Abstract
Background
Heparin-induced thrombocytopenia (HIT) is a dreaded complication of heparin-related products and correlates with a worse outcome in aneurysmal subarachnoid hemorrhage (SAH) patients.
Objective
To study the risk factors and outcomes of SAH patients suspected of having HIT, confirmed as present or absent by the platelet factor 4 (PF4) antibody test.
Methods
All patients with presumed aneurysmal, nontraumatic SAH and having undergone a PF4 test were identified through our research patient database. Charts, laboratory values and images were analyzed retrospectively.
Results
We identified 166 patients with SAH who were tested for HIT; 42 patients (25%) had a positive antibody test. There was no difference in platelet profiles or mean platelet nadirs of HIT+ and HIT- patients (147 ± 93 vs. 153 ± 86 ×109/l, respectively). Univariate analysis identified gender, magnesium prophylaxis, Fisher group 3, clipping versus coiling, presence of angiographic vasospasm, number of vasospasm treatments, and day of HIT testing as potential risk factors associated with HIT. A multivariate analysis indicated that female gender (OR 8.2, 95% CI 2.0-33.2), greater number of vasospasm treatments (OR 1.5, 95% CI 1.2-2.0), later day of HIT testing (OR 1.2, 95% CI 1.1-1.3), and clipping (OR 5.0, 95% CI 1.42-10.0) were independently associated with HIT positivity. HIT+ patients showed more infarcts on CT, longer ICU and hospital stays and worse modified Rankin Scale scores on discharge.
Conclusion
The presence of HIT in SAH has adverse consequences and is more likely in female patients who have undergone aneurysm clipping and require multiple endovascular vasospasm treatments.
Key Words: Subarachnoid hemorrhage, Heparin-induced thrombocytopenia, Gender, Vasospasm, Delayed ischemic neurologic deficit
Introduction
Heparin-induced thrombocytopenia (HIT) type 2 is a known cause of increased morbidity and mortality [1]. The common, nonimmune-mediated HIT type 1, caused by direct platelet aggregation of heparin, occurs in 10% of patients and is clinically innocuous. Whereas, the potentially life-threatening immune-mediated HIT type 2 is estimated to occur in 1-5% of the general population who is exposed to heparin and in <1% of those exposed to low molecular weight heparin (LMWH) [2]. HIT should be suspected after a 50% decrease in platelets, or a platelet count of <150,000/μl, or arterial thrombosis approximately 5-10 days after exposure to heparin or LMWH [3]. HIT is an immune-mediated response in which antibodies form against complexes of heparin and platelet factor 4 (PF4) [4]. Consequently, platelets are activated and cause a prothrombotic state [5]. The risk of thrombosis is elevated (approx. 30 times that of the general population), and it remains elevated for days to weeks, even after heparin is removed and the platelet count has normalized [1]. HIT causes a hyperthrombotic state that can result in stroke, myocardial infarction, pulmonary embolus, deep vein thrombosis (DVT), amputation, multisystem organ failure, and death [2,6].
Less is known about HIT in the subarachnoid hemorrhage (SAH) population, but research on this specific population estimates an incidence between 5 to 15% [7,8]. SAH patients are exposed to heparin or heparin-related products in a variety of forms: indwelling catheters, endovascular procedures, and DVT prophylaxis. Prior univariate analysis by our group suggested that HIT was more common in Fisher group 3 and female gender [8]. In addition, these patients had worse outcomes in terms of an increased stroke rate and higher mortality. However, this older study evaluated the difference between HIT+ patients and all other SAH patients. Furthermore, the previous study has used clinical diagnosis despite the performance of a confirmatory test. We sought to perform a larger analysis on the risk factors and outcomes of patients with the clinical suspicion of HIT who had been tested by PF4 antibody testing (both negative and positive tests). Once clinical suspicion is raised, various choices must be made in order to limit further heparin exposure or instituting antithrombotic therapy. Therefore, this newer study, with a more defined population suspected of HIT, is of greater clinical relevance to the practicing clinician.
Methods
The institutional review board of the Massachusetts General Hospital, Boston, Mass., USA, approved the following retrospective study. In our neurocritical care unit, SAH patients underwent a PF4 antibody testing based on clinical suspicion of HIT, after multiple vasospasm treatments, or if clot formation was observed at the catheter tip during cerebral angiograms. The clinical criteria for HIT were based on the ACCP guidelines [3] and include: (1) occurrence of thrombocytopenia 4-14 days after start of the heparin therapy (earlier if heparin exposure occurred over the previous 100 days); (2) a reduction in platelet count to <100,000/μl or <50% of baseline, and (3) exclusion of other causes of thrombocytopenia, such as infection, drug and autoimmune mediated. Through the hospital-wide patient research database of the Massachusetts General Hospital, we identified all patients with nontraumatic, presumed or confirmed aneurysmal SAH who had undergone a test for type 2 HIT during their hospitalization. HIT assay was a PF4 ELISA with a specificity and sensitivity of 90 and 98%, respectively [9]. HIT testing was repeated at different occasions up to 3 times in cases with a high clinical suspicion.
The search revealed a total of 166 patients admitted from February 1994 to September 2008. A retrospective chart review was performed to collect information on patient demographics, clinical, laboratory and radiographic data. The modified Rankin Scale (mRS) score was calculated at the time of discharge from chart review. All CT imaging data was blindly reviewed to assess the HIT status. Angiographic vasospasm was determined from the clinical procedure notes or if balloon or drug therapy was utilized.
Standard care for patients included daily transcranial Doppler (TCD) for vasospasm screening, heparinized arterial line, central subclavian intravenous line with heparin flushes and DVT prophylaxis with heparin or LMWH. Only patients with suspected vasospasm on TCD underwent angiography for vasospasm confirmation and treatment. CT imaging was not routinely performed at prespecified times, other than at time of admission. CT was only performed if the clinical team had a clinical indication (e.g. hydrocephalus, ventriculostomy, clinical neurologic changes, etc.). Patients with suspected or confirmed HIT were initiated on an argatroban drip and all heparin-based products were immediately discontinued.
Statistical Analysis
Analysis was performed with SPSS 16 version 16.0.2 for Mac (Chicago, Ill., USA). Normality for continuous variables was tested by the Shapiro-Wilk test. Those variables that failed to meet normality were analyzed by Mann-Whitney U (nonparametric) tests. Equality of variance for continuous variables was tested by Levene's test and significance was adjusted accordingly. Platelet profile by hospital day was analyzed by two-way repeated measures ANOVA. Variables with potential to impact outcomes were first assessed by univariate analysis. Those variables that reached statistical significance were then placed into the multivariate analysis. Prior to performing a logistic regression with a forward stepwise method, we assessed for variables that might have high collinearity, or directly vary with one another. Statistical significance for a type I error was set at p ≤ 0.05.
Results
Of the 166 patients with clinical suspicion of HIT, 42 patients (25%) were HIT+ based on the PF4 test. Univariate analysis demonstrated no differences between HIT- and HIT+ patients in the variables of age, admission Hunt-Hess score, Glasgow Coma Scale score, World Federation of Neurosurgery score, FOUR score, Fisher group 3 score, or past medical history (table 1).
Table 1.
Admission demographics
| HIT+ |
Significance | |||
|---|---|---|---|---|
| no | yes | |||
| Age at admission | median | 60 | 59 | n.s. |
| IQR | 49 – 70 | 50 – 68 | ||
| Gender | % female | 66 | 90 | p = 0.002 |
| History of hypertension | % present | 52 | 38 | n.s. |
| History of diabetes | % present | 6 | 7 | n.s. |
| History of coronary artery disease | % present | 10 | 10 | n.s. |
| History of tobacco use | % present | 35 | 31 | n.s. |
| History of alcohol use | % present | 21 | 10 | n.s. |
| Aneurysm size, mm | median | 7 | 6 | n.s. |
| IQR | 5 – 10 | 4 – 8 | ||
| Aneurysm treated only with clipping | % clipped | 56 | 74 | p = 0.038 |
| Hunt-Hess score | median | 3 | 3 | n.s. |
| Admission Glasgow Coma Scale score | median | 12 | 13 | n.s. |
| IQR | 7 – 15 | 10 – 15 | ||
| Admission FOUR score | median | 9 | 14 | n.s. |
| IQR | 5 – 16 | 8 – 16 | ||
| World Federation of Neurosurgery score | median | 3 | 2.5 | n.s. |
| Fisher Hemorrhage score | median | 3 | 3 | n.s. |
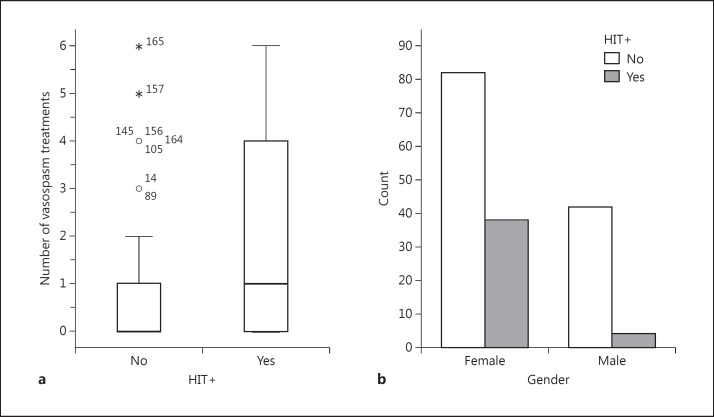
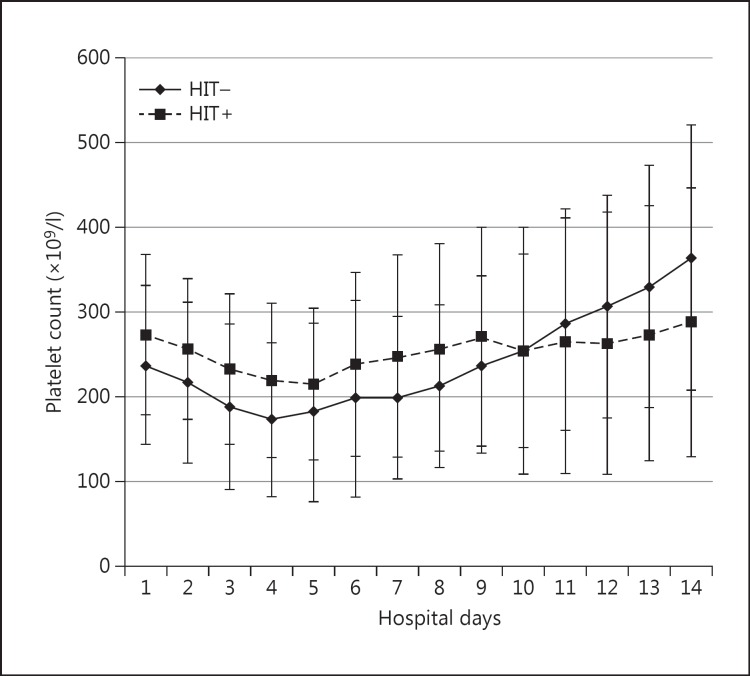
The risk of developing HIT was substantially greater in female patients (fig. 2). Of the 42 HIT+ patients, 38 (90%) were female [χ2(1) = 9.2, p = 0.002, OR 5.8]. The presence of Fisher group 3 increased the odds of HIT positivity by 3.2 times [χ2(1) = 10, p = 0.002]. Furthermore, there was a 2.2 times increase in the development of HIT in patients that underwent aneurysm clipping [χ2(1) = 4.3, p = 0.038]. Interestingly, neither the platelet nadir nor the zenith between the HIT- or HIT+ patients was different [147 ± 93 vs. 153 ± 86 ×109/l (nadir) and 390 ± 173 vs. 415 ± 168 ×109/l (zenith), respectively] (fig. 1). In addition, there was no difference in the decrease of the maximum daily percentage platelet. However, time from admission to ruling in or out the presence of HIT was substantially different. Negative PF4 antibody tests ruling out HIT were performed on hospital day 5 ± 5 days, whereas positive PF4 tests confirming HIT were performed on hospital day 11 ± 6 [t(164) = −6.9, p < 0.001]. We also found that there was a 3.2 times greater risk of being HIT+ if the patient had been treated with a magnesium drip for spasm prophylaxis [χ2(1) = 6.3, p = 0.01]. Angiographic vasospasm was significantly more likely in HIT+ patients than HIT- patients [29 of 42 (69%) vs. 41 of 124 (33%), respectively, (χ2(1) = 16.7, p < 0.0001)]. Furthermore, there were significantly greater numbers of vasospasm treatments for HIT+ patients [1.9 ± 2.1 vs. 0.65 ± 1.3, respectively (U = 1,555, p < 0.0001)] (fig. 2). Interestingly, only 4 of the 29 HIT+ patients with vasospasm treatment had been diagnosed with HIT prior to the treatment, whereas the vast majority had been diagnosed after the initial interventional treatment. However, there was no difference in the day of vasospasm onset (day 6), number of vessels in spasm between the two groups or the distribution of the vessels in spasm.
Fig. 2.
Risk factors for HIT. a Box plot of the number of vasospasm treatments for HIT+ and HIT- patients. The numbers represent individual case outliers (open circle = 1 standard deviation; asterisk = 2 standard deviations). b Total number of male and female patients diagnosed as HIT+ or HIT-.
Fig. 1.
Platelet counts (mean ± standard deviation) of HIT+ and HIT- patients over the first 14 hospital days. A two-way repeated measures ANOVA showed no significant differences between these two patient groups.
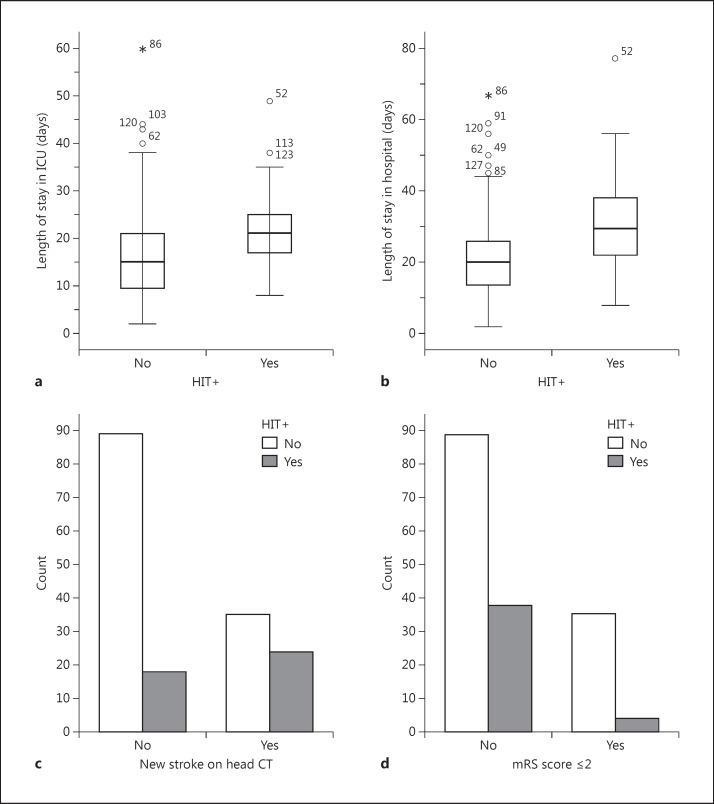
In terms of outcomes (fig. 3), HIT+ patients were 3.4 times more likely to have a new infarction on follow-up CT scan than HIT- patients [χ2(1) = 11, p = 0.001]. HIT+ patients had a greater length of ICU stay [21 ± 8 vs. 16 ± 10 days, respectively (U = 1,530, p < 0.001)] and hospital stay [30 ± 14 vs. 21 ± 13 days, respectively (U = 1,465, p < 0.001)]. HIT+ patients also had worse discharge outcomes with 4 of 42 having a mRS score of ≤2 compared to 35 of 124 HIT- patients [χ2(1) = 6.1, p = 0.01]. This risk remained regardless of dichotomization at an mRS score of 3. There was no significant difference in death between the groups.
Fig. 3.
HIT outcomes. a, b Box plots of the length of ICU and hospital stay, respectively, for HIT+ and HIT- patients, numbers represent individual case outliers (open circle = 1 standard deviation; asterisk = 2 standard deviations). c, d Total number of HIT+ and HIT- patients with new ischemic lesions or good outcome as measured by an mRS score of ≤2, respectively.
We performed a multivariate logistical regression analysis to analyze those factors associated with development of a HIT+ titer. Our model included the following variables: gender, magnesium prophylaxis, Fisher group 3, clipping versus coiling, angiographic spasm, number of vasospasm treatments, and day of HIT testing. Of these variables, the variables that remained significant were female gender (OR 8.2, 95% CI 2.0-33.2), increased number of vasospasm treatments (OR 1.5, 95% CI 1.2-2.0), later hospital day for HIT testing (OR 1.2, 95% CI 1.1-1.3) and clipping (OR 5.0, 95% CI 1.42-10.0) [model χ2(4) = 64.3, p < 0.001]. The Hosmer and Lemeshow test was significant [χ2(8) = 16.5, p = 0.04], indicating that our model differs significantly from the observed data and that perhaps a linear model is not the optimal model.
Discussion
Our multivariate analysis identified four variables that are independently associated with the development of HIT. Of these variables, the most robust association is that of female gender. This finding is confirmed by other studies reflecting the increased risk of HIT in women [8,10]. The cause of the higher HIT risk in women is unclear, but it is hypothesized that HIT affects more women as they have a higher predisposition for autoimmune diseases [10]. Interestingly, the risk of HIT in women in the SAH population appears to be even higher than in other published reports. The increase of HIT in SAH may also be due to the interaction of a higher risk of vasospasm in this group.
Fisher group 3 might be a risk factor for the development of HIT but this conclusion is not supported by our multivariate analysis. Fisher group 3 has been demonstrated to be a risk factor in our previous study comparing HIT+ patients to the general SAH population [8]. The only two other studies in this population did not find a statistically significant association of Fisher 3 group with HIT, but there was a numerical difference. Furthermore, these studies may have lacked sufficient power as there were only 10 HIT+ patients in the Fisher group 3 category in the first study and 18 in the second one [7,11]. It is likely that this association is more a reflection on the risk of vasospasm rather than a direct cause. As such, it may either be due to the fact that patients who undergo endovascular treatment for vasospasm routinely receive continuous intra-arterial infusions of heparinized saline (4,000 U/l in our series) via the intra-arterial catheter, which either has greater risk than exposure to LMWH [3], or to the higher dose exposure [12,13], delivery through an intravenous route [14] or that vasospasm possibly results in higher levels of circulating PF4.
Although the presence of any angiographic vasospasm was a risk in univariate analysis, it appears that the real impact is in those patients that require a second or greater number of intra-arterial therapies [11]. As discussed above, this likely represent an initial exposure to unfractionated heparin and then further exposure on follow-up treatments. Clinically, we have become very sensitive to this possibility, as some of our cases were diagnosed during angiograms where evidence of clot formation at the catheter tip was witnessed. In fact, in some of these cases our ability to predict HIT preceded our detection of the antibody or florid platelet reductions, the typical clinical trigger. In approximately 25% of HIT cases, the thrombotic event precedes the HIT-associated drop in platelet count [3]. As such, if a de novo clot is seen during angiography, our practice is to institute an argatroban drip immediately or in lieu of heparin for the fourth vasospasm treatment and to check for HIT antibodies after multiple vasospasm treatments or sooner if a clot is seen during angiography.
Probably not surprising is the fact that surgical clipping poses a higher risk for HIT development. Indeed, our findings were fully consisted with another recent report which also showed that compared to HIT- patients, HIT+ patients more often undergo aneurysm clipping [11]. It is well established that surgical patients in general have higher risks than medical patients [5]. This is likely due to the tissue injury that accompanies surgery and the release of greater amounts of PF4. It is not known whether SAH in general has a higher or lower release of PF4 relative to other neurosurgical or surgical diseases.
Later testing for HIT was associated with a higher risk of HIT positivity. This likely reflects a combination of longer exposure time to DVT prophylaxis and multiple vasospasm treatments. It is interesting that there were no differences in platelets between groups, suggesting that platelet counts are a poor surrogate for estimating risk for those who will be HIT+. One reason for lack of difference is study inclusion. Patients were only included if HIT was suspected; therefore, even the patients who were HIT-had platelet drops. Others have described a similar platelet nadir around day 4 or 5 after surgery to that of both our HIT+ and HIT- patients [15]. Consequently, this typical postsurgical drop is unlikely to be immune-mediated from heparin and may represent dilution, nonimmune HIT, or other drug reactions. Interestingly, our HIT+ patients generally did not have a dramatic rebound thrombocytosis typically seen after the postsurgical/procedural nadir [15], and HIT+ patients tend to have slight platelet dips and plateaus around conversion time. It is quite possible that some cases may have false-positive HIT or nonfunctional HIT+ antibodies [3]. Furthermore, given our vigilance for this disease, rapid detection has curtailed the time for substantial platelet drops. Even if some of the cases are false-positive or have nonfunctional antibodies, it is quite evident that this population as a whole has substantially worse outcomes by spasm criteria, strokes, length of ICU stay, duration of hospitalization and discharge outcomes.
An interesting and unexpected variable that was significant in univariate analysis but not in multivariate analysis was exposure to magnesium infusion for spasm prophylaxis. As a routine protocol, SAH patients meeting set criteria are started on a continuous infusions of magnesium to elevate the serum magnesium level to 3-4.5 mEq/l. Magnesium is thought to be vasoprotective and reduce the incidence of vasospasm in the SAH population [16,17]. The magnesium protocol was started in 2006 in our institution but patients were included in 1994; consequently, only 20 (12%) patients in our analysis fell into the magnesium treatment time period. There may be some concern about the risk of high-dose magnesium. Some studies have demonstrated increased platelet aggregation [18] at high magnesium levels, and high doses of magnesium (typically at levels used for vasospasm prophylaxis) enhance tissue factor activation [19]. Indeed, cardiac trials using some of the highest magnesium doses have shown adverse outcomes [20]. An analysis of magnesium infusion in the general SAH population should be performed to find out whether this risk will remain or if the risk is still present and whether the proposed benefits will outweigh the risk of HIT.
The main caveat to this study is its retrospective nature. As such, despite established clinical criteria for HIT, we could not control for when or whether a patient was tested. Such difference in testing may enhance the chance of significant false-positive findings or obscure possible significant variables. Differences in when or who is tested may account for timing differences of HIT+ results. Other reasonable possibilities include different causes of early platelet drops (surgical consumption, dilution, etc.), false negatives earlier in the course, or longer heparin exposure. In addition, timing of testing may impact interpretation of the association of vasospasm with HIT positivity. Delayed testing might suggest that vasospasm is a risk for HIT positivity, while early testing might suggest that HIT positivity is a risk for vasospasm. Our data currently indicate the former but one should be very careful about drawing cause-and-effect relationships out of this retrospective data.
Conclusions
HIT within the aneurysmal SAH population has substantial negative ramifications. It appears that traditional risk factors for HIT such as female gender and surgery remain or are substantial for the SAH population. Additional clues to the development of HIT are those patients requiring a second or greater vasospasm treatment and in whom suspicious drops in platelet counts occur during the late spasm window. Additional research is necessary to further identify these associations as likely risks for patients in the hopes of minimizing the damage of HIT and eventually preventing HIT.
References
- 1.Arepally GM, Ortel TL. Clinical practice. Heparin-induced thrombocytopenia. N Engl J Med. 2006;355:809–817. doi: 10.1056/NEJMcp052967. [DOI] [PubMed] [Google Scholar]
- 2.Warkentin TE. Think of HIT. Hematology Am Soc Hematol Educ Program. 2006:408–414. doi: 10.1182/asheducation-2006.1.408. [DOI] [PubMed] [Google Scholar]
- 3.Warkentin TE, Greinacher A, Koster A, et al. Treatment and prevention of heparin-induced thrombocytopenia: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. (8th Edition) 2008;133:340S–380S. doi: 10.1378/chest.08-0677. [DOI] [PubMed] [Google Scholar]
- 4.Greinacher A, Potzsch B, Amiral J, et al. Heparin-associated thrombocytopenia: isolation of the antibody and characterization of a multimolecular PF4-heparin complex as the major antigen. Thromb Haemost. 1994;71:247–251. [PubMed] [Google Scholar]
- 5.Levy JH, Tanaka KA, Hursting MJ. Reducing thrombotic complications in the perioperative setting: an update on heparin-induced thrombocytopenia. Anesth Analg. 2007;105:570–582. doi: 10.1213/01.ane.0000277497.70701.47. [DOI] [PubMed] [Google Scholar]
- 6.Napolitano LM, Warkentin TE, Almahameed A, et al. Heparin-induced thrombocytopenia in the critical care setting: diagnosis and management. Crit Care Med. 2006;34:2898–2911. doi: 10.1097/01.CCM.0000248723.18068.90. [DOI] [PubMed] [Google Scholar]
- 7.Kim GH, Hahn DK, Kellner CP, et al. The incidence of heparin-induced thrombocytopenia Type II in patients with subarachnoid hemorrhage treated with heparin versus enoxaparin. J Neurosurg. 2009;110:50–57. doi: 10.3171/2008.3.17480. [DOI] [PubMed] [Google Scholar]
- 8.Hoh BL, Aghi M, Pryor JC, et al. Heparin-induced thrombocytopenia Type II in subarachnoid hemorrhage patients: incidence and complications. Neurosurgery. 2005;57:243–248. doi: 10.1227/01.neu.0000166539.02280.e5. discussion 243-248. [DOI] [PubMed] [Google Scholar]
- 9.Arepally G, Reynolds C, Tomaski A, et al. Comparison of PF4/heparin ELISA assay with the 14C-serotonin release assay in the diagnosis of heparin-induced thrombocytopenia. Am J Clin Pathol. 1995;104:648–654. doi: 10.1093/ajcp/104.6.648. [DOI] [PubMed] [Google Scholar]
- 10.Warkentin TE, Sheppard JA, Sigouin CS, et al. Gender imbalance and risk factor interactions in heparin-induced thrombocytopenia. Blood. 2006;108:2937–2941. doi: 10.1182/blood-2005-11-012450. [DOI] [PubMed] [Google Scholar]
- 11.Alaraj A, Wallace A, Mander N, et al. Risk factors for heparin-induced thrombocytopenia type II in aneurysmal subarachnoid hemorrhage. Neurosurgery. 2011;69:1030–1036. doi: 10.1227/NEU.0b013e3182284a81. [DOI] [PubMed] [Google Scholar]
- 12.Harbrecht U, Bastians B, Kredteck A, et al. Heparin-induced thrombocytopenia in neurologic disease treated with unfractionated heparin. Neurology. 2004;62:657–659. doi: 10.1212/01.wnl.0000110187.16764.9a. [DOI] [PubMed] [Google Scholar]
- 13.Kawano H, Toyoda K, Miyata S, et al. Heparin-induced thrombocytopenia: a serious complication of heparin therapy for acute stroke. Cerebrovasc Dis. 2008;26:641–649. doi: 10.1159/000166841. [DOI] [PubMed] [Google Scholar]
- 14.Smythe MA, Koerber JM, Mattson JC. The incidence of recognized heparin-induced thrombocytopenia in a large, tertiary care teaching hospital. Chest. 2007;131:1644–1649. doi: 10.1378/chest.06-2109. [DOI] [PubMed] [Google Scholar]
- 15.Hirashima Y, Hamada H, Kurimoto M, et al. Decrease in platelet count as an independent risk factor for symptomatic vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2005;102:882–887. doi: 10.3171/jns.2005.102.5.0882. [DOI] [PubMed] [Google Scholar]
- 16.Zhao XD, Zhou YT, Zhang X, et al. A meta analysis of treating subarachnoid hemorrhage with magnesium sulfate. J Clin Neurosci. 2009;16:1394–1397. doi: 10.1016/j.jocn.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Friedlich D, Agner C, Boulos AS, et al. Retrospective analysis of parenteral magnesium sulfate administration in decreased incidence of clinical and neuroradiological cerebral vasospasm: a single center experience. Neurol Res. 2009;31:621–625. doi: 10.1179/174313209X38232. [DOI] [PubMed] [Google Scholar]
- 18.Serebruany VL, Herzog WR, Schlossberg ML, et al. Bolus magnesium infusion in humans is associated with predominantly unfavourable changes in platelet aggregation and certain haemostatic factors. Pharmacological Res. 1997;36:17–22. doi: 10.1006/phrs.1997.0212. [DOI] [PubMed] [Google Scholar]
- 19.van den Besselaar AM. Magnesium and manganese ions accelerate tissue factor-induced coagulation independently of factor IX. Blood Coagul Fibrinolysis. 2002;13:19–23. doi: 10.1097/00001721-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 20.ISIS-4. a randomised factorial trial assessing early oral captopril, oral mononitrate, and intravenous magnesium sulphate in 58,050 patients with suspected acute myocardial infarction. ISIS-4 (Fourth International Study of Infarct Survival) Collaborative Group. Lancet. 1995;345:669–685. [PubMed] [Google Scholar]