Abstract
Human extravillous trophoblast (EVT) invades the decidua via integrin receptors and subsequently degrades extracellular matrix proteins. In preeclampsia (PE), shallow EVT invasion elicits incomplete spiral artery remodeling, causing reduced uteroplacental blood flow. Previous studies show that preeclamptic decidual cells, but not interstitial EVTs, display higher levels of extracellular matrix–degrading matrix metalloproteinase (MMP)-9, but not MMP-2. Herein, we extend our previous PE-related assessment of MMP-2 and MMP-9 to include MMP-1, which preferentially degrades fibrillar collagens, and MMP-3, which can initiate a local proteolytic cascade. In human first-trimester decidual cells incubated with estradiol, tumor necrosis factor-α (TNF-α) significantly enhanced MMP-1, MMP-3, and MMP-9 mRNA and protein levels and activity measured by real-time quantitative RT-PCR, ELISA, immunoblotting, and zymography, respectively. In contrast, interferon γ (IFN-γ) reversed these effects and medroxyprogesterone acetate elicited further reversal. Immunoblotting revealed that p38 mitogen-activated protein kinase signaling mediated TNF-α enhancement of MMP-1, MMP-3, and MMP-9, whereas IFN-γ inhibited p38 mitogen-activated protein kinase phosphorylation. Unlike highly regulated MMP-1, MMP-3, and MMP-9, MMP-2 mRNA and protein expression was constitutive in decidual cells. Because inflammation underlies PE-associated shallow EVT invasion, these results suggest that excess macrophage-derived TNF-α augments expression of MMP-1, MMP-3, and MMP-9 in decidual cells to interfere with normal stepwise EVT invasion of the decidua. In contrast, decidual natural killer cell–derived IFN-γ reverses such TNF-α–induced MMPs to protect against PE.
Preeclampsia (PE) is a multifactorial disease that affects 6% to 8% of pregnancies in the United States, is responsible for nearly 8% of maternal deaths, and is a leading cause of perinatal morbidity and mortality. Severe PE is a major indication for early, medically indicated preterm birth.1 The diagnosis of PE is usually made after 20 weeks by the appearance of hypertension and proteinuria (maternal syndrome).1
During the first 20 weeks of gestation, extravillous trophoblasts (EVTs) arise from cytotrophoblast at the tips of placental anchoring villi and invade the decidua and upper third of the myometrium. As they navigate through the decidua, EVTs enter and facilitate remodeling of spiral arteries and arterioles into large-bore, low-resistance vessels that increase uteroplacental blood flow to the intervillous space requisite for fetal growth and development.2,3 The onset of PE is strongly associated with shallow decidual EVT invasion, which leads to incomplete vascular transformation and reduced uteroplacental blood flow. The resulting hypoxic placenta4 secretes several putative inducers of endothelial cell activation and angiogenesis (eg, soluble flt-1 and endoglin) into the maternal circulation that elicits vascular damage,5,6 leading to the maternal syndrome.1
Invasion of the decidua by EVT involves sequential attachment to adhesion molecules, followed by their degradation. Relevant integrin (ITG) heterodimers include ITG-α1/ITG-β1 and ITG-α5/ITG-β1, which recognize laminin/collagen IV and fibronectin, respectively, in the decidual extracellular matrix (ECM),7–9 as well as vascular endothelial cadherin, an endothelial cell receptor.10 In addition to newly synthesized basement membrane–type proteins, the decidual ECM also contains significant residual interstitial collagens.11 Degradation of the ECM scaffolding structure is mediated principally by matrix metalloproteinases (MMPs), a family of zinc-requiring enzymes that includes collagenases, gelatinases, and stromelysins.12 Tissue inhibitors of MMPs (TIMPs) regulate MMP catalytic activity.13 The MMPs act in concert with urokinase-type plasminogen activator (uPA) and its specific inhibitor, plasminogen activator inhibitor-1 (PAI-1).14
Previously, our laboratory compared immunostaining of the decidua from women with PE versus gestational age–matched control decidua for the presence of the basement membrane–degrading gelatinases, MMP-2 and MMP-9, as well as their respective inhibitors, TIMP-1 and TIMP-2, and found that PE is accompanied by a significant increase in MMP-9 levels in decidual cells, but not in interstitial EVTs. Unlike MMP-9, no PE-related changes in immunostaining were observed for either MMP-2 or TIMP-1 or TIMP-2 in either decidual cells or interstitial EVTs.15 Significant subsets of PE are associated with underlying maternal infections and/or inflammation,16 accompanied by an excess of decidual macrophages17–20 that are likely sources of elevated levels of the proinflammatory cytokines IL-1β and tumor necrosis factor-α (TNF-α).21
Consistent with the in situ observations described above and strong evidence that the pathogenesis of most cases of PE are initiated in early pregnancy,1 we found that incubation of primary leukocyte-free, first-trimester human decidual cells with either IL-1β or TNF-α markedly enhanced MMP-9 mRNA and protein expression, unaccompanied by significant changes in either MMP-2 or TIMP-1 or TIMP-2 mRNA and protein expression.15
The current study extends our previous PE-related assessment of MMP-2 and MMP-9 to include MMP-1, which preferentially degrades fibrillar collagens, and MMP-3, which can initiate a local proteolytic cascade by degrading a wide array of ECM proteins and by activating the secreted zymogenic form of other MMPs, such as pro–MMP-1 and pro–MMP-9.13,22 We found the following using a two-tiered approach of integrating in situ with in vitro observations: immunoreactive MMP-1 and MMP-3 levels were compared in decidual cells and interstitial EVTs of decidual placental sections from women with PE versus gestational age–matched controls: MMP-1, MMP-3, as well as MMP-2 and MMP-9, were measured in the conditioned medium of primary, leukocyte-free, first-trimester human decidual cells incubated in parallel with estradiol (E2), which was used as the control incubation for E2 + medroxyprogesterone acetate (MPA) to mimic the pregnant steroid milieu. The steroids were added alone or with either TNF-α or interferon γ (IFN-γ) or TNF-α + IFN-γ. Inclusion of IFN-γ, a primary decidual natural killer (dNK) cell product,23 was prompted by our recent observations that co-incubation of first-trimester human decidual cells with IFN-γ and either IL-1β or TNF-α synergistically enhances expression of two chemokines, interferon gamma-induced protein 10 (IP-10; alias CXCL10) and interferon-inducible T cell alpha chemoattractant (ITAC; alias CXCL11), that can selectively recruit the peripheral C-X-C chemokine receptor 3–expressing CD56bright CD16(−) NK cell population to the decidua,24 where they mediate several pregnancy protective effects.25–27
Materials and Methods
Tissues
Immunohistochemical (IHC) studies were performed on placentas and attached fetal membranes obtained under Human Investigation Committee approval at Yale University (New Haven, CT) from uncomplicated (n = 13) or preeclamptic (n = 12) pregnancies after cesarean or vaginal delivery in the absence of signs and symptoms of decidual hemorrhage (abruption), chorioamnionitis, chronic villitis, or other indications of underlying acute or chronic inflammation, as determined from histological examination. Patient information is summarized in Table 1. Gestational age was confirmed by ultrasonography before 20 weeks of gestation. The PE was defined as the occurrence of elevated blood pressure (>140/90 mmHg) and proteinuria (≥0.3 g protein in a 24-hour urine specimen) identified on two occasions 6 hours apart after 20 weeks of gestation in women without hypertension history.
Table 1.
Summary of Patient Information
| Group | Gestational age (weeks) | Maternal age (years) | Fetal weight (grams) |
|---|---|---|---|
| Control (n = 13) | 33.4 ± 1.1 | 24.8 ± 2.5 | 1903.8 ± 271.0 |
| PE (n = 12) | 32.6 ± 0.8 | 29.3 ± 3.1 | 1548.8 ± 212.0 |
| P value | 0.568 | 0.267 | 0.32 |
Data are given as means ± SEM. A Student's t-test was applied to compare gestational age, maternal age, and fetal weight between control and PE groups.
PE, preeclampsia.
Decidual cell cultures were derived from decidual specimens obtained from eight uncomplicated, elective terminations between 6 and 12 weeks’ gestation under Institutional Review Board and Human Investigation Committee approval at Bellevue Hospital (New York, NY) and Yale University School of Medicine (New Haven, CT), respectively. The decidua was separated, and a small portion was formalin fixed and paraffin embedded and then examined histologically for signs of underlying acute and chronic inflammation. The remainder was used to isolate decidual cells.
IHC Analysis
Formalin-fixed, paraffin-embedded sections (5 μm thick) were deparaffinized in xylene and rehydrated in a graded series of ethanol. Immunostaining was preceded by boiling in citrate buffer (10 mmol/L; pH 6.0) for 20 minutes to unmask antigenic sites, followed by immersion in 3% hydrogen peroxide (in 50% methanol/50% distilled water) for 10 minutes to block endogenous peroxidase activity. After several rinses in Tris-buffered saline (TBS; pH 7.6), slides were incubated in a humidified chamber with 5% normal horse or goat serum (Vector Labs, Burlingame, CA) in TBS for 30 minutes at room temperature to block non-specific binding. After removing excess serum, serial sections were incubated with primary antibodies [anti–MMP-1 goat polyclonal and anti–MMP-3 mouse monoclonal antibodies, both at 1:100 dilution (R&D Systems, Minneapolis, MN); anti-vimentin rabbit polyclonal antibody at 1:200 dilution (Labvision, Waltham, MA); and anti-cytokeratin mouse monoclonal antibody at 1:400 dilution (Dako Inc., Carpinteria, CA)] at room temperature for 60 minutes in a humidified chamber. For negative controls, sections were treated with normal mouse IgG1 isotype, normal goat IgG, or normal rabbit IgG antibodies (Vector Labs). Sections were rinsed in TBS 3× for 5 minutes each, then biotinylated horse anti-mouse or horse anti-goat or goat anti-rabbit antibodies (Vector Labs) were added at a 1:400 dilution for 30 minutes at room temperature. After washing 3× in TBS for 5 minutes each, the antigen-antibody complex was labeled with a streptavidin-peroxidase kit or a streptavidin-alkaline phosphatase kit (Vector Labs). The immunoreaction was developed using the chromogens, diaminobenzidine (3,3-diaminobenzidine tetrahydrochloride dehydrate; Vector Labs) for cytokeratin and Fast Red (Vector Labs) for vimentin, MMP-1, and MMP-3. Sections were counterstained with hematoxylin (Sigma-Aldrich, St. Louis, MO) and mounted with Permount (Fisher Chemicals, Springfield, NJ) on glass slides.
The distribution and intensities of MMP-1 and MMP-3 immunostaining were evaluated semiquantitatively: 0 (no staining), 1+ (weak, but detectable, staining), 2+ (moderate or distinct staining), and 3+ (intense staining). For each tissue, an HSCORE value was derived by taking the sum of the percentage of cells that stained at each intensity category and multiplying that value by the weighted intensity of the staining, using the following formula:
| (1) |
where i represents the intensity score and Pi the corresponding percentage of cells. In each slide, five different areas were evaluated microscopically at ×40 objective magnification. The average score was obtained after evaluation by two investigators blinded to the samples (W.M. and M.B.).28
Isolation of Decidual Cells
Tissues were minced and digested with 0.1% collagenase type IV and 0.01% DNase in RPMI 1640 medium containing 20 μg/mL penicillin/streptomycin and 1 μL/mL amphotericin B (Fungizone; Gibco, Grand Island, NY) in a 37°C shaking water bath for 30 minutes. After rinsing with sterile phosphate-buffered saline (PBS), the digestate was washed 3× and subjected to consecutive filtration through 100-, 70-, and 40-μm Millipore filters. Cells were then resuspended in RPMI 1640 medium, grown to confluence on polystyrene tissue culture dishes, harvested using trypsin/EDTA, and analyzed by flow cytometric analysis with anti-CD45 and anti-CD14 monoclonal antibodies (BD Pharmingen, San Diego, CA) to monitor the presence of leukocytes after each passage. After three to four passages, cell cultures were found to be leukocyte free (<1%).
Monolayers of the decidual cells were vimentin positive and cytokeratin negative and displayed decidualization-related morphological and biochemical changes during incubation with MPA, including enhanced expression of prolactin and PAI-1 and inhibited expression of MMP-1 and MMP-3 (data not shown). Cell aliquots were frozen in fetal calf serum/dimethyl sulfoxide (9:1; Sigma-Aldrich) and stored in liquid nitrogen.
Experimental Decidual Cell Incubations
Thawed cells were incubated in basal medium, a phenol red-free mix (1:1, v/v) of Dulbecco’s modified Eagle’s medium (Gibco) and Ham F-12 (Flow Labs, Rockville, MD), with 100 U/mL penicillin, 100 μg/mL streptomycin, and 0.25 μg/mL amphotericin B (Fungizone), supplemented with 10% charcoal-stripped calf serum (BMS). After two additional passages, confluent cultures were incubated in parallel in BMS containing either 10−8 mol/L E2 or E2 + 10−7 mol/L MPA (Sigma-Aldrich). Elevated levels of E2 and progesterone during pregnancy prompted use of E2 as the control for incubations with E2 + MPA; the latter was substituted for progesterone because of its greater stability during culture of primary human endometrial cells.29 After 7 days, the cultures were washed twice with PBS to remove residual serum, then switched to a defined medium (DM) consisting of basal medium plus Insulin-Transferrin-Selenium (Collaborative Research, Waltham, MA), 5 μmol/L FeSO4, 50 μmol/L ZnSO4, 1 nmol/L CuSO4, 20 nmol/L Na2SeO3, trace elements (Gibco), 50 μg/mL ascorbic acid (Sigma-Aldrich), and 50 ng/mL epidermal growth factor (Becton-Dickinson, Bedford, MA), with either vehicle control (0.1% ethanol) or steroids, added with or without 1.0 ng/mL each of TNF-α, IFN-γ, or TNF-α + IFN-γ (R&D Systems). To identify the signaling pathways that regulate MMP expression in the cultured first-trimester decidual cells, the experimental incubations were repeated in the presence of specific inhibitors of the NF-κB pathway (NF-κB activation inhibitor III at 10−5 mol/L), the c-Jun N-terminal kinase mitogen-activated protein kinase (MAPK) pathway (SP600125 at 10−5 mol/L), or the extracellular signal–regulated kinase 1/2 MAPK (PD98059 at 2 × 10−5 mol/L), mammalian target of rapamycin (rapamycin at 10−7 mol/L), or p38 MAPK (SB203580 at 10−5 mol/L) pathway (Calbiochem-EMD; Millipore, San Diego, CA). After the test period (6 hours for RT-PCR analysis, 15 minutes for Western blot signaling pathway analysis, or 24 hours for both Western blot and ELISA measurements of conditioned DM), the cells were harvested by scraping into ice-cold PBS, pelleted, and extracted in ice-cold lysis buffer. Conditioned DM supernatants and cell lysates were stored at −80°C before final RNA or protein extraction.
Reverse Transcription
Total RNA from first-trimester decidual cells was isolated using the Qiagen Mini Kit (Qiagen, Valencia, CA), and cleanup of RNA samples was performed using the RNeasy MinElute Cleanup Kit (Qiagen), according to the manufacturer's instructions. To eliminate genomic DNA contamination, isolated total RNA was treated with DNase I (Qiagen). Reverse transcription was performed using the RETROscript kit (Ambion, Austin, TX) in two steps: Template RNA was initially incubated with random dexamer primers at 85°C for 3 minutes to eliminate any secondary structures, followed by a reverse transcription reaction, which was performed at 42°C for 1 hour in 2 μL of 10× reverse transcriptase buffer, 4 μL of 1.25 mmol/L dNTP mix, 1 μL of RNase inhibitor (10 U/μL), and 1 μL of Moloney murine leukemia virus-RT (100 U/μL). Subsequently, the Moloney murine leukemia virus-RT enzyme was inactivated at 92°C for 10 minutes.
qPCR for MMP-1, MMP-3, and MMP-9
Quantitative real-time PCR (qPCR) was performed using the TaqMan Gene Expression Assay Kits for MMP-1, MMP-3, and MMP-9 (MMP-1, MMP-3, and MMP-9 TaqMan ID numbers Hs00899658_m1, Hs00968305_m1, and Hs00234579_m1, respectively; Applied Biosystems, Grand Island, NY), according to the manufacturer's protocol. In brief, 10 μL of TaqMan 2× Universal PCR Master Mix was combined with 8 μL of nuclease-free water, 1 μL of 20× TaqMan MicroRNA Assay mix, and 1 μL of cDNA in the PCR tube. Amplification used 40 cycles of PCR in Applied Biosystems 7500 Real-Time PCR Detection System (Applied Biosystems), with the following program: initial denaturation at 95°C for 10 minutes, followed by 40 cycles at 95°C for 15 seconds and 60°C for 60 seconds. All reactions were run in triplicate. Expression of the target mRNAs was normalized to β-actin levels, and the 2−ΔΔCT method was used to calculate relative expression levels.
Biochemical Assays
Total cell protein levels were measured by a modified Lowry assay (Bio-Rad Laboratories, Hercules, CA). Commercial ELISA kits were used to measure total, noncomplexed immunoreactive levels, each of MMP-1, MMP-2, MMP-3, and MMP-9, in the decidual cell–conditioned medium, according to the manufacturer’s instructions (Quantikine kits; R&D Systems). The sensitivity of the MMP-1 ELISA is 0.021 ng/mL, and that of the MMP-3 ELISA is 0.009 ng/mL. The MMP-1 assay coefficients of variation for intra-assay and interassay are 4.6% and 9.4%, respectively. The MMP-3 assay coefficients of variation for intra-assay and interassay are 6.1% and 7.8%, respectively. Both MMP-2 and MMP-9 ELISAs have sensitivities of 0.16 ng/mL. The MMP-2 assay coefficients of variation for intra-assay and interassay are 4.8% and 7.7%, respectively. The MMP-9 assay coefficients of variation for intra-assay and interassay are 2.3% and 7.5%, respectively.
Substrate Gel Zymography
Gel zymography, using gelatin as the substrate within the gel, detected the proteolytic activity of MMP-2 and MMP-9, whereas casein was used to detect the proteolytic activity of MMP-1 and MMP-3. Conditioned DM from decidual cell cultures was centrifuged, and for zymograms in which the proforms and latent forms of MMP-2 and MMP-9 were evaluated, the supernatants were pretreated with dimethyl sulfoxide at a concentration of <7% of the total volume, with and without 2 mmol/L aminophenylmercuric acetate (APMA; Sigma-Aldrich) at 37°C for 2 hours; APMA converts latent (pro) forms of the MMPs to their active forms. The reaction was stopped by mixing the samples 1:1 with nonreducing zymogram sample buffer (Bio-Rad Laboratories) and then incubating for 10 minutes at room temperature. This step also blocks the APMA reaction. Samples were loaded onto a 10% gelatin zymogram gel or a 12% casein zymogram gel (Bio-Rad Laboratories), then electrophoresed for 1.75 hours in Tris-glycine SDS running buffer. To enable the enzymes to renature, the gel was incubated for 45 minutes in 2.5% Triton X-100 (Bio-Rad Laboratories) at room temperature and incubated in zymogram development buffer (Bio-Rad Laboratories) for 30 minutes, and then placed in fresh zymogram development buffer overnight at 37°C. The gel was stained with 0.5% Coomassie Brilliant Blue (Sigma-Aldrich) solution of methanol/acetic acid/water (40:10:50, v/v) for 2 hours at room temperature, and then destained with methanol/acetic acid/water (30:10:60, v/v) for 4 hours at room temperature. The presence of clear bands in the gels at the appropriate molecular weights reflects gelatinolytic activity of the latent (pro) and active forms of MMP-2 and MMP-9 and the caseinolytic activity of MMP-1 and MMP-3.
Immunoblotting
Immunoblot analysis was performed on concentrated conditioned DM supernatants, which were diluted 1:6 in nonreducing Laemmli 6× sample buffer (Boston Bioproducts, Boston, MA) and then boiled for 4 minutes. The centrifuged media were subjected to SDS-PAGE on a 7.5% Tris-HCl gel (Bio-Rad Laboratories), with subsequent electroblotting transfer onto a 0.45-μm nitrocellulose membrane (Bio-Rad Laboratories). After transfer, the membrane was blocked overnight in TBS (Fisher, Fairlawn, NJ) with 4% nonfat dry milk and then incubated for 2 hours with mouse anti-human MMP-1, MMP-2, MMP-3, or MMP-9 monoclonal antibodies (R&D Systems). Membranes were rinsed in PBS and 0.1% Tween 20 before and after incubation with horseradish peroxidase–conjugated anti-mouse IgG (ICN Biomedicals, Aurora, OH). Chemiluminescence was detected with electrochemiluminescence reagents (Perkin-Elmer Life Sciences, Boston, MA) and autoradiography film (Amersham Pharmacia, Pittsburgh, PA), according to the manufacturer's instructions. The membranes were then rinsed as above and incubated in stripping buffer (100 mmol/L 2-mercaptoethanol, 2% SDS, and 62.5 mmol/L Tris-HCl, pH 6.7) for 30 minutes at 50°C with subsequent rinsing and reblocking, as indicated above, with β-actin.
Statistical Analysis
MMP ELISA results were examined by the Shapiro-Wilk normality test, followed by the Wilcoxon signed rank test. Immunoblotting, RT-qPCR, and immunostaining HSCORE results were normally distributed, as determined by Kolmogorov-Smirnov test, and analyzed by one-way analysis of variance, followed by testing post hoc with the Holm-Sidak method. In comparisons of control with treatment groups, P < 0.05 is accepted as statistically significant. Statistical calculations used SigmaStat software version 3.0 (Systat Software, San Jose, CA).
Results
Immunostaining for MMP-1 and MMP-3 in Preterm Control and Gestational Age–Matched Preeclamptic Decidua
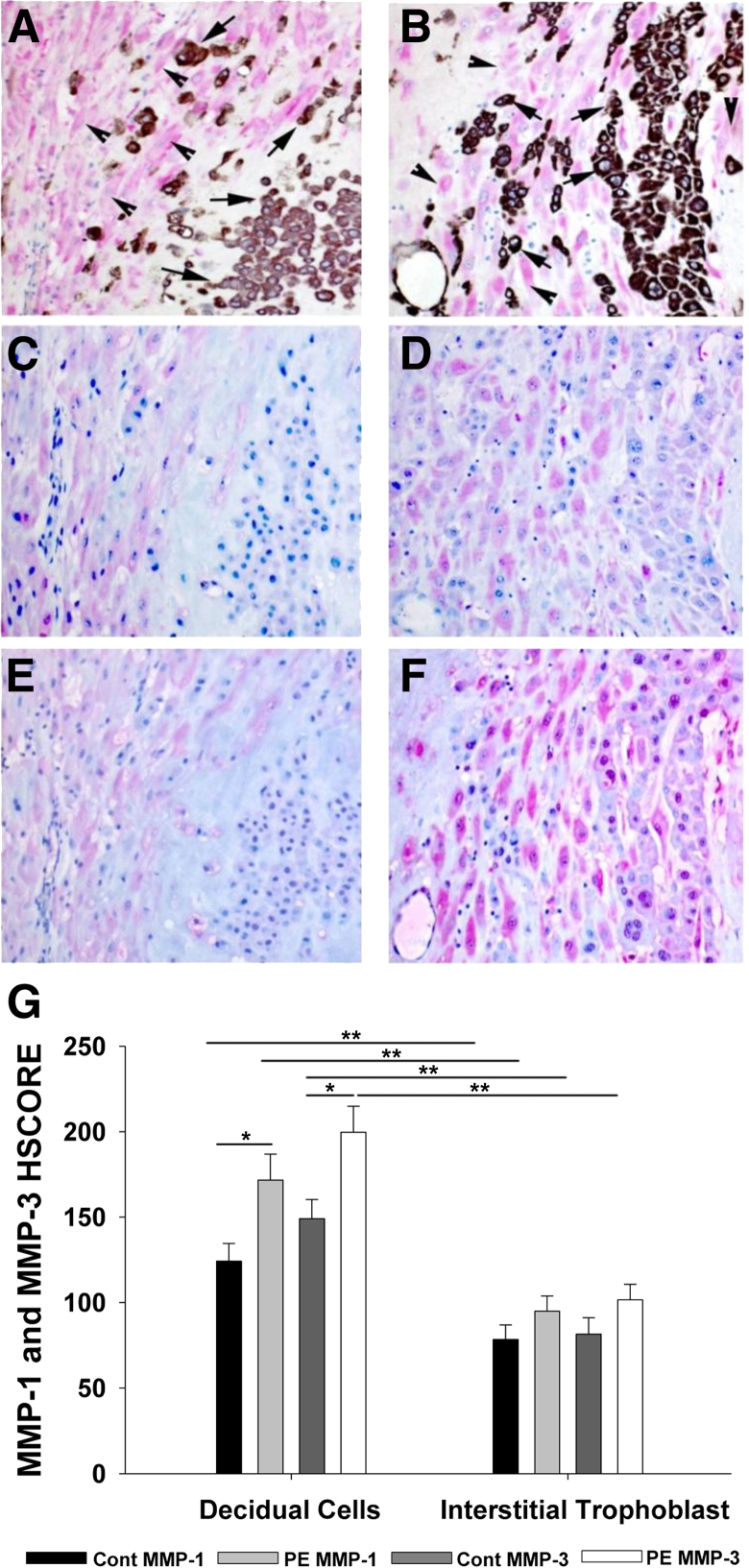
In the immunostained sections of decidua basalis, vimentin-expressing decidual cells were readily distinguished from adjacent cytokeratin-expressing interstitial EVTs (Figure 1, A and B). Both decidual cells and interstitial EVTs expressed immunoreactive MMP-1 (Figure 1, C and D) and MMP-3 (Figure 1, E and F). However, the PE specimens displayed greater immunoreactivity than gestational age–matched controls for MMP-1 (Figure 1, D versus C) and MMP-3 (Figure 1, F versus E). The HSCORE values confirmed this assessment by indicating significantly enhanced PE-related immunostaining in the decidual cells for MMP-1 (mean ± SEM, 171.67 ± 15.17) versus preterm controls (124.23 ± 10.44; P = 0.016) (Figure 1G). In contrast, the HSCORE values for MMP-1 in interstitial EVTs were not significantly different between PE (mean ± SEM, 95.00 ± 8.84) and preterm control (mean ± SEM, 78.46 ± 8.54; P = 0.19) specimens (Figure 1G). Similarly, HSCORES for MMP-3 immunostaining (Figure 1G) in decidual cells of PE specimens (mean ± SEM, 199.67 ± 15.18) were significantly higher than those in preterm controls (149.08 ± 11.29; P = 0.013), and again no significant differences were evident in interstitial EVTs between PE (101.67 ± 9.03) and preterm control (81.54 ± 9.60) specimens (P = 0.14) (Figure 1G). The HSCORE values also indicate that the expression of each MMP was significantly higher in decidual cells than in interstitial EVTs in both preterm control and PE samples (P < 0.001) (Figure 1G).
Figure 1.
The IHC analysis of MMP-1 and MMP-3 expression in decidual tissues from preterm specimens. Decidual cells (arrowheads) and interstitial EVTs (arrows) are distinguished by vimentin (red) and cytokeratin (brown) immunostaining, respectively, in gestational age–matched decidua of women serving as preterm controls (A) or with PE (B). MMP-1 expression (red) in preterm control (C) and preeclamptic decidual cells (D), and MMP-3 expression (red) in preterm control (E) and preeclamptic decidual cells (F). G: HSCORE values for MMP-1 and MMP-3 expression. Both decidual cells and interstitial trophoblasts are compared between preterm controls (Cont; n = 13) and those from PE specimens (n = 12). Data represent means ± SEM. ∗P < 0.02, ∗∗P < 0.002.
MMP Protein Expression in Decidual Cell Monolayer Cultures
MMP-1 and MMP-3 Expression
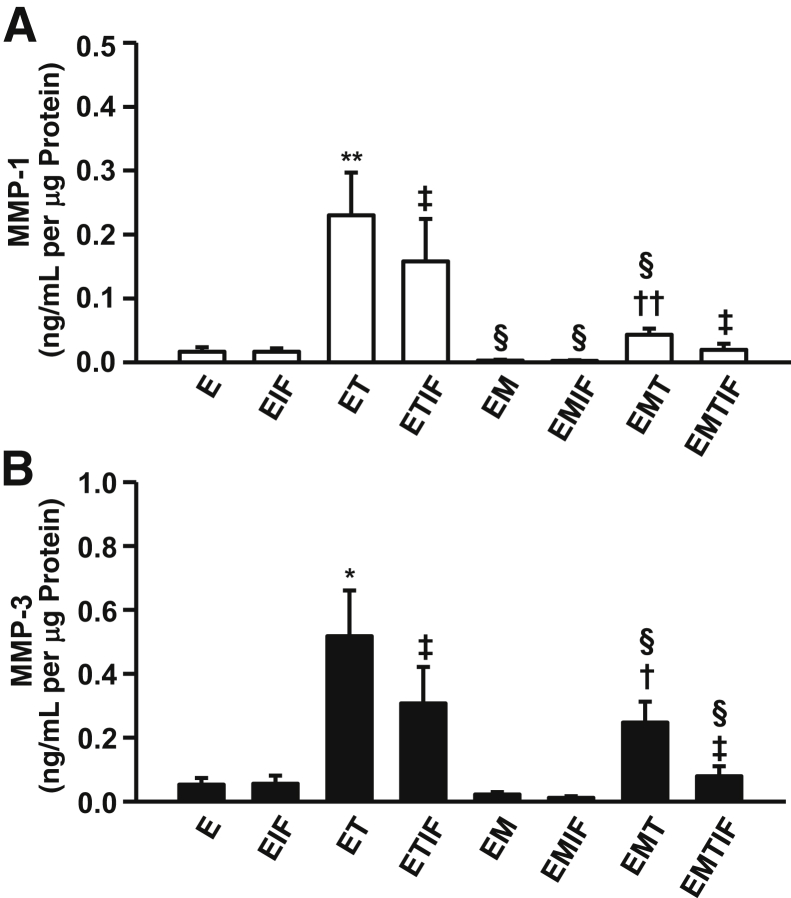
The individual and combined effects of steroids and cytokines on the output of MMP-1 (Figure 2A) and MMP-3 (Figure 2B) by leukocyte-free, first-trimester decidual cells are shown. As detailed in Materials and Methods, E2 was used as the control for parallel incubations with E2 + MPA, which simulates the pregnant steroid milieu.
Figure 2.
Effects of TNF-α or IFN-α or TNF-α + IFN-α incubated with E (E2) or EM (E2 + MPA) on MMP-1 (A) and MMP-3 (B) output by first-trimester decidual cell monolayers. Confluent, leukocyte-free decidual cells were primed for 7 days in BMS with 10−8 mol/L E2, or E2 + 10−7 mol/L MPA, and then switched to DM with corresponding steroid(s), with or without 1 ng/mL each of TNF-α (T) or IFN-α (IF) or TNF-α + IFN-α (TIF) for 24 hours. Results are measured by specific ELISAs in conditioned DM supernatants and normalized to cell protein. Data represent means ± SEM. ∗P < 0.05, ∗∗P < 0.01 versus E; †P < 0.05 , ††P < 0.01 versus EM; ‡P < 0.05 versus corresponding treatment without IF; §P < 0.05 versus corresponding treatment without M (n = 6).
Compared with MMP-1 values obtained by treatment with E2 alone (0.016 ± 0.006 ng/mL per μg of cell protein, n = 6), MMP-1 output was elevated to 0.238 ± 0.055 ng/mL per μg protein (15-fold increase; P < 0.05) by addition of 1 ng/mL of TNF-α (Figure 2A). Despite a lack of a response to 1 ng/mL of IFN-γ alone (0.016 ± 0.004 ng/mL per μg protein), co-incubating IFN-γ with TNF-α blunted the effect of the latter by 43.4% (P < 0.05). Compared with treatment with E2 alone, the addition of MPA significantly reduced MMP-1 output (0.002 ± 0.001 ng/mL per μg of cell protein) by 85.2% (P < 0.05). This MPA-mediated inhibitory effect is also evident in the blunted response to TNF-α alone (81%) or to TNF-α + IFN-γ (85.6%) when each is compared with cultures exposed to E2 alone. Addition of IFN-γ alone also significantly inhibited MMP-1 production induced by TNF-α by 57.13% in cultures exposed to E2 + MPA.
Compared with E2 alone (0.053 ± 0.021 ng/mL per μg of cell protein, n = 6), co-incubation with 1 ng/mL of TNF-α elevated MMP-3 output to 0.517 ± 0.143 ng/mL per μg protein, representing a 10-fold increase (P < 0.05) (Figure 2B). Despite a nonsignificant response to 1 ng/mL of IFN-γ alone (0.056 ± 0.025 ng/mL per μg protein), co-incubation with IFN-γ significantly blunted TNF-α–mediated up-regulation by 40.6% (P < 0.05). In parallel incubations with E2 + MPA, MMP-3 output (0.022 ± 0.007 ng/mL per μg of cell protein) was significantly reduced by 58.3% (P < 0.05) compared with E2 alone. This MPA-mediated inhibitory effect in co-incubations with E2 is also evident in the diminished response to TNF-α alone (52.2% inhibition) or to TNF-α + IFN-γ (74.3% inhibition). Moreover, IFN-γ significantly inhibited MMP-3 production induced by TNF-α with E2 + MPA by 68%.
The decidual cell-conditioned DM displays caseinolytic activity in the molecular weight range corresponding to MMP-1 and MMP-3 (Figure 3). Consistent with the ELISA results (Figure 2), in incubations with E2, the addition of TNF-α markedly enhanced this caseinolytic zone. Although IFN-γ alone elicited a minimal effect, close inspection indicated inhibition of the TNF-α–induced lytic zone during co-incubation with IFN-γ. In the parallel incubation with E2 + MPA, the TNF-α–mediated lytic zone is markedly reduced compared with E2 alone, and co-incubation with IFN-γ virtually eliminates the TNF-α–mediated caseinolytic effect.
Figure 3.
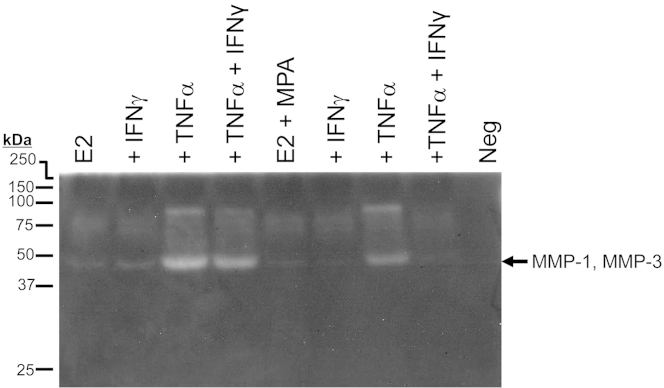
Substrate gel zymography of MMP-1 and MMP-3 activity by first-trimester decidual cell monolayers. Confluent, leukocyte-free decidual cells were incubated for 7 days in BMS containing 10−8 mol/L E2 or E2 + 10−7 mol/L MPA, and then switched to DM with corresponding steroid(s), with or without 1 ng/mL each of TNF-α or IFN-α or TNF-α + IFN-α for 24 hours. Conditioned DM was run on a 12% casein gel zymogram and then subjected to renaturing and staining procedures (as described in Materials and Methods). Proteolytic activity of MMPs is indicated by clear bands in the darkly stained gel. Neg, negative.
MMP-2 and MMP-9 Expression
The effects (separate and interactive) of steroids and cytokines on the output of MMP-2 (Figure 4A) and MMP-9 (Figure 4B) by leukocyte-free, first-trimester decidual cells are shown. We previously reported15 that TNF-α and IL-1β each significantly enhance MMP-9 expression in parallel incubations with E2 or E2 + MPA, whereas MMP-2 expression was unchanged irrespective of experimental condition. Therefore, E2 + MPA, which represents the pregnant steroid milieu, was used as the control incubation. Basal (control) MMP-2 output was similar in all of the experimental treatments (Figure 4A). In contrast with constitutive MMP-2 expression, basal (control) MMP-9 output (0.066 ± 0.016 pg/mL per μg of cell protein) was essentially unchanged during incubation with IFN-γ alone (0.074 ± 0.022 pg/mL per μg of cell protein), but markedly up-regulated by incubation with TNF-α alone to 14.724 ± 6.275 pg/mL per μg of cell protein (225-fold increase) (Figure 4B). As displayed above for the expression of MMP-1 (Figure 2A) and MMP-3 (Figure 2B), co-incubation of the decidual cells with IFN-γ significantly lowered TNF-α–induced effects on MMP-9 levels by 81.07% to 2.787 ± 1.573 pg/mL per μg of cell protein.
Figure 4.
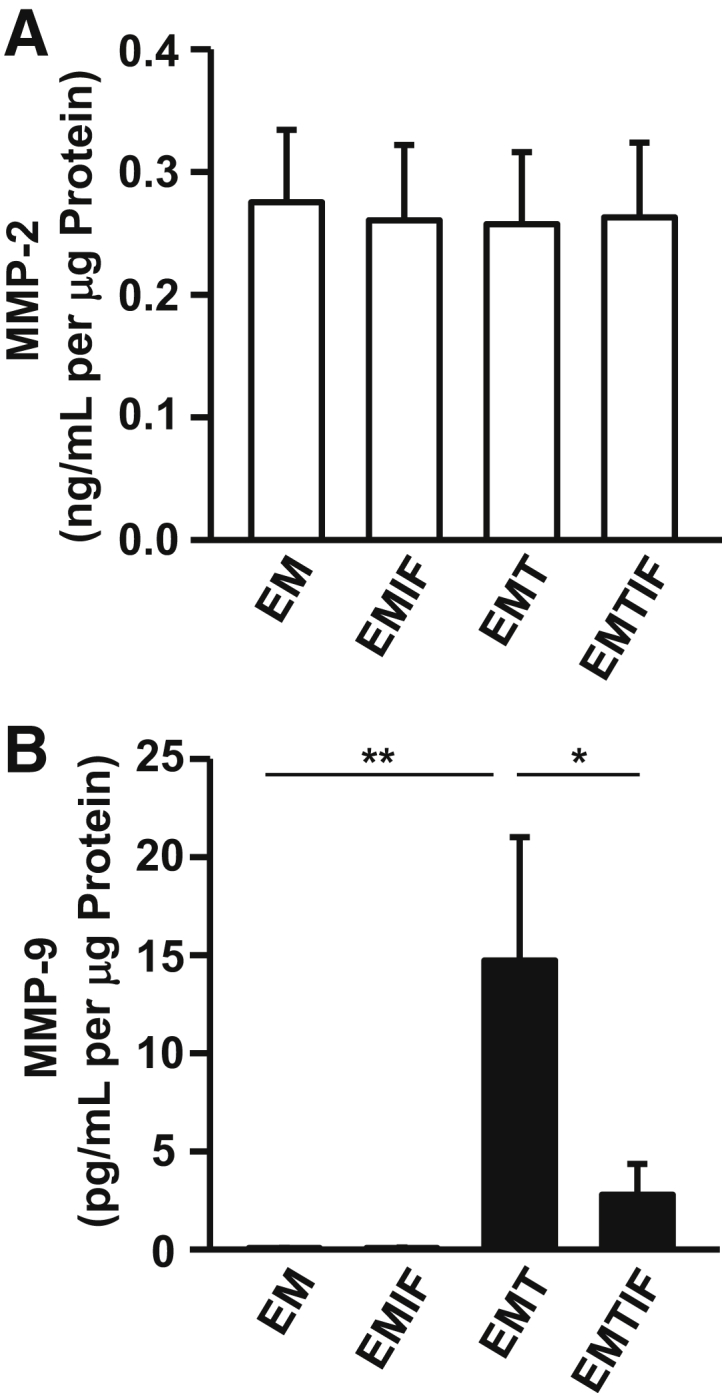
Effects of TNF-α or IFN-α or TNF-α + IFN-α incubated with E2 + MPA on secreted MMP-2 and MMP-9 levels by first-trimester decidual cell monolayers. Confluent, leukocyte-free decidual cells were incubated for 7 days in BMS containing 10−8 mol/L E (E2) + 10−7 mol/L M (MPA), and then switched to DM with corresponding steroid(s), with or without 1 ng/mL each of TNF-α (T) or IFN-α (IF) or TNF-α + IFN-α (TIF) for 24 hours. MMP-2 (A) and MMP-9 (B) levels were measured by specific ELISAs in conditioned DM supernatants and normalized to cell protein (n = 8). Data represent means ± SEM. ∗P < 0.05, ∗∗P < 0.01.
The zymograms were derived from conditioned DM obtained after parallel incubations with E2 + MPA alone (control) or E2 + MPA plus IFN-γ, or TNF-α, or IFN-γ + TNF-α (Figure 5). Decidual cell-conditioned DM contained discrete zones of gelatinolytic activity that corresponded to MMP-2 and MMP-9 at the appropriate molecular weights (72 and 92 kDa, respectively, in the absence of APMA) (Figure 5). Pretreatment of the decidual cell-conditioned medium with APMA shifted virtually all of the pro-MMP forms to the active MMP forms (66 kDa for MMP-2 and 86 kDa for MMP-9). Consistent with the constitutive expression of MMP-2 and the regulated expression of MMP-9, indicated by ELISA (Figure 4), neither TNF-α nor IFN-γ affected the magnitude of MMP-2–mediated proteolysis, whereas expression of both the pro- and active forms of MMP-9 was markedly increased by TNF-α, with this induction blunted by co-incubation with IFN-γ.
Figure 5.
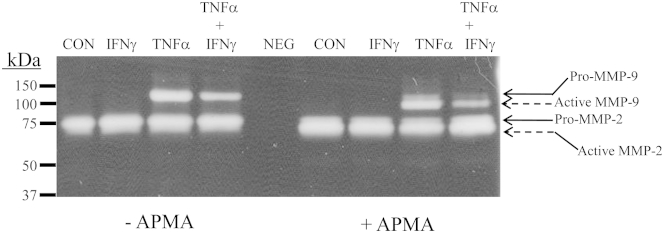
Substrate gel zymography of MMP-2– and MMP-9–mediated gelatinase activity by first-trimester decidual cell monolayers. Confluent, leukocyte-free decidual cells were incubated for 7 days in BMS containing 10−8 mol/L E2 + 10−7 mol/L MPA (control) and then switched to DM with corresponding steroid(s), with or without 1 ng/mL of TNF-α or IFN-α or TNF-α + IFN-α for 24 hours. Aliquots of conditioned DM were subjected to gelatin zymography after pretreatment with and without APMA, which converts the pro-forms (Pro) of the MMPs to their active forms (Act). CON, control; NEG, negative control.
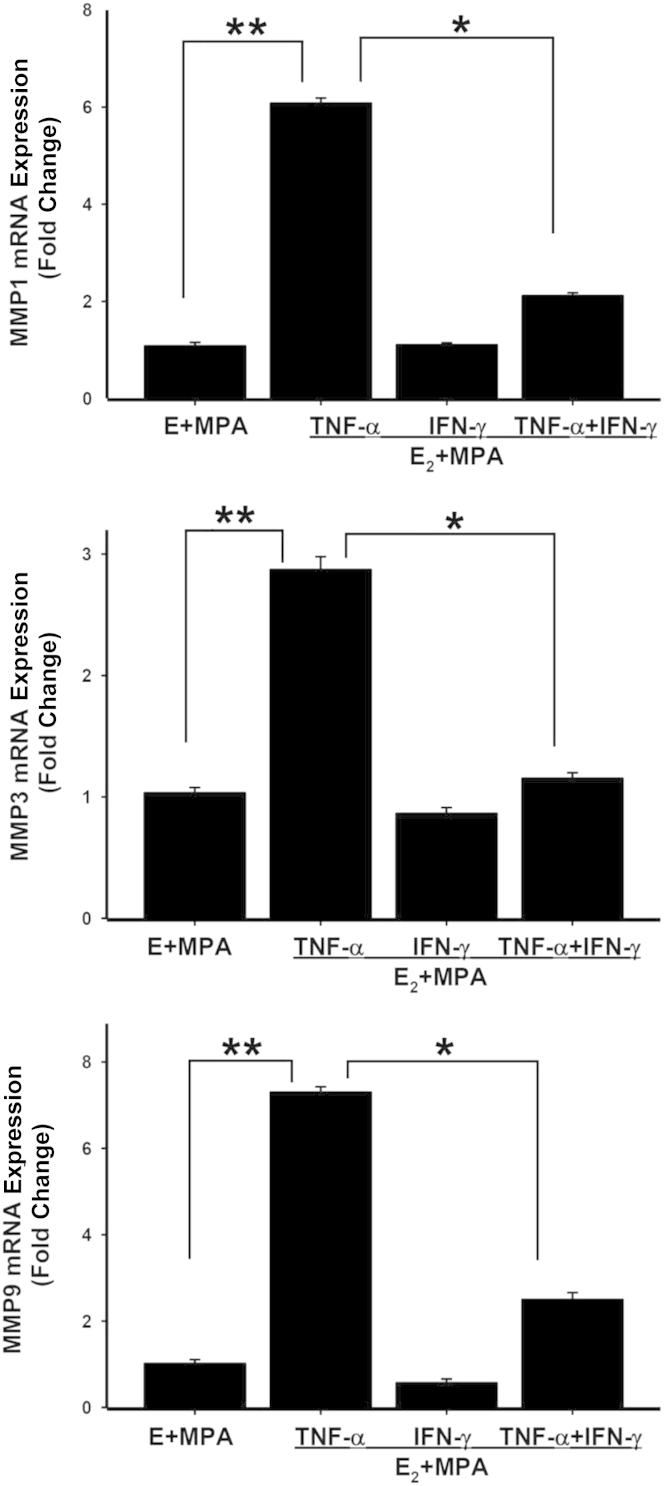
Transcriptional Regulation of TNF-α–Induced MMP-1, MMP-3, and MMP-9 Expression in First-Trimester Decidual Cells by IFN-γ
The RT-PCR results (Figure 6) showed that steady-state mRNA levels for MMP-1, MMP-3, and MMP-9 correspond to changes in protein levels measured by ELISA (Figures 2 and 4) and substrate gel zymography (Figures 3 and 5). Specifically, compared with incubations with E2 + MPA, treatment of the first-trimester decidual cells with TNF-α significantly enhanced MMP-1, MMP-3, and MMP-9 mRNA expression by sixfold, threefold, and sevenfold, respectively. Moreover, the addition of IFN-γ significantly blunted TNF-α–induced MMP-1, MMP-3, and MMP-9 expression by 65%, 60%, and 66%, respectively (Figure 6).
Figure 6.
Transcriptional regulation of MMPs by TNF-α or IFN-α or TNF-α + IFN-α incubated with E (E2) + MPA by first-trimester decidual cells. Confluent, leukocyte-free decidual cells were incubated for 7 days in 10−8 mol/L E2 + 10−7 mol/L MPA in BMS (control) and then switched to DM with corresponding steroid(s), with or without 1 ng/mL of TNF for 6 hours. Extracted RNA subjected to RT-qPCR analysis of MMP-1, MMP-3, and MMP-9 mRNA. Data represent means ± SEM (n = 3). ∗P < 0.05, ∗∗P < 0.01.
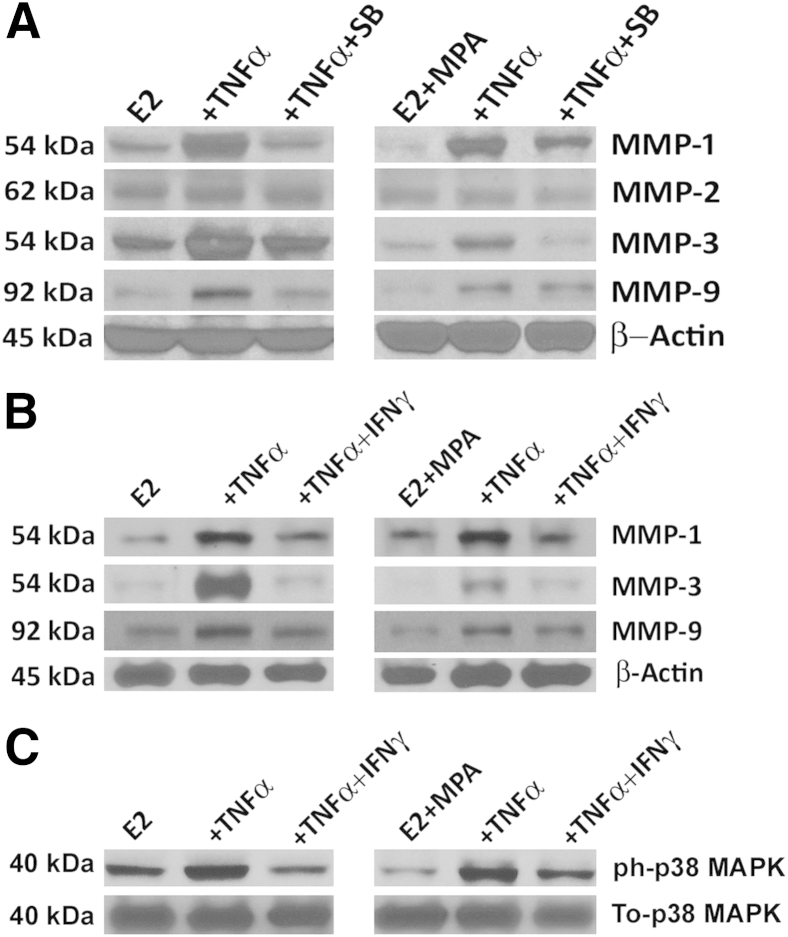
p38 MAPK Signaling Mediates TNF-α Induction of MMP Expression and the Inhibitory Effects of IFN-γ
Cell lysates derived from first-trimester decidual cell monolayers incubated in parallel with E2 or E2 + MPA displayed individual bands migrating with the expected molecular weights for MMP-1 (54 kDa), MMP-3 (54 kDa), MMP-2 (62 kDa), and MMP-9 (92 kDa) (Figure 7A). Compared with the constitutive expression of MMP-2, the magnitude of MMP-1, MMP-3, and MMP-9 expression was enhanced by TNF-α, with this effect blunted by co-incubation with SB203580, a p38 MAPK inhibitor. No such effect was observed for TNF-α, with or without other specific signaling pathway inhibitors for NF-κB (NF-κB III), c-Jun N-terminal kinase MAPK (SP600125), extracellular signal–regulated kinase MAPK (PD98059), or mammalian target of rapamycin (rapamycin) (results not shown).
Figure 7.
Role of p38 MAPK in regulating MMP-1, MMP-2, MMP-3, and MMP-9 expression by TNF-α and IFN-γ in first-trimester cultured decidual cells. Parallel incubation of confluent leukocyte-free decidual cells ± TNF-α (1 ng/mL) ± SB203580 (SB; 10−5 mol/L; p38 MAPK inhibitor) (A); ± TNF-α (1 ng/mL) ± IFN-γ (1 ng/mL) (B); ± TNF-α (1 ng/mL) ± IFN-γ (1 ng/mL) in DM containing E2 alone (10−8 mol/L) or E2 + MPA (10−8 mol/L + 10−7 mol/L) for 24 hours (A and B) or for 15 minutes (C). ph-p38 MAPK, phosphorylated (active) p38 MAPK; To-p38 MAPK, total p38 MAPK (n = 3).
The ELISA results for MMP-1 and MMP-3 (Figure 2) and MMP-9 (Figure 4) were confirmed (Figure 7B) and indicated that IFN-γ inhibited TNF-α induction of each MMP. We postulated that this IFN-γ–mediated anti-inflammatory effect resulted from inhibition of the p38 MAPK signaling pathway. This hypothesis was confirmed as in parallel incubations of decidual cells treated with E2 or with E2 + MPA, TNF-α significantly increased the phosphorylated form of p38 MAPK compared with the respective control incubations (either E2 alone or with E2 + MPA alone) (Figure 7C). In contrast, addition of IFN-γ reduced TNF-α–induced phospho-p38 MAPK. No experimental condition affected total p38 MAPK levels.
Discussion
Human blastocyst-derived EVTs invade a decidua composed of decidual cells (40%) and immune cells dominated by dNK cells (30%) and macrophages (15%).30–32 Conversion of spiral arteries and arterioles to low-resistance, high-capacity vessels that increase uteroplacental blood flow to the developing fetal-placental unit was attributed to fibrinoid-embedded vascular EVTs.33 Newer evidence indicates that dNK cells accumulated around spiral arteries in the absence of EVTs26,34 initiate early vascular remodeling steps, including vascular dilation, endothelial hyperplasia, and vacuolization of medial smooth muscle cells by elaborating MMP-2 and MMP-9,35 IFN-γ, and the angiogenic factors vascular endothelial cell growth factor-C and angiopoietins 1 and 2.36 Trophoblasts influence early vascular remodeling by recruiting circulating NK cells to the decidua. Thus, EVTs lining spiral arteries express stromal cell–derived factor-1 (alias CXCL12), which binds to CCR4 expressed at the surface of the minority CD56brightCD16- circulating NK cells to promote their recruitment.33
Previously, we17 and others37 found that preeclamptic decidua contains a macrophage excess. Macrophage-derived TNF-α is implicated in mediating PE-related shallow EVT invasion of the decidua directly by inducing EVT apoptosis38 and/or indirectly by reciprocally inhibiting expression of trophoblast-bound uPA and increasing levels of PAI-1, which inactivates uPA bound to its receptor at the leading edge of EVT invasion.39 Moreover, macrophage-derived TNF-α stimulates first-trimester decidual cells to secrete factors that activate macrophages,20 which display enhanced activity in inducing EVT apoptosis20 and inhibiting EVT invasion.19
Substantial evidence established that EVT–decidual cell interactions bear the primary responsibility of mediating the later and more decisive changes in spiral artery remodeling.40 Investigations of normal and immortalized first-trimester EVTs indicate that EVT migration and invasion are modulated by several factors expressed by both trophoblasts and decidual cells at the maternal-fetal interface. These include insulin-like growth factor-2 (IGF2), its IGF2 mannose-6 phosphate receptor,41 IGF-binding protein 1,42,43 prostaglandin E2 and its EP1 receptor,44 and transforming growth factor-β isoforms and decorin, which stores transforming growth factor-β in the decidual ECM.45–47
Human EVTs and cancer cells display similar proliferative, angiogenic, and migratory phenotypes48 mediated by several common molecular circuits.49 Both human EVT and tumor cell invasion involve sequential ITG-mediated adherence to, and then proteolysis of, specific proteins in the host tissue ECM.49,50 By comparison, human EVT invasion of decidua is under far more stringent spatial and temporal control because it is limited to the decidua and proximal myometrium and ended by midgestation.49–51 Initial studies stressed the importance in promoting tumor cell invasiveness of the expression of uPA52 and MMPs, particularly MMP-2 and MMP-9,53,54 acting in concert with the respective modulating actions of PAI-152 and TIMPs.54 Recent IHC and in situ hybridization observations in human tissues, supported by studies in transgenic cancer mouse models, switched the emphasis away from tumor cells to the host stromal cells as major contributors of uPA and MMPs during tumor growth, invasion, and metastasis, while indicating that tumor cells act primarily to stimulate adjacent stromal cells to synthesize key regulators of ECM turnover.55,56 Moreover, correcting a misconception that overexpression of specific MMPs, either by tumor cells or adjacent host stromal cells, always promotes tumor growth, reports of tumor formation in transgenic mice with either targeted overexpressed or ablated MMPs revealed that overexpression of specific MMPs at either the primary or metastatic site paradoxically protects against multiple stages of cancer progression.57
By comparison with the complex involvement of MMP expression by both tumor and host cells in determining invasiveness of the former, in PE, the focus of impaired decidual EVT invasion has generally remained fixed on MMP expression by trophoblasts.50,51,58,59 Initially, trophoblasts from preeclamptic pregnancies were reported to express lower levels of MMP-2 and MMP-9 than their normal counterparts.60 Subsequently, integration of microarray analysis, together with double-labeled immunofluorescence staining, revealed that, among several genes implicated in mediating trophoblast differentiation and invasion, MMP-1 expression alone was significantly and strikingly reduced in EVTs in cases of PE and intrauterine growth restriction or PE plus intrauterine growth restriction versus gestational age–matched control specimens.61
Searching for a relationship between PE and MMP expression by the predominant cell types at the maternal-fetal interface, we observed significantly increased immunoreactive MMP-9 in vimentin-positive decidual cells of gestational age–matched preeclamptic versus control decidual sections; we did not observe this in decidual cell–expressed MMP-2, and there were no PE-related changes in immunoreactive MMP-2 or MMP-9 levels in neighboring cytokeratin-positive interstitial EVTs.15 In parallel with previous MMP-9 observations, the current study finds significantly elevated immunoreactive MMP-1 and MMP-3 levels in PE-associated decidual cells, with no changes in adjacent interstitial EVTs. In situ–increased MMP-1, MMP-3, and MMP-9 immunostaining in the preeclamptic decidua is consistent with disruption of ITG-mediated EVT invasion. These observations prompted incubations of leukocyte-free, first-trimester human decidual cells with E2 + MPA to mimic the pregnant steroid milieu, with added TNF-α to mimic the proinflammatory milieu of PE. Consistent with our previous and current IHC observations of preeclamptic versus control decidual specimens, ELISA measurements demonstrated that, unlike constitutively expressed MMP-2 expression, TNF-α significantly increased MMP-9 levels. The current study observes similar TNF-α–augmented decidual cell MMP-1 and MMP-3 expression.
Histological and genetic studies in mice indicate that dNK cell-derived IFN-γ mediates spiral artery remodeling, similar to that described in early pregnant human decidua.62 Reportedly, early pregnant human dNK cells are a major source of IFN-γ.63 We determined that IFN-γ is crucial in mediating decidual cell recruitment of peripheral NK cells via enhanced expression of the chemokines IP-10 and ITAC.23 The well-documented role of NK cells in promoting several pregnancy protective effects in mice64,65 and humans25–27 suggested that IFN-γ may also counteract proinflammatory cytokine-enhanced aberrant MMP expression in first-trimester decidual cells, which are implicated in promoting the shallow EVT invasion of PE. Consistent with this supposition, the current ELISA measurements indicate that, in first-trimester decidual cells incubated with E2 + MPA, co-incubation with IFN-γ virtually eliminated the marked up-regulation of MMP-1, MMP-3, and MMP-9 by TNF-α, as confirmed by substrate gel electrophoresis and RT-qPCR. Immunoblotting revealed that p38 MAPK signaling mediates TNF-α–induced MMP-1, MMP-3, and MMP-9 expression, whereas IFN-γ significantly reverses these TNF-α effects by inhibiting p38 MAPK phosphorylation.
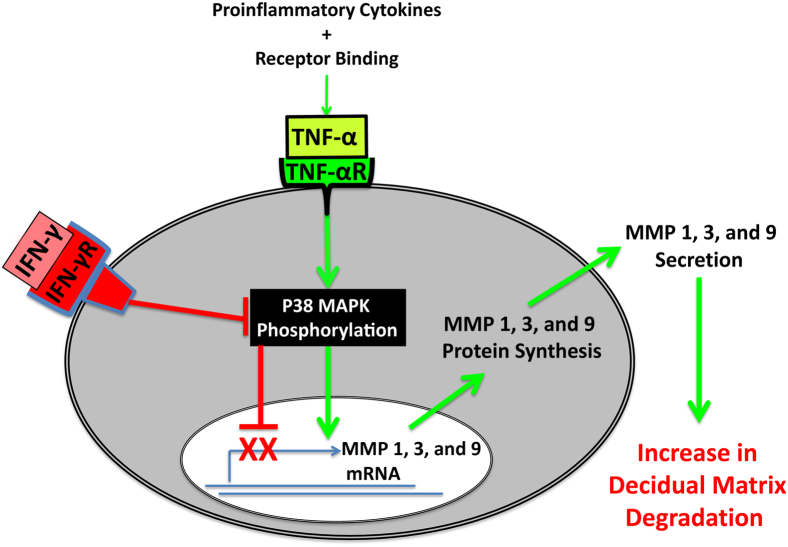
In summary, new in situ and in vitro observations indicate that aberrant TNF-α–augmented expression of MMP-1, MMP-3, and MMP-9 by decidual cells could interfere with normal stepwise ITG-mediated EVT invasion of the decidua to promote the later onset of PE. In addition, IFN-γ reverses this abnormal TNF-α–enhanced expression (Figure 8). These IFN-γ effects are consistent with restoration of normal stepwise EVT invasion of the decidua and suggest a mechanism by which dNK cell–derived IFN-γ counteracts the shallow trophoblast invasion strongly implicated in impaired decidual vascular remodeling, leading to the later development of PE.1
Figure 8.
Schematic representation of molecular mechanisms mediating the separate and interactive regulatory effects of TNF-α and IFN-γ on MMP expression in human first-trimester decidual cells. Binding of TNF-α to its cell surface receptor (TNFαR) increases MMP-1, MMP-3, and MMP-9 expression by stimulating p38 MAPK phosphorylation (activity) in decidual cells, whereas IFN-γ bound to its cell surface receptor blocks TNF-α–induced p38 phosphorylation to protect against MMP-mediated ECM degradation in the decidua.
Footnotes
This work was supported by NIH grants R01 HD033937 (C.J.L.) and 5R01HD056123 (S.J.H.).
Disclosures: None declared.
References
- 1.Pennington K.A., Schlitt J.M., Jackson D.L., Schulz L.C., Schust D.J. Preeclampsia: multiple approaches for a multifactorial disease. Dis Model Mech. 2012;5:9–18. doi: 10.1242/dmm.008516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Damsky C.H., Fisher S.J. Trophoblast pseudo-vasculogenesis: faking it with endothelial adhesion receptors. Curr Opin Cell Biol. 1998;10:660–666. doi: 10.1016/s0955-0674(98)80043-4. [DOI] [PubMed] [Google Scholar]
- 3.Pijnenborg R., Vercruysse L., Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27:939–958. doi: 10.1016/j.placenta.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Caniggia I., Winter J., Lye S.J., Post M. Oxygen and placental development during the first trimester: implications for the pathophysiology of pre-eclampsia. Placenta. 2000;21(Suppl A):S25–S30. doi: 10.1053/plac.1999.0522. [DOI] [PubMed] [Google Scholar]
- 5.Karumanchi S.A., Bdolah Y. Hypoxia and sFlt-1 in preeclampsia: the “chicken-and-egg” question. Endocrinology. 2004;145:4835–4837. doi: 10.1210/en.2004-1028. [DOI] [PubMed] [Google Scholar]
- 6.Borzychowski A.M., Sargent I.L., Redman C.W. Inflammation and pre-eclampsia. Semin Fetal Neonatal Med. 2006;11:309–316. doi: 10.1016/j.siny.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Aplin J.D., Charlton A.K., Ayad S. An immunohistochemical study of human endometrial extracellular matrix during the menstrual cycle and first trimester of pregnancy. Cell Tissue Res. 1988;253:231–240. doi: 10.1007/BF00221758. [DOI] [PubMed] [Google Scholar]
- 8.Loke Y.W., Gardner L., Burland K., King A. Laminin in human trophoblast–decidua interaction. Hum Reprod. 1989;4:457–463. doi: 10.1093/oxfordjournals.humrep.a136926. [DOI] [PubMed] [Google Scholar]
- 9.Damsky C.H., Librach C., Lim K.H., Fitzgerald M.L., McMaster M.T., Janatpour M., Zhou Y., Logan S.K., Fisher S.J. Integrin switching regulates normal trophoblast invasion. Development. 1994;120:3657–3666. doi: 10.1242/dev.120.12.3657. [DOI] [PubMed] [Google Scholar]
- 10.Bulla R., Villa A., Bossi F., Cassetti A., Radillo O., Spessotto P., De Seta F., Guaschino S., Tedesco F. VE-cadherin is a critical molecule for trophoblast-endothelial cell interaction in decidual spiral arteries. Exp Cell Res. 2005;303:101–113. doi: 10.1016/j.yexcr.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 11.Huppertz B., Kertschanska S., Demir A.Y., Frank H.G., Kaufmann P. Immunohistochemistry of matrix metalloproteinases (MMP), their substrates, and their inhibitors (TIMP) during trophoblast invasion in the human placenta. Cell Tissue Res. 1998;291:133–148. doi: 10.1007/s004410050987. [DOI] [PubMed] [Google Scholar]
- 12.Nagase H., Woessner J.F., Jr. Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 13.Nagase H., Visse R., Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Lijnen H.R. Matrix metalloproteinases and cellular fibrinolytic activity. Biochemistry (Mosc) 2002;67:92–98. doi: 10.1023/a:1013908332232. [DOI] [PubMed] [Google Scholar]
- 15.Lockwood C.J., Oner C., Uz Y.H., Kayisli U.A., Huang S.J., Buchwalder L.F., Murk W., Funai E.F., Schatz F. Matrix metalloproteinase 9 (MMP9) expression in preeclamptic decidua and MMP9 induction by tumor necrosis factor alpha and interleukin 1 beta in human first trimester decidual cells. Biol Reprod. 2008;78:1064–1072. doi: 10.1095/biolreprod.107.063743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sibai B., Dekker G., Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 17.Lockwood C.J., Matta P., Krikun G., Koopman L.A., Masch R., Toti P., Arcuri F., Huang S.T., Funai E.F., Schatz F. Regulation of monocyte chemoattractant protein-1 expression by tumor necrosis factor-alpha and interleukin-1beta in first trimester human decidual cells: implications for preeclampsia. Am J Pathol. 2006;168:445–452. doi: 10.2353/ajpath.2006.050082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeh C.C., Chao K.C., Huang S.J. Innate immunity, decidual cells, and preeclampsia. Reprod Sci. 2013;20:339–353. doi: 10.1177/1933719112450330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang S., Chen C.P., Schatz F., Rahman M., Abrahams V., Lockwood C. Pre-eclampsia is associated with dendritic cell recruitment into the uterine decidua. J Pathol. 2008;214:328–336. doi: 10.1002/path.2257. [DOI] [PubMed] [Google Scholar]
- 20.Wu Z.M., Yang H., Li M., Yeh C.C., Schatz F., Lockwood C.J., Di W., Huang S.J. Pro-inflammatory cytokine-stimulated first trimester decidual cells enhance macrophage-induced apoptosis of extravillous trophoblasts. Placenta. 2012;33:188–194. doi: 10.1016/j.placenta.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nathan C.F. Secretory products of macrophages. J Clin Invest. 1987;79:319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Itoh Y., Nagase H. Preferential inactivation of tissue inhibitor of metalloproteinases-1 that is bound to the precursor of matrix metalloproteinase 9 (progelatinase B) by human neutrophil elastase. J Biol Chem. 1995;270:16518–16521. doi: 10.1074/jbc.270.28.16518. [DOI] [PubMed] [Google Scholar]
- 23.Fu B., Li X., Sun R., Tong X., Ling B., Tian Z., Wei H. Natural killer cells promote immune tolerance by regulating inflammatory TH17 cells at the human maternal-fetal interface. Proc Natl Acad Sci U S A. 2013;110:E231–E240. doi: 10.1073/pnas.1206322110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lockwood C.J., Huang S.J., Chen C.P., Huang Y., Xu J., Faramarzi S., Kayisli O., Kayisli U., Koopman L., Smedts D., Buchwalder L.F., Schatz F. Decidual cell regulation of natural killer cell-recruiting chemokines: implications for the pathogenesis and prediction of preeclampsia. Am J Pathol. 2013;183:841–856. doi: 10.1016/j.ajpath.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalkunte S., Chichester C.O., Gotsch F., Sentman C.L., Romero R., Sharma S. Evolution of non-cytotoxic uterine natural killer cells. Am J Reprod Immunol. 2008;59:425–432. doi: 10.1111/j.1600-0897.2008.00595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lash G.E., Robson S.C., Bulmer J.N. Review: functional role of uterine natural killer (uNK) cells in human early pregnancy decidua. Placenta. 2010;31(Suppl):S87–S92. doi: 10.1016/j.placenta.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 27.Manaster I., Mandelboim O. The unique properties of uterine NK cells. Am J Reprod Immunol. 2010;63:434–444. doi: 10.1111/j.1600-0897.2009.00794.x. [DOI] [PubMed] [Google Scholar]
- 28.Lockwood C.J., Kayisli U.A., Stocco C., Murk W., Vatandaslar E., Buchwalder L.F., Schatz F. Abruption-induced preterm delivery is associated with thrombin-mediated functional progesterone withdrawal in decidual cells. Am J Pathol. 2012;181:2138–2148. doi: 10.1016/j.ajpath.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arici A., Marshburn P.B., MacDonald P.C., Dombrowski R.A. Progesterone metabolism in human endometrial stromal and gland cells in culture. Steroids. 1999;64:530–534. doi: 10.1016/s0039-128x(99)00029-x. [DOI] [PubMed] [Google Scholar]
- 30.Dunn C.L., Kelly R.W., Critchley H.O. Decidualization of the human endometrial stromal cell: an enigmatic transformation. Reprod Biomed Online. 2003;7:151–161. doi: 10.1016/s1472-6483(10)61745-2. [DOI] [PubMed] [Google Scholar]
- 31.Kabawat S.E., Mostoufi-Zadeh M., Driscoll S.G., Bhan A.K. Implantation site in normal pregnancy: a study with monoclonal antibodies. Am J Pathol. 1985;118:76–84. [PMC free article] [PubMed] [Google Scholar]
- 32.Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol. 2002;2:656–663. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- 33.Hanna J., Wald O., Goldman-Wohl D., Prus D., Markel G., Gazit R., Katz G., Haimov-Kochman R., Fujii N., Yagel S., Peled A., Mandelboim O. CXCL12 expression by invasive trophoblasts induces the specific migration of CD16- human natural killer cells. Blood. 2003;102:1569–1577. doi: 10.1182/blood-2003-02-0517. [DOI] [PubMed] [Google Scholar]
- 34.Smith S.D., Dunk C.E., Aplin J.D., Harris L.K., Jones R.L. Evidence for immune cell involvement in decidual spiral arteriole remodeling in early human pregnancy. Am J Pathol. 2009;174:1959–1971. doi: 10.2353/ajpath.2009.080995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naruse K., Lash G.E., Innes B.A., Otun H.A., Searle R.F., Robson S.C., Bulmer J.N. Localization of matrix metalloproteinase (MMP)-2, MMP-9 and tissue inhibitors for MMPs (TIMPs) in uterine natural killer cells in early human pregnancy. Hum Reprod. 2009;24:553–561. doi: 10.1093/humrep/den408. [DOI] [PubMed] [Google Scholar]
- 36.Robson A., Harris L.K., Innes B.A., Lash G.E., Aljunaidy M.M., Aplin J.D., Baker P.N., Robson S.C., Bulmer J.N. Uterine natural killer cells initiate spiral artery remodeling in human pregnancy. FASEB J. 2012;26:4876–4885. doi: 10.1096/fj.12-210310. [DOI] [PubMed] [Google Scholar]
- 37.Reister F., Frank H.G., Heyl W., Kosanke G., Huppertz B., Schroder W., Kaufmann P., Rath W. The distribution of macrophages in spiral arteries of the placental bed in pre-eclampsia differs from that in healthy patients. Placenta. 1999;20:229–233. doi: 10.1053/plac.1998.0373. [DOI] [PubMed] [Google Scholar]
- 38.Otun H.A., Lash G.E., Innes B.A., Bulmer J.N., Naruse K., Hannon T., Searle R.F., Robson S.C. Effect of tumour necrosis factor-alpha in combination with interferon-gamma on first trimester extravillous trophoblast invasion. J Reprod Immunol. 2011;88:1–11. doi: 10.1016/j.jri.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Huber A.V., Saleh L., Bauer S., Husslein P., Knofler M. TNFalpha-mediated induction of PAI-1 restricts invasion of HTR-8/SVneo trophoblast cells. Placenta. 2006;27:127–136. doi: 10.1016/j.placenta.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 40.Menkhorst E.M., Lane N., Winship A.L., Li P., Yap J., Meehan K., Rainczuk A., Stephens A., Dimitriadis E. Decidual-secreted factors alter invasive trophoblast membrane and secreted proteins implying a role for decidual cell regulation of placentation. PLoS One. 2012;7:e31418. doi: 10.1371/journal.pone.0031418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zygmunt M., McKinnon T., Herr F., Lala P.K., Han V.K. HCG increases trophoblast migration in vitro via the insulin-like growth factor-II/mannose-6 phosphate receptor. Mol Hum Reprod. 2005;11:261–267. doi: 10.1093/molehr/gah160. [DOI] [PubMed] [Google Scholar]
- 42.Irwin J.C., Suen L.F., Martina N.A., Mark S.P., Giudice L.C. Role of the IGF system in trophoblast invasion and pre-eclampsia. Hum Reprod. 1999;14(Suppl 2):90–96. doi: 10.1093/humrep/14.suppl_2.90. [DOI] [PubMed] [Google Scholar]
- 43.Irwin J.C., Suen L.F., Faessen G.H., Popovici R.M., Giudice L.C. Insulin-like growth factor (IGF)-II inhibition of endometrial stromal cell tissue inhibitor of metalloproteinase-3 and IGF-binding protein-1 suggests paracrine interactions at the decidua:trophoblast interface during human implantation. J Clin Endocrinol Metab. 2001;86:2060–2064. doi: 10.1210/jcem.86.5.7451. [DOI] [PubMed] [Google Scholar]
- 44.Nicola C., Timoshenko A.V., Dixon S.J., Lala P.K., Chakraborty C. EP1 receptor-mediated migration of the first trimester human extravillous trophoblast: the role of intracellular calcium and calpain. J Clin Endocrinol Metab. 2005;90:4736–4746. doi: 10.1210/jc.2005-0413. [DOI] [PubMed] [Google Scholar]
- 45.Caniggia I., Grisaru-Gravnosky S., Kuliszewsky M., Post M., Lye S.J. Inhibition of TGF-beta 3 restores the invasive capability of extravillous trophoblasts in preeclamptic pregnancies. J Clin Invest. 1999;103:1641–1650. doi: 10.1172/JCI6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lysiak J.J., Hunt J., Pringle G.A., Lala P.K. Localization of transforming growth factor beta and its natural inhibitor decorin in the human placenta and decidua throughout gestation. Placenta. 1995;16:221–231. doi: 10.1016/0143-4004(95)90110-8. [DOI] [PubMed] [Google Scholar]
- 47.Xu G., Guimond M.J., Chakraborty C., Lala P.K. Control of proliferation, migration, and invasiveness of human extravillous trophoblast by decorin, a decidual product. Biol Reprod. 2002;67:681–689. doi: 10.1095/biolreprod67.2.681. [DOI] [PubMed] [Google Scholar]
- 48.Louwen F., Muschol-Steinmetz C., Reinhard J., Reitter A., Yuan J. A lesson for cancer research: placental microarray gene analysis in preeclampsia. Oncotarget. 2012;3:759–773. doi: 10.18632/oncotarget.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferretti C., Bruni L., Dangles-Marie V., Pecking A.P., Bellet D. Molecular circuits shared by placental and cancer cells, and their implications in the proliferative, invasive and migratory capacities of trophoblasts. Hum Reprod Update. 2007;13:121–141. doi: 10.1093/humupd/dml048. [DOI] [PubMed] [Google Scholar]
- 50.Cohen M., Meisser A., Bischof P. Metalloproteinases and human placental invasiveness. Placenta. 2006;27:783–793. doi: 10.1016/j.placenta.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 51.Librach C.L., Werb Z., Fitzgerald M.L., Chiu K., Corwin N.M., Esteves R.A., Grobelny D., Galardy R., Damsky C.H., Fisher S.J. 92-kD type IV collagenase mediates invasion of human cytotrophoblasts. J Cell Biol. 1991;113:437–449. doi: 10.1083/jcb.113.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dano K., Behrendt N., Hoyer-Hansen G., Johnsen M., Lund L.R., Ploug M., Romer J. Plasminogen activation and cancer. Thromb Haemost. 2005;93:676–681. doi: 10.1160/TH05-01-0054. [DOI] [PubMed] [Google Scholar]
- 53.Westermarck J., Kahari V.M. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. 1999;13:781–792. [PubMed] [Google Scholar]
- 54.Bjorklund M., Koivunen E. Gelatinase-mediated migration and invasion of cancer cells. Biochim Biophys Acta. 2005;1755:37–69. doi: 10.1016/j.bbcan.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 55.Almholt K., Johnsen M. Stromal cell involvement in cancer. Recent Results Cancer Res. 2003;162:31–42. doi: 10.1007/978-3-642-59349-9_3. [DOI] [PubMed] [Google Scholar]
- 56.Pedersen T.X., Pennington C.J., Almholt K., Christensen I.J., Nielsen B.S., Edwards D.R., Romer J., Dano K., Johnsen M. Extracellular protease mRNAs are predominantly expressed in the stromal areas of microdissected mouse breast carcinomas. Carcinogenesis. 2005;26:1233–1240. doi: 10.1093/carcin/bgi065. [DOI] [PubMed] [Google Scholar]
- 57.Martin M.D., Matrisian L.M. The other side of MMPs: protective roles in tumor progression. Cancer Metastasis Rev. 2007;26:717–724. doi: 10.1007/s10555-007-9089-4. [DOI] [PubMed] [Google Scholar]
- 58.Seval Y., Akkoyunlu G., Demir R., Asar M. Distribution patterns of matrix metalloproteinase (MMP)-2 and -9 and their inhibitors (TIMP-1 and TIMP-2) in the human decidua during early pregnancy. Acta Histochem. 2004;106:353–362. doi: 10.1016/j.acthis.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 59.Hu Y., Dutz J.P., MacCalman C.D., Yong P., Tan R., von Dadelszen P. Decidual NK cells alter in vitro first trimester extravillous cytotrophoblast migration: a role for IFN-gamma. J Immunol. 2006;177:8522–8530. doi: 10.4049/jimmunol.177.12.8522. [DOI] [PubMed] [Google Scholar]
- 60.Campbell S., Rowe J., Jackson C.J., Gallery E.D. Interaction of cocultured decidual endothelial cells and cytotrophoblasts in preeclampsia. Biol Reprod. 2004;71:244–252. doi: 10.1095/biolreprod.103.026716. [DOI] [PubMed] [Google Scholar]
- 61.Lian I.A., Toft J.H., Olsen G.D., Langaas M., Bjorge L., Eide I.P., Bordahl P.E., Austgulen R. Matrix metalloproteinase 1 in pre-eclampsia and fetal growth restriction: reduced gene expression in decidual tissue and protein expression in extravillous trophoblasts. Placenta. 2010;31:615–620. doi: 10.1016/j.placenta.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 62.Zhang J., Chen Z., Smith G.N., Croy B.A. Natural killer cell-triggered vascular transformation: maternal care before birth? Cell Mol Immunol. 2011;8:1–11. doi: 10.1038/cmi.2010.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lash G.E., Otun H.A., Innes B.A., Kirkley M., De Oliveira L., Searle R.F., Robson S.C., Bulmer J.N. Interferon-gamma inhibits extravillous trophoblast cell invasion by a mechanism that involves both changes in apoptosis and protease levels. FASEB J. 2006;20:2512–2518. doi: 10.1096/fj.06-6616com. [DOI] [PubMed] [Google Scholar]
- 64.Ashkar A.A., Croy B.A. Functions of uterine natural killer cells are mediated by interferon gamma production during murine pregnancy. Semin Immunol. 2001;13:235–241. doi: 10.1006/smim.2000.0319. [DOI] [PubMed] [Google Scholar]
- 65.Bilinski M.J., Thorne J.G., Oh M.J., Leonard S., Murrant C., Tayade C., Croy B.A. Uterine NK cells in murine pregnancy. Reprod Biomed Online. 2008;16:218–226. doi: 10.1016/s1472-6483(10)60577-9. [DOI] [PubMed] [Google Scholar]