Abstract
Greater attained height and greater body mass index (BMI; weight (kg)/height (m)2) in young adulthood have been associated with glioma risk, but few studies have investigated the association with body size at birth or during childhood, when the brain undergoes rapid cell growth and differentiation. The Copenhagen School Health Records Register includes data on 320,425 Danish schoolchildren born between 1930 and 1989, with height and weight measurements from ages 7–13 years and parentally recorded birth weights. We prospectively evaluated associations between childhood height and BMI, birth weight, and adult glioma risk. During follow-up (1968–2010), 355 men and 253 women aged ≥18 years were diagnosed with glioma. In boys, height at each age between 7 and 13 years was positively associated with glioma risk; hazard ratios per standard-deviation score at ages 7 (approximately 5.1 cm) and 13 (approximately 7.6 cm) years were 1.17 (95% confidence interval (CI): 1.05, 1.30) and 1.21 (95% CI: 1.09, 1.35), respectively. No associations were observed for childhood height in girls or for BMI. Birth weight was positively associated with risk (per 0.5 kg: hazard ratio = 1.13, 95% CI: 1.04, 1.24). These results suggest that exposures associated with higher birth weight and, in boys, greater height during childhood may contribute to the etiology of adult glioma.
Keywords: adolescence, birth weight, body mass index, brain neoplasms, childhood, glioma, height, prospective studies
Cancers of the brain and nervous system account for an estimated 142,000 deaths worldwide each year (1). The etiology of glioma, the most common type of malignant brain tumor in adulthood, remains largely unknown. However, several lines of evidence suggest that exposures incurred during early life, when the brain and immune system are less developed and the brain undergoes more rapid cell growth and differentiation, may be important in the development of this disease in adulthood (2). Observational studies have shown that season of birth (2, 3), a personal history of asthma or other allergic conditions (4, 5), a personal history of chickenpox and immunoglobulin G antibodies to varciella-zoster virus (6, 7), higher birth order and greater family size (proxies for younger and more frequent exposure to infectious agents during childhood, respectively) (8, 9), and greater physical activity in adolescence (10) have been associated with reduced risk of adult glioma, while right-handedness has been associated with an increased risk (11). High-dose exposure to ionizing radiation, particularly at younger ages, represents the only established external exposure associated with risk of adult glioma (12).
Several epidemiologic studies have found that men and women with greater attained height have an increased risk of adult glioma and, more generally, brain/central nervous system malignancies (10, 13–16). Adult height is often used as a proxy for genetic, hormonal, and environmental exposures associated with early-life growth (17–24), which may be etiologically relevant for the development of cancers such as glioma. For instance, there is some evidence from epidemiologic studies that exposure to infections during childhood may be associated with both a reduced risk of glioma (6–9) and lower height (25). Body mass index (BMI; weight (kg)/height (m)2) in young adulthood (ages 18–21 years), but not in older adulthood, has been positively associated with risk of adult glioma (10, 26), leaving open the possibility that positive energy balance at young ages may contribute to risk of glioma later in life. However, there is little evidence regarding the relationship between body size during periods of rapid growth (e.g., infancy, childhood, or adolescence) and glioma risk in adulthood, since few large observational studies have collected information on body size at these ages. Studies designed to address this will further our understanding about possible etiologically relevant time windows and exposures for glioma development.
The Copenhagen School Health Records Register (CSHRR) contains recorded measurements of weight and height for Danish children who were born between 1930 and 1989 and attended school in the Municipality of Copenhagen from 7 to 13 years of age. These data were linked with glioma incidence data from the Danish Cancer Registry. Birth weights are also included in the CSHRR for children born after 1935. Using these data, we prospectively investigated the relationship between height and BMI during childhood, birth weight, and risk of adult glioma.
METHODS
Study population
The CSHRR is a database of school health records on virtually all Danish children (n = 372,636) who were born between 1930 and 1989 and attended public or private school in the Municipality of Copenhagen (27). The database includes annual height and weight measurements taken at each age between 7 and 13 years up to the 1983 school year; thereafter, children were measured at school entry and exit, or more frequently if the child had special health needs. Measurements were conducted by physicians and nurses. Since 1943, the CSHRR has additionally included birth weights as reported by parents at the first school examination, with memory being aided by a request for parents to bring written documentation of such values recorded at or near the time of birth.
The Danish Civil Registration System, a vital statistics database, was established on April 2, 1968, to collect information on death, emigration, and loss to follow-up (28). By this date, unique government-issued identification numbers had been assigned to all residents of Denmark. These identification numbers were included in the CSHRR database for 329,968 children and enabled linkage with national registers. Because this analysis was register-based, consent from individual participants was not required, in accordance with Danish law. Approval for the use of these data was obtained through the Danish Data Protection Agency.
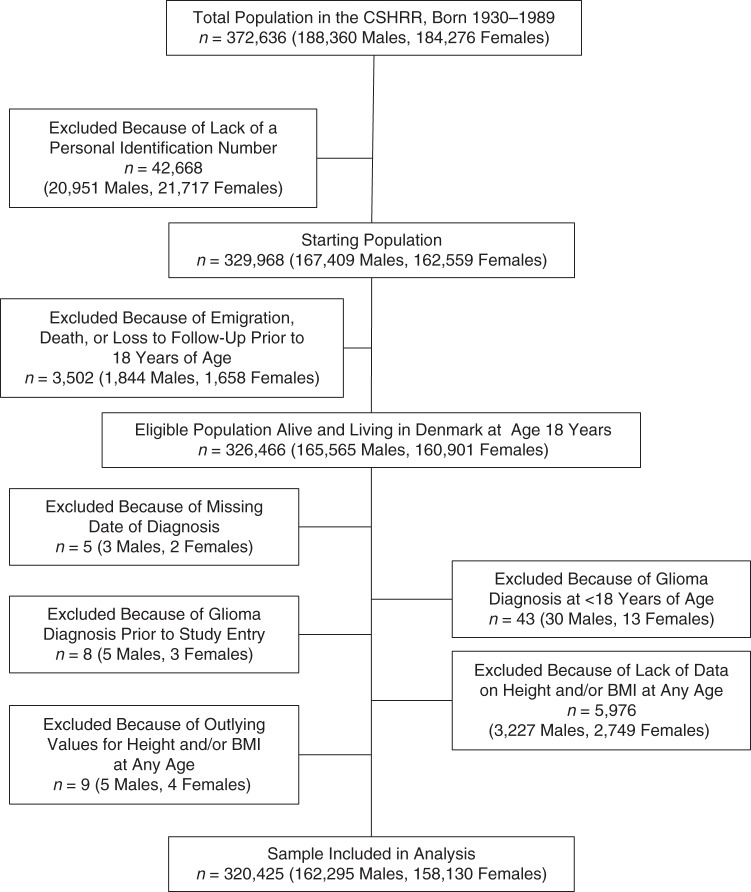
From the CSHRR cohort, our study sample was formed from an eligible population of 326,466 participants (165,565 men and 160,901 women) who were alive and living in Denmark at age 18 years. We excluded 5 subjects with missing dates of glioma diagnosis, 43 subjects who were diagnosed with glioma before age 18 years (because the distribution of cases by histology differs for glioma diagnosed in children and glioma diagnosed in adults (29)), and 8 subjects diagnosed before April 2, 1968 (Figure 1). We also excluded 5,976 subjects with missing data (those without data on height and/or BMI at every age) and 9 subjects with outlying values for height or BMI (z score or standard deviation score (SDS) <−4.5 or >4.5). After the exclusions were applied, our analytical study population included 320,425 participants (162,295 men and 158,130 women). The number of women included in each height or BMI model ranged from 137,195 (87% of the total female population) to 149,580 (95% of the total female population), and the number of men in each model ranged from 138,363 (85% of the total male population) to 153,514 (95% of the total male population). A total of 242,377 participants (76% of the total population) had complete data on height at every age.
Figure 1.
Eligibility criteria for inclusion in a study of associations of childhood height, childhood body mass index (BMI; weight (kg)/height (m)2), and birth weight with adult glioma risk, based on data from the Copenhagen School Health Records Register (CSHRR), Copenhagen, Denmark, 1968–2010.
After 37,828 participants with missing birth weight data and 2,960 participants with birth weights less than 2 kg were excluded from the eligible population, the analytical study population for analyses of birth weight and glioma risk included 244,407 persons (124,768 men and 119,639 women).
Follow-up
Follow-up information starting from April 2, 1968, was obtained via registry linkage using the participants’ identification numbers. Data from the CSHRR were linked with the Danish Civil Registration System vital statistics for information on death, emigration, and loss to follow-up (28) and with the Danish Cancer Registry, which has provided nearly complete coverage of cancer incidence nationwide since its inception in 1942 (27, 30). Glioma was defined as malignant carcinoma of the brain (International Classification of Diseases, Tenth Revision, site codes C71.0–C71.9 and International Classification of Diseases for Oncology, Third Edition, morphology codes 9380–9480; or International Classification of Diseases, Seventh Revision, site code 193 and Danish Cancer Registry codes (for morphology) 093.1–093.7) (31, 32). The morphology codes used to define histological subtypes are shown in Appendix Table 1. Glioma cases with morphological codes outside of these ranges were classified as “other histology” or “malignant glioma, not otherwise specified.”
Follow-up began at age 18 years or on April 2, 1968, whichever occurred last. Subjects were censored at the age of glioma diagnosis, emigration, or death or the date of the latest cancer registry update as of December 31, 2010, whichever occurred first.
Statistical analysis
Associations between anthropometric measures and the risks of total glioma and histological subtypes of glioma were assessed using Cox proportional hazards regression, using attained age as the underlying time metric and stratifying by sex and 5-year birth cohort (12 intervals between 1930 and 1989). Age at measurement and sex-specific anthropometric measures were transformed into standard deviation scores using the lambda mu sigma method to account for different variation and skewness by age at measurement (33). To account for the secular trend of increasing height, we chose age-, sex-, and 5-year birth cohort–specific internal references. The age- and sex-specific internal BMI reference was chosen from a period when the prevalence of obesity was low and stable (calendar years 1955–1960) (27).
We repeated these analyses after mutual adjustment for height and BMI SDS. We observed no significant deviations from the proportional hazards assumption, which was assessed by modeling the time-varying associations of height, BMI, and birth weight by attained age, and no deviations from linearity, which was examined using restricted cubic spline models with 3 knot points (data not shown). Multiplicative interactions by sex and birth cohort were tested by comparing the fit of a model including a cross-product term with the fit of a model that excluded this term using the likelihood ratio test. Results for height and BMI are presented separately by sex to account for differences in growth trajectories during this age period. All tests of statistical significance were 2-sided. Analyses were conducted using Stata statistical software, version 12.1 (StataCorp LP, College Station, Texas).
RESULTS
During follow-up (median, 36.6 years; range, 0.01–42.8 years), 608 subjects (355 men and 253 women) were diagnosed with glioma. The median age at glioma diagnosis was 53 years (range, 18–76) for men and 52 years (range, 19–79) for women. The main histological subtypes of glioma, in order of predominance, were glioblastoma (n = 356 cases (59%); 219 in men and 137 in women), astrocytoma other than glioblastoma (n = 130 cases (21%); 74 in men and 56 in women), oligodendroglioma (n = 75 cases (12%); 39 in men and 36 in women), ependymoma (n = 14 (2%); 7 in men and 7 in women), and medulloblastoma (n = 7 (1%); 5 in men and 2 in women). Twenty-six glioma cases (4%) were classified as not otherwise specified.
The median values and 5th–95th percentile ranges of height and BMI for girls and boys at each age between 7 and 13 years are shown in Table 1. The median birth weights for boys and girls were 3.5 kg and 3.4 kg, respectively.
Table 1.
Median Values and 5th–95th Percentile Ranges for Height and Body Mass Indexa at Each Age Between 7 and 13 Years, Based on Data From the Copenhagen School Health Records Register, Copenhagen, Denmark, 1968–2010
| Age at Measurement, years | Women |
Men |
||||||
|---|---|---|---|---|---|---|---|---|
| Height, cm |
Body Mass Index |
Height, cm |
Body Mass Index |
|||||
| Median | Range (5th–95th Percentiles) | Median | Range (5th–95th Percentiles) | Median | Range (5th–95th Percentiles) | Median | Range (5th–95th Percentiles) | |
| 7 | 121.7 | 113.0–130.4 | 15.3 | 13.5–18.0 | 122.6 | 114.0–131.2 | 15.4 | 13.8–17.7 |
| 8 | 126.9 | 117.9–136.0 | 15.6 | 13.7–18.7 | 127.9 | 118.9–137.0 | 15.7 | 14.0–18.3 |
| 9 | 132.1 | 122.7–142.0 | 16.0 | 13.9–19.6 | 133.1 | 123.8–142.9 | 16.0 | 14.2–19.0 |
| 10 | 137.2 | 127.3–148.0 | 16.4 | 14.2–20.4 | 138.1 | 128.4–148.4 | 16.4 | 14.4–19.9 |
| 11 | 142.9 | 132.0–154.8 | 16.8 | 14.4–21.3 | 142.9 | 132.8–153.8 | 16.8 | 14.7–20.7 |
| 12 | 149.3 | 137.1–161.7 | 17.5 | 14.8–22.2 | 147.8 | 137.0–159.8 | 17.2 | 15.0–21.6 |
| 13 | 155.5 | 143.0–167.0 | 18.3 | 15.3–23.2 | 153.5 | 141.5–167.6 | 17.8 | 15.3–22.3 |
a Weight (kg)/height (m)2.
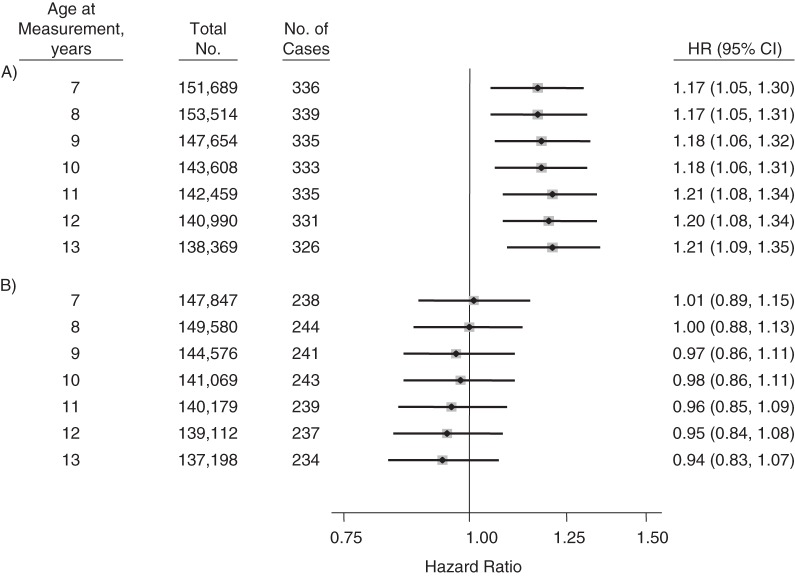
Height (per 1-SDS increase) was positively associated with glioma risk in men at each age of measurement (Figure 2A). For measurements taken at age 7 years, the hazard ratio for glioma per 1-SDS increase in height was 1.17 (95% confidence interval (CI): 1.05, 1.30). The magnitude of the associations slightly increased with age at measurement for boys. By age 13 years, the hazard ratio per 1-SDS increase was 1.21 (95% CI: 1.09, 1.35). A difference of 1 SDS corresponded to approximately 5.1 cm in boys at age 7 years and 7.6 cm in boys at age 13 years. The larger SDS at age 13 years as compared with age 7 years reflects greater variation in height at this age. The associations became stronger after we restricted the outcome to glioblastoma; the hazard ratio per 1-SDS increase in height was 1.23 (95% CI: 1.08, 1.42) at age 7 years and 1.28 (95% CI: 1.12, 1.47) at age 13 years (Table 2). No associations were observed for measured height at any age between 7 and 13 years and risk of glioma in women (Figure 2B). Statistically significant interactions by sex were observed for height measured at each age between 9 and 13 years (P for interaction < 0.05) but not measured at age 7 or 8 years (P values for interaction were 0.10 and 0.06, respectively).
Figure 2.
Hazard ratio (HR) for adult glioma per 1–standard deviation score difference in height at each age from 7 years to 13 years in men (A) and women (B), based on data from the Copenhagen School Health Records Register, Copenhagen, Denmark, 1968–2010. The models used attained age as the time metric, and data were stratified by birth cohort in 5-year intervals. Bars, 95% confidence intervals (CIs).
Table 2.
Hazard Ratiosa,b for Adult Glioblastoma Per 1–Standard Deviation Score Difference in Height and Body Mass Indexc at Each Age Between 7 and 13 Years, Based on Data From the Copenhagen School Health Records Register, Copenhagen, Denmark, 1968–2010
| Variable and Age at Measurement, years | Men |
Women |
||||||
|---|---|---|---|---|---|---|---|---|
| No. in Study Population | No. of Cases | HR | 95% CI | No. in Study Population | No. of Cases | HR | 95% CI | |
| Height | ||||||||
| 7 | 151,689 | 206 | 1.23 | 1.08, 1.42 | 147,847 | 125 | 0.88 | 0.74, 1.05 |
| 8 | 153,514 | 207 | 1.23 | 1.07, 1.42 | 149,580 | 130 | 0.88 | 0.74, 1.05 |
| 9 | 147,654 | 206 | 1.23 | 1.07, 1.41 | 144,576 | 131 | 0.85 | 0.72, 1.01 |
| 10 | 143,608 | 205 | 1.24 | 1.08, 1.42 | 141,069 | 133 | 0.87 | 0.73, 1.04 |
| 11 | 142,459 | 211 | 1.25 | 1.09, 1.43 | 140,179 | 130 | 0.89 | 0.75, 1.06 |
| 12 | 140,990 | 210 | 1.27 | 1.11, 1.46 | 139,112 | 131 | 0.87 | 0.73, 1.03 |
| 13 | 138,369 | 207 | 1.28 | 1.12, 1.47 | 137,198 | 131 | 0.87 | 0.73, 1.03 |
| Body mass index | ||||||||
| 7 | 151,667 | 206 | 1.01 | 0.86, 1.17 | 147,807 | 125 | 0.96 | 0.79, 1.16 |
| 8 | 153,501 | 207 | 1.04 | 0.89, 1.22 | 149,570 | 130 | 0.95 | 0.79, 1.16 |
| 9 | 147,644 | 206 | 1.03 | 0.88, 1.21 | 144,575 | 131 | 0.95 | 0.79, 1.16 |
| 10 | 143,601 | 205 | 1.02 | 0.87, 1.19 | 141,064 | 133 | 0.87 | 0.72, 1.06 |
| 11 | 142,453 | 211 | 1.02 | 0.87, 1.19 | 140,175 | 130 | 0.93 | 0.76, 1.13 |
| 12 | 140,986 | 210 | 1.00 | 0.86, 1.17 | 139,111 | 131 | 0.91 | 0.75, 1.10 |
| 13 | 138,363 | 207 | 1.04 | 0.89, 1.21 | 137,195 | 131 | 1.01 | 0.83, 1.22 |
Abbreviations: CI, confidence intervals; HR, hazard ratio.
a The models used attained age as the time metric, and data were stratified by birth cohort in 5-year intervals and sex.
b Height and body mass index were modeled as continuous variables (per 1–standard deviation score difference).
c Weight (kg)/height (m)2.
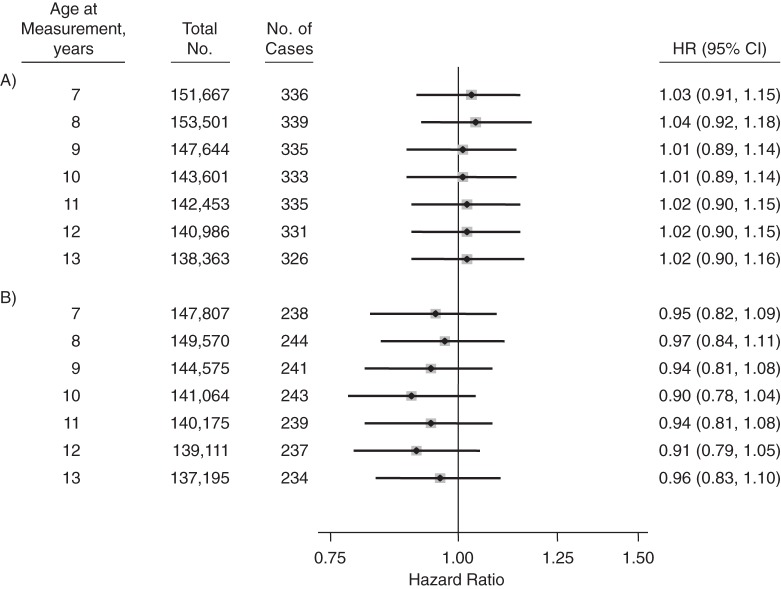
BMI (per 1-SDS increase) at each age of measurement was not associated with glioma risk in either men or women (Figure 3). Restriction of the outcome to glioblastoma yielded similar null results (Table 2). No statistically significant interactions were observed by sex.
Figure 3.
Hazard ratio (HR) for adult glioma per 1–standard deviation score difference in body mass index (weight (kg)/height (m)2) at each age from 7 years to 13 years in men (A) and women (B), based on data from the Copenhagen School Health Records Register, Copenhagen, Denmark, 1968–2010. The models used attained age as the time metric, and data were stratified by birth cohort in 5-year intervals. Bars, 95% confidence intervals (CIs).
Although follow-up information was not captured before 1968, we did not observe significant differences in the associations for height or BMI for persons with complete follow-up (year of birth ≥1950) versus persons with incomplete follow-up (year of birth <1950) (data not shown).
No clear associations were observed for change in height between ages 7 and 10 years, between ages 10 and 13 years, or between ages 7 and 13 years and glioma risk in either men or women (Appendix Table 2).
Positive associations between birth weight (continuous, per 0.5 kg (approximately 1 SDS)) and glioma risk (hazard ratio = 1.13, 95% CI: 1.04, 1.24) were observed in models stratified by birth cohort and including adjustment for sex (data not shown in tables). The magnitude of this association was stronger for men (hazard ratio = 1.19, 95% CI: 1.06, 1.33) than for women (hazard ratio = 1.05, 95% CI: 0.92, 1.21), though results were not statistically significantly different by sex (P for interaction = 0.20).
DISCUSSION
In this large prospective study of Copenhagen schoolchildren born between 1930 and 1989 with annual records of height and weight during childhood, we found that greater height at each age between 7 and 13 years was associated with an increased risk of adult glioma (particularly glioblastoma) in men but not in women. Greater BMI between ages 7 and 13 years was not associated with subsequent glioma risk. Higher birth weight was associated with an increased risk of adult glioma.
Our results, based on childhood measurements of height, are consistent with findings from a recent pooled analysis of case-control and prospective studies in which adult height was positively associated with risk of glioma in men (≥190 cm vs. 170–174 cm: odds ratio (OR) = 1.70, 95% CI: 1.11, 2.61; per 5-cm increase: OR = 1.05, 95% CI: 0.98, 1.12), particularly after the data were restricted to glioblastoma (per 5-cm increase, OR = 1.08, 95% CI: 1.00, 1.18), while the association for women was less clear (per 5-cm increase, glioma OR = 1.02 (95% CI: 0.94, 1.10) and glioblastoma OR = 1.04 (95% CI: 0.94, 1.16)) (16). However, when we compared the odds ratio for adult height in men from the pooled study with the hazard ratio observed for childhood height in boys from the current study, using approximately the same per-unit increase, the results from the current study were stronger. Although adult height may act as a proxy for childhood height and factors associated with growth (34), height and BMI in childhood are only moderately associated (correlations of 0.3–0.6) with adulthood measures (35, 36). Our findings may suggest that height in childhood is a better proxy for exposures important in the etiology of adult glioma than is adult height. However, our results, based on direct measurements of height, could reflect a lower degree of measurement error than in studies relying on self-reports. Differences in study design may be an alternative explanation, since case-control studies are more prone to differential recall and selection biases than are prospective studies.
Exposure to childhood infections may be one possible mechanism explaining the positive association between greater childhood height and adult glioma risk, since childhood infections have been associated with shorter stature in monozygotic twin pairs (25), as well as a reduced risk of adult glioma in some studies (6–9). The increased energy demand that occurs during infection in childhood could divert calories away from growth, and infections may also induce inflammatory responses that inhibit insulin-like growth factor 1 (IGF-1) and growth hormone production, which are important for long bone growth (37, 38). Additional studies are needed to evaluate the temporal nature of the relationship between infection, childhood growth, and glioma risk. However, other factors, including genetics and early-life hormonal and environmental exposures associated with infant, childhood, and adolescent growth (e.g., nutritional status, history of illness or psychosocial stress, maternal smoking) (17–24), could potentially underlie the relationship between height and glioma risk.
The reason for a stronger association between height (in either childhood or adulthood) and glioma risk in men versus women is not clear. As results from the previous pooled analysis suggested (16), the association between adult height and glioma risk may be J- or U-shaped as opposed to linear, with height most likely acting as a proxy for other exposures, some of which may have opposing influences on glioma risk. The positive association we observed in boys may reflect the upward trend in risk at the higher end of the range of adult height, whereas the slight inverse association observed in girls, who tend to be shorter and have a narrower range of height in adulthood, might reflect the downward trend at the lower end of the range of adult height. IGF-1 levels influence childhood and adolescent growth and have been associated with greater attained height, particularly in males (39). IGF-1 has also been shown to induce biological actions which favor tumor growth (40), and elevated IGF-1 levels have been associated with increased risk of several malignancies (e.g., breast, prostate, lung, colon) (41). However, both high and low levels of IGF-1 have been associated with glioma risk (42, 43). Alternatively, the association between height in girls and subsequent glioma risk may have been confounded or masked by exposure to estrogen or factors associated with pubertal development. Estrogen and other sex steroid hormones have been hypothesized to play a role in gliomagenesis because of the higher male:female ratio of glioma incidence, which is apparent starting in childhood and adolescence and increases with age (44, 45). This hypothesis could be tested in future studies by examining height measured earlier in childhood (i.e., prior to puberty-related growth) in relation to subsequent risk of adult glioma.
There is little evidence linking BMI in middle-to-older adulthood with risk of glioma in epidemiologic studies (10, 14, 26, 46, 47). However, some studies have shown that greater BMI in young adulthood is associated with glioma risk. In the National Institutes of Health-AARP (NIH-AARP) Diet and Health Study, a nearly 4-fold increased risk of adult glioma was observed among participants whose BMI at age 18 years was in the obese range (30–34.9) versus the normal-weight range (18.5–24.9) (10). However, these results may have been due to chance, considering the small number of glioma cases (n = 11) in the obese range, or they may have been biased because of inaccurate recall, since BMI at age 18 years was calculated from recalled values for weight and height when participants were between the ages of 52 and 71 years. Nonetheless, the authors also found an inverse association with greater physical activity at age 18 years that was independent of BMI, lending support to a possible role of energy balance. These findings were supported by results from a US-based case-control study of glioma, in which participants were asked to recall their height and usual weight a median of 33 years later, showing a positive albeit weaker association for BMI at age 21 years (26). Our study utilized measured childhood height and weight data, as opposed to recalled data, which reduced the potential for bias due to inaccurate recall.
Although birth weight has been linked with an increased risk of childhood brain tumors, specifically astrocytoma and medulloblastoma/primitive neuroectodermal tumors (48, 49), studies on birth weight and risk of adult glioma are lacking. A recent case-control study relying on recalled exposure information showed no association between birth weight and adult glioma risk (7). However, our observation of an increased risk with higher birth weight, based on reports made at or near the time of birth, should be followed up in future studies. Higher birth weight has been positively associated with circulating maternal levels of estrogen, IGF-1, and leptin (50), but to date there is scant evidence on the possible role of circulating maternal levels of these hormones and adult glioma risk.
The major strengths of this study include the prospective study design, the long length of follow-up, the nearly complete coverage of the study population during follow-up, and the availability of measured (as opposed to recalled) data on height and weight during childhood. Birth weight was either reported by parents or transcribed directly from health booklets given to parents at the child's birth, rather than recalled by the participants later in life. The impact of measurement error introduced by the use of parentally reported birth weights not aided by documentation is likely to have been small, as previous studies have shown very high agreement between hospital records and birth weights recalled by parents less than 15 years after the child's birth (51, 52). Although the study population included virtually all schoolchildren in the Municipality of Copenhagen born between 1930 and 1989, CSHRR records may be missing for years during which children moved to surrounding municipalities or elsewhere outside of Copenhagen. An uncertain number of the records may also have been lost, particularly in the years before the paper versions were made electronic, but there is no evidence of systematic losses. Additional limitations include the lower range of body weight values in this population at the ages at which they were measured, which may have limited our ability to detect associations for very high BMI levels. Nonetheless, this historical cohort is one of the only resources in which a prospective evaluation of this topic is feasible, given the sample size and length of follow-up required. As noted above, we were unable to directly compare the magnitudes of the associations for childhood height and BMI with those for corresponding adult measurements in this population, or to attempt to disentangle the independent associations of childhood measures versus adulthood measures with glioma risk.
We also lacked information on certain potentially confounding factors, specifically exposures in early life that are (or potentially are) associated with both childhood growth and adult glioma risk, including early-life nutritional status, childhood infections and allergies, and maternal smoking. Socioeconomic status, a possible proxy for greater access to medical care and likelihood of disease detection and diagnosis, is another potential confounder in this study. US-based studies have shown higher glioma incidence in areas with a higher socioeconomic position (53, 54), and greater height has been linked to higher socioeconomic status (17, 55). However, this explanation is unlikely, since Denmark has universal and free access to medical care.
In this prospective study, greater height measured at each age between 7 and 13 years was associated with an increased risk of adult glioma (particularly glioblastoma) in men but not in women, while no associations with childhood BMI were observed. Higher birth weight was also associated with an increased risk of this malignancy. These results support a potential role of early-life exposures associated with childhood height and birth size in the development of glioma in adulthood. Because epidemiologic studies have yet to consistently link exposures incurred during adulthood with subsequent risk of glioma, the results of this study add to the existing evidence implicating the prenatal and childhood periods as etiologically relevant for adult glioma.
ACKNOWLEDGMENTS
Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, Maryland (Cari M. Kitahara, Preetha Rajaraman); Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospitals, The Capital Region, Copenhagen, Denmark (Michael Gamborg, Thorkild I. A. Sørensen, Jennifer L. Baker); and Novo Nordisk Foundation Center for Basic Metabolic Research, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (Thorkild I. A. Sørensen, Jennifer L. Baker).
This research was supported in part by the Intramural Research Program of the National Cancer Institute, US National Institutes of Health, and by funding from the European Research Council (ERC) under the European Union's Seventh Framework Programme (2007–2013) (ERC Grant Agreement 281418 awarded to J.L.B.).
Conflict of interest: none declared.
Appendix Table 1.
Morphology Codes Used to Define Histological Subtypes of Glioma, Copenhagen, Denmark, 1968–2010
| Subtype and Classification | Code(s) |
|---|---|
| Glioblastoma | |
| ICD-O-3 | 9440–9442 |
| ICD-7 | 093.3 |
| Astrocytoma other than glioblastoma | |
| ICD-O-3 | 9381, 9384, 9400–9401, 9410–9411, 9420–9421, and 9424 |
| ICD-7 | 093.4 |
| Oligodendroglioma | |
| ICD-O-3 | 9450–9451, 9460, and 9382 |
| ICD-7 | 093.5 |
| Ependymoma | |
| ICD-O-3 | 9383 and 9391–9394 |
| ICD-7 | 093.6 |
| Medulloblastoma | |
| ICD-O-3 | 9470–9472 and 9474 |
| ICD-7 | 093.7 |
Abbreviations: ICD-7, International Classification of Diseases, Seventh Revision; ICD-O-3, International Classification of Diseases for Oncology, Third Edition.
Appendix Table 2.
Hazard Ratio for Adult Glioma per 1–Standard Deviation Score Change in Height Between Ages 7 and 13 Years, Copenhagen, Denmark, 1968–2010a
| Ages at Measurement, years | Men |
Women |
||||
|---|---|---|---|---|---|---|
| No. of Cases | HR | 95% CI | No. of Cases | HR | 95% CI | |
| 7–10 | 323 | 1.12 | 0.75, 1.66 | 233 | 0.98 | 0.66, 1.46 |
| 10–13 | 315 | 1.15 | 0.87, 1.52 | 231 | 0.81 | 0.59, 1.13 |
| 7–13 | 309 | 1.15 | 0.91, 1.44 | 223 | 0.86 | 0.66, 1.11 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
a All analyses were stratified by birth cohort and adjusted for height at the beginning of the period.
REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Efird JT. Season of birth and risk for adult onset glioma. Int J Environ Res Public Health. 2010;7(5):1913–1936. doi: 10.3390/ijerph7051913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenner AV, Linet MS, Shapiro WR, et al. Season of birth and risk of brain tumors in adults. Neurology. 2004;63(2):276–281. doi: 10.1212/01.wnl.0000129984.01327.9d. [DOI] [PubMed] [Google Scholar]
- 4.Chen C, Xu T, Chen J, et al. Allergy and risk of glioma: a meta-analysis. Eur J Neurol. 2011;18(3):387–395. doi: 10.1111/j.1468-1331.2010.03187.x. [DOI] [PubMed] [Google Scholar]
- 5.Wiemels JL, Wiencke JK, Patoka J, et al. Reduced immunoglobulin E and allergy among adults with glioma compared with controls. Cancer Res. 2004;64(22):8468–8473. doi: 10.1158/0008-5472.CAN-04-1706. [DOI] [PubMed] [Google Scholar]
- 6.Wrensch M, Lee M, Miike R, et al. Familial and personal medical history of cancer and nervous system conditions among adults with glioma and controls. Am J Epidemiol. 1997;145(7):581–593. doi: 10.1093/oxfordjournals.aje.a009154. [DOI] [PubMed] [Google Scholar]
- 7.Wrensch M, Weinberg A, Wiencke J, et al. History of chickenpox and shingles and prevalence of antibodies to varicella-zoster virus and three other herpesviruses among adults with glioma and controls. Am J Epidemiol. 2005;161(10):929–938. doi: 10.1093/aje/kwi119. [DOI] [PubMed] [Google Scholar]
- 8.Amirian E, Scheurer ME, Bondy ML. The association between birth order, sibship size and glioma development in adulthood. Int J Cancer. 2010;126(11):2752–2756. doi: 10.1002/ijc.24962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anic GM, Madden MH, Sincich K, et al. Early life exposures and the risk of adult glioma. Eur J Epidemiol. 2013;28(9):753–758. doi: 10.1007/s10654-013-9811-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore SC, Rajaraman P, Dubrow R, et al. Height, body mass index, and physical activity in relation to glioma risk. Cancer Res. 2009;69(21):8349–8355. doi: 10.1158/0008-5472.CAN-09-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inskip PD, Tarone RE, Brenner AV, et al. Handedness and risk of brain tumors in adults. Cancer Epidemiol Biomarkers Prev. 2003;12(3):223–225. [PubMed] [Google Scholar]
- 12.Braganza MZ, Kitahara CM, Berrington de González A, et al. Ionizing radiation and the risk of brain and central nervous system tumors: a systematic review. Neuro Oncol. 2012;14(11):1316–1324. doi: 10.1093/neuonc/nos208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helseth A, Tretli S. Pre-morbid height and weight as risk factors for development of central nervous system neoplasms. Neuroepidemiology. 1989;8(6):277–282. doi: 10.1159/000110195. [DOI] [PubMed] [Google Scholar]
- 14.Benson VS, Pirie K, Green J, et al. Lifestyle factors and primary glioma and meningioma tumours in the Million Women Study cohort. Br J Cancer. 2008;99(1):185–190. doi: 10.1038/sj.bjc.6604445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kabat GC, Anderson ML, Heo M, et al. Adult stature and risk of cancer at different anatomic sites in a cohort of postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2013;22(8):1353–1363. doi: 10.1158/1055-9965.EPI-13-0305. [DOI] [PubMed] [Google Scholar]
- 16.Kitahara CM, Wang SS, Melin BS, et al. Association between adult height, genetic susceptibility and risk of glioma. Int J Epidemiol. 2012;41(4):1075–1085. doi: 10.1093/ije/dys114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batty GD, Shipley MJ, Gunnell D, et al. Height, wealth, and health: an overview with new data from three longitudinal studies. Econ Hum Biol. 2009;7(2):137–152. doi: 10.1016/j.ehb.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Lango Allen H, Estrada K, Lettre G, et al. Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature. 2010;467(7317):832–838. doi: 10.1038/nature09410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berndt SI, Gustafsson S, Mägi R, et al. Genome-wide meta-analysis identifies 11 new loci for anthropometric traits and provides insights into genetic architecture. Nat Genet. 2013;45(5):501–512. doi: 10.1038/ng.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cousminer DL, Berry DJ, Timpson NJ, et al. Genome-wide association and longitudinal analyses reveal genetic loci linking pubertal height growth, pubertal timing and childhood adiposity. Hum Mol Genet. 2013;22(13):2735–2747. doi: 10.1093/hmg/ddt104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gunnell D, Okasha M, Smith GD, et al. Height, leg length, and cancer risk: a systematic review. Epidemiol Rev. 2001;23(2):313–342. doi: 10.1093/oxfordjournals.epirev.a000809. [DOI] [PubMed] [Google Scholar]
- 22.Schroeder DG, Martorell R, Rivera JA, et al. Age differences in the impact of nutritional supplementation on growth. J Nutr. 1995;125(4 suppl):1051S–1059S. doi: 10.1093/jn/125.suppl_4.1051S. [DOI] [PubMed] [Google Scholar]
- 23.Kusin J, Kardjati S, Houtkooper J, et al. Energy supplementation during pregnancy and postnatal growth. Lancet. 1992;340(8820):623–626. doi: 10.1016/0140-6736(92)92168-f. [DOI] [PubMed] [Google Scholar]
- 24.Butler NR, Goldstein H. Smoking in pregnancy and subsequent child development. Br Med J. 1973;4(5892):573–575. doi: 10.1136/bmj.4.5892.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hwang AE, Mack TM, Hamilton AS, et al. Childhood infections and adult height in monozygotic twin pairs. Am J Epidemiol. 2013;178(4):551–558. doi: 10.1093/aje/kwt012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Little RB, Madden MH, Thompson RC, et al. Anthropometric factors in relation to risk of glioma. Cancer Causes Control. 2013;24(5):1025–1031. doi: 10.1007/s10552-013-0178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baker JL, Olsen LW, Andersen I, et al. Cohort profile: the Copenhagen School Health Records Register. Int J Epidemiol. 2009;38(3):656–662. doi: 10.1093/ije/dyn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39(7 suppl):22–25. doi: 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- 29.Ostrom QT, Gittleman H, Farah P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013;15(suppl 2):ii1–ii56. doi: 10.1093/neuonc/not151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gjerstorff ML. The Danish Cancer Registry. Scand J Public Health. 2011;39(7 suppl):42–45. doi: 10.1177/1403494810393562. [DOI] [PubMed] [Google Scholar]
- 31.World Health Organization. International Statistical Classification of Diseases and Related Health Problems, Tenth Revision. 2010 ed . Geneva, Switzerland: World Health Organization; 2011. [PubMed] [Google Scholar]
- 32.Danish National Board of Health. Cancer Incidence in Denmark 2001. Copenhagen, Denmark: Statistics Denmark; 2006. [Google Scholar]
- 33.Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med. 1992;11(10):1305–1319. doi: 10.1002/sim.4780111005. [DOI] [PubMed] [Google Scholar]
- 34.Wei EK, Wolin KY, Colditz GA. Time course of risk factors in cancer etiology and progression. J Clin Oncol. 2010;28(26):4052–4057. doi: 10.1200/JCO.2009.26.9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Power C, Lake JL, Cole TJ. Body mass index and height from childhood to adulthood in the 1958 British born cohort. Am J Clin Nutr. 1997;66(5):1094–1101. doi: 10.1093/ajcn/66.5.1094. [DOI] [PubMed] [Google Scholar]
- 36.Macfarlane GJ, de Silva V, Jones GT. The relationship between body mass index across the life course and knee pain in adulthood: results from the 1958 birth cohort study. Rheumatology (Oxford) 2011;50(12):2251–2256. doi: 10.1093/rheumatology/ker276. [DOI] [PubMed] [Google Scholar]
- 37.McDade TW. Life history, maintenance, and the early origins of immune function. Am J Hum Biol. 2005;17(1):81–94. doi: 10.1002/ajhb.20095. [DOI] [PubMed] [Google Scholar]
- 38.Ahmed SF, Sävendahl L. Promoting growth in chronic inflammatory disease: lessons from studies of the growth plate. Horm Res. 2009;72(suppl 1):42–47. doi: 10.1159/000229763. [DOI] [PubMed] [Google Scholar]
- 39.Crowe FL, Key TJ, Allen NE, et al. A cross-sectional analysis of the associations between adult height, BMI and serum concentrations of IGF-I and IGFBP-1 -2 and -3 in the European Prospective Investigation Into Cancer and Nutrition (EPIC) Ann Hum Biol. 2011;38(2):194–202. doi: 10.3109/03014460.2010.507221. [DOI] [PubMed] [Google Scholar]
- 40.Roberts DL, Dive C, Renehan AG. Biological mechanisms linking obesity and cancer risk: new perspectives. Annu Rev Med. 2010;61:301–316. doi: 10.1146/annurev.med.080708.082713. [DOI] [PubMed] [Google Scholar]
- 41.Jerome L, Shiry L, Leyland-Jones B. Deregulation of the IGF axis in cancer: epidemiological evidence and potential therapeutic interventions. Endocr Relat Cancer. 2003;10(4):561–578. doi: 10.1677/erc.0.0100561. [DOI] [PubMed] [Google Scholar]
- 42.Rohrmann S, Linseisen J, Becker S, et al. Concentrations of IGF-I and IGFBP-3 and brain tumor risk in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev. 2011;20(10):2174–2182. doi: 10.1158/1055-9965.EPI-11-0179. [DOI] [PubMed] [Google Scholar]
- 43.Lönn S, Inskip PD, Pollak MN, et al. Glioma risk in relation to serum levels of insulin-like growth factors. Cancer Epidemiol Biomarkers Prev. 2007;16(4):844–846. doi: 10.1158/1055-9965.EPI-06-1010. [DOI] [PubMed] [Google Scholar]
- 44.Huang K, Whelan EA, Ruder AM, et al. Reproductive factors and risk of glioma in women. Cancer Epidemiol Biomarkers Prev. 2004;13(10):1583–1588. [PubMed] [Google Scholar]
- 45.Inskip PD, Linet MS, Heineman EF. Etiology of brain tumors in adults. Epidemiol Rev. 1995;17(2):382–414. doi: 10.1093/oxfordjournals.epirev.a036200. [DOI] [PubMed] [Google Scholar]
- 46.Wiedmann M, Brunborg C, Lindemann K, et al. Body mass index and the risk of meningioma, glioma and schwannoma in a large prospective cohort study (The HUNT Study) Br J Cancer. 2013;109(1):289–294. doi: 10.1038/bjc.2013.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Michaud DS, Bové G, Gallo V, et al. Anthropometric measures, physical activity, and risk of glioma and meningioma in a large prospective cohort study. Cancer Prev Res (Phila) 2011;4(9):1385–1392. doi: 10.1158/1940-6207.CAPR-11-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oksuzyan S, Crespi CM, Cockburn M, et al. Birth weight and other perinatal factors and childhood CNS tumors: a case-control study in California. Cancer Epidemiol. 2013;37(4):402–409. doi: 10.1016/j.canep.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harder T, Plagemann A, Harder A. Birth weight and subsequent risk of childhood primary brain tumors: a meta-analysis. Am J Epidemiol. 2008;168(4):366–373. doi: 10.1093/aje/kwn144. [DOI] [PubMed] [Google Scholar]
- 50.Troisi R, Potischman N, Hoover RN. Exploring the underlying hormonal mechanisms of prenatal risk factors for breast cancer: a review and commentary. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1700–1712. doi: 10.1158/1055-9965.EPI-07-0073. [DOI] [PubMed] [Google Scholar]
- 51.Tate AR, Dezateux C, Cole TJ, et al. Factors affecting a mother's recall of her baby's birth weight. Int J Epidemiol. 2005;34(3):688–695. doi: 10.1093/ije/dyi029. [DOI] [PubMed] [Google Scholar]
- 52.Adegboye AR, Heitmann B. Accuracy and correlates of maternal recall of birthweight and gestational age. BJOG. 2008;115(7):886–893. doi: 10.1111/j.1471-0528.2008.01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Plascak JJ, Fisher JL. Area-based socioeconomic position and adult glioma: a hierarchical analysis of Surveillance Epidemiology and End Results data. PLOS ONE. 2013;8(4):e60910. doi: 10.1371/journal.pone.0060910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Preston-Martin S. Descriptive epidemiology of primary tumors of the brain, cranial nerves and cranial meninges in Los Angeles County. Neuroepidemiology. 1989;8(6):283–295. doi: 10.1159/000110196. [DOI] [PubMed] [Google Scholar]
- 55.Subramanian SV, Özaltin E, Finlay JE. Height of nations: a socioeconomic analysis of cohort differences and patterns among women in 54 low- to middle-income countries. PLOS ONE. 2011;6(4):e18962. doi: 10.1371/journal.pone.0018962. [DOI] [PMC free article] [PubMed] [Google Scholar]