Abstract
Background
The Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) study validated noninvasive imaging tests of intracranial atherosclerosis against catheter angiography in a prospective, blinded, multicenter setting. Critical evaluation of transcranial Doppler (TCD) and magnetic resonance angiography in the SONIA study standardized their performance and interpretation. We performed a similar analysis of computed tomography angiography (CTA) for the detection of intracranial stenosis.
Methods
Multicenter standardization of image acquisition and blinded, central interpretation of CTA performance were conducted in concert with the Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) trial. Measurements of the intracranial arterial diameter were obtained to derive stenosis values. Correlation with catheter angiography was used to assess CTA performance characteristics.
Results
CTA measurements of intracranial stenosis were obtained in 120 vessel segments, with angiographic correlation in 52. CTA was performed as a noninvasive study prior to conventional angiography. CTA stenoses of 50-99% or a flow gap were identified in 15 of 52 vessel segments, stenoses of <50% in 5 of 52, and normal arterial diameters in 32 of 52 vessel segments. Based on the digital subtraction angiography (DSA) stenosis defined as 50-99%, the positive predictive value (PPV) of CTA was only 46.7% (95% CI 21.3-73.4) and the negative predictive value (NPV) was 73.0% (95% CI 55.9-86.2). For DSA stenosis defined as 70-99%, the PPV of CTA was 13.3% (95% CI 1.7-40.5) and the NPV was 83.8% (95% CI 68.0-93.8).
Conclusions
CTA can accurately rule out the presence of severe stenosis due to intracranial atherosclerosis and may eliminate the need for angiography in many cases. Further prospective, blinded evaluation of CTA and optimization of cutpoints to predict angiographic disease will facilitate future trials of intracranial atherosclerosis.
Key Words: Intracranial atherosclerosis, Stenosis, Computed tomography angiography, Stroke, Diagnosis
Introduction
The Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) study validated noninvasive imaging tests for intracranial atherosclerosis against catheter angiography in a prospective, blinded, multicenter setting [1]. As an ancillary imaging study to the Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) trial [2], the SONIA trial standardized the performance and interpretation of transcranial Doppler (TCD) ultrasonography and magnetic resonance angiography (MRA) and provided a critical evaluation of these noninvasive modalities with respect to conventional angiography. As WASID and the subsequent Stenting and Aggressive Medical Management for Preventing Recurrent stroke in Intracranial Stenosis (SAMMPRIS) trials have not culminated in a novel medical or endovascular treatment for stroke prevention in intracranial atherosclerotic disease (ICAD), the diagnosis and prevention of recurrent stroke remains a formidable challenge in this worldwide disorder [3,4].
Computed tomography angiography (CTA) differs with respect to other noninvasive diagnostic studies such as MRA and TCD. CTA provides images of vascular structures enhanced with intravenous contrast, detailing vessel anatomy within the lumen with simultaneous visualization of the vessel wall. The procedure has inherent advantages and disadvantages. In contrast to MRA, CTA can be performed in patients with pacemakers and has a shorter imaging time, so it is less prone to movement artifacts. Inadequate contrast administration or timing may degrade the quality of CTA images. CTA may be less susceptible to flow artifacts within the vessels, although the presence of vascular stenoses may diminish opacification of distal portions of the vessel due to hemodynamic effects. Postprocessing artifacts may limit the interpretation of CTA. For example, processing to remove bone structures can cause a false decrease in arterial diameter and loss of small or slow-flow vessels. Improper windowing can also produce artifacts.
At the start of the SONIA trial, CTA was in early development based on concurrent advances in helical CT technology. No prospective, blinded assessment of CTA performance after standardization of test performance and measurement had been performed. Referral bias or imaging surveillance of only cases known to harbor prominent stenoses with planned angiography limited evaluation of CTA. Other studies have compared CTA to imaging modalities other than digital subtraction angiography (DSA) but do not include well-described measurement methods and employ no gold standard comparison [5,6,7]. In sum, CTA identification of intracranial stenosis lacks standardized test procedures, measurement methods and adequate validation studies. No agreement exists on the optimal test performance parameters for CTA, and they may vary with equipment. Our objective was to perform a critical analysis of CTA for detection of intracranial stenosis.
Methods
The SONIA and WASID trial designs, methodology, and primary results have been reported elsewhere [1,2]. Test criteria for TCD and MRA sought to identify 50-99% of angiographic stenosis of the middle cerebral, internal carotid, vertebral or basilar arteries. SONIA ascertained whether these test criteria were performed with a reliable positive predictive value (PPV). CTA analysis was not a primary aim of the SONIA trial, and was added as an exploratory aim after the trial began. As with TCD and MRA, CTA sought to identify 50-99% of angiographic stenosis of the middle cerebral, internal carotid, vertebral or basilar arteries. The WASID trial focused on atherosclerosis as the underlying lesion. Occluded vessels on DSA or noninvasive tests were considered to less likely represent atherosclerosis at the time WASID was conducted. Therefore, an occlusion on angiography was not considered a positive finding, and a finding of stenosis on noninvasive tests would be designated as false positive if an occlusion was identified on DSA.
All subjects had experienced a transient ischemic attack or stroke in the prior 90 days. Patients were enrolled in SONIA trial if noninvasive tests had been performed prior to the conventional DSA. Only middle cerebral, internal carotid, vertebral or basilar arteries imaged by both CTA and DSA could be included in this study. Specifically, this was not an intention to diagnose the design. We sought to determine the correlation between CTA and DSA when both tests were able to image the vessel, and not measure how often a vessel and its stenosis were identified with one technique and not the other.
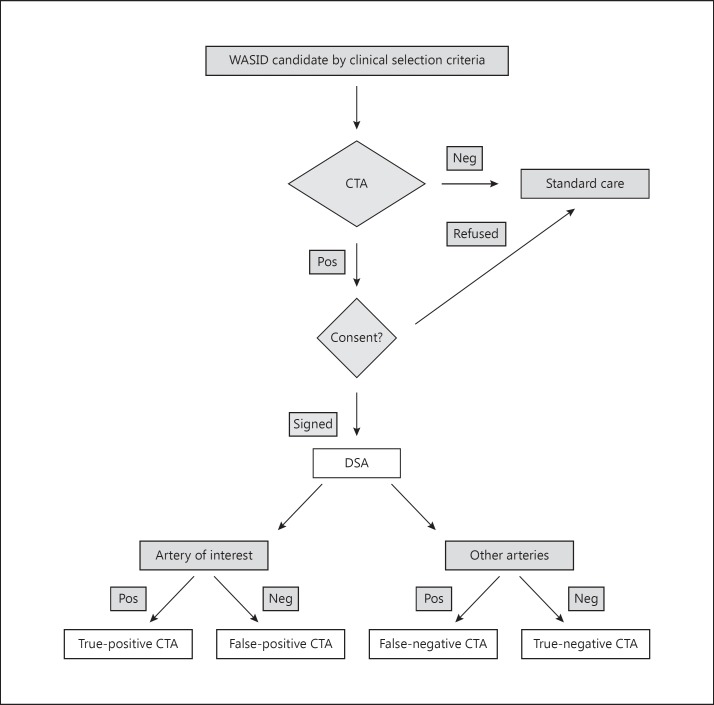
DSA could involve any number of injected vessels, as those decisions were made by the angiographer at local sites. If 50-99% of stenosis was identified by caliper measurements on DSA in the symptomatic artery, subjects were enrolled in the WASID and SONIA trial. If not, such patients were enrolled in the SONIA trial in order to accurately measure the PPV by including patients whose lack of stenosis made them ineligible for the WASID trial. The negative predictive value (NPV) was determined by comparing noninvasive test results and DSA of the contiguous normal vessel or vessels other than the symptomatic artery that did not harbor disease. The SONIA study determined the PPV and NPV of TCD and MRA for detection of stenosis, as defined above. The same approach was used for CTA, but no sample size estimates for CTAs could be established prior to the design of the SONIA trial due to the evolving experience with CTA. The flow diagram in figure 1 illustrates the patient recruitment.
Fig. 1.
Flow diagram of patient recruitment into the CTA cohort of the SONIA trial.
Multicenter standardization of CTA acquisition and blinded, central interpretation of test performance were conducted as part of this study. CTA performance standards included supine patient positioning, acquisition of a localizing image in lateral projection, with gantry and table situated to acquire images extending to the superior aspect of the cranial vault. A test bolus of nonionic contrast was injected at 3 cm3/s into an antecubital vein, acquiring 10 continuous 10-mm thick slices with tube settings of 80 kVp and 80 mA. After an up-front delay of 10 s, or an adjusted delay based on the test injection, helical CT images were obtained using a contrast injection rate of 3 cm3/s, total contrast volume of 90-150 cm3, pitch of 1.5 mm/s, slice collimation of 1 mm, slab thickness of 7.5-9.0 cm, 25-cm field of view, and 512 × 512 matrix, at tube settings of 120 kVp and 240 mA.
All images were submitted in digital form for central adjudication of CTA. Postprocessing was performed on a workstation including reformatting of source images into 1-mm-thick axial images with a 0.5-mm overlap to reconstruct maximum-intensity projection and multiplanar volume reformatted views. Intracranial arterial diameter measurements were obtained to derive stenosis values, utilizing an identical method for measurement of DSA. This WASID method for measurement of intracranial stenosis, utilizes the proximal arterial segment as the normal value, in contrast to the NASCET method, due to differences in anatomy and vascular pathology with respect to extracranial atherosclerosis [8]. Details of the WASID measurement technique have been published previously [9]. CTA flow gap abnormalities were defined by discontinuity of the blood flow column signal, with distal reconstitution. CTA identification of intracranial stenosis of <50% was designated as CTA negative. CTA positivity was defined as 50-99% of stenosis or presence of a flow gap.
Statistical analyses centered on determination of the PPV and NPV for CTA detection of stenosis due to ICAD. A positive CTA was defined as >50% of stenosis or a flow gap. For the purposes of this analysis, occlusions on DSA were considered to reflect an absence of stenosis. Hence, a positive CTA followed by occlusion on angiography was scored as a false positive, and a negative CTA followed by occlusion on DSA was scored as a true negative. The PPV and NPV values were computed as proportions, and exact 95% confidence intervals (CI) were used.
This trial was not registered because enrollment began before July 1, 2005.
Results
CTA measures of intracranial stenosis were obtained in 120 vessel segments, with angiographic correlation on DSA in 52 vessel segments in 21 subjects. The clinical features of this cohort are outlined in table 1. CTA stenoses of ≥50% or a flow gap were identified in 15 of 52 of vessel segments, stenoses of <50% in 5 of 52, and normal arterial diameters in 32 of 52 vessel segments. As detailed in table 2, crosstabulations revealed only modest agreement between CTA and DSA. Based on the CTA threshold of ≥50% stenosis or a flow gap to identify 50-99% of stenosis on DSA, the PPV of CTA was only 46.7% (95% CI 21.3-73.4) and the NPV of CTA was 73.0% (95% CI 55.9-86.2). Using the same CTA threshold of ≥50% stenosis or a flow gap to identify 70-99% of stenosis on DSA, the PPV of CTA was only 13.3% (95% CI 1.7-40.5) and the NPV of CTA was 83.8% (95% CI 68.0-93.8).
Table 1.
Clinical demographics of the SONIA-CTA cohort (n = 20)
| Age, years | |
| Mean±SD | 64.66 ± 11.17 |
| Median | 63.48 |
| Range | 42.27 – 86.98 |
| IQR | 58.98 – 69.78 |
| Sex, n (%) | |
| Male | 15 (75.0) |
| Female | 5 (25.0) |
| Race, n (%) | |
| Asian | 2 (10.0) |
| Black | 3 (15.0) |
| White | 14 (70.0) |
| Other | 1 (5.0) |
Table 2.
Categorization in the degree of maximal luminal stenosis by CTA and DSA
| CTA flow gap (n = 2) | CTA ≥50% stenosis (n = 13) | CTA <50% stenosis (n = 5) | CTA normal (n = 32) | |
|---|---|---|---|---|
| DSA | ||||
| Occlusion | 0 (0.0) | 1 (7.7) | 0 (0.0) | 0 (0.0) |
| 70–99% stenosis | 0 (0.0) | 2 (15.4) | 3 (60.0) | 3 (9.4) |
| 50–69% stenosis | 0 (0.0) | 5 (38.5) | 1 (20.0) | 3 (9.4) |
| <50% stenosis | 1 (50.0) | 2 (15.4) | 1 (20.0) | 5 (15.6) |
| Normal | 1 (50.0) | 3 (23.1) | 0 (0.0) | 21 (65.6) |
Values represent n (%).
Discussion
Our systematic, prospective, blinded evaluation of standardized CTA for the detection of 50-99% of intracranial stenosis with respect to the reference standard of DSA reveals good NPV and only modest PPV. Our sample size was limited due to termination of SONIA in concert with the WASID trial. The low sample size in such a diagnostic test validation study widens the CIs but does not hinder estimates of the PPV and NPV. Thus, even with a larger sample size, CTA would serve well only to exclude the presence of severe intracranial stenosis on DSA.
At the 50% threshold for definition of stenosis, CTA yielded PPV and NPV estimates on a par with other noninvasive tests in the SONIA trial, including TCD and MRA [1]. Concordance of CTA with these other modalities cannot be addressed in this study as only 21 subjects had CTA and either TCD or MRA in the SONIA trial. Interestingly, the 50% threshold on noninvasive tests to define 70-99% of DSA stenoses for entry into the SAMMPRIS trial yielded a PPV of only 13%. The poor PPV of CTA to identify the presence of an intracranial stenosis is in sharp distinction to other reports that describe the performance of CTA, likely due to the standardized, blinded approach and minimization of bias employed in the SONIA trial.
The relatively high NPV of CTA to rule out the presence of 50-99% of intracranial stenosis provides important data for clinicians evaluating patients at risk of stroke. Avoiding the risk and cost of invasive angiography serves an important role in triage. CTA is increasingly used at many centers and many vessels will be normal due to the limited prevalence of ICAD. Even if CTA erroneously rules out stenosis in a given vessel that actually harbors disease, one may question the implications given the current management of ICAD. In the absence of a proven medical or endovascular therapy, such misdiagnosis may be acceptable.
Thus, a screening test with high NPV holds value for clinicians seeking to rule out severe disease and offers patients an optimistic prognosis. Any suggestion of disease must recognize the low PPV and recommend DSA for further evaluation, if useful management changes will be made with the result of DSA. In a clinical trial, in which by definition the ‘best treatment’ is unknown, reliably excluding disease is less relevant. Trial recruitment is optimized with a screening test with high PPV. In our trial, CTA did not appear to offer the ability to provide an optimal screening test for recruitment of patients into a clinical trial.
The reference standard of DSA remains the sole measure to characterize and quantify ICAD. A reliable noninvasive imaging strategy for the diagnosis of ICAD is needed, as the vast majority of patients will not undergo invasive angiography in clinical practice to identify ICAD [10]. DSA is increasingly reserved for only those patients considered for endovascular procedures due to recurrent stroke despite optimal medical therapy. Thus, reliable identification of clinical trial subjects is difficult.
Perhaps more importantly, it must be recognized that these analyses compare CTA and DSA with respect to degree of luminal stenosis, rather than using CTA findings to predict recurrent stroke in the arterial territory. Stroke clinicians need, but do not have, a reliable noninvasive CTA index that identifies clinical risk rather than likelihood of severe anatomic stenosis. In the absence of a definitive treatment for ICAD other than aggressive medical management, further trials of interventional therapy are anticipated. A correlation of CTA findings with risk of recurrent stroke may provide an alternate method for noninvasive tests such as CTA to serve as screening tests for such trials. The percentage of stenosis on CTA is not a strong predictor of recurrent events. In the WASID trial, nearly half of recurrent strokes occurred in patients with 40-69% of stenosis [11]. There is evidence in the coronary literature, and in studies of WASID and SAMMPRIS patients, that hemodynamic assessment of ICAD rather than anatomic assessment with percentage of stenosis is superior for the prediction of recurrent clinical symptoms. Computational fluid dynamics of CT vascular imaging has shown excellent results in identifying the riskiest coronary lesions [12,13], and preliminary work with computational fluid dynamics of intracranial vessels suggests that the same may be true for ICAD. We have found that not all ‘severe’ stenoses characterized by degree of maximal stenosis are hemodynamically significant. The literature on using physiological measures of coronary disease to identify high-risk subjects reveals the superior utility of such an approach [14,15].
Limitations of the current analysis include a limited sample size and evolution of the CTA technique since these data were collected. CTA has advanced in terms of resolution and spatial coverage and would be expected to perform better today than in the past. Regardless of these improvements, poor study design such as lack of blinding or lack of standardized image acquisition and interpretation and intrinsic limitations of the technique related to vessel calcification and timing of imaging may not be overcome by technological changes. On the other hand, the SONIA study design precluded any determination of sensitivity and specificity. Yet, it minimized important biases present in other evaluations of CTA. Such an approach would be useful to continue to develop CTA as a useful screening test for ICAD, especially with the goal of identifying clinical risk rather than anatomic disease severity.
Disclosure Statement
Dr. Liebeskind is a scientific consultant for trial design and conduct to Stryker (modest) and Covidien (modest).
Acknowledgements
The authors extend their gratitude for the efforts of the SONIA Investigators. This work has been supported by NIH – National Institute of Neurological Disorders and Stroke Awards NIH/NINDS R01 NS39131, K23NS054084, K24NS072272, and P50NS044378.
References
- 1.Feldmann E, Wilterdink JL, Kosinski A, Lynn M, Chimowitz MI, Sarafin J, et al. The stroke outcomes and neuroimaging of intracranial atherosclerosis (SONIA) trial. Neurology. 2007;68:2099–2106. doi: 10.1212/01.wnl.0000261488.05906.c1. [DOI] [PubMed] [Google Scholar]
- 2.Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352:1305–1316. doi: 10.1056/NEJMoa043033. [DOI] [PubMed] [Google Scholar]
- 3.Pu Y, Liu L, Wang Y, Zou X, Pan Y, Soo Y, et al. Geographic and sex difference in the distribution of intracranial atherosclerosis in China. Stroke. 2013;44:2109–2114. doi: 10.1161/STROKEAHA.113.001522. [DOI] [PubMed] [Google Scholar]
- 4.Wong LK. Global burden of intracranial atherosclerosis. Int J Stroke. 2006;1:158–159. doi: 10.1111/j.1747-4949.2006.00045.x. [DOI] [PubMed] [Google Scholar]
- 5.Bash S, Villablanca JP, Jahan R, Duckwiler G, Tillis M, Kidwell C, et al. Intracranial vascular stenosis and occlusive disease: evaluation with CT angiography, MR angiography, and digital subtraction angiography. AJNR Am J Neuroradiol. 2005;26:1012–1021. [PMC free article] [PubMed] [Google Scholar]
- 6.Duffis EJ, Jethwa P, Gupta G, Bonello K, Gandhi CD, Prestigiacomo CJ. Accuracy of computed tomographic angiography compared to digital subtraction angiography in the diagnosis of intracranial stenosis and its impact on clinical decision-making. J Stroke Cerebrovasc Dis. 2013;22:1013–1017. doi: 10.1016/j.jstrokecerebrovasdis.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Suwanwela NC, Suwanwela N, Phanthumchinda K. Comparison of transcranial Doppler ultrasound and computed tomography angiography in symptomatic middle cerebral artery stenosis. Australas Radiol. 2000;44:174–177. doi: 10.1046/j.1440-1673.2000.00802.x. [DOI] [PubMed] [Google Scholar]
- 8.Huang J, Degnan AJ, Liu Q, Teng Z, Yue CS, Gillard JH, et al. Comparison of NASCET and WASID criteria for the measurement of intracranial stenosis using digital subtraction and computed tomography angiography of the middle cerebral artery. J Neuroradiol. 2012;39:342–345. doi: 10.1016/j.neurad.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol. 2000;21:643–646. [PMC free article] [PubMed] [Google Scholar]
- 10.Liebeskind DS, Feldmann E. Fractional flow in cerebrovascular disorders. Int Neurol. 2013;1:87–99. doi: 10.1159/000346803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kasner SE, Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation. 2006;113:555–563. doi: 10.1161/CIRCULATIONAHA.105.578229. [DOI] [PubMed] [Google Scholar]
- 12.Koo BK, Erglis A, Doh JH, Daniels DV, Jegere S, Kim HS, et al. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve) study. J Am Coll Cardiol. 2011;58:1989–1997. doi: 10.1016/j.jacc.2011.06.066. [DOI] [PubMed] [Google Scholar]
- 13.Min JK, Koo BK, Erglis A, Doh JH, Daniels DV, Jegere S, et al. Usefulness of noninvasive fractional flow reserve computed from coronary computed tomographic angiograms for intermediate stenoses confirmed by quantitative coronary angiography. Am J Cardiol. 2012;110:971–976. doi: 10.1016/j.amjcard.2012.05.033. [DOI] [PubMed] [Google Scholar]
- 14.De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367:991–1001. doi: 10.1056/NEJMoa1205361. [DOI] [PubMed] [Google Scholar]
- 15.Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van't Veer M, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–224. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]