Abstract
Background
Enrollment of subjects in acute stroke trials is often hindered by narrow timeframes, because a large proportion of patients arrive via transfers from outside facilities rather than primary arrival at the enrolling hospital.
Rationale
Telemedicine networks have been increasingly used for provision of care for acute stroke patients at facilities outside of major academic centers. Treatment decisions made through telemedicine networks in patients with acute ischemic stroke have been shown to be safe, reliable, and effective. With the expanding use of this technology and the impediments to enrolling subjects into clinical trials, this approach can be applied successfully to the field of clinical research.
Methods and conclusions
The Antihypertensive Treatment of Acute Cerebral Hemorrhage II trial is a phase III randomized multicenter trial that has developed a protocol in collaboration with participating sites to implement the use of telemedicine networks for the enrollment of research subjects. The protocol describes the operating procedures and legal and Institutional Review Board perspectives for its implementation.
Introduction
Spontaneous intracerebral hemorrhage (ICH) has a worldwide incidence of 24.6 per 100,000 person-years with approximately 40,000–67,000 cases/year occurring in the United States [1–3]. Despite the high mortality and disability rates associated with ICH[3], there is lack of an effective treatment that reduces mortality and disability.
There are currently at least 33 ongoing clinical trials and observational studies enrolling patients with ICH. Considering the time window for enrollment, 15 (45%) of these trials are recruiting within 4.5–24 h after the onset of symptoms (ClinicalTrials.gov). The difficulties that these time-sensitive trials impose to enroll subjects has been previously noted [4]. One of the main reasons for exclusion is the fact that potentially eligible subjects do not arrive within the time window for enrollment, mainly because patients’ arrival to the hospital is delayed. Additionally, many potentially eligible patients present to nonenrolling medical institutions initially and then are subsequently transferred to the hospital where the study is being conducted. Current estimates from national and regional data suggest that approximately one-fifth of patients with acute ischemic stroke arrive as transfers from outside facilities in the acute setting [5, 6]. For ICH cases a recent single-center prospective study reported a 42% rate of admission via transfer among the ICH cases [7].
The Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH)-II trial is an ongoing phase III randomized multicenter clinical trial of blood pressure (BP) reduction for acute hypertensive response in ICH (ClinicalTrials.gov [NCT01176565]). The description of its methodology and design was previously published [8]. Inclusion and exclusion criteria are outlined in Table 1. Briefly, the ATACH-II trial is a parallel, two-arm study, in which eligible subjects with supratentorial ICH are randomized in a 1:1 ratio to intense systolic blood pressure (SBP) reduction (SBP < 140 mm Hg) or standard SBP reduction (SBP < 180 mm Hg) within 4.5 h of symptom onset. The treatment protocol involves the infusion of intravenous (IV) nicardipine for 24-h postrandomization. The primary study objective is to determine the therapeutic benefit of intensive SBP treatment compared with standard SBP treatment in reducing the proportion of patients with death or significant disability [modified Rankin scale (mRS) of 4–6] at 3 months.
Table 1. ATACH-II inclusion/exclusion criteria for enrollment.
Inclusion Criteria:
|
In this article, we describe the methodology for the utilization of telemedicine in the screening and enrollment of subjects in the ATACH-II trial.
Objective
We address the challenge of patient recruitment in a short-time window in the ATACH-II trial through the incorporation of telemedicine-based screening and enrollment. We expect that this effort will act as a template for this novel technological approach in current and future trials in patients with acute stroke.
Background and rationale
Telemedicine is defined as the use of telecommunication technologies to provide medical information and services [9]. It is used in different medical specialties and its name is usually adapted to reflect those disease-specific telemedicine networks. Telestroke has been defined as the application of telemedicine in stroke care [10], and it is characterized by the remote evaluation of a patient with stroke symptoms by telephone consultation or video-teleconferencing (VTC) and the transfer of imaging data [computed tomography (CT) or magnetic resonance imaging (MRI)] for interpretation and decision making [11].
A typical telestroke network can be organized using the hub and spoke model [12], in which the spoke hospital is usually a community center sometimes located in rural areas without 24/7 coverage by vascular neurologists or other stroke expertise. In this model, the hub hospital is usually a tertiary care facility, commonly a primary or comprehensive stroke center located in urban areas with 24 h availability of trained stroke specialists. Under this model, those patients that present at a spoke hospital are evaluated by the healthcare personnel who then activate the telestroke network through a video-based connection with the hub hospital. At the hub hospital, the vascular neurologist reviews the history and performs a video-based physical examination and evaluates available neuroimages that are transferred to a dedicated image server. An alternative to this approach is to share the screen with the hub site to expedite the imaging evaluation. After evaluation, decisions regarding disposition and medical care are made, often resulting in the transfer of patients to the hub site (where more advanced therapy or inclusion in clinical trials can be offered), or continuity of care at the spoke center.
A recent review from a scientific committee from the American Heart Association/American Stroke Association regarding the use of telemedicine in stroke systems concluded, among other things, that: 1) the National Institutes of Health Stroke Scale (NIHSS) examination by Telestroke is recommended when administered by a stroke specialist when such specialist is not immediately available in the acute setting; 2) the use of teleradiology is recommended for the evaluation of brain CT imaging and identification of exclusions for thrombolytic therapy in acute ischemic stroke; 3) it is recommended that the stroke specialist give their medical opinion in favor of or against the use of thrombolytics via telemedicine when on-site stroke expertise is not immediately available; 4) prehospital telephone communication between emergency medical services (EMS) personnel and stroke specialists can be effective in facilitating enrollment into hyperacute neuroprotective trials [13].
As discussed above, telestroke networks are primarily used in acute ischemic stroke to facilitate the use of thrombolytics and have proven to be safe and effective in the clinical setting [14, 15]. Telestroke is also used in cases of ICH and a recent publication analyzed an 8-year experience, during which 733 patients with ICH were evaluated and their treatment and transport disposition were decided using telestroke. Decision to transfer patients was based on hematoma location, size, and CT scan features in combination with age, relevant clinical data and overall neurological status. The time needed for a neurosurgical consultation from initial admission at the hub hospital was significantly lower when using telestroke compared with the time before the introduction of the telemedicine system (38 min versus 160 min). Also, the use of telestroke made the decision of transferring the patient more efficient, effective, and rapid [16].
Telestroke technology was also implemented in a research setting and proved to be a very successful tool for enrollment of subjects in a clinical site that evaluated it in two acute stroke trials, one of them being a large multicenter trial in ICH, Factor Seven for Acute Hemorrhagic Stroke (FAST) trial [17]. The second trial, Minocycline to Improve Neurological Outcome in Stroke or MINO trial was a study that evaluated IV minocycline in acute ischemic stroke [18]. In this study, the majority of the 28 subjects enrolled in both trials (19% or 68%) were initially identified via telestroke. The FAST trial had a time window of 3 h from symptom onset for the diagnosis with a CT scan, and up to 4 h for randomization. The MINO trial had a window of 6 h for enrollment. Although telestroke was used to screen and identify potential candidates, informed consent forms were faxed to the spoke site on two occasions, signed locally by a legally authorized representative (LAR) then taken to the hub where they were signed by a study investigator. The median time from the arrival of patients at the hub ED to the initiation of study drug in both trials was significantly shorter in those subjects identified by telestroke at spoke hospitals and then transferred, versus those who presented directly to hub ED: 71 min (95% CI, 62–104) versus 140 min (95% CI, 94–188), p = 0.002. This reduction in time to randomization was due to the fact that advanced notification and previous trial discussion help prevent delays in initiating study drug. The investigators concluded that telestroke increases enrollment in acute stroke trials and strongly encouraged the initiation of study drug while the patient is still at the remote spoke hospital [19], which is the main goal for the involvement of telestroke in our trial.
Design and methods
Currently 46 sites in the United States and 20 international sites are participating in ATACH-II. From the US sites, we have identified 18 telestroke networks among those clinical sites participating in the trial. These networks include those that are currently active and being used for clinical purposes and the ones that are being set up and will be active in a short period of time. These telestroke networks cover more than 105 spoke centers. Sites were identified using data from a recently published article on the status of telestroke in the United States [20], by an internal survey sent to all our US sites and by voluntary contact from those sites with active networks that were not identified by the previous two methods, but who became aware of the proposal after internal communications through newsletters and teleconferences.
We developed a Standard Operating Procedures manual for the screening and enrollment of patients in our study using telemedicine technology (Table 2). In our model, the hub hospital is a clinical center currently participating in the ATACH-II trial, while the spoke hospital is a center that is part of an existing local telestroke network as defined previously [12]. The final overall goal is to screen, obtain consent, randomize, and initiate study drug while the patient is still at the spoke hospital in an approach that we call randomize, initiate, and ship. General procedures start when patients arrive at the spoke site with stroke symptoms. Once these patients have been identified with a potential diagnosis of stroke, local standard diagnostic procedures should follow, including acquisition of a noncontrast CT scan of the brain. After the diagnosis of ICH has been made, the telestroke network will be activated to discuss therapeutic options and the ATACH-II inclusion/exclusion criteria with a study investigator located at the hub hospital. If a patient meets enrollment criteria for the study, enrollment in the trial will be discussed with the clinician, the patient and/or LAR. If the subject or LAR provides consent, the study protocol will be followed, explained in more detail as outlined in Table 2.
Table 2.
The step-by-step procedure for enrollment via telestroke networks.
The following outline provides general guidelines that should be used by ATACH‐II clinical sites relying on telemedicine for subject enrollment. Some site-specific adjustments are expected as each ATACH‐II clinical site has a different telemedicine infrastructure in place. Before the implementation of this procedure, the ATACH‐II clinical site must have its local Internal Review Board (IRB) approve the telemedicine procedure that will be implemented.
|
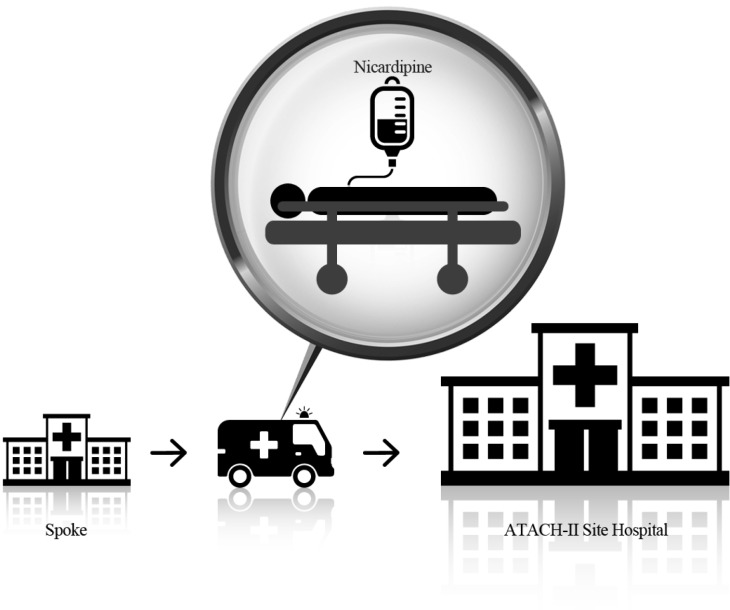
By incorporating this clinical trial in an already established telestroke network, in which patients with ICH are transferred by EMS, we will communicate the assigned treatment goal and BP monitoring frequencies to the EMS crew (Figure 1).
Figure 1. Schematic representation of a research subject being transported after randomization at a spoke hospital.
In anticipation of the incorporation of telestroke into ATACH-II, an update to our existing mobile application in 2012 was made to provide a prescreening tool allowing the preliminary assessment of subjects and the identification of the nearest enrolling site using a global positioning system [21]. The prescreening tool was developed for referring hospital staff, CT technologists, and nursing staff. This feature allows selecting a user type and having inclusion/exclusion criteria for each user type. Users have the ability to mark yes or no for each question, and if all questions are marked yes they will be brought to the site locator to find the nearest clinical trial center and contact information. This application is available on iPhones, Blackberries, and Android-based smart phones. The server is a standard HTTP server and the website does not require use of Adobe Flash or third party JavaScript components.
Conclusions
With the expansion of telestroke across the United States, there is a tremendous opportunity to leverage this resource for diagnosis, expedited treatment, transport, and enrollment of subjects in the ATACH-II trial and in clinical trials of ICH and acute stroke in general. We are actively pursuing telestroke enrollment and hope to demonstrate the feasibility, safety, and benefits of its use in ICH trials. With this project we aim to enhance the basis for the systematic use of telestroke networks in clinical trials related to ICH and other forms of stroke.
References
- van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–76. doi: 10.1016/S1474-4422(09)70340-0. [DOI] [PubMed] [Google Scholar]
- Aguilar MI, Freeman WD. Spontaneous intracerebral hemorrhage. Semin Neurol. 2010;30:555–64. doi: 10.1055/s-0030-1268865. [DOI] [PubMed] [Google Scholar]
- Broderick J, Connolly S, Feldmann E, Hanley D, Kase C, Krieger D, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke. 2007;38:2001–23. doi: 10.1161/STROKEAHA.107.183689. [DOI] [PubMed] [Google Scholar]
- Elkins JS, Khatabi T, Fung L, Rootenberg J, Johnston SC. Recruiting subjects for acute stroke trials: a meta-analysis. Stroke. 2006;37:123–8. doi: 10.1161/01.STR.0000195149.44390.aa. [DOI] [PubMed] [Google Scholar]
- Tekle WG, Chaudhry SA, Hassan AE, Rodriguez GJ, Suri MF, Qureshi AI. Drip-and-ship thrombolytic treatment paradigm among acute ischemic stroke patients in the United States. Stroke. 2012;43:1971–4. doi: 10.1161/STROKEAHA.112.657817. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Chaudhry SA, Rodriguez GJ, Suri MF, Lakshminarayan K, Ezzeddine MA. Outcome of the 'drip-and-ship' paradigm among patients with acute ischemic stroke: results of a statewide study. Cerebrovasc Dis Extra. 2012;2:1–8. doi: 10.1159/000335097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano AR, Winn HR, Gordon E, Frontera JA. Impact of interhospital transfer on complications and outcome after intracranial hemorrhage. Neurocritical Care. 2012;17:324–33. doi: 10.1007/s12028-012-9679-z. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Palesch YY. Antihypertensive treatment of acute cerebral hemorrhage (ATACH) II: Design, methods, and rationale. Neurocritical Care. 2011;15:559–76. doi: 10.1007/s12028-011-9538-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perednia Da AA. Telemedicine technology and clinical applications. JAMA. 1999;273:483–8. [PubMed] [Google Scholar]
- Levine SR, Gorman M. Telestroke: the application of telemedicine for stroke. Stroke. 1999;30:464–9. doi: 10.1161/01.str.30.2.464. [DOI] [PubMed] [Google Scholar]
- Muller-Barna P, Schwamm LH, Haberl RL. Telestroke increases use of acute stroke therapy. Curr Opin Neurol. 2012;25:5–10. doi: 10.1097/WCO.0b013e32834d5fe4. [DOI] [PubMed] [Google Scholar]
- Fisher M. Developing and implementing future stroke therapies: the potential of telemedicine. Ann Neurol. 2005;58:666–71. doi: 10.1002/ana.20659. [DOI] [PubMed] [Google Scholar]
- Schwamm LH, Holloway RG, Amarenco P, Audebert HJ, Bakas T, Chumbler NR, et al. A review of the evidence for the use of telemedicine within stroke systems of care: a scientific statement from the American Heart Association/American Stroke Association. Stroke. 2009;40:2616–34. doi: 10.1161/STROKEAHA.109.192360. [DOI] [PubMed] [Google Scholar]
- Audebert HJ, Kukla C, Clarmann von Claranau S, Kuhn J, Vatankhah B, Schenkel J, et al. Telemedicine for safe and extended use of thrombolysis in stroke: the Telemedic Pilot Project for Integrative Stroke Care (TEMPIS) in Bavaria. Stroke. 2005;36:287–91. doi: 10.1161/01.STR.0000153015.57892.66. [DOI] [PubMed] [Google Scholar]
- Choi JY, Porche NA, Albright KC, Khaja AM, Ho VS, Grotta JC. Using telemedicine to facilitate thrombolytic therapy for patients with acute stroke. Jt Comm J Qual Patient Saf. 2006;32:199–205. doi: 10.1016/s1553-7250(06)32025-9. [DOI] [PubMed] [Google Scholar]
- Angileri FF, Cardali S, Conti A, Raffa G, Tomasello F.2012Telemedicine-assisted treatment of patients with intracerebral hemorrhage Neurosurg Focus 32 E6 [DOI] [PubMed] [Google Scholar]
- Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, et al. Efficacy and safety of recombinant activated factor vii for acute intracerebral hemorrhage. The New England Journal of Medicine. 2008;358:2127–37. doi: 10.1056/NEJMoa0707534. [DOI] [PubMed] [Google Scholar]
- Fagan SC, Waller JL, Nichols FT, Edwards DJ, Pettigrew LC, Clark WM, et al. Minocycline to Improve Neurologic Outcome in Stroke (MINOS): a dose-finding study. Stroke; A Journal of Cerebral Circulation. 2010;41:2283–87. doi: 10.1161/STROKEAHA.110.582601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer JA, Hall CE, Close B, Nichols FT, Gross H, Bruno A, et al. A telestroke network enhances recruitment into acute stroke clinical trials. Stroke. 2010;41:566–9. doi: 10.1161/STROKEAHA.109.566844. [DOI] [PubMed] [Google Scholar]
- Silva GS, Farrell S, Shandra E, Viswanathan A, Schwamm LH. The status of telestroke in the United States: a survey of currently active stroke telemedicine programs. Stroke. 2012;43:2078–85. doi: 10.1161/STROKEAHA.111.645861. [DOI] [PubMed] [Google Scholar]
- Qureshi A, Connelly B, Abbott E, Maland E, Kim J, Blake J. Mobile applications for handheld devices to screen and randomize acute stroke patients in clinical trials. Journal of Vascular and Interventional Neurology. 2012;5:26–9. [PMC free article] [PubMed] [Google Scholar]