Abstract
Transformation of the uterine spiral arteries (SAs) during pregnancy is critical to support the developing fetus, and is impaired in some pregnancy disorders, including preeclampsia. Decidual natural killer (dNK) cells play a role in SA remodeling, although their interactions with fetal trophoblast remain unclear. A uterine artery Doppler resistance index (RI) in the first trimester of pregnancy can be used as a proxy measure of the extent of SA remodeling; we have used this technique to characterize dNK cells from pregnancies with normal (normal RI) and impaired (high RI) SA remodeling, which display least and highest risk of developing preeclampsia, respectively. We examined the impact of dNK cell secreted factors on trophoblast motility, chemoattraction, and signaling pathways to determine the contribution of dNK cells to SA transformation. We demonstrated that the chemoattraction of the trophoblast by dNK cells is impaired in pregnancies with high RI, as is the ability to induce trophoblast outgrowth from placental villous explants. These processes are dependent on activation of the extracellular signal–regulated kinase 1/2 and phosphatidylinositol 3-kinase–Akt signaling pathways, which were altered in trophoblasts incubated with secreted factors from dNK cells from high RI pregnancies. Therefore, by characterizing pregnancies using uterine artery Doppler RI before dNK cell isolation, we have identified that impaired dNK-trophoblast interactions may lead to poor placentation. These findings have implications for pregnancy pathological conditions, such as preeclampsia.
After implantation of the blastocyst, the pregnant uterus (decidua) undergoes physiological changes to ensure a successful pregnancy. Key to this is the transformation of the uterine spiral arteries (SAs) from high-resistance, low-flow vessels to low-resistance, high-flow vessels. This establishes an increased blood flow to the intervillous space, allowing nutrient and gas exchange between the maternal blood flow and fetal placenta.1 SA transformation is under the control of extravillous trophoblast (EVT), specialized fetal cells derived from the placenta that invade into the decidua and remodel the SA by inducing apoptosis of endothelial cells and vascular smooth muscle cells (VSMCs).2,3 The VSMCs undergo induced hypertrophy and disruption of VSMC layers, leading to motility and dedifferentiation of vascular smooth muscle cells.4,5 Trophoblasts then line the SA in place of the absent vascular cells. The essential presence of trophoblasts in SA transformation is inferred from pregnancy disorders, such as preeclampsia and intrauterine growth restriction, that display reduced trophoblast invasion and reduced remodeling of the arteries in the first trimester of pregnancy.6,7 The reason for decreased EVT invasion in these pathological conditions is unknown.
It is increasingly recognized that transformation of SA begins in a trophoblast-independent manner, when only maternal cells are present surrounding the spiral arteries.8 Several different maternal cells exist in the decidua in the first trimester of pregnancy, including leukocytes, of which 70% are decidual natural killer (dNK) cells. These differ from peripheral blood natural killer (pbNK) cells in their surface receptor expression; notably, dNK cells are predominantly CD56brightCD16−, whereas pbNK cells are predominantly CD56dimCD16+. Decidual NK cells are thought to have a cytokine-secreting role as opposed to the cytotoxic role of pbNK cells,9 because dNK cells do not kill trophoblasts in a normal pregnancy,10 despite possessing the same cytotoxic capacity as pbNK cells in terms of expression of the cytolytic proteins, perforin and granzyme.11 Disruption of the vasculature when the SAs are surrounded by dNK cells, but the trophoblasts are absent, has been identified8 and has implicated dNK cells in SA remodeling via both direct interaction with the vascular cells and indirect interaction with trophoblasts.
Decidual NK cells are proposed to contribute directly to SA remodeling by expression of secreted factors that disrupt vascular cell interactions,12 including matrix metalloproteinases that disrupt vascular extracellular matrix connections, therefore enabling migration of VSMCs from the vessel.8,13 Some apoptosis of VSMCs and endothelial cells during remodeling has also been attributed to dNK cells via an Fas-ligand pathway.14 Decidual NK cells may also have indirect effects on remodeling by controlling EVT invasion, by both promotion and inhibition. EVT invasion is dependent on motility and chemotaxis. dNK cells have been demonstrated to increase EVT motility via hepatocyte growth factor secretion,14 and to play a role in chemoattraction of the EVT to the sites of remodeling, in particular through expression of the chemokines IL-8 and CXCL10.15 They have also been reported to decrease trophoblast invasion via an interferon-γ–secreted mechanism.16 However, many chemokines have been identified as secreted by dNK cells, and these may also play a role in the promotion of invasion of EVT through the decidua.17–19
Although there is strong evidence for the role of dNK cells in mouse SA remodeling,20 and human dNK cells have been linked to pregnancy disorders associated with poor SA remodeling,21,22 the key role of dNK cells in human pregnancy is proposed to occur during the first trimester of pregnancy, hampering studies because of a lack of access to tissue. When first-trimester termination of pregnancy samples is used for isolation of dNK cells, the outcome of that pregnancy, if brought to term, and the extent of remodeling are unknown. Uterine artery Doppler resistance index (RI) in the first trimester can be used as a proxy measure of the extent to which remodeling of the spiral arteries has occurred,23,24 and can, therefore, be used as a technique to separate pregnancies of a normal and high RI, which are at least risk (<1%) and most risk (21%) of developing preeclampsia, respectively.14,25 We can, therefore, examine the role of dNK cells isolated from these pregnancies. We have previously demonstrated that dNK cells isolated from high RI pregnancies are less able to induce vascular cell apoptosis.14 In this study, we examined the interactions of dNK cells from normal and high RI pregnancies with trophoblasts, to determine the contribution of dNK to trophoblast-induced SA remodeling.
Materials and Methods
Doppler Ultrasound Characterization
Determination of uterine artery RI was performed in women attending a clinic for termination of pregnancy in the first trimester, as previously described,14,26 at the Fetal Medicine Unit, St. George's Hospital (London, UK). Ethics committee approval and full written consent were obtained. Inclusion criteria were singleton pregnancy, gestational age of 8 to 14 weeks, normal fetal anatomical features, and nuchal translucency thickness with no known maternal medical condition or history of recurrent miscarriage. High-resistance cases were defined as those with bilateral uterine diastolic notches and a mean RI >95th percentile. The presence of diastolic notches reduces the possible effect of an extremely lateral placental insertion, causing a unilateral high RI reading.27 Normal-resistance cases had a mean RI of <95th percentile. These resistance groups represent cases most (21%) and least (<1%) likely to have developed preeclampsia, respectively, had the pregnancy progressed.24,28
dNK Cell Isolation
Decidual tissue was minced and digested in serum-free M199 media containing 2 mg/mL collagenase and 0.1 mg/mL DNase overnight with constant agitation at room temperature. The supernatant was filtered, centrifuged, and resuspended in PBS (2% fetal calf serum) and layered onto Ficoll-Paque (GE Healthcare Life Sciences, Buckinghamshire, UK) to collect the buffy layer. Cells were resuspended in 10 mL dNK cell culture media [phenol red–free RPMI 1640 medium supplemented with 10% (v/v) fetal bovine serum (FBS), containing 2 mmol/L l-glutamine, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 2.5 μg/mL amphotericin] and plated in a 37°C incubator for 15 minutes. Nonadherent cells containing the dNK cell fraction were counted by light microscopy, and dNK cells were purified using negative selection using a MagCellect Human NK Cell Isolation Kit (R&D Systems, Abingdon, UK), according to the manufacturer's instructions. Viability was assessed as measured by the Annexin V Apoptosis Detection Kit and propidium iodide (eBioscience, San Diego, CA) (Supplemental Figure S1). Purity, as measured by CD56+ cells, was, on average, 95.7% ± 0.92% and viability after 6 hours of culture was, on average, 84.6% ± 2.8%.
Cell Culture and Generation of Conditioned Media
Decidual natural killer cells were cultured in dNK culture media supplemented with 5 ng/mL IL-15 and 250 ng/mL stem cell factor (Peprotech, London, UK). The mean gestational ages of dNK cells used in these experiments are listed within the text and were not significantly different between normal and high RI groups. The human EVT cell line, SGHPL-4, was cultured in Hams F10 media supplemented with 10% (v/v) FBS, containing 2 mmol/L l-glutamine, 100 IU/mL penicillin, and 100 μg/mL streptomycin. These cells are well characterized and share many characteristics with isolated primary cells, including the expression of cytokeratin-7 and human leukocyte antigen-G.29,30 All cells were incubated with 95% air and 5% CO2 at 37°C in a humidified incubator.
Conditioned media (CM) were obtained after a 6-hour incubation of dNK cells at a density of 1 × 106 cells/mL in dNK culture media. CM was concentrated fivefold or 20-fold (VivaSpin columns, 3000 mol. wt. cutoff; Sartorius Stedim, Surrey, UK) for use in spheroid invasion and chemotaxis assays, respectively.
Spheroid Invasion Assay
SGHPL-4 spheroids were generated using a hanging drop culture method. A total of 750 cells were placed on the underside of a petri dish lid in a 30-μL drop of Hams F10 media containing 0.5% (v/v) FBS and 25% (v/v) carboxymethylcellulose. Spheroids were left to form for 24 hours before use in experiments, and individual spheroids were then resuspended in 100 μL of fibrin gel (2.5 mg/mL fibrin, 100 U/mL aprotinin, and 1.25 U/mL thrombin) in separate wells of a 96-well plate. A volume of 100 μL of control medium (dNK culture media with IL-15 and stem cell factor supplements) or dNK cell CM was added, spheroids were visualized after 24-hour incubation using an Olympus IX70 inverted microscope (Olympus, Southend on Sea, UK), and images were captured using a Hamamatsu C4742-95 digital camera (Hamamatsu Photonics UK Limited, Hertfordshire, UK). Invasion was measured as the average number and length (μm) of all invasive processes from each spheroid using Adobe Photoshop CS3 Extended version 10.0 (San Jose, CA) and was performed blinded by one observer (A.E.W.).
Chemotaxis Assay
SGHPL-4 cell chemotaxis toward dNK cell conditioned media was measured using the μ-Slide Chemotaxis 2D assay (Ibidi, Martinsried, Germany), according to the manufacturer's instructions. Analysis of chemotaxis and motility was performed by time-lapse microscopy using an Olympus IX70 inverted fluorescence microscope with motorized stage and cooled charge-coupled device camera and enclosed in a heated, humidified chamber at 37°C with 5% CO2 in air. Images were taken every 15 minutes for 24 hours, and time-lapse sequences were analyzed using ImageJ software version 1.43u (NIH, Bethesda, MD) with the Manual Tracking and Chemotaxis Tool version 1.01 plug-ins. Analysis of directional cell movement and cell motility was measured by randomly choosing 20 SGHPL-4 cells in two fields of view, which were manually tracked over the time period. The distance moved (arbitrary units) and number of cells moving toward a chemotactic stimulus (percentage chemotaxis toward stimulus) were recorded. For inhibitor studies, SGHPL-4 were pretreated with 50 μmol/L PD98059, 50 μmol/L LY 294002 (Calbiochem Merck Millipore, Middlesex, UK), or vehicle control for 30 minutes before performing the assay in the presence of the inhibitors.
ELISA Data
The concentration of IL-8, IL-6, and CXCL10 in dNK cell CM was measured using Human Duo-set enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Abingdon, UK), according to the manufacturer's instructions.
Extravillous Trophoblast Explants
EVT explant outgrowth was measured using a modification of a previously described method.31 Briefly, a 96-well plate was coated in Matrigel (BD, Oxford, UK), diluted 1:10 in explant media (Dulbecco's modified Eagle's–F12 media supplemented with 2 mmol/L l-glutamine, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 2.5 μg/mL amphotericin). Villous tip explants were dissected from placental villous tissue and incubated with 120 μL per well of explant medium for 48 hours. Explants displaying EVT outgrowth were then incubated with 120 μL of dNK CM in duplicate. For inhibitor studies, explants were co-incubated with normal RI dNK cell CM and 50 μmol/L PD98059, 50 μmol/L LY 294002, or vehicle control. EVT outgrowth area was measured in images (Olympus IX70 inverted microscope and Hamamatsu C4742-95 digital camera) by manual tracing of total outgrowth area using Adobe Photoshop CS3 Extended version 10.0. The fold increase in outgrowth area was then calculated. Placental tissue used was from pregnancies displaying a normal RI to control for trophoblast background confounding results, and was between 63 and 69 days of gestational age (n = 9) because this is a time point when EVTs are still invasive.32
Western Blot Analysis
SGHPL-4 cells were serum starved for 24 hours in Hams-F10 media containing 0.5% FBS at 37°C before incubation with normal or high RI dNK cell CM (n = 8) for 4 hours. Then, Western blot analysis was performed, as previously described,30 using 1:2500 rabbit anti–phosphorylated-AktSer473 (Cell Signaling Technology, Hertfordshire, UK), 1:1000 rabbit anti–phosphorylated–extracellular signal–regulated kinase (ERK) 1/2 (Cell Signaling Technology), and 1:10,000 mouse anti–α-tubulin (Abcam, Cambridge, UK). Western blots were scanned, and the integrated density of each band was determined using ImageJ software version 1.43u. Results are expressed as a ratio to loading control within the same sample.
Statistical Analysis
Where appropriate, data were analyzed by one-way analysis of variance or Student's t-test, and are presented as means ± SEM.
Results
Trophoblast Invasion and Motility Induced by dNK Cell CM Does Not Differ between Normal and High RI Pregnancies
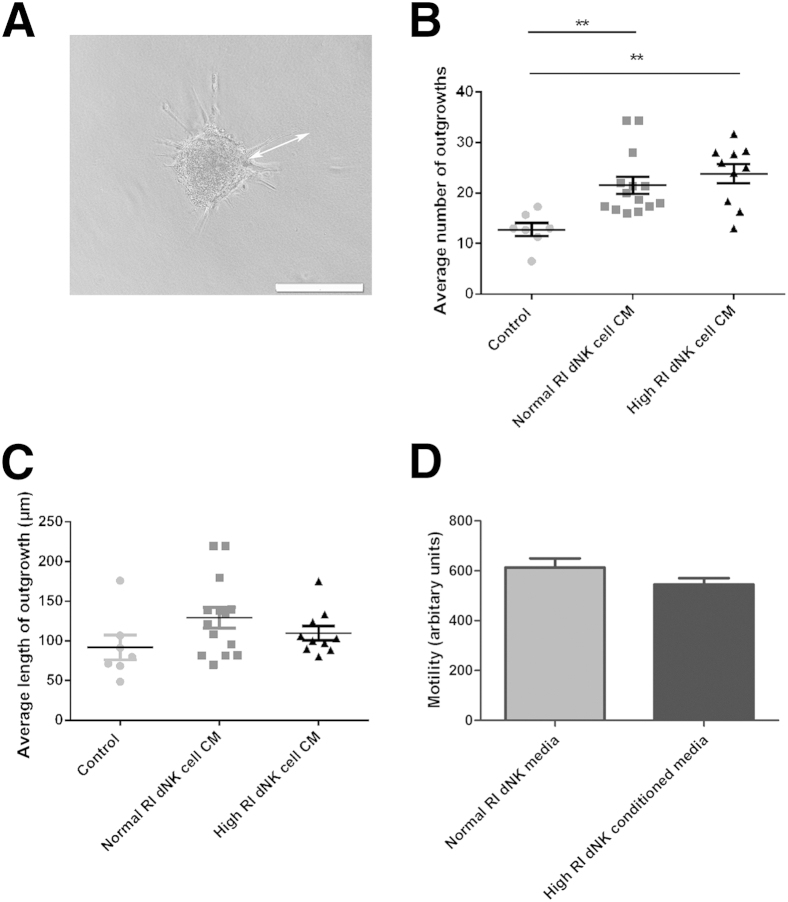
It has been previously demonstrated that dNK-secreted factors induce EVT migration and invasion,15,33 key steps in the recruitment of EVT to the spiral arteries. Because high uterine artery RI in the first trimester may be used as a proxy measure of the extent to which remodeling of the spiral arteries has occurred,24 and is associated with a higher risk of preeclampsia,25 we isolated dNK cells from normal and high uterine artery RI first-trimester terminations of pregnancy. We then examined the effects of dNK cell–secreted factors from individual patients on the invasion of the extravillous trophoblast cell line, SGHPL-4. In a spheroid invasion assay, the length and number of invasive processes formed in response to dNK CM were measured (Figure 1A). Significantly more invasive processes were formed in response to both normal and high RI dNK cell CM compared with control (control, 12.79 ± 1.3; normal RI, 21.5 ± 1.7; high RI, 23.8 ± 1.9; P < 0.01) (Figure 1B). However, there was no difference in number of invasive processes between SGHPL-4 spheroids stimulated with high or normal RI dNK cell CM. The average length of invasive processes did not differ between control media and dNK cell CM (control, 91.78 ± 15.6 μm; normal RI, 129.4 ± 13.06 μm; high RI, 109.8 ± 8.9 μm) (Figure 1C).
Figure 1.
Trophoblast invasion induced by decidual natural killer (dNK) cell conditioned media (CM) does not differ between normal and high RI pregnancies. A: SGHPL-4 cells were cultured to form spheroids as shown and embedded in fibrin gels. The length and number of invasive processes were measured (arrow). B: The average number of invasive process outgrowths of SGHPL-4 cell spheroids increased in response to normal and high resistance index (RI) dNK cell conditioned media from individual patients compared with control culture media. ∗∗P < 0.01. C: The average length of invasive process outgrowths of SGHPL-4 cell spheroids does not differ in response to normal and high RI dNK cell CM from individual patients compared with control culture media. Invasion assay data shown are means ± SEM. Mean gestational ages are as follows: normal, 74.90 ± 2.46 days; high, 74.92 ± 3.31 days. D: Motility of SGHPL-4 cells incubated with normal RI dNK cell CM or high RI dNK cell CM (arbitrary units, as assessed by time-lapse microscopy). Motility assay data shown are means ± SEM. Mean gestational ages are as follows: normal, 73.63 ± 3.7 days; high, 72.1 ± 2.8 days.
A chemotaxis assay was used to measure motility and chemotaxis of SGHPL-4 cells in response to dNK cell CM. Motility of SGHPL-4 was not significantly different when stimulated with normal (n = 8) or high (n = 7) RI dNK CM (normal RI, 613.3 ± 36.19; high RI, 545 ± 25.46) (Figure 1D).
Trophoblast Chemotaxis Is Not Induced by dNK Isolated from High RI Pregnancies
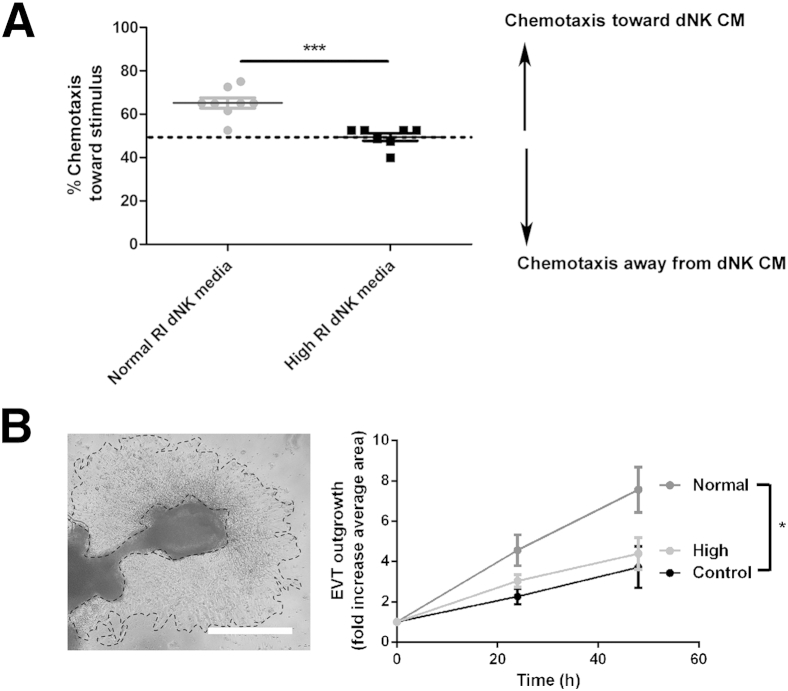
Chemotaxis toward dNK cell CM was examined using high (n = 7) and normal (n = 8) RI dNK CM as a chemoattractant. When cells are undergoing nondirected movement, the expected movement toward a chemoattractant is 50% of cells. When normal RI dNK CM was used as a stimulus, 65.18% ± 2.4% of SGHPL-4 cells were chemoattracted to the CM. This was significantly more chemotactic to SGHPL-4 cells than high RI dNK cell CM, because 49.46% ± 1.7% of SGHPL-4 cells migrated toward high RI dNK CM (P < 0.001) (Figure 2A).
Figure 2.
High resistance index (RI) decidual natural killer (dNK) cell secreted factors do not chemoattract trophoblasts or induce significant extravillous trophoblast (EVT) outgrowth from placental villous tissue. A: SGHPL-4 cells were cultured in chemotaxis chambers to analyze chemotactic capacity of normal RI dNK cell conditioned media (CM) and high RI dNK cell CM. Nondirected movement gives expected chemotaxis of 50% of cells. Normal RI dNK CM is significantly more chemotactic to SGHPL-4 cells than high RI dNK CM. Data shown are means ± SEM. Mean gestational ages are as follows: normal RI dNK cell CM, 73.63 ± 3.7 days; high RI dNK cell CM, 72.1 ± 2.8 days. B: EVT explant outgrowth from villous tissue was incubated with control media, normal RI dNK cell CM, and high RI dNK cell conditioned media, and outgrowth area measured by manual tracing as shown. Normal RI dNK cell CM induced significantly increased EVT outgrowth at 24 and 48 hours compared with control and dNK cell CM. High RI dNK cell CM did not significantly induce explant outgrowth. Scale bar = 500 μm. Data shown are means ± SEM. Mean gestational ages are as follows: normal RI dNK cell CM, 75.42 ± 2.7 days; high RI dNK cell CM, 74.07 ± 2.0 days. ∗P < 0.05; ∗∗∗P < 0.01.
Extravillous Trophoblast Outgrowth Induced by dNK Is Reduced in High-Resistance Pregnancies
To further examine differences in trophoblast responses to normal (n = 15) and high (n = 10) RI dNK cell–secreted factors, extravillous trophoblast outgrowth from villous placental explant tissue cultured in the presence of dNK CM for 48 hours was examined (n = 9). This experiment was conducted to determine whether primary trophoblasts also responded to normal and high RI dNK cell CM differentially, and because outgrowth of primary EVT over Matrigel requires a combination of both invasion through Matrigel and migration. Compared with control media, only normal RI dNK cell CM significantly increased EVT outgrowth at both 24 and 48 hours (24 hours: control, 2.3 ± 0.4-fold; normal, 4.6 ± 0.8-fold; 48 hours: control, 3.7 ± 0.9-fold; normal, 7.5 ± 0.8-fold; P < 0.05) (Figure 2B). High RI dNK cell-conditioned media did not significantly induce explant outgrowth compared with control (24 hours: control, 2.3 ± 0.4-fold; high, 3.0 ± 0.3-fold; 48 hours: control, 3.7 ± 0.9-fold; high, 4.4 ± 0.8-fold) (Figure 2B).
IL-8, IL-6, and CXCL10 Expression by dNK Cells Is Not Different between High and Normal RI Pregnancies
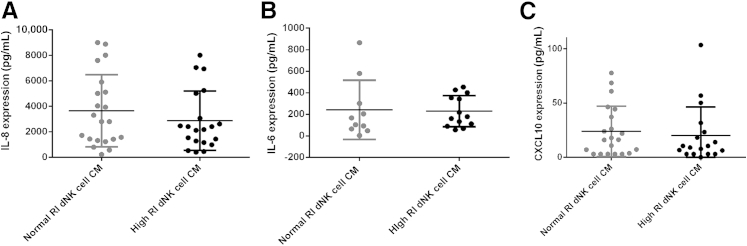
Chemokines that have been previously described as important in controlling trophoblast invasion include IL-8, IL-6, and CXCL10.15,34 Expression of these three chemokines was examined in the dNK cell CM, and expression levels were not found to be significantly different between high and normal RI dNK cell CM [IL-8: normal RI dNK cell, 3655 ± 618 pg/mL; high RI dNK cell, 2868 ± 520 pg/mL (P = 0.338); IL-6: normal RI dNK cell, 242.7 ± 87 pg/mL; high RI dNK cell, 229.9 ± 40.27 pg/mL (P = 0.89); CXCL10: normal RI dNK cell CM, 23.8 ± 5 pg/mL; high RI dNK cell CM, 20.02 ± 6.2 pg/mL (P = 0.635)] (Figure 3).
Figure 3.
Expression of IL-8, IL-6, and CXCL10 is not significantly altered between normal resistance index (RI) and high RI decidual natural killer (dNK) cell conditioned media (CM). Expression of IL-8 (A), IL-6 (B), and CXCL10 (C) was examined by ELISA in normal and high RI dNK cell CM. Expression levels were not found to be significantly different between high and normal RI dNK cell CM. Data shown are means ± SEM. Mean gestational ages are as follows: IL-8 and CXCL10: normal RI dNK cell CM, 75 ± 2.4 days; high RI dNK cell CM, 73.72 ± 1.6 days; IL-6: normal RI dNK cell CM, 79.7 ± 3.8 days; high RI dNK cell CM, 74.2 ± 1.8 days.
CM from Normal RI dNK Cells Phosphorylates ERK1/2 and Aktser473 to a Greater Extent than CM from High RI dNK Cells
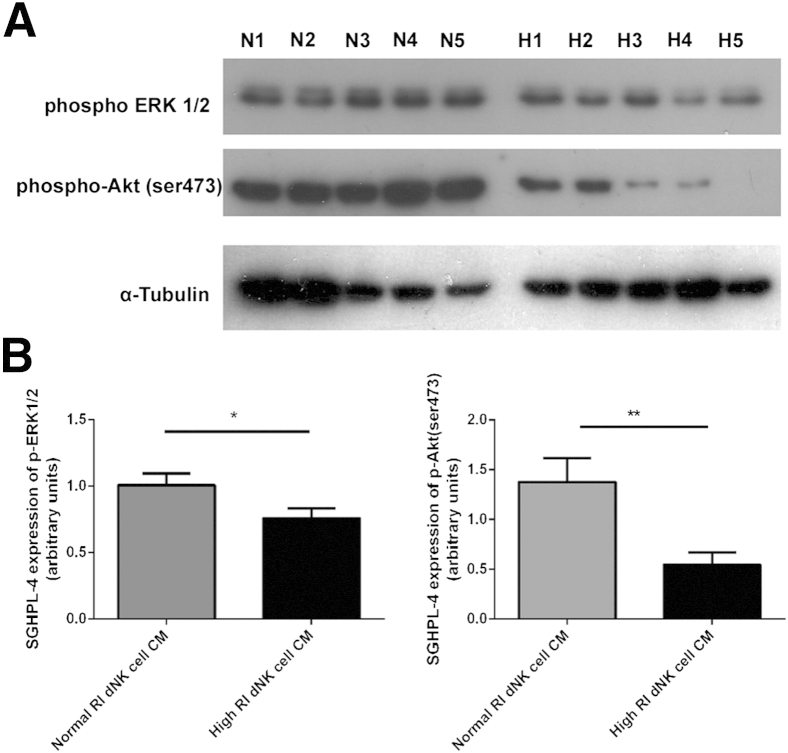
Because the three chemokines, IL-8, IL-6, and CXCL10, showed no difference in expression between normal and high RI dNK cell CM, to examine the mechanisms of reduced trophoblast chemotaxis and EVT outgrowth from villous tissue explants in response to high RI dNK cell CM, the signaling pathways in trophoblast downstream of dNK cell-secreted factors binding to trophoblast receptors were examined. Common signaling pathways previously demonstrated to be important in motility of SGHPL-4 cells include activation of the classic mitogen-activated protein kinase signaling pathway (p42/44 MAPK, or ERK1/2) and the phosphatidylinositol 3-kinase (PI3K)-Akt signaling pathway, which have both also been demonstrated to be important in invasion and motility in other trophoblast models.35,36 SGHPL-4 cells were incubated with dNK CM from normal and high RI pregnancies (n = 8) for 4 hours, and phosphorylation of ERK1/2 and Aktser473 (serine 473) was assessed by using Western blot analysis (Figure 4). SGHPL-4 cells incubated with high RI dNK CM were found to display 1.3-fold lower ERK1/2 phosphorylation (P < 0.05) and 2.5-fold lower Aktser473 phosphorylation (P < 0.01) compared with SGHPL-4 cells incubated with normal RI dNK CM.
Figure 4.
SGHPL-4 cell incubation with normal resistance index (RI) decidual natural killer (dNK) cell conditioned media (CM). differentially phosphorylates ERK1/2 and AKTser473 compared with high RI dNK cell CM. A: SGHPL-4 cells were incubated with normal RI dNK cell CM (N1 to N5) and high RI dNK cell CM (H1 to H5) for 4 hours before total protein was subjected to Western blot analysis for the phosphorylation of ERK1/2 and Akt (n = 8). B: Densitometry of Western blot analysis of phospho-ERK1/2 and phospho-Aktser473 in SGHPL-4 cells incubated with normal RI dNK cell CM and high RI dNK cell CM for 4 hours. Data shown are means ± SEM. ∗P < 0.05, ∗∗P < 0.01.
Trophoblast Chemotaxis and Invasion in Normal-Resistance Pregnancies Are Dependent on ERK1/2 and Akt Signaling
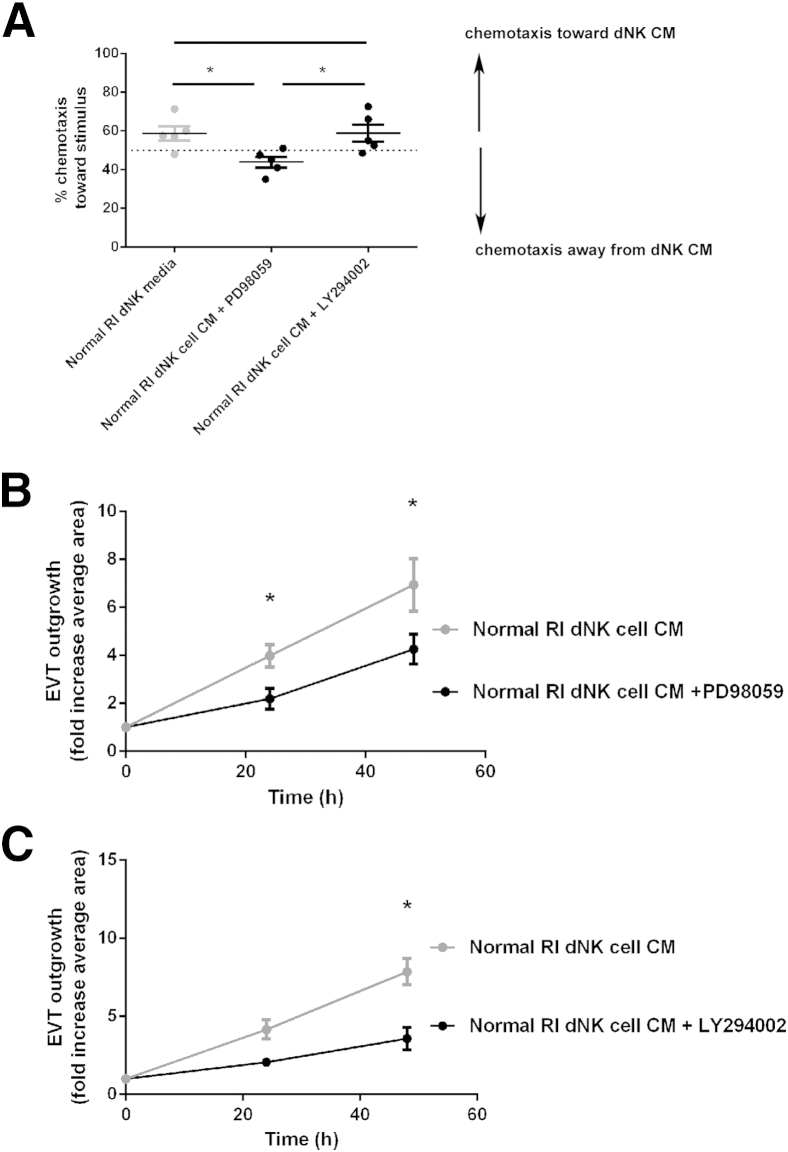
Chemical inhibitors targeting the ERK1/2 and Akt pathways were then used to assess the impact of increased phosphorylation induced by normal RI dNK cell CM on trophoblast chemotaxis and EVT outgrowth from villous explants. Chemotaxis of SHGPL-4 cells in response to normal RI dNK CM was examined in the presence of a specific inhibitor to MEK and, therefore, ERK1/2 phosphorylation and signaling (PD 98059) or a specific inhibitor to PI3K signaling (LY 294002) (n = 5). Compared with vehicle-treated cells, normal RI dNK cell CM–induced chemotaxis was found to be significantly inhibited by PD98059 but not LY29002 (vehicle control, 58.8% ± 3.75%; PD98059, 43.9% ± 2.75%; LY29002, 58.9% ± 4.5%; P < 0.05) (Figure 5A). Inhibition of ERK1/2 and PI3K-Akt signaling was then examined in EVT outgrowth from explants (Figure 5, B and C), and outgrowth induced by normal RI dNK cell CM was found to be significantly decreased in the presence of PD98059 compared with vehicle control at both 24 and 48 hours (at 48 hours: vehicle control, 6.8 ± 1.1-fold increase; PD98059, 4.2 ± 0.6-fold increase; P < 0.05), as was outgrowth induced by normal RI dNK CM in the presence of LY294002 (at 48 hours: vehicle control, 7.8 ± 0.8-fold increase; LY29002, 3.6 ± 0.7-fold increase; P < 0.05).
Figure 5.
The effect of inhibition of the ERK1/2 and PI3K-Akt signaling pathways on trophoblast chemotaxis and extravillous trophoblast (EVT) outgrowth in the presence of normal resistance index (RI) decidual natural killer (dNK) cell conditioned media (CM). A: SGHPL-4 cells were cultured in chemotaxis chambers to analyze chemotactic capacity of normal RI dNK cell CM in the presence of the ERK1/2 inhibitor, PD98059, and the PI3K inhibitor, LY294002. Nondirected movement gives expected chemotaxis of 50% of cells. The ERK1/2 inhibitor, PD98059, significantly decreased chemotaxis compared with normal RI dNK CM with vehicle control or the PI3K inhibitor, LY294002. Data shown are means ± SEM. The mean gestational age of dNK cell CM is 76 ± 3.8 days. B: EVT explant outgrowth from villous tissue incubated with normal RI dNK cell CM in the presence of vehicle control or the ERK1/2 inhibitor, PD98059. EVT outgrowth induced by normal RI dNK cell CM is significantly inhibited in the presence of PD98059 at 24 and 48 hours compared with normal RI dNK cell CM in the presence of vehicle control. C: EVT explant outgrowth from villous tissue incubated with normal RI dNK cell CM in the presence of vehicle control or the PI3K inhibitor, LY294002. EVT outgrowth induced by normal RI dNK cell CM is significantly inhibited in the presence of LY294002 at 48 hours compared with normal RI dNK cell CM in the presence of vehicle control. Data shown are means ± SEM. The mean gestational ages are as follows: normal RI dNK cell CM, 75.42 ± 2.7 days; high RI dNK cell CM, 74.07 ± 2.0 days. ∗P < 0.05.
Discussion
The role of decidual natural killer cells in the first trimester of pregnancy and their potential contribution to pregnancy disorders is difficult to study because of the inability to access first-trimester tissue with a known pregnancy outcome. Herein, we have used a uterine artery Doppler RI as an indirect measure of SA transformation14 to identify key differences in dNK cells from pregnancies with a normal and high RI. A high uterine artery RI is associated with poor spiral artery remodeling and decreased numbers of trophoblast-plugged vessels in the first trimester.24 We have demonstrated that the chemoattraction of trophoblast by dNK cells is impaired in high RI pregnancies, as is the ability of dNK cells to induce EVT outgrowth from placental villous explants, and this process of EVT chemotaxis and outgrowth is dependent on ERK1/2 and Akt signaling. Therefore, spiral artery transformation may be partly limited in high RI pregnancies by the inability of dNK cells to control the migration and invasion of trophoblast into the decidua.
In the decidua, NK cells are the most abundant leukocyte and are found in close contact with trophoblasts.37 An important aspect of the physiological change in pregnancy is the migration of trophoblasts into the decidua, which requires a combination of differentiation to the invasive phenotype, invasion, motility, and chemotaxis of trophoblasts. In the spheroid invasion assay used in this study, dNK cell CM induced trophoblast invasion by increasing the number of invasive cells, rather than the length of the invasive processes. This could indicate that the dNK-secreted factors induce invasion primarily by acting on the ability of EVT to invade, or the differentiation to an invasive phenotype, rather than the distance of cell invasion. However, there was no difference in invasion induced by dNK cell CM from normal and high RI pregnancies on the SGHPL-4 cell line. We have also demonstrated in this study that there was no difference in SGHPL-4 motility when incubated with high or normal RI dNK cell CM, although we have previously shown this under different experimental conditions.14 We then examined EVT outgrowth from placental villous tissue, which represents a combination of proliferation of trophoblast progenitors at the base of the villous tip, differentiation of trophoblast into the invasive extravillous phenotype, and migration. Because this is measured by the area that non-proliferative EVT moves over Matrigel, this is an appropriate assay to use to assess EVT migration and invasion. Both normal and high RI dNK cell CM stimulated explant outgrowth, and this outgrowth was significantly increased when stimulated with normal RI dNK cell CM compared with high RI dNK cell CM. Explant outgrowth has been previously shown to be increased by dNK cells via chemokine-induced alteration of EVT adhesion molecules,38 again indicating induction of differentiation to the invasive phenotype is a key step. Decidual NK cells also increase EVT production of proteases, which allows migration.33 The difference in responsive ability between the SGHPL-4 cells and EVT explants in these assays may be because of the intrinsically invasive properties of the cell line overcoming external effects of the dNK cell secreted factors.29
Chemotaxis is an additional feature allowing migration of EVT through the decidua. Decidual NK cells are often found surrounding the spiral arteries before trophoblast invasion,8 and it is tempting to speculate that they may be inducing chemotaxis of trophoblast into the vicinity of the SA to allow transformation to occur. In this study, dNK cell CM from high RI patients was unable to chemoattract trophoblasts. Decidual NK cells have previously been demonstrated to control trophoblast chemotaxis with secreted factors, and this has been shown to include the chemokines IL-8, CXCL10, and IL-6.15,34,39 Although dNK cells in our studies produced these chemokines, there was no difference in secreted levels between high or normal RI dNK cells and, therefore, this is unlikely to lead to the striking differences in chemotactic ability demonstrated in our study. Between the patients in each group, there was, however, a large range in the amounts of each chemokine secreted, making these results difficult to interpret. A review of the current literature would indicate that there are at least 42 factors produced by dNK cells that could influence trophoblast invasion.40 Because many of these factors, such as vascular endothelial growth factor, may also chemoattract trophoblasts via specific receptors,41 determining the balance of factors present in the dNK cell CM that are altered in high RI dNK cells will be a challenging future study. We, therefore, decided to examine the signaling pathways that are commonly activated in trophoblasts in response to binding of external factors, such as cytokines and growth factors to specific receptors. The directed migration of cells requires localized activation of PI3K at the leading edge of the cell, which has been demonstrated to be upstream of Akt in our trophoblast model.35 The classic MAPK signaling pathway has also been examined in trophoblasts and SGHPL-436,42; thus, both of these signaling pathways were examined. We found that after a 4-hour incubation period with high RI dNK cell CM, SGHPL-4 cells displayed decreased phosphorylation of ERK1/2 and Aktser473 compared with SGHPL-4 cells incubated with normal RI dNK cell CM. We then showed that inhibition of the ERK signaling pathway decreased trophoblast chemotaxis in response to normal RI dNK cell CM; however, a chemical inhibitor of PI3K signaling did not do this. Inhibition of both the ERK and Akt signaling pathways also decreased EVT outgrowth from placental villous tissue explants. The ERK1/2 signaling pathway is crucial in downstream signaling of activation of many receptor types, including chemokine G-protein–coupled receptors and growth factor receptor tyrosine kinases.43 Akt signaling is also important in the regulation of trophoblasts by growth factors and the differentiation of trophoblasts into the invasive phenotype.36 Secreted factors that dNK cells are capable of producing have also previously been shown to decrease EVT outgrowth from explants, showing the variation in this process.16,44,45 Therefore, it is possible that the high RI dNK cell CM activates these signaling pathways in a different manner from normal RI dNK cell CM, leading to decreased trophoblast invasion; however, because of the complexity of the integration of the signaling pathways, the underlying mechanism is still unknown.
Taken together, our results indicate that dNK cells isolated from pregnancies with a high RI, which are at a higher risk of preeclampsia,25 are less able to chemoattract trophoblasts and induce EVT outgrowth from the villi, dependent on ERK1/2 and Akt signaling. Using uterine artery Doppler RI screening before cell isolation has, therefore, identified that dNK cells from high RI pregnancies have altered interactions with trophoblasts, which may lead to poor placentation. We anticipate that these findings will increase our knowledge of maternal-fetal signaling in pathological conditions of pregnancy that develop in the first trimester, and potentially help to develop successful management of these disorders.
Acknowledgments
We thank Prof. Baskaran Thilaganathan and the staff of the Fetal Medicine Unit at St. George's Hospital for their assistance with sample collection.
Footnotes
Supported by the Wellcome Trust project 091550.
Supplemental Data
The effect of longer culture times on decidual natural killer (dNK) cell viability. Representative flow cytometry images of dNK cells cultured for 6 hours (A) and 24 hours (B). Note shift in forward scatter (FSC) and side scatter (SSC) of population implying cell death. Viability was assessed by annexin and propidium iodide (PI) staining; viability, as measured by PI cells, was 84.6% ± 2.8% and 58.8% ± 6.7% of the population at 24 hours.
Supplemental Data
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2013.08.023.
References
- 1.Pijnenborg R., Vercruysse L., Brosens I. Deep placentation. Best Pract Res Clin Obstet Gynaecol. 2011;25:273–285. doi: 10.1016/j.bpobgyn.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Ashton S.V., Whitley G.S., Dash P.R., Wareing M., Crocker I.P., Baker P.N., Cartwright J.E. Uterine spiral artery remodeling involves endothelial apoptosis induced by extravillous trophoblasts through Fas/FasL interactions. Arterioscler Thromb Vasc Biol. 2005;25:102–108. doi: 10.1161/01.ATV.0000148547.70187.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keogh R.J., Harris L.K., Freeman A., Baker P.N., Aplin J.D., Whitley G.S., Cartwright J.E. Fetal-derived trophoblast use the apoptotic cytokine tumor necrosis factor-alpha-related apoptosis-inducing ligand to induce smooth muscle cell death. Circ Res. 2007;100:834–841. doi: 10.1161/01.RES.0000261352.81736.37. [DOI] [PubMed] [Google Scholar]
- 4.Bulmer J.N., Innes B.A., Levey J., Robson S.C., Lash G.E. The role of vascular smooth muscle cell apoptosis and migration during uterine spiral artery remodeling in normal human pregnancy. FASEB J. 2012;26:2975–2985. doi: 10.1096/fj.12-203679. [DOI] [PubMed] [Google Scholar]
- 5.Wallace A.E., Cartwright J.E., Begum R., Laing K., Thilaganathan B., Whitley G.S. Trophoblast-induced changes in C-x-C motif chemokine 10 expression contribute to vascular smooth muscle cell dedifferentiation during spiral artery remodeling. Arterioscler Thromb Vasc Biol. 2013;33:e93–e101. doi: 10.1161/ATVBAHA.112.300354. [DOI] [PubMed] [Google Scholar]
- 6.Pijnenborg R., Anthony J., Davey D.A., Rees A., Tiltman A., Vercruysse L., van Assche A. Placental bed spiral arteries in the hypertensive disorders of pregnancy. Br J Obstet Gynaecol. 1991;98:648–655. doi: 10.1111/j.1471-0528.1991.tb13450.x. [DOI] [PubMed] [Google Scholar]
- 7.Brosens I., Dixon H.G., Robertson W.B. Fetal growth retardation and the arteries of the placental bed. Br J Obstet Gynaecol. 1977;84:656–663. doi: 10.1111/j.1471-0528.1977.tb12676.x. [DOI] [PubMed] [Google Scholar]
- 8.Smith S.D., Dunk C.E., Aplin J.D., Harris L.K., Jones R.L. Evidence for immune cell involvement in decidual spiral arteriole remodeling in early human pregnancy. Am J Pathol. 2009;174:1959–1971. doi: 10.2353/ajpath.2009.080995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El Costa H., Tabiasco J., Berrebi A., Parant O., Aguerre-Girr M., Piccinni M.P., Le Bouteiller P. Effector functions of human decidual NK cells in healthy early pregnancy are dependent on the specific engagement of natural cytotoxicity receptors. J Reprod Immunol. 2009;82:142–147. doi: 10.1016/j.jri.2009.06.123. [DOI] [PubMed] [Google Scholar]
- 10.Tabiasco J., Rabot M., Aguerre-Girr M., El Costa H., Berrebi A., Parant O., Laskarin G., Juretic K., Bensussan A., Rukavina D., Le Bouteiller P. Human decidual NK cells: unique phenotype and functional properties – a review. Placenta. 2006;27(Suppl A):S34–S39. doi: 10.1016/j.placenta.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Le Bouteiller P., Siewiera J., Casart Y., Aguerre-Girr M., El Costa H., Berrebi A., Tabiasco J., Jabrane-Ferrat N. The human decidual NK-cell response to virus infection: what can we learn from circulating NK lymphocytes? J Reprod Immunol. 2011;88:170–175. doi: 10.1016/j.jri.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Harris L.K. IFPA Gabor Than Award lecture: transformation of the spiral arteries in human pregnancy: key events in the remodelling timeline. Placenta. 2011;32(Suppl 2):S154–S158. doi: 10.1016/j.placenta.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 13.Naruse K., Lash G.E., Innes B.A., Otun H.A., Searle R.F., Robson S.C., Bulmer J.N. Localization of matrix metalloproteinase (MMP)-2, MMP-9 and tissue inhibitors for MMPs (TIMPs) in uterine natural killer cells in early human pregnancy. Hum Reprod. 2009;24:553–561. doi: 10.1093/humrep/den408. [DOI] [PubMed] [Google Scholar]
- 14.Fraser R., Whitley G.S., Johnstone A.P., Host A.J., Sebire N.J., Thilaganathan B., Cartwright J.E. Impaired decidual natural killer cell regulation of vascular remodelling in early human pregnancies with high uterine artery resistance. J Pathol. 2012;228:322–332. doi: 10.1002/path.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanna J., Goldman-Wohl D., Hamani Y., Avraham I., Greenfield C., Natanson-Yaron S., Prus D., Cohen-Daniel L., Arnon T.I., Manaster I., Gazit R., Yutkin V., Benharroch D., Porgador A., Keshet E., Yagel S., Mandelboim O. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12:1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- 16.Hu Y., Dutz J.P., MacCalman C.D., Yong P., Tan R., von Dadelszen P. Decidual NK cells alter in vitro first trimester extravillous cytotrophoblast migration: a role for IFN-gamma. J Immunol. 2006;177:8522–8530. doi: 10.4049/jimmunol.177.12.8522. [DOI] [PubMed] [Google Scholar]
- 17.Hannan N.J., Jones R.L., White C.A., Salamonsen L.A. The chemokines, CX3CL1, CCL14, and CCL4, promote human trophoblast migration at the feto-maternal interface. Biol Reprod. 2006;74:896–904. doi: 10.1095/biolreprod.105.045518. [DOI] [PubMed] [Google Scholar]
- 18.Lash G.E., Schiessl B., Kirkley M., Innes B.A., Cooper A., Searle R.F., Robson S.C., Bulmer J.N. Expression of angiogenic growth factors by uterine natural killer cells during early pregnancy. J Leukoc Biol. 2006;80:572–580. doi: 10.1189/jlb.0406250. [DOI] [PubMed] [Google Scholar]
- 19.Saito S., Nishikawa K., Morii T., Enomoto M., Narita N., Motoyoshi K., Ichijo M. Cytokine production by CD16-CD56 bright natural killer cells in the human early pregnancy decidua. Int Immunol. 1993;5:559–563. doi: 10.1093/intimm/5.5.559. [DOI] [PubMed] [Google Scholar]
- 20.Bilinski M.J., Thorne J.G., Oh M.J., Leonard S., Murrant C., Tayade C., Croy B.A. Uterine NK cells in murine pregnancy. Reprod Biomed Online. 2008;16:218–226. doi: 10.1016/s1472-6483(10)60577-9. [DOI] [PubMed] [Google Scholar]
- 21.Hiby S.E., Apps R., Sharkey A.M., Farrell L.E., Gardner L., Mulder A., Claas F.H., Walker J.J., Redman C.W., Morgan L., Tower C., Regan L., Moore G.E., Carrington M., Moffett A. Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA-C2. J Clin Invest. 2010;120:4102–4110. doi: 10.1172/JCI43998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hiby S.E., Walker J.J., O'Shaughnessy K.M., Redman C.W., Carrington M., Trowsdale J., Moffett A. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J Exp Med. 2004;200:957–965. doi: 10.1084/jem.20041214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melchiorre K., Leslie K., Prefumo F., Bhide A., Thilaganathan B. First-trimester uterine artery Doppler indices in the prediction of small-for-gestational age pregnancy and intrauterine growth restriction. Ultrasound Obstet Gynecol. 2009;33:524–529. doi: 10.1002/uog.6368. [DOI] [PubMed] [Google Scholar]
- 24.Prefumo F., Sebire N.J., Thilaganathan B. Decreased endovascular trophoblast invasion in first trimester pregnancies with high-resistance uterine artery Doppler indices. Hum Reprod. 2004;19:206–209. doi: 10.1093/humrep/deh037. [DOI] [PubMed] [Google Scholar]
- 25.Melchiorre K., Wormald B., Leslie K., Bhide A., Thilaganathan B. First-trimester uterine artery Doppler indices in term and preterm pre-eclampsia. Ultrasound Obstet Gynecol. 2008;32:133–137. doi: 10.1002/uog.5400. [DOI] [PubMed] [Google Scholar]
- 26.Whitley G.S., Dash P.R., Ayling L.J., Prefumo F., Thilaganathan B., Cartwright J.E. Increased apoptosis in first trimester extravillous trophoblasts from pregnancies at higher risk of developing preeclampsia. Am J Pathol. 2007;170:1903–1909. doi: 10.2353/ajpath.2007.070006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kofinas A.D., Penry M., Swain M., Hatjis C.G. Effect of placental laterality on uterine artery resistance and development of preeclampsia and intrauterine growth retardation. Am J Obstet Gynecol. 1989;161:1536–1539. doi: 10.1016/0002-9378(89)90920-4. [DOI] [PubMed] [Google Scholar]
- 28.Martin A.M., Bindra R., Curcio P., Cicero S., Nicolaides K.H. Screening for pre-eclampsia and fetal growth restriction by uterine artery Doppler at 11-14 weeks of gestation. Ultrasound Obstet Gynecol. 2001;18:583–586. doi: 10.1046/j.0960-7692.2001.00594.x. [DOI] [PubMed] [Google Scholar]
- 29.Cartwright J.E., Holden D.P., Whitley G.S. Hepatocyte growth factor regulates human trophoblast motility and invasion: a role for nitric oxide. Br J Pharmacol. 1999;128:181–189. doi: 10.1038/sj.bjp.0702757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grant I., Cartwright J.E., Lumicisi B., Wallace A.E., Whitley G.S. Caffeine inhibits EGF-stimulated trophoblast cell motility through the inhibition of mTORC2 and Akt. Endocrinology. 2012;153:4502–4510. doi: 10.1210/en.2011-1930. [DOI] [PubMed] [Google Scholar]
- 31.James J.L., Stone P.R., Chamley L.W. Cytotrophoblast differentiation in the first trimester of pregnancy: evidence for separate progenitors of extravillous trophoblasts and syncytiotrophoblast. Reproduction. 2005;130:95–103. doi: 10.1530/rep.1.00723. [DOI] [PubMed] [Google Scholar]
- 32.James J.L., Stone P.R., Chamley L.W. The regulation of trophoblast differentiation by oxygen in the first trimester of pregnancy. Hum Reprod Update. 2006;12:137–144. doi: 10.1093/humupd/dmi043. [DOI] [PubMed] [Google Scholar]
- 33.Lash G.E., Otun H.A., Innes B.A., Percival K., Searle R.F., Robson S.C., Bulmer J.N. Regulation of extravillous trophoblast invasion by uterine natural killer cells is dependent on gestational age. Hum Reprod. 2010;25:1137–1145. doi: 10.1093/humrep/deq050. [DOI] [PubMed] [Google Scholar]
- 34.Jovanovic M., Vicovac L. Interleukin-6 stimulates cell migration, invasion and integrin expression in HTR-8/SVneo cell line. Placenta. 2009;30:320–328. doi: 10.1016/j.placenta.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 35.LaMarca H.L., Dash P.R., Vishnuthevan K., Harvey E., Sullivan D.E., Morris C.A., Whitley G.S. Epidermal growth factor-stimulated extravillous cytotrophoblast motility is mediated by the activation of PI3-K, Akt and both p38 and p42/44 mitogen-activated protein kinases. Hum Reprod. 2008;23:1733–1741. doi: 10.1093/humrep/den178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knofler M., Pollheimer J. IFPA Award in Placentology lecture: molecular regulation of human trophoblast invasion. Placenta. 2012;33(Suppl):S55–S62. doi: 10.1016/j.placenta.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trundley A., Moffett A. Human uterine leukocytes and pregnancy. Tissue Antigens. 2004;63:1–12. doi: 10.1111/j.1399-0039.2004.00170.x. [DOI] [PubMed] [Google Scholar]
- 38.Jovanovic M., Stefanoska I., Radojcic L., Vicovac L. Interleukin-8 (CXCL8) stimulates trophoblast cell migration and invasion by increasing levels of matrix metalloproteinase (MMP)2 and MMP9 and integrins alpha5 and beta1. Reproduction. 2010;139:789–798. doi: 10.1530/REP-09-0341. [DOI] [PubMed] [Google Scholar]
- 39.Dominguez F., Martinez S., Quinonero A., Loro F., Horcajadas J.A., Pellicer A., Simon C. CXCL10 and IL-6 induce chemotaxis in human trophoblast cell lines. Mol Hum Reprod. 2008;14:423–430. doi: 10.1093/molehr/gan032. [DOI] [PubMed] [Google Scholar]
- 40.Wallace A.E., Fraser R., Cartwright J.E. Extravillous trophoblast and decidual natural killer cells: a remodelling partnership. Hum Reprod Update. 2012;18:458–471. doi: 10.1093/humupd/dms015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lash G.E., Warren A.Y., Underwood S., Baker P.N. Vascular endothelial growth factor is a chemoattractant for trophoblast cells. Placenta. 2003;24:549–556. doi: 10.1053/plac.2002.0923. [DOI] [PubMed] [Google Scholar]
- 42.Cartwright J.E., Tse W.K., Whitley G.S. Hepatocyte growth factor induced human trophoblast motility involves phosphatidylinositol-3-kinase, mitogen-activated protein kinase, and inducible nitric oxide synthase. Exp Cell Res. 2002;279:219–226. doi: 10.1006/excr.2002.5616. [DOI] [PubMed] [Google Scholar]
- 43.Cargnello M., Roux P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. 2011;75:50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Otun H.A., Lash G.E., Innes B.A., Bulmer J.N., Naruse K., Hannon T., Searle R.F., Robson S.C. Effect of tumour necrosis factor-alpha in combination with interferon-gamma on first trimester extravillous trophoblast invasion. J Reprod Immunol. 2011;88:1–11. doi: 10.1016/j.jri.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 45.Lash G.E., Otun H.A., Innes B.A., Kirkley M., De Oliveira L., Searle R.F., Robson S.C., Bulmer J.N. Interferon-gamma inhibits extravillous trophoblast cell invasion by a mechanism that involves both changes in apoptosis and protease levels. FASEB J. 2006;20:2512–2518. doi: 10.1096/fj.06-6616com. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The effect of longer culture times on decidual natural killer (dNK) cell viability. Representative flow cytometry images of dNK cells cultured for 6 hours (A) and 24 hours (B). Note shift in forward scatter (FSC) and side scatter (SSC) of population implying cell death. Viability was assessed by annexin and propidium iodide (PI) staining; viability, as measured by PI cells, was 84.6% ± 2.8% and 58.8% ± 6.7% of the population at 24 hours.