Abstract
The development of medical grade iron oxide nanoparticles (IONP) has renewed interest in hyperthermia cancer therapy. Because of their modifiable size and heating capabilities under an AC magnetic field (alternating magnetic field, AMF), IONPs have the potential to damage or kill cells in a manner more therapeutically efficient than previous hyperthermia techniques. The use of IONPs in hyperthermia cancer therapy has prompted numerous questions regarding the cytotoxic mechanism associated with IONP heat therapy and if such mechanism is different (more or less effective) with respect to conventional hyperthermia techniques.
Keywords: Iron oxide, nanoparticle, alternating magnetic field, AMF, hyperthermia, transmission electron microscopy, TEM, efficacy, MCF-7
In this in vitro study, we determine the immediate and long-term (24 hours) cytotoxic effects of isothermal IONP hyperthermia treatment versus a conventional global heating technique (water bath). Using the same heating time and temperature we showed significantly greater cytotoxicity in IONP-heated cells as opposed to water bath-treated cells. We postulate that the difference in treatment efficacy is due to the spatial relationship of particle-induced thermal damage within cells. Although the exact mechanism is still unclear, it appears likely that intracellular IONPs have to achieve a very high temperature in order to heat the surrounding environment; therefore it is reasonable to assume that particles localized to specific areas of the cell such as the membrane can deliver exacerbated injury to those areas. In this experiment, although detectable global temperature for the particle-heated cells stands comparable to the conventional heat treatment, particle-induced cell death is higher. From the results of this study, we propose that the mechanism of IONP hyperthermia renders enhanced cytotoxicity compared to conventional waterbath hyperthermia at the same measured thermal dose.
2. INTRODUCTION
Biological processes in cells, including cancer cells, are particularly susceptible to changes in temperature. A change in temperature of 6°C, from 37°C to 43°C, can kill a cancer cell provided the cell is exposed to this temperature for a sufficient period of time.1,2 While the biology of thermal damage in tissues is relatively well understood, this knowledge has, in an overall sense, translated relatively poorly into successful clinical cancer therapy-6 treatment. One reason for this is the absence of technology that effectively localizes heat to the tumor without heating the surrounding healthy tissues.7 A second limitation is the inability to accurately measure the heat dose deposited into the tumor relative to surrounding tissue.8–9 Technologies are being developed to address these limitations by coupling energy sources with susceptor materials that selectively deposit heat directly into tumor tissue when the energy activates the susceptor. One such combination comprises alternating magnetic fields (AMF) and magnetic IONPs that deposit heat locally to tissue when activated by the AMF.
Dextran-coated magnetic IONPs have been used to deliver heat selectively to the microenvironment of tumors with external AMF.10 Systemic or local delivery of nanoparticles specifically to cancer tissues in concentrations sufficient to achieve therapeutic thermal dose when activated by external alternating magnetic fields (AMF) has also been demonstrated, and may provide predictive heating by calculated heat dosimetry.5,6 This technique may allow for the targeted heating of cancer tissues using biocompatible IONPs and noninvasive AMF.7, 11
The potential utility of these particles for cancer therapy is explored in this study by comparing the effects of heating cancer cell cultures in vitro by IONP hyperthermia with the effects of a “global” application of conductive heating, i.e. water bath. Using the same heating time and temperature we showed significantly greater cytotoxicity in nanoparticle-treated cells as opposed to water bath-heated cells. We postulate that the difference in treatment efficacy is due to the spatial relationship of particle-induced thermal damage with cells, where particles that localize to specific areas of the cell, such as the membrane, can deliver exacerbated injury to those areas. Therefore, although detectable global temperature for the particle-heated cells stands comparable to the conventional heat treatment, damage on the cellular level is aggravated. The proposed mechanistic difference for IONP hyperthermia speaks to its untapped potential for development as a promising new treatment method.
3. MATERIALS AND METHODS
In an attempt to capture the most accurate and robust sense of cytotoxicty for both heating techniques, we used three independent assessments of cell damage/death (LDH, NADH/NADPH associated bioreduction and Trypan Blue exclusion).
3.1 Nanoparticles
The dextran-coated IONPs used in this study were graciously provided by Triton Biosystems of Chelmsford, MA (currently Aduron Biosystems). All particles were manufactured by micromod Partikeltechnologie GmbH in Rostock, Germany. The nanoparticles consist of 80 nm double layer dextran coated iron oxide at a particle concentration of 37 mg/ml, having high specific absorption rates (SAR) in applied magnetic fields, and were synthesized by high-pressure homogenization according to a core-shell method.12 The specific absorption rates (SAR) was measured at 150 kHz and various AMF amplitudes.12 The particles possess an iron oxide core (density >5 g/cm3), (10 – 60 nm) that is surrounded by a layer of dextran adsorbed to the oxide surface. The iron content of the particles is >50% w/w, and they are separable with a permanent magnet.
3.2 Cell culture and treatments
MCF-7 cells were harvested, counted, centrifuged, and resuspended according to treatment group parameters. Cells harvested for use as the lactate dehydrogenase (LDH) standards were resuspended at a concentration of 500,000 cells/ml. All cells for both water bath and magnetic hyperthermia treatment were resuspended at a concentration of 533,000 cells/ml. 5.16 ml of cell suspension were put into nine different 15 ml conical tubes for treatment. Directly before treatment 338 µl of particles were added to both water bath and magnetic hyperthermia treatment samples for a final concentration of 1 mg Fe/ml suspension. 338 µl of particles were also added to the particle control tube. 338 µl of PBS were added to the other control tubes in order to maintain identical cell concentrations across all groups.
Treatment groups were assigned as follows and maintained at temperatures± 1° C :
W41: Waterbath heating 10 min @ 41° C
W45: Waterbath heating 10 min @ 45° C
W50: Waterbath heating 10 min @ 50° C
P41: Particle heating 10 min @ 41° C
P45: Particle heating 10 min @ 45° C
P50: Particle heating 10 min @ 50° C
C+: Control with no particles, + AMF
CP−: Control with particles, − AMF
C−: Control with no particles, − AMF
For each temperature group, both water bath and IONP hyperthermia treatments were conducted simultaneously. Both samples were exposed to 30° C for five minutes prior to treatment. For the water bath group temperatures were increased at a rate which mimicked the AMF-treated samples.
Aliquots for LDH, MTS and Trypan Blue Exclusion Assays were taken every 2.5 hours (range: 2–3 hours) for 25 hours. TEM samples were taken at the two and eight hour time points for P45 and P50. The initial post treatment sample (the 0 hour time point) was taken immediately following the 10 minute treatment. The entire experiment was completed in duplicate to verify results.
3.3 Lactate dehydrogenase assay
The LDH assay measures the amount of LDH released from cells upon their lysis. The presence of LDH in the supernatant is an indicator of cell damage stemming from the breakdown of the cellular membrane allowing LDH leakage. The assay consists of multiple steps leading to the conversion of a tetrazolium salt into a red formazan product. The absorbance of the red product directly corresponds to the amount of LDH released, and thus the number of dead cells.
The LDH assay components were obtained from the CytoTox 96 Non-Radioactive Cytotoxicity Assay kit, manufactured by Promega Corporation, Madison, WI. 200 µl aliquots of cell suspension were collected from every sample at each time point. Cell suspension aliquots were centrifuged at 14,000 RPM, and 50 µl per well of the supernatant was added in triplicate to flat-bottom 96-well plates. Substrate was prepared as directed by the kit and 50 µl of substrate was added to each well followed by a 30 min incubation covered from light. 50 µl of the assay kit stop solution was then added to each well and the plates were read using PBS as a blank at 490 nm. Readings were compared to a standard ladder curve of fully-lysed cells which underwent the same procedure.
3.4 MTS cell proliferation assay
The MTS Cell Proliferation Assay measures the amount of bioreduction of Owen’s reagent performed by cells through NADH/NADPH produced by dehydrogenase enzymes. Metabolically active cells will produce a formazan red product in the presence of the substrate. The absorbance reading at 490 nm directly corresponds to the number of active cells present in the sample.
The MTS assay components were obtained from the CellTiter 96® AQueous One Solution Cell Proliferation Assay, manufactured by Promega Corporation, Madison, WI. 100 µl cell suspensions per well of each sample time point were loaded in triplicate onto a 96 well plate. 20 µl of the kit’s substrate was added to each well and the plates incubated for 1.5 hours. 25 µl lysis solution was then added to each well to stop the assay and the plates read at 490 nm with PBS as a blank.
3.5 Trypan blue exclusion assay
Trypan Blue is a blue stain which is mixed with a cell suspension to determine cell viability. Those cells which have damaged cell membranes (signifying extreme stress/death) will be unable to exclude the dye and thus appear blue. Viable cells will not take up the dye, and thus appear normal.
50 ul aliquots of cell suspension were collected from every sample vial at each time point. Live cells from nine different representative volumes were then counted using a hemocytometer and a Nikon TMS Model number 212845 microscope. Using the volume of the original sample and a dye dilution factor the total concentration of cells was then calculated for each time point.
3.6 Transmission Electron Microscopy
Transmission Electron Microscopy (TEM) sample preparation materials and images were obtained with assistance of Katherine Connolly at the Dartmouth College Ripple Electron Microscopy Facility. A separate set of cells were treated according to the aforementioned cell culture and treatments section and samples were taken at 2 and 8 hours post-treatment for TEM imaging. 4% buffered glutaraldehyde fixative was added to each 500 µl cell suspension sample. Cells were suspended in ice cold 0.1M sodium cacodylate buffer and stored at 4° C until further processed for imaging.
4. RESULTS
4.1 Treatment temperatures
IONP-AMF and water bath heated sample temperatures were measured by a fiber optic temperature probe. Treatment timings were started once the probe indicated the specified temperature per sample, maintained at the treatment temperature ±0.5° C, and ended 10 minutes thereafter. Because of this, the heating curves for particle and water bath treatments do not differ noticeably: with the exception of the 41°C/10 particle treatment which showed a linear temperature increase before flattening at the treatment temperature for 10 minutes. This situation was due to a setting error on the timer circuit of the field generator. In contrast, the water bath treatments display a gradual temperature increase with decreased rate near the treatment temperature, resulting in later treatment starting times. In comparison to the areas under the heating curves for particle treatments, corresponding water bath treatments appear to have a slightly higher measured thermal dose.
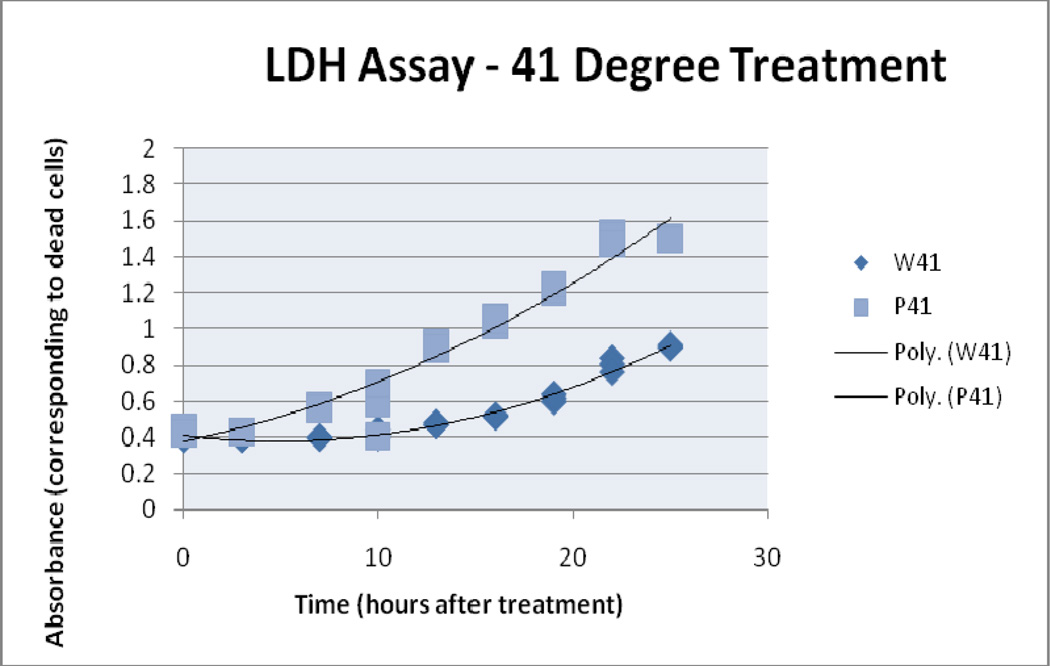
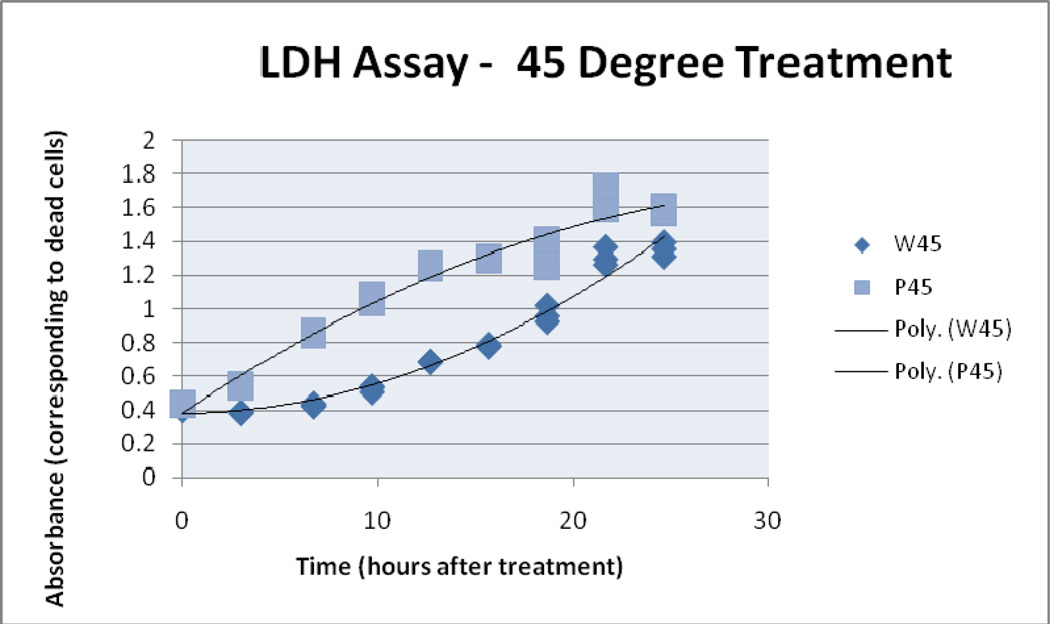
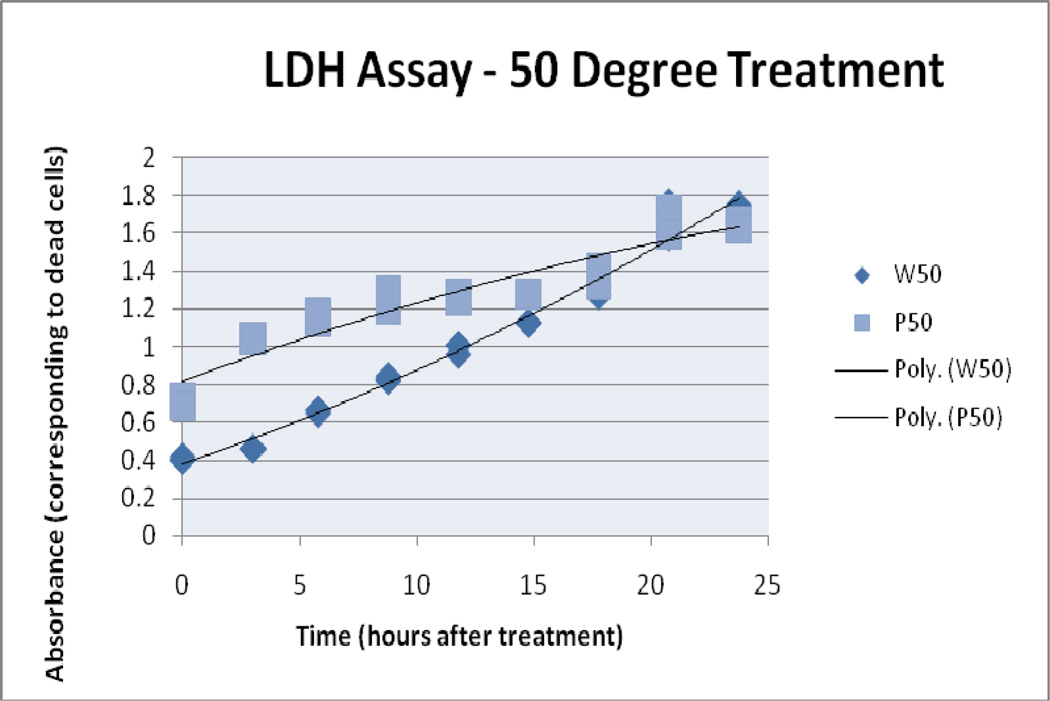
4.2 LDH assay
LDH assay results suggest there was increased cell damage with increased thermal dose. Absorbance readings for the LDH analysis of treatment samples reached their highest value in the end time point of the 50° C/10 water bath sample. The 50° C/10 particle sample begins with a higher LDH reading and then converges with the water bath samples. For these experiments the use of prolonged time points (up to 25 hours post treatment) prevents the direct comparison of LDH and Trypan blue results in terms of percent death, therefore trends are compared instead of the direct values. 41°C/10 AMF and water bath treatment absorbance readings began to diverge after the 3 hour time point concluding with an 0.6 AU separation. Similarly, the 45° C/10 treatment samples separated after the 0 hour time point.
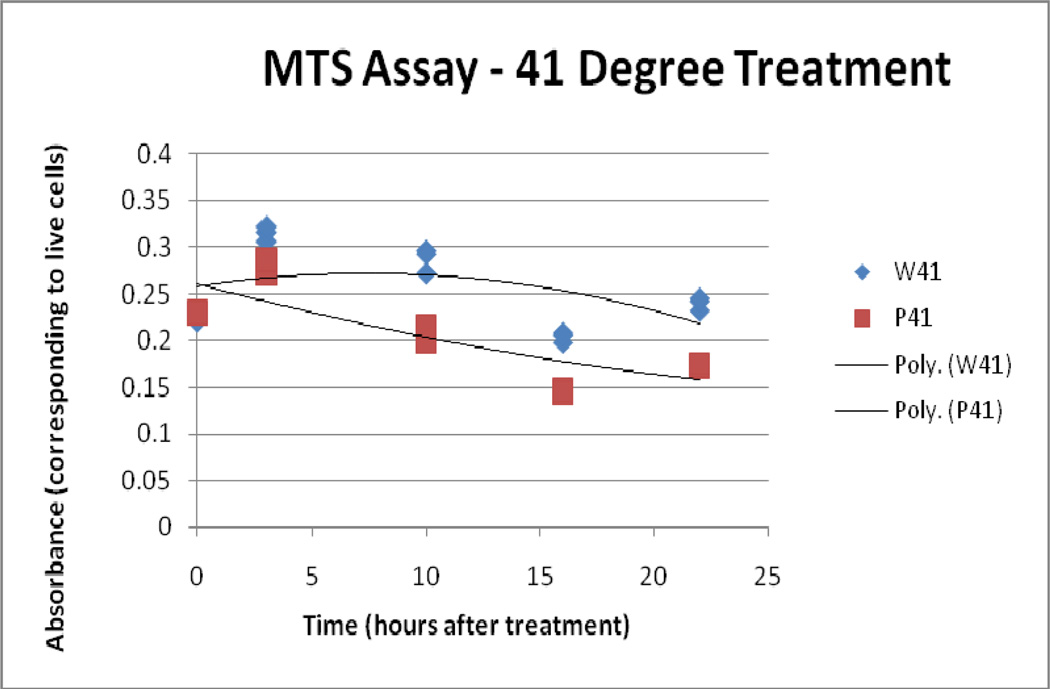
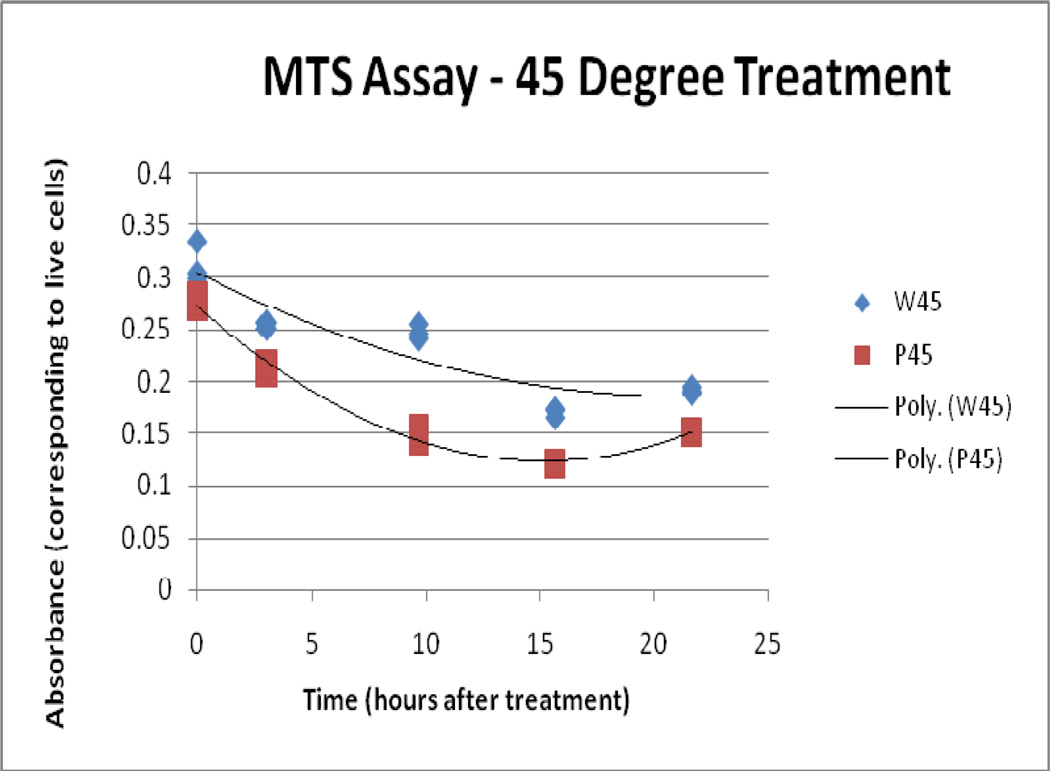
4.3 MTS assay
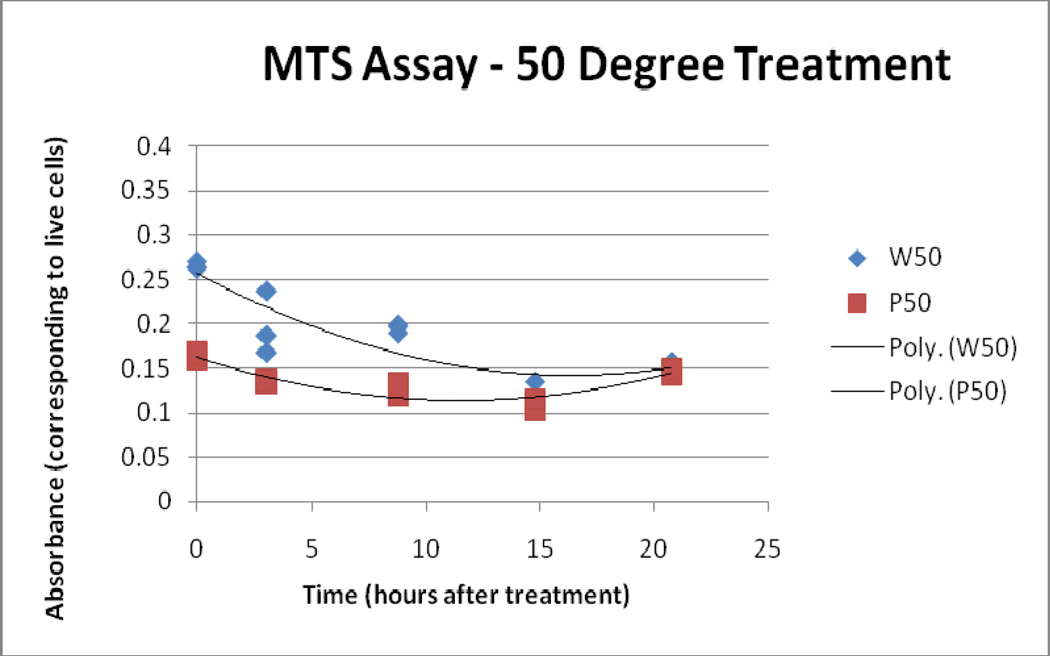
Results from the MTS assay supported the findings of the LDH and Trypan assays. Over time, both the 41°C/10 and 45° C/10 IONP treatments display less bioreductive activity than the 41°C/10 and 45° C/10 water bath-treated samples. The 50° C/10 IONP treatment displays a markedly lower bioreductive activity from the start when compared to the 50° C/10 water bath treatment, and the two converge at the 25 hour time point, showing that they have reached similar levels of total cell death.
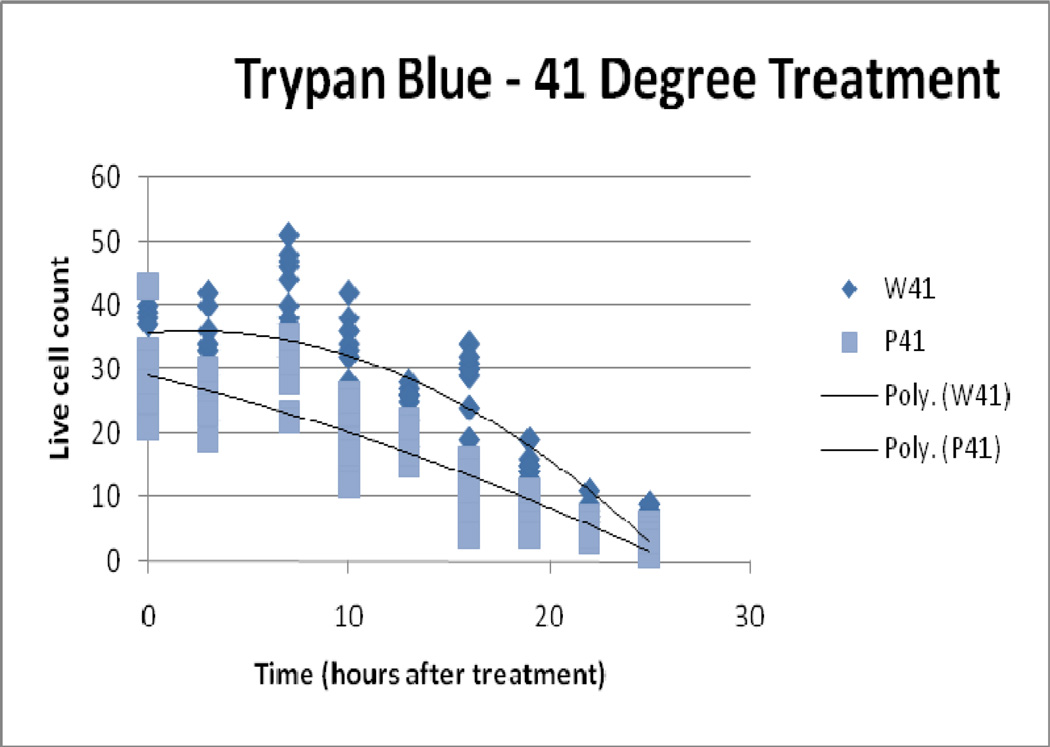
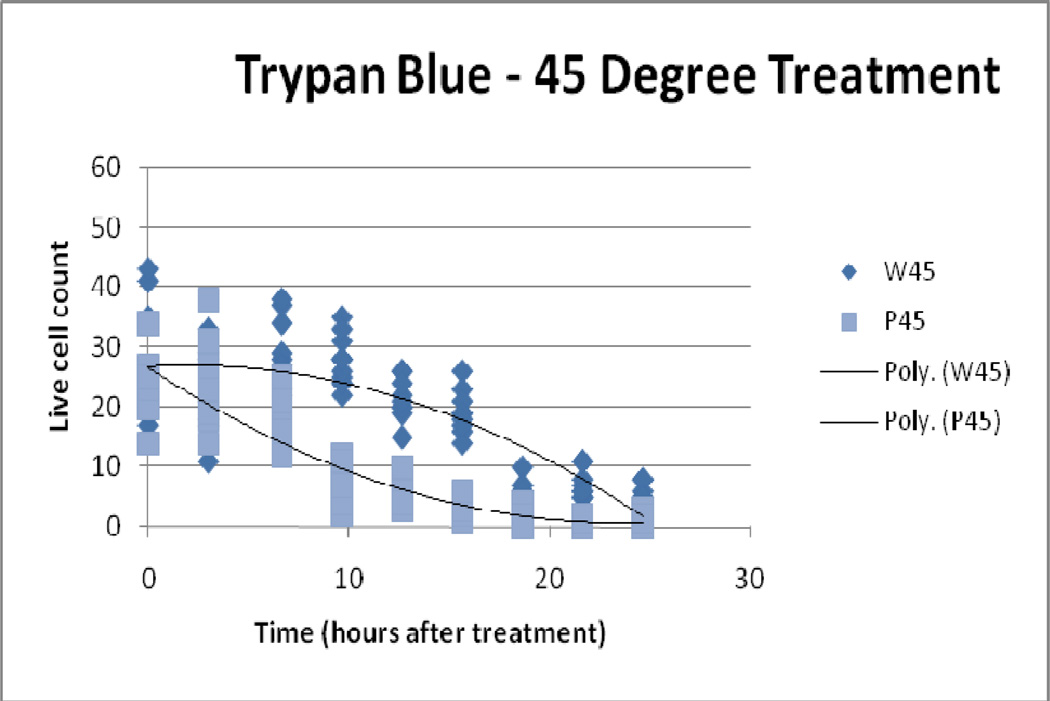
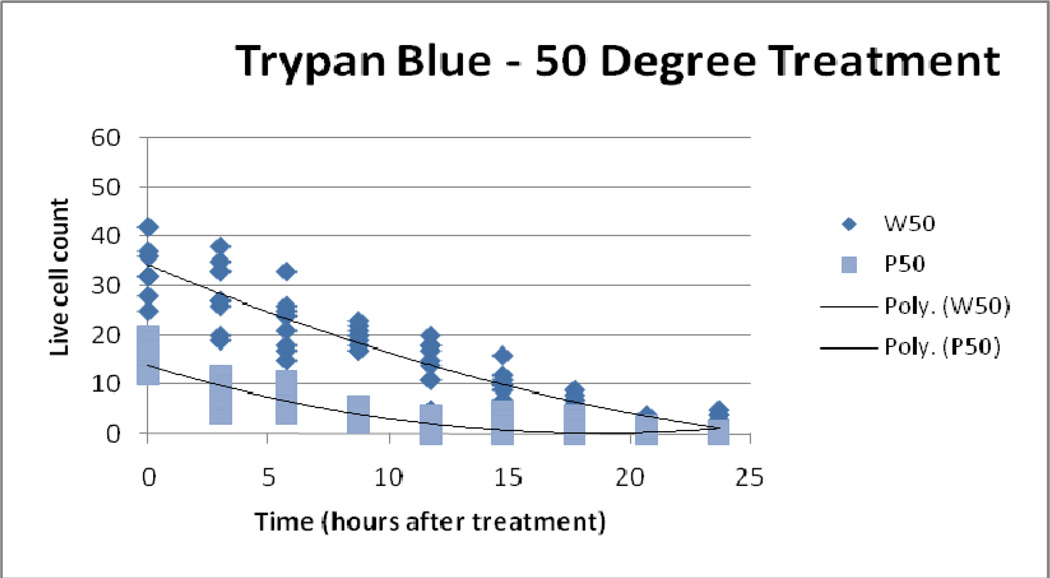
4.4 Trypan blue exclusion assay
Trypan blue assessments demonstrated greater relative cytotoxicity (than LDH and MTS) with increasing thermal dose for both IONP and water bath treatment ( Figure 7, Figure 8, Figure 9). Controls, 41°C/10 and 45° C/10 IONP treatment, and all water bath treatment samples did not display immediate cell death (all approximately 0% death at the 0 hour time point). However a thermal dose of 50° C/10 from IONP treatment produced an average initial cell count of 15 versus an average cell count of 34 from the water bath treatment. As time progressed the difference between particle and water bath treatment cell death increased in the 41°C/10 and 45° C/10 temperature groups. The 50° C/10 isotemperature samples proceeded to reach 100% cell death for both modalities at the 25 hour time point. This effect was expected due to the high thermal dose.
Figure 7.
41° C Treatment, Trypan Blue Assay
Figure 8.
45° C Treatment, Trypan Blue Assay Results
Figure 9.
50° C Treatment, Trypan Blue Assay
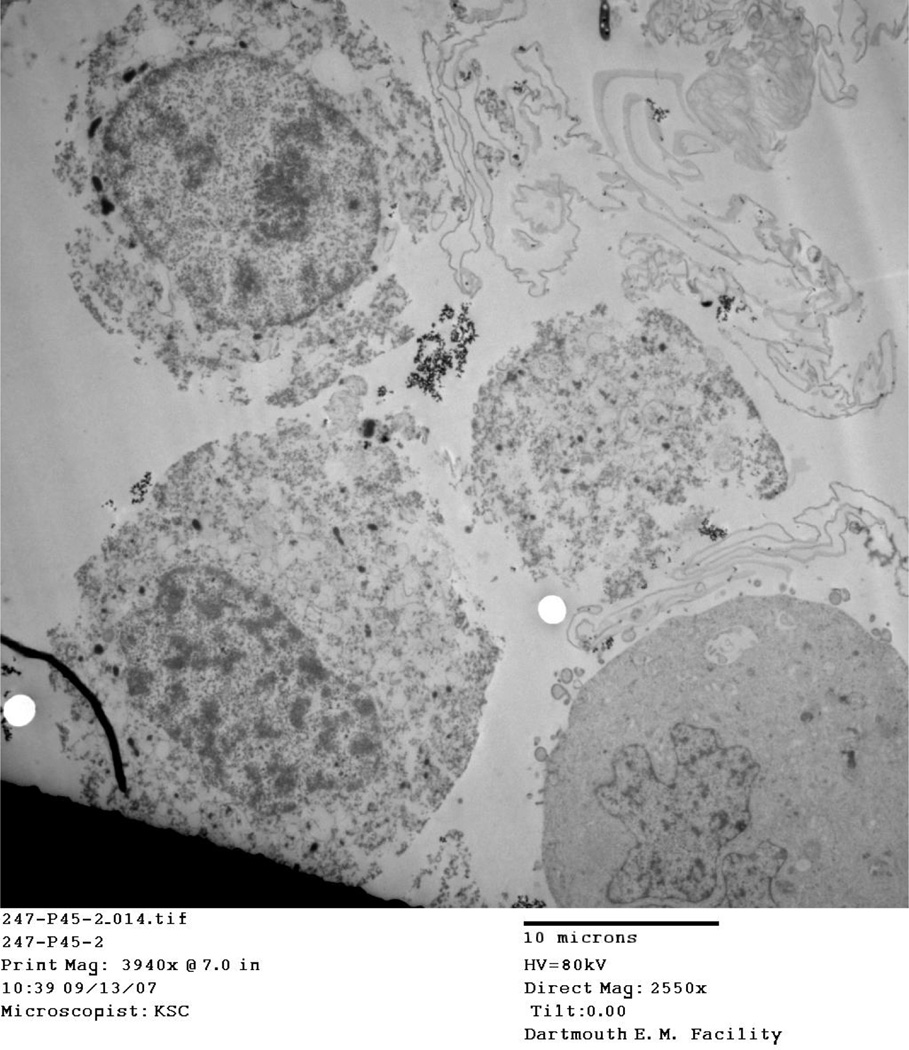
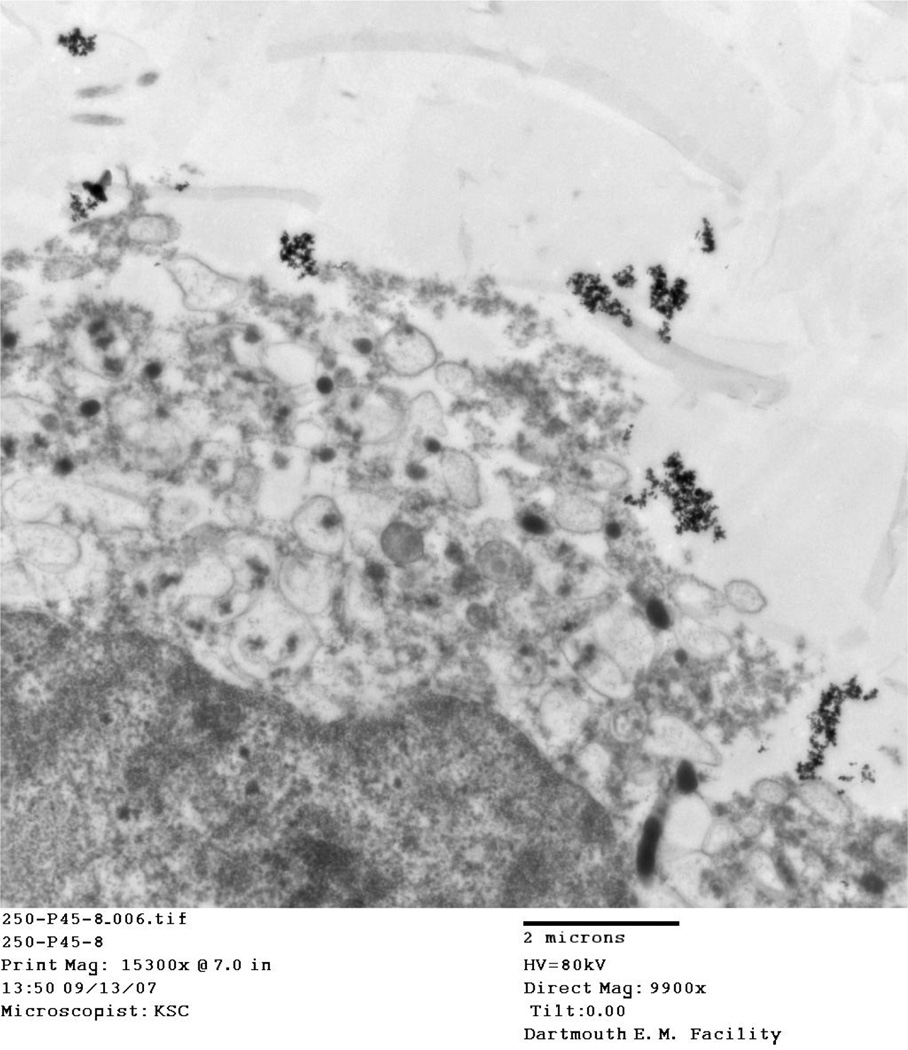
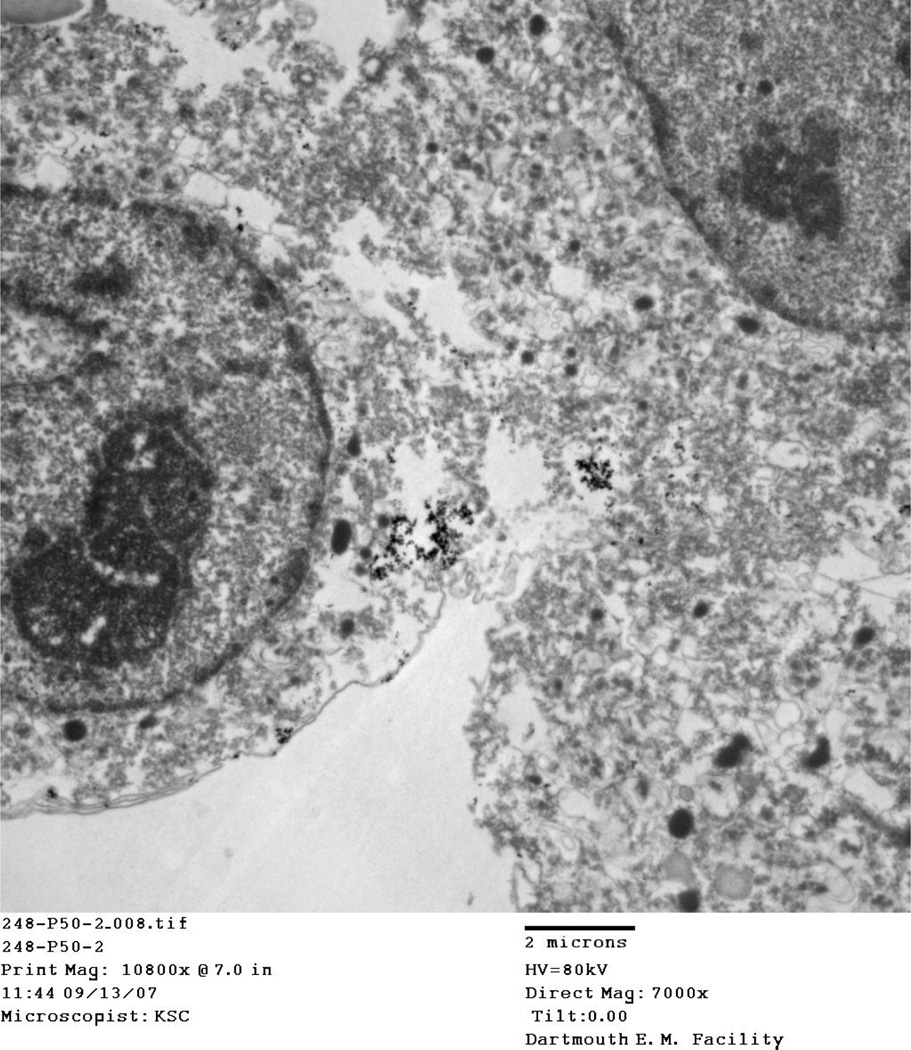
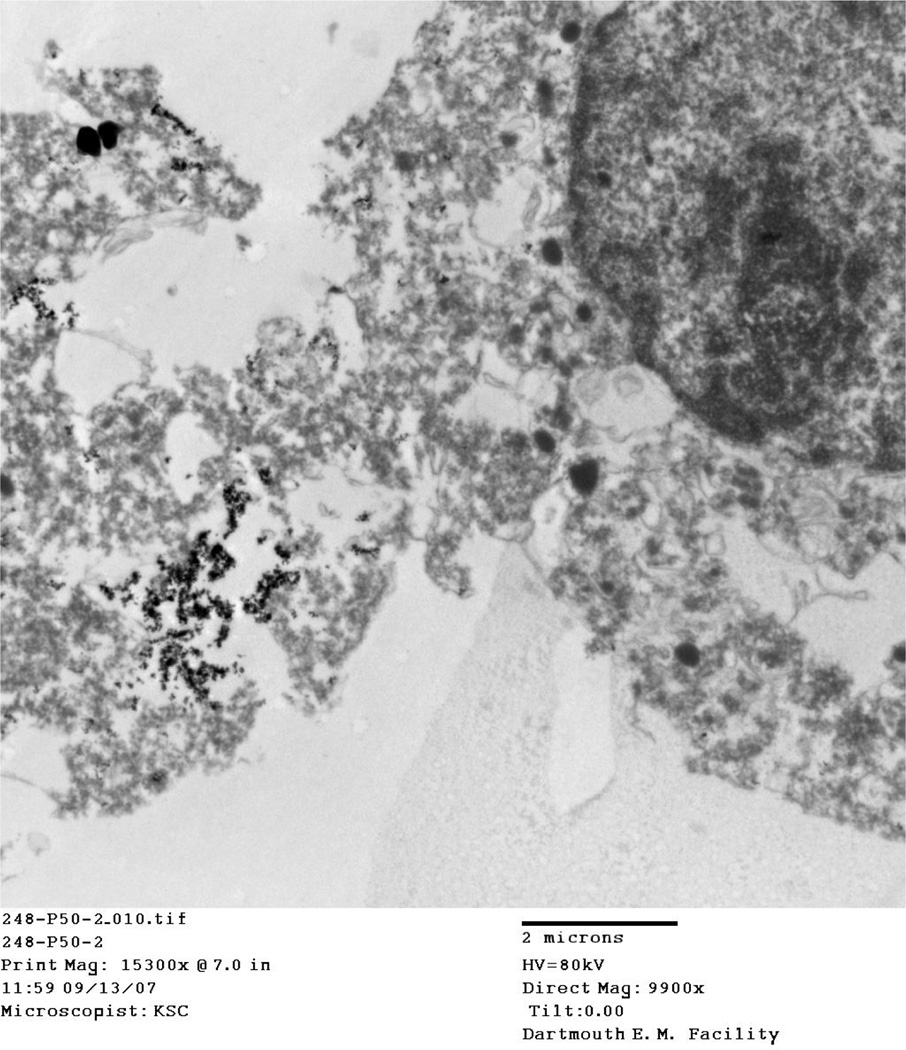
4.5 Location of particles
TEM images taken of IONP associated cells at thermal doses of 45°/10 and 50° C/10 at both 2 and 8 hour time points consistently show aggregated nanoparticles, damaged cellular membranes, and enlarged nuclei. Aggregated IONP can be seen both outside the cell (Figure, Figure ) and inside the cell (Figure 10, Figure 11). Cellular membranes are clearly damaged and separating from the cells as shown in Figure and Figure 10.
Figure 10.
45°C Particle Treatment, 2 hour post injection
Figure 11.
45°C Particle Treatment, 8 hour post injection
Figure 10.
50°C Particle Treatment, 2 hour post injection
Figure 11.
50°C Particle Treatment, 8 hour post injection
5. DISCUSSION and CONCLUSION
In summary, all three cytotoxicity assessments used (LDH, MTS and Trypan blue) demonstrated the thermal dose necessary for morphologic and pathophysiologic cell damage is greater for water bath heating than for IONP hyperthermia.. At certain thermal doses / temperatures, IONP hyperthermia treated cells exhibit an initial cell death that is not seen in water bath hyperthermia treated cells. In addition to this difference in initial cell death, at specific sublethal treatment levels, IONP hyperthermia exhibited a higher overall level of cell injury than water bath hyperthermia treated cells. These findings suggest that IONP hyperthermia treatment induces a localized heat dose that is more effective than a global conductive heat treatment when the same environmental thermal dose is used.. In the first ten hours following treatment, the 41° C/10 min and 45° C/10 min IONP treatment samples exhibit greater cytotoxicity than the water bath treated samples and controls. Although the three cell damage assessment techniques used measure different criteria for cell injury, together their use provides a robust assessment of the comparison of IONP and water bath hyperthermia treatment.
TEM images for the 45° C and 50° C at 2 and 8 hour post-treatment time points displayed cells in various stages of death. Particles viewed on the TEM tended to aggregate outside and inside cells (Figure, Figure, Figure 10, Figure 11). In addition, cellular membranes were commonly observed to have separated from the cell (Figure 10).
The data presented in this paper disagree with the results published by Chan and Jordan indicating that IONP hyperthermia induces no greater cell death than that of water bath hyperthermia.13,14 Some possible explanations for this disagreement include differences in particles used for treatment and differing methods of assaying cytotoxicity. Further experiments must be conducted in order to characterize this observed difference between IONP hyperthermia treatment and water bath hyperthermia treatment.
Figure 1.
41° C Treatment, LDH Assay
Figure 2.
45° C Treatment, LDH Assay
Figure 3.
50° C Treatment, LDH
Figure 4.
41° C Treatment, MTS Assay
Figure 5.
45° C Treatment, MTS Assay
Figure 6.
50° C Treatment, MTS Assay
Acknowledgements
The authors thank Kate Connolly (Dartmouth Medical School) for her expertise and valuable insights in transmission electron microscopy. The authors would also like to thank Aduro Biotech, Berkley, CA (formerly Triton Biosystems), Chelmsford, MA for graciously providing the particles used in this experiment.
REFERENCES
- 1.Overgaard J. In: Hyperthermic Oncology. Overgaard J, editor. Vol. 2. London: Taylor and Francis; 1985. pp. 8–9. ©. [Google Scholar]
- 2.Streffer C, van Beuningen D. In: Hyperthermia and the therapy of malignant tumors. Streffer J, editor. Berlin: Springer; 1987. pp. 24–70. ©. [Google Scholar]
- 3.Zaffaroni N, Fliorentini G, De Giorgi U. Eur. J. Surg. Oncol. 2001;27:340. doi: 10.1053/ejso.2000.1040. [DOI] [PubMed] [Google Scholar]
- 4.Hildebrandt B, Wust P, Ahlers O, et al. Crit. Rev. Oncol. Hematol. 2002;43:33. doi: 10.1016/s1040-8428(01)00179-2. [DOI] [PubMed] [Google Scholar]
- 5.Moroz P, Jones SK, Gray BN. J. Surg. Oncol. 2002;80:149. doi: 10.1002/jso.10118. [DOI] [PubMed] [Google Scholar]
- 6.Moroz P, Jones SK, Gray BN. Int. J. Hyperthermia. 2002;18:267. doi: 10.1080/02656730110108785. [DOI] [PubMed] [Google Scholar]
- 7.DeNardo SJ, DeNardo GL, Natarajan A, Miers LA, Foreman AR, Gruettner C, Adamson GN, Ivkov R. J. Nucl. Med. 2007;48:437. [PubMed] [Google Scholar]
- 8.Dewhirst MW, Jones E, Vujaskovic T, Li C, Prosnitz L. In: Cancer Medicine. Kufe D, Pollock R, Weichselbaum R, et al., editors. Hamilton, Ontario, Canada: BC Decker, Inc; 2003. pp. 623–636. ©. [Google Scholar]
- 9.van der Zee J. Annals of Oncology. 2002;13:1173. doi: 10.1093/annonc/mdf280. [DOI] [PubMed] [Google Scholar]
- 10.DeNardo SJ, DeNardo GL, Miers LA, Natarajan A, Foreman AR, Gruettner C, Adamson GN, Ivkov R. Clin. Cancer Res. 2005;11:7087s. doi: 10.1158/1078-0432.CCR-1004-0022. [DOI] [PubMed] [Google Scholar]
- 11.Hoopes PJ, Strawbridge R, Gibson UJ, Zeng Q, Pierce Z, Savellano M, Tate JA, Ogden JA, Baker I, Ivkov R, Foreman AR, Gaito C, Dulatas L, Tate J, Ogden J. Proc. of SPIE. 2007;6440:6440K. doi: 10.1117/12.706302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gruettner C, Mueller K, Teller J, Westphal F, Foreman A, Ivkov R. J. Magn. Magn. Mater. 2007;311:181. [Google Scholar]
- 13.Jordan A. J. Magn. Magn. Mater. 1999;201:413. [Google Scholar]
- 14.Chan D. J. Magn. Magn. Mater. 1993;122:374. [Google Scholar]