Abstract
Introduction: Although tablet systems are becoming a powerful technology, particularly useful in every application of medical imaging, to date no one has investigated the acceptance and performance of this technology in digital cytology. The specific aims of the work were (1) to design a health technology assessment (HTA) tool to assess, in terms of performance and acceptance, the introduction of tablet technologies (wearable, portable, and non portable) in the e-laboratories of cytology and (2) to test the tool in a first significant application of digital cytology. Materials and Methods: An HTA tool was proposed operating on a domain of five dimensions of investigation comprising the basic information of the product of digital cytology, the perceived subjective quality of images, the assessment of the virtual navigation on the e-slide, the assessment of the information and communication technologies features, and the diagnostic power. Six e-slides regarding studies of cervicovaginal cytology digitalized by means of an Aperio (www.aperio.com) scanner and uploaded onto the www.digitalslide.it Web site were used for testing the methodology on three different network connections. Results: Three experts of cytology successfully tested the methodology on seven tablets found suitable for the study in their own standard configuration. Specific indexes furnished by the tool indicated both a high degree of performance and subjective acceptance of the investigated technology. Conclusions: The HTA tool thus could be useful to investigate new tablet technologies in digital cytology and furnish stakeholders with useful information that may help them make decisions involving the healthcare system. From a global point of view the study demonstrates the feasibility of using the tablet technology in digital cytology.
Key words: : e-health, telepathology, technology, telehealth
Introduction
Background
Digital cytology is a field of digital pathology that integrates medical knowledge and properly designed information and communication technologies (ICT) to investigate the morphological alterations in different degrees of morbidity directly on a digital image called virtual glass (or e-slide).1–3 E-slides are the product of a digitalization workflow by means of a heterogeneous process that starts from the microscope slide. The core element of the digitalization process is the digital scanner whose degree of magnification depends on its scanning resolution. Until recently, e-slides were mainly exchanged using personal computers as clients, which rendered it possible to successfully set up effective networks for teleconsulting and e-learning.1,4,5 In previous studies6,7 we showed the importance of using health technology assessment (HTA) tools in telemedicine and e-health for the assessment of both the acceptance and performance of a telemedicine solution. In 2008, we successfully investigated the acceptance and performance of virtual microscopy using PC platforms with a dedicated HTA tool.8 In recent years, further technological advances have opened up new directions of research for digital cytology, including integration with DICOM (www.dicom.com) and with three-dimensional technologies and utilization of tablet technologies.9–14
Today, tablets are replacing personal computers, especially in multimedia operations of medical images in telemedicine and e-health. Nevertheless, no one has so far investigated the implications of the introduction of tablets in the e-laboratory of cytology (e-cyt-lab). The implications of the changes that tablets could introduce in the e-cyt-lab are wide,15,16 which should be addressed by means of a proper HTA study.
Objective of the Study
The specific aims of the work were (1) to design an HTA tool to assess in terms of performance and acceptance the introduction of tablet technologies (wearable, portable, and nonportable) in the e-cyt-lab and (2) to test the HTA tool in a first significant application.
Materials and Methods
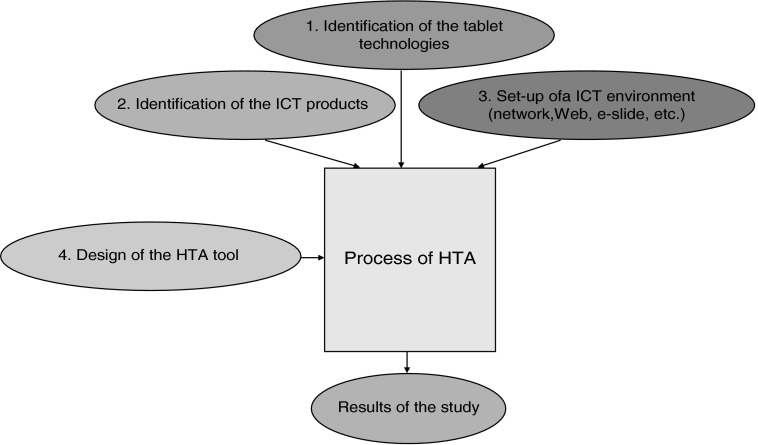
The methodological flow (Fig. 1) involved the following steps:
1. Identification of the tablet technologies potentially useful in the e-cyt-lab
2. Identification of the ICT products potentially useful in the e-cyt-lab
3. Set up of an ICT environment for testing the HTA tool
4. Design of the HTA tool
Fig. 1.
The methodological flow. HTA, health technology assessment; ICT, information and communication technologies.
The first three issues represent the variables of input of the process of HTA.
Tablet Technologies Potentially Useful in the E-CYT-Lab
The available tablet technologies potentially useful to share image information in digital cytology can be grouped as follows:
• Wearable tablets: tablets that can be carried in a pocket (e.g., such as smartphones)
• Portable tablets: tablets that can be carried in a briefcase such as the Apple (Cupertino, CA) iPad®
• Nonportable tablets: very large, touch tablets such as the Xdesk by Epson (Long Beach, CA)
In general, all the above technologies allow for image management through a gesture-based, touchscreen interface (i.e., moving, panning, zooming images, speeding operations, and interacting with the screen in a user-friendly fashion).
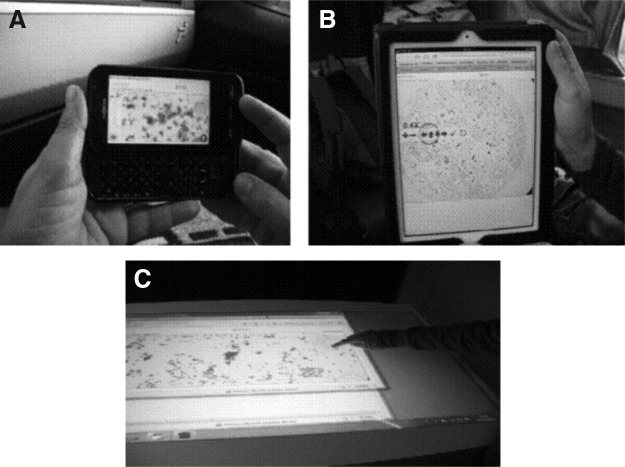
Wearable and portable tablets are an excellent chance both for inside-hospital local area network and outside-hospital wide area network consulting. A scenario of virtual navigation in digital cytology with these two systems is illustrated in Figure 2A and B, respectively.
Fig. 2.
Some tablet technologies as applied to digital cytology: (A) wearable tablet, (B) portable tablet, and (C) cooperation at the Epson Xdesk.
Nonportable tablets are a powerful ICT solution for cooperative analysis and on-site discussion, like, for instance, the Epson XDesk, with a 52-inch screen and a 1024×768 touchscreen display.
Figure 2C shows a scenario of application of this technology.
ICT Products Potentially Useful in the E-CYT-LAB
In the last decade we have witnessed the ever-increasing diffusion of products for digital pathology embedding both digital histology and digital cytology. In an attempt to follow a formal approach at listing the producers of ICT solutions for digital cytology in our HTA study, the following is a list of the producers participating in the 5th International Congress in Virtual Microscopy, held in Venice, Italy, June 6–9, 2012 (www.telepathology2012.com): Aperio (www.aperio.com), Aurora (www.auroramsc.com), Hamamatsu (www.hamamatsu.com), Huron (www.huron-technologies.com), Leica (www.leicabiosystems.com), Menarini (www.menarinidiagnostics.com), Metrizer (www.metrizer.com), NoemaLife (www.noemalife.com), Olympus (www.olympusamerica.com), Philips (www.research.philips.com), Roche (www.roche.com), 3DHistech (www.3dhistech.com), and GE Healthcare (www.gehealthcare.com).
ICT Environment for Testing the HTA Tool
The ICT environment (network, Web, e-slide) for testing the methodology was follows. Six e-slides regarding studies of cervicovaginal cytology were digitalized with an Aperio scanner and uploaded onto the www.digitalslide.it Web site accessed by domain users through password and log-in. Tablets were investigated through three different network connections (Table 1).
Table 1.
The Different Network Connections
| DESCRIPTION | CODE |
|---|---|
| Wireless hot spot connection at 20 Mb/s at a distance of 20 m from the antenna (Fig. 2A) | NW-1 |
| Wireless connection with a SIM card and tablet tools during a ride on a train without Internet WiFi sources (Fig. 2B) | NW-2 |
| The LAN at the Istituto Superiore di Sanità connected to the WAN through a firewall (Fig. 2C) | NW-3 |
LAN, local area network; Mb, megabits; NW, network; WAN, wide area network.
The Design of the HTA Tool
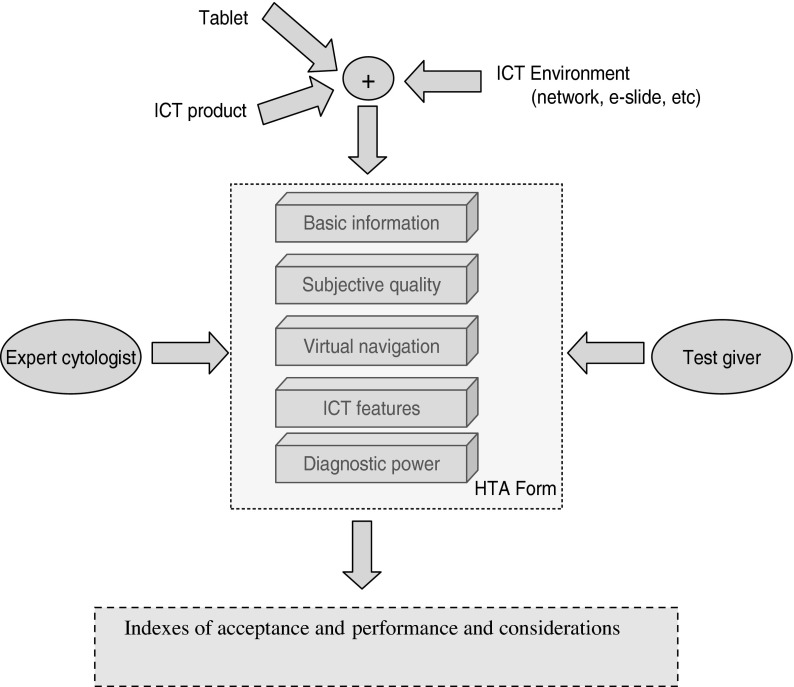
Figure 3 illustrates the flow proposed for the process of HTA. It starts with the input variables: tablet, ICT product, ICT environment (e-slide, network, etc.). A properly designed HTA tool (a form electronically distributed) is then used by experts of cytology as a tool for the technological assessment during an application of digital cytology. The entire process returns, as output, indexes of acceptance and performance and useful considerations.
Fig. 3.
The proposed health technology assessment (HTA) methodological flow. ICT, information and communication technologies.
The study consisted in the submission of the methodology to three experts of cytology supported by three test givers different from the designers of the methodology. The experts, supported by the test givers, tested the methodology on the defined application of digital cytology.
Furthermore, also the so-called client satisfaction about the methodology of the two groups of actors (i.e., the experts of cytology and the test givers) was investigated.
The core element of the methodology was an HTA form that operates on a domain of five dimensions (D1–D5) of investigation (Fig. 3):
D1. Basic information. It contains (1) a list of producers of digital cytology systems (Fig. 4), (2) a list of tablets, and (3) the details of the e-slides proposed in the HTA.
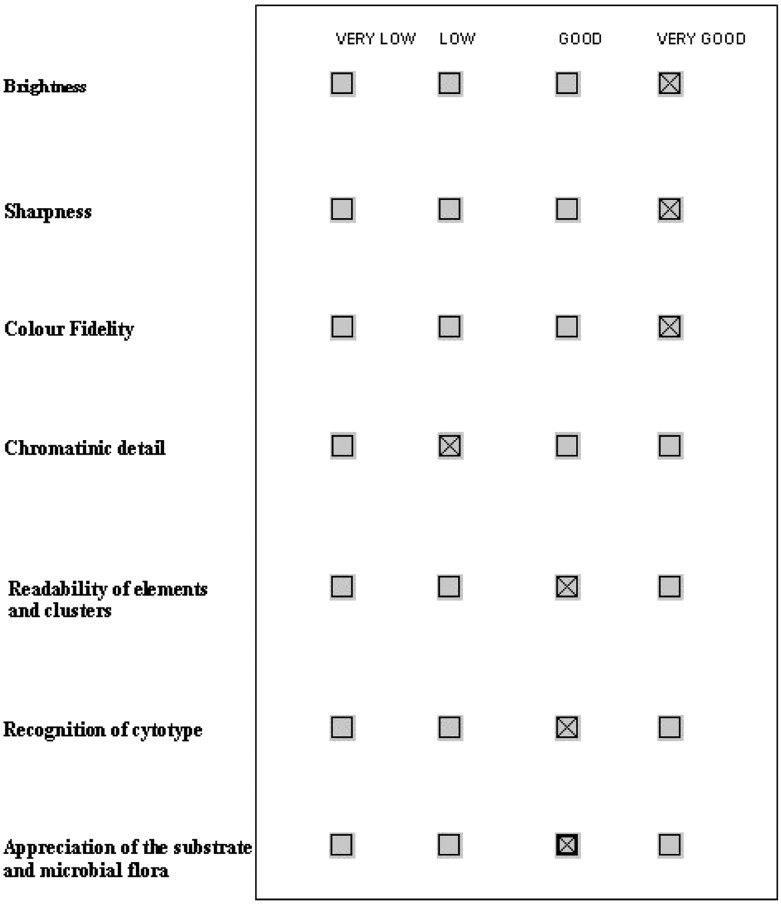
D2. Subjective quality. The section proposes a list of questions about the perceived quality. The answers allow for multiple levels of scoring (Fig. 5).
D3. Virtual navigation. The section is a list of questions on the perceived performance of virtual navigation. The answers allow for multiple levels of scoring (Fig. 6).
D4. ICT features. This section is for a detailed description of the characteristics of the investigated technology. It is compiled with the support of the test giver.
D5. Diagnostic power. The section proposes a list of questions that focus on the diagnostic power of the assessed system: for example, whether a complete diagnosis has been arrived at; if so, the time needed to reach it; the degree of user-friendliness, etc. In this section it is possible to insert personal comments and considerations.
Fig. 4.

Screenshot sample of the compiled section Virtual Microscopy System.
Fig. 5.
Screenshot sample of the compiled section Subjective Quality.
Fig. 6.
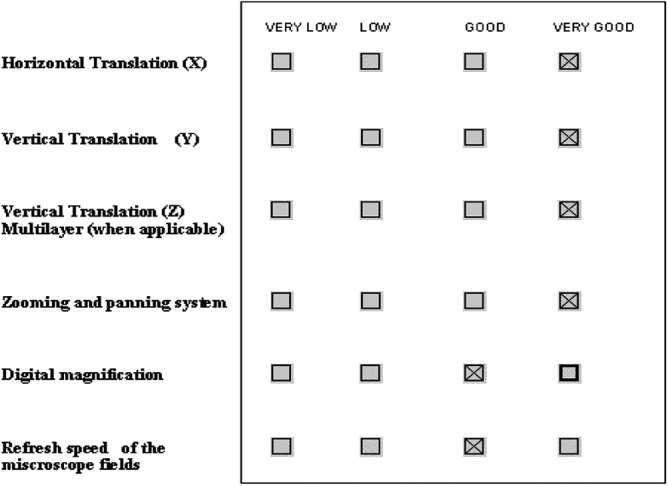
Screenshot sample of the compiled section Virtual Movement and Navigation.
Results
Protocol
Table 2 shows 12 different tablets (available at the time of the study) initially considered in their own standard configuration in the study. Table 3 shows the seven finally chosen tablets because they were directly available for the study in the laboratory and capable to cover the three different groups of tablets: wearable, portable, and nonportable. The portable and wearable tablets were investigated in all the above-described three network conditions; the nonportable tablet, Epson Xdesk, was examined only in the third network condition. All the systems were assessed for acceptance and performance (D1–D4 of the HTA tool). The nonportable system Xdesk was also assessed for diagnostic power (D1–D5 of the HTA tool). Three experts of cytology were recruited. Three test givers (different from the designers) supported the experts. Table 4 for the sake of clarity summarizes the configuration of investigation.
Table 2.
The Initially Investigated Tablets
| WEARABLE TABLETS | PORTABLE TABLETS | NONPORTABLE TABLETS |
|---|---|---|
| LG Optimum Dual | Asus EeePad | Epson Xdesk |
| Nokia c6 | iPad 2 | |
| iPhone 4s | iPad 3 | |
| Samsung Galaxy SII | ||
| Samsung Galaxy SIII | ||
| Samsung Galaxy Next | ||
| Nokia Lumia 610 |
Table 3.
The Finally Chosen Tablets
| WEARABLE TABLETS | PORTABLE TABLETS | NONPORTABLE TABLETS |
|---|---|---|
| LG Optimum Dual | Asus EeePad | Epson Xdesk |
| Nokia c6 | iPad 2 | |
| iPhone 4s | iPad 3 |
Table 4.
Summary of the Configuration of Investigation in the Process of Health Technology Assessment
| TABLET | INVESTIGATED DIMENSIONS OF HTA | NETWORKS | E-SLIDES |
|---|---|---|---|
| LG Optimum Dual | D1, D2, D3, D4 | NW-1, NW-2, NW-3 | 6 |
| Asus EeePad | |||
| Nokia c6 | |||
| iPad 2 | |||
| iPad 3 | |||
| iPhone 4s | |||
| Epson Xdesk | D1, D2, D3, D4, D5 | NW-3 | 6 |
HTA, health technology assessment; NW, network.
Quantitative Outcome
The quantitative outcome, in coherence with the objective, considered all the investigations performed (Table 4); however, the methodology could be also used in specific research studies to find the so-called best of the bunch.
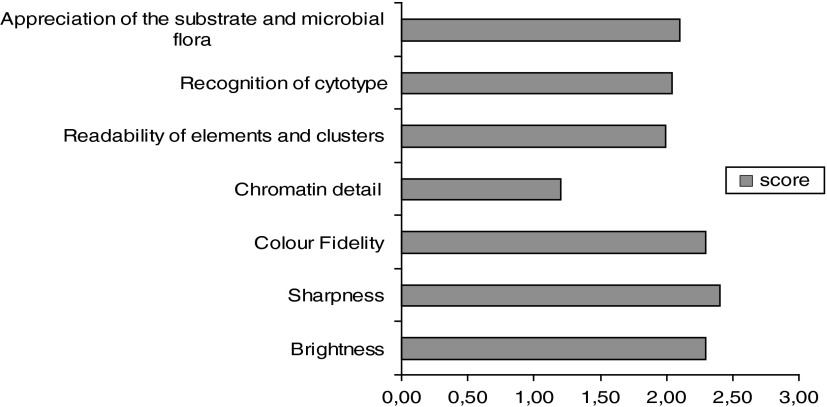
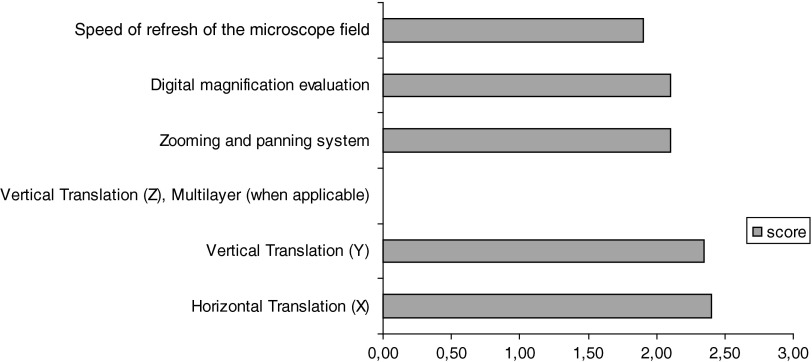
Figure 7 shows the encouraging outcome of the study (in terms of scoring) for D2: Subjective Quality. The only point of weakness was chromatin detail, as confirmed by the subjective comments. Figure 8 shows, again, an encouraging outcome for D3: Virtual Movement and Navigation.
Fig. 7.
Subjective Quality (scoring from 0=minimum to 3=maximum).
Fig. 8.
Virtual Movement and Navigation (scoring from 0=minimum to 3=maximum).
The cytology experts investigated the diagnostic power of the method on the six e-slides with the nonportable tablet Epson Xdesk. The time to reach diagnosis was compared in the two conditions (tablet Xdesk, traditional PC). D5 showed that the time to reach a diagnosis was lower by 13% (mean) when the tablet technology was used, with a statistically significant difference by Student's t test (p<0.01).
The degree of acceptance of the proposed methodology was also investigated in terms of the so-called client satisfaction by test givers and experts. Table 5 shows the high level of acceptance of the methodology.
Table 5.
Acceptance of the Health Technology Assessment Tool
| MEAN SCORE | |||
|---|---|---|---|
| DIMENSION | ASPECT | USERS (CYTOLOGY EXPERTS) | TEST GIVERS |
| 1 | User friendly | 2.5 | 2.8 |
| 2 | Online help | 2.3 | 2.9 |
| 4 | Speed of operations | 2.3 | 2.9 |
| 5 | Failure rate | 2.6 | 2.7 |
Scoring was on a scale from 3=maximum to 0=low.
Documental Outcome and Considerations
Figures 4–6 are sample screenshots of the tool form compiled for the Epson Xdesk: Virtual Microscopy Systems, Subjective Quality, and Virtual Movement and Navigation, respectively.
Recurrent comments were:
• Chromatin detail could be further improved.
• Depending on network traffic, occasional refresh problems have been encountered that impaired virtual navigation (Fig. 9).
• There was good acceptance and usefulness of the gesture-based navigation, which can speed up the operations (Fig. 10).
Fig. 9.
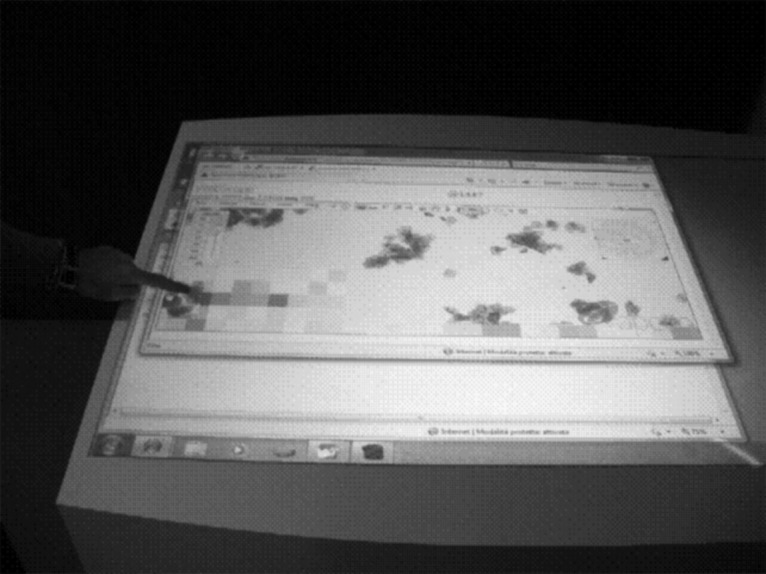
An occasional refresh problem.
Fig. 10.
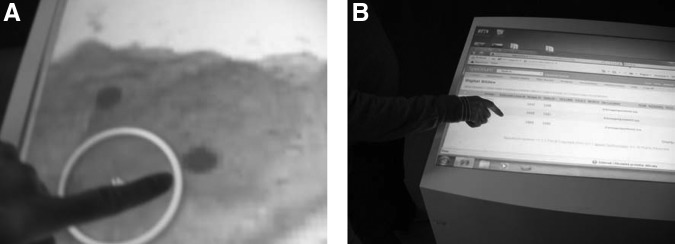
(A and B) The expediency of gesture-based navigation.
Discussion and Conclusions
Digital cytology1,17–19 integrates medical knowledge and properly designed ICT to investigate the morphological alterations in different degrees of morbidity directly on a digital image (e-slide); it is currently playing an important role in the healthcare system to change and improve the workflows4,5,20 in the cytology laboratory. The introduction of tablet technology, ranging from cooperative diagnosis up to teleconsulting and telediagnosis, is vital to the evolution of the healthcare system15,16 for telemedicine and e-health applications of teleimaging. However, to date16 no one has investigated the acceptance and performance of this technology in digital cytology.2 The proposed study focused to the introduction of tablet technologies in the e-cyt-lab. In particular, the study proposed a methodology designed to assess in terms of both performance and acceptance the integration of the tablet technologies with digital cytology.
The core element of the methodology was an HTA tool. This tool was electronically distributed and allowed an assessment of both acceptance and performance of the technology in five dimensions of investigation comprising the basic information of the product of digital cytology, the perceived subjective quality of images, the assessment of the virtual navigation on the e-slide, the assessment of the ICT features, and the diagnostic power. The tool was successfully tested on six e-slides regarding studies of cervicovaginal cytology digitalized with an Aperio scanner and uploaded onto the www.digitalslide.it Web site on seven different tablets. The diagnostic power further investigated on the nonportable system Epson Xdesk was found to be higher than that of traditional personal computers, in terms of speed of diagnosis. The experts and the test givers involved in the study also assigned a high level of acceptance to the methodology itself as client satisfaction.
Added Value
• The first added value of the methodology is the availability of a tool (the HTA tool) useful to investigate new tablet technologies in digital cytology and to furnish stakeholders with useful information that may help them make decisions involving the healthcare system.
• The second added value is the possibility to furnish, by means of the HTA tool, quantitative indexes for each one of the investigated technologies, as, for example, in the case of the assessment of the perceived subjective quality and the assessment the virtual navigation described in Results dedicated to quantitative outcome. These indexes could allow a comparison among the different investigated technologies to detect the so-called best of the bunch.
• The third added value is represented by the possibility to detect by means of the HTA tool also the points of weakness of the technology in one or multiple positions of the telemedicine chain, as, for example, the need of improving the chromatin details highlighted in Results dedicated to the documental outcome.
Final Considerations
The study from a global point of view concretely demonstrates that tablet technologies could be useful in digital cytology and thus answers the primary basic question of “Can a tablet be used in digital cytology?”
The scenarios of use of this technology are thus in the near future going to involve:
1. E-learning. The wearable and portable tablets15,16 are in fact natural candidates for the replacement of traditional personal computers as clients of e-learning applications at home.5,20
2. Teleconsulting. The wearable and portable tablets are in fact suitable for remote consulting.15
3. Cooperative diagnosis. Here nonportable tablets such as the Epson Xdesk system could be useful for cooperative diagnosis. In particular, it would be a valid substitution for the so-called multihead scopes currently used for cooperative diagnosis.16
Acknowledgments
The authors wish to thank Ms. Monica Brocco for the English editing of the manuscript.
Disclosure Statement
No competing financial interests exist.
References
- 1.Giansanti D, Grigioni M, Giovagnoli MR. Virtual microscopy and digital cytology: Fact or fantasy? Preface. Ann Ist Super Sanita 2010;46:113–114 [DOI] [PubMed] [Google Scholar]
- 2.Giansanti D, Grigioni M, D'Avenio G, Morelli S, Maccioni G, Bondi A, Giovagnoli MR. Virtual microscopy and digital cytology: State of the art. Ann Ist Super Sanita 2010;46:115–122 [DOI] [PubMed] [Google Scholar]
- 3.Morelli S, Grigioni M, Giovagnoli MR, Balzano S, Giansanti D. Picture archiving and communication systems in digital cytology. Ann Ist Super Sanita 2010;46:130–137 [DOI] [PubMed] [Google Scholar]
- 4.Giansanti D, Castrichella L, Giovagnoli MR. Telepathology training in a master of cytology degree course. J Telemed Telecare 2008;14:338–341 [DOI] [PubMed] [Google Scholar]
- 5.Giansanti D, Castrichella L, Giovagnoli MR. New models of e-learning for healthcare professionals: A training course for biomedical laboratory technicians. J Telemed Telecare 2007;13:374–376 [DOI] [PubMed] [Google Scholar]
- 6.Giansanti D, Morelli S, Macellari V. Telemedicine technology assessment part II: Tools for a quality control system. Telemed J E Health 2007;13:130–140 [DOI] [PubMed] [Google Scholar]
- 7.Giansanti D, Morelli S, Macellari V. Telemedicine technology assessment part I: Setup and validation of a quality control system. Telemed J E Health 2007;13:118–129 [DOI] [PubMed] [Google Scholar]
- 8.Giansanti D, Castrichella L, Giovagnoli MR. The design of a health technology assessment system in telepathology. Telemed J E Health 2008;14:570–575 [DOI] [PubMed] [Google Scholar]
- 9.Giansanti D, Morelli S, Silvestri S, D'Avenio G, Grigioni M, Giovagnoli MR, Pochini M, Balzano S, Giarnieri E, Carico E. A look inside the future perspectives of the digital cytology. In: Di Giamberardino P, Iacoviello D, Tavares JMRS, Jorge RMN, eds. Computational Modelling of Objects Represented in Images III: Fundamentals, Methods and Applications. Boca Raton, FL: CRC Press, 2012:335–340 [Google Scholar]
- 10.Ash WM, Krzewina L, Kim MK. Quantitative imaging of cellular adhesion by total internal reflection holographic microscopy. Appl Opt 2009;48:144–152 [DOI] [PubMed] [Google Scholar]
- 11.Seet KY, Nieminen TA, Zvyagin AV. Refractometry of melanocyte cell nuclei using optical scatter images recorded by digital Fourier microscopy. J Biomed Opt 2009;14:135–142 [DOI] [PubMed] [Google Scholar]
- 12.Cheong FC, Sun B, Dreyfus R, Amato-Grill J, Xiao K, Dixon L, Grier DG. Flow visualization and flow cytometry with holographic video microscopy. Opt Express 2009;17:1371–1379 [DOI] [PubMed] [Google Scholar]
- 13.Yu L, Mohanty S, Zhang J, Genc S, Kim MK, Berns MW, Chen Z. Digital holographic microscopy for quantitative cell dynamic evaluation during laser microsurgery. Opt Express 2009;17:12031–12038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giansanti D, Boschetto A, Pochini M, Bottini L, Giovagnoli MR. Design of a process for image improvement in digital cytology: A preliminary technology assessement. Comput Methods Biomech Biomed Eng Imaging Visualization 2014. doi: 10.1080/21681163.2014.883940 [DOI] [Google Scholar]
- 15.Giansanti D, Pochini M, Giovagnoli NR. How tablet technology is going to change cooperative diagnosis in the cytology e-laboratory Telemed J E Health 2013;19:991–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giansanti D, Pochini M, Giarnieri E, Giovagnoli MR. Towards the integration of digital cytology in the tablet technologies. Diagn Pathol 2013;8(Suppl 1):S40 [Google Scholar]
- 17.Huisman A, Looijen A, van den Brink SM, van Diest PJ. Creation of a fully digital pathology slide archive by high volume tissue slide scanning. Hum Pathol 2010;41:751–757 [DOI] [PubMed] [Google Scholar]
- 18.Demichelis F, Della Mea V, Forti S, Dalla Palma P, Feltrami CA. Digital storage of glass slides for quality assurance in histopathology and cytopathology. J Telemed Telecare 2002;8:138–142 [DOI] [PubMed] [Google Scholar]
- 19.Bondi A, Pierotti P, Crucitti P, Lega S. The virtual slide in the promotion of cytologic and hystologic quality in oncologic screenings. Ann Ist Super Sanita 2010;46:144–150 [DOI] [PubMed] [Google Scholar]
- 20.Giansanti D, Castrichella L, Giovagnoli MR. Telepathology requires specific training for the technician in the biomedical laboratory. Telemed J E Health 2008;14:801–807 [DOI] [PubMed] [Google Scholar]