Abstract
Experimental data in animals, but also observational studies in humans, suggest that the composition of the gut microbiota differs in obese vs. lean individuals, in patients with vs. without diabetes, or in patients presenting other diseases associated with obesity or nutritional disbalance, such as non-alcoholic fatty liver disease (NAFLD) or cardiovascular diseases. In this review, we describe how changes in the composition and/or activity of the gut microbiota by administration of nutrients with probiotic or prebiotic properties can modulate host gene expression and metabolism and thereby positively influence host adipose tissue development and related metabolic disorders.
State of the Art
Obesity always relates to “a positive energy balance,” implying that the total caloric intake is greater than the total energy expenditure over a relatively long period of time. In addition, the “obesogenic” diet is often rich in fats and poor in dietary fibers and carbohydrates with a low-glycemic index.
Increased attention was focused on the potential implication of the bacteria that colonize our gut, which in ideal conditions live in symbiosis with the host. Several papers and reviews support the idea that a “dysbiosis” (inadequate changes of gut microbiota composition and/or activity related to host disease) characterizes overweight or obese individuals or those with diabetes (1, 2). Regarding the inadequate composition of the gut microbiota, obese and overweight individuals were initially characterized by a change in the Firmicutes-to-Bacteroides ratio (1, 3). Nevertheless, these results were not strictly confirmed by other papers that extend the concept of dysbiosis to other bacterial phyla, genus, or species (for review, see 3). The analysis of the microbial composition of human fecal samples revealed that the bacterial population can be stratified into 3 robust clusters termed “enterotypes,” dominated by Bacteroides, Prevotella, or Ruminococcus, respectively (4). These enterotypes seem independent of nationality, age, gender, and BMI but are influenced by long-term dietary habits (5).
It appears that, in addition to these quantitative modifications of microbial phyla, obesity and some related metabolic diseases might be associated with modifications of microbial gene expression and therefore with the modulation of metabolic functions of the gut microbiota (6, 7). Thanks to the improvement in the exploration of gut microbiota, mainly through the development of metagenomic approaches, scientists are now able to identify and quantify gut microbial genes and to stratify individuals by their “gut bacterial richness.” Le Chatelier et al. (8) reported that, among overweight and obese Danish individuals, those characterized by a low number of gut microbial genes (meaning a low bacterial richness) present adiposity, insulin resistance, dyslipidemia, and inflammatory phenotype to a larger extent than those characterized by a high bacterial richness. Furthermore, Cotillard et al. (9) highlighted that overweight and obese individuals characterized by low bacterial gene richness seem quite resistant to dietary intervention and exhibit persistent inflammation. These results suggest that alterations of bacterial functions associated with obesity could explain the differential response to dietary intervention.
The microbiome is now considered as a new therapeutic target against obesity and its linked diseases (10). In fact, changes in dietary habits and especially an enrichment in some bioactive food components present in whole grain cereals are able to modify the composition of gut microbiota and could be helpful in the prevention of chronic diseases, including obesity and related disorders, such as type 2 diabetes (11). Wu et al. (5) showed that microbiome composition may change 24 h after initiating a high-fat (HF)4/low-fiber or a high-fiber/low-fat diet but that enterotype identity remained stable during a 10-d nutritional intervention. They suggest that nutrients like dietary fibers, which are not digested by host enzymes but are fermented by gut bacteria, could modulate the gut microbiome in a relatively short period of time, independently of the effect of changes in transit time.
Nowadays, gut microbiota modulation appears as an interesting tool in the prevention and/or treatment of the dysbiosis associated with obesity and metabolic disorders. The gut microbiota may be modulated by the administration of antibiotics, prebiotics, or probiotics or by fecal transplantation. This review will focus on the interest of probiotic and prebiotic approaches in the management of obesity and related diseases.
Interest of Probiotic Approaches in the Management of Obesity and Related Diseases
The oral delivery of viable bacterial strains (probiotics) allow their integration, even if transiently, into the gut ecosystem. Both rodent and human studies reveal interesting results issued from probiotic administration in the treatment or the prevention of obesity (3, 12, 13).
Most of the studies regarding the “anti-obesity” effects of probiotics performed in rodents are achieved with members of the genus Lactobacillus. Three papers (14–16) report that the administration of Lactobacillus gasseri BNR17 is able to suppress body-weight and fat-mass gain in high-sucrose diet-induced obese rodents and to reduce fasting glycemia in db/db mice. Another 3 studies (17–19) show that L. gasseri SBT2055 is able to decrease fat mass and adipocyte size in rodents. Furthermore, Miyoshi et al. (18) reveal that L. gasseri SBT2055 administration decreases adipose tissue inflammation and fat accumulation in the liver, 2 phenomena implicated in the complication of obesity, such as insulin resistance and NAFLD. These results suggest that, in addition to effects on body weight and fat mass, the administration of probiotics is able to counteract some metabolic diseases related to obesity. Other in vivo studies show that L. rhamnosus GG or L. sakei NR28 administration is able to decrease body-weight gain and adipose tissue weight in mice. Both strains are able to decrease lipogenic gene expression in the liver (20). Another study confirms that probiotic administration could modulate lipid metabolism. The administration of L. curvatus HY7601, combined or not with L. plantarum KY1032, reduces plasma cholesterol amount and hepatic lipid content (TGs and cholesterol) in diet-induced obese mice (21). However, probiotic administration is not always successful. For example, 1 study performed in ApoE−/− mice with L. reuteri fails to show any improvement of atherosclerotic lesions, despite a decrease in body-weight gain, adipose tissue, and liver weight (22).
In addition to the studies performed with Lactobacillus species, several studies used specific Bifidobacterium strains alone, such as Bifidobacterium longum or B. adolescentis, or a combination of Bifidobacterium species (B. pseudocatenulatum SPM1204, B. longum SPM1205, and B. longum SPM1207). These studies show that Bifidobacterium spp. are able to decrease body-weight gain and adipose tissue in HF diet–induced obese rats (23–25). A recent study also demonstrated that the administration of the strain B. pseudocatenulatum CECT7765 to diet-induced obese mice can ameliorate metabolic and immunologic alterations associated with obesity (26).
Finally, a study performed with a commercial combination of probiotics (VSL#3: Lactobacillus spp., Bifidobacterium spp., Streptococcus thermophilus) demonstrated that VSL#3 administration decreases the hepatic inflammation induced by HF diet in young rats (27).
The anti-obesity effect described in the previously reported studies are not associated with any modification in food consumption, suggesting other specific effects of probiotic-strain ingestion on adipose tissue and weight gain. However, the mechanisms by which probiotics exert their beneficial effects are not elucidated yet. Some host targets, including lipid and glucose metabolism but also inflammation, are modulated by probiotic administration and could in part be implicated in the anti-obesity effect of probiotics. One study (28) showed that L. paracasei ssp. paracasei F19 administration in germ-free mice or HF diet–fed mice is able to increase circulating amounts of a lipoprotein lipase inhibitor (angiopoietin-related protein 4), leading to a decrease in fat storage. Another study (17) also suggested that probiotic administration could decrease dietary fat absorption. Insulin sensitivity may be improved by probiotic administration. Indeed, L. rhamnosus GG administration increases the production of insulin-sensitizing hormone, such as adiponectine, but also decreases gluconeogenesis in the liver (29). Finally, Lactobacillus spp. administration is associated with a decreased expression of proinflammatory genes in white adipose tissue of HF diet–fed mice (30).
In the majority of the studies mentioned above, the gut microbiota analysis after probiotic administration is lacking, so we cannot state that the selected bacteria per se are responsible for the improvement of obesity and related disorders. Furthermore, in a study reporting gut microbiota composition analysis, the authors showed that probiotic administration modifies the abundance of other bacteria. For example, the administration of L. acidophilus NCDC13 is associated with an increase of Bifidobacterium spp. (31).
The number of clinical studies remains limited Table 1. Only very few human intervention studies were designed to analyze the effect of probiotic administration on body fat and weight (3). In fact, the ingestion of L. gasseri SBT2055 allows for the reduction of fat-mass gain, body weight, BMI, waist, hip, and waist-to-hip ratio in the probiotic group compared with placebo group after 12 wk of intervention in overweight adults (32). These results were confirmed in a recent study with a lower dose of the same bacterial strain than the 1 used in the first study (108 vs. 1011 LG2055 CFU/d) (33). Another interventional study showed that the perinatal modulation of the gut microbiota by a probiotic [L. rhamnosus GG (ATCC53103; American Type Culture Collection)] is able to avoid excessive weight gain during the first years of life (34). Even if they are controversial, some papers suggested that probiotic administration might increase the growth of children and promote obesity (35, 36). However, a recent study counteracts this idea, reporting no effect on anthropometric variables or the serum lipoproteins profile after the administration of L. paracasei ssp. paracasei F19 from 4 to 13 mo of age (37). This study also shows that probiotic administration is associated with a decrease of palmitoleic acid (MUFA strongly linked to visceral obesity) and an increase of putrescin (polyamine with importance for gut integrity) amounts in the plasma (37). The effects on the health of the modifications of bacterial-derived metabolites need to be further evaluated to propose a mechanism by which selected probiotics are able to counteract obesity-related metabolic disorders. One trial suggested that the administration of L. acidophilus NCFM is able to prevent the decrease in insulin sensitivity observed in the placebo group, suggesting that this Lactobacillus strain is able to prevent the establishment of metabolic disorders associated with obesity, such as insulin resistance (38). However, other studies conducted with the strain L. casei Shirota fail to show any improvement of the gut permeability or low-grade inflammation in patients with the metabolic syndrome. The authors suggest that the lack of effect is probably due to the too-short duration of the study or the underdosing of the probiotic strain (39, 40). Another recent study (41) reports that the administration of L. salivarius Ls-33 in obese adolescents fails to show any improvement of inflammatory markers or metabolic variable linked to the metabolic syndrome.
TABLE 1.
Probiotic administration in human studies1
| Probiotic and Patients | Design | Dose/duration | Outcome | Reference |
| L. rhamnosus GG | (36) | |||
| Healthy, term infants | Double blind | 107 CFU/g powdered milk | Increased weight and length | |
| Placebo-controlled | Until the age of 6 mo | |||
| L. paracasei ssp. paracasei F19 | (37) | |||
| Healthy, term infants | Double blind | 108 CFU/d | Length, weight, head circumference | |
| Placebo-controlled | From 4 to 13 mo of age | |||
| L. rhamnosus GG | (34) | |||
| Pregnant women and children | Double blind | 1010 CFU/d | BMI and BWG in children | |
| Placebo-controlled | Mothers: 4 wk before delivery | |||
| Children: 6 mo after birth | ||||
| L. rhamnosus GG and B. lactis Bb12 | ||||
| Pregnant women | Double blind | 1010 CFU/d each strain | Over the pregnancy: BWG, adiposity, insulin sensitivity | (80) |
| Placebo-controlled | From trimester 1 of pregnancy until the end of exclusive breastfeeding | In the postpartum period: adiposity | (81) | |
| L. acidophilus L-1 | (43) | |||
| Normal-weight adults | Open trial | 4.9 × 109 to 2.7 × 1010/d | BW, blood lipid profile | |
| Placebo-controlled | 6 wk | |||
| B. breve CBG-C2 and Enterococcus faecalis FK-23 | (82) | |||
| Healthy adults | Double blind | Doses not mentioned | BW, BMI, LDL cholesterol | |
| Placebo-controlled | 8 wk | |||
| L. acidophilus La5 and B. lactis Bb12 | (42) | |||
| Women with BMI < 30 | Open trial | 3.9 × 107/g each strain | BW, BMI, plasma lipid profile | |
| Placebo-controlled | 300 g/d | |||
| 6 wk | ||||
| L. gasseri SBT2055 | (32, 33) | |||
| Overweight and obese adults | Double blind | 108–1011 CFU/d | BWG, BMI, waist-to-hip ratio, adiposity | |
| Placebo-controlled | 12 wk | |||
| L. salivarius Ls-33 | (41) | |||
| Obese adolescents | Double blind | 1010 CFU/d | BW, height, BMI, waist-to-hip ratio | |
| Placebo-controlled | 12 wk | |||
| L. casei Shirota | (39) | |||
| Obese with metabolic syndrome | Open trial | 3 × 6.5 × 109 CFU/d | No effect on body weight, BMI, and gut permeability | |
| Control group without placebo | 3 mo | |||
| L. casei Shirota | (40) | |||
| Obese with metabolic syndrome | Open trial | 3 × 6.5 × 109 CFU/d | Insulin sensitivity, endothelial function, low-grade inflammation | |
| Control group without placebo | 12 wk | |||
| L. gasseri BNR17 | (83) | |||
| Overweight and obese adults | Double blind | 6 × 1010 CFU/d | BW, BMI, adiposity | |
| Placebo-controlled | 12 wk | |||
| Lactobacillus spp. | (84) | |||
| Morbidly obese individuals after Roux-en-Y gastric bypass | Open trial | 2.4 × 109/d | Weight loss | |
| Control group without placebo | 6 mo | |||
| L. plantarum 299v | (85) | |||
| Males with moderately elevated blood cholesterol | Double blind | 5 × 107 CFU/mL | Plasma lipids (TG, total, LDL cholesterol, HDL cholesterol) and glucose | |
| Placebo-controlled | 200 mL/d | |||
| E. faecium and S. thermophilus | (86) | |||
| Healthy overweight and obese adults | Double blind | 6 × 107/mL for E. faecium | BW and fat mass, LDL cholesterol | |
| Placebo-controlled | 1 × 109/mL for S. thermophilus | |||
| 450 mL/d | ||||
| 8 wk | ||||
| L. plantarum 299v | (44) | |||
| Smokers | Double blind | 5 × 107 CFU/mL | BMI, systolic blood pressure and other atherosclerosis risk factors | |
| Placebo-controlled | 400 mL/d | |||
| 6 wk | ||||
| L. acidophilus NCFM | (38) | |||
| Males with type 2 diabetes or impaired/normal glucose tolerance | Double blind | 1010 CFU/d | Insulin sensitivity, systemic inflammation | |
| Placebo-controlled | 4 wk | |||
| L. gasseri SBT2055 | (87) | |||
| Adults with hypertriacylglycerolemia | Single blind | 5 × 1010 CFU/100 g | BW, BMI, NEFA after oral fat-loading test, Hb A1c | |
| Placebo-controlled | 200 g/d | |||
| 4 wk |
The table was designed as follows: the first part describes studies performed with children, the second part describes studies performed with pregnant women, the third part describes studies performed with healthy or normal-weight adults, and the last part describes studies performed with obese individuals or patients with metabolic syndrome. Outcomes are indicated as follows: beneficial, bold; null, normal font; harmful, italics. BW, body weight; BWG, body-weight gain; Hb A1C, hemoglobin A1c; NEFA, non-esterified FA.
Some trials testing the effect of Lactobacillus spp. administration on serum lipids profile or on markers of cardiovascular risk do not allow showing any significant changes in BMI during probiotic administration, which is comprehensive in view of the short duration of the treatment (6–8 wk). Those studies did not highlight major modifications in the serum lipoproteins profile (42, 43). Interestingly, another study (44) demonstrated a reduction in cardiovascular disease risk factors (decreased systolic blood pressure and plasma fibrinogen) by administration of L. plantarum 299v (2 × 1010 CFU/d) in smokers.
Some studies were performed to analyze the effect of probiotic administration on liver damage associated with obesity: NAFLD (45). A mixture of L. bulgaricus and S. thermophilus decreases aminostransferase activity in patients treated with the probiotic mixture, whereas in the placebo group, the amounts of aminotransferases remain unchanged (46). Another study (47) reports a decrease in alanine aminotransferase activity after 8 wk of treatment with L. rhamnosus GG in children with obesity-related liver disease.
Most of the authors reporting the studies mentioned above suggest a strain-specific ability of probiotics to modulate obesity and the associated metabolic disorders. However, the comparison between strains is rarely performed, and the mechanisms by which probiotics exert their beneficial effects remain to be characterized. Additional investigation is needed to assess the potential role of probiotics in body-weight regulation, which remains modest in term of kilograms lost during intervention studies.
Interest of Prebiotic-Type Nutrients in the Management of Obesity and Related Diseases
Would it be possible to link the properties of dietary fibers that specifically modulate the gut microbiota with host functions related to obesity and overfeeding? Some fermentable carbohydrates were initially defined as prebiotics, because they are preferentially fermented by specific types of bacteria, generally recognized as beneficial for the host (48, 49). Bifidobacterium spp. represent an important and complex group of bacteria whose presence is often associated with interesting health effects (50). In the context of obesity, several studies (2, 51, 52) reported that a low number of Bifidobacterium spp. correlated with the development of obesity and/or diabetes. Several fermentable carbohydrates (glucans, galactans, and fructans) are easily and widely fermented by bifidobacteria and promote their development. Several data had the bifidogenic effect of dietary inulin-type fructans (ITF) or arabinoxylans added in the diet of obese mice or rats (53–55).
In fact, promoting bifidobacteria is not the sole consequence of the prebiotic treatment. The pyrosequencing and microarray analysis of the caecal 16S rDNA of ob/ob mice treated or not with prebiotics allow us to point out >100 sequences that were different after prebiotic treatment, with some bacteria being particularly increased and other decreased by >10-fold (56). This allows for the identification of interesting bacteria that are promoted with the ITF prebiotic approach in this particular context. This is the case for Faecalibacterium prausnitzii, which exhibits interesting anti-inflammatory properties and is potentially involved in diabetes-related inflammation (57), or Akkermansia muciniphila, which was shown to be inversely correlated with body weight in pregnant women and preschool children (58, 59). Concerning human data, we confirmed recently, in an intervention study with ITF prebiotics in obese women, that, even if the increase in bifidobacteria remains the major and common signature of the prebiotic approach, a complex modulation of the gut microbial ecology at the phylum and genus–like taxonomic level also occurs during prebiotic treatment in obese women (60).
In obese animals (ob/ob mice, diet-induced obesity, obese Zucker or JCR:LA-cp rats), the dietary supplementation with nondigestible/fermentable carbohydrates, such as ITF or arabinoxylans, is able to lessen adiposity (49). Prebiotic treatment changes the gene expression pattern in the white adipose tissue of obese mice (by acting on peroxisome proliferator-activated receptor γ and G-protein-coupled receptor 43), leading to an increased lipolysis, a decreased adipogenesis, and an increased metabolic response to hormones such as leptin, and all those phenomenon contributing to a lower adiposity (56, 61). In obese animal fed ITF, an increase in anorexigenic peptides [peptide YY (PYY) and glucagon-like peptide-1 (GLP-1)] and a decrease in the orexigenic peptide ghrelin occurs, contributing to the satietogenic effect of the ITF (62, 63). This satietogenic effect was confirmed in a human pilot study showing that oligofructose increases satiety and reduces hunger and prospective food consumption and in another human study in which prebiotic supplementation was associated with an increase in plasma anorexigenic gut peptide concentration (PYY and GLP-1) (64, 65). The underlying mechanism of these effects is the modulation of the gut endocrine function by prebiotics in obese rats or mice that involves an increase in the number of L endocrine cells in the intestine, an effect that is correlated with bacterial changes in the gut (66). However, it remains rather difficult to know by which mechanism the gut microbial environment influences L-cell differentiation. The production of short-chain FAs (SCFAs) (namely acetate and propionate) during prebiotic fermentation could promote the increase in secretion of gut peptides by the endocrine cells (67).
Several substrates with prebiotic properties (fructans and arabinoxylans) are also able to counteract proinflammatory processes linked to obesity (54, 68). The decrease in LPS absorption occurs in prebiotic-treated animals through an improvement of the expression and activity of proteins involved in gut-barrier function, including glucagon-like peptide-2 (GLP-2), which is cosecreted with GLP-1 by endocrine L cells (54).
In addition to the beneficial effects mentioned above, in most of the studies performed, the administration of prebiotics leads to an improvement of fasting and/or post-oral glucose load glycemia (56, 69). The mechanisms by which this effect occurs could involve the secretion of gut peptides with incretin function, such as GLP-1, which participates in the improvement of hepatic insulin resistance (69). Two intervention studies with ITF prebiotics were reported in patients exhibiting hepatic diseases, suggesting that ITF administration is able to improve markers, such as LPS or aminotransferases (70, 71).
The different ways of the actions of prebiotics were not identified exhaustively. One of them is the modification of metabolites produced by the gut bacteria. The main and well-studied metabolites are SCFAs. However, these SCFAs are not the sole metabolites produced by the gut microbiota. Indeed, in view of the very large size of the microbiome (100-fold more genes than the human genome), the gut microbiota have a near-infinite metabolic potential. Many of the metabolites could be produced by the gut microbiota, and many of them are probably still unknown for the moment (72, 73). Among the metabolites identified and potentially related to human health, we can cite bile acid metabolites, choline metabolites, vitamins, polyamines, and lipid metabolites (72).
The bile acids signature is heavily dependent on microbial activities and the bile acid profiles of different tissues (liver, kidney, and heart) and in the plasma are modified by gut microbiota modulation (74). This modification of bile acid profile in host tissues may modulate the host physiologic response, for example, by modifying dietary lipid absorption or by changing host gene expression through interactions with specific nuclear receptors (farnesoid-X receptor and transmembrane G protein-coupled receptor TGR5) (75).
A recent study (76) reports that an HF diet and prebiotic supplementation both increase PUFA-derived bacterial metabolites in host tissues, such as cecal tissue and subcutaneous adipose tissue. We highlighted an increase in rumenic acid (conjugated linoleic acid cis-9,trans-11-18:2) but also in vaccenic acid (trans-11-18:1), with both metabolites being produced during the reduction of linoleic acid by the gut microbiota. Indeed, bacteria are able to metabolize PUFAs, such as linoleic acid and α-linolenic acid into SFAs, to lessen the potential toxicity of those PUFAs (77). In vitro studies show that human gut isolated bacteria are also able to produce these PUFA-derived bacterial metabolites (78, 79). However, the physiologic relevance of these endogenously produced PUFA-derived metabolites on human health remains to be determined.
Conclusions
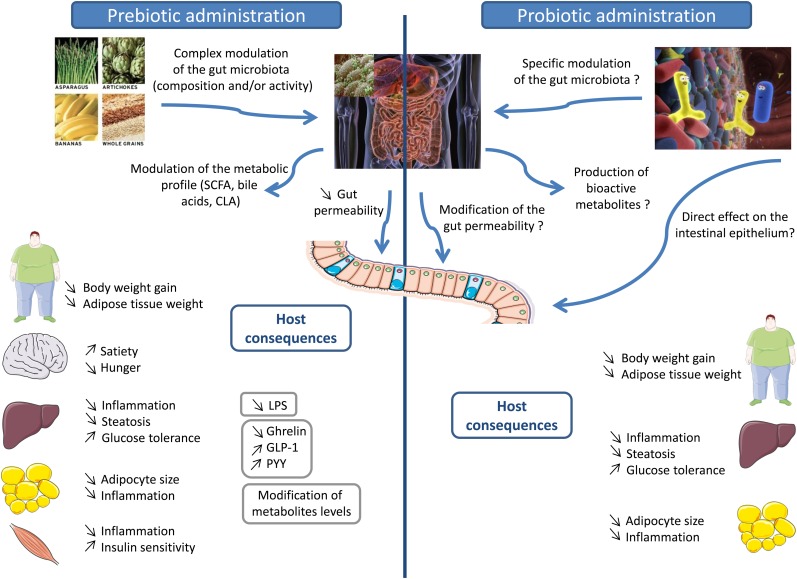
Highly fermentable carbohydrates, such as prebiotics, are able to counteract several metabolic alterations linked to obesity, including hyperglycemia, inflammation, and hepatic steatosis, at least in animal models (Fig. 1). The mechanistic studies suggest that the changes in the gut microbiota occurring on prebiotics can be related to an improvement of gut bacterial functions implicated in the regulation of host energy homeostasis. The promotion of gut hormone release, changes in the gut-barrier integrity, and/or the release of bacterial-derived metabolites could all participate in the improvement of host health in the particular context of overfeeding and obesity. Appropriate human intervention studies with “colonic” nutrients (dietary fibers, prebiotics, and others), which allow for the selective promotion of beneficial bacteria, or with food containing colonic nutrients are essential to confirm the relevance of those nutrients in the nutritional management of overweight and obesity. Administration of live microorganisms (probiotics) seems also able to lessen obesity and related metabolic disorders. However, the mechanisms implicated in the beneficial effects of probiotics are not completely known. Animal studies suggest that regulation of lipid and glucose metabolism, reduction of adipose cell size and inflammation in adipose tissue, and reduction of inflammation in the liver could in part be implicated in the anti-obesity effects of probiotics (Fig. 1). These hypotheses need to be confirmed in human trials. Furthermore, regarding these probiotics, a clarification of the strain and the dose able to counteract obesity and related disorders is necessary before the generalization of the use of these microorganisms.
FIGURE 1.
Effect of prebiotics and probiotics on host pathophysiology related to obesity. Dietary carbohydrates with prebiotic properties change the gut microbiota composition by favoring bacteria involved in the control of gut-barrier function and host immunity. In the gut, prebiotics help to improve the gut-barrier function, a phenomenon that decreases LPS translocation in the host and decreases low-grade inflammation associated with obesity. Prebiotics also promote the production of gut hormones that control appetite (increase satiety and decrease hunger) and glucose homeostasis (improve glucose tolerance). The prebiotic approach also counteracts hepatic steatosis, hepatic insulin resistance, and adiposity by modifying gene expression at the tissue level. Administration of probiotics (live microorganisms) could affect host metabolism in different ways: 1) a direct effect of these microorganisms on the intestinal epithelium; 2) inducing a modulation of the composition of the gut microbiota that can also act on the intestinal epithelium; and finally 3) acting directly on the host tissue. The interaction with host tissues can be mediated by the production of bioactive compounds by the probiotics, such as SCFAs, PUFA-derived bacterial metabolites, or bile acid metabolites. The data available suggest that probiotic administration is associated with a decrease of body weight and adipose tissue weight, a decrease of the adipocyte size, a modulation of glucose and lipid metabolism, an improvement of glucose tolerance, and a decrease of systemic inflammation in adipose tissue and the liver. GLP-1, glucagon-like peptide-1; PYY, peptide YY.
Acknowledgments
All authors read and approved the final version of the manuscript.
Footnotes
Abbreviations used: GLP-1, glucagon-like peptide-1; GLP-2, glucagon-like peptide-2; HF, high-fat; ITF, inulin-type fructan; NAFLD: non-alcoholic fatty liver disease; PYY, peptide YY.
References
- 1.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature 2006;444:1022–3 [DOI] [PubMed] [Google Scholar]
- 2.Wu X, Ma C, Han L, Nawaz M, Gao F, Zhang X, Yu P, Zhao C, Li L, Zhou A, et al. Molecular characterisation of the faecal microbiota in patients with type II diabetes. Curr Microbiol 2010;61:69–78 [DOI] [PubMed] [Google Scholar]
- 3.Sanz Y, Rastmanesh R, Agostoni C. Understanding the role of gut microbes and probiotics in obesity: how far are we? Pharmacol Res 2013;69:144–55 Erratum in: Pharmacol Res 2103;71:69 [DOI] [PubMed] [Google Scholar]
- 4.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, et al. Enterotypes of the human gut microbiome. Nature 2011;473:174–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011;334:105–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010;464:59–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, et al. A core gut microbiome in obese and lean twins. Nature 2009;457:480–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto JM, Kennedy S, et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013;500:541–6 [DOI] [PubMed] [Google Scholar]
- 9.Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, Almeida M, Quinquis B, Levenez F, Galleron N, et al. Dietary intervention impact on gut microbial gene richness. Nature 2013;500:585–8 [DOI] [PubMed] [Google Scholar]
- 10.Cani PD, Delzenne NM. The gut microbiome as therapeutic target. Pharmacol Ther 2011;130:202–12 [DOI] [PubMed] [Google Scholar]
- 11.Gil A, Ortega RM, Maldonado J. Wholegrain cereals and bread: a duet of the Mediterranean diet for the prevention of chronic diseases. Public Health Nutr 2011;14:2316–22 [DOI] [PubMed] [Google Scholar]
- 12.Delzenne NM, Neyrinck AM, Backhed F, Cani PD. Targeting gut microbiota in obesity: effects of prebiotics and probiotics. Nat Rev Endocrinol 2011;7:639–46 [DOI] [PubMed] [Google Scholar]
- 13.Kovatcheva-Datchary P, Arora T. Nutrition, the gut microbiome and the metabolic syndrome. Best Pract Res Clin Gastroenterol 2013;27:59–72 [DOI] [PubMed] [Google Scholar]
- 14.Yun SI, Park HO, Kang JH. Effect of Lactobacillus gasseri BNR17 on blood glucose levels and body weight in a mouse model of type 2 diabetes. J Appl Microbiol 2009;107:1681–6 [DOI] [PubMed] [Google Scholar]
- 15.Kang JH, Yun SI, Park MH, Park JH, Jeong SY, Park HO. Anti-obesity effect of Lactobacillus gasseri BNR17 in high-sucrose diet-induced obese mice. PLoS One 2013;8:e54617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang JH, Yun SI, Park HO. Effects of Lactobacillus gasseri BNR17 on body weight and adipose tissue mass in diet-induced overweight rats. J Microbiol 2010;48:712–4 [DOI] [PubMed] [Google Scholar]
- 17.Hamad EM, Sato M, Uzu K, Yoshida T, Higashi S, Kawakami H, Kadooka Y, Matsuyama H, Abd El-Gawad IA, Imaizumi K. Milk fermented by Lactobacillus gasseri SBT2055 influences adipocyte size via inhibition of dietary fat absorption in Zucker rats. Br J Nutr 2009;101:716–24 [DOI] [PubMed] [Google Scholar]
- 18.Miyoshi M, Ogawa A, Higurashi S, Kadooka Y. Anti-obesity effect of Lactobacillus gasseri SBT2055 accompanied by inhibition of pro-inflammatory gene expression in the visceral adipose tissue in diet-induced obese mice. Eur J Nutr 2014;53:599–606 [DOI] [PubMed] [Google Scholar]
- 19.Sato M, Uzu K, Yoshida T, Hamad EM, Kawakami H, Matsuyama H, Abd El-Gawad IA, Imaizumi K. Effects of milk fermented by Lactobacillus gasseri SBT2055 on adipocyte size in rats. Br J Nutr 2008;99:1013–7 [DOI] [PubMed] [Google Scholar]
- 20.Ji YS, Kim HN, Park HJ, Lee JE, Yeo SY, Yang JS, Park SY, Yoon HS, Cho GS, Franz CM, et al. Modulation of the murine microbiome with a concomitant anti-obesity effect by Lactobacillus rhamnosus GG and Lactobacillus sakei NR28. Benef Microbes 2012;3:13–22 [DOI] [PubMed] [Google Scholar]
- 21.Yoo SR, Kim YJ, Park DY, Jung UJ, Jeon SM, Ahn YT, Huh CS, McGregor R, Choi MS. Probiotics L. plantarum and L. curvatus in combination alter hepatic lipid metabolism and suppress diet-induced obesity. Obesity (Silver Spring) 2013;21:2571–8 [DOI] [PubMed] [Google Scholar]
- 22.Fåk F, Backhed F. Lactobacillus reuteri prevents diet-induced obesity, but not atherosclerosis, in a strain dependent fashion in Apoe−/− mice. PLoS One 2012;7:e46837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.An HM, Park SY. Lee do K, Kim JR, Cha MK, Lee SW, Lim HT, Kim KJ, Ha NJ. Antiobesity and lipid-lowering effects of Bifidobacterium spp. in high fat diet-induced obese rats. Lipids Health Dis 2011;10:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen JJ, Wang R, Li XF, Wang RL. Bifidobacterium longum supplementation improved high-fat-fed-induced metabolic syndrome and promoted intestinal Reg I gene expression. Exp Biol Med (Maywood) 2011;236:823–31 [DOI] [PubMed] [Google Scholar]
- 25.Chen J, Wang R, Li XF, Wang RL. Bifidobacterium adolescentis supplementation ameliorates visceral fat accumulation and insulin sensitivity in an experimental model of the metabolic syndrome. Br J Nutr 2012;107:1429–34 [DOI] [PubMed] [Google Scholar]
- 26. Cano PG, Santacruz A, Trejo FM, Sanz Y. Bifidobacterium CECT 7765 improves metabolic and immunological alterations associated with obesity in high-fat diet-fed mice. Obesity (Silver Spring) 2013;21:231–21. [DOI] [PubMed]
- 27.Esposito E, Iacono A, Bianco G, Autore G, Cuzzocrea S, Vajro P, Canani RB, Calignano A, Raso GM, Meli R. Probiotics reduce the inflammatory response induced by a high-fat diet in the liver of young rats. J Nutr 2009;139:905–11 [DOI] [PubMed] [Google Scholar]
- 28.Aronsson L, Huang Y, Parini P, Korach-Andre M, Hakansson J, Gustafsson JA, Pettersson S, Arulampalam V, Rafter J. Decreased fat storage by Lactobacillus paracasei is associated with increased levels of angiopoietin-like 4 protein (ANGPTL4). PLoS One 2010;5:pii:e13087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim SW, Park KY, Kim B, Kim E, Hyun CK. Lactobacillus rhamnosus GG improves insulin sensitivity and reduces adiposity in high-fat diet-fed mice through enhancement of adiponectin production. Biochem Biophys Res Commun 2013;431:258–63 [DOI] [PubMed] [Google Scholar]
- 30.Park DY, Ahn YT, Park SH, Huh CS, Yoo SR, Yu R, Sung MK, McGregor RA, Choi MS. Supplementation of Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 in diet-induced obese mice is associated with gut microbial changes and reduction in obesity. PLoS One 2013;8:e59470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arora T, Anastasovska J, Gibson G, Tuohy K, Sharma RK, Bell J, Frost G. Effect of Lactobacillus acidophilus NCDC 13 supplementation on the progression of obesity in diet-induced obese mice. Br J Nutr 2012;108:1382–9 [DOI] [PubMed] [Google Scholar]
- 32.Kadooka Y, Sato M, Imaizumi K, Ogawa A, Ikuyama K, Akai Y, Okano M, Kagoshima M, Tsuchida T. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur J Clin Nutr 2010;64:636–43 [DOI] [PubMed] [Google Scholar]
- 33.Kadooka Y, Sato M, Ogawa A, Miyoshi M, Uenishi H, Ogawa H, Ikuyama K, Kagoshima M, Tsuchida T. Effect of Lactobacillus gasseri SBT2055 in fermented milk on abdominal adiposity in adults in a randomised controlled trial. Br J Nutr 2013;110:1696–703 [DOI] [PubMed] [Google Scholar]
- 34.Luoto R, Kalliomaki M, Laitinen K, Isolauri E. The impact of perinatal probiotic intervention on the development of overweight and obesity: follow-up study from birth to 10 years. Int J Obes (Lond) 2010;34:1531–7 [DOI] [PubMed] [Google Scholar]
- 35.Raoult D. Probiotics and obesity: a link? Nat Rev Microbiol 2009;7:616. [DOI] [PubMed] [Google Scholar]
- 36.Vendt N, Grunberg H, Tuure T, Malminiemi O, Wuolijoki E, Tillmann V, Sepp E, Korpela R. Growth during the first 6 months of life in infants using formula enriched with Lactobacillus rhamnosus GG: double-blind, randomized trial. J Hum Nutr Diet 2006;19:51–8 [DOI] [PubMed] [Google Scholar]
- 37.Chorell E, Karlsson Videhult F, Hernell O, Antti H, West CE. Impact of probiotic feeding during weaning on the serum lipid profile and plasma metabolome in infants. Br J Nutr 2013;110:116–26 [DOI] [PubMed] [Google Scholar]
- 38.Andreasen AS, Larsen N, Pedersen-Skovsgaard T, Berg RM, Moller K, Svendsen KD, Jakobsen M, Pedersen BK. Effects of Lactobacillus acidophilus NCFM on insulin sensitivity and the systemic inflammatory response in human subjects. Br J Nutr 2010;104:1831–8 [DOI] [PubMed] [Google Scholar]
- 39.Leber B, Tripolt NJ, Blattl D, Eder M, Wascher TC, Pieber TR, Stauber R, Sourij H, Oettl K, Stadlbauer V. The influence of probiotic supplementation on gut permeability in patients with metabolic syndrome: an open label, randomized pilot study. Eur J Clin Nutr 2012;66:1110–5 [DOI] [PubMed] [Google Scholar]
- 40.Tripolt NJ, Leber B, Blattl D, Eder M, Wonisch W, Scharnagl H, Stojakovic T, Obermayer-Pietsch B, Wascher TC, Pieber TR, et al. Short communication: effect of supplementation with Lactobacillus casei Shirota on insulin sensitivity, beta-cell function, and markers of endothelial function and inflammation in subjects with metabolic syndrome–a pilot study. J Dairy Sci 2013;96:89–95 [DOI] [PubMed] [Google Scholar]
- 41.Gøbel RJ, Larsen N, Jakobsen M, Molgaard C, Michaelsen KF. Probiotics to adolescents with obesity: effects on inflammation and metabolic syndrome. J Pediatr Gastroenterol Nutr 2012;55:673–8 [DOI] [PubMed] [Google Scholar]
- 42.Sadrzadeh-Yeganeh H, Elmadfa I, Djazayery A, Jalali M, Heshmat R, Chamary M. The effects of probiotic and conventional yoghurt on lipid profile in women. Br J Nutr 2010;103:1778–83 [DOI] [PubMed] [Google Scholar]
- 43.de Roos NM, Schouten G, Katan MB. Yoghurt enriched with Lactobacillus acidophilus does not lower blood lipids in healthy men and women with normal to borderline high serum cholesterol levels. Eur J Clin Nutr 1999;53:277–80 [DOI] [PubMed] [Google Scholar]
- 44.Naruszewicz M, Johansson ML, Zapolska-Downar D, Bukowska H. Effect of Lactobacillus plantarum 299v on cardiovascular disease risk factors in smokers. Am J Clin Nutr 2002;76:1249–55 [DOI] [PubMed] [Google Scholar]
- 45.Ma YY, Li L, Yu CH, Shen Z, Chen LH, Li YM. Effects of probiotics on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol 2013;19:6911–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aller R, De Luis DA, Izaola O, Conde R, Gonzalez Sagrado M, Primo D, De La Fuente B, Gonzalez J. Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: a double blind randomized clinical trial. Eur Rev Med Pharmacol Sci 2011;15:1090–5 [PubMed] [Google Scholar]
- 47.Vajro P, Mandato C, Licenziati MR, Franzese A, Vitale DF, Lenta S, Caropreso M, Vallone G, Meli R. Effects of Lactobacillus rhamnosus strain GG in pediatric obesity-related liver disease. J Pediatr Gastroenterol Nutr 2011;52:740–3 [DOI] [PubMed] [Google Scholar]
- 48.Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, Wolvers D, Watzl B, Szajewska H, Stahl B, et al. Prebiotic effects: metabolic and health benefits. Br J Nutr 2010;104(Suppl 2):S1–63 [DOI] [PubMed] [Google Scholar]
- 49.Delzenne NM, Neyrinck AM, Cani PD. Gut microbiota and metabolic disorders: How prebiotic can work? Br J Nutr 2013;109:(Suppl 2):S81–5 [DOI] [PubMed] [Google Scholar]
- 50.Russell DA, Ross RP, Fitzgerald GF, Stanton C. Metabolic activities and probiotic potential of bifidobacteria. Int J Food Microbiol 2011;149:88–105 [DOI] [PubMed] [Google Scholar]
- 51.Kalliomäki M, Collado MC, Salminen S, Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr 2008;87:534–8 [DOI] [PubMed] [Google Scholar]
- 52.Schwiertz A, Taras D, Schafer K, Beijer S, Bos NA, Donus C, Hardt PD. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 2010;18:190–5 [DOI] [PubMed] [Google Scholar]
- 53.Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, Gibson GR, Delzenne NM. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 2007;50:2374–83 [DOI] [PubMed] [Google Scholar]
- 54.Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, Geurts L, Naslain D, Neyrinck A, Lambert DM, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009;58:1091–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neyrinck AM, Possemiers S, Druart C, Van de Wiele T, De Backer F, Cani PD, Larondelle Y, Delzenne NM. Prebiotic effects of wheat arabinoxylan related to the increase in bifidobacteria, Roseburia and Bacteroides/Prevotella in diet-induced obese mice. PLoS One 2011;6:e20944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Everard A, Lazarevic V, Derrien M, Girard M, Muccioli GG, Neyrinck AM, Possemiers S, Van Holle A, Francois P, de Vos WM, et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes 2011;60:2775–86 Erratum in: Diabetes 2011;60:3307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Furet JP, Kong LC, Tap J, Poitou C, Basdevant A, Bouillot JL, Mariat D, Corthier G, Dore J, Henegar C, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes 2010;59:3049–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Santacruz A, Collado MC, Garcia-Valdes L, Segura MT, Martin-Lagos JA, Anjos T, Marti-Romero M, Lopez RM, Florido J, Campoy C, et al. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br J Nutr 2010;104:83–92 [DOI] [PubMed] [Google Scholar]
- 59.Karlsson CL, Onnerfalt J, Xu J, Molin G, Ahrne S, Thorngren-Jerneck K. The microbiota of the gut in preschool children with normal and excessive body weight. Obesity (Silver Spring) 2012;20:2257–61 [DOI] [PubMed] [Google Scholar]
- 60.Dewulf EM, Cani PD, Claus SP, Fuentes S, Puylaert PG, Neyrinck AM, Bindels LB, de Vos WM, Gibson GR, Thissen JP, et al. Insight into the prebiotic concept: lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut 2013;62:1112–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dewulf EM, Cani PD, Neyrinck AM, Possemiers S, Van Holle A, Muccioli GG, Deldicque L, Bindels LB, Pachikian BD, Sohet FM, et al. Inulin-type fructans with prebiotic properties counteract GPR43 overexpression and PPARgamma-related adipogenesis in the white adipose tissue of high-fat diet-fed mice. J Nutr Biochem 2011;22:712–22 [DOI] [PubMed] [Google Scholar]
- 62.Cani PD, Dewever C, Delzenne NM. Inulin-type fructans modulate gastrointestinal peptides involved in appetite regulation (glucagon-like peptide-1 and ghrelin) in rats. Br J Nutr 2004;92:521–6 [DOI] [PubMed] [Google Scholar]
- 63.Cani PD, Neyrinck AM, Maton N, Delzenne NM. Oligofructose promotes satiety in rats fed a high-fat diet: involvement of glucagon-like peptide-1. Obes Res 2005;13:1000–7 [DOI] [PubMed] [Google Scholar]
- 64.Cani PD, Joly E, Horsmans Y, Delzenne NM. Oligofructose promotes satiety in healthy human: a pilot study. Eur J Clin Nutr 2006;60:567–72 [DOI] [PubMed] [Google Scholar]
- 65.Cani PD, Lecourt E, Dewulf EM, Sohet FM, Pachikian BD, Naslain D, De Backer F, Neyrinck AM, Delzenne NM. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am J Clin Nutr 2009;90:1236–43 [DOI] [PubMed] [Google Scholar]
- 66.Cani PD, Hoste S, Guiot Y, Delzenne NM. Dietary non-digestible carbohydrates promote L-cell differentiation in the proximal colon of rats. Br J Nutr 2007;98:32–7 [DOI] [PubMed] [Google Scholar]
- 67.Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012;61:364–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Neyrinck AM, Van Hee VF, Piront N, De Backer F, Toussaint O, Cani PD, Delzenne NM. Wheat-derived arabinoxylan oligosaccharides with prebiotic effect increase satietogenic gut peptides and reduce metabolic endotoxemia in diet-induced obese mice. Nutr Diabetes 2012;2:e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cani PD, Knauf C, Iglesias MA, Drucker DJ, Delzenne NM, Burcelin R. Improvement of glucose tolerance and hepatic insulin sensitivity by oligofructose requires a functional glucagon-like peptide 1 receptor. Diabetes 2006;55:1484–90 [DOI] [PubMed] [Google Scholar]
- 70.Daubioul CA, Horsmans Y, Lambert P, Danse E, Delzenne NM. Effects of oligofructose on glucose and lipid metabolism in patients with nonalcoholic steatohepatitis: results of a pilot study. Eur J Clin Nutr 2005;59:723–6 [DOI] [PubMed] [Google Scholar]
- 71.Malaguarnera M, Vacante M, Antic T, Giordano M, Chisari G, Acquaviva R, Mastrojeni S, Malaguarnera G, Mistretta A, Li Volti G, et al. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig Dis Sci 2012;57:545–53 [DOI] [PubMed] [Google Scholar]
- 72.Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science 2012;336:1262–7 [DOI] [PubMed] [Google Scholar]
- 73.Delzenne NM, Cani PD. Interaction between obesity and the gut microbiota: relevance in nutrition. Annu Rev Nutr 2011;31:15–31 [DOI] [PubMed] [Google Scholar]
- 74.Swann JR, Want EJ, Geier FM, Spagou K, Wilson ID, Sidaway JE, Nicholson JK, Holmes E. Systemic gut microbial modulation of bile acid metabolism in host tissue compartments. Proc Natl Acad Sci USA 2011;108(Suppl 1):4523–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Aguiar Vallim TQ, Tarling EJ, Edwards PA. Pleiotropic roles of bile acids in metabolism. Cell Metab 2013;17:657–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Druart C, Neyrinck AM, Dewulf EM, De Backer FC, Possemiers S, Van de Wiele T, Moens F, De Vuyst L, Cani PD, Larondelle Y, et al. Implication of fermentable carbohydrates targeting the gut microbiota on conjugated linoleic acid production in high-fat-fed mice. Br J Nutr 2013;110:998–1011 [DOI] [PubMed] [Google Scholar]
- 77.Maia MR, Chaudhary LC, Bestwick CS, Richardson AJ, McKain N, Larson TR, Graham IA, Wallace RJ. Toxicity of unsaturated fatty acids to the biohydrogenating ruminal bacterium, Butyrivibrio fibrisolvens. BMC Microbiol 2010;10:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gorissen L, Raes K, Weckx S, Dannenberger D, Leroy F, De Vuyst L, De Smet S. Production of conjugated linoleic acid and conjugated linolenic acid isomers by Bifidobacterium species. Appl Microbiol Biotechnol 2010;87:2257–66 [DOI] [PubMed] [Google Scholar]
- 79.Coakley M, Ross RP, Nordgren M, Fitzgerald G, Devery R, Stanton C. Conjugated linoleic acid biosynthesis by human-derived Bifidobacterium species. J Appl Microbiol 2003;94:138–45 [DOI] [PubMed] [Google Scholar]
- 80.Laitinen K, Poussa T, Isolauri E, Nutrition AMI, Intestinal Microbiota G. Probiotics and dietary counselling contribute to glucose regulation during and after pregnancy: a randomised controlled trial. Br J Nutr 2009;101:1679–87 [DOI] [PubMed] [Google Scholar]
- 81.Ilmonen J, Isolauri E, Poussa T, Laitinen K. Impact of dietary counselling and probiotic intervention on maternal anthropometric measurements during and after pregnancy: a randomized placebo-controlled trial. Clin Nutr 2011;30:156–64 [DOI] [PubMed] [Google Scholar]
- 82.Chang BJ, Park SU, Jang YS, Ko SH, Joo NM, Kim SI, Kim CH, Chang DK. Effect of functional yogurt NY-YP901 in improving the trait of metabolic syndrome. Eur J Clin Nutr 2011;65:1250–5 [DOI] [PubMed] [Google Scholar]
- 83.Jung SP, Lee KM, Kang JH, Yun SI, Park HO, Moon Y, Kim JY. Effect of Lactobacillus gasseri BNR17 on overweight and obese adults: a randomized, double-blind clinical trial. Korean J Fam Med 2013;34:80–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Woodard GA, Encarnacion B, Downey JR, Peraza J, Chong K, Hernandez-Boussard T, Morton JM. Probiotics improve outcomes after Roux-en-Y gastric bypass surgery: a prospective randomized trial. J Gastrointest Surg 2009;13:1198–204 [DOI] [PubMed] [Google Scholar]
- 85.Bukowska H, Pieczul-Mroz J, Jastrzebska M, Chelstowski K, Naruszewicz M. Decrease in fibrinogen and LDL-cholesterol levels upon supplementation of diet with Lactobacillus plantarum in subjects with moderately elevated cholesterol. Atherosclerosis 1998;137:437–8 [DOI] [PubMed] [Google Scholar]
- 86.Agerholm-Larsen L, Raben A, Haulrik N, Hansen AS, Manders M, Astrup A. Effect of 8 week intake of probiotic milk products on risk factors for cardiovascular diseases. Eur J Clin Nutr 2000;54:288–97 [DOI] [PubMed] [Google Scholar]
- 87.Ogawa A, Kadooka Y, Kato K, Shirouchi B, Sato M. Lactobacillus gasseri SBT2055 reduces postprandial and fasting serum non-esterified fatty acid levels in Japanese hypertriacylglycerolemic subjects. Lipids Health Dis 2014;13:36. [DOI] [PMC free article] [PubMed] [Google Scholar]