Abstract
Adolescence is a critical period, because major physical and psychologic changes occur during a very short period of time. Changes in dietary habits may induce different types of nutritional disorders and are likely to track into adulthood. The aim of this review is to describe the key findings related to nutritional status in European adolescents participating in the HELENA (Healthy Lifestyle in Europe by Nutrition in Adolescence) study. We performed a cross-sectional study in 3528 (1845 females) adolescents aged 12.5–17.5 y. Birth weight was negatively associated with abdominal fat mass in adolescents and serum leptin concentrations (in female adolescents), providing additional evidence for a programming effect of birth weight on energy homeostasis control. Breakfast consumption was associated with lower body fat content and healthier cardiovascular profile. Adolescents eat half of the recommended amount of fruit and vegetables and less than two-thirds of the recommended amount of milk and milk products but consume more meat and meat products, fats, and sweets than recommended. For beverage consumption, sugar-sweetened beverages, sweetened milk, low-fat milk, and fruit juice provided the highest amount of energy. Although the intakes of saturated fatty acids (FAs) and salt were high, the intake of polyunsaturated FAs was low. Adolescents spent, on average, 9 h/d of their waking time (66–71% and 70–73% of the registered time in boys and girls, respectively) in sedentary activities. Factors associated with adolescents’ sedentary behavior included the following: 1) age; 2) media availability in the bedroom; 3) sleeping time; 4) breakfast consumption; and 5) season. Sedentary time was also associated with cardiovascular risk factors and bone mineral content. In European adolescents, deficient concentrations were identified for plasma folate (15%), vitamin D (15%), pyridoxal 5′-phosphate (5%), β-carotene (25%), and vitamin E (5%). Scientists and public health authorities should raise awareness of the importance of a healthy and sustainable lifestyle as a foundation of the health of the European population, now and in the future.
Introduction
Adolescence is a critical period in life, because major physical and psychologic changes occur during a very short period of time. Nutritional issues in adolescence are mainly characterized by increased energy and nutrient requirements and changes in dietary habits, which could induce different types of nutrition-related disorders and are likely to track into adulthood (1).
Nutrition related noncommunicable diseases have their origin very early in life, and they develop during childhood and adolescence (2). Apart from genetics, lifestyle behaviors are the main determinants of these diseases, especially dietary patterns and sedentary behaviors (3, 4). During adolescence, the most prevalent nutritional problems are not only obesity and its comorbidities but also some micronutrient deficiencies (5). Literature on lifestyle factors and diseases in European adolescents is scarce (6). Therefore, the HELENA (Healthy Lifestyle in Europe by Nutrition in Adolescence)10 study aimed to investigate different lifestyle factors and health outcomes among European adolescents. The aim of this review is to summarize the main findings of the HELENA study related to the nutritional programming of adolescent nutritional diseases, dietary intake, physical activity, and vitamin status in European adolescents.
Methods
The HELENA cross-sectional study was designed to assess the nutritional status of the adolescent population in Europe. Because the majority of the European adolescents are living in urban areas, we aimed to assess adolescents (aged 12.5–17.5 y) in 10 cities, with >100,000 inhabitants: 1) Athens, Greece; 2) Dortmund, Germany; 3) Ghent, Belgium; 4) Heraklion, Crete; 5) Lille, France; 6) Pécs, Hungary; 7) Rome, Italy; 8) Stockholm, Sweden; 9) Vienna, Austria; and 10) Zaragoza, Spain. Inclusion criteria were as follows: 1) not to participate simultaneously in another clinical trial; 2) be free of any acute infection lasting <1 wk before the inclusion; and 3) have valid data for age, sex, and BMI. A total of 3528 (1845 females) adolescents participated in the study. One-third of the adolescents were also invited to have a blood sampling for additional analysis. In all the cities, the study protocol was approved by the corresponding ethics committees. The adolescents and their parents signed the written informed consent.
The HELENA cross-sectional study was conceived to obtain standardized, reliable and harmonized data on the following: 1) dietary intake; 2) food choices and preferences; 3) anthropometry; 4) serum indicators of lipid and glucose metabolism; 5) vitamin and mineral status; 6) immunoinflammatory markers; 7) physical activity and fitness; and 8) genetic polymorphisms. Detailed information on specific methods will be provided in each section.
Results
Perinatal nutrition—how does it affect adolescents?
Nutritional factors in early life may have long-term physiologic effects in humans. There is evidence that low birth weight is associated with cardiovascular disease risk and with unhealthy body composition later in life (2). It was also suggested that breastfeeding may protect against the development of type 2 diabetes, cardiovascular diseases, and obesity (7).
Assessment of perinatal and early life factors.
We developed a questionnaire for parents to collect information on the adolescents’ birth weight, gestational age, and breastfeeding duration. Parents were specifically asked to recall this information from health booklets, in which perinatal information is recorded by health professionals. The duration of any breastfeeding and of exclusive breastfeeding was coded (Table 1). First, we assessed associations between birth weight or breastfeeding and health outcomes of interest and then the influence of gene and/or environment on these associations.
TABLE 1.
Duration of breastfeeding according to the study centers in adolescents from the HELENA study1
| Study center | Never | <3 mo | 3 to <6 mo | ≥6 mo |
| % | ||||
| Athens, Greece | 12.1 | 27.5 | 30.2 | 30.2 |
| Dortmund, Germany | 25.6 | 26.3 | 19.5 | 28.5 |
| Ghent, Belgium | 27.2 | 31.3 | 24.9 | 16.6 |
| Heraklion, Crete | 28.0 | 43.6 | 22.7 | 5.7 |
| Lille, France | 42.8 | 33.3 | 14.0 | 10.0 |
| Pécs, Hungary | 6.2 | 29.6 | 30.8 | 33.4 |
| Rome, Italy | 16.0 | 26.6 | 24.5 | 32.8 |
| Stockholm, Sweden | 4.5 | 21.8 | 23.1 | 50.6 |
| Vienna, Austria | 7.8 | 32.7 | 23.0 | 36.5 |
| Zaragoza, Spain | 14.3 | 25.8 | 32.7 | 27.2 |
HELENA, healthy lifestyle in Europe by nutrition in adolescence.
Nutritional programming in European adolescents.
We showed that birth weight was negatively associated with abdominal fat mass independently of total fat mass (8). One of the main genetic susceptibility factors for obesity is the so-called fat mass and obesity (FTO) associated gene. We observed that the minor A allele of the FTO rs9939609 polymorphism was significantly associated with higher BMI, body fat percentage, and fat mass index (9).
Birth weight was negatively associated with serum leptin concentrations only in female adolescents, providing additional evidence for a sex-specific programming effect of birth weight on the energy homeostasis control (10). We also showed that physical activity attenuates the negative effect of low birth weight on leptin concentrations in female adolescents (11). Ponderal index at birth interacted with the leptin rs10244329 and rs3828942 polymorphisms and the leptin receptor rs8179183 polymorphism. These results are consistent with a higher susceptibility to the deleterious effect of risk alleles on total adiposity in those adolescents born with a low ponderal index (12). We also observed that physical activity attenuated the effect of low birth weight on insulin resistance in adolescents (13).
We showed that birth weight was significantly associated with serum EPA and DHA in adolescents (14). Insufficient fetal nutrition and growth may partially program the activity and/or expression of the enzymes required for the metabolic endogenous synthesis of these FAs.
Participating to a vast genome-wide association meta-analysis with birth weight available and follow-up study, 7 new loci, some of them linked to type 2 diabetes, adult height, and blood pressure, were also found to be associated with birth weight (15).
Breastfeeding modulates the influence of the PPARγ Pro12Ala polymorphism on BMI of adolescents. PPARγ variants influence the BMI of adolescents who were never breastfed, although it has no influence on adiposity if the individuals were breast-fed, regardless of its duration (16).
Breastfeeding was not associated with the inflammatory status in healthy adolescents (17). We also showed that longer breastfeeding was associated with a higher performance in the standing long-jump test in adolescents (18).
Dietary intake in European adolescents: issues and controversies
Methods used to assess dietary intake.
Dietary intake was assessed via 2 nonconsecutive self-reported 24-h recalls using the HELENA-Dietary Assessment Tool (DIAT) program (19) and an FFQ. The self-administration took place in a computer classroom where the pupils completed the HELENA-DIAT autonomously while field workers were present to give assistance. Consumed foods were translated to nutrients by use of the German Food Code and Nutrient Data Base (Bundeslebensmittelschlüssel, version II.3.1) (20). The multiple source method was used to estimate the habitual dietary intake of nutrients, total energy, and foods, corrected for age, sex, and study center (21, 22). Supplement use was not considered.
Adolescents reported in a questionnaire to what extent they agree (1 for strongly disagree to 7 for strongly agree) to the following statement: “I often skip breakfast.” Adolescents were categorized into 3 groups: 1) “consumers” (1 or 2); 2) “occasional consumers” (3, 4, or 5); and 3) “skippers” (6 or 7).
An overall diet quality index for adolescents was calculated based on the dietary intake information obtained via the 2 24-h dietary recalls. It was based on an existing diet quality index for preschoolers (23). Two dietary indices were calculated, with the only difference between them being the inclusion or not of physical activity (24).
Dietary intake and associated factors.
Only half of the adolescents were breakfast consumers (51% of males vs. 45% of females), and 25% of males were breakfast skippers vs. 33% of females (25). Adolescents who regularly consume breakfast have lower body fat content, higher cardiorespiratory fitness, and a healthier cardiovascular profile, especially in males (26).
Food intake among European adolescents was not optimal compared with food-based dietary guidelines. Adolescents eat half of the recommended amount of fruit and vegetables and less than two-thirds of the recommended amount of milk products but consume much more meat products, fats, and sweets than recommended (27).
When investigating beverage consumption, results showed that water was consumed by the largest percentage of adolescents, followed by sugar-sweetened beverages, fruit juice, and sweetened milk. Sugar-sweetened beverages, sweetened milk, low-fat milk, and fruit juice provided the highest amount of energy. Patterns of energy intake from each beverage varied between countries (28). Multiple linear regression analysis showed that the frequency of sugar-sweetened beverage consumption was significantly associated with the HOMA-IR after controlling for potential confounders (29).
In both sexes, waist circumference and sum of skin folds were inversely associated with consumption of milk and yogurt, and milk- and yogurt-based beverages, whereas a positive association was observed with cardiorespiratory fitness. Moreover, dairy consumption was inversely associated with cardiovascular disease risk score (β = −0.230, P = 0.001) in adolescent girls (30).
The dietary index including physical activity was inversely associated with the HOMA-IR and directly with the quantitative insulin sensitivity check index in females, but not in males, after adjusting for pubertal status, study center, BMI, and cardiorespiratory fitness. Therefore, considering physical activity as part of the dietary index is of relevance because the resulted index is inversely related to insulin resistance independently of potential confounders (31).
The intakes of most nutrients were, in general, in line with the German/Austrian/Swiss Nutrition Societies recommendations. Although the intakes of SFAs and salt were too high, the intake of PUFAs was too low. Furthermore, the intakes of vitamin D, folate, iodine, and fluoride were less than ∼55% of the recommendations (32).
Energy intake was inversely associated with fat mass and positively associated with vigorous physical activity (33). Active youths develop lean bodies while ingesting relatively large amounts of energy and accompanying nutrients needed for healthy growth.
Sedentary behavior in European adolescents
The amount of daily sedentary time in adolescents across Europe is rather unknown. The accelerometer data from the HELENA study provides a great opportunity to determine the sedentary levels of European adolescents and to identify associated factors with excess sedentary time.
Sedentary time assessment.
Sedentary time was assessed using a reliable self-reported sedentary behavior questionnaire (34) and accelerometry (GT1M accelerometer; Actigraph) (35, 36).
Sedentary time of European adolescents participating in the HELENA study.
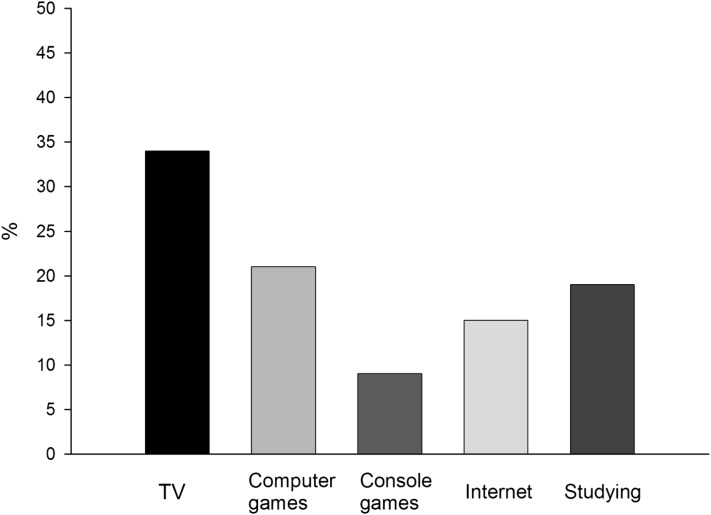
The percentage of self-reported time spent on different sedentary activities in a typical day in adolescents from the HELENA study is presented in Figure 1 (34). Adolescents spent, on average, 9 h/d of their waking time (66–71% and 70–73% of the registered time in boys and girls, respectively) in sedentary activities (35). Based on self-reported measures, we observed that one-third of adolescents spent >2 h/d watching television during weekdays (34).
FIGURE 1.
Percentage of daily time spent on different sedentary activities in a typical weekday in adolescents from the HELENA study (based on results from reference 34). N = 3278 adolescents (1537 males and 1741 females). HELENA, healthy lifestyle in Europe by nutrition in adolescence; TV, television.
The following are factors associated with excess sedentary time.
1) Age: Sedentary time was higher in older adolescents and in those with higher pubertal stage (35). When assessed by questionnaire, we observed that adolescents younger than 15 y were 22% less likely to watch television >2 h/d (34).
2) Media availability in the bedroom: Almost two-thirds of European males and half of females had a television in their bedroom, and 62% of males and 49% of females had a computer in their bedroom (34). Adolescents with a television in the bedroom were 2.7 times more likely to exceed the >2 h/d television recommendations.
3) Sleeping: Adolescents sleeping <8 h/d were more sedentary (∼15 min/d) as assessed by accelerometry and were more likely to exceed the >2 h/d recommendations for television (36). Short sleepers tend to wake up more tired (37) and to reduce their participation in organized sports (38), which may explain the association between short sleep duration and increased sedentary time.
4) Breakfast consumption: Breakfast consumption was associated with sedentary time in both males and females yet with some inconsistencies (39). Male skippers spent, on average, ∼45 min/d more on objectively measured sedentary time compared with regular consumers (39).
5) Season: Physical activity engagement is influenced by many individual, social, and environmental factors, such as the built environment (40). European females were more sedentary during winter compared with spring (Fig. 2), and females from the central–north region of Europe were more sedentary during winter compared with spring. No association between season and sedentary time was observed in adolescents from southern Europe (41).
FIGURE 2.
Objectively measured sedentary time in European females in winter, autumn, and spring (based on results from reference 46). Values are mean estimates. *Different from spring, P < 0.05.
The following are associations of sedentary behavior with other lifestyle behaviors and cardiovascular risk factors.
1) Food consumption and dietary pattern: Adolescents reporting >4 h/d of television viewing, playing computer games, and using the Internet for recreation were more likely to consume sweetened beverages and less likely to consume fruit than those who spent <2 h/d (42). The time spent in these sedentary behaviors were also related with adolescents’ dietary patterns, such as “snacking,” “breakfast,” and “health conscious” (43).
2) Cardiorespiratory fitness: Females who spent ≥69% of their waking hours in sedentary time had lower levels of cardiorespiratory fitness. Meeting the physical activity recommendations (60 min/d of moderate-to-vigorous physical activity) may attenuate the harmful effect of sedentary time on cardiorespiratory fitness (44).
3) Cardiovascular diseases risk factors: We sought to determine the association of time engaged in sedentary behaviors (television viewing and video games) with single risk factors (45) and a clustered cardiometabolic risk (46). We observed no association between television viewing and metabolic risk in adolescents, whereas playing video games >4 h/d (on weekend days) was associated with the presence of a clustered metabolic risk in males (46).
4) Bone mineral content: The sum of the time spent on reported sedentary behaviors was associated with bone mineral content (47). The use of the Internet for non-study activities was negatively associated with whole-body bone mineral content in males, whereas in females, time spent studying was negatively associated with femoral neck bone mineral content (47).
Vitamin status in adolescents: is there a need for intervention?
New physiologic functions beyond the classical ones (48, 49) and the roles vitamins play in the prevention of chronic diseases are under debate (50). In Europe, there is a lack of comparable data on vitamin intake and blood concentrations (51, 52). Reference ranges specifically developed for children and adolescents are missing, because most of the cutoffs used were those defined for adults (51, 53).
Vitamin status measurement.
A subsample (n = 1089) of the HELENA study was randomly selected for blood sampling. A specific handling, transport, and traceability system for biologic samples was developed (54). Concentrations of plasma folate, RBC folate, vitamin B-6 [pyridoxal 5′-phosphate (PLP)], vitamin B-12 (cobalamin and holotranscobalamin), vitamin D (25-hydroxyvitamin D), retinol, β-carotene, α-tocopherol, and vitamin C were measured (55–57).
Vitamin status of European adolescents.
Descriptive values are presented in Table 2 together with currently accepted reference values (49–68). Insufficient concentrations were identified for vitamin D (75%), PLP (20%), plasma folate (35%), and RBC folate (30%). Deficient concentrations were identified for plasma folate (15%), vitamin D (15%), PLP (5%), β-carotene (25%), and vitamin E (5%). A total of 71% of the females had RBC folate concentrations <906 nmol/L. Concentrations above this cutoff are considered maximally protective against folate-dependent neural tube defects. This high percentage of young females identified with low RBC folate concentrations should be considered and requires additional study. As expected, vitamin B-12 status is not a prevalent problem in adolescents (56).
TABLE 2.
Blood vitamin concentrations in adolescents aged 12.5–17.49 y from the HELENA study and reference values used to analyze sufficiency status1
| Vitamin biomarker | Total | n | Males | n | Females | n | Reference values |
| Plasma retinol, μg/L | 356 ± 108 | 933 | 363 ± 104 | 444 | 351 ± 111 | 489 | Incipient deficiency, 10–20 μg/dL (0.7 μmol/L) |
| Deficiency, <10 μg/dL (0.35 μmol/L) (58) | |||||||
| Plasma β-carotene, μg/L | 245 ± 170 | 942 | 237 ± 180 | 449 | 254 ± 159 | 493 | Preventing effect, >0.5 μmol/L |
| Acceptable, >0.3 μmol/L (58, 59) | |||||||
| Plasma α-tocopherol, μg/mL | 9.9 ± 2.1 | 933 | 9.5 ± 2.0 | 444 | 10.3 ± 2.1 | 489 | Adequate, 12–46 μmol/L |
| Primary prevention of cardiovascular disease, >30 μmol/L (60) | |||||||
| Plasma vitamin C, mg/L | 10.3 ± 3.3 | 1053 | 10.0 ± 3.3 | 501 | 10.6 ± 3.3 | 552 | Deficiency, <1 mg/L (10 μmol/L) |
| Insufficiency, <5 mg/L | |||||||
| Adequate, 5–15 mg/L (61) | |||||||
| Plasma vitamin D, nmol/L | 58.8 ± 23.1 | 1006 | 57.4 ± 22.7 | 470 | 60.0 ± 23.4 | 536 | Sufficiency, ≥75 nmol/L |
| Insufficiency, 50–75 nmol/L | |||||||
| Deficiency, 27.5–49.99 nmol/L | |||||||
| Severe deficiency, <27.5 nmol/L (59, 62, 63) | |||||||
| Plasma pyridoxal 5′-phosphate, nmol/L | 49.1 (5.75–899) | 974 | 53.7 (9.84–483) | 448 | 46.2 (5.75–899) | 526 | Sufficiency, >30 nmol/L |
| Insufficiency, 20–30 nmol/L | |||||||
| Deficiency, <20 nmol/L (64, 65) | |||||||
| Plasma folate, nmol/L | 16.0 (4.8–82.9) | 1051 | 15.9 (4.9–82.9) | 499 | 16.0 (4.8–79.8) | 522 | Sufficiency, >13.6 nmol/L (6 μg/L) |
| Insufficiency, 13.6–10.2 nmol/L (4.5 μg/L) | |||||||
| Deficiency, <10.2 nmol/L (4.5 μg/L) (66) | |||||||
| RBC folate, nmol/L | 722 (140–3673) | 1040 | 727 (139–3673) | 495 | 710 (213–2609) | 545 | Deficiency, <566 nmol/L (250 μg/L) (66) |
| Deficiency, <318 nmol/L (140 μg/L) (59) | |||||||
| Optimal for lowering neural tube defect risks, >906 nmol/L (400 μg/L) (67) (only for females) | |||||||
| Plasma cobalamin, pmol/L | 319 (110–1091) | 1051 | 302 (114–961) | 499 | 334 (110–1091) | 522 | Deficiency, <149 pmol/L (59) |
| Plasma holotranscobalamin, pmol/L | 57.8 (16.3–331.8) | 1018 | 58.5 (16.3–318) | 467 | 57.5 (18.4–331.8) | 551 | Deficiency, <30 pmol/L (68) |
Data are shown as means ± SDs or medians (range). HELENA, healthy lifestyle in Europe by nutrition in adolescence.
Vitamin D and folate were identified as the vitamins most at risk in the analyzed adolescents. Median intakes of vitamin D and folate were <50% of reference values, followed by vitamin E (median intakes at 75% of reference values) and vitamins A and C (median intakes at 90% of reference values) (32). All associations between nutrient intake and concentration of biomarkers were significantly positive, and for all nutrients, biomarker status significantly increased with increasing tertiles of nutrient intake (69).
According to our own percentile values, blood concentrations of some vitamins are influenced by age and sex. Sex differences should be considered when analyzing vitamin C, α-tocopherol, vitamin B-12, and vitamin B-6. Age should be considered when analyzing plasma folate, holotranscobalamin, and RBC folate in males and β-carotene, plasma folate, and cobalamin in females. For the analysis of retinol concentrations, age should be considered in both sexes.
Regarding cardiorespiratory fitness, associations were observed with retinol and vitamin C in males and β-carotene and vitamin D in females. Muscular fitness was associated with β-carotene, retinol, and α-tocopherol in males and β-carotene and vitamin D in females (70). A significant relation between vitamin D concentrations and bone mineral content was only observed in physically active adolescents (71).
Conclusions
The HELENA study observations are obtained from a healthy population of urban European adolescents, and the interpretation is mainly descriptive given the cross-sectional design. Nevertheless, a number of important public health reflections can be made.
Lifestyle habits established during childhood and adolescence are likely to track into adulthood. Building further on this premise, the reported observations on health behavior and health status in European adolescents lead to rather pessimistic projections for lifestyle-related mortality and morbidity in the upcoming generations. Sedentary patterns and unbalanced diets seem to be widely present in the European society at the beginning of the 21st century and cast a public health shadow into the future for which, as yet, no clear answer is available. It will make a huge challenge to tackle these problems in a sustainable way, because their drivers are at the heart of the modern economic and societal life.
Our adolescents’ diets clearly suffer from critical imbalances at the level of nutrient intakes and at the level of foods, especially fruit and vegetables, sugar-sweetened beverages, sweet foods, and meat. In addition, there seems to be a major concern regarding adolescents’ breakfast consumption. Food manufacturers, retailers, and public health workers should collaborate and, in concert with a societal debate on this topic, find ways to make appealing changes in the everyday dietary experience of our adolescents as part of a stimulating and easily adoptable lifestyle.
For the first time, data on vitamin status are presented using the same methodology in European adolescents, showing the need for public health authorities to find ways of enhancing particularly vitamin D and folic acid status in identified risk groups.
At the level of physical activity and sedentary patterns, scientists and public health authorities should work together to find creative solutions for a reversing of the generalized “slowing down” of the general population, indeed most critically demonstrated here in European adolescents.
The current recovering from the deep economic and political crisis in Europe should be done in parallel with efforts for integrating a healthy and sustainable lifestyle as a foundation of the health of the European population, now and in the future.
Acknowledgments
The members of the HELENA Study Group are as follows: Coordinator: Luis A. Moreno; Core Group members: Luis A. Moreno, Fréderic Gottrand, Stefaan De Henauw, Marcela González-Gross, and Chantal Gilbert; Steering Committee: Anthony Kafatos (President), Luis A. Moreno, Christian Libersa, Stefaan De Henauw, Jackie Sánchez, Fréderic Gottrand, Mathilde Kersting, Michael Sjöstrom, Dénes Molnár, Marcela González-Gross, Jean Dallongeville, Chantal Gilbert, Gunnar Hall, Lea Maes, and Luca Scalfi; Project Manager: Pilar Meléndez; University of Zaragoza (Spain): Luis A. Moreno, Jesús Fleta, José A. Casajús, Gerardo Rodríguez, Concepción Tomás, María I. Mesana, Germán Vicente-Rodríguez, Adoración Villarroya, Carlos M. Gil, Ignacio Ara, Juan Revenga, Carmen Lachen, Juan Fernández Alvira, Gloria Bueno, Aurora Lázaro, Olga Bueno, Juan F. León, Jesús Ma Garagorri, Manuel Bueno, Iris Iglesia, Paula Velasco, and Silvia Bel; Spanish National Research Council (Spain): Ascensión Marcos, Julia Wärnberg, Esther Nova, Sonia Gómez, Esperanza Ligia Díaz, Javier Romeo, Ana Veses, Mari Angeles Puertollano, Belén Zapatera, and Tamara Pozo; University of Lille 2 (France): Laurent Beghin, Christian Libersa, Frédéric Gottrand, Catalina Iliescu, and Juliana Von Berlepsch; Research Institute of Child Nutrition Dortmund, Rheinische Friedrich Wilhelms University Bonn (Germany): Mathilde Kersting, Wolfgang Sichert-Hellert, and Ellen Koeppen; University of Pécs (Hungary): Dénes Molnar, Eva Erhardt, Katalin Csernus, Katalin Török, Szilvia Bokor, Mrs. Angster, Enikö Nagy, Orsolya Kovács, and Judit Répasi; University of Crete School of Medicine (Greece): Anthony Kafatos, Caroline Codrington, María Plada, Angeliki Papadaki, Katerina Sarri, Anna Viskadourou, Christos Hatzis, Michael Kiriakakis, George Tsibinos, Constantine Vardavas Manolis Sbokos, Eva Protoyeraki, and Maria Fasoulaki; Institute of Nutritional and Food Sciences—Food Physiology, Rheinische Friedrich Wilhelms University (Germany): Peter Stehle, Klaus Pietrzik, Marcela González-Gross, Christina Breidenassel, Andre Spinneker, Jasmin Al-Tahan, Miriam Segoviano, Anke Berchtold, Christine Bierschbach, Erika Blatzheim, Adelheid Schuch, and Petra Pickert; University of Granada (Spain): Manuel J. Castillo Garzón, Ángel Gutiérrez Sáinz, Francisco B. Ortega Porcel, Jonatan Ruiz Ruiz, Enrique García Artero, Vanesa España Romero, David Jiménez Pavón, Cristóbal Sánchez Muñoz, Victor Soto, Palma Chillón, Jose M. Heredia, Virginia Aparicio, Pedro Baena, Claudia M. Cardia, and Ana Carbonell; National Institute for Agricultural Research for Food and Nutrition (Italy): Davide Arcella, Giovina Catasta, Laura Censi, Donatella Ciarapica, Marika Ferrari, Cinzia Le Donne, Catherine Leclerq, Luciana Magrì, Giuseppe Maiani, Rafaela Piccinelli, Angela Polito, Raffaela Spada, and Elisabetta Toti; University of Napoli “Federico II,” Department of Food Science (Italy): Luca Scalfi, Paola Vitaglione, and Concetta Montagnese; Ghent University (Belgium): Ilse De Bourdeaudhuij, Stefaan De Henauw, Tineke De Vriendt, Lea Maes, Christophe Matthys, Carine Vereecken, Mieke de Maeyer, Charlene Ottevaere, and Inge Huybrechts; Medical University of Vienna (Austria): Kurt Widhalm, Katharina Phillipp, Sabine Dietrich, Birgit Kubelka, and Marion Boriss-Riedl; Harokopio University (Greece): Yannis Manios, Eva Grammatikaki, Zoi Bouloubasi, Tina Louisa Cook, Sofia Eleutheriou, Orsalia Consta, George Moschonis, Ioanna Katsaroli, George Kraniou, Stalo Papoutsou, Despoina Keke, Ioanna Petraki, Elena Bellou, Sofia Tanagra, Kostalenia Kallianoti, Dionysia Argyropoulou, Katerina Kondaki, Stamatoula Tsikrika, and Christos Karaiskos; Pasteur Institute of Lille (France): Jean Dallongeville and Aline Meirhaeghe; Karolinska Institute (Sweden): Michael Sjöstrom, Patrick Bergman, María Hagströmer, Lena Hallström, Mårten Hallberg, Eric Poortvliet, Julia Wärnberg, Nico Rizzo, Linda Beckman, Anita Hurtig Wennlöf, Emma Patterson, Lydia Kwak, Lars Cernerud, Per Tillgren, and StefaanSörensen; Asociación de Investigación de la Industria Research Association of the Agrofood Industry (Spain): Jackie Sánchez-Molero, Elena Picó, Maite Navarro, Blanca Viadel, José Enrique Carreres, Gema Merino, Rosa Sanjuán, María Lorente, María José Sánchez, and Sara Castelló Campden BRI (United Kingdom): Chantal Gilbert, Sarah Thomas, Elaine Allchurch, and Peter Burguess; Institute for Food and Biotechnology (Sweden): Gunnar Hall, Annika Astrom, Anna Sverkén, and Agneta Broberg; Meurice Research and Development (Belgium): Annick Masson, Claire Lehoux, Pascal Brabant, Philippe Pate, and Laurence Fontaine; Campden and Chorleywood Food Development Institute (Hungary): Andras Sebok, Tunde Kuti, and Adrienn Hegyi; Productos Aditivos (Spain): Cristina Maldonado and Ana Llorente; Cárnicas Serrano (Spain): Emilio García; Cederroth International (Sweden): Holger von Fircks, Marianne Lilja Hallberg, and Maria Messerer; Lantmännen Food Research and Development (Sweden): Mats Larsson, Helena Fredriksson, Viola Adamsson, and Ingmar Börjesson; European Food Information Council (Belgium): Laura Fernández, Laura Smillie, and Josephine Wills; and Technical University of Madrid (Spain): Marcela González-Gross, Agustín Meléndez, Pedro J. Benito, Javier Calderón, David Jiménez-Pavón, Jara Valtueña, Paloma Navarro, Alejandro Urzanqui, Ulrike Albers, Raquel Pedrero, and Juan José Gómez Lorente. All authors read and approved the final manuscript. The authors thank Christel Bierschbach, Adelheid Schuch, Anke Berchtold, Petra Pickert, Anke Carstensen, Silvia Soria, Sergio Consuegra, Miriam Pedrero, and Rosa María Torres for their contribution to laboratory work and Laura Barrios for support in the statistical analysis of the data. All authors read and approved the final version of the manuscript.
Footnotes
Abbreviations used: DIAT, dietary assessment tool; FTO, fat mass and obesity; HELENA, healthy lifestyle in Europe by nutrition in adolescence; PLP, pyridoxal 5′-phosphate.
References
- 1.Moreno LA. Adolescence. In: Koletzko B, ed. Pediatric Nutrition in Practice. Basel: Karger; 2008. p. 114–7.
- 2.Fernandez-Twinn DS, Ozanne SE. Early life nutrition and metabolic programming. Ann N Y Acad Sci 2010;1212:78–96 [DOI] [PubMed] [Google Scholar]
- 3.Moreno LA, Rodríguez G. Dietary risk factors for development of childhood obesity. Curr Opin Clin Nutr Metab Care 2007;10:336–41 [DOI] [PubMed] [Google Scholar]
- 4.Rey-López JP, Vicente-Rodríguez G, Biosca M, Moreno LA. Sedentary behaviour and obesity development in children and adolescents. Nutr Metab Cardiovasc Dis 2008;18:242–51 [DOI] [PubMed] [Google Scholar]
- 5.Moreno LA, González-Gross M, Kersting M, Molnár D, de Henauw S, Beghin L, Sjöström M, Hagstromer M, Manios Y, Gilbert CC, et al. , on behalf of the HELENA Study Group. Assessing, understanding and modifying nutritional status, eating habits and physical activity in European adolescents. The HELENA Study. Public Health Nutr 2008;11:288–99 [DOI] [PubMed] [Google Scholar]
- 6.Rangul V, Holmen TL, Bauman A, Bratberg GH, Kurtze N, Midthjell K. Factors predicting changes in physical activity through adolescence: the Young-Hunt Study, Norway. J Adolesc Health 2011;48:616–24 [DOI] [PubMed] [Google Scholar]
- 7.Agostoni C, Braegger C, Decsi T, Kolacek S, Koletzko B, Michaelsen KF, Mihatsch W, Moreno LA, Puntis J, Shamir R, et al. ; ESPGHAN Committee on Nutrition. Breast-feeding: a commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr 2009;49:112–25 [DOI] [PubMed] [Google Scholar]
- 8.Labayen I, Ruiz JR, Vicente-Rodríguez G, Turck D, Rodríguez G, Meirhaeghe A, Molnár D, Sjöström M, Castillo MJ, Gottrand F, et al. Early life programming of abdominal adiposity in adolescents; the HELENA study. Diabetes Care 2009;32:2120–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Labayen I, Ruiz JR, Ortega FB, Gottrand F, Huybrechts I, Dallongeville J, Widhalm K, Ferrari M, Buyken A, Kersting M, et al. Body size at birth modifies the effect of fat mass and obesity associated (FTO) rs9939609 polymorphism on adiposity in adolescents: the Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA) study. Br J Nutr 2012;107:1498–504 [DOI] [PubMed] [Google Scholar]
- 10.Labayen I, Ruiz JR, Huybrechts I, Ortega FB, Rodríguez G, DeHenauw S, Breidenassel C, Jiménez-Pavón D, Vyncke KE, Censi L, et al. Sexual dimorphism in the early life programming of serum leptin levels in European adolescents: the HELENA Study. J Clin Endocrinol Metab 2011;96:E1330–4 [DOI] [PubMed] [Google Scholar]
- 11.Labayen I, Ortega FB, Moreno LA, Gonzalez-Gross M, Jimenez-Pavon D, Martínez-Gómez D, Breidenassel C, Marcos A, Molnar D, Manios Y, et al. Physical activity attenuates the negative effect of low birth weight on leptin levels in European adolescents; the HELENA study. Nutr Metab Cardiovasc Dis 2013;23:344–9 [DOI] [PubMed] [Google Scholar]
- 12.Labayen I, Ruiz JR, Moreno LA, Ortega FB, Beghin L, DeHenauw S, Benito PJ, Diaz LE, Ferrari M, Moschonis G, et al. The effect of ponderal index at birth on the relationships between common LEP and LEPR polymorphisms and adiposity in adolescents. Obesity (Silver Spring) 2011;19:2038–45 [DOI] [PubMed] [Google Scholar]
- 13.Ortega FB, Ruiz JR, Hurtig-Wennlöf A, Meirhaeghe A, González-Gross M, Moreno LA, Molnar D, Kafatos A, Gottrand F, Widhalm K, et al. Physical activity attenuates the effect of low birth weight on insulin resistance in adolescents: findings from two observational studies. Diabetes 2011;60:2295–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Labayen I, Moreno LA, Ruiz JR, Ortega FB, Sjostrom M, Huybrechts I, Gonzalez-Gross M, Spinneker A, De Henauw S, Manios Y, et al. Associations of birth weight with serum long chain polyunsaturated fatty acids in adolescents; the HELENA study. Atherosclerosis 2011;217:286–91 [DOI] [PubMed] [Google Scholar]
- 15.Horikoshi M, Yaghootkar H, Mook-Kanamori DO, Sovio U, Taal HR, Hennig BJ, Bradfield JP, St Pourcain B, Evans DM, Charoen P, et al. New loci associated with birth weight identify genetic links between intrauterine growth and adult height and metabolism. Nat Genet 2013;45:76–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verier C, Meirhaeghe A, Bokor S, Breidenassel C, Manios Y, Molnár D, Artero EG, Nova E, De Henauw S, Moreno LA, et al. Breastfeeding modulates the influence of the peroxisome proliferator-activated receptor gamma (PPARG2) Pro12Ala polymorphism on adiposity in adolescents: the HELENA Study. Diabetes Care 2010;33:190–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vérier CM, Duhamel A, Béghin L, Diaz LE, Warnberg J, Marcos A, Gómez-Martínez S, Manios Y, De Henauw S, Sjöström M, et al. Breastfeeding in infancy is not associated with inflammatory status in healthy adolescents. J Nutr 2011;141:411–7 [DOI] [PubMed] [Google Scholar]
- 18.Artero EG, Ortega FB, España-Romero V, Labayen I, Huybrechts I, Papadaki A, Rodriguez G, Mauro B, Widhalm K, Kersting M, et al. Longer breastfeeding is associated with increased lower body explosive strength during adolescence. J Nutr 2010;140:1989–95 [DOI] [PubMed] [Google Scholar]
- 19. Vereecken CA, Covents M, Sichert-Hellert W, Alvira JM, Le Donne C, De Henauw S, De Vriendt T, Phillipp K, Beghin L, Manios Y et al. Development and evaluation of a self-administered computerized 24-h dietary recall method for adolescents in Europe. Int J Obes (Lond) 2008:32(Suppl 5):S26–34. [DOI] [PubMed]
- 20.Dehne LI, Klemm C, Henseler G, Hermann-Kunz E. The German Food Code and Nutrient Data Base (BLS II.2). Eur J Epidemiol 1999;15:355–9 [DOI] [PubMed] [Google Scholar]
- 21.Harttig U, Haubrock J, Knuppel S, Boeing H. The MSM program: web-based statistics package for estimating usual dietary intake using the Multiple Source Method. Eur J Clin Nutr 2011;65(Suppl 1):S87–91 [DOI] [PubMed] [Google Scholar]
- 22.Haubrock J, Nothlings U, Volatier JL, Dekkers A, Ocke M, Harttig U, Illner AK, Knuppel S, Andersen LF, Boeing H. Estimating usual food intake distributions by using the multiple source method in the EPIC-Potsdam Calibration Study. J Nutr 2011;141:914–20 [DOI] [PubMed] [Google Scholar]
- 23.Huybrechts I, Vereecken C, De Bacquer D, Vandevijvere S, Van Oyen H, Maes L, Vanhauwaert E, Temme L, De Backer G, De Henauw S. Reproducibility and validity of a diet quality index for children assessed using a FFQ. Br J Nutr 2010;104:135–44 [DOI] [PubMed] [Google Scholar]
- 24.Vyncke K, Cruz FE, Fajo-Pascual M, Cuenca-Garcia M, De Keyzer W, Gonzalez-Gross M, Moreno LA, Beghin L, Breidenassel C, Kersting M, et al. Validation of the Diet Quality Index for Adolescents by comparison with biomarkers, nutrient and food intakes: the HELENA study. Br J Nutr 2013;109:2067–78 [DOI] [PubMed] [Google Scholar]
- 25.Hallström L, Vereecken CA, Labayen I, Ruiz JR, Le Donne C, Garcia MC, Gilbert CC, Martinez SG, Grammatikaki E, Huybrechts I, et al. Breakfast habits among European adolescents and their association with sociodemographic factors: the HELENA (Healthy Lifestyle in Europe by Nutrition in Adolescence) study. Public Health Nutr 2012;15:1879–89 [DOI] [PubMed] [Google Scholar]
- 26.Hallström L, Labayen I, Ruiz JR, Patterson E, Vereecken CA, Breidenassel C, Gottrand F, Huybrechts I, Manios Y, Mistura L, et al. Breakfast consumption and CVD risk factors in European adolescents: the HELENA (Healthy Lifestyle in Europe by Nutrition in Adolescence) Study. Public Health Nutr 2013;16:1296–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diethelm K, Jankovic N, Moreno LA, Huybrechts I, De Henauw S, De Vriendt T, Gonzalez-Gross M, Leclercq C, Gottrand F, Gilbert CC, et al. Food intake of European adolescents in the light of different food-based dietary guidelines: results of the HELENA (Healthy Lifestyle in Europe by Nutrition in Adolescence) Study. Public Health Nutr 2012;15:386–98 [DOI] [PubMed] [Google Scholar]
- 28.Duffey KJ, Huybrechts I, Mouratidou T, Libuda L, Kersting M, De Vriendt T, Gottrand F, Widhalm K, Dallongeville J, Hallstrom L, et al. Beverage consumption among European adolescents in the HELENA study. Eur J Clin Nutr 2012;66:244–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kondaki K, Grammatikaki E, Jimenez-Pavon D, De Henauw S, Gonzalez-Gross M, Sjostrom M, Gottrand F, Molnar D, Moreno LA, Kafatos A, et al. Daily sugar-sweetened beverage consumption and insulin resistance in European adolescents: the HELENA (Healthy Lifestyle in Europe by Nutrition in Adolescence) Study. Public Health Nutr 2013;16:479–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bel-Serrat S, Mouratidou T, Jimenez-Pavon D, Huybrechts I, Cuenca-Garcia M, Mistura L, Gottrand F, Gonzalez-Gross M, Dallongeville J, Kafatos A, et al. Is dairy consumption associated with low cardiovascular disease risk in European adolescents? Results from the HELENA Study. Pediatr Obes 2013;Jul 15 (Epub ahead of print; DOI:10.1111/j.2047-6310.2013.00187.x) [DOI] [PubMed]
- 31.Jiménez-Pavón D, Sese MA, Huybrechts I, Cuenca-Garcia M, Palacios G, Ruiz JR, Breidenassel C, Leclercq C, Beghin L, Plada M, et al. Dietary and lifestyle quality indices with/without physical activity and markers of insulin resistance in European adolescents: the HELENA study. Br J Nutr 2013;110:1919–25 [DOI] [PubMed] [Google Scholar]
- 32.Diethelm K, Huybrechts I, Moreno L, De Henauw S, Manios Y, Beghin L, Gonzalez-Gross M, Le Donne C, Cuenca-Garcia M, Castillo MJ, et al. Nutrient intake of European adolescents: results of the HELENA (Healthy Lifestyle in Europe by Nutrition in Adolescence) Study. Public Health Nutr 2014;17:486–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cuenca-García M, Ortega FB, Ruiz JR, Labayen I, Moreno LA, Patterson E, Vicente-Rodriguez G, Gonzalez-Gross M, Marcos A, Polito A, et al. More physically active and leaner adolescents have higher energy intake. J Pediatr 2014;164:159–66 [DOI] [PubMed] [Google Scholar]
- 34.Rey-Lopéz JP, Vicente-Rodriguez G, Ortega FB, Ruiz JR, Martinez-Gomez D, De Henauw S, Manios Y, Molnar D, Polito A, Verloigne M, et al. Sedentary patterns and media availability in European adolescents: the HELENA study. Prev Med 2010;51:50–5 [DOI] [PubMed] [Google Scholar]
- 35.Ruiz JR, Ortega FB, Martinez-Gomez D, Labayen I, Moreno LA, De Bourdeaudhuij I, Manios Y, Gonzalez-Gross M, Mauro B, Molnar D, et al. Objectively measured physical activity and sedentary time in European adolescents: the HELENA Study. Am J Epidemiol 2011;174:173–84 [DOI] [PubMed] [Google Scholar]
- 36.Garaulet M, Ortega FB, Ruiz JR, Rey-Lopez JP, Beghin L, Manios Y, Cuenca-García M, Plada M, Diethelm K, Kafatos A, et al. Short sleep duration is associated with increased obesity markers in European adolescents: effect of physical activity and dietary habits. The HELENA study. Int J Obes (Lond) 2011;35:1308–17 [DOI] [PubMed] [Google Scholar]
- 37.Knutson KL. The association between pubertal status and sleep duration and quality among a nationally representative sample of U. S. adolescents. Am J Hum Biol 2005;17:418–24 [DOI] [PubMed] [Google Scholar]
- 38.von Kries R, Toschke AM, Wurmser H, Sauerwald T, Koletzko B. Reduced risk for overweight and obesity in 5- and 6-y-old children by duration of sleep—a cross-sectional study. Int J Obes Relat Metab Disord 2002;26:710–6 [DOI] [PubMed] [Google Scholar]
- 39.Cuenca-García M, Ruiz JR, Ortega FB, Labayen I, Gonzalez-Gross M, Moreno LA, Gomez-Martinez S, Ciarapica D, Hallström L, Wästlund A, et al. Association of breakfast consumption with objectively measured and self-reported physical activity, sedentary time and physical fitness in European adolescents: the HELENA (Healthy Lifestyle in Europe by Nutrition in Adolescence) Study. Public Health Nutr 2013;11:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vanhelst J, Beghin L, Salleron J, Ruiz JR, Ortega FB, De Bourdeaudhuij I, Molnar D, Manios Y, Widhalm K, Vicente-Rodriguez G, et al. A favorable built environment is associated with better physical fitness in European adolescents. Prev Med 2013;57:844–9 [DOI] [PubMed] [Google Scholar]
- 41.Gracia-Marco L, Ortega FB, Ruiz JR, Williams CA, Hagstromer M, Manios Y, Kafatos A, Béghin L, Polito A, De Henauw S, et al. Seasonal variation in physical activity and sedentary time in different European regions. The HELENA study. J Sports Sci 2013;31:1831–40 [DOI] [PubMed] [Google Scholar]
- 42.Santaliestra-Pasías AM, Mouratidou T, Verbestel V, Huybrechts I, Gottrand F, Le Donne C, Cuenca-García M, Díaz LE, Kafatos A, Manios Y, et al. Food consumption and screen-based sedentary behaviors in European adolescents: the HELENA study. Arch Pediatr Adolesc Med 2012;166:1010–20 [DOI] [PubMed] [Google Scholar]
- 43.Santaliestra-Pasías AM, Mouratidou T, Huybrechts I, Beghin L, Cuenca-Garcia M, Castillo MJ, Galfo M, Hallstrom L, Kafatos A, Manios Y, et al. Increased sedentary behaviour is associated with unhealthy dietary patterns in European adolescents participating in the HELENA study. Eur J Clin Nutr 2014;68:300–8 [DOI] [PubMed] [Google Scholar]
- 44.Martinez-Gomez D, Ortega FB, Ruiz JR, Vicente-Rodriguez G, Veiga OL, Widhalm K, Manios Y, Béghin L, Valtueña J, Kafatos A, et al. Excessive sedentary time and low cardiorespiratory fitness in European adolescents: the HELENA study. Arch Dis Child 2011;96:240–6 [DOI] [PubMed] [Google Scholar]
- 45.de Moraes AC, Carvalho HB, Rey-Lopez JP, Gracia-Marco L, Beghin L, Kafatos A, Jiménez-Pavón D, Molnar D, De Henauw S, Manios Y, et al. Independent and combined effects of physical activity and sedentary behavior on blood pressure in adolescents: gender differences in two cross-sectional studies. PLoS One 2013;8:e62006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rey-López JP, Bel-Serrat S, Santaliestra-Pasias A, de Moraes AC, Vicente-Rodriguez G, Ruiz JR, Artero EG, Martínez-Gómez D, Gottrand F, De Henauw S, et al. Sedentary behaviour and clustered metabolic risk in adolescents: the HELENA study. Nutr Metab Cardiovasc Dis 2013;23:1017–24 [DOI] [PubMed] [Google Scholar]
- 47.Gracia-Marco L, Rey-Lopez JP, Santaliestra-Pasias AM, Jimenez-Pavon D, Diaz LE, Moreno LA, Vicente-Rodríguez G. Sedentary behaviours and its association with bone mass in adolescents: the HELENA Cross-Sectional Study. BMC Public Health 2012;12:971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moreno LA, Valtueña J, Pérez-López F, González-Gross M. Health effects related to low vitamin D concentrations: beyond bone metabolism. Ann Nutr Metab 2011;59:22–7 [DOI] [PubMed] [Google Scholar]
- 49.Spinneker A, Sola R, Lemmen V, Castillo MJ, Pietrzik K, González-Gross M. Vitamin B6 status, deficiency and its consequences–an overview. Nutr Hosp 2007;22:7–24 [PubMed] [Google Scholar]
- 50.Kerr MA, Livingstone B, Bates CJ, Bradbury I, Scott JM, Ward M, Pentieva K, Mansoor MA, McNulty H. Folate, related B vitamins, and homocysteine in childhood and adolescence: potential implications for disease risk in later life. Pediatrics 2009;123:627–35 [DOI] [PubMed] [Google Scholar]
- 51.Al-Tahan J, Gonzalez-Gross M, Pietrzik K. B-vitamin status and intake in European adolescents. A review of the literature. Nutr Hosp 2006;21:452–65 [PubMed] [Google Scholar]
- 52.Lambert J, Agostoni C, Elmadfa I, Hulshof K, Krause E, Livingstone B, Socha P, Pannemans D, Samartin S. Dietary intake and nutritional status of children and adolescents in Europe. Br J Nutr 2004;92(Suppl 2):S147–211 [DOI] [PubMed] [Google Scholar]
- 53.Valtueña J, Breidenassel C, Folle K, González-Gross M. Assessment of serum fat-soluble vitamin levels A,D,E and β-carotene provitamin in European adolescents. Differences and variability according to areas. A review. Nutr Hosp 2011;26:280–8 [DOI] [PubMed] [Google Scholar]
- 54.González-Gross M, Breidenassel C, Gómez-Martínez S, Ferrari M, Béghin L, Spinneker A, Díaz LE, Maiani G, Demailly A, Al-Tahan J, et al. Sampling and processing of fresh blood samples within a European multicenter nutritional study: evaluation of biomarker stability during transport and storage. Int J Obes (Lond) 2008;32(Suppl 5):S66–75 [DOI] [PubMed] [Google Scholar]
- 55.González-Gross M, Valtuena J, Breidenassel C, Moreno LA, Ferrari M, Kersting M, De Henauw S, Gottrand F, Azzini E, Widhalm K, et al. Vitamin D status among adolescents in Europe: the Healthy Lifestyle in Europe by Nutrition in Adolescence study. Br J Nutr 2012;107:755–64 [DOI] [PubMed] [Google Scholar]
- 56.González-Gross M, Benser J, Breidenassel C, Albers U, Huybrechts I, Valtueña J, Spinneker A, Segoviano S, Widhalm K, Molnar D, et al. , on behalf of the HELENA Study Group. Gender and age influence blood folate, vitamin B12, vitamin B6, and homocysteine levels in European adolescents: the Helena Study. Nutr Res 2012;32:817–26 [DOI] [PubMed] [Google Scholar]
- 57.Breidenassel C, Valtueña J, González-Gross M, Benser J, Spinneker A, Moreno LA, de Henauw S, Widhalm K, Molnar D, Maiani G, et al. Antioxidant vitamin status (A, E, C, and Beta-carotene) in European adolescents—the HELENA Study. Int J Vitam Nutr Res 2011;81:245–55 [DOI] [PubMed] [Google Scholar]
- 58.Biesalski HK, Fürst P, Kasper H, Kluthe R, Pölert W, Puchstein C, et al. Stähelin HB, Waigand-Brauer M, editors. Ernährungsmedizin. Georg Thieme Verlag. Stuttgart. 2004
- 59. Institute of Medicine, Food and Nutrition Board. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. A report of the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline and Subcommittee on Upper Reference Levels of Nutrients. Washington: National Academy Press; 1998. [PubMed]
- 60. Referenzwerte für die Nährstoffzufuhr. Deutsche Gesellschaft f. Ernährung (DGE), Österreichische Gesellschaft f. Ernährung (ÖGE), Schweizerische Gesellschaft f. Ernährungsforschung, Schweizerische Vereinigung für Ernährung, editors. Frankfurt am Main: Umschau Braus GmbH Verlagsgesellschaft. 2000.
- 61.Pietrzik K, Golly I, Loew D. Handbuch Vitamine. Munich: Elsevier; 2008.
- 62.Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet 1998;351:805–6 [DOI] [PubMed] [Google Scholar]
- 63.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr 2006;84:18–28 Errata in: Am J Clin Nutr 2006;84:1253 and 2007;86:809 [DOI] [PubMed] [Google Scholar]
- 64.Leklem JE. Vitamin B-6: a status report. J Nutr 1990;120(Suppl 11):1503–7 [DOI] [PubMed] [Google Scholar]
- 65.Lui A, Lumeng L, Aronoff GR, Li TK. Relationship between body store of vitamin B6 and plasma pyridoxal-P clearance: metabolic balance studies in humans. J Lab Clin Med 1985;106:491–7 [PubMed] [Google Scholar]
- 66.Pietrzik K, Hages M. Folsäuremangel: Definition, Nachweis und Burteilung der Versorgungslage. In: Pietrzik K, editor. Zuckschwerdt, München (Germany). 1987.
- 67.Daly LE, Kirke PN, Molloy A, Weir DG, Scott JM. Folate levels and neural tube defects. Implications for prevention. JAMA 1995;274:1698–702 [DOI] [PubMed] [Google Scholar]
- 68.Brady J, Wilson L, McGregor L, Valente E, Orning L. Active B12: a rapid, automated assay for holotranscobalamin on the Abbott AxSYManalyzer. Clin Chem 2008;54:567–73 [DOI] [PubMed] [Google Scholar]
- 69.Vandevijvere S, Geelen A, Gonzalez-Gross M, Van’t Veer P, Dallongeville J, Mouratidou T, Dekkers A, Börnhorst C, Breidenassel C, Crispim SP, et al. Evaluation of food and nutrient intake assessment using concentration biomarkers in European adolescents from the Healthy Lifestyle in Europe by Nutrition in Adolescence study. Br J Nutr 2013;109:736–47 [DOI] [PubMed] [Google Scholar]
- 70.Gracia-Marco L, Valtueña J, Ortega FB, Pérez-López FR, Vicente-Rodríguez G, Breidenassel C, Ferrari M, Molnar D, Widhalm K, de Henauw S, et al. HELENA Study Group. Iron and vitamin status biomarkers and its association with physical fitness in adolescents: the HELENA study. J Appl Physiol 2012;113:566–73 [DOI] [PubMed] [Google Scholar]
- 71.Valtueña J, Gracia-Marco L, Vicente-Rodríguez G, González-Gross M, Huybrechts I, Rey-López JP, Mouratidou T, Sioen I, Mesana MI, Martínez AE, et al. ; HELENA Study Group. Vitamin D status and physical activity interact to improve bone mass in adolescents. The HELENA Study. Osteoporos Int 2012;23:2227–37 [DOI] [PubMed] [Google Scholar]