating that they would prefer to die at home.3,4 Some estimates, however, suggest that up to one in four patients with lung cancer will be hospitalized in the ICU prior to death, a finding not consistent with the wish to avert aggressive nonpalliative care.5‐7
Efforts to make the end-of-life care of individuals with lung cancer more concordant with their wishes must first characterize the extent of and reasons for ICU use in this population. On the one hand, the aggressiveness of nonpalliative care may be increasing over time, as reflected by greater use of mechanical ventilation (MV), a therapy associated with close to 90% mortality within 6 months.2,5 On the other hand, growth in palliative and hospice care may have increased the rates of death at home and reduced the use of MV among ICU admissions, or at least limited the use of the ICU to patients with a better prognosis.8,9 Less aggressive nonpalliative critical care may also be reflected by changes in the type of ICU to which patients are being admitted. For example, intermediate ICUs are often used as less intense alternatives to traditional ICUs, yet still provide greater nursing support than is usually available on hospital floors.10,11 To date, little is known about the extent to which ICU admission patterns among patients with lung cancer have changed.
We sought to examine longitudinal trends in ICU admission rates and the reason for ICU admission among hospitalized patients with lung cancer. We used the largest population-based registry of elderly patients with lung cancer in the United States and, based on past work, hypothesized that ICU use among patients with lung cancer would be increasing and that ICU care would be more aggressive, as measured by the use of MV and the use of traditional full-service ICUs vs intermediate ICUs.
Materials and Methods
Design
We conducted a retrospective cohort study examining the Surveillance, Epidemiology, and End Results (SEER)-Medicare registry, a publically available database provided by the National Cancer Institute.12 The SEER program collects extensive information, including cancer type, histology, and stage of disease, for patients given a diagnosis of cancer living in one of the SEER geographic regions. Through its links to Medicare, the SEER-Medicare registry provides a comprehensive way to assess hospital use among a nationally representative sample of elderly patients with cancer.13
Patient Sample
Details of the study cohort were described previously.2 Briefly, patients were eligible if they had received a diagnosis of lung cancer between 1992 and 2005 (n = 324,509) and they were also enrolled in Medicare during that time. We excluded (1) patients whose lung cancer was diagnosed at autopsy or on a death certificate only (n = 7,650), (2) those with an in situ stage (n = 195), (3) patients younger than 66 years old at the time of diagnosis (n = 56,048), (4) those who were eligible for Medicare benefits because of disability (n = 25,078), and (5) those enrolled in Medicare-managed care plans between 1 year prior to and 5 years after a lung cancer diagnosis (n = 59,629) because these patients may have incomplete claims, and are, typically, excluded in studies using Medicare claims.14,15 Finally, those whose discharge date was after their date of death (n = 153) were excluded from all analyses because this likely indicated data entry errors.
Study Variables
We identified an ICU admission as an ICU room charge and/or a procedure code for MV using International Classification of Diseases, Ninth Revision procedure codes (96.7x) in any of the six procedure fields or a diagnosis-related group code indicating MV (475 or 483).16 We excluded ICU admissions that occurred within 30 days (before and after) of the presence of a procedure code for surgical resection of the lung cancer (32.1-32.59).17 To further characterize the intensity of ICU use, we identified the subset of ICU admissions to intermediate ICUs, because admission patterns for these units may differ from more resource-intensive ICUs. In Medicare Provider and Review File (MedPAR), ICU location is coded as the location where the majority of ICU care is provided during the hospitalization.
We obtained age, sex, race, lung cancer histology, and stage from SEER and estimated annual income by linking the patient’s zip code to the 2000 census. We derived the number of comorbidities for each hospitalization from International Classification of Diseases, Ninth Revision, Clinical Modification codes present in the MedPAR discharge abstract using the Deyo modification of the Charlson index.12,18 ICU location (eg, intermediate ICU vs full-service ICU [i.e., medical/surgical/other ICU]) and admission diagnosis (primary diagnosis field) were obtained from MedPAR. We classified the admission diagnosis for each hospitalization with an ICU stay into 285 mutually exclusive categories using Clinical Classification Software developed by the Agency for Health Care Research and Quality.19
Statistical Analysis
We calculated summary statistics for the characteristics of hospitalized patients with and without an ICU stay by ICU type using percentages, means (SDs), and medians (interquartile ranges). For each year, we calculated the incidence of hospitalizations with an ICU stay per 1,000 hospitalizations, stratified by the dominant location of care (intermediate ICU vs full-service ICU) and by whether the ICU stay involved MV, and plotted the rates over time. We used the χ2 test to compare incidence rates between 1992 and 2005 and to compare the absolute change in the top 15 admission diagnoses among hospitalizations in the ICU from 1992 to 2005. To determine the independent relationship between patient characteristics and ICU admission, and full-service ICU vs intermediate ICU admission, we used logistic regression to generate adjusted ORs and respective 95% CIs.
Data management and analysis were performed using SAS 9.2 (SAS Institute Inc) and Stata 13 (StataCorp). We conducted the study under a data-use agreement with the Centers for Medicare and Medicaid Services and received approval from the institutional review board of the Portland Veterans Affairs Medical Center (IRB No. 1 VA No. 07-1309).
Results
Of the 175,756 patients with lung cancer, 49,373 patients (28%) had at least one ICU admission for reasons other than surgical resection of their lung cancer, and 90,675 patients (52%) had at least one non-ICU hospitalization (Table 1). Among patients with an ICU admission, 15,932 involved a stay in an intermediate ICU. Compared with patients hospitalized outside an ICU, patients in an ICU were more often younger, male, and of nonwhite race. Patients in an ICU also had a greater median annual income and a greater number of comorbid conditions, and more often had local or regional disease, relative to patients not in an ICU (Table 1). Patients admitted to intermediate ICU care were slightly older and had a higher income and more comorbidities compared with full-service patients in the ICU.
TABLE 1 ] .
Characteristics of Patients With Lung Cancer Admitted to Hospital Stratified by Location of Hospitalization
| Characteristic | Any Non-ICU Hospitalization (n = 90,675) | Any ICU Admission (n = 49,373) | Full-Service ICU Admission (n = 33,441) | Intermediate-Care ICU Admission (n = 15,932) |
| Age | ||||
| 65-69 y | 15,511 (17.1) | 9,984 (20.2) | 7,234 (21.7) | 2,750 (17.3) |
| 70-74 y | 23,634 (26.1) | 14,384 (29.1) | 10,060 (30.1) | 4,324 (27.1) |
| 75-79 y | 23,634 (26.1) | 13,060 (26.5) | 8,706 (26.0) | 4,354 (27.3) |
| 80-84 y | 16,981 (18.7) | 7,923 (16.0) | 5,039 (15.1) | 2,884 (18.1) |
| 85-89 y | 7,997 (8.8) | 3,159 (6.4) | 1,924 (5.8) | 1,235 (7.8) |
| ≥ 90 y | 2,981 (3.2) | 869 (1.7) | 484 (1.4) | 385 (2.4) |
| Women | 43,223 (47.7) | 22,271 (45.1) | 13,865 (44.0) | 8,406 (47.2) |
| Race | ||||
| Non-Hispanic white | 79,047 (87.5) | 41,650 (84.6) | 28,174 (84.2) | 13,476 (84.8) |
| Black | 6,572 (7.3) | 4,216 (8.6) | 2,931 (8.8) | 1,285 (8.1) |
| Other | 4,714 (5.2) | 3,350 (6.8) | 2,220 (6.6) | 1,130 (7.1) |
| Median annual income | ||||
| < 15,000 USD | 22,714 (26.1) | 9,263 (19.8) | 7,204 (21.5) | 2,059 (13.5) |
| 15,000-29,000 USD | 10,105 (11.6) | 6,211 (13.3) | 4,284 (12.7) | 1,927 (12.7) |
| 30,000-44,990 USD | 22,379 (25.7) | 12,376 (26.4) | 8,243 (24.6) | 4,133 (27.2) |
| 45,000-59,990 USD | 16,244 (18.7) | 9,719 (20.7) | 6,101 (18.2) | 3,618 (23.8) |
| 60,000-74,990 USD | 8,175 (9.4) | 4,914 (10.5) | 3,068 (9.2) | 1,846 (12.1) |
| ≥ 75,000 USD | 7,344 (8.4) | 4,371 (9.3) | 2,734 (8.2) | 1,637 (10.8) |
| Comorbidity (Charlson index) | ||||
| 0 | 71,924 (79.3) | 37,367 (75.5) | 25,487 (76.2) | 11,880 (75.7) |
| 1 | 9,131 (10.1) | 5,312 (10.8) | 3,546 (10.6) | 1,766 (11.1) |
| 2 | 5,436 (6.0) | 3,582 (7.3) | 2,346 (7.0) | 1,236 (7.8) |
| ≥ 3 | 4,184 (4.6) | 3,112 (6.3) | 2,062 (6.2) | 1,050 (6.6) |
| Stage at diagnosis | ||||
| Local | 14,260 (15.7) | 9,782 (19.8) | 6,447 (19.3) | 3,335 (20.9) |
| Regional | 21,105 (23.3) | 13,293 (26.9) | 9,146 (27.3) | 4,147 (26.2) |
| Distant | 47,873 (52.8) | 22,538 (45.6) | 15,234 (45.6) | 7,304 (45.8) |
| Unknown | 7,437 (8.2) | 3,760 (7.6) | 2,641 (7.9) | 1,119 (7.0) |
| Histology | ||||
| NSCLC | 70,758 (78) | 39,645 (80.3) | 26,868 (80.3) | 12,777 (80.2) |
| SCLC | 12,922 (14.3) | 6,488 (13.1) | 4,425 (13.2) | 2,063 (12.9) |
| Other | 6,995 (7.7) | 3,240 (6.6) | 2,148 (6.4) | 1,092 (6.9) |
Data are presented as No. (%). NSCLC = non-small cell lung cancer; SCLC = small cell lung cancer; USD = US dollars.
Factors Associated With ICU Admission
On multivariable analysis, factors independently associated with a greater likelihood of ICU admission included younger age, male sex, nonwhite race, higher income, greater number of comorbidities, and local cancer stage (Table 2). Older age was a strong predictor of reduced ICU use. For example, relative to 65- to 69-year-old patients, patients who were > 90 years old were 58% less likely to be admitted to the ICU (OR, 0.42; 95% CI, 0.38-0.46). However, when older patients were admitted to an ICU, they were more likely to be admitted to intermediate units rather than to full-service ICUs. For example, compared with 65- to 69-year-old patients, the odds of intermediate ICU use were 1.24-fold greater (95% CI, 1.06-1.19) for 70- to 74-year-old patients and increased to 2.14-fold greater (95% CI, 1.84-2.48) for those > 90 years old.
TABLE 2 ] .
Factors Associated With ICU vs Non-ICU Hospital Admission and Intermediate ICU vs Medical/Surgical/Other ICU Admission Among Patients Given a Diagnosis of Lung Cancer Between 1992 and 2005
| Characteristic | ICU vs Non-ICU Hospitalization | P Value | Intermediate ICU vs Full-Service ICU Admission | P Value |
| Age | ||||
| 65-69 y | Reference | … | … | … |
| 70-74 y | 0.93 (0.89-0.96) | < .001 | 1.24 (1.06-1.19) | < .001 |
| 75-79 y | 0.80 (0.77-0.83) | < .001 | 1.28 (1.21-1.36) | < .001 |
| 80-84 y | 0.67 (0.64-0.70) | < .001 | 1.47 (1.37-1.57) | < .001 |
| 85-89 y | 0.55 (0.51-0.58) | < .001 | 1.64 (1.50-1.79) | < .001 |
| ≥ 90 y | 0.42 (0.38-0.46) | < .001 | 2.14 (1.84-2.48) | < .001 |
| Sex | ||||
| Women | Reference | … | … | … |
| Men | 1.10 (1.07-1.12) | < .001 | 0.9 (0.87-0.94) | < .001 |
| Race/ethnicity | ||||
| Non-Hispanic white | Reference | … | … | … |
| Black | 1.22 (1.16-1.28) | < .001 | 1.04 (0.97-1.12) | .29 |
| Other | 1.33 (1.26-1.41) | < .001 | 1.06 (0.98-1.25) | .14 |
| Median annual income | ||||
| < 15,000 USD | Reference | … | … | … |
| 15,000-29,000 USD | 1.47 (1.40-1.54) | < .001 | 1.55 (1.44-1.67) | < .001 |
| 30,000-44,990 USD | 1.36 (1.31-1.41) | < .001 | 1.72 (1.61-1.83) | < .001 |
| 45,000-59,990 USD | 1.48 (1.41-1.54) | < .001 | 2.02 (1.89-2.16) | < .001 |
| 60,000-74,990 USD | 1.48 (1.41-1.56) | < .001 | 2.04 (1.89-2.20) | < .001 |
| ≥ 75,000 USD | 1.50 (1.42-1.57) | < .001 | 2.05 (1.89-2.22) | < .001 |
| Comorbidities | ||||
| 0 | Reference | … | … | … |
| 1 | 1.16 (1.11-1.21) | < .001 | 1.06 (0.99-1.13) | .07 |
| 2 | 1.27 (1.20-1.34) | < .001 | 1.08 (1.00-1.26) | .05 |
| ≥ 3 | 1.46 (1.38-1.54) | < .001 | 1.05 (0.96-1.13) | .29 |
| Lung cancer histology | ||||
| NSCLC | Reference | … | … | … |
| SCLC | 0.91 (0.87-0.94) | < .001 | 1.02 (0.96-1.08) | .56 |
| Other | 0.79 (0.75-0.83) | < .001 | 1.0 (0.92-1.08) | .99 |
| Stage at diagnosis | ||||
| Local | Reference | … | … | … |
| Regional | 0.90 (0.87-0.94) | < .001 | 0.90 (0.84-0.95) | < .001 |
| Distant | 0.61 (0.59-0.63) | < .001 | 0.91 (0.86-0.96) | < .001 |
| Unknown | 0.74 (0.70-0.79) | < .001 | 0.81 (0.75-0.89) | < .001 |
Data are presented as adjusted OR (95% CI). See Table 1 legend for expansion of abbreviations.
Trends in ICU Admission Over Time
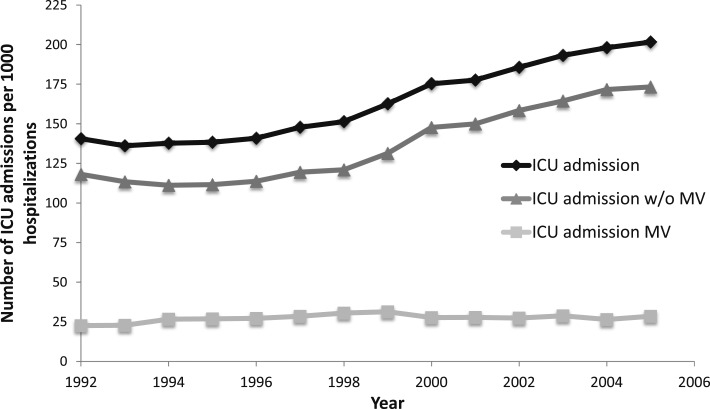
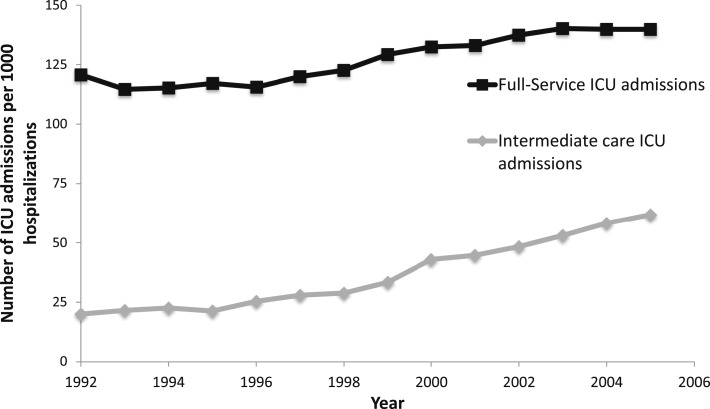
Over the study period, there were significant increases in ICU admission that were largely attributed to patients who were nonventilated (Fig 1). For example, in 1992, the incidence of ICU admission (per 1,000 hospitalizations) was 141 (95% CI, 136-146), and it had increased to 202 (95% CI, 198-206) in 2005 (Fig 1, Table 3). Among the subset of ICU admissions that did not require MV, there was a 1.47-fold (95% CI, 1.40-1.53) increase in ICU admissions between 1992 and 2005. In addition, increases in ICU use were also greatest among patients admitted to intermediate ICUs (Fig 2). Intermediate ICU admissions increased 3.09-fold (95% CI, 2.78-3.44) between 1992 and 2005 compared with a 1.16-fold (95% CI, 1.11-1.21) increase among full-service ICU admissions (Table 3).
Figure 1 –
Rate of ICU admission (overall and with and without MV) per 1,000 hospitalizations. MV = mechanical ventilation.
TABLE 3 ] .
Changes in the Incidence of ICU Admission by Mechanical Ventilation Status and Location Among Individuals Given a Diagnosis of Lung Cancer Between 1992 and 2005
| Incidence Outcome (Not Mutually Exclusive) | Incidence per 1,000 Hospitalizations (95% CI) | Risk Ratio (2005/1992) (95% CI) | P Value | |
| 1992 | 2005 | |||
| ICU admission | 141 (136-146) | 202 (198-206) | 1.43 (1.38-1.49) | < .001 |
| Mechanical ventilation | 23 (21-25) | 29 (27-30) | 1.26 (1.13-1.41) | < .001 |
| No mechanical ventilation | 118 (114-123) | 173 (169-177) | 1.47 (1.40-1.53) | < .001 |
| Nonintermediate ICU | 121 (116-125) | 139.9 (136-144) | 1.16 (1.11-1.21) | < .001 |
| Intermediate ICU | 20 (18-22) | 62 (59-64) | 3.09 (2.78-3.44) | < .001 |
Figure 2 –
Rate of ICU admission by ICU type (full-service ICU, intermediate ICU) per 1,000 hospitalizations.
Changes in Admission Diagnosis
The most frequent primary diagnoses among patients with lung cancer hospitalized in an ICU in 1992 and 2005 are shown in Table 4. The top two most common primary diagnoses, lung cancer (cancer of the bronchus, lung) or other malignancies (secondary malignancies), did not change between 1992 and 2005. However, there were significant increases among respiratory failure, sepsis, and pulmonary heart disease. For example, respiratory failure (respiratory failure, insufficiency, arrest) ranked fourth in 1992 and third in 2005, accounting for a 3.6% (95% CI, 2.4% to 4.7%) point increase in the proportion of annual hospitalizations with an ICU stay with a primary diagnosis of respiratory failure. Of patients receiving a respiratory failure diagnosis, 30.7% were mechanically ventilated in 1992, compared with 40.7% in 2005. Sepsis (septicemia [except in labor]) similarly increased in rank from 12th in 1992 to fifth in 2005, accounting for a 2.9% (95% CI, 2.15% to 3.6%) point increase. Primary diagnoses of congestive heart failure and coronary artery disease decreased in frequency, whereas other diagnoses in the top 15 in 2005 did not show significant changes.
TABLE 4 ] .
Top 15 Admission Diagnosis Categories Coded at Discharge for ICU Admissions Among Patients Given a Diagnosis of Lung Cancer Between 1992 and 2005
| Primary Discharge Diagnosis | Rank by Frequency (% of Total for Year) | Absolute % Point Change, 1992-2005 (95% CI) | |
| 1992 | 2005 | ||
| Cancer of bronchus, lung | 1 (25.7) | 1 (23.7) | −1.9 (−4.2 to 0.3) |
| Secondary malignancies | 2 (11.7) | 2 (12.3) | 0.6 (−1.0 to 2.3) |
| Respiratory failure, insufficiency, arrest | 4 (4.2) | 3 (7.9) | 3.6 (2.4 to 4.7) |
| Pneumonia (except that caused by TB or STD) | 3 (7.3) | 4 (6.9) | −0.4 (−1.7 to 0.9) |
| Septicemia (except in labor) | 12 (1.6) | 5 (4.4) | 2.9 (2.1 to 3.6) |
| Cardiac dysrhythmias | 6 (3.3) | 6 (3.7) | 0.3 (−0.6 to 1.2) |
| Acute myocardial infarction | 7 (3.1) | 7 (2.7) | −0.5 (−1.3 to 0.4) |
| Congestive heart failure, nonhypertensive | 5 (3.9) | 8 (2.4) | −1.6 (−2.4 to −0.6) |
| COPD and bronchiectasis | 9 (2.7) | 9 (2.3) | −0.4 (−1.1 to 0.4) |
| Acute cerebrovascular disease | 11 (1.6) | 10 (1.9) | 0.3 (−0.3 to 1.0) |
| Coronary atherosclerosis and other heart disease | 8 (3.0) | 11(1.7) | −1.3 (−2.1 to −0.5) |
| Pleurisy, pneumothorax, pulmonary collapse | 13 (1.4) | 12 (1.6) | 0.2 (−0.4 to 0.8) |
| Complications of surgical procedures or medical care | 17 (1.1) | 13 (1.5) | 0.3 (−0.2 to 0.9) |
| Pulmonary heart disease | 33 (0.5) | 14 (1.4) | 0.9 (0.5 to 1.3) |
| GI hemorrhage | 10 (1.7) | 15 (1.4) | −0.2 (−0.8 to 0.4) |
| Total % of patients | 72.8 | 75.8 | |
STD = sexually transmitted disease.
Discussion
We demonstrated that between 1992 and 2005, there was close to a 40% increase in the use of ICUs for the care of elderly patients with lung cancer hospitalized for reasons other than resection of their tumor. Increases in ICU admission were greatest among individuals not requiring MV, as well as among those hospitalized in intermediate units rather than in full-service ICUs. Most patients were hospitalized in an ICU for reasons directly related to their underlying lung cancer, but diagnoses of respiratory failure without MV and sepsis appear to be increasing, whereas cardiac causes for admission are decreasing. Finally, although close to one-half of patients with lung cancer admitted to ICUs had distant disease, ICU admission was less likely for older patients with distant disease.
Our study complements existing work that demonstrates increasing use of the ICU over time for patients with cancer.5,6,20 Several studies demonstrated a slightly lower increase in ICU admission than that observed in our analysis. For example, studies from the United States and Canada demonstrated increases in ICU admission from 3.1% to 7.2% to 5.4% to 9.4% in patients who are dying with cancer during the 1990s.5,20 The lower rates and smaller increases in ICU admission observed in these studies likely result from their inclusion of multiple types of cancer with lower mortality rates than lung cancer. Sharma and colleagues6 examined patients with lung cancer and demonstrated similar increases in ICU admission, from 17.5% to 24.7%, between 1993 and 2002. In contrast to our analysis, their study examined only SEER-Medicare patients in the last 6 months of life.
Noting that many patients who are dying with cancer often receive ICU care that they may not want, many experts point toward this literature as evidence that the palliative care and hospice movement has not been wholly successful at eliminating ICU care among such patients,21 even though involving palliative care is encouraged at the time of lung cancer diagnosis.22 Studies of Medicare beneficiaries with stage IV lung cancer suggest that approximately one-half elect hospice care.23 Teno and colleagues7 determined that although the use of hospice among the elderly at the time of death has nearly doubled, from 23% to 42% over the last 10 years, one-third of hospice stays are for < 3 days and are often preceded by an ICU stay. These findings suggest that hospice is not being used to replace high-intensity ICU care, but rather as an “add on” to high-intensity ICU care. However, it is not clear from Teno and colleagues7 whether ICU care in their study occurred in traditional ICUs or in lower-intensity intermediate ICU settings.
Our study provides some of the first insights into the aggressiveness of ICU admission among patients with lung cancer. Over the study period, we observed slightly increased MV rates among ICU admissions and a threefold increase in admission rates to intermediate ICUs. Intermediate ICUs are often used as a less intense and less costly alternative to traditional ICUs in either a “step-up” or a “step-down” role, while still providing access to greater nursing and monitoring than in typical hospital wards.10,11 These units are often used for monitoring of patients at the lowest risk of death, and to free ICU beds for more acutely ill patients. It is possible that physicians, in an effort to minimize overly aggressive ICU care for patients with poor prognoses, are increasingly using the intermediate ICUs to provide nursing or monitoring not available on the wards. Less intense triage may be reflected by our observation that the likelihood of admission to an intermediate ICU was greatest for the oldest patients. Many intermediate ICUs have the capability to deliver noninvasive ventilation,24 which may be an alternative mode of support for patients with lung cancer with respiratory failure who do not wish to be intubated. Although we were unable to examine noninvasive ventilation in our data, a recent study showed a nearly 400% increase in the use of noninvasive ventilation in patients without COPD over the last decade.25 Greater use of noninvasive ventilation may be partially responsible for the observed increase in respiratory failure as the admission diagnosis for patients with lung cancer. Although the continued growth in ICU use among patients with lung cancer is a concern, these findings suggest that providers may be consciously shifting at least a proportion of ICU care toward the less intense intermediate ICUs.
Our findings should be placed in the context of several limitations. First, because we used administrative data, we had limited ability to account for other factors that are known to drive ICU admission. In particular, we could not examine the role of severity of illness and physiologic derangement and we did not have access to other hospital factors, such as ICU or intermediate ICU bed availability, that are known to influence ICU admission decisions.26,27 Importantly, family preferences for intensive care were not measured in the current study.4,26 Second, we were unable to determine if increases in intermediate care use are unique to patients with lung cancer or reflect broader trends in the Medicare population. We were also unable to formally characterize how full-service ICUs differ from intermediate ICUs in terms of availability of life support or nurse staffing. In fact, little is known about the spectrum of services offered in intermediate care throughout the United States.10,11 A related limitation is our inability to distinguish patients who spent their entire ICU time in an intermediate care setting from those who only spent the majority of their time in intermediate care. However, even if patients coded as intermediate care spend time in medical/cardiac/other ICUs, our results still support a significant growth in the amount of time patients with lung cancer are spending in intermediate care settings.
Conclusions
In summary, we found that growth in the use of the ICU for patients with lung cancer over time is greatest among patients admitted to intermediate ICUs, a location where more elderly were admitted relative to other ICU types. Lung cancer was a common reason to be admitted to the ICU, but respiratory failure and sepsis as causes for ICU admission are increasing. ICU use was more common among patients who were younger and those who had greater comorbid illness, as well as those with nondistant disease. These data suggest that providers zare reserving the more intensive services in an ICU for patients with the greatest ability to benefit.
Acknowledgments
Author contributions: C. R. C. and C. G. S. contributed to the conception of the study and drafting or revision of the manuscript for important intellectual content and take full responsibility for the content of the manuscript, including the integrity of the data and the accuracy of the analysis. L. C. F. and R. S. W. contributed to the interpretation of the data and revision of the manuscript for important intellectual content; C. R. C., M. E. O., and C. G. S. contributed to the data analysis; L. C. F., R. S. W., and M. E O. contributed to the revision of the manuscript for important intellectual content; and C. R. C., L. C. F., R. S. W., M. E. O., and C. G. S. contributed to approval of the final manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript. The views expressed here are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the National Institutes of Health.
ABBREVIATIONS
- MedPAR
Medicare Provider and Review File
- MV
mechanical ventilation
- SEER
Surveillance, Epidemiology, and End Results
Footnotes
FUNDING/SUPPORT: This work was supported in part by the Agency for Healthcare Research and Quality [Grant K08 HS020672 to Dr Cooke], the National Cancer Institute [Grant K07 CA138772 to Dr Wiener], the National Heart, Lung and Blood Institute [Grant K23 HL111116 to Dr Feemster], and the Department of Veterans Affairs [to Drs Wiener and Feemster].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Toffart AC, Minet C, Raynard B, et al. Use of intensive care in patients with nonresectable lung cancer. Chest. 2011;139(1):101-108 [DOI] [PubMed] [Google Scholar]
- 2.Slatore CG, Cecere LM, Letourneau JL, et al. Intensive care unit outcomes among patients with lung cancer in the Surveillance, Epidemiology, and End Results-Medicare registry. J Clin Oncol. 2012;30(14):1686-1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Higginson IJ, Sen-Gupta GJ. Place of care in advanced cancer: a qualitative systematic literature review of patient preferences. J Palliat Med. 2000;3(3):287-300 [DOI] [PubMed] [Google Scholar]
- 4.Barnato AE, Herndon MB, Anthony DL, et al. Are regional variations in end-of-life care intensity explained by patient preferences?: A study of the US Medicare population. Med Care. 2007;45(5):386-393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Earle CC, Neville BA, Landrum MB, Ayanian JZ, Block SD, Weeks JC. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol. 2004;22(2):315-321 [DOI] [PubMed] [Google Scholar]
- 6.Sharma G, Freeman J, Zhang D, Goodwin JS. Trends in end-of-life ICU use among older adults with advanced lung cancer. Chest. 2008;133(1):72-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teno JM, Gozalo PL, Bynum JP, et al. Change in end-of-life care for Medicare beneficiaries: site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA. 2013;309(5):470-477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Center for Health Statistics. Health, United States, 2010: with special feature on death and dying. http://www.cdc.gov/nchs/data/hus/hus10.pdf. Accessed December 11, 2013 [PubMed]
- 9.Morrison RS, Maroney-Galin C, Kralovec PD, Meier DE. The growth of palliative care programs in United States hospitals. J Palliat Med. 2005;8(6):1127-1134 [DOI] [PubMed] [Google Scholar]
- 10.Zimmerman JE, Wagner DP, Knaus WA, Williams JF, Kolakowski D, Draper EA. The use of risk predictions to identify candidates for intermediate care units: implications for intensive care utilization and cost. Chest. 1995;108(2):490-499 [DOI] [PubMed] [Google Scholar]
- 11.Junker C, Zimmerman JE, Alzola C, Draper EA, Wagner DP. A multicenter description of intermediate-care patients: comparison with ICU low-risk monitor patients. Chest. 2002;121(4):1253-1261 [DOI] [PubMed] [Google Scholar]
- 12.National Cancer Institute. SEER-Medicare. Medicare claims files. 2011. http://appliedresearch.cancer.gov/seermedicare/medicare/claims.html. Accessed June 25, 2014
- 13.Potosky AL, Riley GF, Lubitz JD, Mentnech RM, Kessler LG. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31(8):732-748 [PubMed] [Google Scholar]
- 14.Schermerhorn ML, O’Malley AJ, Jhaveri A, Cotterill P, Pomposelli F, Landon BE. Endovascular vs. open repair of abdominal aortic aneurysms in the Medicare population. N Engl J Med. 2008;358(5):464-474 [DOI] [PubMed] [Google Scholar]
- 15.Wunsch H, Guerra C, Barnato AE, Angus DC, Li G, Linde-Zwirble WT. Three-year outcomes for Medicare beneficiaries who survive intensive care. JAMA. 2010;303(9):849-856 [DOI] [PubMed] [Google Scholar]
- 16.Quan H, Parsons GA, Ghali WA. Validity of procedure codes in International Classification of Diseases, 9th revision, clinical modification administrative data. Med Care. 2004;42(8):801-809 [DOI] [PubMed] [Google Scholar]
- 17.Bach PB, Cramer LD, Schrag D, Downey RJ, Gelfand SE, Begg CB. The influence of hospital volume on survival after resection for lung cancer. N Engl J Med. 2001;345(3):181-188 [DOI] [PubMed] [Google Scholar]
- 18.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619 [DOI] [PubMed] [Google Scholar]
- 19.Healthcare Cost and Utilization Project - Agency for Healthcare Research and Quality. Clinical classifications software (CCS) for ICD-9-CM. http://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed December 11, 2013
- 20.Ho TH, Barbera L, Saskin R, Lu H, Neville BA, Earle CC. Trends in the aggressiveness of end-of-life cancer care in the universal health care system of Ontario, Canada. J Clin Oncol. 2011;29(12):1587-1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenq G, Tinetti ME. Changes in end-of-life care over the past decade: more not better. JAMA. 2013;309(5):489-490 [DOI] [PubMed] [Google Scholar]
- 22.Ford DW, Koch KA, Ray DE, Selecky PA. Palliative and end-of-life care in lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5_suppl):e498S-512S [DOI] [PubMed] [Google Scholar]
- 23.Mack JW, Chen K, Boscoe FP, et al. Underuse of hospice care by Medicaid-insured patients with stage IV lung cancer in New York and California. J Clin Oncol. 2013;31(20):2569-2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nava S, Sturani C, Hartl S, et al. ; European Respiratory Society Task Force on Ethics and decision-making in end stage lung disease. End-of-life decision-making in respiratory intermediate care units: a European survey. Eur Respir J. 2007;30(1):156-164 [DOI] [PubMed] [Google Scholar]
- 25.Walkey AJ, Wiener RS. Use of noninvasive ventilation in patients with acute respiratory failure, 2000-2009: a population-based study. Ann Am Thorac Soc. 2013;10(1):10-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Escher M, Perneger TV, Chevrolet JC. National questionnaire survey on what influences doctors’ decisions about admission to intensive care. BMJ. 2004;329(7463):425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stelfox HT, Hemmelgarn BR, Bagshaw SM, et al. Intensive care unit bed availability and outcomes for hospitalized patients with sudden clinical deterioration. Arch Intern Med. 2012;172(6):467-474 [DOI] [PubMed] [Google Scholar]