Abstract
Reliable information about the nutritional status is essential to identify potential critical nutrients and the population groups at risk of deficiency, as well as to develop effective public health policies to counteract unfavorable nutrition patterns that contribute to morbidity and mortality. In this review, the important role of biomarkers in the assessment of nutritional status is outlined, major strengths and limitations of established and new biomarkers are described, and important criteria for biomarker selection and development are discussed. Indeed, biomarkers offer a more objective assessment tool than pure dietary approaches that suffer from inadequate data reporting in particular, although biomarkers are often only measured in subsamples because of the higher costs and proband burden they entail. However, biomarkers are subject to individual variability and influences from other factors besides the nutrient of interest. Rapid turnover or tight control of nutrient concentrations in blood (homeostasis) limits their sensitivity as biomarkers, as in the case of many trace elements. The existence of different forms of a micronutrient in the body adds additional complexity. Functional biomarkers, such as enzyme activities, mirror long-term status better but are subject to confounding factors, and some are influenced by several micronutrients, not specific for only 1, so using a combination of biomarkers is advisable. Additionally, the applicability of a biomarker also depends on the existence of adequate reference values and cutoff points for the target population. Therefore, a careful selection is warranted, especially when biomarkers are to be used in larger samples.
Introduction
Nutritional status assessment provides the data necessary to study the effects of nutrition on health and disease, to identify critical nutrients in a specific population and the groups within this collective that are at risk of deficiency, and to develop effective public health policies to prevent and cure nutrition-related diseases. Indeed, diet-related noncommunicable diseases are the most common cause of death worldwide and are associated with obesity and excessive intakes of SFAs and/or free sugars (1). Knowledge of the nutritional status is also required for the formulation of recommendations for nutrient intake (2).
Data on nutritional status at the population level are mostly obtained from epidemiologic surveys that often enable only a rough estimation of the true situation, particularly as a result of misreporting by the study participants and errors in quantification (3). Therefore, the validity of nutritional status assessment is greatly improved by biochemical analyses using nutritional biomarkers. However, in light of the complex influences on nutrient metabolism, they have to be selected carefully (4).
This review presents the current fields of application of biomarkers in the assessment of nutritional status and how they can contribute to the improvement of epidemiologic approaches. The strengths and limitations of some established and new biomarkers are described, and important criteria for biomarker selection and development are discussed.
Current State of Knowledge
Assessment of body weight and the importance of body composition.
Body weight and composition mirror the adequacy of energy intake. BMI is generally considered a versatile tool to assess body weight, especially in light of its easy applicability in the field. However, the use of self-reported data, although they facilitate the assessment of large samples, are often associated with underestimation of body weight and overestimation of height. Thus, data measured under standardized conditions should be given as much preference as possible (5).
Moreover, BMI provides no information on body composition and the relative amounts of lean and fat mass. This can lead to misinterpretations in individuals with a high or low muscle mass, as well as in the general population, especially for those with intermediate BMI ranges in which fat mass is higher than average (6).
Thus, a study in the United States found that 30% of the male and 46% of the female participants were classified as obese according to their body fat content, although their BMI was <30 kg/m2 (7).
The limits of the BMI to detect obesity were also evidenced by the Austrian Nutrition Report 2012 (8). The prevalence of obesity assessed by BMI (≥30 kg/m2) was 9.7% in adult women and 14.9% in men, whereas it was 14.9% and 39.7%, respectively, when taking measured body fat content as a criterion. Similarly, among elderly adults (aged 65–80 y), 37.2% of women and 27.5% of men had a BMI considered overweight (24–29.99 kg/m2), whereas body fat content was too high (i.e., >23% in men and >35% in women) in 62.4% and 54.5%, respectively. In children, the discrepancy between obesity prevalence assessed by BMI and fat content was particularly seen in boys, in which increases in lean body mass contributed more to body weight increases than in girls.
A commonly used approach to estimate body fat mass is the measurement of waist circumference (WC)4. Indeed, the positive correlation between WC and cardiovascular mortality, all-cause mortality, and the risk of cardiometabolic diseases is scientifically well established and is a diagnostic variable for the metabolic syndrome. However, there is a variety of measurement protocols using different body sites (9). The currently used values are based on an increasing cardiometabolic risk associated with a WC >80 cm in women and >94 cm in men and such a high risk of a WC >88 cm in women and >102 cm in men found in a European population from The Netherlands. WC was measured midway between the lowest rib and the iliac crest (10–12). However, a meta-analysis of 120 studies suggested that the relation with disease risk appears to be given regardless of measurement site (13). In turn, a strong ethnic effect on the amount and distribution of body fat was observed. This is especially true for individuals of Asian descent who, at a given BMI and WC, have higher body fat contents and a higher cardiometabolic risk than whites despite a certain variability between different Asian populations (14, 15).
Conversely, in African Americans, visceral adipose tissue is on average lower than in white and Hispanic individuals of the same BMI and WC, but to date, there is no clear evidence of an effect on cardiovascular risk (16). These findings stress the need for defining race-specific cutoff points for WC and BMI. Suggestions were already made for Asian populations. Thus, the WHO defined BMI categories associated with an increased cardiometabolic risk (23–27.5 kg/m2) and a high cardiometabolic risk (≥27.5 kg/m2) for Asian populations (14). Besides, the International Diabetes Federation using WC as a diagnostic criterion for the metabolic syndrome gives separate values for Asians (90 cm in Asian men instead of 94 cm in white men, but 80 cm is valid for both white and Asian women). Both entities acknowledge the possibility of an additional categorization for different Asian subpopulations, such as Japanese and East and South Asians (17).
Excessive adipose tissue in normal-weight individuals was described previously, especially in women, and is associated with an unfavorable metabolic profile comparable with classical obesity. Thus, assessment of body composition is also important in light of the emerging role of visceral adipose tissue, in particular in the development of noncommunicable diseases such as type 2 diabetes mellitus (18, 19).
Although WC allows a reasonable estimate of body fat content, it suffers from a high individual variability. The problem with more accurate laboratory methods is their high technical, operational, and financial expenses, as well as the participant burden. The latter is particularly true for hydrodensitometry or underwater weighing, which is still considered 1 of the gold standards in the evaluation of body composition.
A widely used technology is the biologic impedance analysis based on the differences in the electrical conductivity of different body tissues. The required equipment is inexpensive, easy to use, and transportable, making it suitable for field applications. However, inaccuracies can arise from variations in body hydration and as a result of ethnic-, age-, and gender-related differences. Recently, DXA and air displacement plethysmography gained popularity in body composition assessment. Despite their reliance on assumptions about the density of lean and fat body tissues and tissue hydration, they show a good accuracy, but they are not widely available because of the cost of the equipment and because it is not easily transportable (20, 21).
Currently, there is still a need for accurate methods to assess body composition that are at the same time inexpensive and applicable with minimal effort on large samples in nutrition surveys.
Assessing the intake of food and food components.
Information on the supply of food and nutrients and other food components is commonly obtained through dietary assessment. At the population level, statistical databases, such as the food balance sheets of the FAO or national household budget survey data, provide a rough overview of nutritional supply, but more detailed information on the actual food intake can only be gained from dietary surveys (22, 23). These methods are used for the compilation of multinational reports describing the nutritional situation, thus providing a basis for intervention policies, such as the European Nutrition and Health Report 2004 and 2009 (23, 24) and Comparative Analysis of Food and Nutrition Policies in WHO European Member States, 2003 (25).
Comparable with self-reported anthropometric data, dietary reports are compromised by misreporting, whether inadvertently or deliberately (26).
The heavy impact of misreporting on the validity of energy intake assessment in the frame of the NHANES was outlined recently. Based on the ratio of the reported energy intake to the estimated basal metabolic rate and the comparison of the reported energy intake to the estimated total energy expenditure (TEE), it was concluded that, across all NHANES survey periods, less than half of the population provided plausible energy intake amounts. Part of this seems due to methodologic causes, as supported by a temporary increase in plausible reports after methodologic improvements. However, considering that underreporting was more pronounced in women than in men and in overweight and obese than in normal-weight individuals, deliberate misreporting by the participants seriously weakens the validity of nutritional surveillance data (3).
In line with the previously cited report, in obese individuals, a selective underreporting of fat intake was observed, whereas normal-weight individuals also underreported healthy diets and foods, such as fruit, vegetables, and unsaturated FAs (26, 27).
Misreporting was observed across all age groups and in both males and females. Especially in the elderly, it is important to discern underreporters from true undereaters because food intake often declines with age. Thus, based on weight change, Shahar et al. (28) identified 14% of a sample of 296 elderly individuals (aged 70–79 y) as undereaters (13% of women, 16% of men).
In children, especially at younger ages, overreporting is more common than in adults. Younger children are also less prone to selective misreporting of foods with a special social value, such as sugar- or fat-rich foods that are often underreported and fruits and vegetables that are overreported. In turn, reporting of food intake by the parents generally results in greater accuracy at least for children of normal weight (29).
The extent of underreporting varies between studies, most often ranging from 10% to 20% for energy intake in adults with a tendency toward slightly higher deviations among women than men (30). In the OPEN (Observing Protein and Energy Nutrition) Study, 12–14% of male participants underreported their energy intake in 24-h recalls compared with 16–20% of their female counterparts (31). Furthermore, it is generally more pronounced among overweight and especially obese individuals: in the OPEN Study, men and women with a BMI ≥ 30 kg/m2 underreported their energy intake on average by 16% and 20%, respectively, compared with 7% and 8%, respectively, for nonobese participants (32).
Misreporting arising from inadvertent omission of food items and errors in portion size estimation can be minimized by the use of food models, pictures, or modern information and communication technologies, which allow a more direct and easier recording of the food consumed. Examples include computer-assisted recording of foods and the taking of pictures of the meals with a mobile phone. However, these techniques do not prevent deliberate misreporting (33).
Energy and, to a lesser extent, protein intake are the most versatile markers to identify misreporters. By comparing reported energy intake to the individuals’ measured or estimated TEE, implausibly low or high intake amounts can be discerned. A common approach is the use of the Goldberg cutoffs set within the 95% CI of the TEE based on the formula by Goldberg and colleagues. However, this requires knowledge or at least a good estimate of the physical activity level of the target population because TEE can vary widely (34).
Moreover, defining misreporting based on energy intake might not catch inaccuracies in the assessment of certain nutrients, especially in the case of selective misreporting (30).
The fact that especially large surveys often rely on FFQs to collect data on food consumption can further compromise the accuracy of epidemiologic data on food and nutrient intake considering the limited power of FFQs for quantitative intake evaluation.
When an FFQ is used to assess the food and nutrient intake as part of the nutritional status assessment, it has to be validated against a more precise method. Dietary approaches to validation are less expensive and more widely applicable, which allows for larger subsample sizes. Although dietary records, particularly weighed ones, and 24-h recalls are generally more accurate than FFQs, the measurement errors of these assessment methods are not entirely independent from those of the FFQ, limiting their suitability as reference methods. Thus, 24-h recalls like the FFQ relies on the participant’s memory (35).
This makes validation of an FFQ against established biomarkers preferable to dietary methods (4, 36, 37). Ideally, dietary and biochemical reference methods should be combined because biochemical methods are independent from dietary ones, which allows for a more objective evaluation (38).
As an example, this was applied in the development of a diet quality index in elderly adults based on an FFQ. In this study, higher plasma concentrations of carotenoids were significantly associated with a higher quality index mirroring a higher intake of fruits and vegetables (39).
Moreover, the FFQ has to be adapted for the target group and the purpose of the study. This influences the choice and amount of food items included, as well as the frequency categories and the portion sizes to be selected by the respondent. An existing FFQ can help in the design of a new one but should be revised, updated, validated by using adequate biomarkers, and pretested on the target population (35).
An additional constraint arises from the scarcity of good biomarkers, especially for macronutrient and energy intake. Again, comparing the reported or estimated intake with the TEE is an option. TEE, in turn, is optimally determined through the double-labeled water method, although the high costs and technical requirements of this method limit its applicability (40).
Recently, urinary excretion of sucrose and fructose was proposed as a biomarker for sugar intake based on the fact that small amounts of these saccharides escape metabolism (41).
Dietary assessment also requires comprehensive food composition databases. Their improvement and harmonization is the aim of the International Network of Food Data Systems initiated by the UN in 1984, under the coordination of the FAO of the UN since 1999 and a task force of the International Union of Nutritional Sciences (42). Particularly, data on dietary fiber, trace elements, bioactive plant components, and fortified foods, as well as the composition of traditional and indigenous foods, are still insufficient (43, 44).
Moreover, the composition of natural foods shows a wide variability related to geographical and weather conditions, cultivation techniques, and crop varieties. In prepared foods, differences in recipes add additional complexity (45).
With regards to assessing the nutritional status of individuals, additional difficulties arise from the fact that the reference values for nutrient intake do not necessarily correspond to one’s individual nutritional needs. In fact, most of these values, such as the RDA for the U.S. population, the reference nutrient intake for the UK population, and the reference values for nutrient intake (Zufuhrempfehlungen) of the German-speaking countries, are based on the estimated median requirement (estimated average requirement) of a given collective of healthy individuals to which the equivalent of 2 SDs is added. For nutrients for which requirements are not normally distributed within a population, the SD is replaced by a CV of 20–30%. In this way, the requirements of 97.5% of the population are covered, but an inadequate supply would be expected in 2.5% of the population (2, 46, 47). In this case, but also at population level, more accurate results require the use of biochemical markers of nutrient status.
Nutritional biomarkers: criteria for development and selection.
A nutritional biomarker is a biochemical indicator of intake and/or status of a given nutrient or food component. Status markers are direct markers of past exposure. In turn, functional markers reflect an effect of a nutrient or its absence. As such, some of them can also act as intermediate markers for future disease risk (36, 37).
The development of a nutritional biomarker for a specific nutrient is in most cases based on what is known about the chemistry, absorption, distribution in the body, and metabolism of the latter.
With recent advances in metabolomic techniques enabling the simultaneous measurement of several analytes and large sample amounts, the search for biomarkers can also follow a more inductive approach in that metabolites found in the sample are examined for their suitability as biomarkers (48).
The search for nutritional biomarkers requires well-controlled dietary intervention designs to minimize potential confounding factors and a careful validation of the candidate markers, especially regarding dose–response effects and their specificity, sensitivity, and suitability for various population subgroups (37).
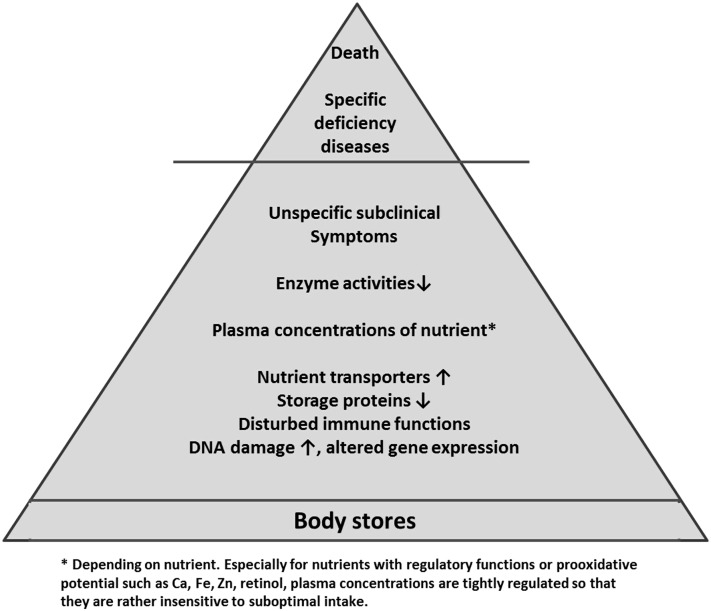
However, although biomarkers allow a more objective assessment of nutrient status, the fact that the effects of dietary compounds on body functions are generally more subtle and less clearly delimitable than those seen after drug administration makes them less efficient than biomarkers used in drug trials. For instance, marginal deficiency states are generally not associated with manifest clinical symptoms, which makes their detection much more challenging than that of a single drug effect. Indeed, the absence of severe deficiency signs does not exclude detrimental effects on the body, underscoring the importance of early diagnosis (49, 50). This hierarchy of effects of nutrient deficiency on the body is depicted in Figure 1.
FIGURE 1.
Hierarchy of biomarkers of the nutrient status of the body. Ca, calcium; Fe, iron; Zn, zinc.
Moreover, testing biomarkers for essential nutrients limits the possibilities to include a nonexposed human control group in randomized controlled intervention trials. This leaves testing the effects of supplementation with a nutrient on the candidate biomarker as the approach of choice (51, 52). However, in many cases, effects are observed in depleted but much less in adequately supplied individuals, reducing the value of the biomarker to detect marginal status.
The choice of biomarker is strongly influenced by the study objectives. For large-scale nutrition surveys, factors such as cost, technical and personnel requirements, feasibility, and participants’ burden are more important determinants than in clinical intervention studies with smaller sample sizes.
Measuring nutrient concentrations in blood or urine is a widely used method for status assessment because these samples are easy obtained. However, blood concentrations of many nutrients, especially those involved in regulatory mechanisms such as calcium, zinc, and retinol, are maintained within narrow ranges regardless of body stores (53, 54). Changes in blood concentrations of such nutrients only occur in progressed/severe deficiency states. In other cases, like for carotenoids, they can vary markedly because of a short half-life and depending on recent intake (55). Moreover, conditions such as acute infections or stress can influence serum concentrations of some nutrients, such as zinc (56). Concentrations in other tissues, such as cell membranes, fat tissue, or bone, fluctuate less, which gives a better view of long-term supply. Unfortunately, some of these samples are difficult to obtain. For the assessment of the status of long-chain PUFAs, the concentration in membrane phospholipids measured in erythrocytes or whole blood were identified as better biomarkers of long-term exposure than plasma concentrations (57, 58).
Moreover, suboptimal nutrient intake over a longer time can affect specific physiologic functions, such as activities of enzymes with nutrients as cofactors, which can serve as functional biomarkers (49).
An overview of plasma concentrations and examples of functional biomarkers for some micronutrients is given in Table 1.
TABLE 1.
Overview of the plasma concentrations and examples of functional biomarkers (nonexhaustive) for selected micronutrients1
| Plasma concentration cutoffs for status assessment | Functional biomarkers | Clinical signs of deficiency |
| Vitamin A | ||
| >1.05 μmol/L, normal | Retinol-binding protein | Night blindness, xerophthalmia, anemia |
| <0.7 μmol/L, markedly deficient | ||
| Vitamin D [as 25(OH)D3] | ||
| >50 nmol/L, desirable | Alkaline phosphatase activity; serum osteocalcin | Decline of bone density, rickets in children, osteomalacia in adults, disturbed immune function |
| <25 nmol/L, deficient | ||
| Vitamin B-6 (as pyridoxal phosphate) | ||
| >30 nmol/L, normal | α-EGOT activity | Rare; neurologic symptoms, seborrhea, dermatitis, eczema, cheilosis, anemia (microcytic, hypochromic) |
| <20 nmol/L, deficient | ||
| Folate (as folic acid) | ||
| ≥13.4 nmol/L, normal | HCys, urinary excretion of FIGLU | Anemia (macrocytic, hyperchromic), cheilosis, glossitis, neurologic symptoms in elderly, higher risk of cardiovascular diseases (through HCys) |
| <6.8 nmol/L, markedly deficient | ||
| In erythrocytes: | ||
| ≥356 nmol/L, normal | ||
| <317 nmol/L, deficient | ||
| Vitamin B-12 | ||
| ≥147 pmol/L, normal | HCys, methylmalonic acid | Anemia (macrocytic, hyperchromic), neurologic symptoms |
| <110 nmol/L, markedly deficient | ||
| Calcium | ||
| Excretion in urine, 2.5–6.0 mmol/d, normal | Excretion of hydroxyproline in urine | Decline of bone density, osteoporosis, rickets in children, osteomalacia in adults |
| Iron | ||
| Ferritin ≥15 μg/L, normal | Hemoglobin, hematocrit, total iron binding capacity | Anemia (microcytic, hypochromic) |
| ≥12 μg/L (in children aged <5 y), normal | ||
| Zinc | ||
| Adults | Activity of zinc superoxide dismutase, zinc binding capacity in thalassemia | Growth retardation in children, disturbed immune function (especially cellular), reduced glucose tolerance, skin lesions, impaired wound healing |
| 13–19 μmol/L, normal | ||
| <11.5 μmol/L, deficient | ||
| Children | ||
| <10 y: <9.9 μmol/L, deficient | ||
| Pregnant women | ||
| Trimester 1: <8.6 μmol/L, deficient | ||
| Trimester 2: 7.6 μmol/L, deficient |
Biomarkers complement dietary assessment.
In the Austrian Nutrition Report 2012 (59), activity of erythrocyte glutamic oxaloacetic transaminase, a functional marker of vitamin B-6 status, better reflected the intake of this nutrient than the plasma concentration of pyridoxal phosphate, especially in adults younger than 65 y. Plasma concentrations were adequate in >80% of adults and elderly and 99% of children, whereas intake was below the recommended amounts in 18% of male and 32% of female children, ∼40% of adults, and approximately half of the elderly. In turn, erythrocyte glutamic oxaloacetic transaminase activity suggested a deficient status in ∼40% of children and adult men, 55% of adult women, and 22% and 26% of elderly women and men, respectively.
The importance of biomarkers for status assessment was also seen for folate in the Austrian Nutrition Report 2012, a national nutrition survey conducted in a representative sample of 1002 individuals from various age groups (59). Although 94–100% of the participants across all age groups did not reach the then-recommended intake value of 400 μg/d, 70–80% of children and adults and almost 70% of the elderly had adequate plasma concentrations of this vitamin. Homocysteine concentrations were higher in individuals with lower folic acid plasma concentrations, confirming the suitability of this metabolite as a biomarker for folate, although it is also associated with vitamin B-12 and vitamin B-6 (59).
Comparable discrepancies between the status assessed from dietary intake and the biochemical status were also reported from other countries. Thus, although according to the NHANES the majority of the U.S. population does not meet the estimated average requirement, the biochemical status based on serum α-tocopherol was deficient in <1% in the Second National Report on Biochemical Indicators of Diet and Nutrition in the U.S. Population 2012 (60). As discussed above, a possible explanation lies in the selective underreporting of fat-rich food that is the major source of vitamin E.
The Importance of Reference Ranges
The correct interpretation of biomarkers requires well-defined reference values for the respective marker. However, in many cases, there is still a lack of consensus on the normal range in healthy adequately supplied individuals. An example that received much attention recently is vitamin D. Although serum concentration of 25-hydroxy vitamin D3 [25(OH)D3] is widely acknowledged as a valid biomarker for vitamin D status that reflects dietary intake and endogenous synthesis, the optimal range of this variable is still the matter of debate. Cutoffs for deficiency are generally set between 25 and 50 nmol/L (10–20 ng/dL) based on the effects on calcium and phosphate metabolism and bone health. However, with the emergence of new physiologic roles for vitamin D, questions about the optimal range arose. Although some evidence exists for associations between 25(OH)D3 serum concentrations and the risk of cardiovascular and other metabolic and autoimmune diseases, benefits from higher concentrations in the healthy general population were not shown unequivocally (61, 62). Furthermore, serum concentrations below the reference range are not necessarily associated with deficiency. Thus, African Americans have on average lower serum 25(OH)D3 concentrations than their white counterparts, but the prevalence of osteoporosis and the occurrence of fragility fractures are also lower (63). To define optimal ranges for serum 25(OH)D3 concentrations for different population groups, additional randomized controlled trials are warranted, taking into account ethnic differences and the influence of age-, gender-, and disease-related aspects (61, 62).
Correct reference ranges and validated sensitive and specific biomarkers also reduce the risk of measurement errors and misclassification that can negatively influence the interpretation of a study outcome.
The influence of genetic variability and differences between nutrient forms.
Not only in the case of vitamin D are biomarkers of nutritional status influenced by an individual’s genetic makeup. Genes involved in the absorption and metabolism of nutrients and other food components determine the concentrations of these compounds in various body tissues. This was shown for single nucleotide polymorphisms in genes involved in the absorption, transport, and metabolism of fat-soluble vitamins, such as genes for apoA, apoB, and apoE, as well as lipoprotein lipase, scavenger-receptor class B type I (SR-BI), and carotene oxygenases that were related to plasma concentrations of α- and γ-tocopherol, α- and β-carotene, lycopene, and β-cryptoxanthin and the response to their dietary intake (64, 65).
Genetic variations also cause differences in nutrient requirements, such as the C677T polymorphism in the methylene tetrahydrofolate reductase (MTHFR) gene, resulting in a less active enzyme and an association with a higher risk of neural tube defects and cardiovascular disease (66). Because MTHFR provides 5-methyl tetrahydrofolate as a methyl donor for the reconversion of homocysteine to methionine, the lower activity of the mutant MTHFR can be compensated by higher folate intake (66).
Another aspect to consider is the fact that nutrients occur in different forms in food and in the body. These isomers generally differ in their biologic activity, calling for conversion factors to define equivalents. Although a single form may reflect total body stores adequately [such as 25(OH)D3 for vitamin D], isoforms can differ in their effects. An example of this is vitamin E, because special functions of γ-tocopherol and the tocotrienols are emerging that are not observed with α-tocopherol, the major bioactive form (67). In some studies, γ-tocopherol proved to be a more potent anticancerogenic agent than α-tocopherol, an effect that may be due to its better ability to scavenge reactive nitrogen species. Being more hydrophilic than α-tocopherol, it exerts its antioxidant effects in a complimentary way in other cell compartments (67). Although studies are less abundant than for α-tocopherol, tocotrienols also showed positive effects on health and the prevention of diseases (68). So far, vitamin E status is generally assessed on the basis of α-tocopherol equivalents that comprise all vitamers as far as these are measured. With regards to the special properties of non-α-tocopherol forms, a better differentiation seems advisable.
New nutritional biomarkers: the potential role of immune functions and gene expression.
Advances in molecular biology extended our insights in the physiology of nutrients, thus offering new potential biomarkers of their status. Indeed, vitamins and minerals, but also FAs, especially PUFAs, and amino acids can influence gene expression and cell proliferation, and this has a particular impact on highly proliferating cells such as those of the immune system. Thus, it was reported that deficiency of trace elements, especially zinc and copper, was associated with reduced secretion of mediators, such as IL-2, IL-1β, and TNF-α, after mitogenic stimulation. Notably, a decrease of IL-2 secretion was already observed after marginal copper deficiency (56, 69). In turn, supplementation with zinc increased the stimulated secretion of IL-1β, IFN-γ, and TNF-α (70). However, although cytokines and other immune function markers may be sensitive markers of nutrient deficiency, they are rather unspecific (70).
In the case of zinc, recent studies identified some potential markers, such as changes in zinc absorption and expression of zinc transporters and the storage protein metallothionein (70, 71). It was reported that zinc absorption is more influenced by current zinc intake than by long-term intake. In fact, although total absorption increases with higher intakes, the opposite is seen for percentage absorption rate (72, 73). This makes nutrient transporters and storage proteins promising candidate biomarkers.
Because sufficient micronutrient supply, especially of antioxidants and compounds involved in DNA synthesis, is necessary for DNA stability, maintenance, and repair, chromosomal damage may also serve as a biomarker of nutritional status. Indeed, oxidative stress is a major cause of DNA damage, whereas folate provides methyl groups for the conversion of uracil to thymine and the maintenance of CpG methylation in DNA. In turn, minerals such as zinc, iron, and magnesium are cofactors or components of DNA repair enzymes. Correlations between markers of DNA damage, such as the formation of micronuclei and micronutrient status (folate, vitamin B-12, iron), were shown in humans (74, 75).
Another relation between nutrients and the genome is through epigenetic mechanisms, especially with the availability of nutrients that provide C1 units, needed for methylation. This was shown by Wolff et al. (76) who reported overexpression of the agouti gene and development of obesity, hyperinsulinemia, higher cancer susceptibility, and reduced life expectancy in mice exposed to methyl-deficient diets in utero. These changes were associated with altered methylation of the agouti gene (77). The Dutch Hunger Winter Study revealed epigenetic effects of nutrient status in humans as well: individuals whose mothers were exposed to famine during pregnancy had altered methylation patterns of regulative loci of candidate genes associated with metabolic and cardiovascular diseases compared with their siblings that developed under normal conditions (78).
With all these potential biomarkers, difficulties arise from the fact that immune functions and gene and protein expression are influenced by a multitude of nutrients, as well as other food components and factors not related to the diet so that alterations of these variables can in most cases not be directly associated with a single nutrient. However, they can be useful to corroborate suspected deficiency states and to evaluate the individual severity of the insufficiency.
Conclusions
Reliable assessment of nutritional status is essential for identifying nutritional issues and at-risk groups in a given population for the development of dietary intervention programs and for monitoring the efficiency of such interventions. Because the validity of data obtained from dietary approaches alone is rather low, nutritional surveys should be complemented by biochemical analyses using appropriate biomarkers. These latter are also required to validate dietary methods such as FFQs or dietary records. Recovery markers are best suited for this purpose, such as the double-labeled water method for the assessment of energy expenditure or urinary nitrogen and potassium excretion as biomarkers for protein and potassium intake, respectively. Biomarkers can also serve to estimate the intake of a food group. Thus, the plasma concentration of carotenoids was identified as a promising marker of fruit and vegetable consumption. However, the high costs of some of these markers, such as the double-labeled water method, limit their use, so that there is a need for biomarkers applicable to large sample sizes that are not overly expensive while still offering a high sensitivity and specificity. Although the determination in plasma or spot urine samples has a high practicability, the concentrations of many nutrients in these media show a high variability.
This underscores the interest in markers from other tissues or functional biomarkers showing less fluctuation.
The fact that functional markers of nutrient status are often also indicators of a higher risk of certain diseases, such as hyperhomocysteinemia and cardiovascular risk or DNA damage and cancer risk, adds to their value. New analytical techniques, especially at the molecular level, offer a multitude of potential biomarkers from the fields of immunology and gene expression. However, although these markers are very sensitive, they often lack specificity, so that a combination of several biomarkers is advisable to differentiate between micronutrients.
There is also a need to optimize the use of existing biomarkers especially by taking biologic/genetic variability into account. This also applies to reference ranges that can differ depending on age, gender, and ethnic background. In particular, there is a lack of specific reference values for children and the elderly.
Advances in analytical procedures especially at the molecular level will help in the development of new accurate and precise biomarkers that are inexpensive and easily applicable even on large samples at the population level.
Acknowledgments
Both authors read and approved the final manuscript.
Footnotes
Abbreviations used: MTHFR, methylene tetrahydrofolate reductase; SR-BI, scavenger-receptor class B type I; TEE, total energy expenditure; WC, waist circumference; 25(OH)D3, 25-hydroxy vitamin D3.
References
- 1.World Health Organization. Global status report on noncommunicable diseases 2010. Geneva: World Health Organization; 2011 [Google Scholar]
- 2.Otten JJ, Hellwig JP, Meyers LD. editors. Institute of Medicine (IOM). Dietary reference intakes: the essential guide to nutrient requirements. Washington: National Academies Press; 2006 [Google Scholar]
- 3.Archer E, Hand GA, Blair SN. Validity of U.S. nutritional surveillance: National Health and Nutrition Examination Survey caloric energy intake data 1971–2010. PLoS One 2013;8:e76632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prentice RL, Tinker LF, Huang Y, Neuhouser ML. Calibration of self-reported dietary measures using biomarkers: an approach to enhancing nutritional epidemiology reliability. Curr Atheroscler Rep 2013;15:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Connor Gorber S, Tremblay M, Moher D, Gorber B. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: a systematic review. Obes Rev 2007;8:307–26 [DOI] [PubMed] [Google Scholar]
- 6.Okorodudu DO, Jumean MF, Montori VM, Romero-Corral A, Somers VK, Erwin PJ, Lopez-Jimenez F. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obes (Lond) 2010;34:791–9 [DOI] [PubMed] [Google Scholar]
- 7.Frankenfield DC, Rowe WA, Cooney RN, Smith JS, Becker D. Limits of body mass index to detect obesity and predict body composition. Nutrition 2001;17:26–30 [DOI] [PubMed] [Google Scholar]
- 8. Hasenegger V, Elmadfa I. Prevalence of overweight and obesity in school-aged children, adults and elderly in Austria (in German). Nutrition 2013;6:237–40.
- 9.Ness-Abramof R, Apovian CM. Waist circumference measurement in clinical practice. Nutr Clin Pract 2008;23:397–404 [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Preventing and managing the global epidemic of obesity. Report of the World Health Organization Consultation on Obesity. Geneva: World Health Organization; 1997 [PubMed] [Google Scholar]
- 11.National Institutes of Health. The practical guide: identification, evaluation and treatment of overweight and obesity in adults. Bethesda (MD): National Institutes of Health; 2000 [Google Scholar]
- 12.Han TS, van Leer EM, Seidell JC, Lean MEJ. Waist circumference action levels in the identification of cardiovascular risk factors: prevalence study in a random sample. BMJ 1995;311:1401–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross R, Berentzen T, Bradshaw AJ, Janssen I, Kahn HS, Katzmarzyk PT, Kuk JL, Seidell JC, Snijder MB, Sørensen TIA, et al. Does the relationship between waist circumference, morbidity and mortality depend on measurement protocol for waist circumference? Obes Rev 2008;9:312–25 [DOI] [PubMed] [Google Scholar]
- 14.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–63 [DOI] [PubMed] [Google Scholar]
- 15.Cameron AJ, Sicree RA, Zimmet PZ, Alberti KG, Tonkin AM, Balkau B, Tuomilehto J, Chitson P, Shaw JE. Cut-points for waist circumference in Europids and South Asians. Obesity (Silver Spring) 2010;18:2039–46 [DOI] [PubMed] [Google Scholar]
- 16.Carroll JF, Chiapa AL, Rodriquez M, Phelps DR, Cardarelli KM, Vishwanatha JK, Bae S, Cardarelli R. Visceral fat, waist circumference, and BMI: Impact of race/ethnicity. Obesity (Silver Spring) 2008;16:600–7 [DOI] [PubMed] [Google Scholar]
- 17.International Diabetes Federation. IDF consensus worldwide definition of the metabolic syndrome [cited 2014 March 28]. Available from: http://www.idf.org/metabolic-syndrome
- 18.De Lorenzo A, Deurenberg P, Pietrantuono M, Di Daniele N, Cervelli V, Andreoli A. How fat is obese? Acta Diabetol 2003;40:S254–7 [DOI] [PubMed] [Google Scholar]
- 19.Marques-Vidal P, Pécoud A, Hayoz D, Paccaud F, Mooser V, Waeber G, Vollenweider P. Normal weight obesity: relationship with lipids, glycaemic status, liver enzymes and inflammation. Nutr Metab Cardiovasc Dis 2010;20:669–75 [DOI] [PubMed] [Google Scholar]
- 20.Wagner DR, Heyward VH. Techniques of body composition assessment: a review of laboratory and field methods. Res Q Exerc Sport 1999;70:135–49 [DOI] [PubMed] [Google Scholar]
- 21.Ball SD, Altena TS. Comparison of the Bod Pod and dual energy x-ray absorptiometry in men. Physiol Meas 2004;25:671–8 [DOI] [PubMed] [Google Scholar]
- 22.Food and Agriculture Organization of the United Nations. Food balance sheets. A handbook. Rome: Food and Agriculture Organization; 2001 [Google Scholar]
- 23.Elmadfa I. editor. European Nutrition and Health Report 2009. Basel (Switzerland): Karger; 2009. p.62. [Google Scholar]
- 24.Elmadfa I, Weichselbaum E. editors. European Nutrition and Health Report 2004. Basel (Switzerland): Karger; 2004. p.58. [Google Scholar]
- 25.WHO Regional Office for Europe. Nutrition and Food Security Programme. Comparative analysis of food and nutrition policies in WHO European Member States. Copenhagen: World Health Organization; 2003 [Google Scholar]
- 26.Suchanek P, Poledne R, Hubacek JA. Dietary intake reports fidelity–fact or fiction? Neuroendocrinol Lett 2011;32:29–31 [PubMed] [Google Scholar]
- 27.Goris AH, Westerterp-Plantenga MS, Westerterp KR. Undereating and underrecording of habitual food intake in obese men: selective underreporting of fat intake. Am J Clin Nutr 2000;71:130–4 [DOI] [PubMed] [Google Scholar]
- 28.Shahar DR, Yu B, Houston DK, Kritchevsky SB, Newman AB, Sellmeyer DE, Tylavsky FA, Lee JS, Harris TB; Health, Aging, and Body Composition Study. Misreporting of energy intake in the elderly using doubly labeled water to measure total energy expenditure and weight change. J Am Coll Nutr. 2010;29:14–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rangan A, Allman-Farinelli M, Donohoe E, Gill T. Misreporting of energy intake in the 2007 Australian Children’s Survey: differences in the reporting of food types between plausible, under- and over-reporters of energy intake. J Hum Nutr Diet 2013;Nov 8 (Epub ahead of print; DOI:10.1111/jhn.12182). [DOI] [PubMed] [Google Scholar]
- 30.Poslusna K, Ruprich J, de Vries JH, Jakubikova M, van’t Veer P. Misreporting of energy and micronutrient intake estimated by food records and 24 hour recalls, control and adjustment methods in practice. Br J Nutr 2009;101:S73–85 [DOI] [PubMed] [Google Scholar]
- 31.Subar AF, Kipnis V, Troiano RP, Midthune D, Schoeller DA, Bingham S, Sharbaugh CO, Trabulsi J, Runswick S, Ballard-Barbash R, et al. Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: the OPEN study. Am J Epidemiol 2003;158:1–13 [DOI] [PubMed] [Google Scholar]
- 32.Lissner L, Troiano RP, Midthune D, Heitmann BL, Kipnis V, Subar AF, Potischman N. OPEN about obesity: recovery biomarkers, dietary reporting errors and BMI. Int J Obes (Lond) 2007;31:956–61 [DOI] [PubMed] [Google Scholar]
- 33.Ngo J, Engelen A, Molag M, Roesle J, García-Segovia P, Serra-Majem L. A review of the use of information and communication technologies for dietary assessment. Br J Nutr 2009;101:S102–12 [DOI] [PubMed] [Google Scholar]
- 34.Goldberg GR, Black AE, Jebb SA, Cole TJ, Murgatroyd PR, Coward WA, Prentice AM. Critical evaluation of energy intake data using fundamental principles of energy physiology: 1. derivation of cut-off limits to identify under-recording. Eur J Clin Nutr 1991;45:569–81 [PubMed] [Google Scholar]
- 35.Cade J, Thompson R, Burley V, Warm D. Development, validation and utilisation of food-frequency questionnaires—a review. Public Health Nutr 2002;5:567–87 [DOI] [PubMed] [Google Scholar]
- 36.Kaaks R, Ferrari P, Ciampi A, Plummer M, Riboli E. Uses and limitations of statistical accounting for random error correlations, in the validation of dietary questionnaire assessments. Public Health Nutr 2002;5:969–76 [DOI] [PubMed] [Google Scholar]
- 37.Freisling H, Elmadfa I, Schuh W, Wagner KH. Development and validation of a food frequency index using nutritional biomarkers in a sample of middle-aged and older adults. J Hum Nutr Diet 2009;22:29–39 [DOI] [PubMed] [Google Scholar]
- 38.Jenab M, Slimani N, Bictash M, Ferrari P, Bingham SA. Biomarkers in nutritional epidemiology: applications, needs and new horizons. Hum Genet 2009;125:507–25 [DOI] [PubMed] [Google Scholar]
- 39.Kuhnle GGC. Nutritional biomarkers for objective dietary assessment. J Sci Food Agric 2012;92:1145–9 [DOI] [PubMed] [Google Scholar]
- 40.Livingstone MB, Black AE. Markers of the validity of reported energy intake. J Nutr 2003;133:895S–920S [DOI] [PubMed] [Google Scholar]
- 41.Tasevska N, Runswick SA, McTaggart A, Bingham SA. Urinary sucrose and fructose as biomarkers for sugar consumption. Cancer Epidemiol Biomarkers Prev 2005;14:1287–94 [DOI] [PubMed] [Google Scholar]
- 42.Food and Agriculture Organization of the United Nations. International Network of Food Data Systems (INFOODS) (cited 2014 March 28). Available from: http://www.fao.org/infoods/infoods/en/
- 43.Pennington JA, Stumbo PJ, Murphy SP, McNutt SW, Eldridge AL, McCabe-Sellers BJ, Chenard CA. Food composition data: the foundation of dietetic practice and research. J Am Diet Assoc 2007;107:2105–13 [DOI] [PubMed] [Google Scholar]
- 44.Williamson C; EuroFIR. Synthesis report No 2: the different uses of food composition databases EuroFIR, Norwich, United Kingdom (2006)
- 45.Pennington JAT. Applications of food composition data: data sources and considerations for use. J Food Compos Anal 2008;21:S3–S12 [Google Scholar]
- 46.German Nutrition Society, Austrian Nutrition Society, Swiss Society for Nutrition Research, Swiss Nutrition Association. Reference values for nutrient intake. 1st ed., 5th corrected reprint. Frankfurt/Main (Germany): Umschau/Braus; 2013 [Google Scholar]
- 47.Department of Health. Dietary reference values for food energy and nutrients in the United Kingdom. London: Her Majesty’s Stationary Office; 1991 [Google Scholar]
- 48.García-Cañas V, Simó C, León C, Cifuentes A. Advances in nutrigenomics research: novel and future analytical approaches to investigate the biological activity of natural compounds and food functions. J Pharm Biomed Anal 2010;51:290–304 [DOI] [PubMed] [Google Scholar]
- 49.Sauberlich HE. Laboratory tests for the assessment of nutritional status. 2nd ed. Boca Raton (FL): CRC Press; 1999 [DOI] [PubMed] [Google Scholar]
- 50.Shenkin A. Micronutrients in health and disease. Postgrad Med J 2006;82:559–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heaney RP. Nutrients, endpoints, and the problem of proof. J Nutr 2008;138:1591–5 [DOI] [PubMed] [Google Scholar]
- 52.Blumberg J, Heaney RP, Huncharek M, Scholl T, Stampfer M, Vieth R, Weaver CM, Zeisel SH. Evidence-based criteria in the nutritional context. Nutr Rev 2010;68:478–84 [DOI] [PubMed] [Google Scholar]
- 53.Panel on Micronutrients, Subcommittees on Upper Reference Levels of Nutrients and of Interpretation and Use of Dietary Reference Intakes, and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington: National Academies Press; 2001 [PubMed] [Google Scholar]
- 54.Ross AC Taylor CL Yaktine AL Del Valle HB editors; Institute of Medicine. Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary reference intakes for calcium and vitamin D. Washington: National Academies Press; 2011 [PubMed] [Google Scholar]
- 55.Al-Delaimy WK, van Kappel AL, Ferrari P, Slimani N, Steghens JP, Bingham S, Johansson I, Wallström P, Overvad K, Tjønneland A, et al. Plasma levels of six carotenoids in nine European countries: report from the European Prospective Investigation into Cancer and Nutrition (EPIC). Public Health Nutr 2004;7:713–22 [DOI] [PubMed] [Google Scholar]
- 56.Ryu MS, Langkamp-Henken B, Chang SM, Shankar MN, Cousins RJ. Genomic analysis, cytokine expression, and microRNA profiling reveal biomarkers of human dietary zinc depletion and homeostasis. Proc Natl Acad Sci USA 2011;108:20970–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Risé P, Eligini S, Ghezzi S, Colli S, Galli C. Fatty acid composition of plasma, blood cells and whole blood: relevance for the assessment of the fatty acid status in humans. Prostaglandins Leukot Essent Fatty Acids 2007;76:363–9 [DOI] [PubMed] [Google Scholar]
- 58.Sun Q, Ma J, Campos H, Hankinson SE, Hu FB. Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. Am J Clin Nutr 2007;86:74–81 [DOI] [PubMed] [Google Scholar]
- 59.Elmadfa I, Hasenegger V, Wagner K, Putz P, Weidl NM, Wottawa D, Kuen T, Seiringer G, Meyer AL, Sturtzel B, et al. Austrian Nutrition Report 2012. 1st ed. (in German, English summary available on request) (cited 2014 March 28). Available from: http://bmg.gv.at/cms/home/attachments/4/5/3/CH1048/CMS1348749794860/oeb12.pdf.
- 60. U.S. Centers for Disease Control and Prevention. Second National Report on Biochemical Indicators of Diet and Nutrition in the U.S. Population 2012. Atlanta: National Center for Environmental Health; 2012.
- 61.Pilz S, Tomaschitz A, März W, Drechsler C, Ritz E, Zittermann A, Cavalier E, Pieber TR, Lappe JM, Grant WB, et al. Vitamin D, cardiovascular disease and mortality. Clin Endocrinol (Oxf) 2011;75:575–84 [DOI] [PubMed] [Google Scholar]
- 62.Rosen CJ, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, et al. IOM committee members respond to Endocrine Society vitamin D guideline. J Clin Endocrinol Metab 2012;97:1146–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aloia JF. African Americans, 25-hydroxyvitamin D, and osteoporosis: a paradox. Am J Clin Nutr 2008;88:545S–50S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Borel P, Moussa M, Reboul E, Lyan B, Defoort C, Vincent-Baudry S, Maillot M, Gastaldi M, Darmon M, Portugal H, et al. Human plasma levels of vitamin E and carotenoids are associated with genetic polymorphisms in genes involved in lipid metabolism. J Nutr 2007;137:2653–9 [DOI] [PubMed] [Google Scholar]
- 65.Borel P. Genetic variations involved in interindividual variability in carotenoid status. Mol Nutr Food Res 2012;56:228–40 [DOI] [PubMed] [Google Scholar]
- 66.Molloy AM, Scott JM. Folates and prevention of disease. Public Health Nutr 2001;4:601–9 [DOI] [PubMed] [Google Scholar]
- 67.Wagner KH, Kamal-Eldin A, Elmadfa I. Gamma-tocopherol—an underestimated vitamin? Ann Nutr Metab 2004;48:169–88 [DOI] [PubMed] [Google Scholar]
- 68.Aggarwal BB, Sundaram C, Prasad S, Kannappan R. Tocotrienols, the vitamin E of the 21st century: Its potential against cancer and other chronic diseases. Biochem Pharmacol 2010;80:1613–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bonham M, O’Connor JM, Hannigan BM, Strain JJ. The immune system as a physiological indicator of marginal copper status? Br J Nutr 2002;87:393–403 [DOI] [PubMed] [Google Scholar]
- 70.Aydemir TB, Blanchard RK, Cousins RJ. Zinc supplementation of young men alters metallothionein, zinc transporter, and cytokine gene expression in leukocyte populations. Proc Natl Acad Sci USA 2006;103:1699–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ryu MS, Lichten LA, Liuzzi JP, Cousins RJ. Zinc transporters ZnT1 (Slc30a1), Zip8 (Slc39a8), and Zip10 (Slc39a10) in mouse red blood cells are differentially regulated during erythroid development and by dietary zinc deficiency. J Nutr 2008;138:2076–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chung CS, Stookey J, Dare D, Welch R, Nguyen TQ, Roehl R, Peerson JM, King JC, Brown KH. Current dietary zinc intake has a greater effect on fractional zinc absorption than does longer term zinc consumption in healthy adult men. Am J Clin Nutr 2008;87:1224–9 [DOI] [PubMed] [Google Scholar]
- 73.King JC. Does zinc absorption reflect zinc status? Int J Vitam Nutr Res 2010;80:300–6 [DOI] [PubMed] [Google Scholar]
- 74.Fenech M. The Genome Health Clinic and Genome Health Nutrigenomics concepts: diagnosis and nutritional treatment of genome and epigenome damage on an individual basis. Mutagenesis 2005;20:255–69 [DOI] [PubMed] [Google Scholar]
- 75.Prá D, Bortoluzzi A, Müller LL, Hermes L, Horta JA, Maluf SW, Henriques JA, Fenech M, Franke SI. Iron intake, red cell indicators of iron status, and DNA damage in young subjects. Nutrition 2011;27:293–7 [DOI] [PubMed] [Google Scholar]
- 76.Wolff GL, Kodell RL, Moore SR, Cooney CA. Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. FASEB J 1998;12:949–57 [PubMed] [Google Scholar]
- 77.Cooney CA, Dave AA, Wolff GL. Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. J Nutr 2002;132:2393S–400S [DOI] [PubMed] [Google Scholar]
- 78.Tobi EW, Lumey LH, Talens RP, Kremer D, Putter H, Stein AD, Slagboom PE, Heijmans BT. DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Hum Mol Genet 2009;18:4046–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.International Zinc Nutrition Consultative Group (IZiNCG); Brown KH, Rivera JA, Bhutta Z, Gibson RS, King JC, Lönnerdal B, Ruel MT, Sandtröm B, Wasantwisut E, Hotz C. International Zinc Nutrition Consultative Group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr Bull 2004;25(1 Suppl 2):S91–203 [PubMed] [Google Scholar]