Abstract
Potato virus Y (PVY) and potato virus X (PVX), the RNA viruses of two different genera results into synergistic interactions on mixed infection. In this study, a N–Wi strain of PVY and a PVX strain that is asymptomatic on potato were used to study their interactions during mixed infection in Nicotiana benthamiana and Nicotiana tabacum with reference to symptom expression, level of coat protein (CP) using ELISA and suppressor gene using real time PCR under high temperature (26–40 °C) and low temperature (5–25 °C) conditions. Both mixed and single infection caused severe necrosis and death of N. benthamiana plants. Single infection of these viruses in N. tabacum showed mild symptoms but mixed infection caused more severe symptoms. Synergistic symptoms were more pronounced under low temperature conditions than at high temperature. In low temperature conditions, the CP level of PVX in N. benthamiana was twofold higher than PVY and both the viruses reached at peak at 28 dpi in single virus infection. When PVY and PVX inoculated together, the CP levels of both the viruses increased and reached to the peak earlier (within 7–14 days) than that in the single virus inoculation. Although, the CP level of PVX was higher than PVY in mixed infection, at later stage (28 dpi) both the CP level declined to the similar level. The level of p25 suppressor gene was higher than HC-Pro in single inoculation. However, under mixed inoculation of PVY and PVX, expression of p25 was declined to the level of HC-Pro when the CP levels of both the virus also were observed to decline. The expression pattern of CP and suppressor gene was different in plants when mixed infection was created by inoculation of one virus followed by the other. This study showed the level of CP and suppressor gene of specific strain of PVY and PVX during their mixed infection in tobacco.
Keywords: PVY, PVX, HC-Pro, p25, Synergism, Mixed infection
Introduction
Potato virus Y (PVY) of the genus Potyvirus and family Potyviridae, an important viral pathogen is distributed all over the world. PVY spreads through several species of aphids in a non-persistent manner. Major symptoms of PVY in potato include mild mosaic on leaves and necrosis of leaves and tubers. Yield losses due to PVY infections are between 40 and 70 % [20]. The genome of PVY consists of a +ssRNA of ~10 kb containing an open reading frame (ORF) encoding a single polyprotein precursor of 350 kDa [33]. The polyprotein is processed by viral and host proteases into seven smaller proteins: P1 Protease (P1), helper component protease (HC-Pro), P3-Protein (P3), cylindrical inclusion (CI), nuclear inclusion A (NIa), nuclear inclusion B (NIb), capsid protein (CP) and two small putative proteins known as 6 kDa protein 1(6K1) and 6 kDa protein 2 (6K2) [4, 7, 8].
Potato virus X (PVX) of the genus Potexvirus and family Alfaflexiviridae is also widely distributed in all potato growing regions of the world. The genome of PVX is +ssRNA of 6.4 kb containing four ORFs, which are expressed through sub genomic transcripts [12, 24, 27, 38]. PVX is transmitted through contact. It causes mild mottle mosaic symptoms in potato and accounts for 15–30 % yield losses [3, 20]. The PVX genome contains a large 5′ ORF-1 coding for the 65K protein, a putative component for RNA replication and it is translated directly from genomic RNA [24, 38]. The ORF-2, -3 and -4 are known as triple gene block (TGB), which encode 25K helicase (p25, TGB1) [12, 38], 12K protein (p12, TGB2) and 8K protein (p8, TGB3), respectively [27]. The TGB proteins are known to involve in cell-to-cell movement of the virus [15, 38]. The ORF-5 encodes CP, which is involved in the encapsidation and movement of the virion [21].
Expression of symptoms due to a viral infection depends on several factors such as the level of host resistance, growth stage of the plant, kind of virus species or strains and environmental conditions. Plant virus encodes silencing suppressor protein that is known to play a major role in defeating the plant defense [1, 42]. HC-Pro, a silencing suppressor protein, is also known as a synergy factor needed for potyvirus infection [1, 18, 31, 32]. HC-Pro is a multifunctional protein, which helps in transmission of the virus through aphids, replication and systemic movement of the virus [13, 39]. p25 of PVX is known as a silencing suppressor protein. It has nucleotide binding and RNA helicase activity [4, 17, 25, 26, 41] and it plays a role in cellular movement of the virus [4, 14].
In nature, mixed infection of plant viruses is a common phenomenon and two or more viruses can cause synergism in plants where more severe forms of symptoms are expressed. In the synergistic situations, either virus titre or the movement or both can be enhanced [35, 43]. Breakdown of resistance by a co-infection with an unrelated virus is also an indication of a synergistic interaction [6, 22, 28, 30]. A number of viruses are reported to induce synergistic interactions in plants [5, 10]. Mixed infection of PVY and PVX causes synergistic interaction resulting in rugose disease in potato, which is characterized by severe crinkling, mottling, stunting and death of plants [37].
Several strains of PVY are known to exist and severity of synergistic interactions with PVX depends on the strain of PVY [29]. In India, PVX and PVY are important viral pathogens of potato, however, hardly any information is available on strains variation and their interactions during mixed infection. We have a previously characterized PVY isolate (JN034046) from potato showing mild mottling [36] and an isolate of PVX (JF430080), which causes no symptoms on potato [23]. The present study reports interactions of mild strains of PVY and PVX with reference to symptom expression and level of CP and suppressor genes during mixed inoculations.
Materials and methods
Virus source
PVY showing mild mottling was isolated form potato field at IARI, New Delhi. The virus isolate named as PVY-Del66 was maintained on Nicotiana benthamiana through sap inoculation. PVY-Del66 was typed as N–Wi strain based on biology, serology and complete genome sequence (JF430080) [36]. PVX (isolate PtDell9) was isolated from IARI field showing no symptoms on potato. The virus was characterized based on host reaction and complete genome sequencing [23].
Sap inoculation
Nicotiana benthamiana and Nicotiana tabacum seedlings were grown in plastic pots (one pot/plant) containing sand 1: peat 2: soil mixture. Single and mixed inoculations of PVY-Del66 isolate and PVX pt-Del19 isolates were performed mechanically to the healthy N. benthamiana and N. tabacum cv. Xanthi seedlings at 3–4 leaf stage. The inoculum was prepared by grinding infected leaves (1:1 = g:ml) in 0.1 M phosphate buffer, pH 7.0 containing 0.2 % sodium sulfite and 0.01 M 2-mercaptoethanol in a chilled pestle and mortar. The extract was filtered using cheesecloth and 1 % of Celite 545 (S.D. Fine Chem. Ltd., Hyderabad, India) was added to it. The inoculum was applied by rubbing the two expanded leaves predusted with Carborundum 600 mesh (HiMedia Laboratories, Mumbai, India).
To study the interaction of PVX and PVY in tobacco, inoculation was conducted at two different temperature regimes, high temperature (26–40 °C) and low temperature (5–25 °C). Five different combinations of inoculations were performed—(1) PVY alone; (2) PVX alone; (3) PVY + PVX: equal quantity of N. benthamiana leaf tissues infected with respective virus were used to prepare the inoculum containing both the viruses; (4) PVY followed by PVX: tobacco plants were inoculated with PVY and then at 7 days post inoculation (dpi) the same leaves of the plants were re-inoculated with PVX; (5) PVX followed by PVY: inoculation was followed as described in combination-4, only the virus was altered. For each combination of inoculation five plants were inoculated in two replications.
Sample collection
For determining level of CP and suppressor gene in single and mixed infection of PVX and PVY at different dpi, single leaf from each of the five inoculated plants were collected to make composite samples.
Enzyme-linked immunsorbent assay (ELISA)
ELISA was conducted for the quantification of the level of CP of PVY using commercially available antibody kit for PVYO strain (Agdia, Inc, Elkhart, IN, USA) and for CP of PVX using in-house polyclonal antibody [23]. The extracts were prepared from the composite leaf samples by grinding the each of the composite sample (1:10 = g: ml; 0.2 M sodium carbonate buffer, pH 9.4). ELISA was performed in three replications and OD at 405 nm was recorded 1 h after the addition of the substrate, p-nitrophenyl phosphate by an ELISA reader (BIOTEK Instruments, USA).
Real time RT-PCR
For the amplification of HC-Pro of PVY, BM270F (5′GGGGTTATGGATTCAATGGTTC3′) and BM417R (5′TCACTTGCGTGCTTGTGTA3′) primers were designed selecting the target regions starting form 986 nt and ending at 1,245 nt (260 bp amplicon size) of PVY-Del66 isolate (JN034046). For the amplification of p25 (TGB1) of PVX, BM296F (5ATGGATATTCTCATCATTAG3′) and BM420R (5′ATCAGCAAAAAGTGCCTGG 3′) primers were designed selecting the target region from 4,486 to 4,785 nt (300 bp amplicon size) of PVX-ptDel9 (JF430080). The primers were checked for non target binding using BLAST programme (1). Actin primers; ActF: 5′ATGCCATTCTCCGTCTTGACTTG 3′ and ActR: 5′GAGTTGTATGTAGTCTCGTGGATT 3′ (amplicon size of 324 bp) were used to estimate the expression of actin housekeeping gene in the samples as an internal control [9, 16]. cDNA was synthesized from total plant leaf RNA extracted using TRIzole (Life Technologies, Grand Island, NY, USA) with RevertAid First Strand cDNA Synthesis Kit (Fisher Scientific-USA, Pittsburgh PA, USA) according to the manufacture’s protocol.
The Real time PCR was carried out using a LightCycler® 480 Real-time PCR system (Roche Diagnostics GmbH, Penzberg, Germany) to find out the level of expression of HC-pro and p25 of PVY and PVX, respectively and actin gene at 14, 21 and 28 dpi in leaf tissues of N. benthamiana. Inoculation was performed with PVY-Del66 and PVX- ptDel9 at low temperature (5 -25 °C) in the combination of the virus as described in the previous section. The PCR reaction mix of 20 μl consisted of 10 μl of KAPA SYBR Master Mix (2X) (KAPA Biosystems, Woburn, MA, USA), 0.5 μl of 10 mM forward and reverse primer each for HC-Pro and p25 suppressor genes, 1 μl of template cDNA (50 ng) and nuclease free water to mark up the volume. The threshold cycle (Ct) at which the significant increase in fluorescence occurred was calculated using software version LCS480 1.5.0.39 provided with the LightCycler® 480. Ct values for HC-Pro and p25 expressions at 14, 21 and 28 dpi were plotted under each treatment. The housekeeping gene (actin) was used as an internal reference.
Results
Symptom expression in single and mixed infections
PVY alone inoculation
Nicotiana benthamiana at low temperature produced mild mottling on the inoculated leaves at 7 dpi. At 14 dpi onward systemic necrotic spots developed, which gradually became severe resulting death of plant at 40 dpi. At high temperature, a similar progress of diseases symptoms was observed but the degree of severity was low compared to those plants inoculated at low temperature. The plants at high temperature showed systemic necrotic spots, however, plants were not killed due to necrosis as found in low temperature. N. tabacum under low temperature produced yellow patches on the inoculated leaves and subsequently systemic vein yellowing and necrotic veins developed. Under high temperature however, N. tabacum produced no symptoms on the inoculated leaves, but at 14 dpi onwards it produced systemic yellow patches or mottling. The plant at later stage (after 28 dpi) developed necrotic vein (Fig. 1).
Fig. 1.
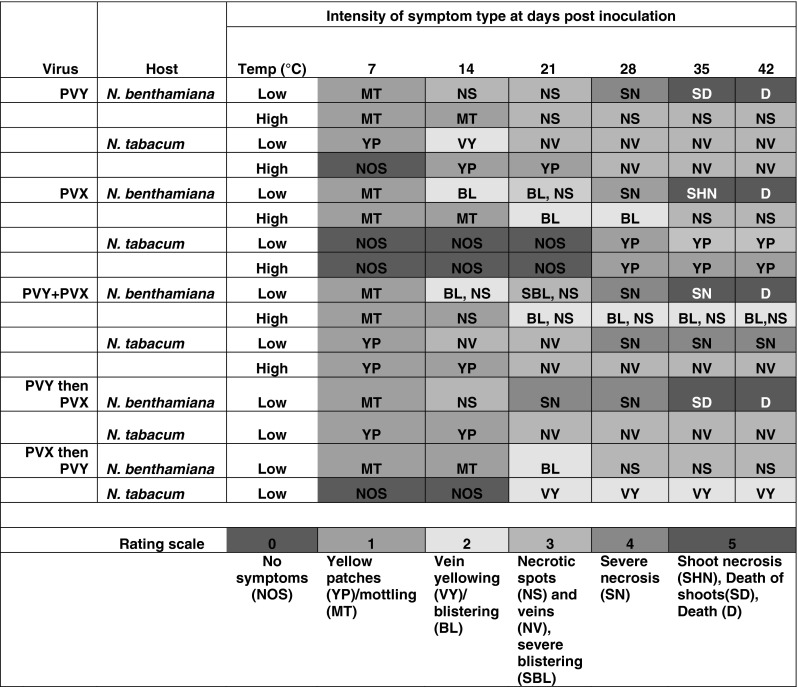
Symptoms expression in Nicotiana benthamiana and N. tabacum following inoculation with potato virus Y-Del66 (PVY) and potato virus X-ptDel9 (PVX) singly and together as mixing both or one followed by the other a week later under low (5–25 °C) and high temperature (26–40 °C) regimes. Symptom intensity expressed in 0–5 rating scale is depicted by colour code and symptom type is shown as abbreviations, where MT mottling, YP yellow patches, NOS no symptom, NS necrotic spots, VY vein yellowing, BL blistering, SBL severe blistering, NV necrotic vein, SN severe necrosis, SHN shoot necrosis, D death
PVX alone inoculation
PVX induced mottling followed by greenish blistering and necrotic spots on N. benthamiana by 21 dpi at low temperature. These plants subsequently developed severe necrosis, which caused death of plants by 40 dpi. Under high temperature, PVX developed mottling and blistering as major symptoms in N. benthamiana, however, some minor necrotic spots developed after 28 dpi. Under high temperature conditions, there was no death of N. benthamiana plants due to infection of PVX. In N. tabacum, PVX induced no symptoms both under high and low temperature conditions till 21 dpi, however, at 21dpi onwards plant developed yellow patch symptoms (Fig. 1).
PVY and PVX mixed inoculation
Mixed inoculation of PVX and PVY to N. benthamiana at low temperature resulted in severe systemic symptoms that comprised of severe blistering, necrosis and death of plants, whereas, under high temperature, both the viruses caused blistering and necrosis only. At both the low and high temperature regimes, N. tabacum produced yellow patches on the inoculated leaves followed by systemic veinal necrosis. At low temperature, mixed inoculation of both the viruses resulted in more pronounced veinal necrosis compared to that at high temperature (Fig. 1).
One virus followed by another
Under low temperature regime, PVY followed by PVX inoculated N. benthamiana plants developed systemic symptoms that included severe necrosis to the death of plants. Whereas, in N. tabacum under this condition produced yellow patches and necrotic veins (Fig. 1).
When PVX followed by PVY inoculation was performed, N. benthamiana produced mottling, blistering and necrosis under low temperature. N. tabacum plants under these conditions developed no symptoms till 14 dpi, however, they developed yellow vein symptoms after 21 dpi (Fig. 1).
Level of CP of PVY and PVX under single and mixed infections
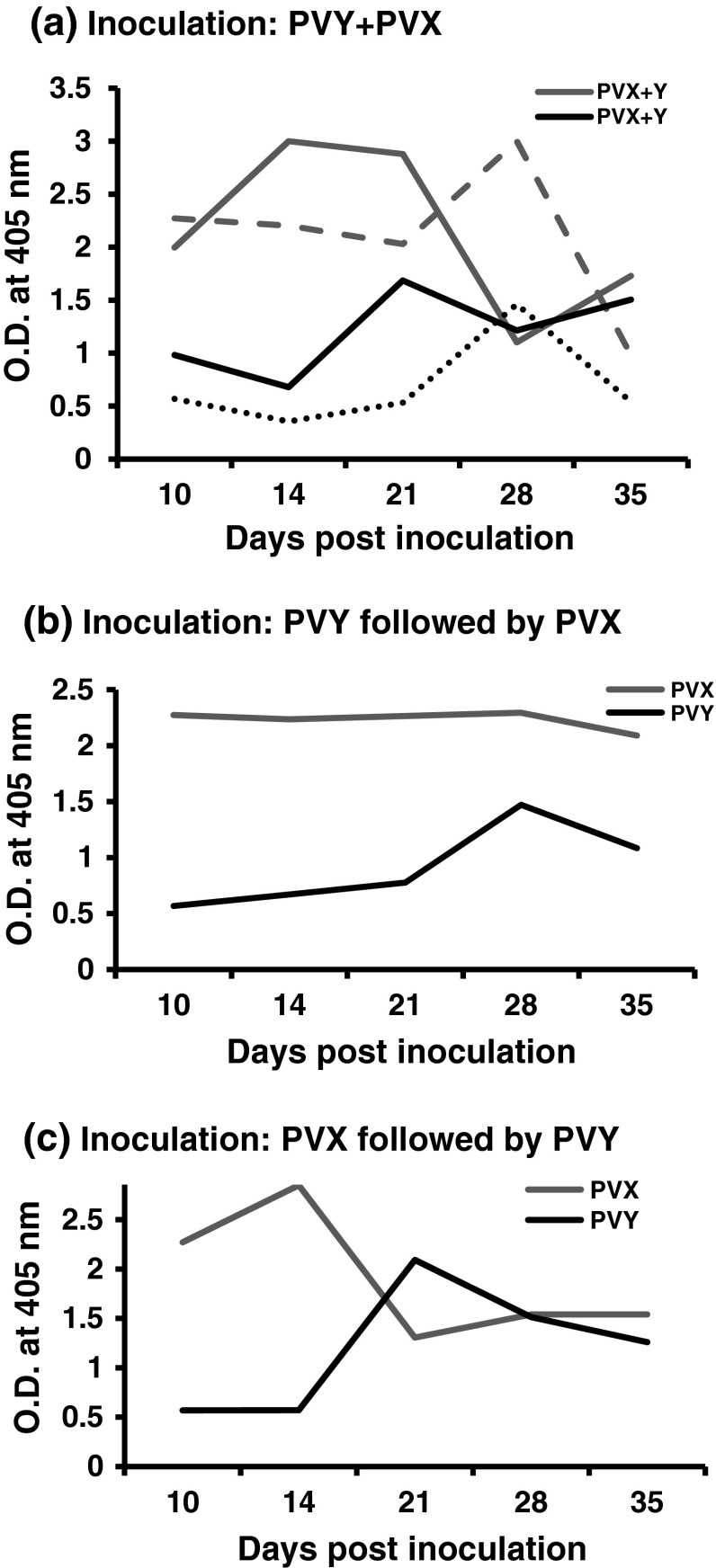
During 10–35 dpi, the ELISA value (OD) of PVX alone in N. benthamiana varied from 0.98 to 3.0, whereas in case of PVY alone, it varied from 0.35 to 1.46. The level of CP in N. benthamiana inoculated with individual virus reached to the highest level at 28 dpi and then declined. At 28 dpi, PVX showed OD value twice than that of PVY. When PVX and PVY were inoculated together (Fig. 2a), the peak OD of PVX shifted to 14–21 dpi and PVY shifted to 21 dpi. Under this conditions OD value for both PVX and PVY declined to the lowest level (OD 1.21–1.10) at 28 dpi and then increased. When the inoculation was performed with PVY followed by PVX (Fig 2b), similar level (OD 0.56–1.4) of PVY was observed over the time as in case of PVY alone infection, whereas, consistently a higher level (OD 2.09–2.29) of PVX was observed under this situation. When the inoculation was performed with PVX followed by PVY (Fig. 2c), the peak OD of 2.84 for PVX was observed at 14 dpi, which declined subsequently. PVY however, showed a peak OD of 2.09 at 21 dpi and then declined.
Fig. 2.
ELISA showing the level of coat protein of potato virus Y (PVY-Del66) and potato virus X (PVX-ptDel9) in Nicotiana benthamiana following inoculation of the viruses singly and together as mixing both or one followed by another under low temperature regime (5–25 °C). At each dpi, a composite sample was made collecting single leaves from each of the five inoculated plants and a representative aliquot was used in ELISA. OD value was recorded 1.0 h after adding the substrate. The brown segmented lines (PVX) and black dotted lines (PVY) in (a) represent single virus inoculation. (Color figure online)
Level of f HC-Pro and p25 under single and mixed infections of PVY and PVX
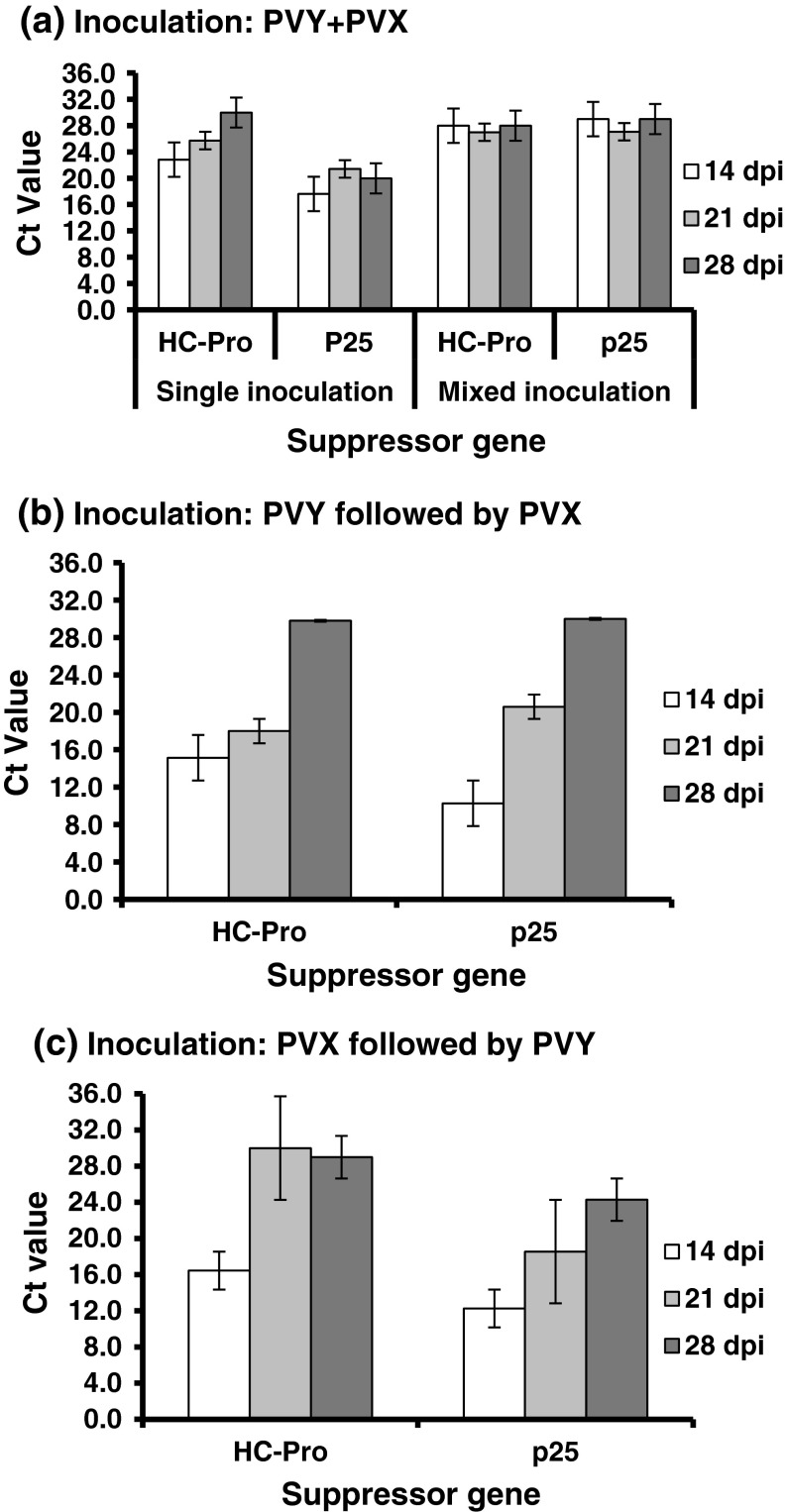
The Ct values of actin in healthy and virus infected (single and mixed infection) N. benthamiana under low temperature conditions, varied from 25.12 to 30.0 at 14–28 dpi, which were statistically insignificant variations, indicating unaltered expression of actin in N. benthamiana during PVY and PVX infection. The Ct value of HC-Pro in PVY infection was 22.85–25.73 at 14–21 dpi, which significantly increased to 30.0 at 28 dpi indicating a decreasing level of HC-Pro expression over the time. The relative level of p25 over 14–28 dpi was observed to be unaltered as it showed Ct value 17.63–21.42. The level of p25 was relatively higher than that of HC-Pro in single infection of the respective virus (Fig. 3).
Fig. 3.
Real time RT-PCR showing the level of expression of HC-Pro and p25 suppressor genes of potato virus X (PVX-ptDel9) and potato virus Y (PVY-Del66) in Nicotiana benthamiana leaves following inoculation of the viruses singly and together as mixing both or one followed by another under low temperature regime (5–25 °C). SYBR Green was used in realtime RT-PCR with HC-Pro (BM270F and BM417R) and p25 (BM296F and BM420R) primers. Ct values for HC-Pro and p25 expressions were obtained at 14, 21 and 28 days post inoculation (dpi). Expression of Actin gene taken as internal reference, was unaltered in healthy plants and in single and mixed infection of PVX and PVY
After simultaneous inoculation of PVX and PVY, the Ct value for the suppressor genes for both the viruses showed similar range of 27.0–29.0. Under inoculation with PVY followed by PVX, Ct values for suppressor genes ranged from 15.14 to 29.80 for HC-Pro and 10.27 to 30.00 for p25. Under this situation HC-Pro level was higher at 14–21 dpi and then significantly declined at 28 dpi. p25 also showed a significantly decreased level of expression at 14, 21 and 28 dpi. Under the inoculation with PVX followed by PVY, Ct values for suppressor genes ranged from 16.45 to 29.0 for HC-Pro and 12.27 to 24.30 for p25 indicating more or less similar level of expression.
Discussion
The present study shows that synergistic symptoms in tobacco were more pronounced under low temperature conditions (5–25 °C) compared to high temperature (26–40 °C) irrespective of tobacco species used and combinations of inoculations of N–Wi strain of PVY and a mild strain of PVX. In low temperature conditions, the severity grade was higher in case of PVY when compared to PVX. Mixed infection by simultaneous inoculation of PVY and PVX or PVY followed by PVX under low temperature, showed no significant synergistic symptomatology as this combination of inoculation as well as single inoculation of individual viruses caused severe form of disease and death of N. benthamiana plants. Whereas, when mixed infection was created by inoculation of PVX followed by PVY symptom progress over the time was not as severe as in the situation of single or mixed infection as above under low temperature regime. The response of N. tabacum to single and mixed infection under both high and low temperature regimes was less intense than that of N. benthamiana. However, pronounced synergistic symptoms due to mixed infection of PVY and PVX were observed in N. tabacum under low temperature. PVY and PVX infection symptoms depend on the seasons [34]. Synergistic symptoms of PVY and PVX were shown to vary depending on strains of PVY. More remarkable synergism was previously observed in infection of NTN strain rather than O strain of PVY with PVX [29].
As PVX and PVY produce higher level of symptoms in tobacco at low temperature, the level of viral CP and suppressor gene were studied under low temperature. Although, PVX produced less pronounced symptoms in both N. benthamiana and N. tabacum compared to PVY, the level of PVX was two-fold higher than PVY in N. benthamiana. The level of CP of both the viruses differed over the time in mixed infection when both the viruses were inoculated together or one followed by the other. PVX when inoculated together with PVY or PVX followed by PVY, it showed higher level of CP at 2 weeks post inoculation and then increased at 4 weeks post inoculation to the level of PVY. PVX showed different pattern CP level under mixed infection where inoculation was conducted as PVY followed by PVX, the level of CP did not decline and was maintained at similar level beyond 3 weeks post inoculation. Previous studies showed an increase of PVX in mixed infection of PVY and PVX compared to the single inoculations [32, 40]. Whereas, González-Jara et al. [11] reported that PVY and PVX mixed infection did not cause a considerable increase in the accumulation of PVX in N. benthamiana though the severe symptoms like necrosis of leaves and stems leading up to the death of plants. Similar observations were also reported for PVX in mixed infections with O and NTN strain of PVY [29]. This study and previous studies show that strain of viruses may play a major role in the accumulation of each virus under mixed infection conditions.
In mixed viral infections, silencing suppressor proteins may help to overcome natural RNA silencing mechanisms of hosts facilitating synergism where one or more viruses that are co-infecting accumulate at higher titers than observed in single virus infections [1, 32]. Potyviral HC-Pro has been shown to play an important role in symptom development and viral replication and suppression of antivirus silencing [1, 2, 19]. In the present study, suppressor gene p25 showed a higher level of expression compared to HC-Pro under single inoculations, which correlated with the higher level of PVX compared to PVY. In the mixed infection, both HC-Pro and p25 declined to the level equivalent to HC-Pro at 28 dpi of single infection of PVY and the level of PVX and PVY was also observed to decline 21 dpi. The pattern of these suppressor gene expressions was different in N. benthamiana plants when PVY infection took place before PVX and vice versa. Under this situation HC-Pro and p25 levels declined over the time. Our study shows expression of suppressor genes under mixed infection of both the viruses declined, which influenced level of each virus in N. benthamiana under low temperature condition. The present study measures different gene activity in a mixed infection of specific strains of PVY and PVX. The finding of this research explains how two different strains of PVY and PVX behave in a synergistic interaction.
Acknowledgments
Fellowship provided by the Council for Agricultural Research Policy, Sri Lanka and the support from the Postgraduate School, Indian Agricultural Research Institute are thankfully acknowledged.
References
- 1.Anandalakshmi R, Pruss GJ, Ge X, Marathe R, Mallory AC, Smith TH, Vance VB. A viral suppressor of gene silencing in plants. Proc Natl Acad Sci USA. 1998;95:13079–13084. doi: 10.1073/pnas.95.22.13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atreya CD, Atreya PL, Thornbury DW, Pirone TP. Site directed mutations in the potyvirus HC-Pro gene affect helper component activity, virus accumulation, and symptom expression in infected tobacco plants. Virology. 1992;191:106–111. doi: 10.1016/0042-6822(92)90171-k. [DOI] [PubMed] [Google Scholar]
- 3.Bai Y, Guo Z, Wang X, Bai D, Zhang W. Generation of double-virus-resistant marker-free transgenic potato plants. Prog Nat Sci. 2009;19:543–548. [Google Scholar]
- 4.Bayne EH, Rakitina DV, Morozov SY, Baulcombe DC. Cell-to-cell movement of Potato Potexvirus X is dependent on suppression of RNA silencing. Plant J. 2005;44:471–482. doi: 10.1111/j.1365-313X.2005.02539.x. [DOI] [PubMed] [Google Scholar]
- 5.Calvert LA, Ghabrial SA. Enhancement by Soybean mosaic virus of bean pod mottle virus titer in doubly infected soybean. Phytopathology. 1983;73:992–997. [Google Scholar]
- 6.Carr RJ, Kim KS. Evidence that Bean golden mosaic virus invades non-phloem tissue in double infection with Tobacco mosaic virus. J Gen Virol. 1983;64:2489–2492. [Google Scholar]
- 7.Carrington JC, Dougherty WG. Small nuclear inclusion protein encoded by a plant potyvirus genome is a protease. J Virol. 1987;61:2540–2548. doi: 10.1128/jvi.61.8.2540-2548.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carrington JC, Dougherty WG. Processing of the tobacco etch virus 49K protease requires autoproteolysis. Virology. 1987;160:355–362. doi: 10.1016/0042-6822(87)90006-7. [DOI] [PubMed] [Google Scholar]
- 9.García-Marcos A, Pacheco R, Martiá˜nez J, González-Jara P, Díaz-Ruíz JR, Tenllado F. Transcriptional changes and oxidative stress associated with the synergistic interaction between Potato virus X and Potato virus Y and their relationship with symptom expression. Mol Plant Microbe Interact. 2009;22:1431–1444. doi: 10.1094/MPMI-22-11-1431. [DOI] [PubMed] [Google Scholar]
- 10.Gil-Salas FM, Peters J, Boonham N, Cuadrado IM, Janssen D. Yellowing disease in zucchini squash produced by mixed infections of cucurbit yellow stunting disorder virus and cucumber vein yellowing virus. Phytopathology. 2011;101:1365–1372. doi: 10.1094/PHYTO-12-10-0343. [DOI] [PubMed] [Google Scholar]
- 11.González-Jara P, Tenllado F, Martínez-García B, Atencio FA, Barajas D, Vargas M, Díaz-Ruiz J, Díaz-Ruíz JR. Host-dependent differences during synergistic infection by potyviruses with potato virus X. Mol Plant Pathol. 2004;5:29–35. doi: 10.1111/j.1364-3703.2004.00202.x. [DOI] [PubMed] [Google Scholar]
- 12.Gorbalenya AE, Koonin EV, Donchenko AP, Blinov VM. A novel super family of nucleoside triphosphate binding motif containing proteins which are probably involved in duplex unwinding in DNA and RNA replication and recombination. FEBS Lett. 1988;235:16–24. doi: 10.1016/0014-5793(88)81226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris K, Smith O, Duffus J. Virus-insect-plant interactions. San Diego: Academic Press; 2001. [Google Scholar]
- 14.Howard AR, Heppler ML, Ju HJ, Krishnamurthy K, Payton ME, Verchot-Lubicz J. Potato virus X TGBp1 induces plasmodesmata gating and moves between cells in several host species whereas CP moves only in N. benthamiana leaves. Virology. 2004;328:185–197. doi: 10.1016/j.virol.2004.06.039. [DOI] [PubMed] [Google Scholar]
- 15.Huisman MJ, Linthorst HJM, Bol JF, Cornelissen BJC. The complete nucleotide sequence of potato virus X and its homologies at the amino acid level with various plus-stranded RNA viruses. J Gen Virol. 1988;69:1789–1798. doi: 10.1099/0022-1317-69-8-1789. [DOI] [PubMed] [Google Scholar]
- 16.Jyothsna P. Satellite DNA-b mediated interference of host gene expression in tomato. Ph.D. Thesis. Dept. of Botany, Ch. Charan Singh University, Meerut, India. 2013.
- 17.Kalinina NO, Rakitina DV, Solovyev AG, Schiemann J, Morozov SY. RNA helicase activity of the plant virus movement proteins encoded by the first gene of the triple gene block. Virology. 2002;296(2):321–329. doi: 10.1006/viro.2001.1328. [DOI] [PubMed] [Google Scholar]
- 18.Kasschau CD, Carrington JC. A counter defensive strategy of plant viruses: suppression of posttranscriptional gene silencing. Cell. 1998;95:461–470. doi: 10.1016/s0092-8674(00)81614-1. [DOI] [PubMed] [Google Scholar]
- 19.Kasschau KD, Carrington JC. Requirement for HC-Pro processing during genome amplification of tobacco etch potyvirus. Virology. 1995;209:268–273. doi: 10.1006/viro.1995.1254. [DOI] [PubMed] [Google Scholar]
- 20.Khurana S. Potato viruses and their management. Dis Fruits Veg. 2004;2:389–440. [Google Scholar]
- 21.Lough TJ, Netzler NE, Emerson SJ, Sutherland P, Carr F, Beck DL, Lucas WJ, Forster RLS. Cell-to-cell movement of potexviruses: evidence for a ribonucleoprotein complex involving the coat protein and first triple gene block protein. Mol Plant Microbe Interact. 2000;13:962–974. doi: 10.1094/MPMI.2000.13.9.962. [DOI] [PubMed] [Google Scholar]
- 22.Malyshenko SI, Kondakova OA, Taliansky ME, Arabekov JG. Plant virus transport function: complementation by helper viruses is non-specific. J Gen Virol. 1989;70:2751–2757. [Google Scholar]
- 23.Mandal B, Kumar A, Rani P, Jain RK. Complete genome sequence, phylogenetic relationships and molecular diagnosis of an indian isolate of potato virus X. J Phytopathol. 2012;160:1–5. [Google Scholar]
- 24.Miroshnichenko NA, Karpova OV, Morozov SY, Rodionova NP, Atabekov JG. Translation arrest of potato virus X RNA in Krebs-2 cell-free system: RNase H cleavage promoted by complementary oligodeoxy nucleotides. FEBS Lett. 1988;234:65–68. doi: 10.1016/0014-5793(88)81304-8. [DOI] [PubMed] [Google Scholar]
- 25.Morozov SY, Solovyev AG, Kalinina NO, Fedorkin ON, Samuilova OV, Schiemann J, Atabekov JG. Evidence for two non overlapping functional domains in the potato virus X 25K movement protein. Virology. 1999;260(1):55–63. doi: 10.1006/viro.1999.9788. [DOI] [PubMed] [Google Scholar]
- 26.Morozov SY, Solovyev AG. Triple gene block: modular design of a multifunctional machine for plant virus movement. J Gen Virol. 2003;84:1351–1366. doi: 10.1099/vir.0.18922-0. [DOI] [PubMed] [Google Scholar]
- 27.Morozov SY, Miroshnichenko NA, Zelenina DA, Fedorkin ON, Solovyev AG, Lukasheva TI, Atabekov JG. Expression of RNA transcripts of potato virus X full length and subgenomic cDNAs. Biochimie. 1990;72:677–684. doi: 10.1016/0300-9084(90)90051-h. [DOI] [PubMed] [Google Scholar]
- 28.Murphy JF, Kyle MM. Alleviation of restricted systemic spread of Pepper mottle potyvirus in Capsium annuum cv. Avelar by coinfection with a cucumovirus. Phytopathology. 1995;85:561–566. [Google Scholar]
- 29.Nie X, Singh M. Response of potato, tobacco and Physalis floridana plants to mixed infection with PVX, PVYNTN and PVYO strains. Can J Plant Pathol. 2013;35(3):390–401. [Google Scholar]
- 30.Okada K, Nagata T, Takebe I. Co-electroporation of rice protoplasts with RNAs of cucumber mosaic and tobacco mosaic viruses. Plant Cell Rep. 1988;7:333–336. doi: 10.1007/BF00269931. [DOI] [PubMed] [Google Scholar]
- 31.Plisson C, Drucker M, Blanc S, German-Retana S, Le Gall O, Thomas D, Bron P. Structural characterization of HC-Pro, a plant virus multifunctional protein. J Biol Chem. 2003;278:23753–23761. doi: 10.1074/jbc.M302512200. [DOI] [PubMed] [Google Scholar]
- 32.Pruss G, Ge X, Shi XM, Carrington JC, Bowman VV. Plant viral synergism: the potyviral genome encodes a broad-range pathogenicity enhancer that transactivates replication of heterologous viruses. Plant Cell. 1997;9:859–868. doi: 10.1105/tpc.9.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riechmann JL, Lain S, Garcia JA. Review article: highlights and prospects of potyvirus molecular biology. J Gen Virol. 1992;73:1–16. doi: 10.1099/0022-1317-73-1-1. [DOI] [PubMed] [Google Scholar]
- 34.Rochow WF, Ross AF. Virus multiplication in plants doubly infected by potato viruses X and Y. Virology. 1955;1:10–27. doi: 10.1016/0042-6822(55)90003-9. [DOI] [PubMed] [Google Scholar]
- 35.Siddiqui SA, Valkonen JPT, Rajamäki ML, Lehto K. The 2b silencing suppressor of a mild strain of Cucumber mosaic virus alone is sufficient for synergistic interaction with Tobacco mosaic virus and induction of severe leaf malformation in 2b-transgenic tobacco plants. Mol Plant Microbe Interact. 2011;24:685–693. doi: 10.1094/MPMI-12-10-0290. [DOI] [PubMed] [Google Scholar]
- 36.Singh S, Mandal B, Jain RK. The complete genome sequence of potato virus Y occurring in India. Abstract in the International conference on plant biotechnology for food security: new frontiers, held at IARI on 21–24 February 2012, New Delhi. Society for Plant Biochemistry and Biotechnology, pp 182.
- 37.Singh RP, Valkonen JPT, Gray SM, Boonham N, Jones RAC, Kerlan C, Schubert J. Discussion paper: the naming of potato virus Y strains infecting potato. Arch Virol. 2008;153:1–13. doi: 10.1007/s00705-007-1059-1. [DOI] [PubMed] [Google Scholar]
- 38.Skryabin KG, Morozov SY, Kraev AS, Rozanov MN, Chernov BK, Lukasheva LI, Atabekov JG. Conserved and variable elements in RNA genomes of potexviruses. FEBS Lett. 1988;240:33–40. doi: 10.1016/0014-5793(88)80335-1. [DOI] [PubMed] [Google Scholar]
- 39.Urcuqui-Inchima S, Walter J, Drugeon G, German-Retana S, Haenni AL, Candresse T, Bernardi F, Le Gall O. Potyvirus helper component-proteinase self interaction in the yeast two hybrid system and delineation of the interaction domain involved. Virology. 1999;258:95–99. doi: 10.1006/viro.1999.9725. [DOI] [PubMed] [Google Scholar]
- 40.Vance VB. Replication of potato virus X RNA is altered in coinfections with potato virus Y. Virology. 1991;182(2):486–494. doi: 10.1016/0042-6822(91)90589-4. [DOI] [PubMed] [Google Scholar]
- 41.Voinnet O, Lederer C, Baulcombe DC. A viral movement protein prevents spread of the gene silencing signal in Nicotiana benthamiana. Cell. 2000;103:157–167. doi: 10.1016/s0092-8674(00)00095-7. [DOI] [PubMed] [Google Scholar]
- 42.Wadsworth S, Dunoyer P. Plant RNA-silencing immunity and viral counter-defence strategies. In: Bouarab K, Brisson N, Daayf F, editors. Molecular plant-microbe interaction. London: CABI; 2009. pp. 1–35. [Google Scholar]
- 43.Zhang XS, Holt J, Colvin J. Synergism between plant viruses: a mathematical analysis of the epidemiological implications. Plant Pathol. 2001;50:732–746. [Google Scholar]