Abstract
It is estimated that >90% of Americans do not consume sufficient dietary vitamin E, as α-tocopherol, to meet estimated average requirements. What are the adverse consequences of inadequate dietary α-tocopherol intakes? This review discusses health aspects where inadequate vitamin E status is detrimental and additional vitamin E has reversed the symptoms. In general, plasma α-tocopherol concentrations <12 μmol/L are associated with increased infection, anemia, stunting of growth, and poor outcomes during pregnancy for both the infant and the mother. When low dietary amounts of α-tocopherol are consumed, tissue α-tocopherol needs exceed amounts available, leading to increased damage to target tissues. Seemingly, adequacy of human vitamin E status cannot be assessed from circulating α-tocopherol concentrations, but inadequacy can be determined from “low” values. Circulating α-tocopherol concentrations are very difficult to interpret because, as a person ages, plasma lipid concentrations also increase and these elevations in lipids increase the plasma carriers for α-tocopherol, leading to higher circulating α-tocopherol concentrations. However, abnormal lipoprotein metabolism does not necessarily increase α-tocopherol delivery to tissues. Additional biomarkers of inadequate vitamin E status are needed. Urinary excretion of the vitamin E metabolite α-carboxy-ethyl-hydroxychromanol may fulfill this biomarker role, but it has not been widely studied with regard to vitamin E status in humans or with regard to health benefits. This review evaluated the information available on the adverse consequences of inadequate α-tocopherol status and provides suggestions for avenues for research.
Introduction
The focus of this review is to evaluate the hypothesis that adequacy of human vitamin E status cannot be assessed from circulating α-tocopherol concentrations but that inadequacy can be determined from “low” values. Although α-tocopherol was discovered in 1922, it was not until the 1980s that α-tocopherol deficiency was described in humans (1). Moreover, it took nearly a decade longer to define the deficiency symptoms in humans, in cases in which the α-tocopherol deficiency disorder was not complicated by additional nutritional or metabolic defects. The question remains, however, as to what are the symptoms of vitamin E inadequacy; >90% of Americans do not consume sufficient dietary vitamin E to meet estimated average requirements (EARs)3 (2, 3), yet they appear to undergo no obvious ill effects and their circulating α-tocopherol concentrations are not obviously abnormal. This observation led to the idea that the EAR is too high and that this apparent low dietary α-tocopherol intake has no biologic significance. Thus, this review evaluates the consequences of low intakes in relation to both genetic and metabolic causes of inadequacy, as well as frank malnutrition.
Current Status of Knowledge
Vitamin E required amounts
The determination of how much of a nutrient is required daily is dependent on specifically assessing not only its function but also defining a biomarker that is indicative of inadequacy that changes with nutrient intakes (4). For vitamin E, results from the in vitro hydrogen peroxide–induced erythrocyte hemolysis test were chosen by the Institute of Medicine (IOM) in 2000 as a marker of vitamin E status because increased peroxide-induced erythrocyte hemolysis was correlated with increased erythrocyte fragility in vitamin E–deficient individuals. Additionally, anemia with increased erythrocyte turnover during vitamin E deficiency was observed in vitamin E–deficient children with cystic fibrosis (5). Moreover, anemia is a symptom of experimental vitamin E deficiency in animals (6–8). The EAR for vitamin E was set in humans on the basis of vitamin E depletion and repletion studies in men with the use of erythrocyte hemolysis as the biomarker (4). The RDA of 15 mg α-tocopherol was extrapolated from that value (4). According to the IOM (4), only α-tocopherol meets human vitamin E requirements because it was the only form that was demonstrated to reverse vitamin E deficiency symptoms in humans, as well as being the only vitamin E form maintained in plasma and tissues, as discussed below.
Plants make 8 different forms of vitamin E, 4 (α-, β-, γ-, and δ-) tocopherols and 4 (α-, β-, γ-, and δ-) tocotrienols. The phytyl tail of natural α-tocopherol is in the RRR-conformation, whereas chemically synthesized α-tocopherol (all racemic) contains 8 stereoisomers [RRR-, RSR-, RSS-, and RRS- (the 2R- forms) and SRR-, SRS-, SSR-, SSS-] (4). In the diet, α-tocopherol is found in foods such as nuts and seeds and in vegetable oils, such as wheat germ, sunflower seed, safflower, and olive. Judicious food choices allow consumption of 15 mg α-tocopherol daily (9). However, most Americans require supplements to attain these recommended α-tocopherol intakes (10). In a study assessing a biomarker of vitamin E status, Lebold et al. (11) found that individuals who are highly motivated and interested in their diets consumed nearly the recommended α-tocopherol amounts (4). However, surveys of vitamin E intakes of the general public found that 90% of men and 96% of women do not consume the EAR of 12 mg α-tocopherol (2). The 2010 Dietary Guidelines (12) did not emphasize that vitamin E is a relatively difficult nutrient to obtain from the diet. For example, a report from the USDA covering the period from 2000 to 2006 states that vitamin E intakes, measured as α-tocopherol equivalents (α-TEs), were “21.1 mg alpha-TE per capita per day in 2006, up from 19.5 mg alpha-TE per capita per day in 2000. The level of vitamin E has generally increased over the series with the highest level in 2006” (13). These intakes apparently easily exceed the RDA. This α-TE value, however, includes intakes of non–α-tocopherol forms, largely γ-tocopherol, in addition to α-tocopherol. The α-TEs were defined in the 1989 RDA (14) but were not used in the 2000 DRI for vitamin E (4); instead milligrams of 2R-α-tocopherol was defined as the vitamin E unit. α-TEs fell out of favor because non–α-tocopherols are rapidly metabolized, do not substitute for α-tocopherol, and cannot be metabolically converted to α-tocopherol by humans and therefore should not be included in measures of vitamin E intake (4). Thus, a key controversy to be addressed by the nutrition community is “What are the adverse consequences of inadequate dietary α-tocopherol intakes?”
α-Tocopherol deficiency and inadequacy
Vitamin E deficiency is seldom found in adults but is more frequently found in children, likely because they have limited stores and are growing rapidly, thereby allowing deficiency symptoms to be readily apparent. This section, therefore, emphasizes findings in children. It should be noted that there are some reports of vitamin E deficiency in adults. For example, after decades of inadequate vitamin E absorption due to short bowel syndrome, a 71-y-old man complained of neurologic abnormalities that were consistent with vitamin E deficiency and responded to vitamin E supplementation (15). Thus, vitamin E is required throughout the life span.
Deficiency symptoms in humans
In humans, severe vitamin E deficiency occurs as a result of genetic defects in the α-tocopherol transfer protein (α-TTP), causing the disorder ataxia with vitamin E deficiency (AVED) (16). The lack of functional α-TTP results in the rapid depletion of plasma α-tocopherol (17, 18), thereby demonstrating that α-TTP is needed to maintain plasma α-tocopherol concentrations.
Fat malabsorption also leads to vitamin E deficiency. Examples of fat malabsorption include genetic defects in the microsomal TG transfer protein or in apoB (abeta- and hypobeta-lipoproteinemia, respectively) and fat-malabsorption syndromes, such as cholestatic liver disease or cystic fibrosis (19).
Human vitamin E deficiency symptoms include a progressive neurologic disorder, spinocerebellar ataxia, which occurs as a result of a dying back of peripheral nerves, specifically sensory neurons (20). As the vitamin E deficiency continues over time, the neurologic defects become so severe such that they that result in ataxia (16). With progressing deficiency in humans, there is also muscle deterioration, and this deterioration can include the heart muscle. Vitamin E deficiency ultimately results in death. In severe vitamin E deficiency, cardiomyopathy was among the symptoms of a vitamin E–deficient child who died of hepatic and cardiac failure (21). Cardiomyopathy is also a symptom of vitamin E deficiency in some patients with AVED (16, 20, 22).
Vitamin E supplements in amounts well over 1000 mg/d have been prescribed for children with vitamin E deficiency. α-Tocopherol supplements are recommended because they prevent the further progression of the neurologic abnormalities, and in some cases reverse them. For example, when given before the onset of abnormalities, supplements prevented neurologic symptoms (23) and stopped the progression of myopathy in individuals with abetalipoproteinemia (24). Similarly, in children with AVED, vitamin E supplements improved symptoms and halted the disease progression (16).
Malnutrition.
Vitamin E deficiency symptoms in humans have been sufficiently well characterized to allow detection of more subtle examples of vitamin E inadequacy. Examples of frank vitamin E deficiency due to low dietary intakes include children in India with severe malnutrition (25, 26). In addition to general malnutrition, severe vitamin E deficiency was recognized in those children because the specific neurologic abnormalities associated with vitamin E deficiency were detected. Vitamin E supplementation was initiated and was found to reverse the symptoms (25, 26), thereby confirming that the neurologic abnormalities were dependent on α-tocopherol status.
Vitamin E inadequacy in children.
Assessing normal plasma α-tocopherol concentrations in children is complicated because α-tocopherol is transported in plasma lipoproteins and concentrations of cholesterol and lipoproteins, as well as of α-tocopherol, increase with age (27). An example in which circulating α-tocopherol concentrations did not reflect dietary intakes was illustrated in a study in which these values in adolescents and their parent or grandparent were compared. Although the estimates of mean ± SEM vitamin E intakes in adolescents (9.2 ± 0.2 mg α-tocopherol/d) were higher than those of the adults (8.4 ± 0.2 mg α-tocopherol/d), plasma α-tocopherol concentrations were lower in adolescents (17 ± 0.4 μmol/L, corrected for cholesterol concentrations) compared with adults (26 ± 0.6 μmol/L) (28). The values for plasma α-tocopherol concentrations in these adolescents were similar to those reported for healthy children in Tunisia (29) or Germany (30). These data emphasize the well-accepted finding that circulating α-tocopherol concentrations do not correlate very highly with dietary α-tocopherol intakes.
Given the close relation between circulating lipids and α-tocopherol, it is important to recognize that both variables may be decreased in malnutrition. Squali Houssainï et al. (31) studied control children compared with severely malnourished children in Morocco. They reported, “In severely malnourished children, albumin, cholesterol and low density lipoprotein (LDL) cholesterol, plasma selenium, vitamin E and zinc were low, whereas inflammatory proteins and triglycerides were high. These features worsened with essential fatty acid deficiency.” Their findings emphasize that malnutrition alters plasma lipid concentrations; thus, correction of plasma α-tocopherol for lipids may mask deficiency states. Decreased cholesterol concentrations were also observed in protein energy malnutrition (32). These cholesterol decreases were also associated with low circulating concentrations of α-tocopherol and increased inflammatory markers, such as IL-6. In contrast, Laryea et al. (33) suggested that the individuals they studied were active, normal Congolese village children and the low plasma α-tocopherol (mean ± SD: 7.3 ± 1.3 μmol/L) should be corrected for low lipids based on the observation that, when reported as tocopherol:lipid ratios, the vitamin E values were within the usual range for children. However, low plasma α-tocopherol concentrations (median: 7.33 μmol/L; range: 2.61–18.42 μmol/L) were also found in children with falciparum malaria infections compared with control children (median: 17.71 μmol/L; range: 6.48–28.08 μmol/L); both groups had similar α-tocopherol:cholesterol ratios [median (range): 4.61 (1.24–7.20) vs. 5.15 (1.80–8.92) μmol/mmol] because the children with malaria had depressed cholesterol concentrations (mean ± SD: 1.89 ± 0.62 vs. 3.47 ± 0.59 mmol/L in controls) (34). These data suggest that both malnutrition and infectious diseases can lower circulating cholesterol and its lipoprotein carriers. Thus, correction of plasma α-tocopherol concentrations for lipids is not appropriate in cases in which circulating lipids are below normal concentrations.
Circulating α-tocopherol concentrations <12 μmol/L were defined by the IOM to be in the deficient/inadequate range for healthy adults (4). For comparison, European children in the Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA) study were reported to have mean ±SD circulating α-tocopherol concentrations of 23 ± 4.9 μmol/L (35), whereas pediatric reference intervals for circulating α-tocopherol from 1136 healthy U.S. children aged 7 to 17 y ranged from 11 to 30 μmol/L (36); and in U.S. children aged 7 mo to 9 y, values ranged from 12 to 40 μmol/L with a mean of ∼20 μmol/L (37). Thus, the ranges of circulating α-tocopherol in healthy U.S. and European children were above the deficiency cutoff value of 12 μmol/L. However, there have been some reports of children in the United States with circulating α-tocopherol below this cutoff (38), suggesting low intakes. Indeed, some reports claim that dietary vitamin E intakes in U.S. children are generally below recommended values, except for those children taking supplements (39).
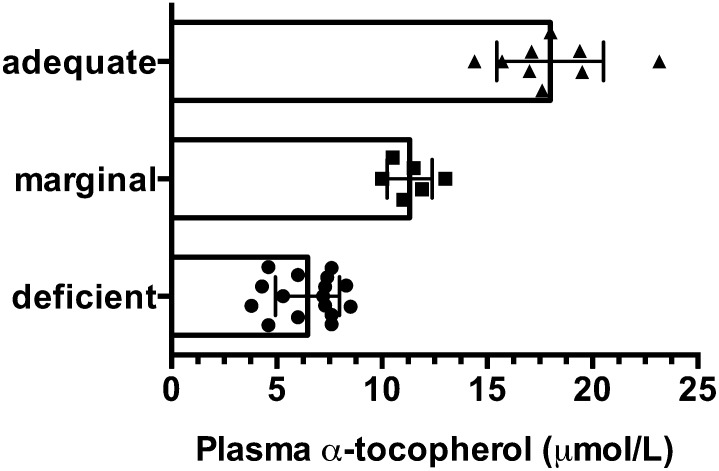
Taken together, these findings suggest that circulating α-tocopherol concentrations below the cutoff of 12 μmol/L are likely indicative of inadequacy if not frank vitamin E deficiency. Numerous reports worldwide have shown that such concentrations are frequently reported in children (Fig. 1). These low circulating α-tocopherol concentrations are caused by the combination of consumption of diets low in vitamin E, along with inadequate intakes of fat, protein, and calories. These latter dietary components are necessary for fat absorption and transport, which are required elements for vitamin E absorption and its lipoprotein transport, as reviewed elsewhere (40).
FIGURE 1.
Plasma α-tocopherol concentrations are shown from various reports of children with potentially low vitamin E status. The bars and error bars indicate overall means ± SDs of all data points, and the individual data points represent mean or median values from specific research reports. Plasma α-tocopherol concentrations fell into 3 categories: deficient (<9 μmol/L), marginal (9–12 μmol/L), and adequate (>12 μmol/L). References for these reports are as follows: deficient (25, 31–34, 128–138), marginal (38, 139–143), adequate (28–30, 41–43, 144–146).
Obesity and metabolic syndrome.
In contrast to malnourished children, many studies have shown that obese children do not have low plasma α-tocopherol concentrations; however, when their values were corrected for their circulating lipids, the α-tocopherol:lipid ratios were significantly lower than those in the control group because the obese children had elevated circulating cholesterol and TG concentrations (41–44). Vitamin E supplements in apparently adequately nourished obese children decrease oxidative stress markers (45), suggesting that obese children routinely consume inadequate amounts of antioxidants to prevent oxidative stress. Furthermore, it is likely that this increased oxidative stress is a consequence of chronic inflammation, which is seen secondary to obesity (46) and is a risk factor for other complications of obesity.
An extreme example is nonalcoholic fatty liver disease, which is a major cause of liver dysfunction and is increasing in children due to the increasing prevalence of obesity and type 2 diabetes. The severe negative effects associated with nonalcoholic fatty liver disease include progression to nonalcoholic steatohepatitis (NASH), liver cirrhosis, and ultimately liver cancer (47). Vitamin E supplementation decreases histologic evidence of NASH (48); therefore, supplementation has been tried in children with promising results (49). D'Adamo et al. (50) reported in obese children that 600 mg α-tocopherol daily doubled plasma concentrations from a mean (±SD) of 32.7 ± 1.5 μmol/L to 63 ± 14 μmol/L. The authors did not provide lipid-corrected values, but serum total cholesterol was, on average, 180 mg/dL (4.65 μmol/L) and TGs were 83 mg/dL (0.94 μmol/L). After 6 mo of vitamin E treatment, serum alanine aminotransferase decreased with vitamin E supplementation, indicating improved liver function. Supplementation also decreased urinary prostaglandin F2α, insulin, and fasting glucose concentrations, as well as their lipid profiles, and high-sensitivity C-reactive protein. It is unclear how many of these changes are due to the diet and behavior intervention rather than to vitamin E supplementation.
The findings in obese children raise the question as to whether the increased inflammation observed with obesity increases vitamin E requirements. Notably, interventions with vitamin E supplements in children (49) and in adults (48) with NASH had beneficial effects, especially with regard to serum alanine aminotransferase measures of liver dysfunction (51). In adults, Sanyal et al. (48) reported, “Vitamin E therapy, as compared with placebo, was associated with a significantly higher rate of improvement in nonalcoholic steatohepatitis (43% vs. 19%, P = 0.001)….” Taken together, these data suggest that obese children likely are consuming inadequate amounts of vitamin E, despite their apparently elevated circulating α-tocopherol concentrations.
To investigate whether antioxidant supplements could mitigate impaired inflammatory and antioxidant status, Murer et al. (45) studied overweight or obese children and adolescents (n = 44; mean ± SD age: 12.7 ± 1.5 y) participating in a lifestyle modification program, who were given daily antioxidants (vitamin E, 400 IU; vitamin C, 500 mg; selenium, 50 mg) or placebo for 4 mo. They then measured a number of variables, including the urinary vitamin E metabolite α-carboxy-ethyl-hydroxychromanol (α-CEHC). We previously proposed that the vitamin E metabolite could serve as biomarker of vitamin E adequacy because daily urinary α-CEHC excretion was reflective of adequacy when its excretion exceeded 1.39 μmol/g creatinine (11). Murer et al. (45) reported that the median urinary α-CEHC excretion in their obese and overweight children was low throughout the study in the placebo group [median (range) for baseline vs. postintervention:1.2 (0.01–2.9) vs. 1.2 (0.3–15.9) μmol/g creatinine], whereas in the antioxidant group it was low before supplementation but increased dramatically after antioxidant supplementation [1.8 (0.5–19.2) vs. 16.3 (0.01–81.2) μmol/g creatinine; P < 0.001 for intervention]. These data suggest that, despite apparently normal plasma α-tocopherol concentrations in the study participants, urinary α-CEHC excretion suggests inadequate vitamin E status. In support of this statement, vitamin E supplementation in these children also decreased F2-isoprostanes but not markers of inflammation (45).
The findings in obese participants emphasize that obesity does not necessarily reflect adequate micronutrient intakes, and vitamin E status may be inadequate for normal liver function in these individuals because they have increased oxidative stress. These findings are especially important because lipid peroxidation has been shown to cause dysregulation of liver lipoprotein secretion, which was prevented by increases in vitamin E intake in experimental animal studies (52, 53). Taken together, these data emphasize the importance of adequate vitamin E status in obese individuals to maintain healthy liver function and potentially prevent the progression of fatty liver to more serious forms of the disease. They further raise the question of whether liver dysfunction is thus a symptom of vitamin E inadequacy.
Circulating α-tocopherol concentrations as a biomarker of vitamin E status
As is apparent from the previous discussion, circulating α-tocopherol concentrations are very difficult to interpret. In normal healthy adults who consume a variety of foods, including nuts, seeds, and whole grains, plasma α-tocopherol concentrations average ∼20 μmol/L, whereas those individuals who consume supplements or fortified foods have concentrations that average ∼30 μmol/L or more (11). However, as a person ages, plasma lipid concentrations also increase, and these increases in lipids also increase the plasma carriers for α-tocopherol, leading to higher circulating concentrations. However, abnormal lipoprotein metabolism does not necessarily increase α-tocopherol delivery to tissues.
The experimental findings in obese children described above highlight the difficulty in assessing vitamin E status by measuring only circulating α-tocopherol. Another example is in individuals with cholestatic liver disease, who have high circulating lipids. Their plasma α-tocopherol concentrations are apparently within normal ranges; however, their α-tocopherol to lipid ratios are low, and most important, tissue α-tocopherol concentrations are at deficient levels (54). Thus, if plasma lipids are elevated, then correction of α-tocopherol for lipid concentrations is appropriate to assess adequacy. In this case, adequate values for α-tocopherol:lipid ratios should be similar to those in individuals with normal circulating lipid concentrations (55).
This close relation of plasma α-tocopherol to lipids has led some investigators, who evaluated poorly nourished children with low circulating lipids, to postulate on the basis of these ratios that the children’s α-tocopherol status was adequate. However, if both plasma lipids and α-tocopherol are abnormally low, then correction of circulating α-tocopherol concentrations for plasma lipids will yield a value indicating a normal α-tocopherol:lipid ratio. This assumption of adequate vitamin E status is likely invalid, because the low lipids reflect the inadequacy of the plasma carriers for delivery of vitamin E to tissues. Moreover, direct measurements of tissue α-tocopherol concentrations, or other surrogate markers of vitamin E status, have not been used to test the assumption that a normal circulating α-tocopherol:lipid ratio, which is caused by both low α-tocopherol and low lipids, reflects an adequate vitamin E status. The prevalence of stunting and anemia in malnourished children, who have limited intakes of both energy and micronutrients, suggests that these children lack important nutritional factors, including vitamin E (56).
Additional markers of inadequate vitamin E status are needed. Adipose tissue α-tocopherol concentrations have been used to assess vitamin E status (57–59). El-Sohemy et al. (58) reported that adipose tissue α-tocopherol concentrations were not well correlated with plasma α-tocopherol concentrations, but they did appear to reflect long-term vitamin E status. We found that in children suffering from severe burn injury, adipose tissue α-tocopherol concentrations rapidly (within 1 mo) become depleted, suggesting that this tissue can serve as an α-tocopherol storage site, releasing α-tocopherol upon increased metabolic demands (60). Additional studies are needed to evaluate intakes relative to tissue α-tocopherol concentrations and long-term health benefits.
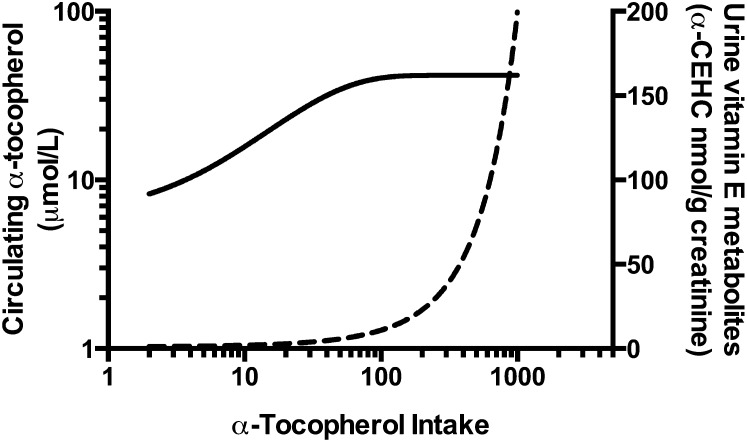
Urinary α-CEHC may fulfill this biomarker role (11, 61), but this marker of vitamin E status has not been widely studied with regard to vitamin E status in humans. Vitamin E metabolism is a hot topic and has been extensively studied with regard to non–α-tocopherol intakes in both humans and in experimental animals. Non–α-tocopherols are readily converted to their respective CEHC forms, even during α-tocopherol deficiency (62, 63). Thus, hepatic vitamin E metabolism is a major regulator of the forms of vitamin E found in the body. The reader is directed to a recent review on this topic (64). Hypothetically, once liver α-tocopherol concentration reaches a threshold level, additional α-tocopherol will be metabolized; thus, plasma α-tocopherol reaches a “plateau,” whereas α-CEHC excretion increases exponentially (Fig. 2).
FIGURE 2.
Hypothetical relation between dietary α-tocopherol intakes, plasma α-tocopherol concentrations, and urinary excretion of α-carboxy-ethyl-hydroxychromanol (α-CEHC). These hypothetical relations were based on data from published reports (11, 45, 61) with the assumption that as dietary α-tocopherol intakes increase, plasma α-tocopherol concentrations (solid line, left y-axis) reach a plateau. This nexus is the point at which the urinary α-CEHC excretion (dashed line, right y-axis) begins an exponential increase.
Anemia has traditionally been a marker of poor vitamin E status, but anemia in pregnant women in Bangladesh was not only associated with decreased plasma α-tocopherol concentrations but also with deficiencies of some other micronutrients (65), emphasizing that anemia is not necessarily caused by the lack of just 1 micronutrient. Thus, the multifactorial nature of nutritional status must be taken into account when evaluating adequacy, adding to the complexity of evaluating vitamin E status in free-living individuals.
Consequences of low vitamin E status
It is notoriously difficult to show adverse consequences of vitamin E deficiency in experimental animals, as well as in humans. In patients with cystic fibrosis, anemia and decreased erythrocyte survival (5) were widely accepted as signs of vitamin E deficiency until reports of neurologic abnormalities that responded to vitamin E supplementation were discovered (66–68). The neurologic abnormalities at the outset of vitamin E deficiency are so subtle that they are difficult to assess, but with progression of the deficiency they become readily demonstrable (69, 70). This section of the review will therefore address health aspects in which inadequate vitamin E status is detrimental and where supplemental vitamin E has been shown to be beneficial for health, including pregnancy and neurologic diseases.
Is vitamin E deficiency an important cause of spontaneous embryonic death?
Vitamin E was discovered nearly 100 y ago because female rats fed a vitamin E–deficient diet resorbed their fetuses early in pregnancy (71); the cause of the embryonic failure has never been fully characterized. We investigated embryonic vitamin E deficiency in a vertebrate model, the zebrafish (Danio rerio), and discovered that α-tocopherol and α-TTP have critical roles in embryonic development. We based our research on the observation that α-TTP is expressed in the human yolk sac (72), that zebrafish embryos abundantly express α-TTP by 48 h postfertilization (hpf), and that α-TTP increases with oxidative stress in zebrafish embryos (73). We discovered that α-tocopherol–deficient adult zebrafish could spawn and produce viable fertilized eggs, but within days the embryos suffered developmental impairment and increased mortality (74). The impaired brain formation in α-TTP knockdown zebrafish embryos raises the possibility that low vitamin E status has adverse events in early central nervous system development in other animals, including humans. Jishage et al. (75) showed that if the mother mouse did not express α-TTP and was not vitamin E supplemented, embryos (regardless of α-TTP status) developed neural tube defects and failed to come to term. Although the study by Jishage et al. focused on mouse maternal α-TTP deficiency, the embryonic phenotype and link to central nervous system development are similar to our findings in the zebrafish. In support of this notion, previous studies showed a clear association between maternal vitamin E status during gestation and cognitive function of the offspring in experimental animal models (76, 77).
Importantly, we found that in the zebrafish embryo, α-TTP knockdown caused head malformation before 15 hpf (78). This phenomenon coincides with the timing for increased synthesis of highly peroxidizable lipids by the embryo, evidenced by increased gene expression in the head/brain of 2 FA elongase enzymes, elongation of very long-chain fatty acid (Elovl)4 (79) and Elovl5 (80). When we measured specific PUFA concentrations in zebrafish embryos between 24 and 72 hpf, we found that both α-tocopherol and DHA concentrations decreased in vitamin E–deficient embryos but not in control embryos. Moreover, arachidonic acid concentrations decreased 3 times faster in α-tocopherol–deficient embryos (21 pg/h) compared with vitamin E–sufficient E embryos (7 pg/h) (P < 0.0001) (81). At 36 hpf, vitamin E-deficient embryos contained double the 5-hydroxy-eicosatetraenoic acids and 7-hydroxy-DHA concentrations, whereas other detectable hydroxy-lipids remained unchanged (81). Thus, vitamin E deficiency during embryogenesis depleted both omega-3 and omega-6 FAs (DHA and arachidonic acid, respectively) and increased hydroxy-FAs derived from these PUFAs, suggesting that α-tocopherol is necessary to protect these critical FAs during development of the nervous system. Our studies show that the target zone that is most sensitive to α-tocopherol depletion is the head/brain/eye; without delivery of α-tocopherol, the brain fails to develop properly (78).
This absolute requirement for α-tocopherol by the zebrafish embryo takes place during a time analogous to the first 20 d of human embryonic gestation, a time during pregnancy before a woman knows she is pregnant. This time frame is 10–15 hpf for the zebrafish embryo (82), 9.5 d for rats (83), and 17–19 d for humans (84–86). Thus, the requirement for vitamin E very early in human pregnancy is analogous to situations of inadequate folic acid status.
The importance of α-tocopherol for preventing neural tube defects in humans can be surmised from studies in which multivitamins were compared with folic acid supplements. Specifically, folic acid supplements were not as effective in preventing neural tube defects as folic acid/multivitamin combinations, as shown in a review of 5 human trials (87). In a Hungarian trial to evaluate neural tube defects, the multivitamin contained 15 mg vitamin E along with other vitamins (88). The importance of vitamin E in preventing neural tube defects is emphasized by the findings from a study of neural tube defects and maternal micronutrient intakes, including 954 cases (300 with anencephaly, 654 with spina bifida) and 6268 controls (89). A decreased risk of spina bifida was associated with increased intakes of preconception supplements containing antioxidant vitamins E and C, as well as other micronutrients (89). The importance of vitamin E in the nervous system was also supported by a study in China that showed that higher maternal and cord blood α-tocopherol concentrations at birth were associated with improved cognitive function when the child was assessed at age 2 y (90). And conversely, low plasma α-tocopherol concentrations were associated with poorer cognitive function in patients with cystic fibrosis at diagnosis (91, 92).
Vitamin E in pregnancy.
The role of vitamin E in pregnancy is of increasing concern because it is clear that adequate nutritional status for the first 1000 d of life is necessary for subsequent adult health and well-being, given that stunting cannot be reversed after this critical window (93). Moreover, a study in Egypt emphasized that vitamin E is a key missing micronutrient in stunted children (56). The authors showed that 78.2% of stunted children were vitamin E deficient, with plasma α-tocopherol concentrations of 7.7 μmol/L compared with 14.1 μmol/L in control children (56). Fares et al. (94) reported that vitamin A, E, and D deficiencies were very common in very-low-birth-weight Tunisian neonates and were associated with pre-eclampsia (94). However, pre-eclampsia risk was not changed by vitamin E and C supplements in a number of studies in Western countries (95–99). The lack of benefit of vitamin E supplements in pre-eclampsia may be a result of the relative adequacy of vitamin E status of the women studied. For example, Poston et al. (99) reported that the circulating α-tocopherol:cholesterol ratios were >6 μmol/mmol in the placebo group and >9 μmol/mmol in the vitamin E and C supplement group; these ratios indicate that even the placebo group was well nourished with respect to vitamin E. Taken together, these data indicate that low vitamin E status may increase pre-eclampsia risk, but women with adequate vitamin E status do not benefit further from vitamin E supplements. The definitions of what is “low” and “adequate” vitamin E status are not clearly delineated and merit further research.
Worldwide, the adequacy of α-tocopherol status during pregnancy is unclear and not frequently measured, and thus the utility of vitamin E supplements in improving outcomes has been variable. In situations in which α-tocopherol status was documented to be low, vitamin E supplements had beneficial outcomes. For example, multivitamin supplements containing vitamin E reduced adverse pregnancy outcomes in HIV-positive women in Tanzania (100, 101). However, by using a cutoff of <11.6 μmol/L for plasma α-tocopherol concentrations, the prevalence of low vitamin E status was 5.9% of nonpregnant women of reproductive age in the northern Persian Gulf region, leading the authors to conclude that most women had an adequate vitamin E status. Additionally, in a study in the United States (n = 9968; n = 4992 in the vitamin group and n = 4976 in the placebo group), where at baseline pregnant women were taking 22 IU vitamin E in a daily multivitamin (equal to the RDA), additional vitamin E supplements (400 IU) were not beneficial in reducing the risk of preterm births (102). By contrast, vitamin E supplements were associated with a decreased incidence of preterm births in a Hungarian population study (103). Although it is apparent that the vitamin E status of pregnant women must be adequate to successfully bear a child, these findings suggest that vitamin E supplements in excess of the RDA to adequately nourished women do not provide additional benefits.
Neurologic disease and cognitive impairment with age.
Given the importance of vitamin E in the developing nervous system and for the protection of peripheral nerves, as supported by studies in vitamin E–deficient humans and in experimental animals, it seems likely that vitamin E would also protect the nervous system with aging. There are some experimental data to support this hypothesis, especially with regard to Alzheimer disease. Vitamin E supplements were found to have benefit in slowing Alzheimer disease progression (104, 105), but they did not seem to prevent Alzheimer disease occurrence (106). A recent meta-analysis found that patients with Alzheimer disease compared with cognitively intact elderly controls had significantly lower plasma α-tocopherol concentrations (P < 0.001) (107). Moreover, higher ventricular cerebrospinal fluid α-tocopherol concentrations, measured postmortem in 230 participants from the Religious Orders Study, were associated with a lower density of neuritic plaques and with higher performance on tests of perceptual speed measured before death (108). Furthermore, compared with cognitively normal individuals, patients with either Alzheimer disease or mild cognitive impairment had lower circulating concentrations of all forms of vitamin E and both disorders were associated with increased oxidized vitamin E (109).
In experimental vitamin E deficiency in mice, axonal degeneration was observed in the hippocampus, an important area for memory and cognition (110). The combination of vitamin E deficiency and α-TTP deficiency in mice caused atrophy and decreased branching of Purkinje neurons, which was associated with deficits in motor coordination and cognitive functions that were normalized upon vitamin E supplementation (111). Additionally in mice, impaired vitamin E delivery to the brain resulting from a knockout of the phospholipid transfer protein also resulted in increased memory impairment 1 wk after abeta25–35 peptide injection (112). This impairment could be prevented by vitamin E supplementation (112). These experimental findings are consistent with a report in elderly humans showing that a lifelong dietary pattern that results in nutrient intakes that provide increased circulating concentrations of vitamins B, C, D, and E is associated with a larger brain size (as assessed by MRI) and higher cognitive function (113).
Given the importance of vitamin E in protecting unsaturated FAs, it is not surprising that patients with Alzheimer disease have increased concentrations of circulating lipid peroxidation products (114). Importantly, phosphatidylcholine 16:0/22:6 (DHA-PC 38:6), which contains the highly oxidizable FA DHA, was identified as 1 of 10 phospholipids that were depleted in the plasma of human participants who went on to develop Alzheimer disease (115). By contrast, individuals who were in the top quartile of plasma DHA-PC concentrations among the Framingham Heart Study participants had a significant 47% reduction in the risk of developing all-cause dementia (116). Taken together, these findings suggest that vitamin E protects critical FAs in the brain from lipid peroxidation and that improved brain vitamin E status is protective for cognitive function. Interestingly, vitamin E supplements (300 mg daily for 615 d compared with 30 mg for 361 d) were found to double brain α-tocopherol concentrations in a study carried out in 2 terminally ill patients (117).
Conclusions and Speculations
This review evaluated the information available on the adverse consequences of inadequate α-tocopherol status. In general, plasma α-tocopherol concentrations <12 μmol/L are associated with increased infection, anemia, stunting of growth, and poor outcomes during pregnancy for both the infant and the mother. When low dietary amounts of α-tocopherol are consumed, tissue α-tocopherol needs exceed amounts available, leading to increased damage to target tissues. Hypothetically, these low α-tocopherol intakes in humans lead first to anemia because of the relatively rapid turnover of erythrocytes and their exposure to oxygen and their high iron contents. Further damage might be expected in other tissues with rapid turnover. Potentially, intestinal cells are spared because they are exposed to other dietary antioxidants, as well as to low oxygen concentrations. The nervous system is a special case because α-tocopherol is retained in the brain, likely as a result of brain expression of α-TTP (111). With continued extrahepatic tissue α-tocopherol depletion, peripheral nerves are at risk (5), likely due to their high PUFA contents compared with surrounding tissues. Sensory compared with motor neurons are likely more at risk because the information flow in sensory neurons is from the periphery to the brain, whereas in motor neurons the flow is in the opposite direction, potentially moving α-tocopherol toward the periphery. Severe, or perhaps chronic, vitamin E depletion ultimately decreases brain α-tocopherol, leading to damage and, in the elderly, cognitive impairment.
The adequacy of the middle range of α-tocopherol intakes is difficult to define. Plasma α-tocopherol concentrations between 12 and 20 μmol/L can be raised with increases in dietary intake, suggesting that hepatic α-TTP is not saturated. Studies in experimental animals suggested that hepatic α-TTP maintains circulating α-tocopherol, redistributing it and potentially allowing tissue α-tocopherol depletion (118). In this case, α-tocopherol returning from the periphery to the liver is not metabolized but is salvaged by hepatic α-TTP and returned to the plasma (119), where it could be taken up by tissues with lipoprotein receptors. This process tends to increase circulating α-tocopherol concentrations and normalize them at the expense of depletion of tissue α-tocopherol.
Hepatic α-tocopherol trafficking, disposition, and metabolism are not well understood or characterized. The well-known lack of correlation between dietary vitamin E intakes and circulating α-tocopherol [for examples, see (58, 120, 121)] in this middle range of intakes speaks to the efficiency of the regulatory controls governing circulating α-tocopherol concentrations. These processes serve to protect circulating lipids, which are readily oxidized and potentially exposed to higher oxygen concentrations, as well as reactive oxygen species and free metals. Here the special case of fatty liver disease is of interest because the progression of this disorder to more serious forms of the disease is dependent on oxidative damage to lipids (122), suggesting that inadequate vitamin E intakes may promote disease progression.
Supplements providing vitamin E intakes in excess of 100-fold dietary intakes increase plasma concentrations by ∼2- to 4-fold above baseline values (123–126). The limitation on plasma concentrations appears to be a result of increased hepatic vitamin E metabolism and excretion, as discussed previously (40). Intakes of 12–15 mg α-tocopherol/d are sufficient in normal healthy adult individuals to provide adequate vitamin E status on the basis of the health benefits associated with these intakes (127).
Acknowledgments
The sole author had responsibility for all parts of the manuscript. Brittany Manzek provided excellent technical help with the literature review.
Footnotes
Abbreviations used: α-CEHC, α-carboxy-ethyl-hydroxychromanol; α-TE, α-tocopherol equivalent; α-TTP, α-tocopherol transfer protein; AVED, ataxia with vitamin E deficiency; EAR, estimated average requirement; hpf, hours postfertilization; Elovl, dlongation of very long-chain fatty acid; IOM, Institute of Medicine; NASH, nonalcoholic steatohepatitis.
References
- 1.Niki E, Traber MG. A history of vitamin E. Ann Nutr Metab 2012;61:207–12 [DOI] [PubMed] [Google Scholar]
- 2.Moshfegh A, Goldman J, Cleveland L. What we eat in America, NHANES 2001–2002: usual nutrient intakes from food compared to dietary reference intakes. USDA, Agricultural Research Service [cited 2014 Apr 8]. Available from: http://www.ars.usda.gov/SP2UserFiles/Place/12355000/pdf/0102/usualintaketables2001–02.pdf.
- 3.Maras JE, Bermudez OI, Qiao N, Bakun PJ, Boody-Alter EL, Tucker KL. Intake of alpha-tocopherol is limited among US adults. J Am Diet Assoc 2004;104:567–75 [DOI] [PubMed] [Google Scholar]
- 4.Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington: National Academies Press; 2000 [PubMed] [Google Scholar]
- 5.Farrell PM, Bieri JG, Fratantoni JF, Wood RE, di Sant'Agnese PA. The occurrence and effects of human vitamin E deficiency: a study in patients with cystic fibrosis. J Clin Invest 1977;60:233–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ausman LM, Hayes KC. Vitamin E deficiency anemia in Old and New World monkeys. Am J Clin Nutr 1974;27:1141–51 [DOI] [PubMed] [Google Scholar]
- 7.Jacob HS, Lux SEt. Degradation of membrane phospholipids and thiols in peroxide hemolysis: studies in vitamin E deficiency. Blood 1968;32(4):549–68 [PubMed] [Google Scholar]
- 8.Oski FA, Barness LA. Hemolytic anemia in vitamin E deficiency. Am J Clin Nutr 1968;21:45–50 [DOI] [PubMed] [Google Scholar]
- 9.Gao X, Wilde PE, Lichtenstein AH, Bermudez OI, Tucker KL. The maximal amount of dietary alpha-tocopherol intake in U.S. adults (NHANES 2001–2002). J Nutr 2006;136:1021–6 [DOI] [PubMed] [Google Scholar]
- 10.Fulgoni VL III, Keast DR, Bailey RL, Dwyer J. Foods, fortificants, and supplements: where do Americans get their nutrients? J Nutr 2011;141:1847–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lebold KM, Ang A, Traber MG, Arab L. Urinary alpha-carboxyethyl hydroxychroman can be used as a predictor of alpha-tocopherol adequacy, as demonstrated in the Energetics Study. Am J Clin Nutr 2012;96:801–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. USDA, U.S. Department of Health and Human Services. Dietary guidelines for Americans. 2010 [cited 2014 Apr 15]. Available from: http://www.cnpp.usda.gov/Publications/DietaryGuidelines/2010/PolicyDoc/PolicyDoc.pdf.
- 13.Hiza HAB, Bente L. Nutrient content of the U.S. food supply: developments between 2000 and 2006. USDA, Center for Nutrition Policy and Promotion; 2011 [cited 2014 Jan 24]. Home Economics Research Report No. 59. Available from: http://www.cnpp.usda.gov/Publications/FoodSupply/Final_FoodSupplyReport_2006.pdf.
- 14. Food and Nutrition Board, National Research Council. Recommended Dietary Allowances. 10th ed. Washington: National Academies Press; 1989. [PubMed]
- 15.Traber MG, Schiano TD, Steephen AC, Kayden HJ, Shike M. Efficacy of water-soluble vitamin E in the treatment of vitamin E malabsorption in short-bowel syndrome. Am J Clin Nutr 1994;59:1270–4 [DOI] [PubMed] [Google Scholar]
- 16.Di Donato I, Bianchi S, Federico A. Ataxia with vitamin E deficiency: update of molecular diagnosis. Neurol Sci 2010;31:511–5 [DOI] [PubMed] [Google Scholar]
- 17.Traber MG, Sokol RJ, Burton GW, Ingold KU, Papas AM, Huffaker JE, Kayden HJ. Impaired ability of patients with familial isolated vitamin E deficiency to incorporate alpha-tocopherol into lipoproteins secreted by the liver. J Clin Invest 1990;85:397–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morley S, Cecchini M, Zhang W, Virgulti A, Noy N, Atkinson J, Manor D. Mechanisms of ligand transfer by the hepatic tocopherol transfer protein. J Biol Chem 2008;283(26):17797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Traber MG. Vitamin E. 10th ed. In: Erdman J, Macdonald I, Zeisel S, editors. Present knowledge in nutrition: Singapore: International Life Sciences Institute. Wiley-Blackwell; 2012. p. 214–29. [Google Scholar]
- 20.El Euch-Fayache G, Bouhlal Y, Amouri R, Feki M, Hentati F. Molecular, clinical and peripheral neuropathy study of Tunisian patients with ataxia with vitamin E deficiency. Brain 2014;137:402–10 [DOI] [PubMed] [Google Scholar]
- 21.Saito K, Matsumoto S, Yokoyama T, Okaniwa M, Kamoshita S. Pathology of chronic vitamin E deficiency in fatal familial intrahepatic cholestasis (Byler disease). Virchows Arch A Pathol Anat Histol 1982;396:319–30 [DOI] [PubMed] [Google Scholar]
- 22.Marzouki N, Benomar A, Yahyaoui M, Birouk N, Elouazzani M, Chkili T, Benlemlih M. Vitamin E deficiency ataxia with (744 del A) mutation on alpha-TTP gene: genetic and clinical peculiarities in Moroccan patients. Eur J Med Genet 2005;48:21–8 [DOI] [PubMed] [Google Scholar]
- 23.Zamel R, Khan R, Pollex RL, Hegele RA. Abetalipoproteinemia: two case reports and literature review. Orphanet J Rare Dis 2008;3:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hegele RA, Angel A. Arrest of neuropathy and myopathy in abetalipoproteinemia with high-dose vitamin E therapy. Can Med Assoc J 1985;132:41–4 [PMC free article] [PubMed] [Google Scholar]
- 25.Kalra V, Grover J, Ahuja GK, Rathi S, Khurana DS. Vitamin E deficiency and associated neurological deficits in children with protein-energy malnutrition. J Trop Pediatr 1998;44:291–5 [DOI] [PubMed] [Google Scholar]
- 26.Kalra V, Grover JK, Ahuja GK, Rathi S, Gulati S, Kalra N. Vitamin E administration and reversal of neurological deficits in protein-energy malnutrition. J Trop Pediatr 2001;47:39–45 [DOI] [PubMed] [Google Scholar]
- 27.Ford ES, Schleicher RL, Mokdad AH, Ajani UA, Liu S. Distribution of serum concentrations of alpha-tocopherol and gamma-tocopherol in the US population. Am J Clin Nutr 2006;84:375–83 [DOI] [PubMed] [Google Scholar]
- 28.Kabagambe EK, Baylin A, Irwig MS, Furtado J, Siles X, Kim MK, Campos H. Costa Rican adolescents have a deleterious nutritional profile as compared to adults in terms of lower dietary and plasma concentrations of antioxidant micronutrients. J Am Coll Nutr 2005;24:122–8 [DOI] [PubMed] [Google Scholar]
- 29.Fares S, Chahed MK, Feki M, Beji C, Traissac P, El Ati J, Kaabachi N. Status of vitamins A and E in schoolchildren in the centre west of Tunisia: a population-based study. Public Health Nutr 2011;14:255–60 [DOI] [PubMed] [Google Scholar]
- 30.Laryea MD, Biggemann B, Cieslicki P, Wendel U. Plasma tocopherol and tocopherol to lipid ratios in a normal population of infants and children. Int J Vitam Nutr Res 1989;59:269–72 [PubMed] [Google Scholar]
- 31.Squali Houssaïni FZ, Foulon T, Payen N, Iraqi MR, Arnaud J, Groslambert P. Plasma fatty acid status in Moroccan children: increased lipid peroxidation and impaired polyunsaturated fatty acid metabolism in protein-calorie malnutrition. Biomed Pharmacother 2001;55:155–62 [DOI] [PubMed] [Google Scholar]
- 32.Sauerwein RW, Mulder JA, Mulder L, Lowe B, Peshu N, Demacker PN, van der Meer JW, Marsh K. Inflammatory mediators in children with protein-energy malnutrition. Am J Clin Nutr 1997;65:1534–9 [DOI] [PubMed] [Google Scholar]
- 33.Laryea MD, Mayatepek E, Brunninger P, Doehring-Schwerdtfeger E, Leichsenring M, Bremer HJ. Vitamin E status of Congolese children in a rural area. Int J Vitam Nutr Res 1990;60:107–11 [PubMed] [Google Scholar]
- 34.Das BS, Thurnham DI, Das DB. Plasma alpha-tocopherol, retinol, and carotenoids in children with falciparum malaria. Am J Clin Nutr 1996;64:94–100 [DOI] [PubMed] [Google Scholar]
- 35.Breidenassel C, Valtuena J, Gonzalez-Gross M, Benser J, Spinneker A, Moreno LA, de Henauw S, Widhalm K, Molnar D, Maiani G, et al. Antioxidant vitamin status (A, E, C, and beta-carotene) in European adolescents—the HELENA study. Int J Vitam Nutr Res 2011;81:245–55 [DOI] [PubMed] [Google Scholar]
- 36.Johnson-Davis KL, Moore SJ, Owen WE, Cutler JM, Frank EL. A rapid HPLC method used to establish pediatric reference intervals for vitamins A and E. Clin Chim Acta 2009;405:35–8 [DOI] [PubMed] [Google Scholar]
- 37.Brady H, Lamb MM, Sokol RJ, Ross CA, Seifert JA, Rewers MJ, Norris JM. Plasma micronutrients are associated with dietary intake and environmental tobacco smoke exposure in a paediatric population. Public Health Nutr 2007;10:712–8 [DOI] [PubMed] [Google Scholar]
- 38.Kim YN, Lora KR, Giraud DW, Driskell JA. Nonsupplemented children of Latino immigrants have low vitamin E intakes and plasma concentrations and normal vitamin C, selenium, and carotenoid intakes and plasma concentrations. J Am Diet Assoc 2006;106:385–91 [DOI] [PubMed] [Google Scholar]
- 39.Bailey RL, Fulgoni VL III, Keast DR, Lentino CV, Dwyer JT. Do dietary supplements improve micronutrient sufficiency in children and adolescents? J Pediatr 2012;161:837–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Traber MG. Mechanisms for the prevention of vitamin E excess. J Lipid Res 2013;54:2295–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuno T, Hozumi M, Morinobu T, Murata T, Mingci Z, Tamai H. Antioxidant vitamin levels in plasma and low density lipoprotein of obese girls. Free Radic Res 1998;28:81–6 [DOI] [PubMed] [Google Scholar]
- 42.Strauss RS. Comparison of serum concentrations of alpha-tocopherol and beta-carotene in a cross-sectional sample of obese and nonobese children (NHANES III). J Pediatr 1999;134:160–5 [DOI] [PubMed] [Google Scholar]
- 43.Molnár D, Decsi T, Koletzko B. Reduced antioxidant status in obese children with multimetabolic syndrome. Int J Obes Relat Metab Disord 2004;28:1197–202 [DOI] [PubMed] [Google Scholar]
- 44.Gunanti IR, Marks GC, Al-Mamun A, Long KZ. Low serum concentrations of carotenoids and vitamin E are associated with high adiposity in Mexican-American children. J Nutr 2014;144:489–95 [DOI] [PubMed] [Google Scholar]
- 45.Murer SB, Aeberli I, Braegger CP, Gittermann M, Hersberger M, Leonard SW, Taylor AW, Traber MG, Zimmermann MB. Antioxidant supplements reduced oxidative stress and stabilized liver function tests but did not reduce inflammation in a randomized controlled trial in obese children and adolescents. J Nutr 2014;144:193–201 [DOI] [PubMed] [Google Scholar]
- 46.López-Alcaraz F, Del Toro-Equihua M, Orta-Duarte M, Flores-Ruelas Y, Sanchez-Ramirez CA. Higher levels of C-reactive protein associated with higher adiposity in Mexican schoolchildren. Nutr Hosp 2014;29:531–6 [DOI] [PubMed] [Google Scholar]
- 47.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology 2006;43(2, Suppl 1):S99–112 [DOI] [PubMed] [Google Scholar]
- 48.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010;362:1675–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, Abrams SH, Scheimann AO, Sanyal AJ, Chalasani N, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA 2011;305:1659–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.D'Adamo E, Marcovecchio ML, Giannini C, de Giorgis T, Chiavaroli V, Chiarelli F, Mohn A. Improved oxidative stress and cardio-metabolic status in obese prepubertal children with liver steatosis treated with lifestyle combined with Vitamin E. Free Radic Res 2013;47:146–53 [DOI] [PubMed] [Google Scholar]
- 51.Hoofnagle JH, Van Natta ML, Kleiner DE, Clark JM, Kowdley KV, Loomba R, Neuschwander-Tetri BA, Sanyal AJ, Tonascia J. Vitamin E and changes in serum alanine aminotransferase levels in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2013;38:134–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Caviglia JM, Gayet C, Ota T, Hernandez-Ono A, Conlon DM, Jiang H, Fisher EA, Ginsberg HN. Different fatty acids inhibit apoB100 secretion by different pathways: unique roles for ER stress, ceramide, and autophagy. J Lipid Res 2011;52:1636–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pan M, Cederbaum AI, Zhang YL, Ginsberg HN, Williams KJ, Fisher EA. Lipid peroxidation and oxidant stress regulate hepatic apolipoprotein B degradation and VLDL production. J Clin Invest 2004;113:1277–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sokol RJ, Heubi JE, Iannaccone ST, Bove KE, Balistreri WF. Vitamin E deficiency with normal serum vitamin E concentrations in children with chronic cholestasis. N Engl J Med 1984;310:1209–12 [DOI] [PubMed] [Google Scholar]
- 55.Traber MG, Jialal I. Measurement of lipid-soluble vitamins-further adjustment needed? Lancet 2000;355:2013–4 [DOI] [PubMed] [Google Scholar]
- 56.Ibrahim SA, Abd el-Maksoud A, Nassar MF. Nutritional stunting in Egypt: which nutrient is responsible? East Mediterr Health J 2002;8:272–80 [PubMed] [Google Scholar]
- 57.Handelman GJ, Epstein WL, Peerson J, Spiegelman D, Machlin LJ, Dratz EA. Human adipose alpha-tocopherol and gamma-tocopherol kinetics during and after 1 y of alpha-tocopherol supplementation. Am J Clin Nutr 1994;59:1025–32 [DOI] [PubMed] [Google Scholar]
- 58.El-Sohemy A, Baylin A, Ascherio A, Kabagambe E, Spiegelman D, Campos H. Population-based study of alpha- and gamma-tocopherol in plasma and adipose tissue as biomarkers of intake in Costa Rican adults. Am J Clin Nutr 2001;74:356–63 [DOI] [PubMed] [Google Scholar]
- 59.Kardinaal AFM, Vantveer P, Brants HAM, Vandenberg H, Vanschoonhoven J, Hermus RJJ. Relations between antioxidant vitamins in adipose tissue, plasma, and diet. Am J Epidemiol 1995;141:440–50 [DOI] [PubMed] [Google Scholar]
- 60.Traber MG, Leonard SW, Traber DL, Traber LD, Gallagher J, Bobe G, Jeschke MG, Finnerty CC, Herndon D. α-Tocopherol adipose tissue stores are depleted after burn injury in pediatric patients. Am J Clin Nutr 2010;92:1378–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Imai E, Tsuji T, Sano M, Fukuwatari T, Shibata K. Association between 24 hour urinary alpha-tocopherol catabolite, 2,5,7,8-tetramethyl-2(2′-carboxyethyl)-6-hydroxychroman (alpha-CEHC) and alpha-tocopherol intake in intervention and cross-sectional studies. Asia Pac J Clin Nutr 2011;20:507–13 [PubMed] [Google Scholar]
- 62.Traber MG, Siddens LK, Leonard SW, Schock B, Gohil K, Krueger SK, Cross CE, Williams DE. Alpha-tocopherol modulates Cyp3a expression, increases gamma-CEHC production, and limits tissue gamma-tocopherol accumulation in mice fed high gamma-tocopherol diets. Free Radic Biol Med 2005;38:773–85 [DOI] [PubMed] [Google Scholar]
- 63.Grebenstein N, Schumacher M, Graeve L, Frank J. alpha-Tocopherol transfer protein is not required for the discrimination against gamma-tocopherol in vivo but protects it from side-chain degradation in vitro. Mol Nutr Food Res 2014;58:1052–60 [DOI] [PubMed] [Google Scholar]
- 64.Jiang Q. Natural forms of vitamin E: metabolism, antioxidant and anti-inflammatory activities and the role in disease prevention and therapy. Free Radic Biol Med 2014;72C:76–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shamim AA, Kabir A, Merrill RD, Ali H, Rashid M, Schulze K, Labrique A, West KP, Jr, Christian P. Plasma zinc, vitamin B(12) and alpha-tocopherol are positively and plasma gamma-tocopherol is negatively associated with Hb concentration in early pregnancy in north-west Bangladesh. Public Health Nutr 2013;16:1354–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cynamon HA, Milov DE, Valenstein E, Wagner M. Effect of vitamin E deficiency on neurologic function in patients with cystic fibrosis. J Pediatr 1988;113:637–40 [DOI] [PubMed] [Google Scholar]
- 67.Elias E, Muller DPR, Scott J. Association of spinocerebellar disorders with cystic fibrosis or chronic childhood cholestasis and very low serum vitamin E. Lancet 1981;2:1319–21 [DOI] [PubMed] [Google Scholar]
- 68.Sitrin MD, Lieberman F, Jensen WE, Noronha A, Milburn C, Addington W. Vitamin E deficiency and neurologic disease in adults with cystic fibrosis. Ann Intern Med 1987;107:51–4 [DOI] [PubMed] [Google Scholar]
- 69.Sokol RJ, Guggenheim MA, Iannaccone ST, Barkhaus PE, Miller C, Silverman A, Balistreri WF, Heubi JE. Improved neurologic function after long-term correction of vitamin E deficiency in children with chronic cholestasis. N Engl J Med 1985;313:1580–6 [DOI] [PubMed] [Google Scholar]
- 70.Sokol RJ, Kayden HJ, Bettis DB, Traber MG, Neville H, Ringel S, Wilson WB, Stumpf DA. Isolated vitamin E deficiency in the absence of fat malabsorption—familial and sporadic cases: characterization and investigation of causes. J Lab Clin Med 1988;111:548–59 [PubMed] [Google Scholar]
- 71.Evans HM, Bishop KS. On the existence of a hitherto unrecognized dietary factor essential for reproduction. Science 1922;56:650–1 [DOI] [PubMed] [Google Scholar]
- 72.Jauniaux E, Cindrova-Davies T, Johns J, Dunster C, Hempstock J, Kelly FJ, Burton GJ. Distribution and transfer pathways of antioxidant molecules inside the first trimester human gestational sac. J Clin Endocrinol Metab 2004;89:1452–8 [DOI] [PubMed] [Google Scholar]
- 73.Usenko CY, Harper SL, Tanguay RL. Fullerene C60 exposure elicits an oxidative stress response in embryonic zebrafish. Toxicol Appl Pharmacol 2008;229:44–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miller GW, Labut EM, Lebold KM, Floeter A, Tanguay RL, Traber MG. Zebrafish (Danio rerio) fed vitamin E-deficient diets produce embryos with increased morphologic abnormalities and mortality. J Nutr Biochem 2012;23:478–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jishage K, Arita M, Igarashi K, Iwata T, Watanabe M, Ogawa M, Ueda O, Kamada N, Inoue K, Arai H, et al. alpha-Tocopherol transfer protein is important for the normal development of placental labyrinthine trophoblasts in mice. J Biol Chem 2001;276:1669–72 [DOI] [PubMed] [Google Scholar]
- 76.Shichiri M, Yoshida Y, Ishida N, Hagihara Y, Iwahashi H, Tamai H, Niki E. alpha-Tocopherol suppresses lipid peroxidation and behavioral and cognitive impairments in the Ts65Dn mouse model of Down syndrome. Free Radic Biol Med 2011;50:1801–11 [DOI] [PubMed] [Google Scholar]
- 77.Lockrow J, Prakasam A, Huang P, Bimonte-Nelson H, Sambamurti K, Granholm AC. Cholinergic degeneration and memory loss delayed by vitamin E in a Down syndrome mouse model. Exp Neurol 2009;216:278–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miller GW, Ulatowski L, Labut EM, Lebold KM, Manor D, Atkinson J, Barton CL, Tanguay RL, Traber MG. The alpha-tocopherol transfer protein is essential for vertebrate embryogenesis. PLoS ONE 2012;7:e47402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Monroig O, Rotllant J, Cerda-Reverter JM, Dick JR, Figueras A, Tocher DR. Expression and role of Elovl4 elongases in biosynthesis of very long-chain fatty acids during zebrafish Danio rerio early embryonic development. Biochim Biophys Acta 2010;1801:1145–54 [DOI] [PubMed] [Google Scholar]
- 80.Tan SH, Chung HH, Shu-Chien AC. Distinct developmental expression of two elongase family members in zebrafish. Biochem Biophys Res Commun 2010;393:397–403 [DOI] [PubMed] [Google Scholar]
- 81.Lebold KM, Kirkwood JS, Taylor AW, Choi J, Barton CL, Miller GW, Du JL, Jump DB, Stevens JF, Tanguay RL. Novel liquid chromatography-mass spectrometry method shows that vitamin E deficiency depletes arachidonic and docosahexaenoic acids in zebrafish (Danio rerio) embryos. Redox Biol 2013;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn 1995;203:253–310 [DOI] [PubMed] [Google Scholar]
- 83. Altman PL, Katz DD. Growth, including reproduction and morphological development. Prepared under the auspices of the Committee on Biological Handbooks, FASEB. Washington: Federation of American Societies for Experimental Biology; 1962.
- 84.O'Rahilly R. Early human development and the chief sources of information on staged human embryos. Eur J Obstet Gynecol Reprod Biol 1979;9:273–80 [DOI] [PubMed] [Google Scholar]
- 85.Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. N Engl J Med 1999;340:1796–9 [DOI] [PubMed] [Google Scholar]
- 86.Gilbert SF. Developmental biology. 9th ed. Sunderland (MA): Sinauer Associates; 2010. [Google Scholar]
- 87.Czeizel AE, Dudas I, Paput L, Banhidy F. Prevention of neural-tube defects with periconceptional folic acid, methylfolate, or multivitamins? Ann Nutr Metab 2011;58:263–71 [DOI] [PubMed] [Google Scholar]
- 88.Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med 1992;327:1832–5 [DOI] [PubMed] [Google Scholar]
- 89.Chandler AL, Hobbs CA, Mosley BS, Berry RJ, Canfield MA, Qi YP, Siega-Riz AM, Shaw GM. National Birth Defects Prevention Study. Neural tube defects and maternal intake of micronutrients related to one-carbon metabolism or antioxidant activity. Birth Defects Res A Clin Mol Teratol 2012;94(11):864–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen K, Zhang X, Wei XP, Qu P, Liu YX, Li TY. Antioxidant vitamin status during pregnancy in relation to cognitive development in the first two years of life. Early Hum Dev 2009;85:421–7 [DOI] [PubMed] [Google Scholar]
- 91.Koscik RL, Farrell PM, Kosorok MR, Zaremba KM, Laxova A, Lai HC, Douglas JA, Rock MJ, Splaingard ML. Cognitive function of children with cystic fibrosis: deleterious effect of early malnutrition. Pediatrics 2004;113:1549–58 [DOI] [PubMed] [Google Scholar]
- 92.Koscik RL, Lai HCJ, Laxova A, Zaremba KM, Kosorok MR, Douglas JA, Rock MJ, Splaingard ML, Farrell PM. Preventing early, prolonged vitamin E deficiency: an opportunity for better cognitive outcomes via early diagnosis through neonatal screening. J Pediatr 2005;147:S51–6 [DOI] [PubMed] [Google Scholar]
- 93.Dewey KG, Begum K. Long-term consequences of stunting in early life. Matern Child Nutr 2011;7 Suppl 3:5–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fares S, Sethom MM, Khouaja-Mokrani C, Jabnoun S, Feki M, Kaabachi N. Vitamin A, E. , and D deficiencies in Tunisian very low birth weight neonates: prevalence and risk factors. Pediatr Neonatol 2014;55(3):196–201 [DOI] [PubMed] [Google Scholar]
- 95.Weissgerber TL, Gandley RE, McGee PL, Spong CY, Myatt L, Leveno KJ, Thorp JM, Jr, Mercer BM, Peaceman AM, Ramin SM, et al. Haptoglobin phenotype, preeclampsia risk and the efficacy of vitamin C and E supplementation to prevent preeclampsia in a racially diverse population. PLoS ONE 2013;8:e60479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weissgerber TL, Gandley RE, Roberts JM, Patterson CC, Holmes VA, Young IS, McCance DR. Haptoglobin phenotype, pre-eclampsia, and response to supplementation with vitamins C and E in pregnant women with type-1 diabetes. BJOG 2013;120:1192–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Basaran A, Basaran M, Topatan B. Combined vitamin C and E supplementation for the prevention of preeclampsia: a systematic review and meta-analysis. Obstet Gynecol Surv 2010;65:653–67 [DOI] [PubMed] [Google Scholar]
- 98.Beazley D, Ahokas R, Livingston J, Griggs M, Sibai BM. Vitamin C and E supplementation in women at high risk for preeclampsia: a double-blind, placebo-controlled trial. Am J Obstet Gynecol 2005;192:520–1 [DOI] [PubMed] [Google Scholar]
- 99.Poston L, Briley AL, Seed PT, Kelly FJ, Shennan AH; Vitamins in Pre-eclampsia Trial Consortium. Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): randomised placebo-controlled trial. Lancet 2006;367:1145–54 [DOI] [PubMed] [Google Scholar]
- 100.Fawzi WW, Msamanga GI, Spiegelman D, Urassa EJ, McGrath N, Mwakagile D, Antelman G, Mbise R, Herrera G, Kapiga S, et al. Randomised trial of effects of vitamin supplements on pregnancy outcomes and T cell counts in HIV-1-infected women in Tanzania. Lancet 1998;351:1477–82 [DOI] [PubMed] [Google Scholar]
- 101.Kawai K, Kupka R, Mugusi F, Aboud S, Okuma J, Villamor E, Spiegelman D, Fawzi WW. A randomized trial to determine the optimal dosage of multivitamin supplements to reduce adverse pregnancy outcomes among HIV-infected women in Tanzania. Am J Clin Nutr 2010;91:391–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hauth JC, Clifton RG, Roberts JM, Spong CY, Myatt L, Leveno KJ, Pearson GD, Varner MW, Thorp JM, Jr, Mercer BM, et al. Vitamin C and E supplementation to prevent spontaneous preterm birth: a randomized controlled trial. Obstet Gynecol 2010;116:653–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bártfai L, Bartfai Z, Nedeczky I, Puho EH, Banhidy F, Czeizel AE. Rate of preterm birth in pregnant women with vitamin E treatment: a population-based study. J Matern Fetal Neonatal Med 2012;25:575–80 [DOI] [PubMed] [Google Scholar]
- 104.Dysken MW, Sano M, Asthana S, Vertrees JE, Pallaki M, Llorente M, Love S, Schellenberg GD, McCarten JR, Malphurs J, et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM-AD VA cooperative randomized trial. JAMA 2014;311:33–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, Woodbury P, Growdon J, Cotman DW, Pfeiffer E, et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer's disease. N Engl J Med 1997;336:1216–22 [DOI] [PubMed] [Google Scholar]
- 106.Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, Galasko D, Jin S, Kaye J, Levey A, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med 2005;352:2379–88 [DOI] [PubMed] [Google Scholar]
- 107.Lopes da Silva S, Vellas B, Elemans S, Luchsinger J, Kamphuis P, Yaffe K, Sijben J, Groenendijk M, Stijnen T. Plasma nutrient status of patients with Alzheimer's disease: systematic review and meta-analysis. Alzheimers Dement 2013; [DOI] [PubMed] [Google Scholar]
- 108.Hensley K, Barnes LL, Christov A, Tangney C, Honer WG, Schneider JA, Bennett DA, Morris MC. Analysis of postmortem ventricular cerebrospinal fluid from patients with and without dementia indicates association of vitamin E with neuritic plaques and specific measures of cognitive performance. J Alzheimers Dis 2011;24:767–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mangialasche F, Xu W, Kivipelto M, Costanzi E, Ercolani S, Pigliautile M, Cecchetti R, Baglioni M, Simmons A, Soininen H, et al. Tocopherols and tocotrienols plasma levels are associated with cognitive impairment. Neurobiol Aging 2012;33:2282–90 [DOI] [PubMed] [Google Scholar]
- 110.Fukui K, Kawakami H, Honjo T, Ogasawara R, Takatsu H, Shinkai T, Koike T, Urano S. Vitamin E deficiency induces axonal degeneration in mouse hippocampal neurons. J Nutr Sci Vitaminol (Tokyo) 2012;58:377–83 [DOI] [PubMed] [Google Scholar]
- 111.Ulatowski L, Parker R, Warrier G, Sultana R, Butterfield DA, Manor D. Vitamin E is essential for Purkinje neuron integrity. Neuroscience 2014;260:120–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Desrumaux C, Pisoni A, Meunier J, Deckert V, Athias A, Perrier V, Villard V, Lagrost L, Verdier JM, Maurice T. Increased amyloid-beta peptide-induced memory deficits in phospholipid transfer protein (PLTP) gene knockout mice. Neuropsychopharmacology 2013;38:817–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bowman GL, Silbert LC, Howieson D, Dodge HH, Traber MG, Frei B, Kaye JA, Shannon J, Quinn JF. Nutrient biomarker patterns, cognitive function, and MRI measures of brain aging. Neurology 2012;78:241–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Butterfield DA, Bader Lange ML, Sultana R. Involvements of the lipid peroxidation product, HNE, in the pathogenesis and progression of Alzheimer's disease. Biochim Biophys Acta 2010;1801:924–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mapstone M, Cheema AK, Fiandaca MS, Zhong X, Mhyre TR, Macarthur LH, Hall WJ, Fisher SG, Peterson DR, Haley JM, et al. Plasma phospholipids identify antecedent memory impairment in older adults. Nat Med 2014;20(4):415–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schaefer EJ, Bongard V, Beiser AS, Lamon-Fava S, Robins SJ, Au R, Tucker KL, Kyle DJ, Wilson PW, Wolf PA. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: the Framingham Heart Study. Arch Neurol 2006;63:1545–50 [DOI] [PubMed] [Google Scholar]
- 117.Burton GW, Traber MG, Acuff RV, Walters DN, Kayden H, Hughes L, Ingold KU. Human plasma and tissue alpha-tocopherol concentrations in response to supplementation with deuterated natural and synthetic vitamin E. Am J Clin Nutr 1998;67:669–84 [DOI] [PubMed] [Google Scholar]
- 118.Traber MG, Shimoda K, Murakami K, Leonard SW, Enkhbaatar P, Traber LD, Traber DL. Burn and smoke inhalation injury in sheep depletes vitamin E: kinetic studies using deuterated tocopherols. Free Radic Biol Med 2007;42:1421–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Traber MG, Rader D, Acuff RV, Ramakrishnan R, Brewer HB, Kayden HJ. Vitamin E dose-response studies in humans with use of deuterated RRR-alpha-tocopherol. Am J Clin Nutr 1998;68:847–53 [DOI] [PubMed] [Google Scholar]
- 120.Hodge AM, Simpson JA, Fridman M, Rowley K, English DR, Giles GG, Su Q, O'Dea K. Evaluation of an FFQ for assessment of antioxidant intake using plasma biomarkers in an ethnically diverse population. Public Health Nutr 2009;12:2438–47 [DOI] [PubMed] [Google Scholar]
- 121.Talegawkar SA, Johnson EJ, Carithers T, Taylor HA, Jr, Bogle ML, Tucker KL. Total alpha-tocopherol intakes are associated with serum alpha-tocopherol concentrations in African American adults. J Nutr 2007;137:2297–303 [DOI] [PubMed] [Google Scholar]
- 122.Depner CM, Traber MG, Bobe G, Kensicki E, Bohren KM, Milne G, Jump DB. A metabolomic analysis of omega-3 fatty acid-mediated attenuation of western diet-induced nonalcoholic steatohepatitis in LDLR−/− mice. PLoS ONE 2013;8:e83756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Roberts LJ Jr, Oates JA, Linton MF, Fazio S, Meador BP, Gross MD, Shyr Y, Morrow JD. The relationship between dose of vitamin E and suppression of oxidative stress in humans. Free Radic Biol Med 2007;43:1388–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dimitrov NV, Meyer C, Gilliland D, Ruppenthal M, Chenoweth W, Malone W. Plasma tocopherol concentrations in response to supplemental vitamin E. Am J Clin Nutr 1991;53:723–9 [DOI] [PubMed] [Google Scholar]
- 125.Jialal I, Fuller CJ, Huet BA. The effect of alpha-tocopherol supplementation on LDL oxidation: a dose-response study. Arterioscler Thromb Vasc Biol 1995;15:190–8 [DOI] [PubMed] [Google Scholar]
- 126.Devaraj S, Adams-Huet B, Fuller CJ, Jialal I. Dose-response comparison of RRR-alpha-tocopherol and all-racemic alpha-tocopherol on LDL oxidation. Arterioscler Thromb Vasc Biol 1997;17:2273–9 [DOI] [PubMed] [Google Scholar]
- 127.Wright ME, Lawson KA, Weinstein SJ, Pietinen P, Taylor PR, Virtamo J, Albanes D. Higher baseline serum vitamin E concentrations are associated with lower total and cause-specific mortality in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Am J Clin Nutr 2006;84:1200–7 [DOI] [PubMed] [Google Scholar]
- 128.Becker K, Botticher D, Leichsenring M. Antioxidant vitamins in malnourished Nigerian children. Int J Vitam Nutr Res 1994;64:306–10 [PubMed] [Google Scholar]
- 129.Shen YM, Wu JF, Hsu HY, Ni YH, Chang MH, Liu YW, Lai HS, Hsu WM, Weng HL, Chen HL. Oral absorbable fat-soluble vitamin formulation in pediatric patients with cholestasis. J Pediatr Gastroenterol Nutr 2012;55:587–91 [DOI] [PubMed] [Google Scholar]
- 130.Behera S, Dixit S, Bulliyya G, Kar SK. Fat-soluble antioxidant vitamins, Iron overload and chronic malnutrition in beta-Thalassemia Major. Indian J Pediatr 2014;81:270–4 [DOI] [PubMed] [Google Scholar]
- 131.Drewel BT, Giraud DW, Davy SR, Driskell JA. Less than adequate vitamin E status observed in a group of preschool boys and girls living in the United States. J Nutr Biochem 2006;17:132–8 [DOI] [PubMed] [Google Scholar]
- 132.Adelekan DA, Thurnham DI, Adeodu OO, Ojofeitimi EO. Plasma ferritin concentration in relation to vitamin A and E status of children with severe oedematous malnutrition. Ann Trop Paediatr 1991;11:175–80 [DOI] [PubMed] [Google Scholar]
- 133.Bell EF, Hansen NI, Brion LP, Ehrenkranz RA, Kennedy KA, Walsh MC, Shankaran S, Acarregui MJ, Johnson KJ, Hale EC, et al. Serum tocopherol levels in very preterm infants after a single dose of vitamin E at birth. Pediatrics 2013;132(6):e1626–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mai TtT, Hung NtK, Kawakami M, Kawase M, van Chuyen N. Micronutrient status of primary school girls in rural and urban areas of South Vietnam. Asia Pac J Clin Nutr 2003;12(2):178–85 [PubMed] [Google Scholar]
- 135.Laditan AA, Ette SI. Plasma alpha-tocopherol (vitamin E) levels and tocopherol-lipid ratio among children with protein-energy malnutrition (PEM). Ann Trop Paediatr 1982;2:85–8 [DOI] [PubMed] [Google Scholar]
- 136.Allen LH, Rosado JL, Casterline JE, Lopez P, Munoz E, Garcia OP, Martinez H. Lack of hemoglobin response to iron supplementation in anemic mexican preschoolers with multiple micronutrient deficiencies. Am J Clin Nutr 2000;71:1485–94 [DOI] [PubMed] [Google Scholar]
- 137.Metzger A, Mukasa G, Shankar AH, Ndeezi G, Melikian G, Semba RD. Antioxidant status and acute malaria in children in Kampala, Uganda. Am J Trop Med Hyg 2001;65:115–9 [DOI] [PubMed] [Google Scholar]
- 138.Ahmed HM, Laryea MD, el-Karib AO, el-Amin EO, Biggemann B, Leichsenring M, Mayatepek E, Bremer HJ. Vitamin E status in Sudanese children with protein-energy malnutrition. Z Ernahrungswiss 1990;29:47–53 [DOI] [PubMed] [Google Scholar]
- 139.Fazio-Tirrozzo G, Brabin L, Brabin B, Agbaje O, Harper G, Broadhead R. A community based study of vitamin A and vitamin E status of adolescent girls living in the Shire Valley, Southern Malawi. Eur J Clin Nutr 1998;52:637–42 [DOI] [PubMed] [Google Scholar]
- 140.Ashour MN, Salem SI, El-Gadban HM, Elwan NM, Basu TK. Antioxidant status in children with protein-energy malnutrition (PEM) living in Cairo, Egypt. Eur J Clin Nutr 1999;53:669–73 [DOI] [PubMed] [Google Scholar]
- 141.Nasr MR, Ali S, Shaker M, Elgabry E. Antioxidant micronutrients in children with thalassaemia in Egypt. East Mediterr Health J 2002;8:490–5 [PubMed] [Google Scholar]
- 142.Griffiths MJ, Ndungu F, Baird KL, Muller DP, Marsh K, Newton CR. Oxidative stress and erythrocyte damage in Kenyan children with severe Plasmodium falciparum malaria. Br J Haematol 2001;113:486–91 [DOI] [PubMed] [Google Scholar]
- 143.López G, Galvan M. [Relation of total cholesterol in serum tocopherols, probabilistic study in Mexican children.] Arch Latinoam Nutr 2011;61:127–34 [PubMed] [Google Scholar]
- 144.Walter PB, Fung EB, Killilea DW, Jiang Q, Hudes M, Madden J, Porter J, Evans P, Vichinsky E, Harmatz P. Oxidative stress and inflammation in iron-overloaded patients with beta-thalassaemia or sickle cell disease. Br J Haematol 2006;135:254–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Stephensen CB, Marquis GS, Jacob RA, Kruzich LA, Douglas SD, Wilson CM. Vitamins C and E in adolescents and young adults with HIV infection. Am J Clin Nutr 2006;83:870–9 [DOI] [PubMed] [Google Scholar]
- 146.Martín-Gallán P, Carrascosa A, Gussinye M, Dominguez C. Estimation of lipoperoxidative damage and antioxidant status in diabetic children: relationship with individual antioxidants. Free Radic Res 2005;39:933–42 [DOI] [PubMed] [Google Scholar]