Abstract
Introduction
Physical replicas of organs are used increasingly for preoperative planning. The quality of these models is generally accepted by surgeons. In view of the strong trend towards minimally invasive and personalised surgery, however, the aim of this investigation was to assess qualitatively the accuracy of such replicas, using skull models as an example.
Methods
Skull imaging was acquired for three cadavers by computed tomography using clinical routine parameters. After digital three-dimensional (3D) reconstruction, physical replicas were produced by 3D printing. The facsimilia were analysed systematically and compared with the best gold standard possible: the macerated skull itself.
Results
The skull models were far from anatomically accurate. Non-conforming rendering was observed in particular for foramina, sutures, notches, fissures, grooves, channels, tuberosities, thin-walled structures, sharp peaks and crests, and teeth.
Conclusions
Surgeons should be aware that preoperative models may not yet render the exact anatomy of the patient under consideration and are advised to continue relying, in specific conditions, on their own analysis of the native computed tomography or magnetic resonance imaging.
Keywords: Surgical planning, Preoperative replicas, Anatomy, Skull
Tangible replicas of human organs based on computed tomography (CT) are gaining increasing application for preoperative planning in different surgical specialties. 1–6 The quality of these models, obtained by rapid prototyping technologies, is currently generally accepted by surgeons although limitations have been reported. 4,7–9
Considering the marked trend towards minimally invasive surgery and interventional radiology, the standards that preoperative replicas need to meet in terms of anatomical accuracy are likely to increase substantially in the future. Such replicas could be of particular importance for neurosurgical planning when it comes to the treatment of lesions that are difficult to access (ie intrinsic of the skull base) such as chordomas. These tumours are notoriously osteodestructive and they exhibit a very complex growth pattern by which they may arrive close to or engulf relevant neurovascular structures, the carotid or basilar arteries, for instance. These approaches require diligent and sometimes joint planning by neurosurgeons and ear, nose and throat surgeons. The combination of microsurgical techniques and bond drilling in the depth, and of endoscopic techniques, remains challenging for the surgical team involved.
Against this background, it seemed judicious to draw up an inventory of anatomical structures that are especially subject to incorrect rendering. As replicas are used frequently in craniomaxillofacial and neurological surgery, the skull was assessed in this study.
Methods
Craniofacial CT was performed using clinical routine parameters (Somatom Sensation 64, Siemens, Erlangen, Germany) on three bodies donated to our anatomy department. After digital three-dimensional (3D) reconstruction of the skull with Mimics software (Materialise, Leuven, Belgium), replicas were obtained by 3D printing (ZPrinter® 310 Plus; Z Corporation, Burlington, MA, US). The anatomy rendered in these facsimiles was then assessed qualitatively and systematically, region by region, structure by structure, according to the current international anatomical nomenclature, 10 and compared with the best gold standard possible, the original skull that had meanwhile been extracted from the cadaveric head and prepared by anatomical maceration techniques.
Results
The following structures systematically showed non-conforming rendering when compared with the actual skull:
Foramina (eg infraorbital, ovale, jugular, greater palatine, mental or stylomastoid) were mostly reproduced in a way that was too narrow and often occluded, sometimes altered beyond recognition (eg foramina of the cribriform plate and granular foveolae for the arachnoid granulations). Sutures were blurred and sometimes not visible at all (eg a metopic suture in one of the individuals investigated). The same was true for incisurae (frontal and supraorbital notch), fissurae (eg superior orbital and petrotympanic fissure) and sulci (including grooves for middle meningeal artery branches, superior and inferior petrosal sinus, mylohyoid nerve and vessels). Channels were mostly sealed, even large ones (eg optic canal, external and internal acoustic meatus, hypoglossal and carotid canals). Delicately walled structures (eg roof of the tympanic cavity, floor and dorsum of the sella turcica, maxillary tuberosity) displayed defects not present in the actual skull. Illustrative examples regarding the floor and medial wall of the orbit are shown in Figure 1. Spike-shaped structures or those ending as subtle crests were dull edged (eg middle clinoid process, intrajugular process, pterygoid hamulus, Spix’s spine). Tuberosities were often smoothed down (eg masseteric and pterygoid tuberosity, mental spines). Teeth displayed metal artefacts in the presence of dental fillings (Fig 2).
Figure 1. Example of an anatomical structure consistently rendered incorrectly in preoperative replicas: typical artefactual defects in the floor (asterisk) and medial wall of the orbit. As a consequence, assessment of the infraorbital groove and canal is impossible in the patient under consideration.
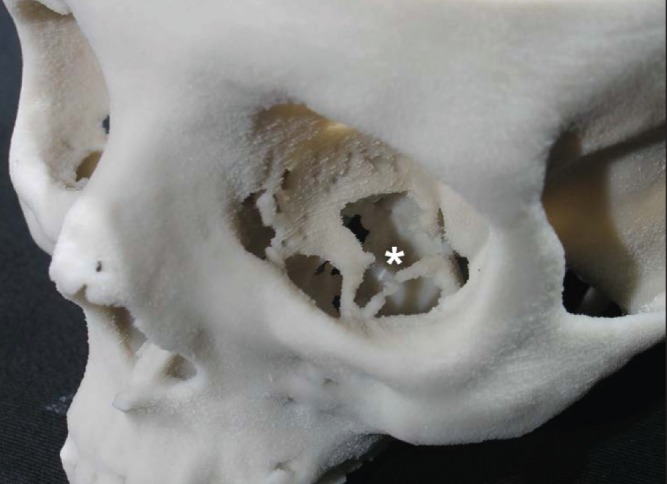
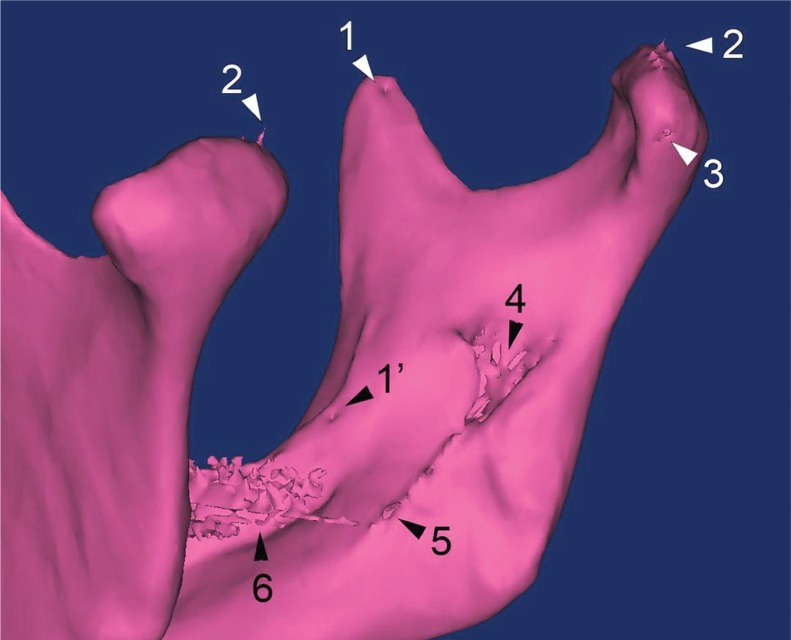
Figure 2. Mandibular reconstruction illustrating several flaws that can occur in preoperative replicas: 1) pseudo-nutrient foramen at the coronoid process (as opposed to a real one medially to the crista buccinatoria [1’]); 2) spike artefacts on the articular surface; 3) incompletely rendered surface of the neck; 4) fragmented lateral wall of the mandibular foramen; 5) defects in the mylohyoid groove; 6) teeth artefacts due to metal fillings.
Discussion
Our results demonstrate that preoperative skull replicas currently obtained in clinical routine practice do not satisfy the requirements for precise anatomical analysis. The rendering of many structures is qualitatively incorrect. These include foramina, sutures, notches, fissures, sulci, canals, spines and tuberosities. Anatomical variants (such as a persistent frontal suture or a bridging sphenoidal lingula), important in the trend towards individualised surgery, were also evaluated and were only reproduced correctly in some instances. Conversely, variants not present in the original skull sometimes appeared in the replica (eg accessory nutrient foramina).
The reasons behind these deficiencies are manifold. Concerning CT parameters, Choi et al emphasised the importance of section thickness and isotropic voxel acquisition to limit the partial volume averaging effect. 11 In the digital 3D reconstruction stage, the major source of inaccuracy is introduced when selecting the threshold values. 9 During the production of the model by rapid prototyping technologies, limiting factors include layer thickness and residual polymerisation phenomena. 8,9,12,13
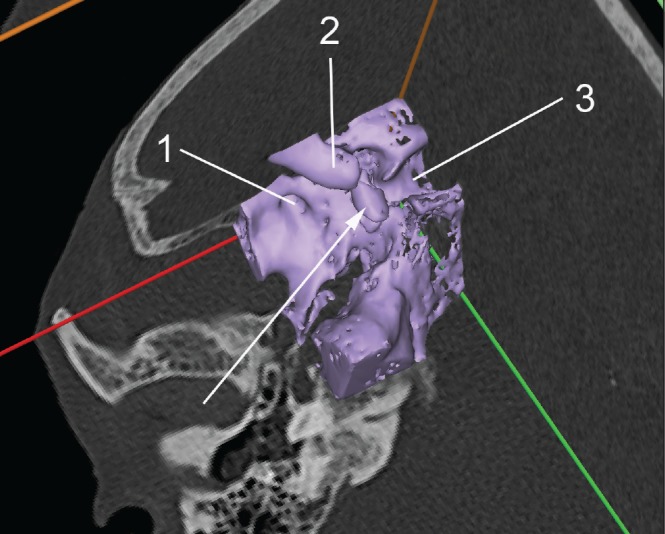
Furthermore, automated, threshold-based reconstructions, as used in clinical routine, do not distinguish between bony structures and non-osseous calcifications. Figure 3 shows an example of a calcified atherosclerotic lesion in the wall of the parasellar internal carotid artery. This fact may seem self-evident but it reminds us that segmentation of CT data does still require interpretation of the original radiological images and should not be delegated as pure routine work to anatomically untrained and clinically unconcerned personnel.
Figure 3. Inappropriate reconstruction of a non-osseous structure (arrow) for a replica intended to render the bony skull base: 1) foramen rotundum; 2) anterior clinoid process; 3) sella turcica. The arrow points at a calcified atherosclerotic lesion in the wall of the parasellar internal carotid artery (segment C4).
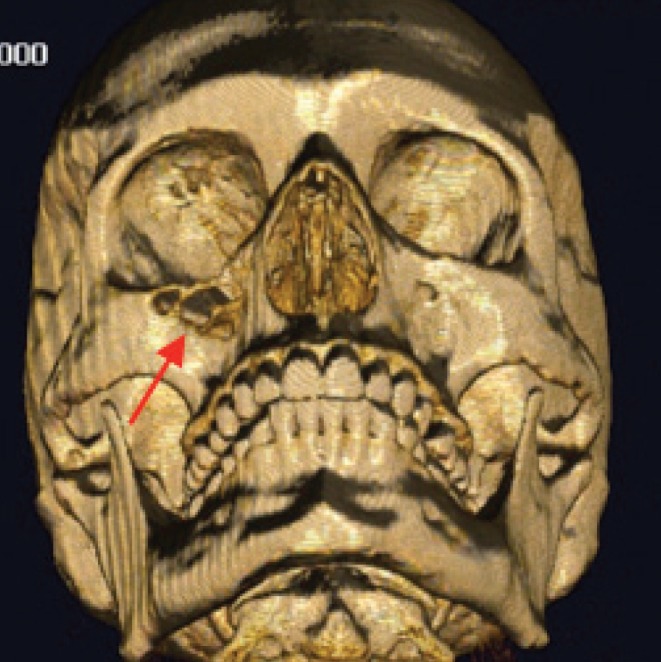
The evidence provided in this study that 3D printed models, from a morphological point of view, are not as reliable as possibly presumed mandates that surgeons maintain a critical attitude towards preoperative replicas. As an example, Figure 4 illustrates a case of bone destruction by a malignant melanoma of the right maxillary sinus, which, in the corresponding printed model, could have been considered artefactual instead of being recognised as defects due to neoplastic invasion. Artefactual holes, when occurring near real foramina, may also be mistaken for the latter and lead to incorrect localisation (such as the infraorbital or greater palatine foramen). In broad terms, using current 3D replicas as an example, we suggest that clinical standards are to be maintained, and not completely replaced by uncritical belief in informatics tools.
Figure 4. Non-artefactual holes in the anterior wall of the right maxillary sinus (arrow) in a patient suffering from a malignant melanoma, in contrast with artefactual defects, in the roof of the right orbit, for instance.
Conclusions
This study demonstrates that current preoperative 3D models do not yet accurately render the anatomy of a given patient. This paper is intended to function as a timely reminder for the surgical community, who should be aware of the potential discrepancies between 3D replicas and reality. Clinicians are encouraged to continue to rely on their own critical judgement and on their own analysis of native CT or magnetic resonance imaging.
References
- 1.Schmauss D, Schmitz C, Bigdeli AKet al Three-dimensional printing of models for preoperative planning and simulation of transcatheter valve replacement. Ann Thorac Surg 2012; 93: e31–e33. [DOI] [PubMed] [Google Scholar]
- 2.Conversano F, Franchini R, Demitri Cet al Hepatic vessel segmentation for 3D planning of liver surgery. Acad Radiol 2011; 18: 461–470. [DOI] [PubMed] [Google Scholar]
- 3.Edwards SP. Computer-assisted craniomaxillofacial surgery. Oral Maxillofac Surg Clin North Am 2010; 22: 117–134. [DOI] [PubMed] [Google Scholar]
- 4.Rengier F, Mehndiratta A, von Tengg-Kobligk Het al 3D printing based on imaging data: review of medical applications. Int J Comput Assist Radiol Surg 2010; 5: 335–341. [DOI] [PubMed] [Google Scholar]
- 5.Guarino J, Tennyson S, McCain Get al Rapid prototyping technology for surgeries of the pediatric spine and pelvis: benefits analysis. J Pediatr Orthop 2007; 27: 955–960. [DOI] [PubMed] [Google Scholar]
- 6.Brown GA, Firoozbakhsh K, DeCoster TAet al Rapid prototyping: the future of trauma surgery? J Bone Joint Surg Am 2003; 85 Suppl 4: 49–55. [PubMed] [Google Scholar]
- 7.Stumpel LJ. Deformation of stereolithographically produced surgical guides: an observational case series report. Clin Implant Dent Relat Res 2012; 14: 442–453. [DOI] [PubMed] [Google Scholar]
- 8.Williams JV, Revington PJ. Novel use of an aerospace selective laser sintering machine for rapid prototyping of an orbital blowout fracture. Int J Oral Maxillofac Surg 2010; 39: 182–184. [DOI] [PubMed] [Google Scholar]
- 9.Winder J, Bibb R. Medical rapid prototyping technologies: state of the art and current limitations for application in oral and maxillofacial surgery. J Oral Maxillofac Surg 2005; 63: 1,006–1,015. [DOI] [PubMed] [Google Scholar]
- 10.Federative Committee on Anatomical Terminology. Terminologia Anatomica. Stuttgart: Thieme; 1998. [Google Scholar]
- 11.Choi JY, Choi JH, Kim NKet al Analysis of errors in medical rapid prototyping models. Int J Oral Maxillofac Surg 2002; 31: 23–32. [DOI] [PubMed] [Google Scholar]
- 12.Dimitrov D, van Wijck W, Schreve K, de Beer N. Investigating the achievable accuracy of three dimensional printing. Rapid Prototyping Journal 2006; 12: 42–52. [Google Scholar]
- 13.Ibrahim D, Broilo L, Heitz Cet al Dimensional error of selective sintering, three-dimensional printing and PolyJet models in the reproduction of mandibular anatomy. J Craniomaxillofac Surg 2009; 37: 167–173. [DOI] [PubMed] [Google Scholar]