Abstract
Sarcopenia is characterized by decreases in both muscle mass and muscle function. The loss of muscle mass, which can precede decrements in muscle function, is ultimately rooted in an imbalance between the rates of muscle protein synthesis and breakdown that favors a net negative balance (i.e., synthesis < breakdown). A preponderance of evidence highlights a blunted muscle protein synthetic response to dietary protein, commonly referred to as “anabolic resistance,” as a major underlying cause of the insipid loss of muscle with age. Dietary strategies to overcome this decreased dietary amino acid sensitivity include the ingestion of leucine-enriched, rapidly digested proteins and/or greater protein ingestion in each main meal to maximally stimulate muscle anabolism. Anabolic resistance is also a hallmark of a sedentary lifestyle at any age. Given that older adults may be more likely to experience periods of reduced activity (either voluntarily or through acute illness), it is proposed that inactivity is the precipitating factor in the development of anabolic resistance and the subsequent progression from healthy aging to frailty. However, even acute bouts of activity can restore the sensitivity of older muscle to dietary protein. Provided physical activity is incorporated into the daily routine, muscle in older adults should retain its capacity for a robust anabolic response to dietary protein comparable to that in their younger peers. Therefore, through its ability to “make nutrition better,” physical activity should be viewed as a vital component to maintaining muscle mass and function with age.
Introduction
Adequate quantity and quality of skeletal muscle mass are vital for overall health and function. In addition to its obvious role in converting chemical energy to mechanical work, skeletal muscle is a major disposal site for blood glucose and FAs and is a significant contributor to the basal metabolic rate (1). Skeletal muscle is the only appreciable “storage reservoir” for body amino acids and, as such, is called upon during periods of reduced energy and/or physiologic stress (e.g., acute inflammation). Finally, a reduced amount of lean body mass has been reported to be a predisposing factor for the risk of hospitalization and is associated with a greater length of stay (2). Therefore, the protection of this vital tissue is important to maintain overall health and a high standard of living with advancing age.
Aging is associated with a gradual and insipid loss of lean body mass that, when accompanied by decrements in muscle strength and/or performance, is classically defined as sarcopenia (3). Although multiple definitions of sarcopenia exist (3–6), the transition from healthy aging to severe sarcopenia (i.e., frailty) is a progressive condition that generally occurs along a continuum but is necessarily accompanied by significant losses of muscle mass. Given that the loss of lean mass can precede that of functional limitations (3) and may represent the proverbial “canary in the coal mine” for the development of sarcopenia, a wealth of research has delved into the etiology of muscle loss with the aim of developing effective strategies to attenuate its loss or, more preferably, augment its mass.
Dietary Protein Requirements
Dietary protein is an essential macronutrient for the maintenance of lean body mass and overall health in individuals of all ages. Provided energy needs are met, the current RDA for older adults is not different from that for their younger counterparts, with daily intakes of 0.8 g/(kg ⋅ d) suggested to be sufficient to meet the metabolic demand for this macronutrient (7). This amount of protein would generally be regarded as “adequate,” with daily intakes below this 0.8 g/(kg ⋅ d) threshold categorized as “suboptimal.” However, recent consensus statements and expert opinions have questioned the adequacy of current protein requirement guidelines by providing evidence that protein intakes greater than the RDA are associated with better health outcomes (e.g., muscle mass and function, bone health, immune function, etc.) in older adults (8–10). As such, the prevailing view is that protein intake should be viewed in terms of what is “optimal” this can extend to both what is required acutely to maximally stimulate muscle protein synthesis (discussed in more detail below) and/or chronically (in terms of daily protein intake) to maximize health outcomes in the elderly (e.g., retention of muscle mass and function) (8).
Metabolic Regulation of Skeletal Muscle Mass
Skeletal muscle proteins are continuously being remodeled or “turned over” through the simultaneous processes of protein synthesis and breakdown. The continuous turnover of skeletal proteins ensures that a functional pool of proteins exists as old and potentially damaged proteins are targeted for degradation by the ubiquitin-proteosome system and are subsequently broken down into their constituent amino acids. These amino acids liberated through degradation can subsequently be exported from the cell to serve as substrates for the synthesis of other body proteins and/or gluconeogenesis. Alternatively, and perhaps more importantly from a muscle net balance perspective (i.e., the algebraic difference between protein synthesis and protein breakdown), amino acids from the free pool can be reutilized to synthesize new skeletal muscle proteins. Although the loss of muscle mass with aging is multifactorial in nature, it is ultimately rooted in an imbalance in the rates of protein synthesis and protein breakdown that favors a net negative protein balance. With a turnover rate of ∼1–2%/d, an average older adult with ∼18 kg of muscle mass could expect to break down and resynthesize ∼180–360 g of muscle protein in a typical 24-h period. Given that muscle loss with age can occur at a modest ∼1%/y (the equivalent of ∼100 g/y or ∼0.3 g/d) (11), even slight differences in the rates of protein synthesis and breakdown could translate into meaningful differences in net protein balance that, over time, could contribute to the risk of developing sarcopenia. Some studies suggest that basal rates of muscle protein synthesis may be suppressed somewhat with age (12, 13), although this finding is not universal (14, 15). However, accumulating evidence points to a significant role for an attenuated anabolic effect of meal feeding in the progression of muscle loss with aging (8), which is primarily manifest through a suppressed stimulation of muscle protein synthesis with dietary amino acid ingestion. Therefore, this review focuses on the role nutrition plays in stimulating muscle anabolism with feeding and how activity, and especially inactivity, can significantly affect the sensitivity of aging muscle to dietary nutrients.
The “Anabolic Resistance” of Aging
Underlying physiology.
Dietary protein (and subsequently amino acid) ingestion is one of the most robust physiologic stimuli to enhance muscle net protein balance, an effect that is primarily mediated by an essential amino acid–induced stimulation of muscle protein synthesis (16). Although protein ingestion stimulates muscle protein synthesis regardless of age (17), a preponderance of evidence suggests that older skeletal muscle is less efficient at assimilating dietary protein–derived amino acids into new muscle proteins (8). For example, with optimal protein or amino acid ingestion, older adults were reported to display a robust increase in muscle protein synthesis that is generally equivalent to their younger counterparts (18–20); this suggests that, under appropriate nutritional conditions, otherwise healthy older muscle for the most part retains the capacity to respond to protein intake. However, at relatively suboptimal protein intakes, older adults typically display an attenuated muscle protein synthetic response when compared with their younger peers (21). The reduced sensitivity of skeletal muscle tissue to dietary protein–derived amino acids is often referred to as “anabolic resistance.” Although some evidence suggests that older adults may have an attenuated capacity for insulin-induced suppression of muscle protein breakdown (22), the anabolic resistance of muscle protein synthesis is believed to be the major underlying mechanism of the normal loss of muscle mass with advancing age (8).
The mechanisms underpinning the anabolic resistance of muscle protein synthesis are multifactorial but are ultimately rooted in an inability to enhance mRNA translation, the rate-controlling step for protein synthesis that is primarily regulated by the mammalian target of rapamycin (mTOR) signaling pathway (23) in response to dietary amino acids. Splanchnic extraction has variably been reported to be greater in older adults (24, 25), which could reduce the appearance of dietary amino acids (especially after suboptimal protein intakes) in the circulation after food intake. Compounding this possibly reduced dietary amino acid availability is recent evidence suggesting that insulin-induced microvascular perfusion is blunted in response to carbohydrate (i.e., insulin) and essential amino acid ingestion with age (26–28). This compromised capillary dilation, which may be mediated by elevated concentrations of the vasoconstrictor endothelin-1, ultimately impairs dietary amino acid delivery to and uptake by skeletal muscle and may be a direct contributor to the anabolic resistance in older adults (26, 27). Research has begun to investigate the potential role of amino acid transporters in the anabolic response to food intake (29), given that these proteins not only play a role in the transport (both influx and efflux) of amino acids across the muscle membrane but also appear to act as a nutrient receptor [i.e., a “transceptor” (30)], the latter of which may help initiate anabolic signaling in a feed-forward manner. Limited research has revealed that basal amino acid transporter protein expression does not appear to be appreciably influenced by aging per se (31, 32), although without a complete picture as to the location (e.g., intracellular vs. intramembranous) and/or functionality (i.e., transport activity/capacity) of these proteins in older muscle, their contribution to the anabolic resistance of aging cannot be ruled out. Finally, the translational machinery of older muscle may have a reduced capacity for and/or sensitivity to amino acids, as suggested by observations of a reduced ribosomal protein content (15) and/or dysregulated mTOR (or 1 of its primary downstream effectors ribosomal protein S6 kinase) signaling in response to exogenous amino acids and/or insulin in older muscle (15, 33). Therefore, there are multiple points of control from the ingestion of dietary protein to the subsequent incorporation of its constituent amino acids into muscle proteins that may be subject to age-related alterations; these aspects are currently the focus of major research programs aiming to unravel the relative contribution of 1 or all of these (or potentially other) factors to the anabolic resistance of aging, but this is beyond the scope of the present review.
Role of physical inactivity.
There is an increasing awareness of the role that physical activity/inactivity plays in the development of anabolic resistance across life conditions, including in older adults (34). It is well known that the absence of activity (e.g., through limb immobilization or bed rest) results in losses of muscle mass and muscle strength in otherwise healthy young and older adults alike (35, 36). Although this form of extreme inactivity may reduce basal rates of muscle protein synthesis (37) and the cumulative response over 24 h (36), studies have reported a reduced sensitivity of muscle protein synthesis to exogenous amino acids that, in the absence of overt increases in muscle protein breakdown (34), likely underpins the characteristic loss of muscle mass with immobilization (38–40). The precise mechanism or mechanisms underlying the anabolic resistance of inactivity (i.e., immobilization or bed rest) are not entirely clear but may be related in part to blunted mTOR-related signaling (38, 39) [perhaps secondary to altered amino acid transporter expression (39)] and/or an attenuated insulin-induced vasodilatory response (41). Nevertheless, even inconspicuous forms of inactivity such as a restriction of daily step counts from ∼6000 to ∼1500 were recently shown to negatively affect insulin sensitivity, reduce lean mass, and increase fat mass in both young and older adults (42, 43). Similar to observations after complete immobility (38, 39), this decrease in habitual step count was also shown to blunt the dietary protein–induced stimulation of muscle protein synthesis in older adults, which presumably underpins the concomitant loss of muscle mass over 2 wk (42). The observation that immobilization or reduced activity recapitulates the characteristic anabolic resistance of aging in both healthy young (38, 40) and older (39, 42) individuals and can result in losses of both muscle mass and strength (35, 44) could be viewed as an acceleration of the “biologic age” of skeletal muscle.
With these aforementioned negative consequences of inactivity in mind, it is somewhat alarming that habitual daily step counts generally decline with age (45) and that the majority of free-living older adults, especially those who are physically frail, report at least 1 episode of “bed rest” in the past month (46). Older adults are also more likely to be hospitalized with acute illness that is often associated with standardized periods of bed rest during treatment (47). Given that older adults were reported to have an attenuated ability to recover from inactivity-induced losses of muscle mass and strength (48), these brief periods of sedentary living have ultimately been proposed to accelerate the sarcopenia of aging (47). A reduction in self-selected habitual activity was also reported after a period of bed rest in older adults (44), which may ultimately compromise the ability to reverse any development of anabolic resistance during the immobilized period in the absence of a targeted rehabilitation strategy.
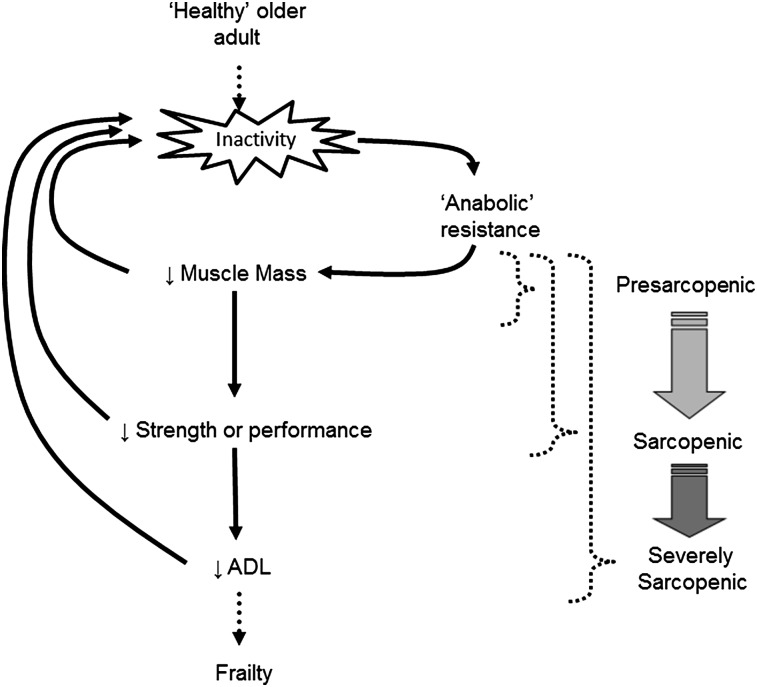
It was proposed recently by others (49) and is echoed here that inactivity is the precipitating factor in the development of anabolic resistance, which, when not addressed through dietary and/or lifestyle alterations, can lead to the loss of muscle mass, muscle function, and ultimately, to the development and/or progression of sarcopenia (8) (Fig. 1). For example, a period of voluntary (e.g., sedentary lifestyle) or involuntary (e.g., illness) inactivity can lead to anabolic resistance in skeletal muscle and an associated decline in lean body mass (39, 42), which is characteristic of individuals in a “presarcopenic” state (3). These individuals would subsequently require a greater protein intake to maximally stimulate muscle protein synthesis or otherwise risk losing further muscle mass. However, if older adults do not voluntarily reverse the sedentary behavior, as was reported previously through self-selected inactivity (44, 46), they may subsequently perpetuate the anabolic resistance; this would ultimately precipitate a further loss of muscle mass and, eventually, muscle strength or function, a combination that would generally categorize them as being sarcopenic (3, 4). Once both muscle mass and muscle function are compromised it is a precipitous decline into functional dependence and frailty (50, 51), otherwise labeled as “severe sarcopenia” (3). Therefore, with this hypothesis in mind, the balance of the present review addresses potential nutrition and/or lifestyle solutions to maintain the sensitivity of older muscle to dietary nutrients as a means to combat anabolic resistance and the potential devolution into physical frailty (Fig. 2).
FIGURE 1.
Schematic of the proposed impact of physical inactivity as a main contributor to the progression of sarcopenia (for further explanation, see text). Inactivity may be voluntary (e.g., sedentary lifestyle) or involuntary (e.g., illness or forced bed rest) but ultimately alters the trajectory away from “healthy” aging through the development of “anabolic resistance” and toward a downward spiral into frailty. Stages of sarcopenia are overlaid with the proposed schematic and correspond to the classifications outlined in the European Working Group on Sarcopenia in Older People (3): “presarcopenic,” loss of muscle mass; “sarcopenic,” loss of muscle mass and muscle strength or performance; “severely sarcopenic,” loss of muscle mass, muscle strength, and muscle performance (e.g., activities of daily living). ADL, activities of daily living.
FIGURE 2.
Theoretical representation of the relation between protein intake and maximal postprandial muscle protein synthesis. “Optimal” corresponds to the amount of protein required to maximally stimulate postprandial muscle protein synthesis [i.e., corresponding to the breakpoint in the dose-response curve, as used previously with daily protein requirements (96)]. Factors that can affect the optimal intake by shifting the dose-response curve (outlined in solid lines and denoted as “healthy”) to the left (i.e., increased “sensitivity” to dietary protein) include an active lifestyle (e.g., regular exercise), greater leucine content of the ingested dietary amino acids, and/or rapidly digested proteins. Conversely, the optimal protein intake can be shifted to the right (i.e., decreased “sensitivity” to dietary protein, commonly referred to as “anabolic resistance”) by an inactive lifestyle (e.g., decreased habitual activity or complete immobility) or ingestion of “lower quality” (e.g., lower leucine content) protein (outlined in dashed lines and denoted as “resistant”).
Nutritional Countermeasures for Anabolic Resistance
Protein amount.
The attenuation of lean body mass loss with age would ultimately require the optimal postprandial stimulation of muscle protein synthesis. Given the relative anabolic resistance of muscle protein synthesis to dietary protein, especially to “suboptimal” amounts, elderly adults generally require a greater protein intake to maximize postprandial muscle protein synthesis (8). In fact, preliminary data from our laboratory suggest that a relative protein intake of ∼0.40 g/kg is required to maximally stimulate postprandial myofibrillar protein synthesis in otherwise healthy older adults (D. R. Moore et al., unpublished results); these results would generally be in line with the observation that 35 g (∼0.45 g/kg) of whey protein stimulates mixed muscle protein synthesis in older adults, whereas 10 and 20 g (∼0.13 and ∼0.28 g/kg, respectively) do not (52). Interestingly, in our laboratory, maximal rates of myofibrillar protein synthesis were similar between young and older adults, yet were achieved at greater relative protein intakes in the latter (∼0.40 vs. ∼0.24 g/kg) (D. R. Moore et al., unpublished results); these results are generally consistent with previous observations that anabolic resistance can be overcome with higher exogenous amino acid ingestion (18–20), suggesting that “optimal” (i.e., greater) protein intake in older adults can elicit an equivalent anabolic response to that in young adults.
Individuals who more frequently elicit a maximal stimulation of muscle protein synthesis throughout a daily meal feeding cycle would be more likely to maintain muscle mass and possibly function. This could explain in part the greater retention of lean body mass in older adults who consume more than the current RDA for protein [i.e., ≥1.2 g/(kg ⋅ d)] relative to those who habitually consume a suboptimal amount [i.e., <0.8 g/(kg ⋅ d)] (53). Additionally, limited evidence suggests that relatively higher protein intakes [i.e., ≥1.2 g/(kg ⋅ d)] are associated with greater muscle strength and quality (i.e., force/kg muscle) than lower intakes [i.e., ≤1.2 g/(kg ⋅ d)] (54, 55). Therefore, older adults aiming to maintain muscle mass (and possibly function) would likely benefit from an optimal protein ingestion (∼0.39 g/kg in our laboratory) to maximize muscle protein synthesis at each main meal of the day, an approach that would increase daily protein intake above the current RDA but bring it in line with recent expert recommendations (8, 9).
Protein type.
Dietary proteins can differ in their constituent amino acid contents and/or rate of digestion, both of which are factors that can affect their anabolic effects within skeletal muscle. Of particular importance for the optimal stimulation of muscle protein synthesis appears to be the leucine content of a protein because this essential amino acid is not only a substrate for protein synthesis but also serves as a key anabolic signal to skeletal muscle tissue through its ability to activate (as suggested by changes in protein phosphorylation) enzymes within the mTOR signaling pathway (23, 56). Although supplemental leucine can increase the anabolic effects of suboptimal amounts of amino acids (57) and/or different protein sources (58), its anabolic properties also extend to proteins that are naturally enriched in this amino acid. For example, whey protein (∼11% leucine) was shown to elicit a greater increase in postprandial muscle protein synthesis in older adults than both soy (∼8% leucine) (59) and casein (∼8% leucine) (60, 61). However, this greater postprandial muscle protein synthetic response after the ingestion of whey protein may also be related in part to its rapid digestion and absorption kinetics. As evidence of this, hydrolyzing casein enhances dietary protein digestion and amino acid absorption rates and subsequent plasma amino acid availability and tends to support greater rates of muscle protein synthesis in older adults than native micellar casein (which typically clots in the acidic pH of the stomach) (61). This somewhat enhanced anabolism occurs despite these protein sources having identical amino acid profiles and leucine content. Interestingly, a comparative analysis of the anabolic effects of micellar casein, hydrolyzed casein, and whey protein in older adults revealed a stepwise increase in muscle protein synthesis between the protein sources and a correlation between peak blood leucine concentration and the maximal protein synthetic response (61).
Studies investigating the effects of dietary amino acids on muscle protein synthesis generally provide the protein or amino acids independent of other macronutrients (e.g., carbohydrates and fats) and in liquid form (20, 52, 59–62); this approach is effective at isolating the impact of dietary protein per se on muscle protein metabolism but may not entirely reflect the response after meal intake (i.e., with additional macronutrients and/or in solid form). For example, the food matrix can affect digestion and absorption with liquid protein sources eliciting a more rapid increase and a greater peak amino acid concentration than solid foods of equivalent protein content (63). However, the inclusion of additional energy in the form of carbohydrates does not appear to affect the utilization of dietary amino acids from high-quality protein sources (e.g., whey/casein) for the synthesis of muscle proteins in young or older adults (64–66). Nevertheless, whole-food protein sources that are more easily masticated and digested (e.g., minced beef compared with beef steak) result in a more rapid dietary amino acid appearance in the blood and a greater net whole-body protein retention in older adults (67, 68). Interestingly, this greater whole-body net protein balance is not consistently mirrored by a stimulation of muscle protein synthesis (67), a finding that is in apparent contrast with previous reports using beef protein ingestion (18). Therefore, additional research is warranted to determine how protein source, food matrix, and macronutrient profile affect the anabolic properties of dietary protein in both healthy and “anabolically resistant” older adults. However, a general rule for dietary solutions (e.g., supplemental nutrition) aimed at eliciting a maximal anabolic response in skeletal muscle of older adults would be to target leucine-enriched, rapidly digested proteins, preferably in a liquid form.
Protein timing.
Western diets are typically unbalanced in the pattern of protein intake (69), which extends into older adulthood independent of habitual protein intake (70). Given that older adults typically display an anabolic resistance to low amounts of dietary amino acids, this unbalanced daily protein ingestion could lead to a suboptimal stimulation of muscle protein synthesis after the morning meal, which generally feature low protein intakes (70). This unbalanced protein ingestion with suboptimal morning intakes may explain in part the previously reported loss of lean body mass in older adults who were apparently consuming adequate daily protein (53). This has led to the suggestion that a balanced ingestion with sufficient dietary protein intake to maximize muscle protein synthesis at each of the 3 main meals (i.e., breakfast, lunch, and dinner) would be the most efficient means to consume the daily protein intake and ultimately help attenuate the loss of lean mass with age (10); this concept has recently garnered support in young individuals because a balanced (i.e., 3 × 30 g) daily protein ingestion pattern supports a greater 24-h muscle protein synthetic response than does an unbalanced (i.e., 15, 20, and 65 g) pattern (71).
It was recently reported that malnourished, hospitalized older adults gained lean mass with a dietary intake pattern that provided the majority (i.e., ∼70%) of their daily protein intake of ∼1.3 g/(kg ⋅ d) in a single midday meal, whereas there was no change in body composition with a more balanced 4-meal approach (72). This would seem at odds with the suggestion that a balanced protein distribution would be more favorable for the stimulation of muscle protein synthesis and retention of muscle mass (10, 71). It is possible that a balanced 4-meal approach, which would have resulted in a suboptimal (i.e., ≤21 or ≤0.31 g/kg) protein ingestion at each meal, was insufficient to maximally stimulate muscle protein synthesis at any meal of the day, especially in this frail older population (72). On the other hand, morning protein supplementation, which would presumably help balance daily protein ingestion over 3 main meal-intake occasions (70), was reported to improve markers of physical function in frail older adults over a 6-mo intervention period (73); however, it is unclear if these results were a reflection of a more balanced and/or greater daily protein ingestion. Presently, it is unclear what effect manipulating daily protein feeding patterns may have on prefrail or healthy older adults in contrast to the previously reported observations in frail and malnourished individuals (72, 73). Therefore, further research is required to elucidate the most efficient means to consume the daily protein intake and to what extent this may represent a viable nutritional therapy to counteract the “normal” sarcopenia of aging.
Lifestyle Countermeasures for Anabolic Resistance
Habitual activity.
Older (∼70 y) lifelong endurance athletes have levels of muscle mass and strength that are generally indistinguishable from individuals almost half their age (∼40 y) (74, 75). However, individuals need not adhere to intense exercise modalities (e.g., prolonged endurance exercise) to reap the benefits of an active lifestyle because even moderate activity levels are associated with greater muscle mass and function (76, 77). Therefore, individuals who are habitually active and generally possess greater muscle mass and function could be viewed as having muscle that is of lower “biological age” (i.e., more similar to individuals of a lower chronological age).
It is clear that periods of inactivity are characterized by decreases in muscle mass and muscle strength, with the initial development of anabolic resistance likely being a major precipitating factor in this progression (39, 42, 78). As a corollary to this, the adherence to an active lifestyle would maintain the sensitivity of older skeletal muscle to dietary amino acids to help stave off subsequent aging-associated muscle loss. For example, a moderate 45-min walk (i.e., ∼2300 steps) has been shown to enhance the muscle protein synthetic response to ingested amino acids for up to 16 h, an effect that may be mediated through an enhanced insulin-induced microvascular perfusion and subsequent delivery of amino acids to the muscle (27, 78). This demonstrates that, at the very least, “habitual activity” may not need to be particularly intense in nature for older muscle to obtain a benefit from a nutritional standpoint. Interestingly, the increase in amino acid sensitivity with the previously mentioned walking protocol occurred in parallel with ∼6300 steps/d compared with the habitual ∼4000 steps/d (27), which highlights that even subtle differences in habitual activity could play a significant role in combating any inactivity-induced anabolic resistance. Given this, it is perhaps not surprising that recommendations for older adults to maintain “physical health” (including normal muscle function) suggest that a minimum of ∼7000 steps/d be targeted (76). Nevertheless, self-selected free-living activities of higher metabolic intensity (i.e., ≥3 times the resting oxygen consumption or metabolic equivalents) would be of greater benefit for overall health (76), with exercises such as cycling reported to increase muscle mass in older adults (79, 80). Additionally, activities that engage a greater amount of muscle mass (e.g., gardening, swimming) that are ≥3 metabolic equivalents could translate into a greater whole-body sensitivity to amino acids, thereby likely preserving a greater proportion of whole-body lean mass with age. Therefore, older adults should be encouraged to adhere to an active lifestyle to help “keep their muscles young,” at least from an amino acid sensitivity standpoint.
Resistance exercise.
Although an active lifestyle can maintain muscle health and its nutrient sensitivity, targeted exercise programs are likely warranted to more effectively mitigate or even reverse the deleterious loss of muscle mass with age. Resistance exercise is unquestionably an effective means to stimulate muscle protein synthesis (81, 82) and enhance muscle mass (83) and muscle strength (84) in older adults. Performing even moderate resistance exercise before consumption of a protein-containing meal allows for a greater utilization of dietary amino acids for the synthesis of skeletal muscle proteins (85). This enhanced sensitivity to dietary amino acids persists for up to 24 h in young adults and, provided exercise is performed to a high degree of voluntary effort (i.e., one that would elicit significant, if not maximal, muscle fiber recruitment), is independent of the absolute weight lifted (i.e., “light” weights are equally effective as “heavy” weights) (86). Because even moderate walking can improve the amino acid–induced stimulation of muscle protein synthesis for up to 16 h in older adults (27), it is likely that this enhanced nutrient sensitivity with resistance exercise would also be present in older adults. The significance of an enhanced exercise-induced amino acid sensitivity of muscle protein synthesis is that more efficient dietary protein use could help increase the anabolic effect of suboptimal acute protein intakes, which, as highlighted previously, is a common feature of the breakfast of most older adults (70).
It was recently shown that resistance exercise enhances the expression of select amino acid transporters for up to 24 h after an acute bout of exercise in both young and older adults (31); although the intracellular localization and/or activity of these transporters is presently unknown, the greater protein expression 24 h after resistance exercise in both young and older muscle (31) may contribute in part to the enhanced amino acid sensitivity we previously observed in young adults (86). Additionally, chronic resistance exercise (i.e., training) improves leg blood flow responses to meal consumption (presumably mediated by enhanced insulin sensitivity) in older adults and ameliorates the normal age-related decline in nutritive blood flow (87). Given that mild aerobic-based exercise acutely enhances insulin-induced microvascular perfusion (27, 78), greater anabolic sensitivity with resistance exercise may also be mediated in part by an enhanced nutritive muscle blood flow. Therefore, through its ability to increase nutritive blood flow, dietary protein use, and muscle protein synthesis, the combined approach of resistance exercise and protein ingestion is ultimately the most effective means to maintain or enhance musculoskeletal mass and quality with aging (88). More important, there is a growing paradigm shift toward exercise that is of high voluntary effort in order to enhance muscle fiber recruitment rather than one highlighted by heavy external loads (89, 90). Iterations of this light-load, high-effort training can indeed stimulate robust increases in muscle protein synthesis (91, 92) and can elicit gains in muscle hypertrophy and strength similar to more “traditional” heavy-load resistance training (93, 94), which ultimately would be beneficial to older populations given the lower stresses placed on aging joints.
In conclusion, anabolic resistance is likely the main precipitating factor in the gradual loss of muscle mass with age. Interestingly, a decreased sensitivity to dietary protein ingestion is common to many conditions that feature a reduction in habitual muscle use (e.g., sedentary lifestyle and/or immobilization). Given that sedentary behavior reduces (39, 42), whereas physical activity increases (27, 85), the muscle protein synthetic response to dietary protein ingestion in older adults, this begs the question as to whether the anabolic resistance of aging could perhaps more accurately be described as the anabolic resistance of inactivity. Therefore, in addition to nutritional strategies aimed at combating this anabolic resistance, such as greater meal (and hence daily) protein intakes and/or the ingestion of rapidly digested, leucine-enriched protein sources, lifestyle modifications that increase physical activity should be viewed as paramount to maintaining nutrient sensitivity in older muscle. In this way, physical activity should be viewed as a tool to help “make nutrition better” to maintain or enhance musculoskeletal health with age.
Acknowledgments
The helpful edits of Dr. Nicholas Burd during the preparation of this manuscript are greatly appreciated. The sole author had responsibility for all parts of the manuscript.
References
- 1.Johnstone AM, Murison SD, Duncan JS, Rance KA, Speakman JR. Factors influencing variation in basal metabolic rate include fat-free mass, fat mass, age, and circulating thyroxine but not sex, circulating leptin, or triiodothyronine. Am J Clin Nutr 2005;82:941–8 [DOI] [PubMed] [Google Scholar]
- 2.Pichard C, Kyle UG, Morabia A, Perrier A, Vermeulen B, Unger P. Nutritional assessment: lean body mass depletion at hospital admission is associated with an increased length of stay. Am J Clin Nutr 2004;79:613–8 [DOI] [PubMed] [Google Scholar]
- 3.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morley JE, Abbatecola AM, Argiles JM, Baracos V, Bauer J, Bhasin S, Cederholm T, Coats AJ, Cummings SR, Evans WJ, et al. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc 2011;12:403–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International Working Group on Sarcopenia. J Am Med Dir Assoc 2011;12:249–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muscaritoli M, Anker SD, Argiles J, Aversa Z, Bauer JM, Biolo G, Boirie Y, Bosaeus I, Cederholm T, Costelli P, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) "cachexia-anorexia in chronic wasting diseases" and "nutrition in geriatrics". Clin Nutr 2010;29:154–9 [DOI] [PubMed] [Google Scholar]
- 7.WHO. Protein and amino acid requirements in human nutrition. World Health Organ Tech Rep Ser 2007;(935):1–265 [PubMed] [Google Scholar]
- 8.Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JE, Phillips SM, Siegel C, Stehle P, Teta D, et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE study group. J Am Med Dir Assoc 2013;14:542–59 [DOI] [PubMed] [Google Scholar]
- 9.Volpi E, Campbell WW, Dwyer JT, Johnson MA, Jensen GL, Morley JE, Wolfe RR. Is the optimal level of protein intake for older adults greater than the recommended dietary allowance? J Gerontol A Biol Sci Med Sci 2013;68:677–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paddon-Jones D, Rasmussen BB. Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care 2009;12:86–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB. The loss of skeletal muscle strength, mass, and quality in older adults: the Health, Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci 2006;61:1059–64 [DOI] [PubMed] [Google Scholar]
- 12.Henderson GC, Dhatariya K, Ford GC, Klaus KA, Basu R, Rizza RA, Jensen MD, Khosla S, O'Brien P, Nair KS. Higher muscle protein synthesis in women than men across the lifespan, and failure of androgen administration to amend age-related decrements. FASEB J 2009;23:631–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Short KR, Vittone JL, Bigelow ML, Proctor DN, Nair KS. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am J Physiol Endocrinol Metab 2004;286:E92–101 [DOI] [PubMed] [Google Scholar]
- 14.Volpi E, Sheffield-Moore M, Rasmussen BB, Wolfe RR. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA 2001;286:1206–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 2005;19:422–4 [DOI] [PubMed] [Google Scholar]
- 16.Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr 2003;78:250–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koopman R, van Loon LJ. Aging, exercise, and muscle protein metabolism. J Appl Physiol 2009;106:2040–8 [DOI] [PubMed] [Google Scholar]
- 18.Symons TB, Schutzler SE, Cocke TL, Chinkes DL, Wolfe RR, Paddon-Jones D. Aging does not impair the anabolic response to a protein-rich meal. Am J Clin Nutr 2007;86:451–6 [DOI] [PubMed] [Google Scholar]
- 19.Chevalier S, Goulet ED, Burgos SA, Wykes LJ, Morais JA. Protein anabolic responses to a fed steady state in healthy aging. J Gerontol A Biol Sci Med Sci 2011;66:681–8 [DOI] [PubMed] [Google Scholar]
- 20.Koopman R, Walrand S, Beelen M, Gijsen AP, Kies AK, Boirie Y, Saris WH, van Loon LJ. Dietary protein digestion and absorption rates and the subsequent postprandial muscle protein synthetic response do not differ between young and elderly men. J Nutr 2009;139:1707–13 [DOI] [PubMed] [Google Scholar]
- 21.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr 2005;82:1065–73 [DOI] [PubMed] [Google Scholar]
- 22.Wilkes EA, Selby AL, Atherton PJ, Patel R, Rankin D, Smith K, Rennie MJ. Blunting of insulin inhibition of proteolysis in legs of older subjects may contribute to age-related sarcopenia. Am J Clin Nutr 2009;90:1343–50 [DOI] [PubMed] [Google Scholar]
- 23.Drummond MJ, Dreyer HC, Fry CS, Glynn EL, Rasmussen BB. Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signaling. J Appl Physiol 2009;106:1374–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volpi E, Mittendorfer B, Wolf SE, Wolfe RR. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol 1999;277:E513–20 [DOI] [PubMed] [Google Scholar]
- 25.Boirie Y, Gachon P, Beaufrere B. Splanchnic and whole-body leucine kinetics in young and elderly men. Am J Clin Nutr 1997;65:489–95 [DOI] [PubMed] [Google Scholar]
- 26.Timmerman KL, Lee JL, Fujita S, Dhanani S, Dreyer HC, Fry CS, Drummond MJ, Sheffield-Moore M, Rasmussen BB, Volpi E. Pharmacological vasodilation improves insulin-stimulated muscle protein anabolism but not glucose utilization in older adults. Diabetes 2010;59:2764–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Timmerman KL, Dhanani S, Glynn EL, Fry CS, Drummond MJ, Jennings K, Rasmussen BB, Volpi E. A moderate acute increase in physical activity enhances nutritive flow and the muscle protein anabolic response to mixed nutrient intake in older adults. Am J Clin Nutr 2012;95:1403–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitchell WK, Phillips BE, Williams JP, Rankin D, Smith K, Lund JN, Atherton PJ. Development of a new Sonovue contrast-enhanced ultrasound approach reveals temporal and age-related features of muscle microvascular responses to feeding. Physiol Rep 2013;1:e00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drummond MJ, Glynn EL, Fry CS, Timmerman KL, Volpi E, Rasmussen BB. An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am J Physiol Endocrinol Metab 2010;298:E1011–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hundal HS, Taylor PM. Amino acid transceptors: gate keepers of nutrient exchange and regulators of nutrient signaling. Am J Physiol Endocrinol Metab 2009;296:E603–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drummond MJ, Fry CS, Glynn EL, Timmerman KL, Dickinson JM, Walker DK, Gundermann DM, Volpi E, Rasmussen BB. Skeletal muscle amino acid transporter expression is increased in young and older adults following resistance exercise. J Appl Physiol 2011;111:135–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dickinson JM, Drummond MJ, Coben JR, Volpi E, Rasmussen BB. Aging differentially affects human skeletal muscle amino acid transporter expression when essential amino acids are ingested after exercise. Clin Nutr 2013;32:273–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guillet C, Prod'homme M, Balage M, Gachon P, Giraudet C, Morin L, Grizard J, Boirie Y. Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. FASEB J 2004;18:1586–7 [DOI] [PubMed] [Google Scholar]
- 34.Phillips SM, Glover EI, Rennie MJ. Alterations of protein turnover underlying disuse atrophy in human skeletal muscle. J Appl Physiol 2009;107:645–54 [DOI] [PubMed] [Google Scholar]
- 35.Yasuda N, Glover EI, Phillips SM, Isfort RJ, Tarnopolsky MA. Sex-based differences in skeletal muscle function and morphology with short-term limb immobilization. J Appl Physiol 2005;99:1085–92 [DOI] [PubMed] [Google Scholar]
- 36.Kortebein P, Ferrando A, Lombeida J, Wolfe R, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA 2007;297:1772–4 [DOI] [PubMed] [Google Scholar]
- 37.Gibson JN, Halliday D, Morrison WL, Stoward PJ, Hornsby GA, Watt PW, Murdoch G, Rennie MJ. Decrease in human quadriceps muscle protein turnover consequent upon leg immobilization. Clin Sci (Lond) 1987;72:503–9 [DOI] [PubMed] [Google Scholar]
- 38.Glover EI, Phillips SM, Oates BR, Tang JE, Tarnopolsky MA, Selby A, Smith K, Rennie MJ. Immobilization induces anabolic resistance in human myofibrillar protein synthesis with low and high dose amino acid infusion. J Physiol 2008;586:6049–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drummond MJ, Dickinson JM, Fry CS, Walker DK, Gundermann DM, Reidy PT, Timmerman KL, Markofski MM, Paddon-Jones D, Rasmussen BB, et al. Bed rest impairs skeletal muscle amino acid transporter expression, mTORC1 signaling, and protein synthesis in response to essential amino acids in older adults. Am J Physiol Endocrinol Metab 2012;302:E1113–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wall BT, Snijders T, Senden JM, Ottenbros CL, Gijsen AP, Verdijk LB, van Loon LJ. Disuse impairs the muscle protein synthetic response to protein ingestion in healthy men. J Clin Endocrinol Metab 2013;98:4872–81 [DOI] [PubMed] [Google Scholar]
- 41.Sonne MP, Alibegovic AC, Hojbjerre L, Vaag A, Stallknecht B, Dela F. Effect of 10 days of bedrest on metabolic and vascular insulin action: a study in individuals at risk for type 2 diabetes. J Appl Physiol 2010;108:830–7 [DOI] [PubMed] [Google Scholar]
- 42.Breen L, Stokes KA, Churchward-Venne TA, Moore DR, Baker SK, Smith K, Atherton PJ, Phillips SM. Two weeks of reduced activity decreases leg lean mass and induces "anabolic resistance" of myofibrillar protein synthesis in healthy elderly. J Clin Endocrinol Metab 2013;98:2604–12 [DOI] [PubMed] [Google Scholar]
- 43.Olsen RH, Krogh-Madsen R, Thomsen C, Booth FW, Pedersen BK. Metabolic responses to reduced daily steps in healthy nonexercising men. JAMA 2008;299:1261–3 [DOI] [PubMed] [Google Scholar]
- 44.Kortebein P, Symons TB, Ferrando A, Paddon-Jones D, Ronsen O, Protas E, Conger S, Lombeida J, Wolfe R, Evans WJ. Functional impact of 10 days of bed rest in healthy older adults. J Gerontol A Biol Sci Med Sci 2008;63:1076–81 [DOI] [PubMed] [Google Scholar]
- 45.Bassett DR, Jr, Wyatt HR, Thompson H, Peters JC, Hill JO. Pedometer-measured physical activity and health behaviors in U.S. adults. Med Sci Sports Exerc 2010;42:1819–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gill TM, Allore H, Guo Z. The deleterious effects of bed rest among community-living older persons. J Gerontol A Biol Sci Med Sci 2004;59:755–61 [DOI] [PubMed] [Google Scholar]
- 47.English KL, Paddon-Jones D. Protecting muscle mass and function in older adults during bed rest. Curr Opin Clin Nutr Metab Care 2010;13:34–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suetta C, Hvid LG, Justesen L, Christensen U, Neergaard K, Simonsen L, Ortenblad N, Magnusson SP, Kjaer M, Aagaard P. Effects of aging on human skeletal muscle after immobilization and retraining. J Appl Physiol 2009:107:1172–80 [DOI] [PubMed] [Google Scholar]
- 49.Burd NA, Gorissen SH, van Loon LJ. Anabolic resistance of muscle protein synthesis with aging. Exerc Sport Sci Rev 2013;41:169–73 [DOI] [PubMed] [Google Scholar]
- 50.Simonsick EM, Guralnik JM, Volpato S, Balfour J, Fried LP. Just get out the door! Importance of walking outside the home for maintaining mobility: findings from the women's health and aging study. J Am Geriatr Soc 2005;53:198–203 [DOI] [PubMed] [Google Scholar]
- 51.Landi F, Onder G, Carpenter I, Cesari M, Soldato M, Bernabei R. Physical activity prevented functional decline among frail community-living elderly subjects in an international observational study. J Clin Epidemiol 2007;60:518–24 [DOI] [PubMed] [Google Scholar]
- 52.Pennings B, Groen B, de Lange A, Gijsen AP, Zorenc AH, Senden JM, van Loon LJ. Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men. Am J Physiol Endocrinol Metab 2012;302:E992–9 [DOI] [PubMed] [Google Scholar]
- 53.Houston DK, Nicklas BJ, Ding J, Harris TB, Tylavsky FA, Newman AB, Lee JS, Sahyoun NR, Visser M, Kritchevsky SB. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr 2008;87:150–5 [DOI] [PubMed] [Google Scholar]
- 54.Filion ME, Barbat-Artigas S, Dupontgand S, Fex A, Karelis AD, ubertin-Leheudre M. Relationship between protein intake and dynapenia in postmenopausal women. J Nutr Health Aging 2012;16:616–9 [DOI] [PubMed] [Google Scholar]
- 55.Lemieux FC, Filion ME, Barbat-Artigas S, Karelis AD, Aubertin-Leheudre M. Relationship between different protein intake recommendations with muscle mass and muscle strength. Climacteric 2013. In press [DOI] [PubMed] [Google Scholar]
- 56.Smith K, Barua JM, Watt PW, Scrimgeour CM, Rennie MJ. Flooding with L-[1–13C]leucine stimulates human muscle protein incorporation of continuously infused L-[1–13C]valine. Am J Physiol 1992;262:E372–6 [DOI] [PubMed] [Google Scholar]
- 57.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab 2006;291:E381–7 [DOI] [PubMed] [Google Scholar]
- 58.Wall BT, Hamer HM, de Lange A, Kiskini A, Groen BB, Senden JM, Gijsen AP, Verdijk LB, van Loon LJ. Leucine co-ingestion improves post-prandial muscle protein accretion in elderly men. Clin Nutr 2013;32:412–9 [DOI] [PubMed] [Google Scholar]
- 59.Yang Y, Churchward-Venne TA, Burd NA, Breen L, Tarnopolsky MA, Phillips SM. Myofibrillar protein synthesis following ingestion of soy protein isolate at rest and after resistance exercise in elderly men. Nutr Metab (Lond) 2012;9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burd NA, Yang Y, Moore DR, Tang JE, Tarnopolsky MA, Phillips SM. Greater stimulation of myofibrillar protein synthesis with ingestion of whey protein isolate v. micellar casein at rest and after resistance exercise in elderly men. Br J Nutr 2012;108:958–62 [DOI] [PubMed] [Google Scholar]
- 61.Pennings B, Boirie Y, Senden JM, Gijsen AP, Kuipers H, van Loon LJ. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am J Clin Nutr 2011;93:997–1005 [DOI] [PubMed] [Google Scholar]
- 62.Koopman R, Crombach N, Gijsen AP, Walrand S, Fauquant J, Kies AK, Lemosquet S, Saris WH, Boirie Y, van Loon LJ. Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein. Am J Clin Nutr 2009;90:106–15 [DOI] [PubMed] [Google Scholar]
- 63.Conley TB, Apolzan JW, Leidy HJ, Greaves KA, Lim E, Campbell WW. Effect of food form on postprandial plasma amino acid concentrations in older adults. Br J Nutr 2011;106:203–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Staples AW, Burd NA, West DW, Currie KD, Atherton PJ, Moore DR, Rennie MJ, Macdonald MJ, Baker SK, Phillips SM. Carbohydrate does not augment exercise-induced protein accretion versus protein alone. Med Sci Sports Exerc 2011;43:1154–61 [DOI] [PubMed] [Google Scholar]
- 65.Glynn EL, Fry CS, Drummond MJ, Dreyer HC, Dhanani S, Volpi E, Rasmussen BB. Muscle protein breakdown has a minor role in the protein anabolic response to essential amino acid and carbohydrate intake following resistance exercise. Am J Physiol Regul Integr Comp Physiol 2010;299:R533–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gorissen SH, Burd NA, Hamer HM, Gijsen AP, Groen BB, van Loon LJ. Carbohydrate co-ingestion delays dietary protein digestion and absorption but does not modulate postprandial muscle protein accretion. J Clin Endocrinol Metab 2014. In press [DOI] [PubMed] [Google Scholar]
- 67.Pennings B, Groen BB, van Dijk JW, de Lange A, Kiskini A, Kuklinski M, Senden JM, van Loon LJ. Minced beef is more rapidly digested and absorbed than beef steak, resulting in greater postprandial protein retention in older men. Am J Clin Nutr 2013;98:121–8 [DOI] [PubMed] [Google Scholar]
- 68.Rémond D, Machebeuf M, Yven C, Buffiere C, Mioche L, Mosoni L, Patureau MP. Postprandial whole-body protein metabolism after a meat meal is influenced by chewing efficiency in elderly subjects. Am J Clin Nutr 2007;85:1286–92 [DOI] [PubMed] [Google Scholar]
- 69.de Castro JM, Bellisle F, Feunekes GIJ, Dalix AM, De Graaf C. Culture and meal patterns: a comparison of the food intake of free-living American, Dutch, and French students. Nutr Res 1997;17:807–29 [Google Scholar]
- 70.Tieland M, Borgonjen-Van den Berg KJ, van Loon LJ, de Groot LC. Dietary protein intake in community-dwelling, frail, and institutionalized elderly people: scope for improvement. Eur J Nutr 2012;51:173–9 [DOI] [PubMed] [Google Scholar]
- 71.Mamerow MM, Mettler JA, English KL, Casperson SL, Arentson-Lantz E, Sheffield-Moore M, Layman DK, Paddon-Jones D. Dietary protein distribution positively influences 24-h muscle protein synthesis in healthy adults. J Nutr 2014. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bouillanne O, Curis E, Hamon-Vilcot B, Nicolis I, Chretien P, Schauer N, Vincent JP, Cynober L, Aussel C. Impact of protein pulse feeding on lean mass in malnourished and at-risk hospitalized elderly patients: a randomized controlled trial. Clin Nutr 2013;32:186–92 [DOI] [PubMed] [Google Scholar]
- 73.Tieland M, van de Rest O, Dirks ML, van der Zwaluw N, Mensink M, van Loon LJ, de Groot LC. Protein supplementation improves physical performance in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc 2012;13:720–6 [DOI] [PubMed] [Google Scholar]
- 74.Crane JD, Macneil LG, Tarnopolsky MA. Long-term aerobic exercise is associated with greater muscle strength throughout the life span. J Gerontol A Biol Sci Med Sci 2013;68:631–8 [DOI] [PubMed] [Google Scholar]
- 75.Wroblewski AP, Amati F, Smiley MA, Goodpaster B, Wright V. Chronic exercise preserves lean muscle mass in masters athletes. Phys Sportsmed 2011;39:172–8 [DOI] [PubMed] [Google Scholar]
- 76.Aoyagi Y, Shephard RJ. Sex differences in relationships between habitual physical activity and health in the elderly: practical implications for epidemiologists based on pedometer/accelerometer data from the Nakanojo Study. Arch Gerontol Geriatr 2013;56:327–38 [DOI] [PubMed] [Google Scholar]
- 77.DiPietro L. Physical activity in aging: changes in patterns and their relationship to health and function. J Gerontol A Biol Sci Med Sci 2001;56 Spec No 2:13–22 [DOI] [PubMed] [Google Scholar]
- 78.Fujita S, Rasmussen BB, Cadenas JG, Drummond MJ, Glynn EL, Sattler FR, Volpi E. Aerobic exercise overcomes the age-related insulin resistance of muscle protein metabolism by improving endothelial function and Akt/mammalian target of rapamycin signaling. Diabetes. 2007;56:1615–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Harber MP, Konopka AR, Undem MK, Hinkley JM, Minchev K, Kaminsky LA, Trappe TA, Trappe S. Aerobic exercise training induces skeletal muscle hypertrophy and age-dependent adaptations in myofiber function in young and older men. J Appl Physiol 2012:113;1495–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Harber MP, Konopka AR, Douglass MD, Minchev K, Kaminsky LA, Trappe TA, Trappe S. Aerobic exercise training improves whole muscle and single myofiber size and function in older women. Am J Physiol Regul Integr Comp Physiol 2009;297:R1452–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yarasheski KE, Zachwieja JJ, Bier DM. Acute effects of resistance exercise on muscle protein synthesis rate in young and elderly men and women. Am J Physiol 1993;265:E210–4 [DOI] [PubMed] [Google Scholar]
- 82.Hasten DL, Pak-Loduca J, Obert KA, Yarasheski KE. Resistance exercise acutely increases MHC and mixed muscle protein synthesis rates in 78–84 and 23–32 yr olds. Am J Physiol Endocrinol Metab 2000;278:E620–6 [DOI] [PubMed] [Google Scholar]
- 83.Peterson MD, Rhea MR, Sen A, Gordon PM. Resistance exercise for muscular strength in older adults: a meta-analysis. Ageing Res Rev 2010;9:226–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peterson MD, Sen A, Gordon PM. Influence of resistance exercise on lean body mass in aging adults: a meta-analysis. Med Sci Sports Exerc 2011;43:249–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pennings B, Koopman R, Beelen M, Senden JM, Saris WH, van Loon LJ. Exercising before protein intake allows for greater use of dietary protein-derived amino acids for de novo muscle protein synthesis in both young and elderly men. Am J Clin Nutr 2011;93:322–31 [DOI] [PubMed] [Google Scholar]
- 86.Burd NA, West DW, Moore DR, Atherton PJ, Staples AW, Prior T, Tang JE, Rennie MJ, Baker SK, Phillips SM. Enhanced amino acid sensitivity of myofibrillar protein synthesis persists for up to 24 h after resistance exercise in young men. J Nutr 2011;141:568–73 [DOI] [PubMed] [Google Scholar]
- 87.Phillips B, Williams J, Atherton P, Smith K, Hildebrandt W, Rankin D, Greenhaff P, Macdonald I, Rennie MJ. Resistance exercise training improves age-related declines in leg vascular conductance and rejuvenates acute leg blood flow responses to feeding and exercise. J Appl Physiol 2012;112:347–53 [DOI] [PubMed] [Google Scholar]
- 88.Cermak NM, Res PT, de Groot LC, Saris WH, van Loon LJ. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr 2012;96:1454–64 [DOI] [PubMed] [Google Scholar]
- 89.Carpinelli RN. The size principle and a critical analysis of the unsubstantiated heavier-is-better recommmendation for resistance training. J Exerc Sci Fit 2008;6:67–86 [Google Scholar]
- 90.Phillips SM, Winett RA. Uncomplicated resistance training and health-related outcomes: evidence for a public health mandate. Curr Sports Med Rep 2010;9:208–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Burd NA, West DW, Staples AW, Atherton PJ, Baker JM, Moore DR, Holwerda AM, Parise G, Rennie MJ, Baker SK, et al. Low-load high volume resistance exercise stimulates muscle protein synthesis more than high-load low volume resistance exercise in young men. PLoS ONE 2010;5:e12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fry CS, Glynn EL, Drummond MJ, Timmerman KL, Fujita S, Abe T, Dhanani S, Volpi E, Rasmussen BB. Blood flow restriction exercise stimulates mTORC1 signaling and muscle protein synthesis in older men. J Appl Physiol 2010;108:1199–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mitchell CJ, Churchward-Venne TA, West DW, Burd NA, Breen L, Baker SK, Phillips SM. Resistance exercise load does not determine training-mediated hypertrophic gains in young men. J Appl Physiol 2012;113:71–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Takarada Y, Takazawa H, Sato Y, Takebayashi S, Tanaka Y, Ishii N. Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J Appl Physiol 2000;88:2097–106 [DOI] [PubMed] [Google Scholar]
- 95.Humayun MA, Elango R, Ball RO, Pencharz PB. Reevaluation of the protein requirement in young men with the indicator amino acid oxidation technique. Am J Clin Nutr 2007;86:995–1002 [DOI] [PubMed] [Google Scholar]