Abstract
HIV-associated neurocognitive disorders (HAND) is a group of syndromes of varying degrees of cognitive impairment affecting up to 50 % of HIV-infected individuals. The neuropathogenesis of HAND is thought to be driven by HIV invasion and productive replication within brain perivascular macrophages and endogenous microglia, and to some degree by restricted infection of astrocytes. The persistence of HAND in individuals experiencing suppression of systemic HIV viral load with antiretroviral therapy (ART) is incompletely explained, and suggested factors include chronic inflammation, persistent HIV replication in brain macrophages, effects of aging on brain vulnerability, and comorbid conditions including hepatitis C (HCV) co-infection, substance abuse, and CNS toxicity of ART, among other factors. This review discusses several of these conditions: chronic inflammation, co-infection with HCV, drugs of abuse, aging, and antiretroviral drug effects. Effectively managing these co-morbid conditions in individuals with and without HAND is critical for improving neurocognitive outcomes and decreasing HIV-associated morbidity.
Keywords: Inflammation, Human immunodeficiency virus, HIV, HIV associated neurocognitive disorders, HAND, Immune activation, Neurotoxicity, Hepatitis C, HCV, Aging, Drug abuse, Neuropathogenesis, MRI, Magnetic resonance imaging, fMRI, Functional magnetic resonance imaging, Neurocognitive testing, Microbial translocation, Antiretroviral therapy, ART
Introduction
Current estimates by the World Health Organization state that approximately 34 million individuals are currently infected with human immunodeficiency virus (HIV), which is associated with a high prevalence of neurocognitive dysfunction even in individuals receiving combination antiretroviral therapy (cART). HIV-associated neurocognitive disorders, or HAND, is the term used to describe a group of syndromes (asymptomatic neuropsychological impairment (ANI), HIV-associated mild neurocogntive disorder (MND), and HIV-associated dementia (HAD), of varying severity of impairment of cognition and associated functioning in HIV-infected individuals [1]. The effectiveness of ART in suppressing HIV replication to levels below the limit of detection in most infected individuals has resulted not only in increased longevity but also increased risk for end-organ dysfunction that can contribute to effects of HAND in aging individuals [2••]. Among these co-morbid effects of aging is the dysfunction of extra-neural organ systems, including heart, liver, and kidney. Other significant co-morbidity factors that are not necessarily linked to aging are chronic inflammation, co-infection, particularly with hepatitis C, substance abuse, and potential central nervous system neurotoxicity of ART. The persistent HAND risk in ART-treated individuals with effective suppression of systemic HIV load represents a major therapeutic challenge that requires targeting of persistent pathological host inflammatory responses to HIV infection as well as consideration of morbidity factors not directly resulting from HIV infection [3].
Chronic Immune Activation, Inflammation and Systemic HIV Disease Progression
A growing body of evidence has defined chronic inflammation associated with immune activation as a major contributor to systemic and CNS disease progression in HIV-infected individuals despite the life-saving benefits of ART [4••]. Although ART significantly reduces morbidity and mortality in HIV-infected individuals, lower life expectancy remains, particularly in individuals beginning ART after becoming immunosuppressed (CD4 T-lymphocyte counts<200 cells/µl) [5, 6]. Such chronic immune activation is thought to result from increased microbial translocation across the damaged gastrointestinal tract, which might harbor more pathogenic bacterial populations than in non-infected individuals [7••, 8••]. In HIV-infected humans and simian immunodeficiency virus (SIV)-infected macaques gut microbial translocation results from depletion of mucosal CD4+ T lymphocytes, mucosal immune activation and inflammation, damage to mucosal epithelium, and transmigration of microbial products into the mesenteric lymph nodes and distal sites through the portal and systemic circulation [7••]. The translocated microbial products include lipopolysaccharide (LPS) [7••, 9, 10], which associates with LPS-binding protein (LBP) in a complex that binds to monocyte CD14 and toll-like receptor 4 (TLR4). The resulting immune activation is associated with induction of NF-κB activation and cytokine production and associated events [11].
Sustained immune activation and associated inflammation are thought to drive HIV pathogenesis despite effective suppression of systemic HIV replication [9, 10, 12]. Elevated plasma levels of the associated biomarkers IL-6, D-dimer, C-reactive protein (CRP), and fibrinogen correlate strongly with HIV mortality in such individuals [13]. Monocyte activation is associated with elevated blood levels of LPS, soluble CD14 (sCD14), and monocyte-associated CD14 and CD16 [14]. The CD16+ monocyte subset is more permissive for HIV infection than the CD16-subset [15], and the level of circulating CD14+/CD16+ monocytes correlates with an increased risk of disease progression [14]. Interactions of LPS derived from gut microbial translocation with LBP and monocyte CD14 can activate monocytes and LBP/TLR interactions can activate T-lymphocytes and induce effector T-lymphocyte sequestration in lymphoid tissues and T-lymphocyte turnover. The associated T-lymphocyte turnover could generate new memory CD4+ T lymphocytes, thus providing additional targets for HIV replication [7••, 16]. Depletion of both CD4+ and CD8+ T-lymphocyte populations, which is strongly associated with disease progression, occurs in late stages of disease and is associated with and proposed to be caused by immune activation [7••].
Because systemic immune activation is driven by HIV replication, suppression of HIV replication with ART suppresses such immune activation; however this suppression is often incomplete [7••, 9, 17, 18]. The presence of viral ‘blips’, defined as periodic detection of viral RNA in plasma which spontaneously returns to undetectable levels in chronically ‘suppressed’ ART-treated individuals has been associated with increased expression of immune activation markers and accelerated disease progression [19•]. The significance of these ‘blips’ remains undetermined, although their association with immune activation in the systemic and CNS compartments is receiving intense scrutiny [19•, 20••, 21]. Nonetheless, the persistence of immune activation despite suppression of HIV replication with our without viral ‘blipping’ remains a major obstacle to preventing further disease progression in ART-treated individuals. Successful therapeutic targeting of persistent immune activation has now been proposed as a critical goal for further improving long-term survival, lowering risk of associated end-organ diseases, and improving quality of life in HIV-infected cART-treated individuals [7••, 16].
Chronic Immune Activation, Inflammation and CNS HIV Disease Progression
Chronic systemic immune activation likely reflects combined effects of translocated microbial products in the circulation and by HIV replication in CD4+ T-lymphocytes (when ART suppression is incomplete), while CNS effects of infection are predominantly driven by HIV replication in macrophages/microglia, and possibly astrocytes. Productive HIV replication in the brain occurs primarily within perivascular monocyte-derived macrophages (MDM) and microglia, while restricted, non-productive replication occurs in astrocytes (reviewed in [22]). Persistent CNS HIV infection drives immune activation of resident macrophages/microglia, pervasive reactive astrocytosis, perivascular inflammation and infiltration of monocytic cells, which can result in HIV-encephalitis (HIVE), particularly in individuals not receiving ART. The introduction of ART has decreased morbidity/mortality of HIV infection, the severity of neurocognitive impairment and associated neuroinflammation in HAND, and the prevalence of encephalitis [22]. Despite these beneficial effects of ART, neuroinflammation and neuropathological damage persists [23, 24], and persistent systemic and CNS markers of monocyte/macrophage activation predict neurocognitive impairment [25–27], even in individuals with excellent virological suppression [28, 29, 30•, 31].
In summary, current research highlights the central role of macrophages/microglia and persistent neuroinflammation in HAND in individuals receiving ART. Although ART has decreased the prevalence of the severest form of HAND, HIV-associated dementia (HAD), a significant and perhaps increasing proportion of patients on ART (30–50 %) suffer from HAND, despite reduced plasma viremia, improved immunological parameters, and decreased progression to AIDS [32–34, 35••]. The high prevalence of HAND and other neurologic manifestations of HIV-infection despite the introduction of ART indicates a critical need for adjunctive therapies that target the persistent inflammation within the CNS compartment.
ART CNS Neurotoxicity
Whether CNS-targeted therapy based upon putative level of CNS ART-penetrance (CNS penetration-effectiveness/CPE), antiviral activity, or even neurotoxicity of a particular drug class will prove effective in limiting HAND is unclear. Early studies of ART effects on neurocognitive test performance suggested that ART regimens containing drugs with relatively higher CPE could have increased benefit in protection against neurocognitive impairment [36]. Other studies have either no benefit to selected ‘neuroactive’ ART regimens or even neurotoxic effects. Two multi-center clinical trials (ACTG 5170, ACTG 736) have suggested possible neurotoxicity of ART, as reflected in improvement in neuropsychological test performance after ART interruption (ACTG 5170 [37]) and poorer performance in patients on ART regimens with a higher CPE (ACTG 736) [38]. A recent study showed that virologically suppressed patients (>one year on a stable regimen, undetectable viral loads) receiving protease-inhibitors (PIs) as ‘boosted monotherapy’ or as triple therapy with two nucleoside reverse transcriptase inhibitors showed similar patterns of neuropsychological test performance in 14 measures [39•]. These results were interpreted as evidence against the number of neuroactive drugs used in a regimen, rather than effective systemic viral load suppression, as an indicator or predictor of neuroprotection. Nonetheless, the issue of chronic ART neurotoxicity as a co-factor for exacerbating HAND remains as a critical and unsettled question in long-term management of HIV infection.
Hepatitis C Co-infection as a Risk for Worsening HAND
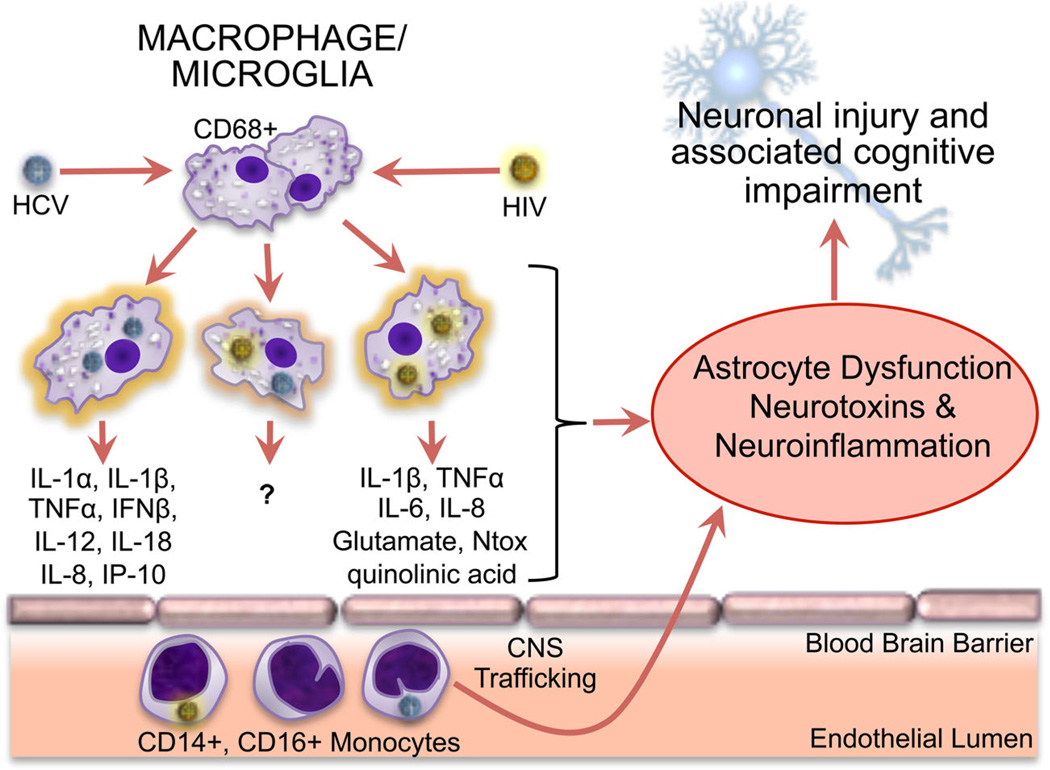
Detection of hepatitis C virus (HCV) genomes and active HCV replication within the CNS has prompted great interest in HCV infection as a contributor to neurocognitive impairment in HIV-infected individuals [40, 41••, 42]. One recent estimate of the prevalence of HCV infection across all populations within the US at 2 % [43] and other studies estimate that 20–30 % of HIV+ individuals worldwide are co-infected with HCV [44, 45]. These high rates of co-infection emphasize the potentially high risk of HCV increasing the morbidity associated with HAND when both viruses infect the brain. Indeed, replicating HCV has been detected in autopsied brain tissue predominantly in CD68+ macrophages/microglia, and to a lesser degree in astrocytes, which suggests a shared HCV/HIV reservoir within the brain [46] (Fig. 1). Furthermore, brain macrophage HCV infection is associated with increased macrophage expression of pro-inflammatory cytokines [47], which is also a feature shared with HIV infection of macrophages that can contribute to neurodegeneration.
Fig. 1.
CNS inflammatory effects of HIV and HCV infection of monocytes, macrophages, and microglia. CD14+/CD16+ monocytes can be infected by HCV and HIV and migrate across the endothelial barrier into the brain. Macrophages and microglia within the brain can support productive infection with HIV and HCV, resulting in the production of pro-inflammatory cytokines and neurotoxins (glutamate, Ntox, quinolinic acid). Those associated with HCV infection are depicted in the left-most macrophage image, and those associated with HIV infection are depicted in the right-most macrophage image. Such pro-inflammatory cytokines and neurotoxins have been shown to induce neuronal injury through direct and indirect (astrocyte-mediated) mechanisms
The prevalence of neurocognitive dysfunction attributable to HCV infection alone is difficult to determine and probably highly variable, with estimates ranging from ~10–49 % [43, 44], while the prevalence of neurocognitive dysfunction in HIV/HCV co-infection has been estimated as high as ~63 % [48]. Individuals seropositive for HCV are twice as likely to have global neuropsychological impairment than seronegative individuals, independent of HIV infection [45]. Such impairment is commonly associated with metabolic and structural brain abnormalities, as detected in magnetic resonance spectroscopy (MRS) and magnetic resonance imaging (MRI) studies. Several MRS studies have found metabolic alterations consistent with neuronal injury and gliosis (decreased N-acetyl-aspartate/creatine and increased choline/creatine ratios) in the brains of HCV+/HIV− individuals with cognitive impairment but without hepatic failure [42, 49]. Furthermore, hyperperfusion in the basal ganglia, suggestive of neuroinflammation, has been detected in HCV+ individuals by brain perfusion-weighted neuroimaging [49]. An MRS study of 15 HCV+/HIV− individuals treated with pegylated interferon/ribavirin demonstrated decreased brain choline/creatine and myoinositol/creatine ratios in individuals with sustained HCV suppression, suggesting a potential brain neuroprotective/antiinflammatory response with anti-HCV treatment [41••]. One structural brain MRI analysis of 251 HIV+ individuals without significant co-moribidities for cognitive impairment other than HIV infection demonstrated a greater loss of white matter volume in HCV+/HIV+ individuals in comparison with HCV−/HIV+ individuals [50]. Notably, a significant correlation between plasma lipopolysaccharide levels and HAND was demonstrated in an HIV/HCV co-infected cohort, despite no significant correlation in HIV− or HCV− monoinfected subcohorts [51]. These studies clearly demonstrate that HCV infection is associated with functionally significant injury to the brain that can potentially exacerbate the injurious effects of HIV infection, and they further suggest that induced neuroinflammation is a shared major contributor.
Although the association between HIV and HCV co-infection and worsened neurocognitive functioning has been observed in numerous studies, the relationship between active HCV replication (as indicated by detectable plasma HCV RNA) and worsening neurocognitive function has been questioned. A study of 24 HCV+ individuals with newly detectable plasma HCV RNA within 12 months of study showed worse neurocognitive performance in comparison with 57 individuals with HIV monoinfection [52]. This was associated with MRS evidence of glial activation/inflammation in the basal ganglia. Contrasting results were seen when The AIDS Clinical Trials Group (ACTG) Longtidunal Linked Randomized Trials (ALLRT) study examined the relationship between HCV replication and neurocognitive performance in 517 HIV-infected individuals, of which 172 demonstrated detectable plasma HCV RNA (345 individuals had no detectable plasma HCV RNA). Although both groups performed below norms, there was no discordance in neurocognitive function between the two groups [53, 54], suggesting that active HCV replication, at least that outside of the CNS, does not drive neurocogntive dysfunction. Nonetheless, consistent with other studies of HCV seropositive (HCV+) patients, an earlier ACTG study confirmed that HCV infection, as indicated by HCV antibody detection (HCV seropositive) in HIV+ individuals is correlated with worse neurocognitive performance than that in HIV+ individuals not co-infected with HCV [55].
Thus, considerable evidence supports a significant negative impact of HCV infection on HAND and the high prevalence of HAND and HIV/HCV co-infection requires that effective suppression of both HIV and HCV be a high priority. The recent FDA approval (December 16, 2013) of the anti-HCV drug, Sofosbuvir, a nucleotide polymerase inhibitor, for HCV monoinfection and HCV/HIV co-infection represents a major step in the struggle to cure HCV infection [56••]. This approval could potentially represent a highly effective means of preventing neurocognitive deterioration in HIV+ patients newly infected with HCV and it also raises the question of the possibility of improving cognitive performance in HAND individuals with co-infection. A therapeutic trial of Sofosbuvir as treatment for or prevention of neurocognitive impairment in HIV+/HCV+ individuals is clearly indicated.
Drug Abuse and HAND
Substance abuse often represents a co-morbid condition with HIV infection, and evidence supports additive or synergistic effects of substance abuse on the persistence and severity of neurocognitive dysfunction in patients with HAND [57]. Under strict diagnostic criteria, a diagnosis of HAND requires the exclusion of other co-morbid conditions that may contribute to neurocognitive impairment, including drug abuse [1]. However, drug abuse is common in HIV infected populations and is a major co-morbidity risk for neurocognitive impairment in HIV+ individuals [58, 59]. In the United States approximately one in four HIV+ individuals studied from 2005 to 2009 required treatment for alcohol or drug abuse [60]. Thus, studying the interactions between drug abuse, HIV infection, and neurocognitive function is of high clinical importance. Although, the potential direct neurotoxic effects of drugs of abuse are not the focus of this review, drug effects that may synergize with HIV-mediated neurocognitive impairment are discussed (Table 1).
Table 1.
Effects of cocaine, methamphetamine, opioids, and dopamine on HIV, SIV, and neuroinflammation
| Cocaine | Meth | Opioids | Dopamine | |
|---|---|---|---|---|
| Risk of neurocognitive impairment in HIV+ individuals with current or recent use of selected drug | ↑ | ↑ | ↑ | N/A |
| HIV replication in human macrophages | ↑/− | ↑ | ↑ | ↑ |
| Monocyte/macrophage CCR5 expression | ↑ | ↑ | ↑ | ? |
| Monocyte/macrophages CXCR4 expression | ↑ | ↑ | ↑/− | ? |
| HIV replication in human T-lymphocytes | ↑↑ | ↑/− | ↑ | ↑ |
| CSF/brain SIV viral load in macaque model | ↓/− | ↑ | ↑ | ↑ |
| Proinflammatory cytokine release from macrophages/microglia/astrocytes | ↑ | ↑ | ↑ | some ↑ some ↓ |
| Anti-inflammatory cytokine release from macrophages/microglia/ astrocytes | ↑/− | ? | ↓/− | ↓ |
| Blood brain Barrier breakdown following exposure in rodent models | ↑ | ↑ | ↑ during withdrawal | ? |
Symbols used: ↑ increased; ↓ decreased; ↑/− increase reported in some studies, but not others; ↓/− decrease reported in some studies, but not others; ? no current data; N/A not applicable
Numerous studies have implicated cocaine, methamphetamine (meth), and opioid use in exacerbating the risk for neuronal injury and neurocognitive impairment in HIV+ patients [57, 61, 62••, 63]. However, a recent large cohort study found that participants with histories of substance use (alcohol, cocaine, cannabis, opiates, methamphetamine) did not have higher rates of neurocognitive impairment or functional impairment in everyday life [62••].Most of these participants were not current users and less than a third reported using illicit substances within the last year. The authors propose that sustained periods of drug abstinence may be sufficient for full or partial reversal of substance use effects on neurocognition. These findings are consistent with another longitudinal study demonstrating improvement in neurocognitive function in long-term abstinent meth users (average of 13 months) compared to non-abstinent meth users [64]. These results suggest that drugs of abuse may have a limited legacy effect on neurocognitive impairment in HIV+ individuals and that current and recent substance use may be more relevant to modulating HIV neuropathogenesis.
Cocaine, meth, and opioids have been shown to increase HIV infection/replication in primary human macrophages in vitro [65–67]. Cocaine and opioids also increase HIV infection and replication in T-lymphocytes in vitro [68, 69]. The effects of meth on T-lymphocyte HIV replication remain controversial due to variations in models, viral strains, exposure time, and drug concentrations used [70•]. Some studies suggest that dopamine, which is elevated within the CNS in response to these drugs of abuse, can enhance macrophage HIV infection and replication as well as alter macrophage cytokine production [71, 72]. These findings are consistent with studies demonstrating that treatment of SIV-infected rhesus macaques with selegiline, an inhibitor of dopamine catabolism through inhibition of MAO activity, or L-DOPA, the precursor of dopamine, resulted in elevated brain viral loads [73, 74]. In the SIV-macaque model, methamphetamine and opioid exposure increased both CSF/brain viral loads and macrophage influx into the CNS [75, 76]. In contrast, cocaine exposed SIV-infected macaques did not show elevated CNS viral replication or alterations in inflammatory markers; notably, the low dose cocaine-treated animals had significantly lower CSF viral RNA [77]. The relative contribution of the direct drug effects or indirect dopamine effects on CNS viral loads and macrophage influx is not known. Taken together these data suggest that some drugs of abuse may increase HIV replication within CNS macrophage/microglia, which likely results in enhanced neuroinflammation, HIV-infected macrophage/microglia neurotoxin production, and associated neuronal dysfunction.
Further supporting a role for drugs of abuse increasing neuroinflammation and associated neurodegeneration, the pathological diagnosis of HIV-encephalitis is more common in HIV+ individuals who abuse drugs compared to HIV+ controls [78, 79]. The relative additive and synergistic effects of drugs of abuse on neuroinflammation in HIV+ individuals is not known. Both in vitro and in vivo data suggest that cocaine, meth, and opioids promote increased proinflammatory cytokine release and activation of microglia, perivascular macrophages, and astrocytes [78, 79]. Increased activation of these glial cells within the CNS drives oxidative stress, excitotoxicity, and inflammatory neuronal injury. Furthermore, distinct mechanisms have been identified for augmenting neurotoxin production by astrocytes. Exposure to opioids (morphine) or meth has been shown to inhibit astrocyte regulation of extracellular glutamate, an excitotoxin strongly implicated in HAND [80]. Cocaine may potentiate astrocyte toxicity through apoptotic pathways involving reactive oxygen species generation, mitochondrial membrane potential loss, and activation of the inflammatory NF-κB pathway [81]. In conclusion, drugs of abuse may induce neuronal injury through potentiation of glia pro-inflammatory signaling and through perturbation of astrocyte homeostatic functioning; additionally, these insults may be further promoted through drug-induced increases in CNS HIV replication.
During HIV infection, both viral and host factors alter the stability and function of the blood brain barrier (BBB) and this is believed to be a critical driver of HIV neuroinflammation and neuropathogenesis [82•]. Drugs of abuse have been proposed to exacerbate neurocognitive impairment in HIV-infection through further disruption of the BBB, resulting in enhanced immune cell infiltration, elevated CNS inflammation, and alteration in cellular homeostasis. In rodent models, cocaine and meth exposure increase BBB breakdown [83]. Cocaine and meth also alter tight junction and cell adhesion molecule expression, permeability, and immune cell migration across brain microvascular endothelia [84, 85]. In contrast, chronic opioid (morphine) exposure did not significantly alter BBB integrity in rats [86]; however, spontaneous morphine withdrawal in rats results in significant BBB breakdown in multiple brain regions, including the cerebral cortex, hypothalamus, hippocampus, and cerebellum [85]. The HIV and drug-mediated BBB dysfunction promotes immune cell infiltration (HIV-infected and non-infected) into the CNS and promotes neuroinflammation, which in turn may drive further loss of BBB integrity.
Lastly, the treatment of drug abuse is likely to improve drug adherence to ART treatment, which remains the best currently available intervention for limiting the severity of neurocognitive impairment and associated neuroinflammation in HAND. Current drug use and alcohol abuse are associated with poorer adherence to ART regimens as well as a decreased or absent access and/or use of HIV care [87]. These obstacles are an important barrier to appropriate treatment of HIV-infection and associated comorbidities, including neurocognitive impairment, in HIV+ drug users. Treating opioid addiction with methadone maintenance or buprenorphine has been shown to increase adherence to ART therapy and improve patient outcomes in HIV+ individuals with opioid abuse history [88]. A review of 20 studies examining the effect of substance use on HIV disease progression found that drug use associated with more rapid HIV disease progression, even after controlling for ART adherence [89]. Thus beyond the effects of drug abuse on ART adherence, drug abuse may affect HIV systemic and CNS disease progression, in part through the mechanisms outlined above.
Thus, drugs of abuse are strongly associated with worsening neuronal injury and neurocognitive impairment in HIV+ individuals. These drugs of abuse likely act through multiple mechanisms to promote neuronal dysfunction including i) increasing HIV infection and replication, ii) reducing BBB integrity, iii) stimulating neuroinflammation, iv) augmenting neurotoxin production and handling by astrocytes and macrophages, and v) decreasing ART regimen adherence. Effective treatment of neurological complications in the HIV+ population will require special clinical attention to drug use and abuse.
Aging and HAND
By 2015 more than 50%of the HIV+ population in the United States will be older than 50 years of age and therefore at increased risk for neurocognitive dysfunction when compared with age-matched seronegative populations [2••]. As ART-treated patients age, they are at risk for associated CNS diseases and systemic diseases that can contribute to cognitive dysfunction, including renal failure and cardiovascular/atherosclerotic disease. Among the CNS diseases prevalent in aging, Alzheimer’s disease (AD) and Parkinson’s disease express some pathological features that have been seen in HIV+ brain tissue [90, 91]. Thus, the brain in aging HIV+ individuals might be particularly vulnerable because of the combined effects of age-associated systemic diseases that indirectly affect the brain, and/or direct brain effects of HIV infection and aging (reviewed in [4••]).
Neuropathological and Biochemical Changes in the Aging HIV-Infected Brain
The neuropathological changes in the aging HIV+ brain and the relationship to neurocognitive dysfunction have received considerable attention but a consistent picture has not emerged. Demonstrations of AD- and PD-like brain neuropathology in ART-treated individuals include elevated levels of hyperphosphorylated Tau (p-Tau), intra- and extracellular β-amyloid (Aβ), and alpha-synuclein [91–93]. An association between the apolipoprotein E (APOE) e4 allele and increased diffuse β-amyloid plaques in HIV-infected brain was recently demonstrated in a study of 160 brains from HIV+ individuals (age range 27–67 years) [94]. This study further demonstrated that age increased age also independently increased the likelihood of plaque deposition. A recent and much smaller brain autopsy study of ten ART-treated individuals (and ten seronegative controls; all patients between 28–58 years of age) showed that brain β-amyloid deposition was increased in HIV+ intravenous drug users, but this did not correlate with brain viral load [95]. Correlations among lower expression of markers of autophagy, increased expression of markers of brain inflammation, reduction of the Beclin 1 protein that forms a critical core for the autophagosome, and older age in individuals with HIVE was demonstrated in a recent neuropathological study [96]. Although β-amyloid deposition was not studied in those cases, the known association between Beclin 1 deficiency, low expression of autophagy markers, and increased risk for β-amyloid deposition in AD brain [97••] suggests the possibility of similar associations in HIVE brain, but whether such associations exist in nonencephalitic HAND cases is not yet reported. Thus, the issue of aberrant processing of amyloid, synuclein, and tau proteins in the HIV-infected brain and the relationship to aging in ART-treated individuals remains unresolved and additional studies are needed to address the potential roles for such aberrant protein processing in HIV neuropathogenesis.
Neurocognitive Performance in Aging HIV+ Cohorts
Determining whether effects of HIV and aging on neurocognitive functioning are truly independent or additive has been a priority of recent longitudinal neuropsychological and neuroimaging studies. A study of 54 HIV+ and 30 HIV-individuals (40–74 years of age) assessed longitudinally over one year for performance on tests of verbal and visuopatial learning and memory [98]. There were significant effect of age group and HIV × age group interactions on learning and memory performance between baseline and one year-follow up examination. The investigators concluded that a decline in performance in HIV+ individuals when compared with HIV-controls was enhanced through an additive age effect. Recently summarized data from the HIV Neurobehavioral Research Center in San Diego showed that global neurocognitive performance declined more rapidly with age in HIV+ individuals than in HIV− matched controls [2••]. The difference in rate of decline of performance vs. age (slope of regression line) indicated that the effects of aging on neurocognitive performance are amplified by HIV infection. The domains of performance that are particularly vulnerable include the speed of information processing and executive functioning.
Application of Neuroimaging to Brain Effects of HIV and Aging
Neuroimaging studies, including MRI, fMRI, MRS, and diffusion tensor imaging (DTI), have provided additional definitive evidence of enhanced vulnerability of the brain in HIV-infected individuals as they age (reviewed in [99]). The distinct advantages of applied neuroimaging are that individuals can be examined repeatedly under different conditions and compared in cross-sectional cohorts or in longitudinally-followed cohorts with complementary neuropsychological and biomarker testing.
Numerous neuroimaging studies of HIV+ individuals have demonstrated regional and global brain atrophy injury (gray and white matter), altered expression of cellular markers of neuronal integrity (N-acetyl aspartate) and glial activation (myoinositol), altered cerebral blood flow and physiological activation states, and microstructural white matter changes that can be attributed to HIV infection as well as aging. Furthermore, a recent CSF metabolomics analysis demonstrated elevated levels of products of metabolic waste (phenyacetylglutamine, lactate, 3-hydroxybutyrate), glutamate, glial activation markers, and mitochondrial metabolites in HAND patients on ART, which suggests similar biochemical brain disturbances in both aging and HAND [100]. Therefore, one can evaluate the natural history of HIV CNS infection, correlations between structural and functional brain disturbances and clinical performance, responses to therapy, and validity of biomarkers with neuroimaging techniques applied cross-sectionally and longitudinally in selected cohorts. Such studies can effectively complement neuropathological analyses to identify critical pathways of cellular injury and response that could potentially be targeted for therapeutics.
The effects of brain aging on functional physiological responses in HIV-infected brain have been recently explored with functional MRI (fMRI) techniques. Impairment of normal recruitment of brain regions for functional responses that can compensate for local brain dysfunction has been demonstrated in HIV infected individuals. Functional MRI was used in a study of 122 individuals (59 seronegative, 29 HIV+/HAND, 37 HIV+/neurocognitive normal) to determine whether subject age affected brain activation during attention-requiring tasks independently from HIV infection [101•]. Strict HAND diagnostic criteria were used, and normalized NP data derived from a seronegative cohort of 342 individuals were adjusted for age and education. The study demonstrated that HAND patients demonstrate an age-dependent decline in functional brain activation in response to certain neuropsychological test demands, which indicates an inability to compensate for declining brain functioning. Another recent study of 52 HIV+ and 52 HIV− individuals using resting-state functional connectivity MRI showed that deficits in functional connectivity between different brain regions in HIV+ individuals resemble those affected in normal aging [102]. These HIV and age effects were independent, which was interpreted as consistent with a premature brain aging effect of HIV.
These and other studies utilizing neuropsychological performance testing, neuroimaging, and neuropathological analyses strongly support a strong correlation between accelerated neurocognitive decline driven by HIV infection in aging individuals, even those with what is considered ‘effective’ suppression of systemic HIV replication. These studies also further emphasize the need for developing new strategies, involving current ART and possibly adjunctive therapies, for protecting the brain against injury in the aging HIV+ population.
Conclusions
HIV associated neurocognitive disorders remain enigmatic despite intensive research aimed at understanding the factors and processes that contribute to their persistence in ART-treated individuals. Among the more significant discoveries in the last several years are the correlations between chronic, persistent inflammation, systemic disease progression, and HAND. These associations of inflammation and immune activation with disease progression in different body compartments suggests that shared, inflammation- and immune-modulating processes should be therapeutically targeted to fully protect the individual from disease progression. Identifying such targets and testing currently available drugs, as well as continuing to develop new drugs, are worthy goals. However, full protection against neurocognitive decline in HIV+ ART-treated patients will also require effective targeting of the common co-morbid conditions that also affect such patients: co-infection with HCV, substance abuse, potential ART toxicity, and aging. Among these, the problem of HCV co-infection is currently addressable and we should be prepared to implement neuroprotection studies of new anti-HCV drugs for individuals with HIV/HCV co-infection. The likelihood of benefit to the CNS seems high. Effectively addressing the other HAND co-morbidities will continue to present major challenges in the foreseeable future, but unquestionable progress is being realized.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest Alexander J. Gill and Dennis L. Kolson declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Alexander J. Gill, Email: agill@mail.med.upenn.edu.
Dennis L. Kolson, Email: kolsond@mail.med.upenn.edu.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Canizares S, Cherner M, Ellis RJ. HIV and aging: effects on the central nervous system. Semin Neurol. 2014;34:27–34. doi: 10.1055/s-0034-1372340. This is a comprehensive review of published (and some unpublished) studies HIV, aging, and associated risk factors, including APOE e4 allele status, metabolic syndrome and other factors. The strengths and weaknesses of published studies are effectively discussed.
- 3.Price RW, Spudich SS, Peterson J, et al. Evolving character of chronic central nervous system HIV infection. Semin Neurol. 2014;34:7–13. doi: 10.1055/s-0034-1372337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hearps AC, Martin GE, Rajasuriar R, et al. Inflammatory comorbidities in HIV+ individuals: learning lessons from healthy ageing. Curr HIV/AIDS Rep. 2014;11:20–34. doi: 10.1007/s11904-013-0190-8. This review thoroughly discusses the interactions among inflammation, immune activation and associated HIV morbidity. The role for microbial translocation is integrated and a particularly thoughtful discussion of therapeutic targeting of the inflammatory response is presented.
- 5.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372:293–299. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marchetti G, Tincati C, Silvestri G. Microbial translocation in the pathogenesis of HIV infection and AIDS. Clin Microbiol Rev. 2013;26:2–18. doi: 10.1128/CMR.00050-12. A comprehensive review of the process of microbial translocation across the gut mucosa, associated host responses, and the implications for SIV and HIV disease pathogenesis.
- 8. Mutlu EA, Keshavarzian A, Losurdo J, et al. A compositional look at the human gastrointestinal microbiome and immune activation parameters in HIV infected subjects. PLoS Pathog. 2014;10:e1003829. doi: 10.1371/journal.ppat.1003829. Analysis of the GI microbiome in HIV+ and HIV-individuals revealed less diversity, loss of commensal bacteria, and gain of some potential pathogenic bacterial taxa in HIV+ individuals. This study validates several earlier studies and further supports the hypothesis that changes in the gut microbiome due to HIV infection directly link to pathogenic immune activation. Therapeutic implications are discussed.
- 9.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 10.Ancuta P, Kamat A, Kunstman KJ, et al. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One. 2008;3:e2516. doi: 10.1371/journal.pone.0002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamann L, Alexander C, Stamme C, et al. Acute-phase concentrations of lipopolysaccharide (LPS)-binding protein inhibit innate immune cell activation by different LPS chemotypes via different mechanisms. Infect Immun. 2005;73:193–200. doi: 10.1128/IAI.73.1.193-200.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neuhaus J, Jacobs DR, Jr, Baker JV, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201:1788–1795. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tien PC, Choi AI, Zolopa AR, et al. Inflammation and mortality in HIV-infected adults: analysis of the FRAM study cohort. J Acquir Immune Defic Syndr. 2010;55:316–322. doi: 10.1097/QAI.0b013e3181e66216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han J, Wang B, Han N, et al. CD14(high)CD16(+) rather than CD14(low)CD16(+) monocytes correlate with disease progression in chronic HIV-infected patients. J Acquir Immune Defic Syndr. 2009;52:553–559. doi: 10.1097/qai.0b013e3181c1d4fe. [DOI] [PubMed] [Google Scholar]
- 15.Ellery PJ, Tippett E, Chiu YL, et al. The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J Immunol. 2007;178:6581–6589. doi: 10.4049/jimmunol.178.10.6581. [DOI] [PubMed] [Google Scholar]
- 16.Douek DC. Disrupting T-cell homeostasis: how HIV-1 infection causes disease. AIDS Rev. 2003;5:172–177. [PubMed] [Google Scholar]
- 17.Wallet MA, Rodriguez CA, Yin L, et al. Microbial translocation induces persistent macrophage activation unrelated to HIV-1 levels or T-cell activation following therapy. AIDS. 2010;24:1281–1290. doi: 10.1097/QAD.0b013e328339e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gori A, Tincati C, Rizzardini G, et al. Early impairment of gut function and gut flora supporting a role for alteration of gastrointestinal mucosa in human immunodeficiency virus pathogenesis. J Clin Microbiol. 2008;46:757–758. doi: 10.1128/JCM.01729-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Castro P, Plana M, Gonzalez R, et al. Influence of episodes of intermittent viremia (“blips”) on immune responses and viral load rebound in successfully treated HIV-infected patients. AIDS Res Hum Retroviruses. 2013;29:68–76. doi: 10.1089/aid.2012.0145. A retrospective analysis of a randomized, double-blinded, placebo-controlled study of HIV-infected individuals on stable cART and receiving vaccinations. All patients had monthly viral loads. Planned cART interruption for 6 months after 12 months of cART during the study. Those with ‘blips’ above 200 copies/ml during cART had higher viral rebound, worse CD4+ T cell recovery, and increased levels of immune activation markers.
- 20. Grennan JT, Loutfy MR, Su D, et al. Magnitude of virologic blips is associated with a higher risk for virologic rebound in HIV-infected individuals: a recurrent events analysis. J Infect Dis. 2012;205:1230–1238. doi: 10.1093/infdis/jis104. A large observational cohort study (3550 HIV+ patients with confirmed virological suppression) that demonstrated a correlation between HIV viral load ‘blip’ amplitude and subsequent virologic rebound. This study emphasizes the importance of determining ‘effective’ virological suppression to potentially determine future risk of disease progression.
- 21.Canestri A, Lescure FX, Jaureguiberry S, et al. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis. 2010;50:773–778. doi: 10.1086/650538. [DOI] [PubMed] [Google Scholar]
- 22.Gelman BBMDJ. HIV-1 neuropathology. In: Gendelman HEGI, Everall IP, Fox HS, Gelbard HA, Lipton SA, Swindells S, editors. The neurology of AIDS. Oxford: Oxford University Press; 2012. pp. 518–535. [Google Scholar]
- 23.Anthony IC, Ramage SN, Carnie FW, et al. Accelerated Tau deposition in the brains of individuals infected with human immunodeficiency virus-1 before and after the advent of highly active anti-retroviral therapy. Acta Neuropathol. 2006;111:529–538. doi: 10.1007/s00401-006-0037-0. [DOI] [PubMed] [Google Scholar]
- 24.Silva AC, Rodrigues BS, Micheletti AM, et al. Neuropathology of AIDS: an autopsy review of 284 cases from Brazil comparing the findings Pre- and Post-HAART (Highly Active Antiretroviral Therapy) and Pre- and postmortem correlation. AIDS Res Treat. 2012;2012:186850. doi: 10.1155/2012/186850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neaton JD, Neuhaus J, Emery S. Soluble biomarkers and morbidity and mortality among people infected with HIV: summary of published reports from 1997 to 2010. Curr Opin HIV AIDS. 2010;5:480–490. doi: 10.1097/COH.0b013e32833ed75d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nixon DE, Landay AL. Biomarkers of immune dysfunction in HIV. Curr Opin HIVAIDS. 2010;5:498–503. doi: 10.1097/COH.0b013e32833ed6f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyons JL, Uno H, Ancuta P, et al. Plasma sCD14 is a biomarker associated with impaired neurocognitive test performance in attention and learning domains in HIV infection. J Acquir Immune Defic Syndr. 2011;57:371–379. doi: 10.1097/QAI.0b013e3182237e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burdo TH, Lo J, Abbara S, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis. 2011;204:1227–1236. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burdo TH, Lentz MR, Autissier P, et al. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J Infect Dis. 2011;204:154–163. doi: 10.1093/infdis/jir214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Burdo TH, Woods A, Letendre S, et al. Elevated sCD163 is a Marker of Neurocognitive Impairment in HIV-infected Individuals on Effective ART. AIDS. 2013;27:1387–1395. doi: 10.1097/QAD.0b013e32836010bd. Thirty four virally suppressed HIV+ patients (15 with HAND, 19 with normal neurocognitive testing) and 34 HIV negative matched controls were assessed for plasma sCD163 at consecutive visits 7–32 months apart. Neuropyschological impairment correlated significantly with plasma sCD163 levels, consistent with a causal link between monocyte activation and neurocognitive dysfunction in the setting of viral suppression.
- 31.Kamat A, Lyons JL, Misra V, et al. Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. J Acquir Immune Defic Syndr. 2012;60:234–243. doi: 10.1097/QAI.0b013e318256f3bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simioni S, Cavassini M, Annoni JM, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010;24:1243–1250. doi: 10.1097/QAD.0b013e3283354a7b. [DOI] [PubMed] [Google Scholar]
- 33.Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heaton RK, Clifford DB, Franklin DR, Jr, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mothobi NZ, Brew BJ. Neurocognitive dysfunction in the highly active antiretroviral therapy era. Curr Opin Infect Dis. 2012;25:4–9. doi: 10.1097/QCO.0b013e32834ef586. This review summarizes findings of CHARTER studies and discusses the ‘paradoxical’ lack of efficacy of HAART in effectively preventing HAND. Particular attention is given to possible CNS ART neurotoxicity.
- 36.Letendre S, Marquie-Beck J, Capparelli E, et al. Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65:65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robertson KR, Su Z, Margolis DM, et al. Neurocognitive effects of treatment interruption in stable HIV-positive patients in an observational cohort. Neurology. 2010;74:1260–1266. doi: 10.1212/WNL.0b013e3181d9ed09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marra CM, Zhao Y, Clifford DB, et al. Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS. 2009;23:1359–1366. doi: 10.1097/QAD.0b013e32832c4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Perez-Valero I, Gonzalez-Baeza A, Estebanez M, et al. Neurocognitive impairment in patients treated with protease inhibitor monotherapy or triple drug antiretroviral therapy. PLoS One. 2013;8:e69493. doi: 10.1371/journal.pone.0069493. This is the first prospective study of PI monotherapy in comparison to triple ART associations with neurocognitive functioning. No differences in functioning were seen, suggesting that PI monotherapy does not have an increased risk for neurotoxicity.
- 40.Parikh N, Nonnemacher MR, Pirrone V, et al. Substance abuse, HIV-1 and hepatitis. Curr HIV Res. 2012;10:557–571. doi: 10.2174/157016212803306023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Byrnes V, Miller A, Lowry D, et al. Effects of anti-viral therapy and HCV clearance on cerebral metabolism and cognition. J Hepatol. 2012;56:549–556. doi: 10.1016/j.jhep.2011.09.015. This study describes evidence that effective HCV antiviral treatment (sustained HCV suppression) can improve neurocognitive functioning and MRS parameters of neuronal injury and glial activation in HIV/HCV co-infection. It is a limited, small study that should prompt thoughtful discussions and larger definitive trials.
- 42.Weissenborn K, Tryc AB, Heeren M, et al. Hepatitis C virus infection and the brain. Metab Brain Dis. 2009;24:197–210. doi: 10.1007/s11011-008-9130-5. [DOI] [PubMed] [Google Scholar]
- 43.Chak E, Talal AH, Sherman KE, et al. Hepatitis C virus infection in USA: an estimate of true prevalence. Liver Int. 2011;31:1090–1101. doi: 10.1111/j.1478-3231.2011.02494.x. [DOI] [PubMed] [Google Scholar]
- 44.Sherman KE, Thomas D, Chung RT. Human immunodeficiency virus and liver disease forum 2012. Hepatology. 2014;59:307–317. doi: 10.1002/hep.26638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Letendre SL, ChernerM, Ellis RJ, et al. The effects of hepatitis C, HIV, and methamphetamine dependence on neuropsychological performance: biological correlates of disease. AIDS. 2005;19(Suppl 3):S72–S78. doi: 10.1097/01.aids.0000192073.18691.ff. [DOI] [PubMed] [Google Scholar]
- 46.Wilkinson J, Radkowski M, Laskus T. Hepatitis C virus neuroinvasion: identification of infected cells. J Virol. 2009;83:1312–1319. doi: 10.1128/JVI.01890-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilkinson J, Radkowski M, Eschbacher JM, et al. Activation of brain macrophages/microglia cells in hepatitis C infection. Gut. 2010;59:1394–1400. doi: 10.1136/gut.2009.199356. [DOI] [PubMed] [Google Scholar]
- 48.Hinkin CH, Castellon SA, Levine AJ, et al. Neurocognition in individuals co-infected with HIV and hepatitis C. J Addict Dis. 2008;27:11–17. doi: 10.1300/j069v27n02_02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bladowska J, Zimny A, Knysz B, et al. Evaluation of early cerebral metabolic, perfusion and microstructural changes in HCV-positive patients: a pilot study. J Hepatol. 2013;59:651–657. doi: 10.1016/j.jhep.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 50.Jernigan TL, Archibald SL, Fennema-Notestine C, et al. Clinical factors related to brain structure in HIV: the CHARTER study. J Neurovirol. 2011;17:248–257. doi: 10.1007/s13365-011-0032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vassallo M, Dunais B, Durant J, et al. Relevance of lipopolysaccharide levels in HIV-associated neurocognitive impairment: the Neuradapt study. J Neurovirol. 2013;19:376–382. doi: 10.1007/s13365-013-0181-y. [DOI] [PubMed] [Google Scholar]
- 52.Garvey LJ, Pavese N, Ramlackhansingh A, et al. Acute HCV/HIV coinfection is associated with cognitive dysfunction and cerebral metabolite disturbance, but not increased microglial cell activation. PLoS One. 2012;7:e38980. doi: 10.1371/journal.pone.0038980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clifford DB, Smurzynski M, Park LS, et al. Effects of active HCV replication on neurologic status in HIV RNA virally suppressed patients. Neurology. 2009;73:309–314. doi: 10.1212/WNL.0b013e3181af7a10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garvey LJ, Pflugrad H, Thiyagarajan A, et al. Effects of active HCV replication on neurologic status in HIV RNA virally suppressed patients. Neurology. 2010;74:779. doi: 10.1212/WNL.0b013e3181d2aff9. author reply 779–780. [DOI] [PubMed] [Google Scholar]
- 55.Clifford DB, Evans SR, Yang Y, et al. The neuropsychological and neurological impact of hepatitis C virus co-infection in HIV-infected subjects. AIDS. 2005;19(Suppl 3):S64–S71. doi: 10.1097/01.aids.0000192072.80572.43. [DOI] [PubMed] [Google Scholar]
- 56. Muir AJ. The rapid evolution of treatment strategies for hepatitis C. Am J Gastroenterol. 2014;109:628–635. doi: 10.1038/ajg.2014.66. This review describes the groundbreaking discovery of the first anti-HCV drug to win FDA approval for treatment of HIV/HCV co-infected individuals. This interferon-sparing drug may be used as monotherapy or combined therapy with other HCV inhibitors with the realistic hope of high efficacy in curing HCV infection.
- 57.Martin-Thormeyer EM, Paul RH. Drug abuse and hepatitis C infection as comorbid features of HIV associated neurocognitive disorder: neurocognitive and neuroimaging features. Neuropsychol Rev. 2009;19:215–231. doi: 10.1007/s11065-009-9101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nath A. Human immunodeficiency virus-associated neurocognitive disorder: pathophysiology in relation to drug addiction. Ann N Y Acad Sci. 2010;1187:122–128. doi: 10.1111/j.1749-6632.2009.05277.x. [DOI] [PubMed] [Google Scholar]
- 59.Beyrer C, Wirtz AL, Baral S, et al. Epidemiologic links between drug use and HIV epidemics: an international perspective. J Acquir Immune Defic Syndr. 2010;55(Suppl 1):S10–S16. doi: 10.1097/QAI.0b013e3181f9c0c9. [DOI] [PubMed] [Google Scholar]
- 60.Substance Abuse and Mental Health Services Administration CfBHSaQ. The NSDUH Report: HIV/AIDS and Substance Use. Rockville: 2010. [Google Scholar]
- 61.Dutta R, Roy S. Mechanism(s) involved in opioid drug abuse modulation of HAND. Curr HIV Res. 2012;10:469–477. doi: 10.2174/157016212802138805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Byrd DA, Fellows RP, Morgello S, et al. Neurocognitive impact of substance use in HIV infection. J Acquir Immune Defic Syndr. 2011;58:154–162. doi: 10.1097/QAI.0b013e318229ba41. This is large cohort study by the CHARTER group examed the relationship between substance abuse history and neurocognitive impairment. The majority of the individuals with substance abuse had sustained abstinence from drug use at time of neurocognitive analysis. The study found no significant effect of substance use status on neurocognitive impairment, suggesting that drugs of abuse may have a limited legacy effect on neurocognitive impairment in HIV+ individuals.
- 63.Banerjee A, Strazza M, Wigdahl B, et al. Role of mu-opioids as cofactors in human immunodeficiency virus type 1 disease progression and neuropathogenesis. J Neurovirol. 2011;17:291–302. doi: 10.1007/s13365-011-0037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Iudicello JE, Woods SP, Vigil O, et al. Longer term improvement in neurocognitive functioning and affective distress among methamphetamine users who achieve stable abstinence. J Clin Exp Neuropsychol. 2010;32:704–718. doi: 10.1080/13803390903512637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang X, Wang Y, Ye L, et al. Modulation of intracellular restriction factors contributes to methamphetamine-mediated enhancement of acquired immune deficiency syndrome virus infection of macrophages. Curr HIV Res. 2012;10:407–414. doi: 10.2174/157016212802138797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reynolds JL, Law WC, Mahajan SD, et al. Morphine and galectin-1 modulate HIV-1 infection of human monocyte-derived macrophages. J Immunol. 2012;188:3757–3765. doi: 10.4049/jimmunol.1102276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dave RS, Khalili K. Morphine treatment of human monocyte-derived macrophages induces differential miRNA and protein expression: impact on inflammation and oxidative stress in the central nervous system. J Cell Biochem. 2010;110:834–845. doi: 10.1002/jcb.22592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mantri CK, Pandhare Dash J, Mantri JV, et al. Cocaine enhances HIV-1 replication in CD4+ T cells by down-regulating MiR-125b. PLoS One. 2012;7:e51387. doi: 10.1371/journal.pone.0051387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim SG, Jung JB, Dixit D, et al. Cocaine exposure enhances permissiveness of quiescent T cells to HIV infection. J Leukoc Biol. 2013;94:835–843. doi: 10.1189/jlb.1112566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mantri CK, Mantri JV, Pandhare J, et al. Methamphetamine inhibits HIV-1 replication in CD4+ T cells by modulating anti-HIV-1 miRNA expression. Am J Pathol. 2014;184:92–100. doi: 10.1016/j.ajpath.2013.09.011. This study presents evidence of an inhibitory effect of methamphetamine on HIV replication in CD4+ T lymphocytes through miRNA regulation, in contrast to other published reports of methamphetamine enhancement of HIV replication. These conflicting results are discussed in detail, and appropriate caution is urged when extrapolating in vitro results in such studies to pathogenic mechanisms in vivo.
- 71.Gaskill PJ, Calderon TM, Coley JS, et al. Drug induced increases in CNS dopamine alter monocyte, macrophage and T cell functions: implications for HAND. J Neuroimmune Pharmacol. 2013;8:621–642. doi: 10.1007/s11481-013-9443-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gaskill PJ, Calderon TM, Luers AJ, et al. Human immunodeficiency virus (HIV) infection of human macrophages is increased by dopamine: a bridge between HIV-associated neurologic disorders and drug abuse. Am J Pathol. 2009;175:1148–1159. doi: 10.2353/ajpath.2009.081067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Czub S, Koutsilieri E, Sopper S, et al. Enhancement of central nervous system pathology in early simian immunodeficiency virus infection by dopaminergic drugs. Acta Neuropathol. 2001;101:85–91. doi: 10.1007/s004010000313. [DOI] [PubMed] [Google Scholar]
- 74.Czub S, Czub M, Koutsilieri E, et al. Modulation of simian immunodeficiency virus neuropathology by dopaminergic drugs. Acta Neuropathol. 2004;107:216–226. doi: 10.1007/s00401-003-0801-3. [DOI] [PubMed] [Google Scholar]
- 75.Marcondes MC, Flynn C, Watry DD, et al. Methamphetamine increases brain viral load and activates natural killer cells in simian immunodeficiency virus-infected monkeys. Am J Pathol. 2010;177:355–361. doi: 10.2353/ajpath.2010.090953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bokhari SM, Hegde R, Callen S, et al. Morphine potentiates neuropathogenesis of SIV infection in rhesus macaques. J Neuroimmune Pharmacol. 2011;6:626–639. doi: 10.1007/s11481-011-9272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weed M, Adams RJ, Hienz RD, et al. SIV/macaque model of HIV infection in cocaine users: minimal effects of cocaine on behavior, virus replication, and CNS inflammation. J Neuroimmune Pharmacol. 2012;7:401–411. doi: 10.1007/s11481-011-9281-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Buch S, Yao H, Guo M, et al. Cocaine and HIV-1 interplay in CNS: cellular and molecular mechanisms. Curr HIV Res. 2012;10:425–428. doi: 10.2174/157016212802138823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu X, Silverstein PS, Singh V, et al. Methamphetamine increases LPS-mediated expression of IL-8, TNF-alpha and IL-1beta in human macrophages through common signaling pathways. PLoS One. 2012;7:e33822. doi: 10.1371/journal.pone.0033822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cisneros IE, Ghorpade A. HIV-1, methamphetamine and astrocyte glutamate regulation: combined excitotoxic implications for neuro-AIDS. Curr HIV Res. 2012;10:392–406. doi: 10.2174/157016212802138832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang Y, Yao H, Lu Y, et al. Cocaine potentiates astrocyte toxicity mediated by human immunodeficiency virus (HIV-1) protein gp120. PLoS One. 2010;5:e13427. doi: 10.1371/journal.pone.0013427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Strazza M, Pirrone V, Wigdahl B, et al. Breaking down the barrier: the effects of HIV-1 on the blood-brain barrier. Brain Res. 2011;1399:96–115. doi: 10.1016/j.brainres.2011.05.015. A review of the effects of HIV infection on the BBB, specifically addressing the role of HIV proteins, monocyte chemotaxis across the BBB, the compounding effects of drug abuse.
- 83.Martins T, Baptista S, Goncalves J, et al. Methamphetamine transiently increases the blood-brain barrier permeability in the hippocampus: role of tight junction proteins and matrix metalloproteinase-9. Brain Res. 2011;1411:28–40. doi: 10.1016/j.brainres.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 84.Dhillon NK, Peng F, Bokhari S, et al. Cocaine-mediated alteration in tight junction protein expression and modulation of CCL2/CCR2 axis across the blood-brain barrier: implications for HIV-dementia. J Neuroimmune Pharmacol. 2008;3:52–56. doi: 10.1007/s11481-007-9091-1. [DOI] [PubMed] [Google Scholar]
- 85.Sharma HS, Ali SF. Alterations in blood-brain barrier function by morphine and methamphetamine. Ann N Y Acad Sci. 2006;1074:198–224. doi: 10.1196/annals.1369.020. [DOI] [PubMed] [Google Scholar]
- 86.Yousif S, Saubamea B, Cisternino S, et al. Effect of chronic exposure to morphine on the rat blood-brain barrier: focus on the P-glycoprotein. J Neurochem. 2008;107:647–657. doi: 10.1111/j.1471-4159.2008.05647.x. [DOI] [PubMed] [Google Scholar]
- 87.Mellins CA, Havens JF, McDonnell C, et al. Adherence to antiretroviral medications and medical care in HIV-infected adults diagnosed with mental and substance abuse disorders. AIDS Care. 2009;21:168–177. doi: 10.1080/09540120802001705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Roux P, Carrieri MP, Villes V, et al. The impact of methadone or buprenorphine treatment and ongoing injection on highly active antiretroviral therapy (HAART) adherence: evidence from the MANIF2000 cohort study. Addiction. 2008;103:1828–1836. doi: 10.1111/j.1360-0443.2008.02323.x. [DOI] [PubMed] [Google Scholar]
- 89.Carrico AW. Substance use and HIV disease progression in the HAART era: implications for the primary prevention of HIV. Life Sci. 2011;88:940–947. doi: 10.1016/j.lfs.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 90.Deeks SG. Immune dysfunction, inflammation, and accelerated aging in patients on antiretroviral therapy. Top HIV Med. 2009;17:118–123. [PubMed] [Google Scholar]
- 91.Xu J, Ikezu T. The comorbidity of HIV-associated neurocognitive disorders and Alzheimer's disease: a foreseeable medical challenge in post-HAART era. J Neuroimmune Pharmacol. 2009;4:200–212. doi: 10.1007/s11481-008-9136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Achim CL, Adame A, Dumaop W, et al. Increased accumulation of intraneuronal amyloid beta in HIV-infected patients. J Neuroimmune Pharmacol. 2009;4:190–199. doi: 10.1007/s11481-009-9152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Khanlou N, Moore DJ, Chana G, et al. Increased frequency of alpha-synuclein in the substantia nigra in human immunodeficiency virus infection. J Neurovirol. 2009;15:131–138. doi: 10.1080/13550280802578075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Soontornniyomkij V, Moore DJ, Gouaux B, et al. Cerebral beta-amyloid deposition predicts HIV-associated neurocognitive disorders in APOE epsilon4 carriers. AIDS. 2012;26:2327–2335. doi: 10.1097/QAD.0b013e32835a117c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Smith DB, Simmonds P, Bell JE. Brain viral burden, neuroinflammation and neurodegeneration in HAART-treated HIV positive injecting drug users. J Neurovirol. 2014;20:28–38. doi: 10.1007/s13365-013-0225-3. [DOI] [PubMed] [Google Scholar]
- 96.Fields J, Dumaop W, Rockenstein E, et al. Age-dependent molecular alterations in the autophagy pathway in HIVE patients and in a gp120 tg mouse model: reversal with beclin-1 gene transfer. J Neurovirol. 2013;19:89–101. doi: 10.1007/s13365-012-0145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Salminen A, Kaarniranta K, Kauppinen A, et al. Impaired autophagy and APP processing in Alzheimer's disease: the potential role of Beclin 1 interactome. Prog Neurobiol. 2013;106–107:33–54. doi: 10.1016/j.pneurobio.2013.06.002. An exhaustive review of autophagy, APP processing and the unique role for Beclin-1, a critical component of the autophagosome. Although dealing primarily with AD, it presents an interesting discussion of the implications for several viral diseases, including HIV CNS infection.
- 98.Seider TR, Luo X, Gongvatana A, et al. Verbal memory declines more rapidly with age in HIV infected versus uninfected adults. J Clin Exp Neuropsychol. 2014;36:356–367. doi: 10.1080/13803395.2014.892061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Holt JL, Kraft-Terry SD, Chang L. Neuroimaging studies of the aging HIV-1-infected brain. J Neurovirol. 2012;18:291–302. doi: 10.1007/s13365-012-0114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cassol E, Misra V, Dutta A, et al. Cerebrospinal fluid metabolomics reveals altered waste clearance and accelerated aging in HIV patients with neurocognitive impairment. AIDS. 2014;28 doi: 10.1097/QAD.0000000000000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Chang L, Holt JL, Yakupov R, et al. Lower cognitive reserve in the aging human immunodeficiency virus-infected brain. Neurobiol Aging. 2013;34:1240–1253. doi: 10.1016/j.neurobiolaging.2012.10.012. This large (n = 122 patients) fMRI study demonstrated poorer regional brain compensation through recruitment of functional attention networks in HIV infected individuals under demand test conditions. Synergistic effects of HIV and aging were proposed.
- 102.Thomas JB, Brier MR, Snyder AZ, et al. Pathways to neurodegeneration: effects of HIV and aging on resting-state functional connectivity. Neurology. 2013;80:1186–1193. doi: 10.1212/WNL.0b013e318288792b. [DOI] [PMC free article] [PubMed] [Google Scholar]