Abstract
Antipsychotic drugs are used to treat dementia-related symptoms in older adults, and observational studies show higher risks of death and stroke associated with the use of first-generation antipsychotic drugs (FGAs) compared with second-generation antipsychotic drugs (SGAs). However, the extent to which stroke explains the differential mortality risk between FGA use and SGA use in older adults is unclear. We followed those who initiated use of antipsychotic drugs (9,777 FGA users and 21,164 SGA users) aged 65 years or older, and who were enrolled in Medicare and either the New Jersey or Pennsylvania pharmacy assistance program during 1994 to 2005, over 180 days for the outcomes of stroke and death. We estimated direct and indirect effects by comparing 180-day mortality risks associated with the use of FGAs versus SGAs as mediated by stroke on the risk ratio scale, as well as the proportion mediated on the risk difference scale. FGA use was associated with marginally higher risks of stroke (risk ratio =1.24, 95% confidence interval (CI): 1.01, 1.53) and death (risk ratio = 1.15, 95% CI: 1.08, 1.22) compared with SGA use, but stroke explained little (2.7%) of the observed difference in mortality risk. The indirect effect was null (risk ratio = 1.004, 95% CI: 1.000, 1.008), and the direct effect was equal to the total effect of antipsychotic drug type (FGA vs. SGA) on mortality risk (risk ratio = 1.15, 95% CI: 1.08, 1.22). These results suggest that the difference in mortality risk between users of FGAs and SGAs may develop mostly through pathways that do not involve stroke.
Keywords: aged, antipsychotic drugs, cerebrovascular disease, death, mediation analysis, mortality risk, pharmacoepidemiology, stroke
Older adults with dementia are often prescribed antipsychotic drugs “off label” to treat behavioral and psychiatric symptoms, such as hallucinations, agitation, and aggression (1). Although short-term use of antipsychotic drugs in older adults can modestly improve symptoms of agitation and aggression (2), it can also pose significant health risks, including higher risk of death.
Randomized trials have demonstrated that second-generation antipsychotic drugs (SGAs) are associated with a 1.6- to 1.7-fold increase in risk of death compared with placebo (3, 4), and several retrospective cohort studies show even higher mortality risk associated with first-generation antipsychotic drugs (FGAs) (5–8). In turn, regulatory agencies issued warnings to communicate the higher mortality risk associated with the use of both FGAs and SGAs (3, 9). Elucidating the mechanisms by which FGAs produce greater mortality risk than SGAs could help reduce the overall risk of death associated with the use of antipsychotic drugs. Nevertheless, after nearly a decade of research, we still do not know which medical events are responsible for the mortality risk difference between FGAs and SGAs.
One plausible contributor to the mortality risk difference is antipsychotic drug–induced stroke. In 2002 and 2004, results from placebo-controlled randomized trials raised concerns that the SGAs risperidone and olanzapine were associated with higher risk of cerebrovascular events (10, 11). Antipsychotic medications may affect neurotransmitter systems in ways that may increase the risk of stroke, including orthostatic hypotension, anticholinergic effects, and impaired mobility from extrapyramidal symptoms. These side effects develop soon after initiation and occur more frequently with the use of FGAs than with SGAs (12).
Evidence from some (13–19), but not all (20–24), observational studies showed higher risk of stroke associated with use of FGAs compared with SGAs. These results suggest that differences in stroke risk between FGAs and SGAs may exist, but they cannot tell us the extent to which this relationship explains the mortality risk difference, nor what the mortality risk difference would be if we could prevent strokes after starting antipsychotic therapy. In this paper, we confirm the higher stroke and mortality risks for FGA users versus SGA users and then apply recent advances in mediation analysis to examine these questions directly using longitudinal claims data on older US Medicare beneficiaries.
METHODS
Mediation analysis framework
We used the causal mediation framework to decompose the total effect of antipsychotic drug type on mortality risk into the natural direct effect and the natural indirect effect as mediated by stroke (25, 26) (Figure 1). On the risk-difference scale, the ratio of the natural indirect effect to the total effect equals the proportion of the total effect mediated by stroke. We also estimated a controlled direct effect to address the policy-relevant question of what the effect of antipsychotic drug type on mortality risk would be if we were able to prevent stroke from occurring after antipsychotic drug initiation, perhaps through prophylactic monitoring or intervention.
Figure 1.
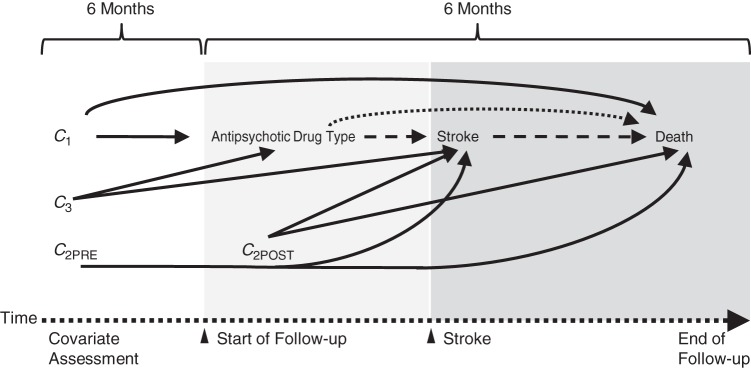
Illustrative directed acyclic graph showing mediation of the effect of antipsychotic drug type (first-generation vs. second-generation) on death from stroke over 180 days of follow-up. C1, mortality risk factors associated with antipsychotic drug type initiation (assessed before start of follow-up); C2PRE: dual risk factors for death and stroke (assessed before start of follow-up); C2POST, dual risk factors for death and stroke (assessed after start of follow-up); C3, stroke risk factors associated with antipsychotic drug type initiation (assessed before start of follow-up). The dotted arrow represents the direct effect; the dashed arrow represents the indirect effect; and the solid arrows represent sources of noncausal association from confounding by C1, C2, and C3.
Data source
Prescription dispensing records were obtained for patients enrolled in the New Jersey Pharmacy Assistance for the Aged and Disabled or the Pennsylvania Pharmaceutical Assistance Contract for the Elderly programs from January 1, 1994, to December 31, 2005. These programs accept individuals earning up to twice the federal poverty level, have low copays, and offer relatively comprehensive drug coverage with open formularies (27, 28).
Data on prescription drug use were extracted from pharmacy records and linked via personal identifiers to Medicare claims data containing information on inpatient and outpatient diagnoses, related procedures, dates of service, short-term rehabilitative stays, and death records from the Social Security Administration Death Master File (http://www.ssdmf.com). Institutional review board approval was granted by Brigham and Women's Hospital (Boston, Massachusetts). This project was covered under a signed data use agreement with the Centers for Medicare and Medicaid Services (Woodlawn, Maryland).
Population and study design
We assembled a retrospective cohort of persons aged 65 years or older who filled a new prescription for an oral antipsychotic drug (listed in Web Appendix 1, available at http://aje.oxfordjournals.org/) and who had no antipsychotic drug dispensing in the preceding 180 days. We retained individuals who, in the 180 days prior to this index date, were continuously enrolled in Medicare and either New Jersey Pharmacy Assistance for the Aged and Disabled or Pennsylvania Pharmaceutical Assistance Contract for the Elderly and who had at least 1 health service encounter or prescription fill. We excluded persons who had at least 2 health care encounters for diagnoses for cancer or other psychiatric or neurological conditions (based on International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes) or whose initial antipsychotic drug dispensing suggested schizophrenia treatment or pro re nata use (Web Appendix 2). Follow-up began on the first dispensing date and lasted for 180 days or until death. We also formed a propensity-matched subcohort for descriptive and confirmatory analyses (Web Appendices 3 and 4).
Stroke and death during follow-up
We assessed strokes occurring between the index prescription date (inclusive) and the end of follow up (180 days) or death. Using the ICD-9-CM, we classified individuals as having a stroke if any of the following codes were present as the primary diagnosis on an inpatient Medicare claim during follow-up: intracranial hemorrhage (code 430.xx, 431.xx, or 432.xx; positive predictive value ≥ 80%) or ischemic stroke (code 433.x1 or 434.x1; positive predictive value ≥ 70%) (29). Vital status and dates of death were obtained from the Social Security Death Master File.
Patient characteristics
Patient characteristics included demographic factors, health service and medication use, medical and psychiatric illnesses, and indicators of frailty; these were defined as present when a related ICD-9-CM code appeared in the 180 days preceding the index date. We also assessed stroke risk factors after antipsychotic drug initiation until the occurrence of stroke, death, or the end of follow-up (Web Appendices 5 and 6).
Statistical analysis
We calculated the baseline prevalence of each covariate and the prevalence difference by comparing FGA users with SGA users (Web Table 1). For each antipsychotic drug group, we calculated the difference in covariate prevalence by comparing patients who developed stroke during follow-up with those who did not (Web Table 2). We estimated crude and adjusted risk ratios (using the propensity-matched subcohort) for the effect of antipsychotic drug type on stroke and for the effect of antipsychotic drug type on mortality risk over 14, 30, 60, 120, and 180 days of follow-up. To estimate direct and indirect effects on the risk ratio scale and the proportion mediated on the risk difference scale (30), we combined parameters from a logistic regression model for stroke conditional on antipsychotic drug type and baseline patient characteristics and a Poisson regression model for death conditional on these covariates and stroke allowing for exposure-mediator interaction (26). We used 500 bootstrap samples and the percentile method to produce 95% confidence intervals. These analyses were repeated at 30, 60, 120, and 180 days of follow-up and for subgroups defined by (treated) dementia, cerebrovascular disease, and duration of use. We repeated the mediation analysis for drug-specific comparisons, examined ischemic and hemorrhagic stroke separately as mediators, and further adjusted for calendar year (Web Appendices 7–9). We performed sensitivity analyses for a binary unmeasured confounder of the stroke-death relationship and for various ICD-9-CM–based definitions of stroke (Web Appendices 10 and 11).
RESULTS
Patients
We identified 9,777 FGA and 21,164 SGA initiators who were aged 65 years or older and who received at least 30 days’ drug supply at the start of follow-up (Web Figure 1). The full cohort was mostly female (80.6%) and older (mean = 82.0 years), and approximately one-third or more had medical claims related to cardiovascular disorders and dementia; they used, on average 7.1 different medications (Web Table 1). FGA users appeared to be healthier than SGA users in terms of chronic disease and psychiatric diagnoses in the full cohort, but they were similar in the propensity score–matched cohort (Web Table 2; Web Figure 2). Most of the cohort discontinued use of antipsychotic medications during the course of follow-up (79.5% of FGA users and 70.7% of SGA users).
Differential risk of stroke and death between FGA and SGA users
The crude mortality risk was high for both FGA users (14.8%) and SGA users (13.1%) over the 180 days of follow-up (Table 1), whereas the risk of stroke was low for both groups (1.41% and 1.67%, respectively). The excess mortality risk comparing those who experienced stroke (n =462) during follow-up with those who did not was 23.7% and was similar for both groups. FGA users were more likely to experience a stroke during 180 days of follow-up after adjustment for baseline covariates (for undifferentiated stroke, odds ratio = 1.24, 95% confidence interval (CI): 1.01, 1.53; for ischemic stroke, odds ratio = 1.25, 95% CI: 0.99, 1.58; for hemorrhagic stroke, odds ratio = 1.17, 95% CI: 0.72, 1.89), as well as death from any cause (risk ratio = 1.15, 95% CI: 1.08, 1.22).
Table 1.
Risk of Stroke and Death Over 180 Days of Follow-up Among Adults Aged 65 Years or Older With Dual Enrollment in Medicare and Pharmacy Assistance Programs in New Jersey or Pennsylvania, 1994–2005
| Outcome and Length of Follow-up, days | Full Cohort |
Matched Cohort (1:1) |
||||
|---|---|---|---|---|---|---|
| SGA (n = 21,164) |
FGA (n = 9,777) |
FGA vs. SGA (n = 8,892 Pairs) |
||||
| No. of Events | Risk, % | No. of Events | Risk, % | Risk Ratioa | 95% CI | |
| Stroke | ||||||
| 14 | 36 | 0.17 | 23 | 0.24 | 1.13 | 0.57, 2.20 |
| 30 | 80 | 0.38 | 54 | 0.55 | 1.31 | 0.85, 2.04 |
| 60 | 131 | 0.62 | 91 | 0.93 | 1.52 | 1.07, 2.15 |
| 120 | 220 | 1.04 | 135 | 1.38 | 1.44 | 1.09, 1.89 |
| 180 | 299 | 1.41 | 163 | 1.67 | 1.30b | 1.02, 1.65 |
| Death | ||||||
| 14 | 213 | 1.01 | 140 | 1.43 | 1.22 | 0.93, 1.59 |
| 30 | 501 | 2.37 | 290 | 2.97 | 1.20 | 1.00, 1.44 |
| 60 | 1,004 | 4.74 | 570 | 5.83 | 1.20 | 1.06, 1.37 |
| 120 | 1,931 | 9.12 | 1,059 | 10.83 | 1.19 | 1.08, 1.30 |
| 180 | 2,764 | 13.06 | 1,442 | 14.80 | 1.12c | 1.04, 1.20 |
Abbreviations: CI, confidence interval; FGA, first-generation antipsychotic drug; SGA, second-generation antipsychotic drug.
a Estimated in the FGA versus SGA 1:1 propensity score–matched cohort unless otherwise noted (see Web Appendices 3 and 4 for details). The propensity model score included demographic characteristics, health services usage, comorbid chronic and psychiatric conditions, and concomitant medication use occurring before the index date, as listed in Web Table 1.
b The corresponding risk ratio for stroke over 180 days of follow-up in the full cohort, estimated (as an odds ratio) via logistic regression with the same covariates used in the propensity score model, was 1.24 (95% CI: 1.01, 1.53).
c The corresponding risk ratio for death over 180 days of follow-up in the full cohort, estimated via Poisson regression with the same covariates used in the propensity score model was 1.15 (95% CI: 1.08, 1.22). Nonparametric bootstrapping (n = 500 samples) was used to obtain the 95% confidence interval.
Stroke as a potential mediator
Over the course of follow-up, the controlled direct effect and total effect of antipsychotic drug type on mortality risk were the same (risk ratio = 1.15, 95% CI: 1.08, 1.22) (Table 2). Although the natural indirect effect did not change appreciably over the study period (risk ratio = 1.004, 95% CI: 1.000, 1.008), the proportion mediated increased slightly to 2.70% by 180 days of follow-up, due mostly to the declining total effect. The results were similar for patients with or without history of cerebrovascular disease, (treated) dementia, or recent nursing home stays (Web Tables 3 and 4). We observed similar results when comparing haloperidol users with olanzapine, risperidone, and quetiapine users, when evaluating ischemic and hemorrhagic strokes separately as mediators, and when further adjusting for potential confounders assessed after antipsychotic drug initiation, including statin and anticoagulant use (Web Table 5). An attenuated indirect effect and an enlarged total effect (risk ratios ranging from 1.28 to 1.41) but similar and nonsignificant stroke effect were observed after stratifying by duration of use (Web Table 6). When we used alternate ICD-9-CM–based definitions, the proportion mediated was as high as 6.7% (Web Table 7).
Table 2.
Direct and Indirect Effects of Exposure (to FGAs vs. SGAs) on Mortality Risk, Mediated by Stroke, Over 180 Days of Follow-up Among Adults Aged 65 Years or Older With Dual Enrollment in Medicare and Pharmacy Assistance Programs in New Jersey or Pennsylvania, 1994–2005
| Analysis | Controlled Direct Effect |
Natural Direct Effect |
Natural Indirect Effect |
Total Effect |
Proportion Mediated, % |
||||
|---|---|---|---|---|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | ||
| Unadjusteda | 1.13 | 1.08, 1.21 | 1.13 | 1.07, 1.20 | 1.004 | 0.999, 1.009 | 1.13 | 1.07, 1.20 | 3.27 |
| Multivariable adjustedb |
|||||||||
| Days since initiation | |||||||||
| 14 | 1.43 | 1.11, 1.81 | 1.42 | 1.10, 1.80 | 1.000 | 1.000, 1.000 | 1.42 | 1.10, 1.80 | −0.01 |
| 30 | 1.25 | 1.06, 1.47 | 1.25 | 1.06, 1.47 | 1.001 | 1.000, 1.003 | 1.25 | 1.07, 1.47 | 0.38 |
| 60 | 1.22 | 1.09, 1.36 | 1.22 | 1.10, 1.36 | 1.005 | 1.001, 1.012c | 1.23 | 1.11, 1.36 | 2.73 |
| 120 | 1.21 | 1.12, 1.31 | 1.21 | 1.12, 1.30 | 1.004 | 1.001, 1.012c | 1.21 | 1.13, 1.31 | 2.34 |
| 180 | 1.15 | 1.08, 1.22 | 1.15 | 1.08, 1.22 | 1.004 | 1.000, 1.008c | 1.15 | 1.08, 1.22 | 2.70 |
| Stroke subtypea | |||||||||
| Ischemic | 1.15 | 1.08, 1.22 | 1.17 | 1.08, 1.22 | 1.003 | 1.000, 1.006 | 1.15 | 1.08, 1.22 | 2.02 |
| Hemorrhagic | 1.15 | 1.08, 1.22 | 1.15 | 1.08, 1.22 | 1.000 | 0.999, 1.002 | 1.15 | 1.08, 1.22 | 0.22 |
Abbreviations: CI, confidence interval; FGA, first-generation antipsychotic drug; RR, risk ratio; SGA, second-generation antipsychotic drug.
a All analyses were conducted over 180 days since initiation unless noted otherwise.
b Multivariable analyses adjusted for demographic characteristics, health services usage, comorbid chronic and psychiatric conditions, and concomitant medication use occurring before the index date, as listed in Web Table 1.
c The lower confidence bound for indirect effect is above the null.
DISCUSSION
Although our study found higher risks of stroke and death over 180 days for FGA users, only a small portion (2.70%) of the effect of antipsychotic drug type on mortality risk was explained by stroke. Stroke prevention efforts would likely decrease the 180-day mortality risk in this population, but our results suggest that the mortality risk gap between FGA users and SGA users would persist.
Our finding of higher risks of stroke and death for FGA users compared with SGA users is consistent with much of the previous literature. Prior studies showed that the relative risk of death was highest in the first month after antipsychotic drug initiation (hazard ratio = 1.55); it decreased over time, but remained elevated at 180 days (hazard ratios ranging from 1.27 to 1.37) (6) and was unlikely to be explained by unmeasured confounding (31).
With regard to stroke, only some studies of initiators found higher risks for FGA users compared with SGA users. Three cohort studies showed higher risk of cerebrovascular events for FGA users than SGA users in the first 180 days of follow-up (hazard ratios ranging from 1.20 to 2.34) (16, 17, 19). In contrast, another 3 cohort studies (20, 22, 32) found no difference in risk, but this discrepancy may be attributable to differences in study design (33), longer follow-up, or artificial (and potentially informative) censoring of patients who switched or discontinued their medications.
Despite evidence for modest effects of antipsychotic drug type on risks of both death and stroke, these 2 effects were only slightly related. Only 2.7% of the difference in mortality risk by antipsychotic drug type could be explained by differences in stroke onset. One potential explanation is measurement error in the ICD-9-CM–based algorithm used to identify stroke, which, if nondifferential, would tend to bias the indirect effect toward the null (34). The use of less restrictive algorithms to improve sensitivity increased the percent mediated from 2.7% to 6.7%.
Medicare insurance claims databases do not contain information on important behavioral cardiovascular risk factors. The sensitivity analyses for a binary unmeasured confounder (35) showed that, although strong unmeasured confounding of the stroke-death relationship could fully explain the observed indirect effect, downward bias from protective risk factors for stroke would be minimal (Web Figure 3). Adjustment for stroke risk factors assessed during follow-up and stratification by the duration of use yielded results that were similar to those of the main analyses (Web Tables 4 and 5). The mediation analysis framework we used requires that no stroke-death confounders be affected by antipsychotic drug type. In sensitivity analyses for violations of this condition (36), the small indirect effect we observed would be subject to such violations, but the large direct effect would be robust; our qualitative conclusions would not change.
Our study has a number of strengths. We approximated a new user design, which is suited for the evaluation of short-term drug effects (37), and the temporal ordering of covariate assessment, antipsychotic drug initiation, stroke occurrence, and death was clear. Mediation analyses typically suffer from unmeasured mediator-outcome confounders; we adjusted for a rich set of patient characteristics. The many sensitivity analyses support our overall conclusion that stroke does not explain much of the observed mortality risk difference between FGA users and SGA users.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Division of Pharmacoepidemiology, Brigham and Women's Hospital, Boston, Massachusetts (John W. Jackson, Sebastian Schneeweiss); Department of Epidemiology, Harvard School of Public Health, Boston, Massachusetts (John W. Jackson, Tyler J. VanderWeele, Deborah Blacker, Sebastian Schneeweiss); Division of Pharmacoepidemiology, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts (John W. Jackson, Sebastian Schneeweiss); Department of Biostatistics, Harvard School of Public Health, Boston, Massachusetts (Tyler J. VanderWeele); Partners TeleStroke Program, Department of Neurology, Massachusetts General Hospital, Boston, Massachusetts (Anand Viswanathan); and Gerontology Research Unit, Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts (Deborah Blacker).
J.W.J. was supported by the National Institute of Mental Health (grant T32 MH017119) and the Horace W. Goldsmith Fellowship at Harvard University; T.J.V. was supported by the National Institutes of Health (grant R01 ES 017876); D.B. was supported by a grant from the Alzheimer's Association.
J.W.J. completed this work at the Division of Pharmacoepidemiology and Pharmacoeconomics at Brigham and Women's Hospital and is now based at the Harvard School of Public Health.
Conflict of interest: S.S. is the primary investigator of unrelated research grants from Pfizer, Inc., Novartis AG, and Boehringer Ingelheim. This was not a commissioned study, and no source of funding had any impact on its conception, conduct, reporting, or interpretation.
REFERENCES
- 1.Rabins PV, Blacker D, Rovner BW, et al. American Psychiatric Association practice guideline for the treatment of patients with Alzheimer's disease and other dementias. Second edition. Am J Psychiatry. 2007;164(12 suppl):5–56. [PubMed] [Google Scholar]
- 2.Maher AR, Maglione M, Bagley S, et al. Efficacy and comparative effectiveness of atypical antipsychotic medications for off-label uses in adults: a systematic review and meta-analysis. JAMA. 2011;306(12):1359–1369. doi: 10.1001/jama.2011.1360. [DOI] [PubMed] [Google Scholar]
- 3.US Food and Drug Administration. Public Health Advisory: Deaths with Antipsychotics in Elderly Patients with Behavioral Disturbances. http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm053171.htm. Published April 11, 2005. Accessed September 12, 2014.
- 4.Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294(15):1934–1943. doi: 10.1001/jama.294.15.1934. [DOI] [PubMed] [Google Scholar]
- 5.Pratt N, Roughead EE, Ryan P, et al. Antipsychotics and the risk of death in the elderly: an instrumental variable analysis using two preference based instruments. Pharmacoepidemiol Drug Saf. 2010;19(7):699–707. doi: 10.1002/pds.1942. [DOI] [PubMed] [Google Scholar]
- 6.Schneeweiss S, Setoguchi S, Brookhart A, et al. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. CMAJ. 2007;176(5):627–632. doi: 10.1503/cmaj.061250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huybrechts KF, Gerhard T, Crystal S, et al. Differential risk of death in older residents in nursing homes prescribed specific antipsychotic drugs: population based cohort study. BMJ. 2012;344:e977. doi: 10.1136/bmj.e977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sikirica S, Marino M, Gagne JJ, et al. Risk of death associated with the use of conventional vs. atypical antipsychotic medications: evaluating the use of the Emilia-Romagna Region database for pharmacoepidemiological studies. J Clin Pharm Ther. 2014;39(1):38–44. doi: 10.1111/jcpt.12099. [DOI] [PubMed] [Google Scholar]
- 9.European Medicines Agency. CHMP Assessment on Conventional Antipsychotics. London, United Kingdom: European Medicines Agency; 2008 http://www.ema.europa.eu/docs/en_GB/document_library/Report/2010/01/WC500054057.pdf. Published November 20, 2008. Accessed November 18, 2013. [Google Scholar]
- 10.Wooltorton E. Risperidone (Risperdal): increased rate of cerebrovascular events in dementia trials. CMAJ. 2002;167(11):1269–1270. [PMC free article] [PubMed] [Google Scholar]
- 11.Wooltorton E. Olanzapine (Zyprexa): increased incidence of cerebrovascular events in dementia trials. CMAJ. 2004;170(9):1395. doi: 10.1503/cmaj.1040539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeste DV, Sable JA, Salzman C. Treatment of late-life disordered behavior, agitation, and psychosis. In: Salzman C, editor. Clinical Geriatric Psychopharmacology. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. pp. 129–170. [Google Scholar]
- 13.Wu CS, Wang SC, Gau SS, et al. Association of stroke with the receptor-binding profiles of antipsychotics—a case-crossover study. Biol Psychiatry. 2013;73(5):414–421. doi: 10.1016/j.biopsych.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Laredo L, Vargas E, Blasco AJ, et al. Risk of cerebrovascular accident associated with use of antipsychotics: population-based case-control study. J Am Geriatr Soc. 2011;59(7):1182–1187. doi: 10.1111/j.1532-5415.2011.03479.x. [DOI] [PubMed] [Google Scholar]
- 15.Kleijer BC, van Marum RJ, Egberts AC, et al. Risk of cerebrovascular events in elderly users of antipsychotics. J Psychopharmacol. 2009;23(8):909–914. doi: 10.1177/0269881108093583. [DOI] [PubMed] [Google Scholar]
- 16.Sacchetti E, Trifirò G, Caputi A, et al. Risk of stroke with typical and atypical anti-psychotics: a retrospective cohort study including unexposed subjects. J Psychopharmacol. 2008;22(1):39–46. doi: 10.1177/0269881107080792. [DOI] [PubMed] [Google Scholar]
- 17.Wang PS, Schneeweiss S, Setoguchi S, et al. Ventricular arrhythmias and cerebrovascular events in the elderly using conventional and atypical antipsychotic medications. J Clin Psychopharmacol. 2007;27(6):707–710. doi: 10.1097/JCP.0b013e31815a882b. [DOI] [PubMed] [Google Scholar]
- 18.Kolanowski A, Fick D, Waller JL, et al. Outcomes of antipsychotic drug use in community-dwelling elders with dementia. Arch Psychiatr Nurs. 2006;20(5):217–225. doi: 10.1016/j.apnu.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Finkel S, Kozma C, Long S, et al. Risperidone treatment in elderly patients with dementia: relative risk of cerebrovascular events versus other antipsychotics. Int Psychogeriatr. 2005;17(4):617–629. doi: 10.1017/S1041610205002280. [DOI] [PubMed] [Google Scholar]
- 20.Huybrechts KF, Schneeweiss S, Gerhard T, et al. Comparative safety of antipsychotic medications in nursing home residents. J Am Geriatr Soc. 2012;60(3):420–429. doi: 10.1111/j.1532-5415.2011.03853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Percudani M, Barbui C, Fortino I, et al. Second-generation antipsychotics and risk of cerebrovascular accidents in the elderly. J Clin Psychopharmacol. 2005;25(5):468–470. doi: 10.1097/01.jcp.0000178414.14685.c4. [DOI] [PubMed] [Google Scholar]
- 22.Gill SS, Rochon PA, Herrmann N, et al. Atypical antipsychotic drugs and risk of ischaemic stroke: population based retrospective cohort study. BMJ. 2005;330(7489):445. doi: 10.1136/bmj.38330.470486.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liperoti R, Gambassi G, Lapane KL, et al. Cerebrovascular events among elderly nursing home patients treated with conventional or atypical antipsychotics. J Clin Psychiatry. 2005;66(9):1090–1096. doi: 10.4088/jcp.v66n0901. [DOI] [PubMed] [Google Scholar]
- 24.Herrmann N, Mamdani M, Lanctôt KL. Atypical antipsychotics and risk of cerebrovascular accidents. Am J Psychiatry. 2004;161(6):1113–1115. doi: 10.1176/appi.ajp.161.6.1113. [DOI] [PubMed] [Google Scholar]
- 25.Vanderweele TJ, Vansteelandt S. Conceptual issues concerning mediation, interventions and composition. Stat Interface. 2009;2(4):457–468. [Google Scholar]
- 26.Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18(2):137–150. doi: 10.1037/a0031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Commonwealth of Pennsylvania. Pharmaceutical Assistance Contract for the Elderly. Harrisburg, PA: Commonwealth of Pennsylvania; 2013. 6 Pa Code § 22.21 http://www.pacode.com/secure/data/006/006toc.html . Accessed July 1, 2013. [Google Scholar]
- 28.State of New Jersey Department of Health. Pharmaceutical Assistance to the Aged and Disabled (PAAD). http://www.state.nj.us/humanservices/doas/home/paaddetail.html. Accessed August 6, 2013.
- 29.Andrade SE, Harrold LR, Tjia J, et al. A systematic review of validated methods for identifying cerebrovascular accident or transient ischemic attack using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(suppl 1):100–128. doi: 10.1002/pds.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vanderweele TJ, Vansteelandt S. Odds ratios for mediation analysis for a dichotomous outcome. Am J Epidemiol. 2010;172(12):1339–1348. doi: 10.1093/aje/kwq332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schneeweiss S, Setoguchi S, Brookhart MA, et al. Assessing residual confounding of the association between antipsychotic medications and risk of death using survey data. CNS Drugs. 2009;23(2):171–180. doi: 10.2165/00023210-200923020-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan MC, Chong CS, Wu AY, et al. Antipsychotics and risk of cerebrovascular events in treatment of behavioural and psychological symptoms of dementia in Hong Kong: a hospital-based, retrospective, cohort study. Int J Geriatr Psychiatry. 2010;25(4):362–370. doi: 10.1002/gps.2347. [DOI] [PubMed] [Google Scholar]
- 33.Pratt N, Roughead EE, Salter A, et al. Choice of observational study design impacts on measurement of antipsychotic risks in the elderly: a systematic review. BMC Med Res Methodol. 2012;12:72. doi: 10.1186/1471-2288-12-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogburn EL, VanderWeele TJ. Analytic results on the bias due to nondifferential misclassification of a binary mediator. Am J Epidemiol. 2012;176(6):555–561. doi: 10.1093/aje/kws131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hafeman DM. Confounding of indirect effects: a sensitivity analysis exploring the range of bias due to a cause common to both the mediator and the outcome. Am J Epidemiol. 2011;174(6):710–717. doi: 10.1093/aje/kwr173. [DOI] [PubMed] [Google Scholar]
- 36.VanderWeele TJ, Chiba Y. Sensitivity analysis for direct and indirect effects in the presence of exposure-induced mediator-outcome confounders. Epidemiol Biostat Public Health. 2014;11(2):e9027. doi: 10.2427/9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915–920. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


