Abstract
We assessed CD30 expression in patients with acute lymphoblastic leukemia (ALL) of either T-cell or B-cell lineage to examine potential benefit of anti-CD30-targeted therapy in this group of patients. Bone marrow specimens of 34 patients with T- and 44 with B-acute lymphoblastic leukemia (ALL) were assessed for CD30 expression by multicolor flow cytometry immunophenotypic analysis. Of these 78 patients, 75(96%) were adults and 63(81%) had refractory/relapse disease. Using an arbitrary 20% cutoff, 13/34 (38%) T-ALL and 6/44 (14%) BALL cases were considered to express CD30. In 5 T-ALL patients with sequential bone marrow tested, increased CD30 expression was observed during the course of high dose chemotherapy (p=0.025). Philadelphia chromosome/BCR-ABL1 fusion was positive in 14/44 B-ALL and 2/32 of T-ALL which showed no significant correlation with CD30 expression. In summary, we detected CD30 expression in approximately one third of T-ALL patients, and less frequently in B-ALL (p=0.017). In T-ALL, CD30 expression is upregulated during high dose chemotherapy. These data indicate that anti-CD30-targeted therapy may be a potential option for T-ALL patients with refractory/relapsed disease.
Keywords: CD30, flow cytometry, acute lymphoblastic leukemia, BCR-ABL1
Introduction
Modern multiagent chemotherapy regimens have increased the cure rate in both children and adults with acute lymphoblastic leukemia (ALL). The improved cure rate, however, comes at the expense of substantial toxicity in adults [1] and multiple late side effects in children related to the intensity of therapy [2]. In addition, an appreciable subset of patients experience disease relapse after achieving complete remission and relapse typically indicates chemotherapy-refractory disease. Monoclonal antibodies directed at cell-surface antigens offer a targeted approach for treating leukemia and other cancers. In B-cell acute lymphoblastic leukemia (BALL), anti-CD20 monoclonal antibodies have been shown to improve survival when used in the frontline setting [3]. Antibodies directed at CD19 [4] and CD22 [5] offer a less toxic and highly effective approach to eradicate minimal residual disease (MRD), prolong relapse-free survival and have shown effectiveness in patients with relapsed disease. In precursor T-cell acute lymphoblastic leukemia (T-ALL), management is based predominantly on intensive chemotherapy and central nervous system (CNS) prophylaxis followed by stem cell transplant [6]. Antibody-directed therapy in T-ALL is far less intensively explored than B-ALL. With the exception of alemtuzumab (anti-CD52) [7], overall experience with monoclonal antibody-direct therapy in T-ALL is very limited.
Brentuximab vedotin, also known as SGN-35 or ADCETRIS®, is an anti-CD30 chimeric antibody conjugated by a protease-cleavable linker to monomethylauristatin E, an agent that disrupts microtubules[8]. Brentuximab vedotin has been shown to induce durable responses in patients with refractory and relapsed Hodgkin lymphoma and relapsed anaplastic large cell lymphoma with well tolerated side effects [9,10]. The drug received accelerated approval by the United States Food and Drug Administration for patients with relapsed Hodgkin lymphoma and anaplastic large cell lymphoma. We recently showed substantial CD30 expression by myeloblasts in patients with acute myeloid leukemia (AML) and high-grade myelodysplastic syndromes (MDS). CD30 was expressed more frequently in patients with refractory AML being actively treated compared with patients with untreated AML[11]. These data raise the possibility that anti-CD30-targeted therapy may be a potential option for this patient group. In the present study, we extend the investigation to patients with ALL and compare CD30 expression in association with different subtypes of ALL, cytogenetic data, and disease status. In our previous study on MDS/AML, we compared flow cytometry with immunohistochemistry performed on paraffin embedded bone marrow biopsy (decalcified) and/or clot (without decalcification) in over 80 cases[11]. Immunohistochemistry staining was consistently much weaker and focal in all cases examined. Since immunohistochemistry[12,13] was not an optimal method in measuring CD30 expression on blasts, we focused the current study using flow cytometry assay only.
Study Design and Results
Patients and bone marrow samples
The study was conducted in accordance with protocols approved by the Institutional Review Board (IRB) at The University of Texas MD Anderson Cancer Center (MDACC). Between August 2011 and March 2013, we assessed CD30 expression by flow cytometry immunophenotyping in 78 consecutive ALL patients who had bone marrow (BM) samples evaluated at the clinical flow cytometry laboratory. Every case was assessed by a comprehensive panel of flow cytometry markers as well as cytochemistry myeloperoxidase/nonspecific esterase stains as part of the routine clinical work-up. All cases fulfilled the diagnostic criteria of ALL; and cases of mixed phenotype leukemia were not included. For patients with recurrent/relapse disease, prior treatment included Hyper-CVAD (hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone) or modified hyperCVAD for Philadelphia chromosome (Ph)-negative ALL; and hyper-CVAD plus tyrosine kinase inhibitors for Ph-positive ALL; as well as hematopoietic stem cell transplant in some patients. For recurrent/refractory disease, Hyper-CVAD, other salvage chemotherapy regimens and/or various investigating agents were used. These 78 patients included 34 patients with TALL and 44 with B-ALL. In every case we had bone marrow smears available for review. The median blast percentage enumerated from a total 500 cell differential count were 61% (range, 3–97) in T-ALL and 86% (range, 2–99) in B-ALL.
The patient demographic information is shown in Table 1. In brief, patients with T-ALL had a median age of 36 years (range, 6–72 years) with one patient younger than 15 years of age. Patients with B-ALL had a median age of 47 years (range, 4–85years) with two patients younger than 15 years. One case B-ALL and one case of T-ALL presented as blast phase of chronic myelogenous leukemia (CML), and the remaining were de novo ALL. Notably, in this study cohort, only 3/34 (9%) T-ALL and 12/44 (27%) B-ALL patients presented as untreated disease; whereas 31/34 (91%) T-ALL and 32/44 (73%) B-ALL patients had refractory/relapse disease.
Table 1.
Acute Lymphoblastic Leukemia: patient Characteristics and in surface CD30 expression by flow cytometry immunophenotypic analysis
| T-ALL (n=34) | B-ALL (n=44) | p | |
|---|---|---|---|
| Age, median(range), years | 36(6–72) | 47(4–85) | |
| Gender, male/female | 26/8 | 24/20 | |
| Lymphoblasts, median (range)(%) | |||
| • By morphology | 61(3–97) | 86(2–99) | |
| • By flow cytometry | 52(2–98) | 52(2–92) | |
| CD30 expression in all cases, | |||
| median (range)(%) | 3.3 (0.1–86.8) | 2.6 (0.0–68.8) | NS |
| Patients with ≥20%CD30 expression | 13 (38%) | 6 (14%) | 0.017 |
| • CD30+ blasts %, median (range) | 40.0(22.7–86.8) | 39.1(24.9–68.8) | NS |
| • MFI ratio | 3.3 (1.8–37.9) | 4.9 (3.0–8.8) | NS |
| CD30 expression by a 20% cutoff according to disease status and cytogenetics | |||
| Untreated patients | 0/3 (0%) | 1/12 (8%) | * NS |
| Relapsed/persistent patient | 13/31(42%) | 5/32 (16%) | |
| Patients with a normal karyotype | 4/15 (27%) | 2/13 (15%) | * NS |
| Patients with an abnormal karyotype | 9/19 (47%) | 4/31(13%) | |
| Patients with BCR-ABL1 | 1/2 (50%) | 3/14 (21%) | * NS |
| Patients without BCR-ABL1 | 12/32 (38%) | 3/30 (10%) | |
ALL: acute lymphoblastic leukemia. MFI: Mean fluorescence intensity;
NS: not significant. Comparisons were performed between B-ALL and T-ALL; within B-ALL and T-ALL subgroups; or combining B-ALL and T-ALL by disease status and cytogenetic data, calculated by the expression percentage or number of positive cases.
By conventional chromosomal analysis, 19/34 (56%) T-ALL and 31/44 (70%) B-ALL had an abnormal karyotype. BCR/ABL1 by FISH was positive in 2 (6%) T-ALL and 14(32%) B-ALL patients. MLL gene rearrangement was seen in one B-ALL. Real time PCR confirmed the presence of BCR/ABL1 fusion products in all 16 Philadelphia positive (Ph) ALL patients, including 2/34 T-ALL and 14/44 B-ALL. In 14 T-ALL patients tested for mutation, 1 patient had a FLT3-ITD and 2 patients had CEBPA mutations, 1 patient each had a mutation of IDH1, IDH2, or NRAS (total cases with mutation: 4/14, 29%). In 16 B-ALL patients tested, one patient had IDH1 and 2 patients had NRAS mutations (total cases with mutation: 3/16, 19%).
Flow cytometry immunophenotyping
BM aspirates were collected in EDTA-anti-coagulated tubes and processed within 24 hours of collection at our CLIA (Clinical Laboratory Improvement Amendments) certified laboratory. Routine flow cytometry immunophenotypic analysis for T-ALL and B-ALL included a comprehensive panel of markers including: CD1a, CD2, CD3, CD4, CD5, CD7, CD8, CD10, CD13, CD14, CD15, CD19, CD20, CD22, CD25, CD33, CD34, CD36, CD38, CD41, CD45, CD52, CD56, CD58, CD64, CD79a, CD81, CD123, HLADR, TdT, MPO, cytoplasmic CD3 and cytoplasmic IgM. Different combinations of these markers were used according to the disease either as initial diagnostic or follow-up BM sample; and according to B- or T-cell lineage of the ALL. Routine flow cytometry immunophenotyping confirmed the presence of lymphoblasts in all cases. To ensure an accurate assessment, we only included cases with blasts ≥ 2% of total cells by flow cytometry assessment. CD30-PE (HRS clone) was purchased from Beckman Coulter and all other antibodies were purchased from BD Biosciences. The panel for CD30 assessment on T-ALL was as follows: Tube1, autofluorescent control, CD7-APC/ CD45-V500 without CD30; Tube2, CD3-FITC; CD30-PE; CD34-PerCP-Cy5-5; CD7-APC; CD45-V500. The panel for CD30 on B-ALL was as follows: Tube 1, autofluorescent control, CD19-APC/CD45-V500 without CD30-PE; Tube2, CD30-PE; CD34-PerCP-Cy5-5; CD19-APC; CD45-V500. A total of 200,000 events per tube were acquired on FACSCanto II instruments (BD Biosciences). Data analysis was performed using FCS Express software (De Novo Software, Los Angeles, CA).
CD30 expression on lymphoblasts: T-lymphoblasts were identified by CD45 dim+/CD7+ (Figure 1 upper panel) and B-lymphoblasts by CD45dim+CD19+ (Figure 1 lower panel). Surface CD30 expression was assessed specifically on lymphoblasts. The intensity of expression was measured by mean fluorescence intensity (MFI) as compared with autofluorescence control. Fisher’s exact test or chi-square was used for comparison of categorical variables and the t test or one-way analysis of variance (ANOVA) was applied for numerical comparisons. A p value of <0.05 was considered to be statistically significant. For the entire study group, the median percentage of T-lymphoblasts positive for CD30 was 3.3% (range, 0.1%–86.8%) with a median MFI ratio of 1.7 (range, 0.4–37.9) whereas, the median percentage of B-lymphoblasts positive for CD30 was 2.6% (range, 0.0%–68.8%) with a median MFI ratio of 1.2 (range, 0.5–8.8). Without knowing what level of CD30 expression would be significant for potential Brentuximab vedotin target therapy, a 20% cutoff, which is generally adopted in flow cytometry to call an expression “positive”, was used. Overall, by a 20% cutoff, 13 of 34 (38%) T-ALL and 6 of 44 (14%) cases were positive for CD30 expression. Focusing only on CD30 positive cases, the median percentage of positive lymphoblasts in T-ALL was 40.0% (range, 22.7–86.8) with a MFI 3.3 (range, 1.8–37.9) and in B-ALL, 39.1% (range, 24.9–68.8) with a MFI of 4.9 (range, 3.0–8.8) in B-ALL. Overall, CD30 expression was more frequently observed in T-ALL than B-ALL using a 20% cutoff (p=0.017).
Figure 1.
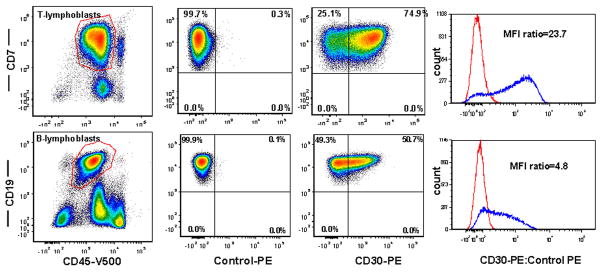
Flow cytometry analysis of surface CD30 expression. T-lymphoblasts are identified by CD45dim+CD7+ (upper panel) and B-lymphoblasts are identified by CD45dim+CD19+ (lower panel). CD30 expression is assessed specifically on lymphoblasts over auto-fluorescence control and presented as percent of CD30 expression as well as mean fluorescence Intensity (MFI) ratio.
We observed no difference in CD30 expression between patients with Ph-positive ALL (14 BALL and 2 T-ALL) versus Ph-negative ALL (32 T-ALL and 30 B-ALL) (Table 1). We also compared CD30 expression by disease status. In T-ALL, we observed no CD30 expression in all 3 newly diagnosed (no therapy) patients, but in13 of 31 (42%) patients who presented with persistent, refractory, or relapsed disease, the lymphoblasts were positive (>20%) for CD30. In 5 T-ALL patients, we performed CD30 assessment on follow-up BM samples at a median interval of 2 months (range, 1–7 months) and observed increased CD30 expression in all 5 cases (p=0.025) (Figure 1). For 4 B-ALL patients (three with <5% and one with 18% CD30 expression) we performed flow cytometry immunophenotypic analysis in sequential BM samples; no increase in CD30 expression in any of the cases. Furthermore, in B-ALL patients CD30 expression did not differ between patients with untreated versus refractory (to high dose chemotherapy) disease (Table 1).
Discussion
In this study, we detected surface CD30 expression by lymphoblasts in a subset of patients with acute lymphoblastic leukemia. Using a 20% cutoff, CD30 was expressed more frequently in TALL), 38%, than in B-ALL, 14%. The frequency and intensity of CD30 expression in T-ALL is similar to what we reported previously in AML and high-grade myelodysplastic syndromes (MDS)[11,12]. In AML/MDS, we observed more frequent CD30 expression in patients with refractory/persistent disease as compared with untreated patients, and therefore we hypothesized that CD30 expression could be upregulated by various therapies. In T-ALL, although only 3 patients presented with untreated disease, we were able to show increased CD30 expression by lymphoblasts in sequential BM samples from 5 patients with persistent/refractory T-ALL following high-dose chemotherapy. This finding provides direct evidence that CD30 expression can be up-regulated by leukemia therapy in T-ALL patients. A similar finding has been reported in patients with B-ALL; CD20 expression is up-regulated during induction therapy in a substantial proportion of patients, and the process is continued and sustained until end-induction, which may be attributable to the actions of glucocorticoids [14]. Our patients with MDS/AML in previous study[11] were mostly treated with induction chemotherapy or hypomethylating agents without dexamethasone, arguing against a pure glucocorticoid effect on upregulated CD30 expression.
Unlike the case for AML/MDS, where we observed higher CD30 expressions in cases with high-risk cytogenetic abnormalities, we found no correlation between CD30 expression and cytogenetic data in T-ALL. Patients with AML/MDS with a poor cytogenetic risk score more frequently presented with refractory disease; in contrast, the importance of karyotypic abnormalities is less well established in T-ALL patients [15]. In contrast with T-ALL, CD30 was expressed less frequently by lymphoblasts in B-ALL. CD30 expression by neoplasms derived from B-cell lineage varies greatly, from 100% in classical Hodgkin lymphoma, to approximately 15–20% in diffuse large B cell lymphoma not otherwise specified (DLBCL NOS)[16], to less than 5% in plasma cell neoplasms [13]. Due to a low frequency of expression, comparisons of CD30 expression with treatment status in B-ALL or cytogenetic alterations may not be meaningful. However, CD30 expression was not upregulated in 4 patients in whom sequential BM samples were examined. In addition, CD30 expression showed no difference between cases of ALL with and without BCR-ABL1.
In summary, using flow cytometry immunophenotypic analysis we show that a substantial subset of patients with T-ALL have lymphoblasts that express surface CD30. CD30 expression by T-lymphoblasts also appears to be upregulated in patients who are treated with high-dose chemotherapy. The data we present here raise the possibility that anti-CD30-targeted therapy may be a potential option for patients with T-ALL, particularly those patients with refractory or relapsed disease.
Figure 2.
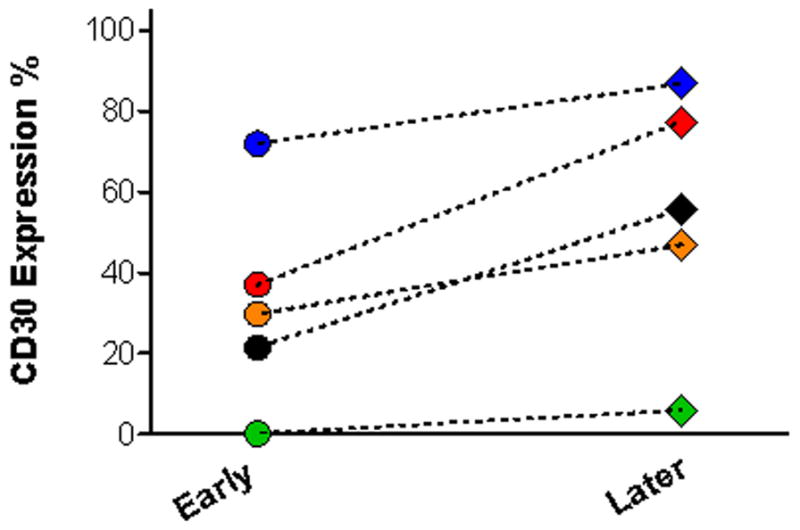
Flow cytometry analysis of CD30 expression in 5 patients with persistent precursor T-lymphoblastic leukemia (T-ALL) after receiving intense chemotherapy. Follow-up bone marrow samples were collected in a median interval of 2 months (1–7 months), CD30 expression is upregulated in all 5 patients (p=0.025).
Acknowledgments
The authors thank the staff members of the flow cytometry laboratory at MD Anderson Cancer Center for their support.
Footnotes
Disclosure of Potential Conflicts of Interest
Dr. Sa A. Wang received research funding from Seattle Genetics, Inc. and supported by the joint financial resources of Seattle Genetics, Inc. and Millennium: The Takeda Oncology Company.
References
- 1.Huguet F, Leguay T, Raffoux E, et al. Pediatric-inspired therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: the GRAALL-2003 study. J Clin Oncol. 2009;27:911–918. doi: 10.1200/JCO.2008.18.6916. [DOI] [PubMed] [Google Scholar]
- 2.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 3.Thomas DA, O’Brien S, Faderl S, et al. Chemoimmunotherapy with a modified hyper-CVAD and rituximab regimen improves outcome in de novo Philadelphia chromosome-negative precursor B-lineage acute lymphoblastic leukemia. J Clin Oncol. 2010;28:3880–3889. doi: 10.1200/JCO.2009.26.9456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topp MS, Kufer P, Gokbuget N, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29:2493–2498. doi: 10.1200/JCO.2010.32.7270. [DOI] [PubMed] [Google Scholar]
- 5.Kantarjian H, Thomas D, Jorgensen J, et al. Inotuzumab ozogamicin, an anti-CD22-calecheamicin conjugate, for refractory and relapsed acute lymphocytic leukaemia: a phase 2 study. Lancet Oncol. 2012;13:403–411. doi: 10.1016/S1470-2045(11)70386-2. [DOI] [PubMed] [Google Scholar]
- 6.Fielding AK, Banerjee L, Marks DI. Recent developments in the management of T-cell precursor acute lymphoblastic leukemia/lymphoma. Curr Hematol Malig Rep. 2012;7:160–169. doi: 10.1007/s11899-012-0123-4. [DOI] [PubMed] [Google Scholar]
- 7.Tibes R, Keating MJ, Ferrajoli A, et al. Activity of alemtuzumab in patients with CD52-positive acute leukemia. Cancer. 2006;106:2645–2651. doi: 10.1002/cncr.21901. [DOI] [PubMed] [Google Scholar]
- 8.Katz J, Janik JE, Younes A. Brentuximab Vedotin (SGN-35) Clin Cancer Res. 2011;17:6428–6436. doi: 10.1158/1078-0432.CCR-11-0488. [DOI] [PubMed] [Google Scholar]
- 9.Younes A, Bartlett NL, Leonard JP, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 363:1812–1821. doi: 10.1056/NEJMoa1002965. [DOI] [PubMed] [Google Scholar]
- 10.Rothe A, Sasse S, Goergen H, et al. Brentuximab vedotin for relapsed or refractory CD30-positive hematologic malignancies: the GHSG experience. Blood. 2012 doi: 10.1182/blood-2012-05-430918. [DOI] [PubMed] [Google Scholar]
- 11.Zheng W, Medeiros LJ, Hu Y, et al. CD30 Expression in High-Risk Acute Myeloid Leukemia and Myelodysplastic Syndromes. Clin Lymphoma Myeloma Leuk. 2013 doi: 10.1016/j.clml.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fathi AT, Preffer FI, Sadrzadeh H, et al. CD30 expression in acute myeloid leukemia is associated with FLT3-internal tandem duplication mutation and leukocytosis. Leuk Lymphoma. 2013;54:860–863. doi: 10.3109/10428194.2012.728596. [DOI] [PubMed] [Google Scholar]
- 13.Zheng W, Liu D, Fan X, et al. Potential therapeutic biomarkers in plasma cell myeloma: A flow cytometry study. Cytometry B Clin Cytom. 2013 doi: 10.1002/cyto.b.21083. [DOI] [PubMed] [Google Scholar]
- 14.Dworzak MN, Schumich A, Printz D, et al. CD20 up-regulation in pediatric B-cell precursor acute lymphoblastic leukemia during induction treatment: setting the stage for anti-CD20 directed immunotherapy. Blood. 2008;112:3982–3988. doi: 10.1182/blood-2008-06-164129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marks DI, Paietta EM, Moorman AV, et al. T-cell acute lymphoblastic leukemia in adults: clinical features, immunophenotype, cytogenetics, and outcome from the large randomized prospective trial (UKALL XII/ECOG 2993) Blood. 2009;114:5136–5145. doi: 10.1182/blood-2009-08-231217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campuzano-Zuluaga G, Cioffi-Lavina M, Lossos IS, Chapman-Fredricks JR. Frequency and Extent of CD30 Expression in Diffuse Large B-Cell Lymphoma and its Relation to Clinical and Biologic Factors: A retrospective study of 167 cases. Leuk Lymphoma. 2013 doi: 10.3109/10428194.2013.778407. [DOI] [PubMed] [Google Scholar]


