Abstract
BACKGROUND:
The risk factors for acute episodes of respiratory disease in current and former smokers who do not have COPD are unknown.
METHODS:
Eight thousand two hundred forty-six non-Hispanic white and black current and former smokers in the Genetic Epidemiology of COPD (COPDGene) cohort had longitudinal follow-up (LFU) every 6 months to determine acute respiratory episodes requiring antibiotics or systemic corticosteroids, an ED visit, or hospitalization. Negative binomial regression was used to determine the factors associated with acute respiratory episodes. A Cox proportional hazards model was used to determine adjusted hazard ratios (HRs) for time to first episode and an acute episode of respiratory disease risk score.
RESULTS:
At enrollment, 4,442 subjects did not have COPD, 658 had mild COPD, and 3,146 had moderate or worse COPD. Nine thousand three hundred three acute episodes of respiratory disease and 2,707 hospitalizations were reported in LFU (3,044 acute episodes of respiratory disease and 827 hospitalizations in those without COPD). Major predictors included acute episodes of respiratory disease in year prior to enrollment (HR, 1.20; 95% CI, 1.15-1.24 per exacerbation), airflow obstruction (HR, 0.94; 95% CI, 0.91-0.96 per 10% change in % predicted FEV1), and poor health-related quality of life (HR, 1.07; 95% CI, 1.06-1.08 for each 4-unit increase in St. George’s Respiratory Questionnaire score). Risks were similar for those with and without COPD.
CONCLUSIONS:
Although acute episode of respiratory disease rates are higher in subjects with COPD, risk factors are similar, and at a population level, there are more episodes in smokers without COPD.
More than 100 million people have a smoking history in the United States, and 20% of the population currently smokes.1 Many of these people experience episodes of acute respiratory disease characterized by increased or new shortness of breath, cough, and/or change in sputum quantity or quality.2 The cost of treating these acute episodes exceeds $30 billion per year.3 The current medical knowledge and literature are confusing because these acute episodes of respiratory disease have multiple terminologies such as acute bronchitis,4 exacerbations of chronic bronchitis,5 and acute exacerbations of COPD,6 despite the fact that the pathophysiology (bacterial and viral infections) and treatment (corticosteroids and antibiotics) are similar. Furthermore, most studies of acute episodes of respiratory disease in current and former smokers include only patients with significant airflow limitation (ie, moderate or more severe COPD) despite population surveys indicating that most current and former smokers do not meet the spirometric criteria for COPD.1 This large understudied group may pose a significant, underrecognized health-care burden, and there is an unmet need to define and quantitate their risk of acute episodes of respiratory disease.
Most current knowledge of acute episodes of respiratory disease in current and former smokers comes from studies of patients with COPD. These episodes are referred to as acute exacerbations of COPD and are associated with decreased quality of life,7,8 increased lung function decline,9 and higher mortality.10‐12 Multiple cross-sectional studies have identified common factors associated with acute exacerbations of COPD such as severe airflow obstruction, poor health-related quality of life,13 gastroesophageal reflux,14 and chronic bronchitis.15 Age, sex, BMI, a history of cardiovascular disease, theophylline use, and a lack of influenza vaccine are also independent risk factors.16‐18 Most of these cross-sectional studies did not include smokers without COPD and few contained a large number of underrepresented populations (eg, blacks).
The Genetic Epidemiology of COPD (COPDGene) study has multiple unique features that make it ideal for studying these unmet needs: (1) it is one of the largest prospective studies of subjects with COPD and also those who are at risk of, but do not meet the spirometric criteria for, COPD; (2) it includes a large number of blacks; and (3) its subjects have been well characterized clinically and by quantitative high-resolution chest CT scan.
Materials and Methods
Study Population
The COPDGene study consists of 10,300 subjects at 21 centers across the United States.19 COPDGene was approved by the institutional review board at each participating center, and all subjects provided written informed consent. The current analysis was approved by the National Jewish Health Institutional Review Board (AS-1887). Subjects were enrolled from January 2008 to April 2011. At the time of enrollment, all subjects were 45 to 80 years old, had a history of smoking for at least 10 pack-years, and had not had an acute respiratory exacerbation for at least 30 days prior to enrollment. Additional characteristics of the study population and study methodology have been described previously.15,19,20 Although originally designed as a single-visit cross-sectional study, the COPDGene cohort was converted into a longitudinal study in its second year. Of the 10,300 subjects originally enrolled, 8,246 participated in a longitudinal follow-up (LFU), defined as at least one follow-up contact at least 6 months after initial enrollment. LFU was conducted every 6 months by telephonic, web-based inquiry as described previously.21 A research coordinator contacted those subjects who did not complete a telephonic or web-based follow-up.
Clinical Definitions
COPD was defined as a postbronchodilator FEV1/FVC ratio < 0.70. COPD was further classified as I to IV based on Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines,22 where GOLD I: FEV1/FVC < 0.7 and FEV1 ≥ 80%; GOLD II: FEV1/FVC < 0.7 and 50% ≤ FEV1, 80% predicted; GOLD III: FEV1/FVC < 0.7 and 30% ≤ FEV1, 50% predicted; GOLD IV: FEV1/FVC < 0.7 and FEV1 < 30% predicted. Current or ex-smokers at risk of COPD but without spirometric evidence of airflow obstruction (FEV1/FVC ≥ 0.70) were classified as control subjects (formerly, GOLD 0). Subjects with FEV1/FVC ≥ 0.70 and FEV1 < 80% were considered unclassified (GOLD U).23 Emphysema was quantified by the percent of lung voxels < −950 Hounsfield units on the inspiratory images of CT scan. Gas trapping was quantified by the percent of lung voxels < −856 Hounsfield units on the expiratory images. The pulmonary artery was measured in the tubular portion and the aorta was measured at the arch, independently by two observers who were blinded to the clinical data. The mean measurement was recorded, and when the two observers varied by > 10%, a third measurement was made and the median was taken.
Acute episodes of respiratory disease were assessed by asking, “Since we last spoke, have you had an episode of increased cough and phlegm or shortness of breath, which lasted 48 hours or more?” If the subject answered yes, he/she was further asked whether he/she had received antibiotics or corticosteroids. Only events that were associated with antibiotics or corticosteroids were counted. Additional questions asked at each LFU contact included whether the subject went to an ED or was hospitalized. A severe episode was a report of hospitalization for an acute episode of respiratory disease. The total number of episodes was defined as the sum during all follow-up periods. The time to an episode was determined using the date of enrollment and the date at which the episode was first reported.
Statistical Analysis
Unless otherwise specified, analyses were conducted using SAS version 9.3 (SAS Institute Inc) or R version 2.14 (R Development Core Team). Episodes in the year prior to enrollment and during LFU were modeled with negative binomial regression, with offset for exposure time and a zero-inflation model to account for the excess number of subjects who reported no acute episodes of respiratory disease. Cox proportional hazards multiple regression was used to determine hazard ratios (HRs) for time to first episode. Variable selection for multiple variable modeling is discussed in e-Appendix 1 (603.4KB, pdf) . The stepwise multiple regression models variable selection method used an entry probability of < 0.15 and an exit probability of > 0.05.
Results
Subject Characteristics
Demographic and baseline characteristics of the COPDGene LFU subjects by spirometry classification are reported in Table 1. A total of 36,231 follow-up surveys were completed in 8,246 subjects followed for an average of 3 ± 1 years. The median number of surveys completed during LFU was five (all groups) with slightly fewer average surveys in the GOLD U group (4.35 ± 2.12 per year), compared with the control group (4.66 ± 2.19 per year) and the COPD group (4.74 ± 2.02 per year) (P < .001). During this time, 9,303 moderate and 2,708 severe (hospitalized) acute episodes of respiratory disease were reported. Two thousand four hundred seven subjects (29%) reported at least one moderate exacerbation, and 1,108 (13%) reported at least one severe respiratory exacerbation during follow-up.
TABLE 1 ] .
Subject Characteristics (N = 8,246)
| Characteristics | Control Subjects (n = 3,444) | GOLD U (n = 998) | COPD (n = 3,804) | P Value |
| Age, y | 58 ± 9 | 58 ± 8 | 64 ± 8 | < .001 |
| Female sex, % | 51 | 57 | 48 | < .001 |
| Current smoker, % | 52 | 57 | 39 | < .001 |
| Smoking history, pack-y | 37 ± 20 | 43 ± 24 | 52 ± 27 | < .001 |
| GERD, % | 23 | 29 | 30 | < .001 |
| Race (non-Hispanic white), % | 67 | 64 | 81 | < .001 |
| Physiology | ||||
| BMI, kg/m2 | 29 ± 6 | 32 ± 7 | 30 ± 6 | < .001 |
| Postbronchodilator FEV1, L | 2.85 ± 0.68 | 2.03 ± 0.49 | 1.63 ± 0.77 | < .001 |
| Postbronchodilator FEV1, % predicted | 97 ± 11 | 70 ± 8 | 57 ± 23 | … |
| Postbronchodilator FEV1/FVC | 0.79 ± 0.05 | 0.76 ± 0.05 | 0.52 ± 0.13 | … |
| Pre- and postbronchodilator change in FEV1, % | 3.9 ± 6.5 | 3.9 ± 10.6 | 8.5 ± 12.4 | < .001 |
| Distance walked in 6 min, m | 460 ± 106 | 388 ± 111 | 381 ± 121 | < .001 |
| BODE index | 0.4 ± 0.8 | 1.2 ± 1.4 | 2.5 ± 2.1 | < .001 |
| Using oxygen on enrollment, % | 1 | 5 | 25 | < .001 |
| HRCT scan measurements | ||||
| Emphysema,a % | 2 ± 3 | 1 ± 3 | 12 ± 12 | < .001 |
| Gas trapping,b % | 11 ± 10 | 11 ± 9 | 36 ± 21 | < .001 |
| Pi10c | 3.64 ± 0.11 | 3.72 ± 0.13 | 3.70 ± 0.14 | < .001 |
| WAd | 60.0 ± 2.8 | 62.4 ± 3.1 | 62.3 ± 3.2 | < .001 |
| Patient-reported outcomes | ||||
| MRC dyspnea score | 0.7 ± 1.1 | 1.5 ± 1.5 | 1.9 ± 1.5 | < .001 |
| SF-36 General Healthe | 69 ± 21 | 56 ± 23 | 54 ± 24 | < .001 |
| SGRQ | 16 ± 17 | 29 ± 23 | 36 ± 23 | < .001 |
| Acute episodes of respiratory disease 12 mo prior to entry | ||||
| Moderate and severe | 0.14 ± 0.54 | 0.35 ± 0.86 | 0.65 ± 1.18 | < .001 |
| Severe (hospitalized) | 0.02 ± 0.23 | 0.07 ± 0.34 | 0.16 ± 0.57 | < .001 |
| Acute episodes of respiratory disease during LFU | ||||
| Years followed | 3.0 ± 1.2 | 2.7 ± 1.1 | 2.9 ± 1.1 | < .001 |
| Moderate and severe, No./y | 0.20 ± 0.73 | 0.34 ± 0.91 | 0.65 ± 1.26 | < .001 |
| Severe, No./y | 0.06 ± 0.39 | 0.11 ± 0.42 | 0.21 ± 0.66 | < .001 |
Data are presented as mean ± SD for continuous measures and % for dichotomous variables. Control subjects: FEV1 ≥ 80% predicted; GOLD U: FEV1/FVC ≥ 0.7 and FEV1% < 80. P values represent the probability that GOLD group variable means are the same. BODE = BMI, airflow obstruction, dyspnea, exercise capacity; GERD = gastroesophageal reflux disease; GOLD = Global Initiative for Chronic Obstructive Lung Disease; HRCT = high-resolution CT; LAA = lung attenuation area; LFU = longitudinal follow-up; MRC = Medical Research Council; SF-36 = 36-Item Short Form Health Survey; SGRQ = St. George’s Respiratory Questionnaire; U = unclassified.
LAA % < −950 Hounsfield units on inspiration.
LAA % < −856 on expiration.
Square root of wall area percent for 10-μm airway.
Segmental wall area percent.
Only 47% of cohort had this measurement.
Factors Predictive of Exacerbations and Hospitalizations
On enrollment, many variables were significantly associated with a history of acute episodes of respiratory disease in the previous year by both random forest analysis (e-Table 1 (603.4KB, pdf) ) and univariate negative binomial regression with zero inflation (e-Table 2 (603.4KB, pdf) ). Similar associations were observed for ED visits (data not shown) and hospitalizations for acute episodes of respiratory disease (e-Table 3 (603.4KB, pdf) ). Multivariate regression revealed that the strongest predictors of a future acute episode of respiratory disease in LFU were baseline St. George’s Respiratory Questionnaire (SGRQ) score, airflow limitation (FEV1 % predicted), and number of acute episodes of respiratory disease within the previous year (Table 2). Other factors that were independently predictive in some subgroups included female sex, a history of gastroesophageal reflux disease (GERD), chronic bronchitis, a parental history of COPD, oxygen use, and gas trapping. Black race was independently associated with ED visits and hospitalizations for acute episodes of respiratory disease. The identical variables were also statistically significant when the total number of moderate and severe exacerbations were modeled using multivariable negative binomial regression with zero inflation (data not shown).
TABLE 2 ] .
Factors Independently Associated With Acute Episodes of Respiratory Disease on LFU
| Time Period and Factor | All Subjects | Control Subjects | GOLD U | COPD (GOLD I, II, III, IV) | ||||
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Moderate to severe episodes | ||||||||
| Female sex | 1.33 (1.22-1.46) | < .0001 | 1.66 (1.37-2.02) | < .0001 | 1.08 (0.81-1.45 | .5803 | 1.27 (1.14-1.41) | < .0001 |
| GERD | 1.31 (1.20-1.43 | < .0001 | 1.36 (1.13-1.64) | .0014 | 1.21 (0.91-1.61) | .1774 | 1.29 (1.16-1.45) | < .0001 |
| Chronic bronchitis | 1.15 (1.03-1.28) | .0091 | 0.99 (0.77-1.27) | .9131 | 1.57 (1.13-2.18) | .0076 | 1.14 (1.01-1.29) | .0335 |
| Parent with history of COPD | 1.21 (1.09-1.35) | .0004 | 1.22 (0.95-1.56) | .1114 | 0.93 (0.63-2.37) | .7135 | 1.28 (1.12-1.45) | .0002 |
| Oxygen use | 1.12 (1.01-1.27) | .0582 | 1.0.03 (0.57-1.86) | .9263 | 1.42 (0.88-2.27) | .1078 | 1.14 (1.01-1.30) | .0397 |
| Previous diagnosis of asthma | 1.44 (1.30-1.59) | < .0001 | 1.92 (1.54-2.40) | < .0001 | 1.32 (0.95-1.84) | .1023 | 1.30 (1.15-1.47) | .0198 |
| SGRQ, per 4-point increase | 1.07 (1.06-1.08) | < .0001 | 1.09 (1.07-1.11) | < .0001 | 1.05 (1.02-1.08) | .0003 | 1.05 (1.04-1.07) | < .0001 |
| Episodes previous year, per event | 1.20 (1.15-1.24) | < .0001 | 1.43 (1.30-1.57) | < .0001 | 1.19 (1.07-1.33) | .0016 | 1.19 (1.14-1.23) | < .0001 |
| FEV1, per 10%, % predicted | 0.94 (0.91-0.96) | < .0001 | 0.99 (0.86-1.01) | .0911 | 1.00 (0.85-1.18) | .9700 | 0.95 (0.91-1.98) | .0059 |
| Gas trapping per 10% | 1.07 (1.04-1.10) | < .0001 | 1.00 (0.91-1.09) | .9282 | 1.07 (0.93-1.23) | .3402 | 1.08 (1.04-1.12) | < .0001 |
| Hospitalized episodes | ||||||||
| Hospitalization previous year, per event | 1.34 (1.24-1.44) | < .0001 | 1.39 (1.11-1.75) | .0045 | 1.29 (0.88-1.91) | .1947 | 1.35 (1.25-1.46) | < .0001 |
| FEV1, per 10%, % predicted | 0.84 (0.81-0.87) | < .0001 | 1.10 (0.94-1.29) | .2475 | 0.95 (0.75-1.19) | .6961 | 0.83 (0.80-0.88) | < .0001 |
| SGRQ, per 4-point increase | 1.10 (1.08-1.11) | < .0001 | 1.12 (1.08-1.17) | < .0001 | 1.14 (1.09-1.19) | < .0001 | 1.08 (1.06-1.10) | < .0001 |
| GERD | 1.19 (1.03-1.37) | .0194 | 1.48 (0.98-2.24) | .0606 | 1.35 (0.84-2.29) | .2159 | 1.15 (0.97-1.35) | .1058 |
| Oxygen use | 1.31 (1.10-1.55) | .0017 | 2.99 (1.31-6.77) | .0089 | 1.36 (0.65-2.84) | .4101 | 1.25 (1.05-1.49) | .0138 |
| Black | 1.34 (1.14-1.58) | .0005 | 1.90 (1.25-2.90) | .0028 | 1.44 (0.89-2.33) | .1421 | 1.25 (1.02-1.53) | .0278 |
| Previous diagnosis of asthma | 1.41 (1.21-1.65) | < .0001 | 2.48 (1.61-3.83) | < .0001 | 0.98 (0.57 0 1.68) | .9417 | 1.34 (1.13-1.59) | .0010 |
| Parent with COPD | 1.35 (1.15-1.59) | .0002 | 1.50 (0.90-2.51) | .1198 | 0.87 (0.43-1.77) | .6965 | 1.40 (1.17-1.67) | .0002 |
HR= hazard ratio. See Table 1 legend for expansion of other abbreviations.
Although the time to the first acute episode of respiratory disease was significantly shorter (P < .0001) for subjects with advanced COPD (GOLD II-IV) compared with control subjects (GOLD 0) and subjects with mild COPD (GOLD I) (e-Fig 1 (603.4KB, pdf) ), the HRs for risk factors associated with moderate to severe acute episodes of respiratory disease were similar (Table 2). GOLD U subjects had intermediate disease-free survival times compared with control subjects and shared major risk factors with other groups (eg, history of a previous acute episode of respiratory disease and high SGRQ score).
Scoring System for Predicting Future COPD Acute Episode of Respiratory Disease
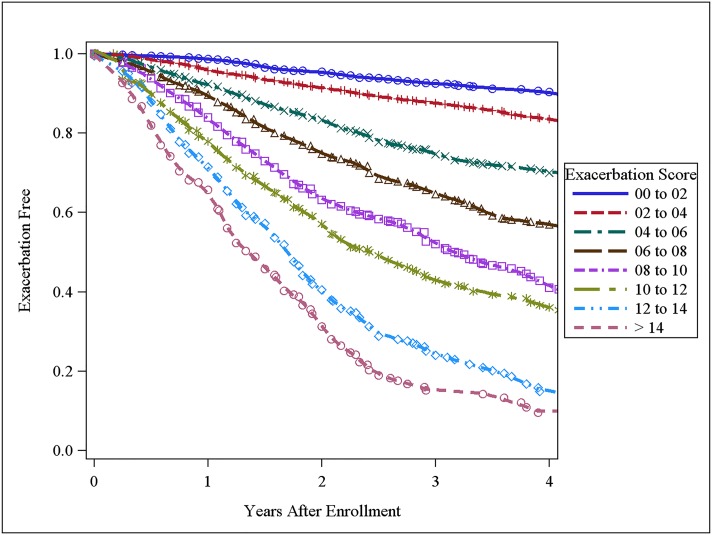
The three major risk factors for acute episodes of respiratory disease were used to develop an acute episode of respiratory disease risk score for all subjects. Parameter estimates from Cox proportional hazards models were used to weight each variable (exacerbation frequency in prior year × 0.23334 + SGRQ × 0.02017 − FEV1 % × 0.00917). For manual scoring, the formula was scaled from 0 to 20 points (Table 3). Disease-free survival times were shorter with higher scores (Fig 1). A higher acute episode of respiratory disease score was associated with a higher observed disease rate for both moderate (Table 4) and severe acute episodes of respiratory disease (e-Table 4 (603.4KB, pdf) ). A similar acute episode of respiratory disease score was associated with similar observed acute episode of respiratory disease rates, regardless of level of airflow obstruction.
TABLE 3 ] .
Variables and Point Values Used for Exacerbation Score
| Points Toward Score | ||||||||
| Component | 0 | 1 | 2 | 3 | 4 | 4 | 6 | 7 |
| FEV1% | ≥ 90% | 80%-89% | 70%-79% | 60%-69% | 50%-59% | 40%-49% | 30%-29% | < 30% |
| SGRQ | < 20 | 20-29 | 30-39 | 40-49 | 50-59 | 60-69 | 70-79 | ≥ 80 |
| Exacerbations in preceding year | 0 | 1 | 2 | 3 | 4 | ≥ 5 | … | … |
See Table 1 legend for expansion of abbreviations.
Figure 1 –
Acute episode of respiratory disease-free survival times by score.
TABLE 4 ] .
Annual Rate of Moderate or Severe Episodes (Actual)
| Score | Control Subjects | GOLD U | COPD GOLD I, II, III, IV |
| 0-2 | 0.1 ± 0.3 (1,020) | … | 0.1 ± 0.4 (100) |
| 2-4 | 0.1 ± 0.4 (1,489) | 0.1 ± 0.4 (306) | 0.1 ± 0.4 (619) |
| 4-6 | 0.3 ± 1.1 (523) | 0.2 ± 0.6 (256) | 0.3 ± 0.7 (637) |
| 6-8 | 0.4 ± 1.0 (268) | 0.3 ± 0.7 (192) | 0.5 ± 0.9 (701) |
| 8-10 | 1.1 ± 1.7 (102) | 0.4 ± 0.8 (116) | 0.7 ± 1.1 (678) |
| 10-12 | 1.0 ± 1.2 (26) | 0.9 ± 1.4 (81) | 1.0 ± 1.6 (556) |
| 12-14 | … | 1.7 ± 2.4 (35) | 1.5 ± 1.6 (292) |
| > 14 | … | … | 2.0 ± 2.3 (220) |
Data are presented as mean ± SD (No. subjects per group). There was a minimum of 25 subjects per group. See Table 1 legend for expansion of abbreviation.
Discussion
“Acute bronchitis,” “acute exacerbations of COPD,” and “acute exacerbations of chronic bronchitis” are terms that are often used interchangeably in medical literature. “Acute bronchitis” has been proposed as an all-encompassing term; however, it emphasizes large airways disease when many episodes are predominantly lower airways disease. Because these terms have similar clinical presentation, pathophysiology, and treatment, we use the term “acute episode of respiratory disease” in this study to collectively refer to all three. We found that some current and former smokers without airflow obstruction have acute episode of respiratory disease rates similar to those of current and former smokers with COPD. These subjects have risk factors similar to those of subjects with COPD, such as poor health-related quality of life, prior acute episodes of respiratory disease, and GERD. Despite their high risk, these subjects are often excluded from clinical trials in which acute episodes of respiratory disease are the primary end point. If one estimates that there may be 30 million current and former smokers without evidence of obstructive lung disease and an estimated 12.9 million subjects with COPD in the United States,1 our data suggest that there are similar total numbers of acute episodes of respiratory disease treated with corticosteroids or antibiotics in smokers with COPD as in those without (8 million vs 6 million).
The most common reason for excluding current and former smokers without obstructive lung disease from observational and interventional studies is that they are thought to be at low risk of acute episodes of respiratory disease. Although this is true in general, our results show that there are some subjects who have high rates similar to those with COPD. We devised an acute episode of respiratory disease score as one method of identifying high-risk subjects who do not have moderate or severe COPD. The exacerbation score illustrates several important risk factor features. First, the strongest predictive factors for acute episodes of respiratory disease can be ascertained with a medical history and inexpensive physiologic measurements (ie, spirometry). A history of acute episodes of respiratory disease, poor health-related quality of life, and reduced spirometry (eg, FEV1 %) are the largest contributers to these prognostic risk scores. Second, these first two risk factors may be present even in those with normal spirometry. Third, GOLD U subjects, who compose a growing proportion of the population and are rarely included in clinical studies, have significantly higher acute episode of respiratory disease rates and different risk factors compared with other control groups. For instance, in GOLD U subjects, female sex is not a risk factor, but chronic bronchitis is a risk factor, compared with control subjects. Finally, large studies such as this can identify statistically significant risk factors such as GERD; however, many of these risk factors contribute only small amounts to the overall risk compared with prior episodes, poor health-related quality of life, and reduced spirometry.
The major differences between our study and similar studies such as the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study14 and other longitudinal studies16‐18 were that COPDGene included smokers without a history of COPD, had more subjects overall, and included a substantial number of blacks. Despite these differences, the major independent predictors of acute episodes of respiratory disease in subjects with COPD were similar: an acute episode of respiratory disease in the previous year, a low SGRQ score, and impaired spirometry. However, there are several differences worthy of mention. Female sex was associated with acute episodes of respiratory disease in multivariate analysis in COPDGene, whereas in ECLIPSE, a significant relationship between female sex and acute episodes of respiratory disease was found only in univariate analysis. Additionally, a parental history of COPD was not included in the exacerbation model in ECLIPSE. Finally, we found that black race was an independent risk factor for severe episodes in COPDGene, whereas in ECLIPSE, the influence of race was not discussed.
Although COPD mortality rates have been increasing in blacks, there are scant data for prediction of exacerbations in this population. In a cross-sectional study of 50 blacks and 278 non-Hispanic whites, blacks appeared to have a greater loss in lung function per pack-year of cigarettes smoked.24 In a retrospective study of the first 1,293 COPDGene subjects (including 224 blacks), a history of more severe exacerbations was noted in blacks.25 In this prospective study, black race was one of the strongest predictive variables for a history of severe episodes, although it was not a statistically significant risk factor for moderate episodes. To our knowledge, this is the largest prospective study of COPD acute episodes of respiratory disease in black smokers (2,110 subjects, including 581 with GOLD II-IV COPD).
A parental history of COPD exacerbations is known to be associated with worse lung function26,27; however, there are few data on the ability of family history to predict COPD exacerbations prospectively. In a retrospective study of the first 1,597 control subjects and GOLD II to IV subjects in COPDGene, it was noted that a parental history of COPD was independently associated with exacerbations when adjusted for covariates.28 In the COPDGene LFU study, a parental history of COPD was the second- or third-strongest predictive factor for both moderate and severe episodes, independent of prior exacerbations. Interestingly, a parental history of COPD was predictive of future moderate and severe episodes only in subjects with COPD. This suggests that the effect of family history may be related to the severity of disease beyond that which can be measured by spirometry or by high-resolution chest CT scan assessment of emphysema.
Over the past decade, sex differences in COPD prevalence and mortality have reversed. Once thought to be a disease of older white men, COPD is now being diagnosed more frequently in women. More women are being hospitalized for COPD acute episodes of respiratory disease, with hospitalization rates similar to those of men by 1995.29 In the United States, the total number of COPD-related deaths in women has exceeded that of men since 2000.1 In this study, female sex conferred a risk of COPD acute episodes of respiratory disease independent of other covariates. The reasons for these associations are unclear but they may be related to sex differences in symptom reporting or differences in disease biology.
GERD has long been associated with respiratory disease, and the link between GERD and COPD is becoming increasingly apparent. A population-based survey found patients with GERD to have more respiratory symptoms compared with the general population.30 An increased prevalence of GERD has also been found in patients with COPD,31‐34 but only recently has GERD emerged as a risk factor for COPD exacerbations. A small study of 86 patients with COPD found that those with GERD had a greater history of exacerbations compared with those without GERD.35 Most recently, the ECLIPSE study found a history of GERD to be an independent risk factor for COPD exacerbations during prospective evaluation.14 The mechanistic link between GERD and exacerbations of respiratory disease is unknown but it may be related to alterations in gastric flora with acid suppression.36
The major strengths of this study are the large population of subjects including blacks and the spectrum of disease severity; however, some limitations should be acknowledged. Acute episodes of respiratory disease were self-reported only every 6 months using both automated and coordinator-assisted methods. Another possible limitation is how events were defined: for this study, we defined an acute episode of respiratory disease as a “flare up” of symptoms treated with either corticosteroids or antibiotics. Because antibiotics were used more frequently than steroids, this may have led to an overrepresentation of events compared with studies that require corticosteroid therapy in their definition. Because the risk factors for severe episodes were similar to those for moderate episodes, it seems likely that true exacerbations lie on a continuum of severity. Other limitations include the fact that pulmonary artery measurements were made in the tubular portion, as opposed to measurements at the bifurcation. Although the tubular measurements were associated with exacerbations in univariate analysis, the pulmonary artery to aorta ratio was not independently associated with acute episodes of respiratory disease in multivariable analysis, as has been reported recently in GOLD II to IV subjects.37 Additionally, although change in FEV1 after bronchodilator was not a major risk factor, there were no airway responsiveness measurements (eg, methacholine challenge). The study also had only baseline spirometry measurements; thus, we do not know if rapid decline in lung function is an independent risk factor for exacerbations. There are also no data on access to medical care; thus, we do not know if increased use of ERs and hospitals by blacks may be because of poor access to outpatient clinics vs more severe exacerbations. Finally, there are no study-wide biomarker assessments in COPDGene. Thus, we were unable to assess whether WBC count, fibrinogen, or C-reactive protein were independent risk factors for acute episodes of respiratory disease.38
Conclusions
In summary, the COPDGene LFU study demonstrates that acute episodes of respiratory disease are common in current and former smokers without COPD, particularly when there is a history of prior events and poor respiratory health status. Furthermore, a large number of current and former smokers do not meet the typical criteria for entry into clinical trials (FEV1/FVC < 0.7 and FEV1% > 60% predicted) and have a similar exacerbation risk to those with more severe COPD. Identification of the subjects using an exacerbation risk score or by other means may be useful in identifying high risk and early disease, for targeted therapy (eg, azithromycin, roflumilast), as well as for risk stratification for health-care providers, insurers, and the pharmaceutical industry.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: R P. B. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. R. P. B., A. A. A. W., E. R., J. D. C., E. K. S., and G. R. W. contributed to the study concept and design; R. P. B., V. K., B. J. M., D. A. L., G. R. W., N. M., G. J. D. C., J. R., X. S., M. K. H., D. D., A. A., A. C., M. D., C. P. H., N. M., F. M., H. P. N., D. N., E. R., F. S., A. S., R. A. W., C. Wendt, and C. Wilson contributed to the acquisition of data; R. P. B., A. A. A. W., V. K., E. R., and R. A. W. contributed to the drafting of the manuscript; A. A. A. W., R. P. B., and C. Wilson contributed to the statistical analysis; and all authors contributed to the critical revision of the manuscript for important intellectual content.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Kim has participated in clinical trials sponsored by Boehringer-Ingelheim, GlaxoSmithKline, MedImmune, and Roche Pharmaceuticals. Dr Make has participated in advisory boards for AstraZeneca; Boehringer-Ingelheim; GlaxoSmithKline; Pfizer Inc; Sunovion Pharmaceuticals Inc; Forest Pharmaceuticals, Inc; Merck; Ikaria; Philips Respironics; Aerocrine; Theravance, Inc; and Covidien Ltd. Dr Lynch is a consultant to Perceptive Imaging, Inc; Boehringer-Ingelheim; Genentech; Gilead Sciences, Inc; Veracyte; Intermune; and MedImmune. Dr Han has participated in research sponsored by GlaxoSmithKline with monies paid to her institution. She has consulted for GlaxoSmithKline, MedImmune, and Ikaria and has participated on speakers bureaus for GlaxoSmithKline; Boehringer-Ingelheim; Pfizer Inc; Forest Pharmaceuticals, Inc; Novartis; and Grifols. Dr Comellas has consulted for Vida Diagnostics, Inc. Dr Dransfield has served as a consultant for GlaxoSmithKline, Boehringer-Ingelheim, and Ikaria. His institution has received research grant support from the National Heart, Lung and Blood Institute; GlaxoSmithKline; and Forest Pharmaceuticals, Inc; and has received contracted support for enrollment in clinical trials from Aeris Therapeutics; Boehringer-Ingelheim; Boston Scientific; Centocor Ortho Biotech, Inc; GlaxoSmithKline; Forest Pharmaceuticals, Inc; Otsuka America Pharmaceutical, Inc; Pearl Therapeutics; Pfizer Inc; and Pulmonx. Dr Hersh has received lecture fees from Novartis and has served as a consultant to CSL Behring. Dr MacIntyre has received NIH funding for the COPDGene project. Dr Martinez has participated in steering committees in COPD or idiopathic pulmonary fibrosis sponsored by Actelion Pharmaceuticals Ltd; Bayer; Centocor Ortho Biotech, Inc; Forest Pharmaceuticals, Inc; Gilead Sciences, Inc; Jannsen Pharmaceuticals, Inc; GlaxoSmithKline, Nycomed/Takeda; and Promedior. He has participated in advisory boards for COPD or idiopathic pulmonary fibrosis for Amgen; AstraZeneca; Carden Jennings Publishing Co, Ltd; CardioMEMS; CSA Medical Inc; Forest Pharmaceuticals, Inc; Ikaria; Jannsen Pharmaceuticals, Inc; MedImmune; Merck; Pearl Therapeutics; Nycomed/Takeda; Pfizer Inc; and Vertex. Dr Martinez has participated on data safety monitoring committees sponsored by GlaxoSmithKline and Stromedix Inc. He has helped with Food and Drug Administration presentations for Boehringer-Ingelheim, GlaxoSmithKline, and Ikaria. He has spoken on COPD for Bayer; Forest Pharmaceuticals, Inc; GlaxoSmithKline; Nycomed/Takeda; and Prescott Medical Communications Group. He has participated in advisory teleconferences sponsored by the American Institute for Research, Axon, Cory Path, Grey Healthcare Group, Merion, and Sudler & Hennessey. He has received royalties from Informa Healthcare. Dr Niewoehner has received fees from Boehringer-Ingelheim, Novartis, and GlaxoSmithKline for serving on end point committees of clinical trials. He has received fees from AstraZeneca and Gilead Sciences, Inc, for serving on advisory boards. Dr Sciurba has served as a pharmaceutical consultant. Dr Silverman has received grant support from GlaxoSmithKline for studies of COPD genetics. Drs Bowler, Regan, Willams, Santorico, Hokanson, Washko, Bercz, Soler, Marchetti, Criner, Ramsdell, Demeo, Anzueto, Crapo, Wells, Nath, Sharafkhaneh, van Beek, Wendt, and Wise, and Ms Wilson have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Collaborators: Jeffrey Curtis, MD; Ella Kazerooni, MD; Nicola Hanania, MD; Philip Alapat, MD; Venkata Bandi, MD; Kalpalatha Guntupalli, MD; Elizabeth Guy, MD; William Lunn, MD; Antara Mallampalli, MD; Charles Trinh, MD; Mustafa Atik, MD; Dawn DeMeo, MD; Craig Hersh, MD; Francine Jacobson, MD, MPH; R. Graham Barr, MD, DrPH; Byron Thomashow, MD; John Austin, MD; Neil MacIntyre, Jr., MD; Lacey Washington, MD; H. Page McAdams, MD; Richard Rosiello, MD; Timothy Bresnahan, MD; Charlene McEvoy, MD, MPH; Joseph Tashjian, MD; Robert Wise, MD; Nadia Hansel, MD, MPH; Robert Brown, MD; Richard Casaburi, MD; Janos Porszasz, MD, PhD; Hans Fischer, MD, PhD; Matt Budoff, MD; Amir Sharafkhaneh, MD; Dennis Niewoehner, MD; Tadashi Allen, MD; Kathryn Rice, MD; Marilyn Foreman, MD; Gloria Westney, MD; Eugene Berkowitz, MD, PhD; Russell Bowler, MD, PhD; Adam Friedlander, MD; Eleonora Meoni, MD; Gerard Criner, MD; Victor Kim, MD; Nathaniel Marchetti, DO; Aditi Satti, MD; A. James Mamary, MD; Robert Steiner, MD; Chandra Dass, MD; William Bailey, MD; Mark Dransfield, MD; Lynn Gerald, PhD, MSPH; Hrudaya Nath, MD; Joe Ramsdell, MD; Paul Ferguson, MS, RCP; Paul Friedman, MD; Geoffrey McLennan, MD, PhD; Edwin JR van Beek, MD, PhD; Fernando Martinez, MD; MeiLan Han, MD; Deborah Thompson, PhD; Ella Kazerooni, MD; Christine Wendt, MD; Tadashi Allen, MD; Frank Sciurba, MD; Joel Weissfeld, MD, MPH; Carl Fuhrman, MD; Jessica Bon, MD; Antonio Anzueto, MD; Sandra Adams, MD; Carlos Orozco, MD; C. Santiago Restrepo, MD; Amy Mumbower, MD; James Crapo, MD; Edwin Silverman, MD, PhD; Barry Make, MD; Elizabeth Regan, MD; Jonathan Samet, MD; Amy Willis, MA; Douglas Stinson; Terri Beaty, PhD; Barbara Klanderman, PhD; Nan Laird, PhD; Christoph Lange, PhD; Iuliana Ionita; Stephanie Santorico, PhD; Edwin Silverman, MD, PhD; David Lynch, MD; Joyce Schroeder, MD; John Newell, Jr., MD; John Reilly, MD; Harvey Coxson, PhD; Philip Judy, PhD; Eric Hoffman, PhD; Raul San Jose Estepar, PhD; George Washko, MD; Rebecca Leek; Jordan Zach; Alex Kluiber; Anastasia Rodionova; Tanya Mann; Robert Crapo, MD; Robert Jensen, PhD; Homayoon Farzadegan, PhD; James Murphy, PhD; Douglas Everett, PhD; Carla Wilson; John Hokanson, MPH, PhD.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Other contributions: The authors thank the COPDGene investigators and Christina Schnell, BA (including her invaluable secretarial support) for data collection and their support of writing this manuscript. A list of participating institutions is available in e-Appendix 1 (603.4KB, pdf) .
Additional information: The e-Appendix, e-Figure, and e-Tables can be found in the Supplemental Materials section of the online article.
ABBREVIATIONS
- COPDGene
Genetic Epidemiology of COPD
- ECLIPSE
Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints
- GERD
gastroesophageal reflux disease
- GOLD
Global Initiative for Chronic Obstructive Lung Disease
- HR
hazard ratio
- LFU
longitudinal follow-up
- SGRQ
St. George’s Respiratory Questionnaire
Footnotes
FUNDING/SUPPORT: This study was supported by the National Heart, Lung and Blood Institute [Grants R01 HL 08 9856 and R01 HL 08 9897], National Center for Research Resources/National Institutes of Health [Grant UL1 RR025780], and National Institute of Nursing Research [NR013377].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
Contributor Information
for the COPDGene investigators:
Jeffrey Curtis, Ella Kazerooni, Nicola Hanania, Philip Alapat, Venkata Bandi, Kalpalatha Guntupalli, Elizabeth Guy, William Lunn, Antara Mallampalli, Charles Trinh, Mustafa Atik, Dawn DeMeo, Craig Hersh, Francine Jacobson, R. Graham Barr, Byron Thomashow, John Austin, Neil MacIntyre, Jr., Lacey Washington, H. Page McAdams, Richard Rosiello, Timothy Bresnahan, Charlene McEvoy, Joseph Tashjian, Robert Wise, Nadia Hansel, Robert Brown, Richard Casaburi, Janos Porszasz, Hans Fischer, Matt Budoff, Amir Sharafkhaneh, Dennis Niewoehner, Tadashi Allen, Kathryn Rice, Marilyn Foreman, Gloria Westney, Eugene Berkowitz, Russell Bowler, Adam Friedlander, Eleonora Meoni, Gerard Criner, Victor Kim, Nathaniel Marchetti, Aditi Satti, A. James Mamary, Robert Steiner, Chandra Dass, William Bailey, Mark Dransfield, Lynn Gerald, Hrudaya Nath, Joe Ramsdell, Paul Ferguson, Paul Friedman, Geoffrey McLennan, Edwin JR van Beek, Fernando Martinez, MeiLan Han, Deborah Thompson, Ella Kazerooni, Christine Wendt, Tadashi Allen, Frank Sciurba, Joel Weissfeld, Carl Fuhrman, Jessica Bon, Antonio Anzueto, Sandra Adams, Carlos Orozco, C. Santiago Restrepo, Amy Mumbower, James Crapo, Edwin Silverman, Barry Make, Elizabeth Regan, Jonathan Samet, Amy Willis, Douglas Stinson, Terri Beaty, Barbara Klanderman, Nan Laird, Christoph Lange, Iuliana Ionita, Stephanie Santorico, Edwin Silverman, David Lynch, Joyce Schroeder, John Newell, Jr., John Reilly, Harvey Coxson, Philip Judy, Eric Hoffman, Raul San Jose Estepar, George Washko, Rebecca Leek, Jordan Zach, Alex Kluiber, Anastasia Rodionova, Tanya Mann, Robert Crapo, Robert Jensen, Homayoon Farzadegan, James Murphy, Douglas Everett, Carla Wilson, and John Hokanson
References
- 1.Ford ES, Mannino DM, Wheaton AG, Giles WH, Presley-Cantrell L, Croft JB. Trends in the prevalence of obstructive and restrictive lung function among adults in the United States: findings from the National Health and Nutrition Examination surveys from 1988-1994 to 2007-2010. Chest. 2013;143(5):1395-1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global strategy for diagnosis, management and prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease website. http://www.goldcopd.com. Accessed September 6, 2013 [Google Scholar]
- 3.Strassels SA, Smith DH, Sullivan SD, Mahajan PS. The costs of treating COPD in the United States. Chest. 2001;119(2):344-352 [DOI] [PubMed] [Google Scholar]
- 4.Smucny J, Fahey T, Becker L, Glazier R. Antibiotics for acute bronchitis. Cochrane Database Syst Rev. 2004;(4):CD000245. [DOI] [PubMed] [Google Scholar]
- 5.Anzueto A, Miravitlles M, Ewig S, Legnani D, Heldner S, Stauch K. Identifying patients at risk of late recovery (≥ 8 days) from acute exacerbation of chronic bronchitis and COPD. Respir Med. 2012;106(9):1258-1267 [DOI] [PubMed] [Google Scholar]
- 6.Stoller JK. Clinical practice. Acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002;346(13):988-994 [DOI] [PubMed] [Google Scholar]
- 7.Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(5 pt 1):1418-1422 [DOI] [PubMed] [Google Scholar]
- 8.Quint JK, Baghai-Ravary R, Donaldson GC, Wedzicha JA. Relationship between depression and exacerbations in COPD. Eur Respir J. 2008;32(1):53-60 [DOI] [PubMed] [Google Scholar]
- 9.Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847-852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGhan R, Radcliff T, Fish R, Sutherland ER, Welsh C, Make B. Predictors of rehospitalization and death after a severe exacerbation of COPD. Chest. 2007;132(6):1748-1755 [DOI] [PubMed] [Google Scholar]
- 11.Kim S, Clark S, Camargo CA., Jr Mortality after an emergency department visit for exacerbation of chronic obstructive pulmonary disease. COPD. 2006;3(2):75-81 [DOI] [PubMed] [Google Scholar]
- 12.Groenewegen KH, Schols AM, Wouters EF. Mortality and mortality-related factors after hospitalization for acute exacerbation of COPD. Chest. 2003;124(2):459-467 [DOI] [PubMed] [Google Scholar]
- 13.Donaldson GC, Wedzicha JA. COPD exacerbations. 1: epidemiology. Thorax. 2006;61(2):164-168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hurst JR, Vestbo J, Anzueto A, et al. ; Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128-1138 [DOI] [PubMed] [Google Scholar]
- 15.Kim YI, Schroeder J, Lynch D, et al. ; COPDGene Investigators. Gender differences of airway dimensions in anatomically matched sites on CT in smokers. COPD. 2011;8(4):285-292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niewoehner DE, Lokhnygina Y, Rice K, et al. Risk indexes for exacerbations and hospitalizations due to COPD. Chest. 2007;131(1):20-28 [DOI] [PubMed] [Google Scholar]
- 17.Briggs A, Spencer M, Wang H, Mannino D, Sin DD. Development and validation of a prognostic index for health outcomes in chronic obstructive pulmonary disease. Arch Intern Med. 2008;168(1):71-79 [DOI] [PubMed] [Google Scholar]
- 18.Schembri S, Anderson W, Morant S, et al. A predictive model of hospitalisation and death from chronic obstructive pulmonary disease. Respir Med. 2009;103(10):1461-1467 [DOI] [PubMed] [Google Scholar]
- 19.Regan EA, Hokanson JE, Murphy JR, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7(1):32-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim V, Han MK, Vance GB, et al. ; COPDGene Investigators. The chronic bronchitic phenotype of COPD: an analysis of the COPDGene Study. Chest. 2011;140(3):626-633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stewart JI, Moyle S, Criner GJ, et al. ; For The Copdgene Investigators. Automated telecommunication to obtain longitudinal follow-up in a multicenter cross-sectional COPD study. COPD. 2012;9(5):466-472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fabbri LM, Hurd SS; GOLD Scientific Committee. Global Strategy for the Diagnosis, Management and Prevention of COPD: 2003 update. Eur Respir J. 2003;22(1):1-2 [DOI] [PubMed] [Google Scholar]
- 23.Washko GR, Hunninghake GM, Fernandez IE, et al. ; COPDGene Investigators. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med. 2011;364(10):897-906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dransfield MT, Davis JJ, Gerald LB, Bailey WC. Racial and gender differences in susceptibility to tobacco smoke among patients with chronic obstructive pulmonary disease. Respir Med. 2006;100(6):1110-1116 [DOI] [PubMed] [Google Scholar]
- 25.Han MK, Curran-Everett D, Dransfield MT, et al. ; COPDGene Investigators. Racial differences in quality of life in patients with COPD. Chest. 2011;140(5):1169-1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silverman EK, Speizer FE, Weiss ST, et al. Familial aggregation of severe, early-onset COPD : candidate gene approaches. Chest. 2000;117(5 Suppl 1):273S-274S [DOI] [PubMed] [Google Scholar]
- 27.DeMeo DL, Carey VJ, Chapman HA, et al. Familial aggregation of FEF(25-75) and FEF(25-75)/FVC in families with severe, early onset COPD. Thorax. 2004;59(5):396-400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hersh CP, Hokanson JE, Lynch DA, et al. ; COPDGene Investigators. Family history is a risk factor for COPD. Chest. 2011;140(2):343-350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic obstructive pulmonary disease surveillance—United States, 1971-2000. MMWR Surveill Summ. 2002;51(6):1-16 [PubMed] [Google Scholar]
- 30.Nordenstedt H, Nilsson M, Johansson S, et al. The relation between gastroesophageal reflux and respiratory symptoms in a population-based study: the Nord-Trøndelag health survey. Chest. 2006;129(4):1051-1056 [DOI] [PubMed] [Google Scholar]
- 31.Casanova C, Baudet JS, del Valle Velasco M, et al. Increased gastro-oesophageal reflux disease in patients with severe COPD [published correction appears in Eur Respir J. 2004;24(6):1074]. Eur Respir J. 2004;23(6):841-845 [DOI] [PubMed] [Google Scholar]
- 32.Weaver EM. Association between gastroesophageal reflux and sinusitis, otitis media, and laryngeal malignancy: a systematic review of the evidence. Am J Med. 2003;115(Suppl 3A):81S-89S [DOI] [PubMed] [Google Scholar]
- 33.Mokhlesi B, Morris AL, Huang CF, Curcio AJ, Barrett TA, Kamp DW. Increased prevalence of gastroesophageal reflux symptoms in patients with COPD. Chest. 2001;119(4):1043-1048 [DOI] [PubMed] [Google Scholar]
- 34.Andersen LI, Jensen G. Prevalence of benign oesophageal disease in the Danish population with special reference to pulmonary disease. J Intern Med. 1989;225(6):393-402 [DOI] [PubMed] [Google Scholar]
- 35.Rascon-Aguilar IE, Pamer M, Wludyka P, et al. Role of gastroesophageal reflux symptoms in exacerbations of COPD. Chest. 2006;130(4):1096-1101 [DOI] [PubMed] [Google Scholar]
- 36.Ruddell WS, Axon AT, Findlay JM, Bartholomew BA, Hill MJ. Effect of cimetidine on the gastric bacterial flora. Lancet. 1980;1(8170):672-674 [PubMed] [Google Scholar]
- 37.Wells JM, Washko GR, Han MK, et al. ; COPDGene Investigators; ECLIPSE Study Investigators. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med. 2012;367(10):913-921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomsen M, Ingebrigtsen TS, Marott JL, et al. Inflammatory biomarkers and exacerbations in chronic obstructive pulmonary disease. JAMA. 2013;309(22):2353-2361 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement