Abstract
Stroke is the leading cause of adult disability and the fourth most common cause of death in the United States. Inflammation is thought to play an important role in stroke pathology, but the factors that promote inflammation in this setting remain to be fully defined. An understudied but important factor is the role of meningeal-located immune cells in modulating brain pathology. Although different immune cells traffic through meningeal vessels en route to the brain, mature mast cells do not circulate but are resident in the meninges. With the use of genetic and cell transfer approaches in mice, we identified evidence that meningeal mast cells can importantly contribute to the key features of stroke pathology, including infiltration of granulocytes and activated macrophages, brain swelling, and infarct size. We also obtained evidence that two mast cell-derived products, interleukin-6 and, to a lesser extent, chemokine (C-C motif) ligand 7, can contribute to stroke pathology. These findings indicate a novel role for mast cells in the meninges, the membranes that envelop the brain, as potential gatekeepers for modulating brain inflammation and pathology after stroke.
Stroke, the leading cause of adult disability and the fourth most common cause of death in the Unites States,1,2 occurs when there is insufficient blood flow to the brain, and the resultant injury initiates a cascade of inflammatory events, including immune cell infiltration into the brain.3–5 This post-stroke inflammation is a critical determinant of damage and recovery after stroke; understanding the interplay between the immune system and the brain after stroke holds much promise for therapeutic intervention.4–7 However, successfully exploiting this therapeutic potential requires a detailed understanding of the interplay between the immune system and the brain after stroke.4
An understudied but important aspect of this interplay is the role of meningeal-located immune cells in modulating brain pathology. The meninges have long been recognized as an anatomical barrier that protects the central nervous system (CNS). However, accumulating evidence suggests that the meninges are important for communication between the CNS and immune system during health and disease.8–10 All blood vessels pass through the meningeal subarachnoid space before entering the brain, and this vascular connection and the close proximity of the meninges to the underlying parenchymal nervous tissue make them ideally located to act as a gatekeeper to modulate immune cell trafficking to the CNS. To support this gatekeeper function is evidence that the meninges modulate brain infiltration of T cells, neutrophils, and monocytes during meningitis and autoimmune conditions,11–14 with immune cells observed in some instances accumulating in the meninges before they infiltrate into the parenchyma.11,13
Emerging evidence suggests that the actions of immune cells resident in the meninges are important for this gatekeeper function.11,12,15 Mast cells (MCs), best known as proinflammatory effector cells, can play critical roles in the development of inflammation in many disease settings.16–18 MCs reside in high numbers within the meninges, but their function in this site has not been fully investigated in stroke pathology. Unlike most immune cells, mature MCs do not circulate in the blood but are long-term residents of tissues, often in perivascular locations, and can rapidly perform their functions in situ. CNS MCs are found in the brain parenchyma and the meninges of rodents and humans.18 It has been proposed that brain parenchymal MCs can enhance brain neutrophil numbers after stroke and can exacerbate stroke pathology.19–24 However, much of the evidence to support such conclusions is indirect. For example, some of the studies that implicate MCs in stroke pathology used pharmacologic approaches to interfere with MC activation,19,20,22 but such drugs can have effects on other cell types.25 Moreover, the role of the meningeal MCs in modulating post-stroke inflammation and pathology is unknown. Finally, little is understood about which among the many MC-derived mediators may be important in stroke pathology.17,26
To address these questions, we used genetic and cell transfer approaches to study the role of MCs in the pathology of ischemic stroke in mice. Specifically, we tested a c-kit–mutant mouse model (ie, WBB6F1-KitW/W-v mice) which is profoundly MC deficient and can be repaired of this deficiency by engraftment of in vitro-derived MCs from wild-type (WT) mice. This MC knock-in approach enables the MC-dependent effects in the mutant mice to be separated from effects due to other abnormalities associated with their mutation,11,17,26,27 because only the MC deficiency is repaired by MC engraftment. Furthermore, one can investigate the mechanisms by which MCs influence stroke pathology by engrafting MCs from transgenic mice that lack specific MC-associated products. We also tested our newly described Cpa3-Cre; Mcl-1fl/fl mice, in which MC (and basophil) numbers are reduced constitutively via Cre-mediated depletion of the anti-apoptotic factor, myeloid cell leukemia sequence 1 (Mcl-1), in the affected lineages.28 Cpa3-Cre; Mcl-1fl/fl mice lack the other abnormalities associated with the c-kit mutations in WBB6F1-KitW/W-v mice.28
With the use of these in vivo models, we identified meningeal MCs as important contributors to key features of stroke pathology, including increased numbers of brain granulocytes and activated macrophages, brain swelling, and infarct size. We also obtained evidence that two potentially proinflammatory MC-derived products, IL-6 and, to a lesser extent, chemokine (C-C motif) ligand 7 (CCL7), can contribute to pathology in this setting.
Materials and Methods
Mice
Male c-kit–mutant genetically MC-deficient (WB/Rej-KitW/J × C57BL/6J-KitW-v/J)F1-KitW/Wv (WBB6F1-KitW/W-v) mice and their congenic WT (WBB6F1-Kit+/+) littermates were purchased from The Jackson Laboratory (Bar Harbor, ME). KitW/W-v mice have a profound deficiency in MCs29 and certain other hematological abnormalities; however, only the MC deficiency is repaired by MC engraftment.17,26,30 KitW/W-v mice have lower levels of neutrophils than the corresponding WT mice in the bone marrow (BM), blood, and spleen and have a mild anemia.27 W is a null allele of Kit and Wv is a point mutation in the cytoplasmic tail of the receptor.17,26 Cpa3-Cre;Mcl-1fl/fl mice are severely deficient in MCs and also have a marked deficiency in basophils.28 In these mice, Cre recombinase is expressed under the control of carboxypeptidase A3 (Cpa3) promoter. Mcl-1 is an intracellular anti-apoptotic factor that is required for MC survival. C57BL/6-Cpa3-Cre; Mcl-1+/+ mice were used as WT controls for Cpa3-Cre;Mcl-1fl/fl mice. IL6–knock-out (KO) mice (B6.129S2-Il6tm1Kopf/J) were purchased from The Jackson Laboratory. CCL7-KO mice31 on a C57BL/6 background were initially developed and were a kind gift from Israel F. Charo (University of California San Francisco, San Francisco, CA). All of the animal procedures were approved by Stanford University Administrative Panel on Laboratory Animal Care.
MC–Knock-In Mouse Model
The MC deficiency in WBB6F1-KitW/W-v mice was selectively repaired by systemic (intravenously through retro-orbital injection under isoflurane anesthesia) or by meningeal administration of mouse BM-derived cultured MCs (BMCMCs) generated in vitro, as indicated. As described before,32 the femoral and tibial BM cells from WBB6F1-Kit+/+, C57BL/6-Kit+/+, C57BL/6-IL6-KO, and C57BL/6-CCL7-KO mice were cultured in 20% medium conditioned by the growth of the WEHI-3 mouse myelomonocytic cell line (containing IL-3) for 4 to 5 weeks. Before engraftment, >95% of cultured cells were identified as BMCMCs by May-Grünwald-Giemsa stain. For systemic engraftment, 107 BMCMCs in 100 μL of phosphate-buffered saline were injected retro-orbitally into 9- to 11-week-old WBB6F1-KitW/W-v mice (50 μL into each retro-orbital side). For meningeal engraftment, 106 BMCMCs or vehicle alone (as a control) were injected into 9- to 11-week-old WBB6F1-KitW/W-v mice, as described.15 The mice were used for the experiments 8 to 10 weeks after either type (ie, i.v. or meningeal) of engraftment. In experiments that used such MC-engrafted mice, WT mice and MC-deficient mice used in the same experiments were the same age and housed with the same conditions of husbandry.
Induction of Experimental Stroke
The mice were subjected to a filament occlusion model of cerebral ischemia as described.6 The mice were habituated in the surgery room overnight, and all of the surgeries were initiated early in the morning. Briefly, mice were anesthetized by 1.5% to 2% isoflurane in a mixture of 1 L/minute of air and 0.2 L/minute of oxygen. The left external and common carotid arteries were permanently ligated. A hole was made in the common carotid artery, and a 7-0, silicon rubber-coated, reusable monofilament (70SPRe2045; Doccol Inc., Sharon, MA) was inserted and advanced toward the internal carotid artery 9 to 10 mm after the carotid bifurcation to occlude the left middle cerebral artery. The core body temperature was measured by a rectal probe and maintained at 37°C throughout the surgery. Thirty minutes after its insertion, the filament was removed to permit reperfusion. The surgical wound was closed, and the mice were returned to their cages with free access to water and food. We measured cerebral blood flow by using a laser Doppler flow meter. The probe was placed onto the skull 2 mm posterior and 5 to 6 mm lateral to the bregma on the left side. No differences were found in the presurgical weights of mice in any experiment. A noninvasive CODA Monitor system (Kent Scientific, Torrington, CT) was used to measure blood pressures and pulse rates. Arterial blood gas and lactate levels were measured by iSTAT CG4+ cartridges and a handheld blood analyzer (Abbott Laboratories, Abbott Park, IL).
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) was performed at Stanford Small Animal Imaging Facility by using the GE Healthcare (Waukesha, WI) Micro-Signa software environment version 12M5 with a Varian 7 Tesla magnet, Research Resonance Instruments BFG-150 to 90 gradient insert. The mice were anesthetized with 2% isoflurane in 2 L/minute of medical grade oxygen while the respiratory rates were monitored, and the surface body temperature was also monitored and kept at 34°C throughout the imaging. An in-house 2-cm diameter surface coil was placed on the mouse skull to obtain the images. The T2-weighted (T2W) imaging protocol parameters were as follows: a two-dimensional fast spin echo sequence with echo time (TE) = 82.5 ms, repetition time (TR) = 4000 ms, echo train length = 8, axial slice thickness = 0.6 mm with no spacing, field of view = 3 cm, matrix = 128 × 128, number of excitations (NEX) = 10. The dicom files were opened in OsiriX version 3.3.2 (OsiriX Foundation, Geneva, Switzerland), and the regions of interest were manually delineated. The hyperintense areas of the stroke region and the total ipsilateral and contralateral hemisphere areas (excluding the ventricles) were measured in 11 consecutive slices, starting approximately 2.5 mm anterior and extending toward −3.5 mm posterior to the bregma.
Assessment of Infarct Size and Brain Swelling
The brain swelling was calculated by the following formula that used T2W−MRI obtained at 3 days after stroke:
| (1) |
The MRI infarct sizes were calculated by the following formula that used T2W-MRI obtained at 3 days or 2 weeks after stroke:
| (2) |
The histological infarct size at 2 weeks after stroke was calculated by using the same formula that used measurements from silver-stained sections. For silver staining, the mice were perfused transcardially with 30 mL of cold 0.9% NaCl, followed by 30 mL of 3% balanced (pH 7.4) formalin solution. The heads were kept overnight in 3% balanced formalin solution, and then the brains were transferred into a 20% sucrose/3% formalin solution until they sank. Then, 30-μm sections were cut with a cryostat. One in every 16 sections (11 sections per brain) were stained with silver stain33 and scanned at 1200 dpi. The total ipsilateral, contralateral, and infarct areas were measured with ImageJ version 1.44o (NIH, Bethesda, MD).
Isolation of Immune Cells from Brain and Blood
After induction of deep anesthesia, blood was collected through cardiac puncture in EDTA syringes (50 μL of 2 mmol/L EDTA for 1 mL of blood). The mice were perfused with 30 mL of cold saline, the brains were removed immediately, the hemispheres were split, and ipsilateral hemispheres were collected in phosphate-buffered saline (Gibco, Carlsbad, CA) on ice. The ipsilateral hemispheres were passed through a 70-μL cell strainer in Hanks' balanced salt solution (Gibco). Then, the homogenates were incubated in 1 mL of 2 U/mL of Liberase CI (Roche, Indianapolis, IN) in Hanks' balanced salt solution for 1 hour at 37°C, then centrifuged (490 × g for 20 minutes without brake) >30% Percoll. The cells were collected as the pellet and washed with 10% fetal bovine serum (Gibco) in Dulbecco's modified Eagle medium (Gibco). The red blood cells in blood were lyzed by lysis buffer (7.47 g of ammonium chloride and 2.04 g of Tris base in 1 L of ddH2O, pH 7.6), and the cells were washed with 10% fetal bovine serum in Dulbecco's modified Eagle medium and kept on ice until staining.
Flow Cytometric Analysis
Cells were first stained with 0.1% Live/Dead-Aqua (Invitrogen, Carlsbad, CA) to exclude the dead cells from the analysis and then blocked with 1% anti-mouse CD16/32 (93) (eBioscience, San Diego, CA) antibody and 10% mouse serum (Jackson ImmunoResearch Laboratories Inc., West Grove, PA) in a staining buffer [5% bovine serum albumin (Sigma-Aldrich, St. Louis, MO) and 2 mmol/L EDTA (Sigma-Aldrich) in phosphate-buffered saline]. The cells were incubated with the following antibodies for 20 minutes at 4°C: anti-mouse CD45 (30-F11), CD11b (M1/70), Gr1 (RB6-8C5), F4/80 (BM8), CD3e (17A2), CD8b (eBioH35 to 17.2), NK1.1 (PK136) (all from eBioscience), and CD4 (RM4-5) (Invitrogen). The flow cytometric analysis was performed on a Becton Dickinson LSR-II (Stanford Shared FACS Facility), and the data were analyzed with FlowJo version 9.7.5 (TreeStar Inc., Ashland, OR). The gates were set based on the unstained cells, and the compensation was achieved by single-color stained BD-CompBeads (BD Biosciences, San Jose, CA).
Histology and Quantification of MCs
After perfusing each mouse under deep isoflurane anesthesia with 30 mL of cold 0.9% NaCl and 30 mL of cold 3% buffered formalin, the heads were removed and incubated overnight in 3% buffered formalin solution. Then, the cranial bones then were removed carefully so that the dura remained on the brain surface. The dura was removed from the brain surface by using a fine-tip forceps, placed onto a slide with a drop of water, and then spread out under a dissection microscope to make a whole-mount preparation. The brain sections were obtained as described in the Assessment of Infarct Size and Brain Swelling section. Toluidine blue-stained brain MCs were quantified in one of every four sections taken throughout the brain, starting approximately 1.8 mm anterior of bregma to approximately −2.5 mm posterior of bregma. This approximately spans the region from where the corpus callosum first appears in coronal sections to mid-late hippocampus. This includes the area where the highest numbers of MCs reside in the brain (the area between hippocampus and thalamus). Thirty-two sections were counted for each brain.
Statistical Analysis
All values are expressed as means ± SEM. The normality of the data was determined by Kolmogorov-Smirnov test for each group. For comparisons of more than two groups, we used one-way analysis of variance followed by post hoc Tukey test or Student's t-test if the data were parametric, or the Kruskal-Wallis test with post hoc Dunn test or U-test if the data were nonparametric. For comparison of two groups, we used a two-tailed Student's t-test with Welch correction if the data were parametric and a two-tailed U-test if the data were nonparametric. Prism version 4.0c (GraphPad Software Inc., San Diego, CA) was used. P < 0.05 was considered statistically significant.
Results
MCs Can Increase Infarct Size after Stroke
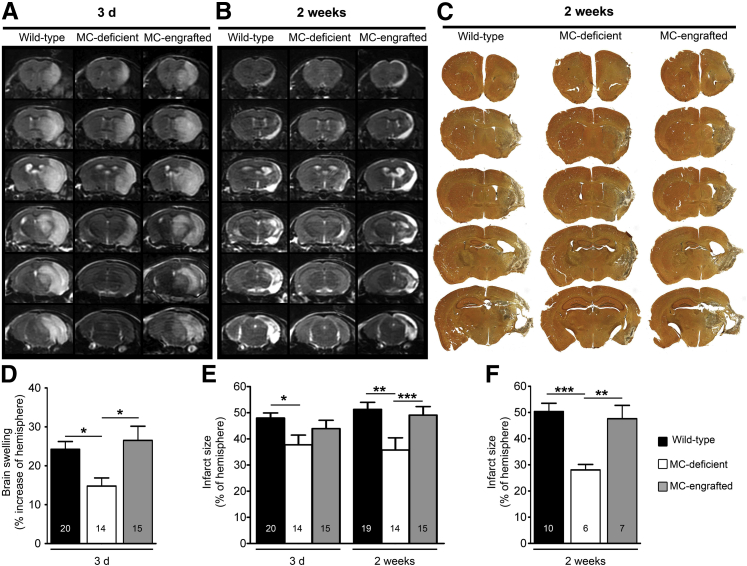
We first subjected MC-deficient WBB6F1-KitW/W-v mice and the corresponding WT mice (ie, WBB6F1-Kit+/+) to stroke and used T2W-MRI to measure brain swelling and infarct size. Compared with the corresponding WT mice, the MC-deficient mice exhibited significantly less brain swelling and smaller infarcts at 3 days after stroke (Figure 1, A, D, and E) and significantly smaller infarct size at 2 weeks after stroke, whether quantified by T2W-MRI (Figure 1, B and E) or histology (Figure 1, C and F). To evaluate whether this reduction in stroke pathology could be reversed by selective repair of the MC deficiency, MC-deficient mice were engrafted intravenously with BMCMCs from WT (ie, WBB6F1-Kit+/+) mice. These MC-engrafted mice exhibited brain swelling and infarct sizes similar to those of the WT mice (Figure 1). By contrast, measurements of cerebral blood flow (Supplemental Figure S1), blood pressure, arterial blood gases, blood glucose, and blood lactate (Supplemental Tables S1 and S2) before, during, or after stroke surgery found no statistically significant differences among the WT, MC-deficient, and MC-engrafted animals. Together, our data indicate that MCs can exacerbate stroke pathology in WBB6F1-KitW/W-v mice without having marked effects on cerebral blood flow, blood pressure, heart rate, or various measures of metabolic function.
Figure 1.
MCs can contribute to infarct size and brain swelling after stroke. Representative T2W-MRI of brains of WT (WBB6F1-Kit+/+) mice, MC-deficient (WBB6F1-KitW/W-v) mice, and MC-engrafted (WBB6F1-Kit+/+ BMCMCs→WBB6F1-KitW/W-v) mice at 3 days (A) or 2 weeks (B) after stroke. C: Representative silver-stained serial coronal sections of brains of WT, MC-deficient, and MC-engrafted mice at 2 weeks after stroke. Quantification from T2W-MRI scans of brain swelling at 3 days (D) and infarct size at 3 days and 2 weeks (E) after stroke. F: Quantification of infarct size from brain sections obtained 2 weeks after stroke. Data are expressed as means ± SEM (D–F). Data were pooled from eight (E) and three (F) experiments, each of which gave similar results. The number of mice in each group is indicated in each bar. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.005.
MCs Can Influence Numbers of Brain Leukocytes after Stroke
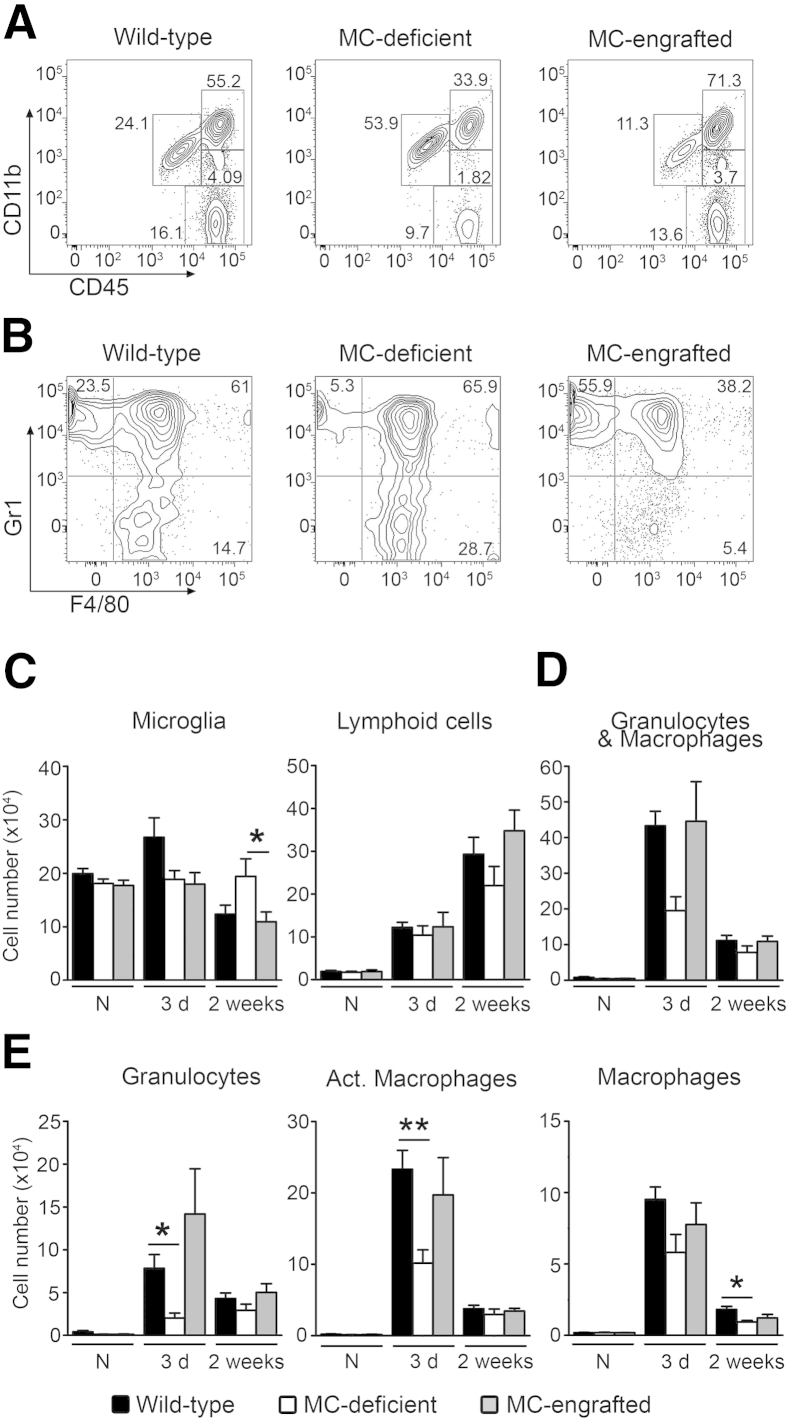
We used flow cytometry of leukocytes recovered from mouse brains to quantify the effects of MCs on populations of brain leukocytes at baseline and at 3 days or 2 weeks after stroke (Figure 2, A and B, and Supplemental Figure S2A). There were few or no differences in numbers of microglia (CD11b+CD45low) or lymphoid cells (CD11bnegativeCD45high) among MC-deficient mice and their corresponding WT and intravenously MC-engrafted groups, either before or 3 days or 2 weeks after stroke, and only one such difference (higher numbers of microglia at 2 weeks after stroke in WBB6F1-KitW/W-v mice) reached statistical significance (Figure 2C). In contrast, MC-deficient mice exhibited fewer cells in the granulocyte and macrophage population (CD11bhighCD45high cells) at 3 days after stroke than did their corresponding WT or MC-engrafted groups (Figure 2, A and D). Although those differences did not achieve statistical significance, we analyzed the granulocyte and macrophage population further by using Gr1 and F4/80 markers to identify three subpopulations: granulocytes (Gr1+F4/80−), activated macrophages (Gr1+F4/80+), and macrophages (Gr1−F4/80+) (Figure 2B and Supplemental Figure S2B). We found fewer granulocytes and activated macrophages in the brains of MC-deficient WBB6F1-KitW/W-v mice at 3 days after stroke than in the corresponding WT or MC-engrafted groups (Figure 2, C and E). Together, our results suggest that MCs may contribute to increases in numbers of brain granulocytes and activated macrophages in this stroke model.
Figure 2.
MCs contribute to infiltration of granulocytes and macrophages into the brain after stroke. Representative flow cytometric plots of CD11b-CD45 (A) and Gr1-F4/80 (B) brain immune cells at 3 days after stroke in MC-deficient (WBB6F1-KitW/W-v) mice and their corresponding WT and MC-engrafted mice. Quantification of the indicated immune cell populations before (N) or 3 days or 2 weeks after stroke in MC-deficient WBB6F1-KitW/W-v mice and the corresponding WT mice and MC-engrafted KitW/W-v mice (C–E). Data are expressed as means ± SEM. n = 9 to 10 animals per group for naive; n = 8 to 12 animals for 3 days; n = 8 to 9 animals for 2 weeks. ∗P < 0.05, ∗∗P < 0.01. Act., activated; N, naive.
MC-Deficient Cpa3-Cre; Mcl-1fl/fl Mice Have Reduced Brain Pathology after Stroke
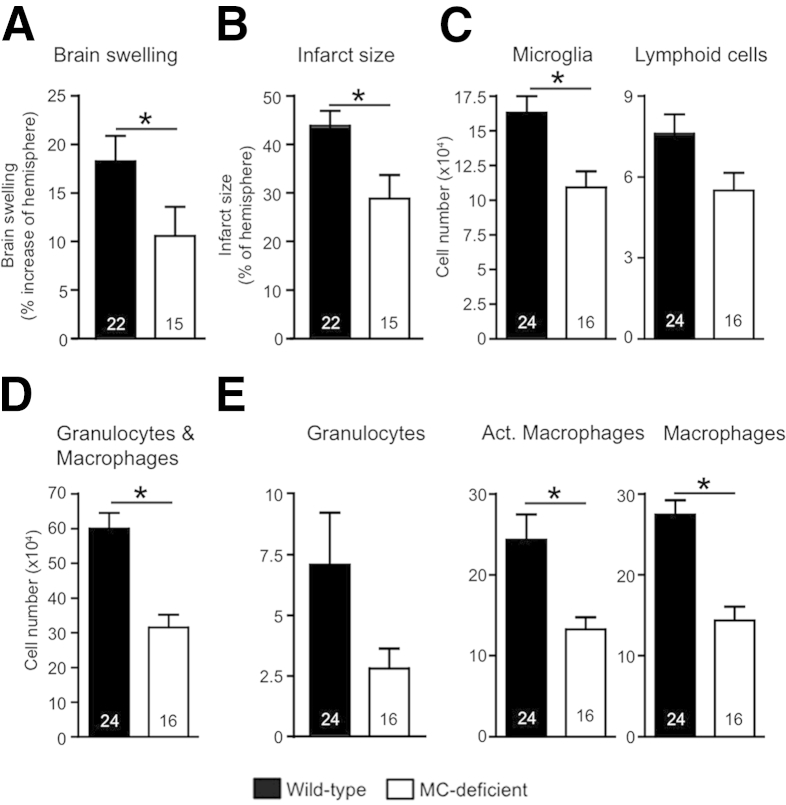
In addition to their MC deficits, MC-deficient mice that have abnormalities of c-kit structure or expression also have additional hematopoietic deficiencies. For example, c-kit–mutant mice have defects in certain hematopoietic and other cell populations.17,27 This fact has engendered considerable recent discussion and debate about the value of these mice for ascertaining MC functions in vivo.30,34,35 For this reason, Reber et al30 have recommended using at least two different lines of mice with distinct mechanisms of MC depletion, in addition to using selective MC engraftment of MC-deficient mice, to provide strong support for conclusions about MC function in models of disease or protective host responses. We therefore also investigated the involvement of MCs in stroke pathology by using Cpa3-Cre; Mcl-1fl/fl mice, which have a severe deficiency of MCs by a mechanism that is independent of mutations at c-kit.28 These MC-deficient mice have markedly reduced MC numbers in all tissues examined (including lungs, skin, liver, and the dura mater, which is part of the meninges) except for the spleen, where the numbers of MCs in Cpa3-Cre; Mcl-1fl/fl mice are similar to that of the corresponding WT controls.28 Furthermore, they lack other non–MC-related abnormalities found in the c-kit–mutant MC-deficient mice; for example, although Cpa3-Cre; Mcl-1fl/fl mice have reduced numbers of blood basophils, these mice have normal numbers of blood neutrophils.28 At 3 days after stroke, Cpa3-Cre; Mcl-1fl/fl mice had significantly less brain swelling, smaller infarcts, and fewer brain microglia, lymphoid cells, granulocytes, and macrophages than the corresponding control Cpa3-Cre; Mcl-1+/+ mice (Figure 3). These data are consistent with those obtained in experiments performed with the c-kit–mutant WBB6F1-KitW/W-v MC-deficient mice and provide further support for an important role for MCs in exacerbating brain pathology after ischemic stroke.
Figure 3.
MC-deficient Cpa3-Cre; Mcl-1fl/fl mice have reduced pathology after stroke. Quantification at 3 days after stroke of brain swelling (A) and infarct size (B) and numbers of microglia and lymphoid cells (C); granulocytes and macrophages (D); granulocytes, Act. macrophages, and macrophages (E) in Cpa3-Cre; Mcl-1+/+ mice (which have normal numbers of MCs and basophils) and Cpa3Cre; Mcl-1fl/fl mice (which have markedly reduced numbers of MCs and also reduced numbers of basophils). Data are expressed as means ± SEM. The number of mice in each group is indicated in each bar. ∗P < 0.05. P = 0.07 for lymphoid cells (C) and P = 0.08 for granulocytes (E). Act., activated.
Importance of Meningeal MCs in Stroke-Induced Pathology
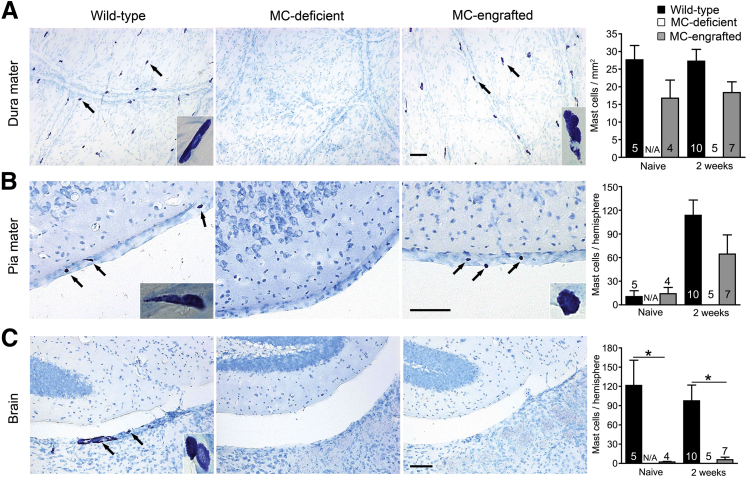
We next sought to identify the specific MC populations in the CNS which might contribute to pathology in stroke. MCs reside in both the brain parenchyma and the meninges in WT mice.36–38 To compare the numbers and anatomical distribution of MCs in the CNS of WT and MC-engrafted mice, we quantified the MCs in the meninges (dura and pia mater) and brain parenchyma of WT and MC-engrafted mice before and after stroke. Both WT mice and MC-engrafted mice had similar numbers of MCs in the dura and pia mater both before and 2 weeks after stroke (Figure 4, A and B). Interestingly, the density of MCs in mouse dura mater (15 to 27 cells/mm2), calculated according to our data, are similar to that reported for MCs in the dura mater of humans (11 to 23 cells/mm2).39 In contrast to the meninges, the brain parenchyma of the MC-engrafted mice had either no or substantially fewer MCs than corresponding WT mice (Figure 4C). These data strongly suggest that brain parenchymal MCs are not responsible for the MC-dependent exacerbation of stroke pathology we observed and instead suggest a potential role for meningeal MCs in modulating the response to ischemic injury.
Figure 4.
Location of MCs in the CNS. Representative toluidine blue-stained images of and quantification of MCs in dura mater (A), pia mater (B), and brain parenchyma (C) of MC-deficient WBB6F1-KitW/W-v mice and the corresponding WT mice and MC-engrafted KitW/W-v mice. MCs stain purple with toluidine blue; arrows indicate representative MCs. Insets show magnified images of MCs in each tissue. Data are expressed as means ± SEM. The number of mice in each group is indicated in (or over) each bar. ∗P < 0.05. Scale bars: 100 μm (A–C). N/A, not applicable.
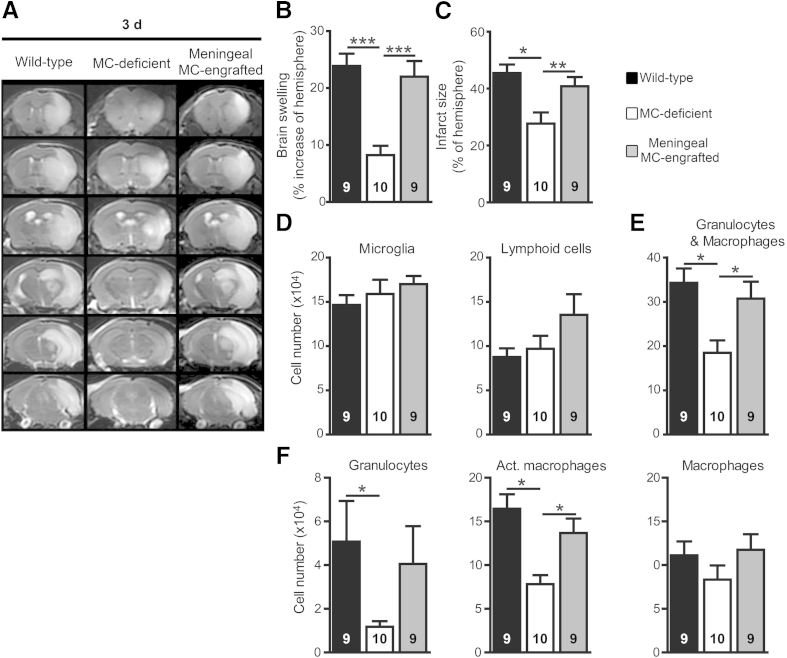
To test this idea, we engrafted BMCMCs locally into the meninges as described,11,15 rather than systemically by i.v. transfer as was done in the previous experiments. After meningeal engraftment of MCs, MC-engrafted WBB6F1-KitW/W-v mice developed significantly more brain swelling and larger infarcts at 3 days after stroke than the MC-deficient mice and resembled the WT mice in both of these features (Figure 5, A–C). Furthermore, both WT and meningeal MC-engrafted WBB6F1-KitW/W-v mice had similar numbers of microglia and lymphoid cells but significantly more brain granulocytes and activated macrophages 3 days after stroke than the MC-deficient group (Figure 5, D–F). These results were similar to those observed with systemic (i.v.) MC-engraftment in WBB6F1-KitW/W-v mice (Figures 1 and 2) and are consistent with the conclusion that meningeal MCs are sufficient to elicit the MC-dependent effects on lesion size and tissue infiltration with granulocytes and macrophages observed after stroke.
Figure 5.
Meningeal MCs are sufficient to enhance pathology after stroke. Representative T2W-MRI images (A); quantification of brain swelling (B) and infarct size (C); and numbers of microglia and lymphoid cells (D); granulocytes and macrophages (E); granulocytes, Act. macrophages, and macrophages (F) in brain 3 days after stroke in MC-deficient WBB6F1-KitW/W-v mice and the corresponding WT mice and meningeal MC-engrafted KitW/W-v mice. Data are expressed as means ± SEM. The number of mice per group is indicated in each bar. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.005. P = 0.07 for MC-deficient versus MC-engrafted groups for granulocytes (F). Act., activated.
Identification of MC-Derived Factors that Influence Stroke Pathology
MCs elicit their biological effects through secretion of a variety of different factors, including cytokines and chemokines.17 We selected two candidate MC-derived factors with biological properties that have the potential to influence stroke pathology (ie, IL-6 and CCL7) and tested them via the MC engraftment approach. IL-6 has been reported to be an important biomarker of disease severity in patients after ischemic stroke, and cerebrospinal fluid and plasma IL-6 levels correlate highly with infarct volume.40–42 However, prior experimental studies in animals showed detrimental, beneficial, or even no effects of IL-6 on stroke pathology,43–48 and it has been suggested that the post-stroke effects of IL-6 may depend on its cellular source.47 CCL7 is a monocyte chemoattractant that is reported to be involved in several disease entities and that is important for the release of monocytes from the BM.31,49 Although the role of CCL7 in stroke pathology is not known, it has been reported that CCL7 is induced after stroke in rats.50 To test whether IL-6 and/or CCL7 of MC origin might play a role in the MC-dependent effects observed after stroke, we engrafted BMCMCs from KO mice for each factor into the meninges of the MC-deficient WBB6F1-KitW/W-v mice. Control groups included MC-deficient mice engrafted in the meninges with WT BMCMCs from either WBB6F1-Kit+/+ mice (the same background as the WBB6F1-KitW/W-v mice) or C57BL/6 mice (the same background as the IL–6- and CCL7-KO mice).
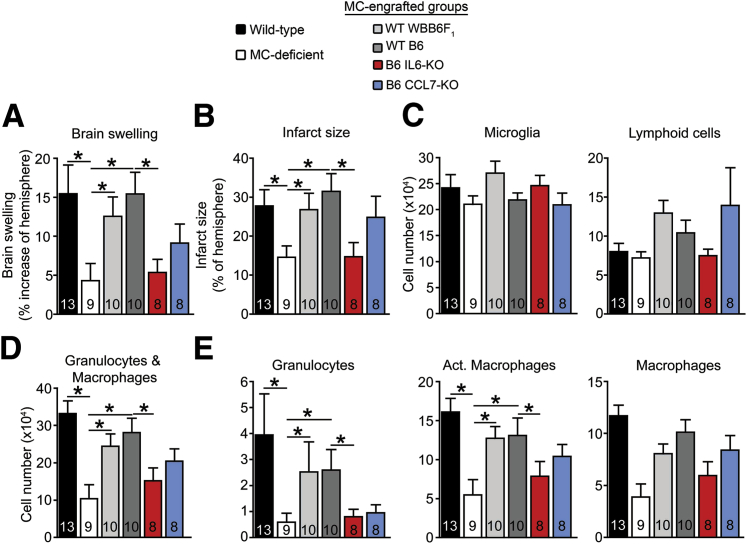
At 3 days after stroke, WBB6F1-KitW/W-v mice engrafted in the meninges with WT BMCMCs of either WBB6F1 or C57BL/6 origin exhibited features of stroke pathology that were similar to each other and to those of the WT (ie, WBB6F1-Kit+/+) mice (Figure 6), with significantly greater brain swelling, larger infarcts, and more brain granulocytes and activated macrophages than in the MC-deficient mice (Figure 6, A, B, D, and E). These findings thus confirmed our previous results (Figure 5), indicating that meningeal MCs can exacerbate stroke outcome. In contrast, the pathology in IL–6-KO MC-engrafted mice resembled that in the MC-deficient mice, with significantly less brain swelling, smaller infarcts, and fewer granulocytes and activated macrophages than in the WT MC-engrafted mice (Figure 6, A, B, D, and E). CCL7-KO MC-engrafted mice had trends for less brain swelling and fewer granulocytes than the WT MC-engrafted mice, but these differences did not reach statistical significance (Figure 6, A, B, D, and E). Numbers of MCs in the dura were similar among the WT mice and the various meningeal MC-engrafted KitW/W-v groups, suggesting that the differing extent of pathology among the groups was not simply because of differing levels of meningeal MC engraftment (Supplemental Figure S3). Although we found MCs in the spleens of meningeal MC-engrafted KitW/W-v mice (indicating that some of the MCs reached sites outside of the CNS), the numbers were substantially lower than those of systemically (i.v.) engrafted mice (data not shown). Together these data suggested that MC-secreted IL-6 plays a major role in MC-dependent exacerbation of injury after stroke, whereas the role of MC-secreted CCL7 was less prominent.
Figure 6.
MC-expressed IL-6 contributes to MC-dependent exacerbation of stroke pathology. Quantification at 3 days after stroke of brain swelling (A) and infarct size (B) from T2W-MRI images and numbers of microglia and lymphoid cells (C); granulocytes and macrophages (D); granulocytes, Act. macrophages, and macrophages (E) in the brain in the indicated groups of MC-deficient WBB6F1-KitW/W-v mice, WT (WBB6F1-Kit+/+) mice, and MC-deficient WBB6F1-KitW/W-v mice engrafted in the meninges with WT BMCMCs from WBB6F1-Kit+/+ (WT WBB6F1) or C57BL/6 (WT B6) mice, or BMCMCs derived from B6 mice genetically lacking (KO) IL-6 or CCL7 (MC-engrafted mice). Data are expressed as means ± SEM. The number of mice per group is indicated in each bar. ∗P < 0.05. P = 0.1 for WT B6 MC-engrafted versus B6 CCL7-KO MC-engrafted groups for granulocytes (E). Act., activated.
Discussion
This study provides evidence to indicate that meningeal MCs can make important contributions to key features of stroke pathology, including increasing numbers of brain granulocytes and activated macrophages and exacerbating infarct size and brain swelling. Furthermore, our data indicate that MC-derived IL-6, and perhaps CCL7, can contribute to the mechanisms by which MCs exacerbate stroke pathology. These data imply a role for the meninges in modulating brain pathology in stroke, and we have begun to delineate the molecular pathways involved.
We used different types of MC-deficient mice to study the role of MCs after stroke, which is important because conclusions about the effects of MCs in certain disease models can vary according to the type of MC-deficient mice analyzed.30,34,35,51–56 We compared results obtained in well-established c-kit–mutant MC-deficient mouse models and in a new, c-kit–independent, cre-mediated MC-deficient mouse. As shown in the Results section, evidence derived from the WBB6F1-KitW/W-v and Cpa3-Cre; Mcl-1fl/fl mouse models of MC deficiency indicates that MCs can exacerbate stroke pathology. When we performed experiments using this model of stroke comparing C57BL/6 WT mice, MC-deficient C57BL/6-KitW−sh/W−sh mice, and C57BL/6-KitW−sh/W−sh mice engrafted i.v. with WT BMCMCs, we obtained results for infarct size at 3 days or 2 weeks, and for granulocyte infiltration of the infarcted area at day 3 after infarction, that were similar to those for the corresponding experiments with WBB6F1 WT mice, MC-deficient KitW/W-v mice or WT BMCMC-engrafted KitW/W-v mice [i.e., lower values for the mast cell-deficient mice than for the WT or MC-engrafted Kit mutant mice (data not shown)]. However, unlike the findings we obtained in the other two types of MC-deficient mice, most of the differences between the results for the three different C57BL/6 mouse groups did not reach the P < 0.05 level of statistical significance, perhaps because of phenotypic differences between C57BL/6 WT and KitW−sh/W−sh mice other than the MC deficiency of the KitW−sh/W−sh mice.30,34,35
Moreover, we provide strong evidence that meningeal MCs were sufficient to elicit the detrimental effects of MCs after stroke. First, although CNS MCs are normally found in both the meninges and brain parenchyma in WT mice, they were only found in the meninges of the MC-engrafted mice. This implies that brain MCs are not necessary for the MC-dependent effects observed and that meningeal MCs may be sufficient to exacerbate stroke outcome. Second, targeted repair of the meningeal MC deficiency strongly suggests that meningeal MCs are sufficient to elicit the effects seen.
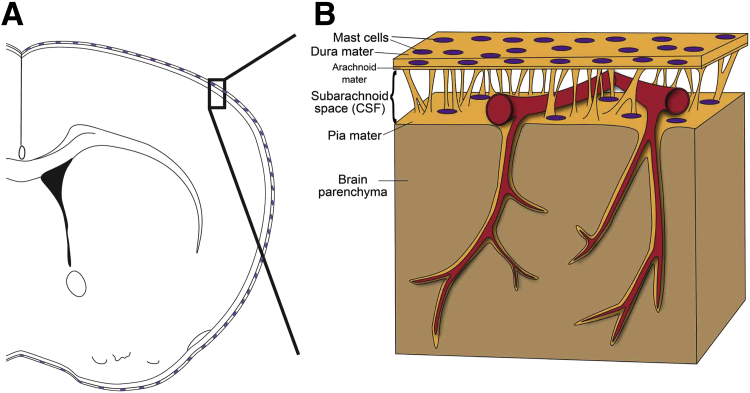
To our knowledge, these data represent the first evidence that immune cells located in the meninges can modulate post-stroke brain pathology and require a shift in current understanding of post-stroke inflammation. Given the perivascular location of MCs and that blood flow to the brain traverses the meninges (Figure 7), meningeal MCs are ideally located to modulate immune cell trafficking to the brain during stroke. Notably, evidence suggests that the meninges can play a role in regulating immune cell trafficking in other disease models such as experimental autoimmune encephalomyelitis,11,14,15 supporting the general idea that the meninges can function as a gatekeeper to modulate brain inflammation.57,58 Moreover, given the relative accessibility of the meninges (eg, via intrathecal injection—a well-established route for drug delivery for treatment of malignancies that involve the meninges59–61), targeting of meningeal MCs offers a potential therapeutic strategy for overcoming the hurdle of delivering drugs to the injured brain and represents an approach that can potentially limit unwanted systemic immunomodulatory side effects of such treatment. On the basis of our identification of meningeal MCs as key effectors of stroke pathology in our mouse model, and in light of the recent development of techniques to ameliorate inflammation after brain injury via transcranial approaches,62 we think that it will be of interest to determine whether there is evidence that meningeal MCs can exacerbate stroke pathology in humans and therefore might represent targets of novel immunomodulatory strategies for limiting stroke pathology.
Figure 7.
A: Scheme shows how the brain is enveloped by the meninges that contain MCs in both the dura mater and pia mater. B: Before entering the brain parenchyma, blood vessels course on the surface of the brain between the dura mater and pia mater. Therefore, as a resident immune cell in the meninges, the MC has the potential to influence blood vessels and to function as a gatekeeper to influence brain inflammation and pathology.
MCs function through the secretion of many mediators, including various cytokines and chemokines.17 We found that MCs which could not produce IL-6 failed to orchestrate meningeal MC-dependent exacerbation of stroke pathology, despite similar numbers and meningeal distribution of IL-6–deficient versus WT-engrafted MCs after their injection into the meninges of MC-deficient mice. These results indicate that MC-derived IL-6 is one MC-derived product that has an important role in mediating the effects of MCs on stroke pathology. This detrimental effect of MC-derived IL-6 after stroke is of considerable interest, because prior work has described detrimental, beneficial, or even no effects of IL-6 on stroke pathology,43–48 and it has been suggested that the post-stroke effects of IL-6 may depend on its cellular source.47 We think that our data are the first to identify the role of IL-6 derived from a specific cell type in a particular anatomical location in stroke pathology. By contrast, we found that MC production of CCL7, a known monocyte chemoattractant,31 had a less striking role after stroke than did MC-derived IL-6. MC-derived IL-6 exacerbated key measured parameters of stroke pathology (ie, brain swelling and infarct size, and numbers of brain granulocytes and activated macrophages), whereas MC-produced CCL7 had its most notable effect (albeit not a statistically significant one) on brain granulocyte numbers, with little effect on infarct size.
Given that cerebrospinal fluid IL-6 levels correlate with infarct size in patients after a stroke,42 our findings suggest that enhanced production of MC-derived IL-6 in this setting may contribute to the observed positive correlation between cerebrospinal fluid IL-6 levels and infarct size. However, an important question that remains to be investigated is how meningeal MCs are activated to secrete IL-6 and presumably other mediators after stroke. The fact that the effects of MCs on brain pathology were observed as early as 3 days after stroke suggests that meningeal MCs may be activated via innate signals,26 which could include products of complement activation, ligands of Toll-like receptors, and/or effects of damage-associated molecular patterns that may originate from tissue damage in the brain parenchyma.
Another question that remains to be answered is how IL-6 (and other products of MC activation, because we have directly studied only two of the many potential mediators produced by MCs in this setting) ultimately exacerbate brain pathology. One can speculate about several potential and not mutually exclusive mechanisms, including effects of secreted factors that increase cell adhesion molecules on brain blood vessel endothelial cells and/or local production of a spectrum of chemoattractants, that together increase the migration of granulocytes and other leukocytes to the area of damage; effects of MC products on leukocyte function; direct toxic effects of certain mediators on brain parenchymal cells (neurons, glia, etc.); and effects of MC-derived products that activate microglial cells to cause damage or orchestrate immune responses that result in parenchymal injury.
Conclusion
We have identified evidence that meningeal MCs can exacerbate stroke outcome in mice, highlighting a novel function for the meninges after stroke as a gatekeeper to modulate brain inflammation and pathology. By finding that IL-6 production can represent one mechanism of action of MCs in this setting, we have begun to delineate the MC-dependent molecular pathways involved in modulating the response to stroke. These findings also suggest that targeting the meninges and/or MC-produced IL-6 might have therapeutic potential in stroke.
Acknowledgments
We thank Chen Liu and Chang Ho Song for help with histology; Ching-Cheng Chen and Stanford Shared FACS Facility staff for flow cytometric advice; Laura J. Pisani, Raphael Guzman, and the Stanford Small Animal Imaging Facility for help with MRI scanning; Scott Hamilton for statistical analysis advice; Elizabeth Hoyte for figure preparation; Cindy Samos for help with manuscript preparation; Drs. Katrin Andreasson and Marion Buckwalter for critical reading of the manuscript; David Kunis for lab management; and Israel Charo (UCSF Gladstone Institute) for the CCL7-KO mice.
Footnotes
Supported in part by NIH grants NS080062 and NS37520-08 (G.K.S.) and AI070813, AI023990, and CA072074 (S.J.G.); Russell and Elizabeth Siegelman (G.K.S.); Bernard and Ronni Lacroute (G.K.S.); William Randolph Hearst Foundation (G.K.S.); NHMRC Career Development Fellowship and NHMRC project grants (M.A.G.); and Stanford School of Medicine Dean's Fellowship (Neizer Funds) (A.A.).
T.M.B. and G.K.S. contributed equally to this work as senior authors.
Disclosures: None declared.
Contributor Information
Michele A. Grimbaldeston, Email: michele.grimbaldeston@unisa.edu.au.
Stephen J. Galli, Email: sgalli@stanford.edu.
Tonya M. Bliss, Email: tbliss1@stanford.edu.
Gary K. Steinberg, Email: gsteinberg@stanford.edu.
Supplemental Data
Laser Doppler flow meter measurements of cerebral blood flow during and after stroke surgery in MC-deficient (WBB6F1-KitW/W-v) mice, WT (WBB6F1-Kit+/+) mice, and MC-engrafted (WBB6F1-Kit+/+ BMCMCs→WBB6F1-KitW/W-v) mice. Pooled analysis of the two independent experiments was performed, each of which gave similar results. Data are expressed as means ± SEM. n = 4 mice per group. CCA, common carotid artery; ECA, external carotid artery.
Identification of leukocyte populations by flow cytometry. Representative CD11b-CD45 (A) and Gr1-F4/80 (B) flow cytometric plots of brain immune cells obtained 3 days after stroke. The CD11bhighCD45high population of granulocytes and macrophages population (A) was further analyzed for expression of Gr1 and F4/80 (B).
MC distribution and counts in dura maters of WT (WBB6F1-Kit+/+) mice, MC-deficient (WBB6F1-KitW/W-v) mice, and MC-deficient (WBB6F1-KitW/W-v) mice after their meningeal engraftment with different populations of WBB6F1 or C57BL/6 (B6) BMCMCs. A: Representative images of toluidine blue-stained whole mounts of dura mater from mice from each indicated group. B: Quantification of MCs in the dura mater (prepared as in A) of the indicated groups. No statistically significant differences were found between any of the groups. Data are expressed as means ± SEM. The number of mice per group is indicated in each bar. Scale bar = 100 μm (A).
References
- 1.Go A.S., Mozaffarian D., Roger V.L., Benjamin E.J., Berry J.D., Borden W.B., American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donnan G.A., Fisher M., Macleod M., Davis S.M. Stroke. Lancet. 2008;371:1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 3.Macrez R., Ali C., Toutirais O., Le Mauff B., Defer G., Dirnagl U., Vivien D. Stroke and the immune system: from pathophysiology to new therapeutic strategies. Lancet Neurol. 2011;10:471–480. doi: 10.1016/S1474-4422(11)70066-7. [DOI] [PubMed] [Google Scholar]
- 4.Iadecola C., Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moskowitz M.A., Lo E.H., Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arac A., Brownell S.E., Rothbard J.B., Chen C., Ko R.M., Pereira M.P., Albers G.W., Steinman L., Steinberg G.K. Systemic augmentation of αB-crystallin provides therapeutic benefit twelve hours post-stroke onset via immune modulation. Proc Natl Acad Sci U S A. 2011;108:13287–13292. doi: 10.1073/pnas.1107368108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becker K.J. Modulation of the postischemic immune response to improve stroke outcome. Stroke. 2010;41:S75–S78. doi: 10.1161/STROKEAHA.110.592881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shechter R., London A., Schwartz M. Orchestrated leukocyte recruitment to immune-privileged sites: absolute barriers versus educational gates. Nat Rev Immunol. 2013;13:206–218. doi: 10.1038/nri3391. [DOI] [PubMed] [Google Scholar]
- 9.Androdias G., Reynolds R., Chanal M., Ritleng C., Confavreux C., Nataf S. Meningeal T cells associate with diffuse axonal loss in multiple sclerosis spinal cords. Ann Neurol. 2010;68:465–476. doi: 10.1002/ana.22054. [DOI] [PubMed] [Google Scholar]
- 10.Derecki N.C., Cardani A.N., Yang C.H., Quinnies K.M., Crihfield A., Lynch K.R., Kipnis J. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med. 2010;207:1067–1080. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christy A.L., Walker M.E., Hessner M.J., Brown M.A. Mast cell activation and neutrophil recruitment promotes early and robust inflammation in the meninges in EAE. J Autoimmun. 2012;42:50–61. doi: 10.1016/j.jaut.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Kivisakk P., Imitola J., Rasmussen S., Elyaman W., Zhu B., Ransohoff R.M., Khoury S.J. Localizing central nervous system immune surveillance: meningeal antigen-presenting cells activate T cells during experimental autoimmune encephalomyelitis. Ann Neurol. 2009;65:457–469. doi: 10.1002/ana.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim J.V., Kang S.S., Dustin M.L., McGavern D.B. Myelomonocytic cell recruitment causes fatal CNS vascular injury during acute viral meningitis. Nature. 2009;457:191–195. doi: 10.1038/nature07591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartholomaus I., Kawakami N., Odoardi F., Schlager C., Miljkovic D., Ellwart J.W., Klinkert W.E., Flugel-Koch C., Issekutz T.B., Wekerle H., Flugel A. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature. 2009;462:94–98. doi: 10.1038/nature08478. [DOI] [PubMed] [Google Scholar]
- 15.Sayed B.A., Christy A.L., Walker M.E., Brown M.A. Meningeal mast cells affect early T cell central nervous system infiltration and blood-brain barrier integrity through TNF: a role for neutrophil recruitment? J Immunol. 2010;184:6891–6900. doi: 10.4049/jimmunol.1000126. [DOI] [PubMed] [Google Scholar]
- 16.Abraham S.N., St John A.L. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol. 2010;10:440–452. doi: 10.1038/nri2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galli S.J., Grimbaldeston M., Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol. 2008;8:478–486. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao K.N., Brown M.A. Mast cells: multifaceted immune cells with diverse roles in health and disease. Ann N Y Acad Sci. 2008;1143:83–104. doi: 10.1196/annals.1443.023. [DOI] [PubMed] [Google Scholar]
- 19.Strbian D., Karjalainen-Lindsberg M.L., Kovanen P.T., Tatlisumak T., Lindsberg P.J. Mast cell stabilization reduces hemorrhage formation and mortality after administration of thrombolytics in experimental ischemic stroke. Circulation. 2007;116:411–418. doi: 10.1161/CIRCULATIONAHA.106.655423. [DOI] [PubMed] [Google Scholar]
- 20.Strbian D., Karjalainen-Lindsberg M.L., Tatlisumak T., Lindsberg P.J. Cerebral mast cells regulate early ischemic brain swelling and neutrophil accumulation. J Cereb Blood Flow Metab. 2006;26:605–612. doi: 10.1038/sj.jcbfm.9600228. [DOI] [PubMed] [Google Scholar]
- 21.Strbian D., Tatlisumak T., Ramadan U.A., Lindsberg P.J. Mast cell blocking reduces brain edema and hematoma volume and improves outcome after experimental intracerebral hemorrhage. J Cereb Blood Flow Metab. 2007;27:795–802. doi: 10.1038/sj.jcbfm.9600387. [DOI] [PubMed] [Google Scholar]
- 22.Jin Y., Silverman A.J., Vannucci S.J. Mast cell stabilization limits hypoxic-ischemic brain damage in the immature rat. Dev Neurosci. 2007;29:373–384. doi: 10.1159/000105478. [DOI] [PubMed] [Google Scholar]
- 23.Biran V., Cochois V., Karroubi A., Arrang J.M., Charriaut-Marlangue C., Heron A. Stroke induces histamine accumulation and mast cell degranulation in the neonatal rat brain. Brain Pathol. 2008;18:1–9. doi: 10.1111/j.1750-3639.2007.00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu W., Xu L., Pan J., Zheng X., Chen Z. Effect of cerebral ischemia on brain mast cells in rats. Brain Res. 2004;1019:275–280. doi: 10.1016/j.brainres.2004.05.109. [DOI] [PubMed] [Google Scholar]
- 25.Oka T., Kalesnikoff J., Starkl P., Tsai M., Galli S.J. Evidence questioning cromolyn's effectiveness and selectivity as a ‘mast cell stabilizer' in mice. Lab Invest. 2012;92:1472–1482. doi: 10.1038/labinvest.2012.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsai M., Grimbaldeston M., Galli S.J. Mast cells and immunoregulation/immunomodulation. Adv Exp Med Biol. 2011;716:186–211. doi: 10.1007/978-1-4419-9533-9_11. [DOI] [PubMed] [Google Scholar]
- 27.Nigrovic P.A., Gray D.H., Jones T., Hallgren J., Kuo F.C., Chaletzky B., Gurish M., Mathis D., Benoist C., Lee D.M. Genetic inversion in mast cell-deficient Wsh mice interrupts corin and manifests as hematopoietic and cardiac aberrancy. Am J Pathol. 2008;173:1693–1701. doi: 10.2353/ajpath.2008.080407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lilla J.N., Chen C.C., Mukai K., BenBarak M.J., Franco C.B., Kalesnikoff J., Yu M., Tsai M., Piliponsky A.M., Galli S.J. Reduced mast cell and basophil numbers and function in Cpa3-Cre; Mcl-1fl/fl mice. Blood. 2011;118:6930–6938. doi: 10.1182/blood-2011-03-343962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grimbaldeston M.A., Chen C.C., Piliponsky A.M., Tsai M., Tam S.Y., Galli S.J. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005;167:835–848. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reber L.L., Marichal T., Galli S.J. New models for analyzing mast cell functions in vivo. Trends Immunol. 2012;33:613–625. doi: 10.1016/j.it.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsou C.L., Peters W., Si Y., Slaymaker S., Aslanian A.M., Weisberg S.P., Mack M., Charo I.F. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grimbaldeston M.A., Nakae S., Kalesnikoff J., Tsai M., Galli S.J. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat Immunol. 2007;8:1095–1104. doi: 10.1038/ni1503. [DOI] [PubMed] [Google Scholar]
- 33.Vogel J., Mobius C., Kuschinsky W. Early delineation of ischemic tissue in rat brain cryosections by high-contrast staining. Stroke. 1999;30:1134–1141. doi: 10.1161/01.str.30.5.1134. [DOI] [PubMed] [Google Scholar]
- 34.Brown M.A., Hatfield J.K. Mast cells are important modifiers of autoimmune disease: with so much evidence, why is there still controversy? Front Immunol. 2012;3:147. doi: 10.3389/fimmu.2012.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodewald H.R., Feyerabend T.B. Widespread immunological functions of mast cells: fact or fiction? Immunity. 2012;37:13–24. doi: 10.1016/j.immuni.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Dropp J.J. Mast cells in mammalian brain. Acta Anat (Basel) 1976;94:1–21. doi: 10.1159/000144540. [DOI] [PubMed] [Google Scholar]
- 37.Dropp J.J. Mast cells in the human brain. Acta Anat (Basel) 1979;105:505–513. doi: 10.1159/000145157. [DOI] [PubMed] [Google Scholar]
- 38.Orr E.L. Dural mast cells: source of contaminating histamine in analyses of mouse brain histamine levels. J Neurochem. 1984;43:1497–1499. doi: 10.1111/j.1471-4159.1984.tb05416.x. [DOI] [PubMed] [Google Scholar]
- 39.Varatharaj A., Mack J., Davidson J.R., Gutnikov A., Squier W. Mast cells in the human dura: effects of age and dural bleeding. Childs Nerv Syst. 2012;28:541–545. doi: 10.1007/s00381-012-1699-7. [DOI] [PubMed] [Google Scholar]
- 40.Acalovschi D., Wiest T., Hartmann M., Farahmi M., Mansmann U., Auffarth G.U., Grau A.J., Green F.R., Grond-Ginsbach C., Schwaninger M. Multiple levels of regulation of the interleukin-6 system in stroke. Stroke. 2003;34:1864–1869. doi: 10.1161/01.STR.0000079815.38626.44. [DOI] [PubMed] [Google Scholar]
- 41.Vila N., Castillo J., Davalos A., Chamorro A. Proinflammatory cytokines and early neurological worsening in ischemic stroke. Stroke. 2000;31:2325–2329. doi: 10.1161/01.str.31.10.2325. [DOI] [PubMed] [Google Scholar]
- 42.Beridze M., Sanikidze T., Shakarishvili R., Intskirveli N., Bornstein N.M. Selected acute phase CSF factors in ischemic stroke: findings and prognostic value. BMC Neurol. 2011;11:41. doi: 10.1186/1471-2377-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clark W.M., Rinker L.G., Lessov N.S., Hazel K., Hill J.K., Stenzel-Poore M., Eckenstein F. Lack of interleukin-6 expression is not protective against focal central nervous system ischemia. Stroke. 2000;31:1715–1720. doi: 10.1161/01.str.31.7.1715. [DOI] [PubMed] [Google Scholar]
- 44.Dugan L.L., Ali S.S., Shekhtman G., Roberts A.J., Lucero J., Quick K.L., Behrens M.M. IL-6 mediated degeneration of forebrain GABAergic interneurons and cognitive impairment in aged mice through activation of neuronal NADPH oxidase. PLoS One. 2009;4:e5518. doi: 10.1371/journal.pone.0005518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loddick S.A., Turnbull A.V., Rothwell N.J. Cerebral interleukin-6 is neuroprotective during permanent focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1998;18:176–179. doi: 10.1097/00004647-199802000-00008. [DOI] [PubMed] [Google Scholar]
- 46.Matsuda S., Wen T.C., Morita F., Otsuka H., Igase K., Yoshimura H., Sakanaka M. Interleukin-6 prevents ischemia-induced learning disability and neuronal and synaptic loss in gerbils. Neurosci Lett. 1996;204:109–112. doi: 10.1016/0304-3940(96)12340-5. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki S., Tanaka K., Suzuki N. Ambivalent aspects of interleukin-6 in cerebral ischemia: inflammatory versus neurotrophic aspects. J Cereb Blood Flow Metab. 2009;29:464–479. doi: 10.1038/jcbfm.2008.141. [DOI] [PubMed] [Google Scholar]
- 48.Yamashita T., Sawamoto K., Suzuki S., Suzuki N., Adachi K., Kawase T., Mihara M., Ohsugi Y., Abe K., Okano H. Blockade of interleukin-6 signaling aggravates ischemic cerebral damage in mice: possible involvement of Stat3 activation in the protection of neurons. J Neurochem. 2005;94:459–468. doi: 10.1111/j.1471-4159.2005.03227.x. [DOI] [PubMed] [Google Scholar]
- 49.Jia T., Serbina N.V., Brandl K., Zhong M.X., Leiner I.M., Charo I.F., Pamer E.G. Additive roles for MCP-1 and MCP-3 in CCR2-mediated recruitment of inflammatory monocytes during Listeria monocytogenes infection. J Immunol. 2008;180:6846–6853. doi: 10.4049/jimmunol.180.10.6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang X., Li X., Yaish-Ohad S., Sarau H.M., Barone F.C., Feuerstein G.Z. Molecular cloning and expression of the rat monocyte chemotactic protein-3 gene: a possible role in stroke. Brain Res Mol Brain Res. 1999;71:304–312. doi: 10.1016/s0169-328x(99)00203-x. [DOI] [PubMed] [Google Scholar]
- 51.Bennett J.L., Blanchet M.R., Zhao L., Zbytnuik L., Antignano F., Gold M., Kubes P., McNagny K.M. Bone marrow-derived mast cells accumulate in the central nervous system during inflammation but are dispensable for experimental autoimmune encephalomyelitis pathogenesis. J Immunol. 2009;182:5507–5514. doi: 10.4049/jimmunol.0801485. [DOI] [PubMed] [Google Scholar]
- 52.Feyerabend T.B., Weiser A., Tietz A., Stassen M., Harris N., Kopf M., Radermacher P., Moller P., Benoist C., Mathis D., Fehling H.J., Rodewald H.R. Cre-mediated cell ablation contests mast cell contribution in models of antibody- and T cell-mediated autoimmunity. Immunity. 2011;35:832–844. doi: 10.1016/j.immuni.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 53.Hendrix S., Kramer P., Pehl D., Warnke K., Boato F., Nelissen S., Lemmens E., Pejler G., Metz M., Siebenhaar F., Maurer M. Mast cells protect from post-traumatic brain inflammation by the mast cell-specific chymase mouse mast cell protease-4. FASEB J. 2012;27:920–929. doi: 10.1096/fj.12-204800. [DOI] [PubMed] [Google Scholar]
- 54.Lindsberg P.J., Strbian D., Karjalainen-Lindsberg M.L. Mast cells as early responders in the regulation of acute blood-brain barrier changes after cerebral ischemia and hemorrhage. J Cereb Blood Flow Metab. 2010;30:689–702. doi: 10.1038/jcbfm.2009.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Piconese S., Costanza M., Musio S., Tripodo C., Poliani P.L., Gri G., Burocchi A., Pittoni P., Gorzanelli A., Colombo M.P., Pedotti R. Exacerbated experimental autoimmune encephalomyelitis in mast-cell-deficient KitW-sh/W-sh mice. Lab Invest. 2011;91:627–641. doi: 10.1038/labinvest.2011.3. [DOI] [PubMed] [Google Scholar]
- 56.Sayed B.A., Walker M.E., Brown M.A. Cutting edge: mast cells regulate disease severity in a relapsing-remitting model of multiple sclerosis. J Immunol. 2011;186:3294–3298. doi: 10.4049/jimmunol.1003574. [DOI] [PubMed] [Google Scholar]
- 57.Engelhardt B., Ransohoff R.M. Capture, crawl, cross: the T cell code to breach the blood-brain barriers. Trends Immunol. 2012;33:579–589. doi: 10.1016/j.it.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 58.Theoharides T.C., Donelan J., Kandere-Grzybowska K., Konstantinidou A. The role of mast cells in migraine pathophysiology. Brain Res Brain Res Rev. 2005;49:65–76. doi: 10.1016/j.brainresrev.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 59.Beauchesne P. Intrathecal chemotherapy for treatment of leptomeningeal dissemination of metastatic tumours. Lancet Oncol. 2010;11:871–879. doi: 10.1016/S1470-2045(10)70034-6. [DOI] [PubMed] [Google Scholar]
- 60.Shapiro W.R., Johanson C.E., Boogerd W. Treatment modalities for leptomeningeal metastases. Semin Oncol. 2009;36:S46–S54. doi: 10.1053/j.seminoncol.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 61.Papisov M.I., Belov V.V., Gannon K.S. Physiology of the intrathecal bolus: the leptomeningeal route for macromolecule and particle delivery to CNS. Mol Pharm. 2013;10:1522–1532. doi: 10.1021/mp300474m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roth T.L., Nayak D., Atanasijevic T., Koretsky A.P., Latour L.L., McGavern D.B. Transcranial amelioration of inflammation and cell death after brain injury. Nature. 2014;505:223–228. doi: 10.1038/nature12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Laser Doppler flow meter measurements of cerebral blood flow during and after stroke surgery in MC-deficient (WBB6F1-KitW/W-v) mice, WT (WBB6F1-Kit+/+) mice, and MC-engrafted (WBB6F1-Kit+/+ BMCMCs→WBB6F1-KitW/W-v) mice. Pooled analysis of the two independent experiments was performed, each of which gave similar results. Data are expressed as means ± SEM. n = 4 mice per group. CCA, common carotid artery; ECA, external carotid artery.
Identification of leukocyte populations by flow cytometry. Representative CD11b-CD45 (A) and Gr1-F4/80 (B) flow cytometric plots of brain immune cells obtained 3 days after stroke. The CD11bhighCD45high population of granulocytes and macrophages population (A) was further analyzed for expression of Gr1 and F4/80 (B).
MC distribution and counts in dura maters of WT (WBB6F1-Kit+/+) mice, MC-deficient (WBB6F1-KitW/W-v) mice, and MC-deficient (WBB6F1-KitW/W-v) mice after their meningeal engraftment with different populations of WBB6F1 or C57BL/6 (B6) BMCMCs. A: Representative images of toluidine blue-stained whole mounts of dura mater from mice from each indicated group. B: Quantification of MCs in the dura mater (prepared as in A) of the indicated groups. No statistically significant differences were found between any of the groups. Data are expressed as means ± SEM. The number of mice per group is indicated in each bar. Scale bar = 100 μm (A).