Abstract
Objective
Approximately 18,000 patients suffer from a subarachnoid hemorrhage (SAH) in the United States annually. SAH is a form of stroke and comprises 1%–5% of all strokes. Nearly 50% of all SAH cases end in fatality within 30 days of presentation; one of eight patients die before reaching a hospital. Those who survive often have neurological or cognitive impairment.
Methods
This case report describes the course of two patients who presented to the emergency department with aneurismal subarachnoid hemorrhage and received external ventricular drainage and endovascular treatment of their aneurysm.
Results
Both patients required treatment with Eptifibatide drip after endovascular approach and their SAH in the basal cisterns resolved by day 5. Neither patient developed signs of clinical or subclinical vasospasm.
Comments
Eptifibatide drip facilitated resolution of the thick clot in the subarachnoid space early enough to eliminate the direct toxicity of oxyhemoglobin on the cerebral arteries and arachnoid granulations, thus preventing vasospasm and eliminating the necessity for a long-term shunt.
Keywords: Aneurismal subarachnoid hemorrhage, transcranial Doppler, endovascular therapy, aneurismal coils, stroke, intracranial stent
Background
Nontraumatic subarachnoid hemorrhage is a neurologic emergency characterized by the extravasation of blood into the spaces covering the central nervous system [19]. The leading cause for this clinical entity is aneurismal rupture [19]. According to multiple reviews and case series, more than 90% of patients with a Fisher scale of 3 further develop clinical vasospasm resulting in delayed ischemic neurological deficit (DID) [4]. Disruption of the thick clots surrounding the basilar cisterns and their drainage with an external ventricular drainage (EVD) within the first 5 days prevents vasospasm and reduces the need for a long-term ventriculoperitoneal shunt (VPS). Eptifibatide drip after securing the aneurysm may be helpful in clearing subarachnoidal blood in patients who undergo EVD. However, there is a paucity of data to support the safety and efficacy of this potential adjunctive treatment.
Methods and results
We present two patients who were admitted with aneurismal subarachnoid hemorrhage. Patients’ blood pressures were controlled as per protocol to a systolic blood pressure of below 160. Both patients were treated with endovascular approach to secure the aneurysm within 24 h of presentation. They developed signs of hydrocephalus that required an EVD to control intracranial pressure, which was placed without any complications. Both patients also received Eptifibatide drip after the procedure for a total of 96 h. TCD was done on a daily basis after day 2.
Patient 1: A 38-year-old African American woman with history of unresponsive hypertension presented after sudden onset of severe headache and collapsing in a parking lot. She was brought to the emergency department and found to have SAH related to a left MCA ruptured aneurysm (Figure 1). Modified Fisher Scale [24] was 3 and Modified Hess and Hunt [25] were 3. After intubation, options were discussed with the patient’s family, as she was lethargic. They elected to proceed with endovascular therapy. Three ORBIT GALAXY coils were deployed to secure her aneurysm (Codman & Shurtleff, Inc. Raynham, MA). In this patient, SAH was complicated with hydrocephalus for which an EVD was inserted. Because the coils were slightly protruding outside the neck of the aneurysm, the patient was started on Eptifibatide drip to reduce the risk of thrombosis out of the aneurysm neck.
Figure 1.
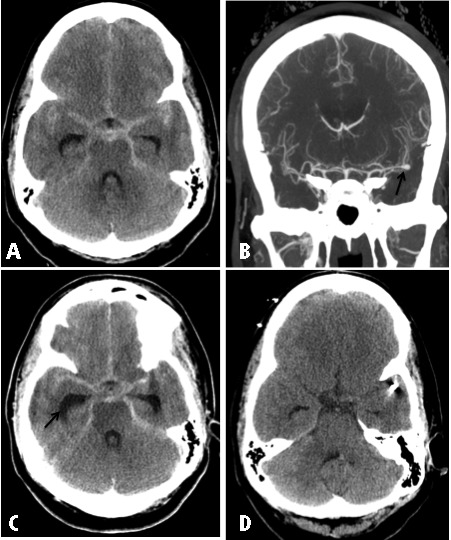
(A) Axial noncontrast CT of the head upon presentation showing extensive more than 10 mm thickness of blood in the basilar cistern. (B) Coronal maximum intensity projections showing left MCA aneurysm at the bifurcation. (C) Postendovascular coiling noncontrast CT scan showing evidence of hydrocephalus and the extensive bleeding in the basilar cisterns. (D) Five days later noncontrast CT scan showing resolution of blood product after treating patient with Eptifibatide drip.
Patient 2: A 53-year-old Hispanic woman with history of unresponsive hypertension presented with sudden onset of the worst headache of her life and found to have left vertebral fusiform aneurysm (Figure 2) that was managed endovascularly by deploying 2 Enterprise stents (Codman & Shurtleff, Inc. Raynham, MA) using flow diversion approach and achieving immediate improvement of aneurysm configuration. The patient was started on Eptifibatide drip. Her modified Fisher score was 4 and modified Hess and Hunt were 4. Before the endovascular procedure, she required EVD placement secondary to hydrocephalus.
Figure 2.
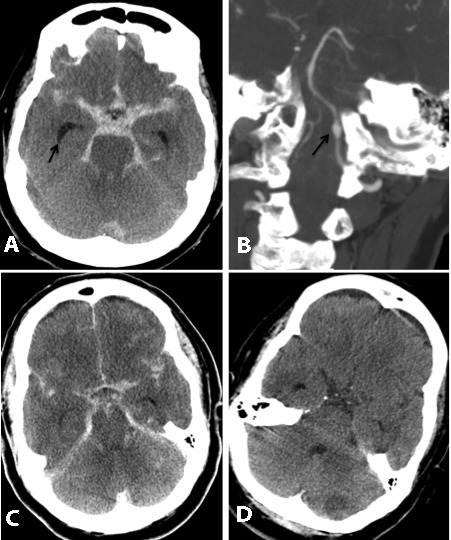
(A) Initial noncontrast CT scan showing extensive more than 10 mm thickness blood in the basilar cisterns. (B) Coronal maximum intensity projections showing fusiform aneurysm of the left vertebral artery. (C) Postendovascular treatment with two enterprise stents showing the extensive bleed and resolution of the hydrocephalus after inserting the EVD before endovascular procedure. (D) Two days noncontrast CT scan after starting Eptifibatide drip poststenting showing almost complete resolution of the blood from basilar cisterns.
Both patients cleared most of the subarachnoidal blood by day 5 and stayed in the Neurocritical Care Unit (NCCU) for a total of 14 days without developing signs of vasospasm nor DID. Daily TCD follow up did not show evidence of subclinical vasospasm and mean MCA velocities remained under 110 cm/s. They had their EVD removed by day 11 after having clear CSF drainage; none of the two patients required a long-term shunt.
Discussion
Van Ginjn in a recent review in Lancet reported that aneurysms are the cause of subarachnoid hemorrhage in 85% of cases [20]. The case fatality after aneurysmal hemorrhage is 50%; one in eight patients with subarachnoid hemorrhage dies before reaching a hospital [20]. Rebleeding of SAH occurs in 20% of patients in the first 2 weeks. The peak incidence of rebleeding occurs 1 day after SAH. This may be from lysis of the aneurismal clot and is the most imminent danger [20]. Thus, the initial step in the therapeutic plan is occlusion of the aneurysm. This is achieved by endovascular obliteration using platinum spirals (coiling) though some patients require a direct neurosurgical approach (clipping). Fisher CM et al reported that in the presence of subarachnoid blood clots larger than 5 × 3 mm (measured on the reproduced images) or layers of blood 1 mm or more thick in fissures and vertical cisterns, severe spasm followed almost invariably (23 of 24 cases) [4].
Outcome in SAH is worse for patients with extensive clots in the basal cisterns than for those with a thin diffuse hemorrhage. Hydrocephalus may develop within the first 24 h due to obstruction of CSF outflow in the ventricular system by clotted blood. Delayed ischemic deficit (DID) is due in part to a delayed and reversible vasculopathy, impaired autoregulatory function, and hypovolemia causing a regional reduction of cerebral perfusion to the point of causing ischemia [13, 18]. Histologically, there are structural alterations in endothelial and smooth muscle cells in the arterial wall [15]. The presence of oxyhemoglobin in the subarachnoid space seems to be necessary to produce these changes [2, 7, 8, 16]. The specific mechanisms leading to vasoconstriction, however, are unknown. This risk is reduced with oral nimodipine and by maintaining circulatory volume [1]. Angiographic vasospasm is detected in 50%–70% of patients with SAH [21] and DID developed in 20% of patients [3]. Lindegaard et al showed in their work that vasospastic MCAs usually demonstrate velocities of greater than 120 cm/s on TCD, with the velocities being inversely related to arterial diameter [6]. In addition, velocities of >200 cm/s are predictive of a residual MCA lumen diameter of <1 mm (normal MCA diameter is approximately 3 mm). The velocities alone cannot determine whether a patient has symptomatic cerebral vasospasm [17].
Antagonists of the platelet GP IIb–IIIa receptor inhibit the binding of fibrinogen, von Willebrand factor, fibronectin, and vitronectin to platelets, thereby suppressing the formation of fresh platelet thrombi and deposition of fibrin on formed platelet thrombi [10]. Eptifibatide is a competitive platelet GP IIb–IIIa receptor inhibitor that was first introduced in 1998. It is cine-aspartate amino acid sequence and is characterized by high specificity and potency, a shorter half-life, and a more consistent platelet inhibitory effect over time [5, 11, 14], all of which are properties that give this drug a potential pharmacological edge over others within this class. Several investigators have reported intravenous use of GP IIb–IIIa inhibitors Eptifibatide in acute ischemic stroke, either to treat reocclusion or to improve recanalization and reperfusion to ischemic brain [9, 12]. Although several studies and reports showed the possible safety of using Eptifibatide drip, Park et al reported potential life-threatening complication by using the longer acting intra-arterial abciximab [23]. In this study, we describe the off-label, compassionate use of intravenous Eptifibatide in patients with aneurysmal subarachnoid hemorrhage who had their aneurysm secured and EVD inserted.
Both patients in this report received low-dose intravenous Eptifibatide (a 135 μg/kg single-dose bolus, then a 0.5 μg/kg/min infusion) [22] for medical reasons related to either stent deployment or coils being close to the neck. They were continued on Eptifibatide drip for a total of 96 h and received an EVD for hydrocephalus before starting Eptifibatide drip. In addition, both patients had a modified Fisher score of 3 or 4. They cleared blood from the subarachnoid space within 2–4 days with follow up without the development of clinical or subclinical vasospasm confirmed using TCD with mean velocities less than 110 cm/s in both patients. Both patients also had a history of uncontrolled hypertension and their pressures were difficult to control blood pressure during the period of hospitalization. Nimodipine 60 mg per protocol was used, and this could have prevented symptomatic vasospasm. We believe that Eptifibatide drip aided in disrupting the thick clot in the subarachnoid space early enough to avoid the toxicity of oxyhemoglobin on the cerebral arteries, thus preventing vasospasm and the need for a long-term shunt.
Previously, there has been no role identified for the use of Eptifibatide drip in treating or clearing thick subarachnoid hemorrhage clot, but it seems that the bioavailability of Eptifibatide drip in CSF is enough to help disrupt the thick clot in the subarachnoid space early enough to help eliminate the toxicity of oxyhemoglobin on cerebral arteries.
References
- Bambakidis NC, Selman WR. Subarachnoid hemorrhage. In: Suarez JI, editor. Critical Care Neurology and Neurosurgery. Totowa, NJ: Humana Press; 2004. pp. 365–77. [Google Scholar]
- Borsody M, Burke A, Coplin W, Miller-Lotan R, Levy A. Haptoglobin and the development of cerebral artery vasospasm after subarachnoid hemorrhage. Neurology. 2006;66:634–40. doi: 10.1212/01.wnl.0000200781.62172.1d. [DOI] [PubMed] [Google Scholar]
- Claassen J, Bernardini GL, Kreiter K, Bates J, Du Y, Copeland D, et al. Effect of cisternal and ventricular blood on risk of delayed cerebral ischemia after subarachnoid hemorrhage . Stroke. 2001;32:2012–20. doi: 10.1161/hs0901.095677. [DOI] [PubMed] [Google Scholar]
- Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning . Neurosurgery. 1980;6:1–9. doi: 10.1227/00006123-198001000-00001. [DOI] [PubMed] [Google Scholar]
- Goa AL, Noble S. Eptifibatide: a review of its use in patients with acute coronary syndromes and/or undergoing percutaneous coronary intervention . Drugs. 1999;57:439–62. doi: 10.2165/00003495-199957030-00015. [DOI] [PubMed] [Google Scholar]
- Lindegaard KF, Nornes H, Bakke SJ, Sorteberg W, Nakstad P. Cerebral vasospasm after subarachnoid haemorrhage investigated by means of transcranial Doppler ultrasound . Acta Neurochir Suppl (Wien) 1988;42:81–4. doi: 10.1007/978-3-7091-8975-7_16. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Weir BK. A review of hemoglobin and the pathogenesis of cerebral vasospasm . Stroke. 1991;22:971–82. doi: 10.1161/01.str.22.8.971. [DOI] [PubMed] [Google Scholar]
- Mayberg MR. Cerebral vasospasm Neurosurg . Clin North Am. 1998;3:615–27. [PubMed] [Google Scholar]
- Memon MZ, Natarajan SK, Sharma J, Mathews MS, Snyder KV, Siddiqui AH, et al. Safety and feasibility of intraarterial eptifibatide as a revascularization tool in acute ischemic. stroke J Neurosurg. 2011;114:1008–13. doi: 10.3171/2010.8.JNS10318. [DOI] [PubMed] [Google Scholar]
- Murugappan S, Shankar H, Kunapuli SP. Platelet receptors for adenine nucleotides and thromboxane. A2 Semin Thromb Hemost. 2004;30:411–8. doi: 10.1055/s-2004-833476. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Siddiqui AM, Hanel RA, Xavier AR, Kim SH, Kirmani JF, et al. Saftey of high-dose intravenous epitifibatide as an adjunct to internal carotid artery angioplasty and stent placement: a prospective registry . Neurosurgery. 2004;54:307–17. doi: 10.1227/01.neu.0000103224.90865.2e. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Hussein HM, Janjua N, Harris-Lane P, Ezzeddine MA. Postprocedure intravenous eptifibatide following intra-arterial reteplase in patients with acute ischemic . stroke J Neuroimaging. 2008;18:50–5. doi: 10.1111/j.1552-6569.2007.00185.x. [DOI] [PubMed] [Google Scholar]
- Sarrafzadeh AS, Haux D, Ludemann L, Amthauer H, Plotkin M, Kuchler I, et al. Cerebral ischemia in aneurismal subarachnoid hemorrhage: a correlative microdialysis-PET study . stroke. 2004;35:638–43. doi: 10.1161/01.STR.0000116101.66624.F1. [DOI] [PubMed] [Google Scholar]
- Scarborough RM. Development of eptifibatide. Am Heart J. 1999;138:1093–104. doi: 10.1016/s0002-8703(99)70075-x. [DOI] [PubMed] [Google Scholar]
- Smith RR, Clower BR, Grotendorst GM, Yabuno N, Cruse JM. Arterial wall changes in early human vasospasm. Neurosurgery. 1985;16:171–6. doi: 10.1227/00006123-198502000-00008. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Muramatsu M, Kojima T, Taki W. Intracranial heme metabolism and cerebral vasospasm after aneurysmal subarachnoid hemorrhage . stroke. 2003;34:2796–800. doi: 10.1161/01.STR.0000103743.62248.12. [DOI] [PubMed] [Google Scholar]
- Torbey MT, Hauser TK, Bhardwaj A, Williams MA, Ulatowski JA, Mirski MA, et al. Effect of age on cerebral blood flow velocity and incidence of vasospasm after aneurismal subarachnoid hemorrhage . stroke . 2003;32:2005–11. doi: 10.1161/hs0901.094622. [DOI] [PubMed] [Google Scholar]
- Vajkoczy P, Horn P, Thome C, Munch E, Schmiedek P. Regional cerebral blood flow monitoring in the diagnosis of delayed ischemia following aneurysmal subarachnoid hemorrhage . J Neurosurg. 2003;98:1227–34. doi: 10.3171/jns.2003.98.6.1227. [DOI] [PubMed] [Google Scholar]
- Van Gijn J, Rinkel GJ. Subarachnoid haemorrhage: diagnosis, causes and management . Brain. 2001;124:249–78. doi: 10.1093/brain/124.2.249. [DOI] [PubMed] [Google Scholar]
- Van Gijn J, Rinkel GJ. Subarachnoid haemorrhage . Lancet. 2007;369:306–18. doi: 10.1016/S0140-6736(07)60153-6. [DOI] [PubMed] [Google Scholar]
- Weir B, Grace M, Hansen J, Rothberg C. Time course of vasospasm in man . J Neurosurg . 1978;48:173–8. doi: 10.3171/jns.1978.48.2.0173. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Ali Z, Suri MF, Kim SH, Fessler RD, Ringer AJ, Guterman LR, Hopkins LN. Open-label phase I clinical study to assess the safety of intravenous eptifibatide in patients undergoing internal carotid artery angioplasty and stent placement . Neurosurgery. 2001;48:998–1005. doi: 10.1097/00006123-200105000-00005. [DOI] [PubMed] [Google Scholar]
- Park JH, Kim JE, Sheen SH, Jung CK, Kwon BJ, Kwon O, Oh CW, Han MH, Han DH. Intraarterial abciximab for treatment of thromboembolism during coil embolization of intracranial aneurysms: outcome and fatal hemorrhagic complications . J Neurosurg. 2008;108:450–457. doi: 10.3171/JNS/2008/108/3/0450. [DOI] [PubMed] [Google Scholar]
- Frontera JA, Claassen J, Schmidt JM, Wartenberg KE, Temes R, Connolly ES, Jr, MacDonald RL, Mayer SA. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: the modified fisher scale . Neurosurgery . 2006;59:21–7. doi: 10.1227/01.neu.0000243277.86222.6c. [DOI] [PubMed] [Google Scholar]
- Hunt WE, Kosnik EJ. Timing and perioperative care intracranial aneurysm surgery . Clin Neursurg. 1974;21:79–89. doi: 10.1093/neurosurgery/21.cn_suppl_1.79. [DOI] [PubMed] [Google Scholar]


