Abstract
Rationale
PREGS and DHEAS are pro-mnesic, anti-amnesic and neuroprotective steroids in rodents. In Alzheimer's disease (AD) patient brains, their low concentrations are correlated with high levels of Aβ and tau proteins. The unnatural enantiomer ent-PREGS enhanced memory in rodents. We investigated here whether ent-PREGS and ent-DHEAS could be neuroprotective in AD models.
Objective
The effects of PREGS, ent-PREGS, DHEAS and ent-DHEAS against Aβ25-35 peptide-induced toxicity were examined in vitro on B104 neuroblastoma cells and in vivo in mice.
Methods
B104 cells pretreated with the steroids before Aβ25-35 were analyzed by flow cytometry measuring cell viability and death processes. Mice injected intracerebroventricularly with Aβ25-35 and the steroids, were analyzed for their memory abilities. Additionally, lipid peroxidation levels in the hippocampus were measured.
Results
ent-PREGS and PREGS significantly attenuated the Aβ25-35-induced decrease in cell viability. Both steroids prevented the Aβ25-35-induced increase in late apoptotic cells. PREGS further attenuated the ratio of necrotic cells. ent-DHEAS and DHEAS significantly reduced the Aβ25-35-induced toxicity and prevented the cells from entering late apoptosis and necrosis. All steroids stimulated neurite outgrowth per se and prevented the Aβ25-35-induced decrease. In vivo, ent-PREGS and ent-DHEAS significantly attenuated the Aβ25-35-induced decrease in memory (spontaneous alternation and passive avoidance) and an increase in lipid peroxidation levels. In contrast to the natural steroids, both enantiomers prevented amnesia when injected 6 h before Aβ25-35 in contrast to the natural steroids.
Conclusion
The unnatural steroids ent-PREGS and ent-DHEAS are potent neuroprotective agents and could be effective therapeutical tools in AD.
Keywords: enantiomer, pregnenolone sulfate, dehydroepiandrosterone sulfate, neuroprotection, memory, Alzheimer's disease, neurosteroid, β-amyloid toxicity, learning and memory, oxidative stress
INTRODUCTION
Alzheimer's disease (AD) is the most common neurodegenerative disease in the elderly population (Selkoe 1997; Hardy and Gwinn-Hardy 1998). Amyloid-β (Aβ) species accumulating in the brain of AD patients as β-amyloid plaques are key actors in the pathogenesis (Blennow et al. 2006; Deshpande et al. 2006). The synthetic Aβ25-35 fragment, the biologically active region of Aβ protein (Yankner et al. 1990; Pike et al. 1995), has been shown to disturb cellular integrity and function (for review, see (Kaminsky et al. 2010). It induces neurodegeneration both in vitro and in vivo (Malouf 1992; Pike et al. 1995; Stepanichev et al. 2003; Meunier et al. 2006; Zussy et al. 2011). One of the earliest fundamental events associated with its toxicity is oxidative damage (Miranda et al. 2000; Butterfield et al. 2001). Aβ-associated free radical oxidative stress causes lipid peroxidation in brain cell membranes, resulting in cell death that underlies cognitive deficits (Mark et al. 1996; Sayre et al. 1997; Butterfield et al. 2002a, b). Aβ25-35 induced neurotoxicity is also associated with other changes including perturbation of calcium homeostasis (Mattson et al. 1992; Harkany et al. 1999) and apoptosis (Forloni et al. 1996). Aβ25-35 impairs memory formation after central administration in rodents (Maurice et al. 1998; Stepanichev et al. 2003; Holscher et al. 2007).
Preventing or protecting neuronal dysfunction and death has become an important component for alleviating memory impairments in AD. We have previously demonstrated that the concentrations of PREGS and dehydroepiandrosterone sulfate (DHEAS) are significantly reduced in the brain of AD patients as compared to non-demented controls and are correlated negatively with high Aβ levels and hyperphosphorylated Tau proteins (Weill-Engerer et al. 2002). PREGS and DHEAS differentially regulate neuronal cell survival, in both in vitro and in vivo Aβ peptide-induced AD models. PREGS exacerbates the decrease in cell viability induced by Aβ25-35 peptide in pheochromocytoma PC12 cell cultures (Akan et al. 2009). However, it shows neuroprotective action in mice centrally injected with the peptide (Yang et al. 2012). PREGS protects hippocampal neurogenesis in the APP/PS1 transgenic AD mouse model (Xu et al. 2012). In mouse cerebral cortex neuronal cultures, DHEAS selectively enhances dendrite growth (Compagnone and Mellon 1998). PREGS and DHEAS display both promnesiant and anti-amnesiant activities in rodents (for review, see (Vallée et al. 2001a; Maurice et al. 2006). In particular, they dose-dependently attenuated the memory deficits provoked by intracerebroventricular (i.c.v.) administration of Aβ25-35 peptide in mice (Maurice et al. 1998) or memory deficits measured in APP/PS1 transgenic mice (Xu et al. 2012).
The synthetic enantiomers of PREGS, ent-PREGS, and of DHEAS, ent-DHEAS, (Nilsson et al. 1998) have been used in pharmacological and electrophysiological studies as tools to provide insight into the enantioselectivity of steroid actions and the existence of chiral specific recognition sites on neurotransmitter receptor coupled channels such as γ-amino-butyric acid and N-methyl-D-aspartate (NMDA) receptors (Covey 2009; Covey et al. 2001). But some recent data showed a similar, if not higher, efficacy of steroid enantiomers over natural steroids in several pharmacological tests. PREGS potentiation of NMDA receptors is known to improve learning (Mathis et al. 1996; Akwa et al. 2001; Petit et al. 2011). Using a two-trial arm recognition task in a Y-maze, we have shown that ent-PREGS is more active than PREGS, the effective i.c.v. doses in rats and mice being roughly 10 × lower than that of PREGS (Akwa et al. 2001). In addition, ent-PREGS acts independently of NMDA receptor activity (Akwa et al. 2001; Petit et al. 2011). By contrast, PREGS is an order of magnitude more effective than ent-PREGS in reversing scopolamine-induced amnesia in rats (Vallée et al. 2001b). To the best of our knowledge, the action of ent-DHEAS upon memory function has not been reported whereas DHEAS is established as a memory enhancer in rodents (Flood et al. 1988; Maurice et al. 1997; Markowski et al. 2001; Farr et al. 2004).
Neither ent-PREGS nor ent-DHEAS have been evaluated for their capacity as neuroprotective agents in rodent models of AD and related neurodegenerative processes. The aim of the present study was therefore to investigate the effects of synthetic ent-PREGS against Aβ25-35-induced toxicity in B104 neuroblastoma cell cultures, as compared to that of natural PREGS, with a focus on cell survival and neurite outgrowth. In vivo, we examined the ability of ent-PREGS to attenuate the oxidative stress and learning impairments induced by i.c.v. administration of Aβ25-35 peptide, using the spontaneous alternation and passive avoidance tests in mice. Behavioral studies were extended to ent-DHEAS, and the duration of actions of the enantiomers was compared to that of the natural steroids.
EXPERIMENTAL PROCEDURES
Preparation of steroids and Aβ peptides
PREGS was purchased from Steraloids (Newport, RI, USA). DHEAS was from Sigma-Aldrich (Saint Quentin-Fallavier, France) ent-PREGS and ent-DHEAS (ammonium salts) were chemically synthesized as previously reported (Nilsson et al. 1998). For experiments in B104 neuroblastoma cells, the concentrations of ent-PREGS and PREGS were obtained by successive dilutions of a 2 mg/ml stock solution in serum free culture medium. Final concentration of ethanol in culture medium was less than 0.1%. For studies in mice, ent-PREGS and ent-DHEAS were dissolved in sterile doubly distilled water. PREGS and DHEAS were solubilized in 5% dimethylsulfoxide (DMSO).
Lyophilized Aβ25-35 used in B104 cell cultures was from Bachem (Weil am Rhein, France). Aβ25-35 (5 mg) was initially dissolved in 0.5 ml of sterile deionized H2O (10 μg/μl), vortexed and stored at −80°C until use. Dilutions were further performed in free serum culture medium to obtain appropriate concentrations. For experiments in mice, lyophilized Aβ25–35 peptide (SC489C) and scrambled Aβ25–35 peptide (SC492) were from NeoMPS (Strasbourg, France). They were dissolved in sterile distilled water at a concentration of 3 mg/ml and stored at −20°C until use. Before being injected, peptides were incubated at 1 mg/ml in sterile distilled water at 37°C for 4 days as previously described (Maurice et al. 1996, 1998). Aβ25-35 was used in its aggregated form in both in vitro and in vivo studies. Indeed, this truncated Aβ-fragment unlike the full-length peptide rapidly forms fibrils and exhibits toxicity immediately upon its solubilisation in water (Yankner et al. 1990; Pike et al. 1995).
Cell culture
We used the B104 neuroblastoma cell line which originates in the rat central nervous system (Schubert et al. 1974). Cells were a gift from Dr A. Meiniel (INSERM U384, Faculty of Medicine, Clermont-Ferrand, France). They have the advantage over primary cortical neuronal cell cultures of a fast growth rate. They display numerous neuronal characteristics such as electrical membrane excitability (Schubert et al, 1986), expression of neurotransmitters/receptors (Hales and Tyndale 1994;Tyndale, 1994) and 14-3-2 neuron-specific protein (Schubert, 1974). These features make them an attractive model for the study of human neurological disease and for testing neurotoxicity of putative drugs. Cells were plated in poly-l-lysine coated plates (6 or 24 wells) and grown in a controlled environment with a humidified atmosphere containing 5% CO2 at 37°C, in complete culture medium containing RPMI 1640 medium supplemented with 10% fetal calf serum, 5% horse serum and a mixture of 1% penicillin/l-glutamine/streptomycin (Gibco, Life Technologies, Saint-Aubin, France).
Experiments on B104 neuroblastoma cell cultures
Steroid effects on B104 cell viability: dose-response study
Experiments were performed in order to determine whether ent-PREGS and ent-DHEAS were toxic to B104 cells as compared to PREGS and DHEAS, respectively. After initial 24 h plating with 8×104 cells / well in 6-well plates, the complete culture medium was replaced by free serum medium containing variable concentrations of each steroid ranging from 0.25 μM to 20 μM or no steroid (control). Cell survival was evaluated 24 h later by flow cytometry.
Aβ25-35 toxicity on B104 cells
In order to determine the minimum concentration of Aβ25-35 that reduced cell viability in steroid neuroprotection experiments, a preliminary dose-response study were carried out. After an initial 24 h cell plating, B104 cells was treated with 5, 10 and 20 μM of peptide in serum free medium, for 24 h. The percentage of viable cells was determined by flow cytometry.
Steroid neuroprotection against Aβ25-35 toxicity
To test the potential neuroprotective effects of the steroids against Aβ peptide toxicity, B104 cells were seeded on poly-l-lysine coated plates at 8×104 in 6-well plates. After an initial 24 h cell plating, the complete culture medium was replaced with fresh medium containing increasing doses of steroids and incubated for 24 h. The culture medium was then replaced by serum free medium containing the same above steroid treatments. Aβ25-35 was then added 30 min after steroid treatments and cells were incubated for an additional 24 h. The percentages of viable, apoptotic and necrotic cells were calculated by flow cytometry.
Steroid effects on neurite outgrowth
Low-density cultures of B104 neuroblastoma cells are suitable for the analysis of the enhanced neurite outgrowth (El Bitar et al. 1999). Three thousands cells in complete culture medium were seeded and allowed to attach for 24 h. They were incubated with ent-PREGS, PREGS, ent-DHEAS or DHEAS or no steroid (control), in the presence or absence of Aβ25-35 in free serum culture medium for 3, 5 or 7 days. Cells were examined on each indicated day of culture under a Nikon Labophot 2 photonic microscope. Images were captured with an Infinity2 camera equipped with Infinity software. Tracing and quantification of the longest neurite per cell was made by using the NeuronJ software. Three randomly chosen fields per well were chosen in which the longest neurite per cell was examined in 3 wells having the same treatment. The experiment was repeated 4 times for ent-PREGS and 3 times for PREGS.
Flow cytometric assay
Flow cytometry was utilized to detect the percentage of intact, apoptotic and necrotic cells. Cells were washed with PBS, harvested by trypsinization, centrifuged at 400 g for 5 min, and assayed with the Alexa-Fluor488®/annexin-V dead cell apoptosis kit (Invitrogen, Life Technologies) according to instructions of the manufacturer. Staining was detected on a FACSCalibur flow cytometer (Becton-Dickinson, Heidelberg, Germany). At least 10,000 cells per treatment condition were analyzed on CellQuest Pro software (Becton-Dickinson). Cells in early apoptosis were annexin-V/Alexa-Fluor488® positive and PI negative. Late apoptotic cells were annexin-V positive/ PI. Necrotic cells were only stained by PI. Living cells show little or no fluorescence.
Animals
Male Swiss mice aged 8-9-weeks old and weighing 32-35 g, were used (Depré, Saint-Doulchard, France). They were group-housed in the animal facility building of the University of Montpellier 2, with free access to food and water, except during experiments. They were kept in a temperature and humidity controlled animal facility on a 12 h/12 h light/dark cycle (lights off at 7:00 pm). Behavioural experiments were carried out between 9:00 am and 4:00 pm, in a sound-attenuated and air-regulated room, to which mice were habituated for at least 30 min. All animal procedures were conducted in strict adherence to the European Union Directive of September 22, 2010 (2010/63/UE).
Peptides and steroid injections
Aβ peptides (9 nmol), steroids (0.05, 0.2, 0.5 and 2 nmol) or vehicle (V) were simultaneously administered intracerebroventricularly (i.c.v.) in mice, under isoflurane 2.5% anaesthesia, through a 28-gauge stainless-steel needle, 3 mm long. An injection volume of 3 μl was delivered gradually within 30 s and the needle left in place for an additional 30 s before being removed, as previously described (Maurice et al. 1996, 1998). Mice (n = 10-12 per group) were examined for memory alteration one week after treatments, by an experimenter blind to the treatments. In the experiment depicted in Fig. 14, under isoflurane anesthesia, mice were implanted with a polyethylene cannula, 0.75 mm inner diameter and 6 mm length (Phymep, Paris, France), fixed using acrylic cement. The tip of the cannula was placed onto the right ventricle, with stereotaxic coordinates from the Bregma being, in mm, A −0.5, L −1, V 2.5. Injections began 36 h after surgery. Steroids were injected at −12 h, −6 h and simultaneously before Aβ25-35 peptide.
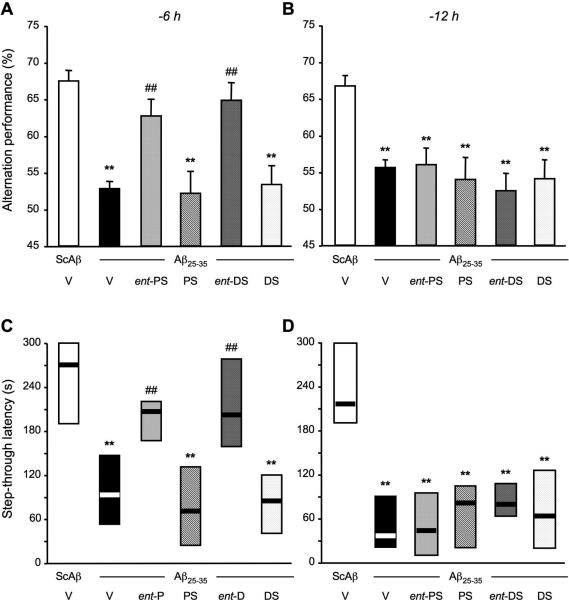
Figure 14.
Comparison of the protective efficacy of ent-PREGS, ent-DHEAS and their respective natural stereoisomers pre-injected 6 h (A, C) or 12 h (B, D) prior to Aβ25-35 peptide in mice: (A, B) Spontaneous alternation performances on day 7 and (C, D) passive avoidance response on days 8-9. Mice were cannulated and administered i.c.v. with distilled water (V) or ent-PREGS (ent-PS), PREGS, ent-DHEAS (ent-DS) or DHEAS (each at 0.5 nmol) either 6 h or 12 h before the Aβ25-35 peptide (9 nmol). The i.c.v. injection of ScAβ (9 nmol) was used as control. F(5,69) = 10.0, p < 0.0001, n = 11-12 per group, in (A); F(5,71) = 5.92, p < 0.0001, n = 12 per group, in (B); H = 27.9, p < 0.0001, n = 11-12, in (C); H = 24.3, p < 0.001, n = 12, in (D). ** p < 0.01 vs. the (ScAβ+V)-treated group; ## p < 0.01 vs. the (Aβ25-35+V)-treated group; Dunnett's test in (A, B), Dunn's test in (C, D).
Experimental protocol
On day 7 after injections, mice were examined for spatial working memory performances using the spontaneous alternation test in the Y-maze. Non-spatial long-term memory was then evaluated using the step-through passive avoidance test, with training and retention sessions carried out on day 8 and 9, respectively. At the end of behavioural experiments, mice were euthanized by decapitation and their hippocampi collected to measure the levels of lipid peroxidation.
Spontaneous alternation performances in the Y-maze
The spatial working memory was examined by measuring spontaneous alternation behaviour in the Y-maze (Maurice et al. 1998). The maze was made of grey polyvinylchloride. Each arm was 40 cm long, 13 cm high, 3 cm wide at the bottom, 10 cm wide at the top and converged at an equal angle. Each mouse was placed at the end of one arm and allowed to move freely through the maze during an 8 min session. The series of arm entries, including possible returns into the same arm, was recorded visually by a trained experimenter. An alternation was defined as entries into all three arms on consecutive occasions. The number of maximum alternations was, therefore, the total number of arm entries minus two and the percentage of alternation was calculated as (actual alternations/maximum alternations) × 100.
Step-through type passive avoidance response
The non-spatial long-term memory was assessed using the step-through passive avoidance procedure (Maurice et al. 1998; Meunier et al. 2006). The apparatus consisted of an illuminated compartment with white polyvinylchloride walls and a darkened compartment with black polyvinylchloride walls (each 15 cm × 20 cm and 15 cm high) and a grid floor. A guillotine door separated each compartment. The white compartment was illuminated during the experimental period by a 60 W lamp positioned 40 cm it. Scrambled footshocks (0.3 mA for 3 s) were delivered to the grid floor using a shock generator scrambler (Lafayette Instruments, Lafayette, MA, USA). The guillotine door was initially closed during the training session. Each mouse was placed into the white compartment. After 5 s, the door was raised. When the mouse entered the darkened compartment and placed all its paws on the grid floor, the door was gently closed and the scrambled foot shock was delivered for 3 s. The step-through latency (i.e., latency spent to enter the dark compartment) and shock sensitivity were measured. The latter was estimated as: 0, no reaction; 1, flinching reactions; and 2, fliching and vocalization reactions. None of the treatment used in this study affected the shock sensitivity of the mice (data not shown). After 24 h (on day 9 after peptide injection), each mouse was placed again into the white compartment. After 5 s, the door was raised. The step-through latency was recorded up to 300 s.
Lipid peroxidation measures
The quantification of lipid peroxidation in tissue extracts is based on Fe(III)/xylenol orange complex formation according to Hermes-Lima et al (Hermes-Lima et al. 1995) and as previously reported (Meunier et al. 2006; Villard et al. 2009). Brain were weighed and kept in liquid nitrogen until assayed. After being thawed, brains were homogenized in cold methanol (1/5 w/v), centrifuged at 1,000 g for 5 min and the supernatant collected. The homogenate was added to a solution containing FeSO4 1 mM, H2SO4 0.25 M, xylenol orange 1 mM and incubated for 30 min in a dark chamber at room temperature. Absorbance was measured at 580 nm (A5801) and 10 μl of cumene hydroperoxide (CHP) was added to the sample and incubated for 30 min at room temperature, to determine the maximal oxidation level. Absorbance was measured at 580 nm (A5802). The level of lipid peroxidation was determined as CHP equivalents according to: CHP equivalents= A5801/A5802 × (CHP (nmol)) × dilution, and expressed as CHP equivalents per wet tissue weight.
Statistical analyses
Data were expressed as mean ± SEM. Statistical analyses were performed using InVivoStat® or Prism® sofware. For the experiments on neuroprotection in B104 cells, data were analysed using a one-way ANOVA (F values) according to treatment, or a two-way ANOVA with treatment and concentration as independent factors, followed by Planned Comparisons on the predicted means to compare the levels of the selected effect. Multiple means were compared using Fisher's protected least significant difference (PLSD) test. For neurotrophicity experiments in B104 cells, the lengths of the longest neurite were calculated by using NeuronJ® software and analyzed using a two-way ANOVA with day and treatment as independent factors. For mice experiments, measures of spontaneous alternation and lipid peroxidation were analyzed using one-way ANOVA, followed by the Dunnett's post hoc multiple comparison test. Passive avoidance latencies did not show a normal distribution as upper cutoff times were set. They were thus expressed as median value and interquartile range and were analyzed using a Kruskal-Wallis non-parametric ANOVA (H values), group comparisons being made with Dunn's non parametric multiple comparisons tests. The level of statistical significance was p < 0.05. For reading clarity, all statistical values are detailed in the figure legends.
RESULTS
Dose-response effects of ent-PREGS and PREGS on B104 neuroblastoma cell viability
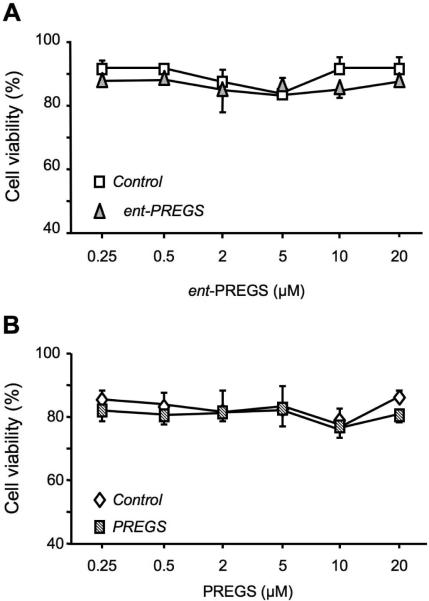
ent-PREGS was incubated at 0.25, 0.5, 2, 5, 10, 20 μM with parallel controls (without steroid), in low density (3×103 cells) cultured B104 cells for 24 h (Fig. 1A). The percentage of viable cells was measured by flow cytometry and we observed that ent-PREGS did not affect the B104 cells viability in the 0.25-20 μM concentration range, as compared to controls (Fig. 1A). Seventy-five to 88 % of viable cells were observed in ent-PREGS treated cell cultures as in controls. Under the same conditions, PREGS also did not affect cell viability in the same concentration range, as compared to controls (Fig. 1B).
Figure 1.
Dose-response of ent-PREGS and PREGS on B104 cell viability. The percentage of viable cells was quantified by flow cytometry. Two-way ANOVA: F(1,40) = 2.16, p > 0.05 for treatment, F(5,40) = 0.74, p > 0.05 for concentration, F(5,40) = 0.12, p > 0.05 for the treatment × concentration interaction in (A); F(1,20) = 2.64, p > 0.05 for treatment, F(5,20) = 0.66, p > 0.05 for concentration, F(5,20) = 0.50, p > 0.05 for the interaction in (B).
Dose response of Aβ25-35 peptide on B104 neuroblastoma cell viability
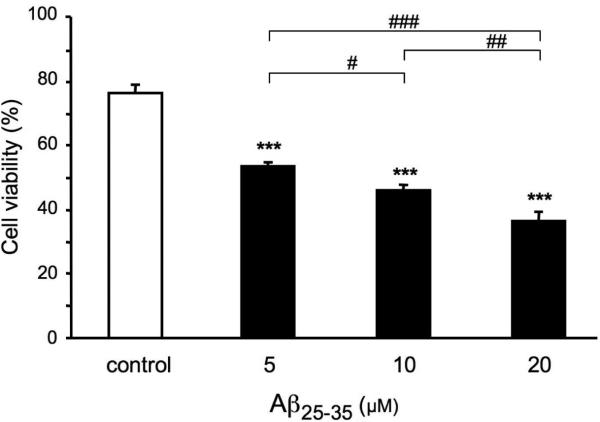
Treatment of the B104 cells for 24 h with Aβ25-35 peptide elicited a dose-dependent reduction in viability, as compared to control cells (Fig. 2). Significant decreases in the percentage of viable cells were already observed with the lowest dose of Aβ25-35.
Figure 2.
Effects of Aβ25-35 peptide on B104 cell viability. Cells were treated with Aβ25-35 peptide (5, 10 and 20 μM) or without peptide (control) for 24 h. The percentage of viable cells was determined by flow cytometric analysis. One-way ANOVA: F(3,8) = 79.6, p < 0.001. *** p < 0.001 vs control cells, # p < 0.05, ## p < 0.01, ### p < 0.001 among the indicated groups, Fisher's PLSD test.
Neuroprotective effect of ent-PREGS or PREGS against Aβ25-35-induced decrease in B104 neuroblastoma cell viability
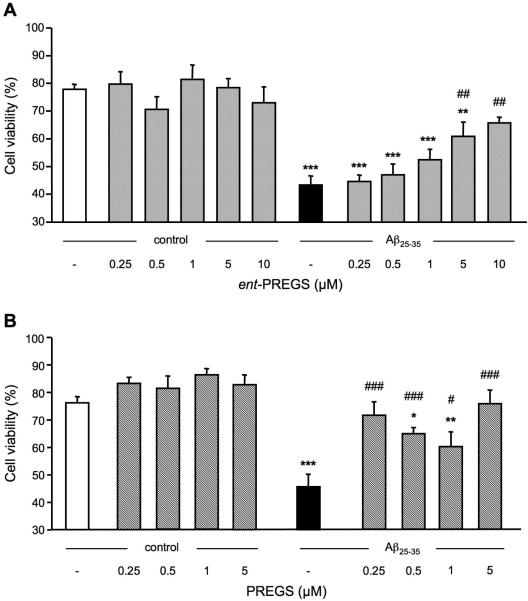
The neuroprotective effect of ent-PREGS was analyzed on the reduction of cell viability provoked by Aβ25-35. ent-PREGS was added at variable concentrations to cell cultures alone or prior to the peptide. As shown in Fig. 3A, the enantiomer failed to affect cell viability in the 0.25-10 μM concentration range. Exposure to Aβ25-35 peptide induced a significant decrease of B104 cell viability, as compared with untreated control cells (p < 0.001; Fig. 3A). Pre-treatment with ent-PREGS attenuated the Aβ25-35-induced decrease in cell viability in a dose-dependent manner, with significant effects at 5 and 10 μM (p < 0.01; Fig. 3A).
Figure 3.
Prevention of Aβ25-35-induced decrease in B104 cell viability by ent-PREGS (A) or PREGS (B). Cells were pre-treated with increasing concentrations of ent-PREGS or PREGS for 30 min, followed or not by exposure to Aβ25-35 (5 μM) for 24 h. Cell viability was determined by flow cytometry analysis. One-way ANOVA: F(11,37) = 9.96, p < 0.001 in (A); F(9,33) = 12.0, p < 0.001 in (B). * p < 0.05, ** p < 0.01, *** p < 0.001 vs. control cells; # p < 0.05, ## p < 0.01, ### p < 0.001 vs. Aβ25-35-treated cells, Fisher's PLSD test.
The effect of PREGS pretreatment on the decrease of cell viability induced by Aβ25-35 was determined. As shown in Fig. 3B, PREGS in the 0.25-5 μM concentration range failed to affect cell viability alone. Treatment with Aβ25-35-induced a significant decrease of B104 cell viability as compared to untreated control cells (p < 0.001; Fig. 3B). This decrease was significantly attenuated by PREGS at all concentrations tested. Particularly, PREGS at the lowest (0.25 μM) and highest concentration tested (5 μM) led to complete prevention of Aβ25-35 toxicity, since group data were not statistically different from control cell data.
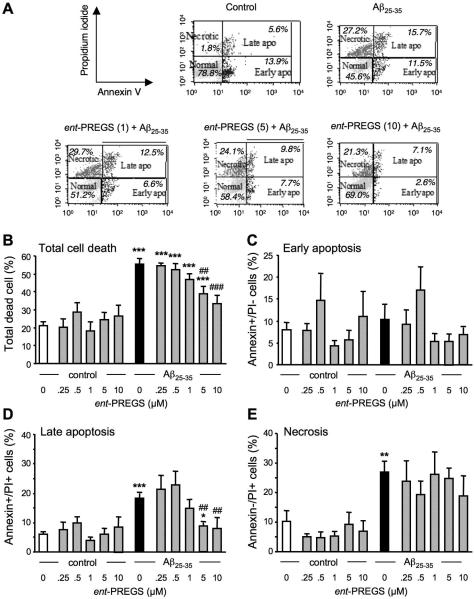
ent-PREGS prevents Aβ25-35 peptide induced late apoptotic death in B104 neuroblastoma cells
We analyzed the ent-PREGS effect on each type of cell death induced by Aβ25-35. Both necrosis (Behl, 1994) and apoptosis (Loo, 1993) have been linked to Aβ25-35-induced toxicity. The use of annexin-V as the Alexa-fluor conjugate in combination with propidium iodide allows one to distinguish between early- and late apoptotic cells, and secondary necrotic cells, by flow cytometry as illustrated in a typical experiment shown in Fig. 4A. We first calculated the percentage of total dead cells by summing that of apoptotic and necrotic cells following the treatments of ent-PREGS (at different concentrations) in the presence or absence of Aβ25-35, and in untreated controls cells (Fig. 4B). Aβ25-35 significantly increased the percentage of total dead cells by three-fold (p < 0.001). ent-PREGS prevented in a concentration-dependent manner Aβ25-35-induced death and this effect was significant at the concentrations of 5 and 10 μM (p < 0.001 Fig. 4B). The percentage of cells in early apoptotic phase was not significantly different whatever the treatment (Fig. 4C). However, there was a highly significant effect of treatment during late apoptosis (Fig. 4D). The percentage of cells in late apoptosis was highly significantly increased by the Aβ25-35 treatment (p < 0.001). The ent-PREGS co-treatment prevented this increase in a concentration-dependant manner, significantly at 5 and 10 μM (p < 0.01; Fig. 4D). Aβ25-35 significantly enhanced the percentage of necrotic cells. Interestingly, ent-PREGS (0.25-10 μM) failed to prevent this Aβ25-35 effect (Fig. 4E).
Figure 4.
Cytometric analysis of the prevention by ent-PREGS (0.25-10 μM) of Aβ25-35-induced B104 cell death: (A) representative Annexin-V-Alexa 488/propidium iodide (PI) double stainings of B104 cells treated with Aβ25-35 peptide ± ent-PREGS (1, 5, 10 μM). The percentages of cells analyzed by flow cytometry in each quadrant under each condition are indicated: lower left, Alexa 488−/PI−, normal intact cells; lower right, Alexa 488+/PI−, early apoptototic cells; upper left, Alexa 488−/PI+, necrotic cells; and upper right, Alexa 488+/PI+, late apoptotic cells. Graphs show the quantification of the percentages of dead cells (B), early apoptotic cells (C), late apoptotic cells (D), and necrotic cells (E). One-way ANOVA: F(11,37) = 14.9, p < 0.001 in (B); F(11,37) = 0.90, p > 0.05 in (C); F(11,37) = 6.39, p < 0.001 in (D); F(11,37) = 2.77, p < 0.01 in (E). * p < 0.05, ** p < 0.01, *** p < 0.001 vs. control cells; ## p < 0.01, ### p < 0.001 vs. Aβ25-35-treated cells; Fisher's PLSD test.
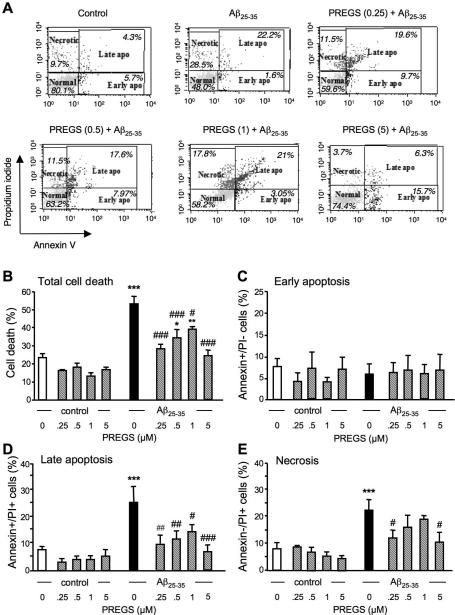
PREGS prevents Aβ25-35 peptide induced late apoptotic and necrotic death in B104 neuroblastoma cells
We tested the PREGS effects under the same conditions as used for ent-PREGS. Figure 5A illustrates a typical experiment showing the percentage of cells in early or late apoptosis, and necrotic cells as determined by flow cytometry in untreated control B104 cell cultures and after treatment by Aβ25-35 without or with PREGS. Aβ25-35 significantly increased the percentage of total dead cells comparing to control (p < 0.001; Fig. 5B). PREGS dose-dependently prevented the death induced by Aβ25-35 and this effect was significant for all concentrations tested, in the 0.25-5 μM concentration-range. However, the percentage of dead cells in cultured cells treated with both Aβ25-35 peptide and PREGS remained significantly higher than in control cells, for 0.5 and 1 μM (Fig. 5B). The PREGS treatment alone had no effect as compared with control cultures. No significant effect of the treatments on cells in early apoptotic phase was measured (Fig. 5C). However, a highly significant effect of the treatments was measured on cells during late apopoptis (Fig. 5D). The percentage of cells in late apoptosis was significantly increased by Aβ25-35. It was significantly lowered by PREGS at all concentrations tested, to the level of control cultures. The percentage of necrotic cells was significantly increased by Aβ25-35 (Fig. 5E). Pre-treatment with PREGS attenuated the peptide effect, significantly at 0.25 and 5.0 μM (Fig. 5E).
Figure 5.
Cytometric analysis of the prevention by PREGS (0.25-5μM) of Aβ25-35-induced B104 cell death: (A) representative Annexin-V-Alexa 488/propidium iodide (PI) double stainings of B104 cells treated with Aβ25-35 peptide ± PREGS. The percentages of cells analyzed by flow cytometry in each quadrant under each condition are indicated: lower left, Alexa 488−/PI−, normal intact cells; lower right, Alexa 488+/PI−, apoptototic cells; upper left, Alexa 488−/PI+, necrotic cells; and upper right, Alexa 488+/PI+, late apoptotic cells. Graphs show the quantifications of the percentages of dead cells (B), early apoptotic cells (C), late apoptotic cells (D), and necrotic cells (E). One-way ANOVA: F(9,33) = 12.0, p < 0.001 in (B); F(9,33) = 0.15, p > 0.05 in (C); F(9,33) = 4.62, p < 0.001 in (D); F(9,33) = 4.32, p < 0.001 in (E). * p < 0.05, ** p < 0.01, *** p < 0.001 vs. control cells; # p < 0.05, ## p < 0.01, ### p < 0.001 vs. Aβ25-35-treated cells; Fisher's PLSD test.
Neuroprotective effect of ent-DHEAS or DHEAS against Aβ25-35-induced decrease in B104 neuroblastoma cell viability
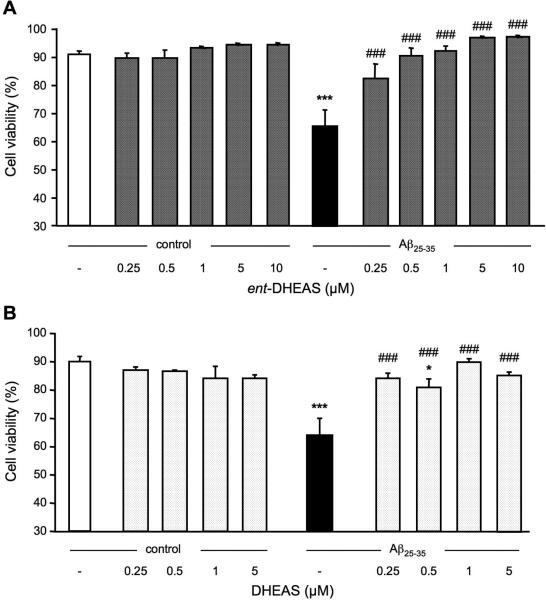
The neuroprotective effect of ent-DHEAS was analyzed for the reduction of cell viability induced by Aβ25-35. ent-DHEAS was added at variable concentrations to cell cultures alone or prior to the peptide. As shown in Fig. 6A, the enantiomer did not affect cell viability in the 0.25-10 μM concentration range. Exposure to Aβ25-35-induced a significant decrease of B104 cell viability, as compared with untreated control cells (p < 0.001; Fig. 6A). Pre-treatment with ent-DHEAS attenuated the Aβ25-35-induced decrease in cell viability with significant effects at all concentrations tested (p < 0.001; Fig. 6A).
Figure 6.
Prevention of Aβ25-35-induced decrease in B104 cell viability by ent-DHEAS (A) or DHEAS (B). Cells were pre-treated with increasing concentrations of ent-DHEAS or DHEAS for 30 min, followed or not by exposure to Aβ25-35 (20 μM) for 24 h. Cell viability was determined by flow cytometry analysis. One-way ANOVA: F(11,27) = 6.75, p < 0.001 in (A); F(9,23) = 6.36, p < 0.001 in (B). *** p < 0.001 vs. control cells; ### p < 0.001 vs. Aβ25-35-treated cells, Fisher's PLSD test.
The effect of DHEAS pretreatment on the decrease of cell viability induced by Aβ25-35 was determined. As shown in Fig. 6B, DHEAS in the 0.25-5 μM concentration range failed to affect cell viability. Treatment with Aβ25-35 induced a significant decrease of B104 cell viability as compared to untreated control cells (p < 0.001; Fig. 6B). This decrease was significantly attenuated by DHEAS at all concentrations tested (p<0.001, Fig.6B).
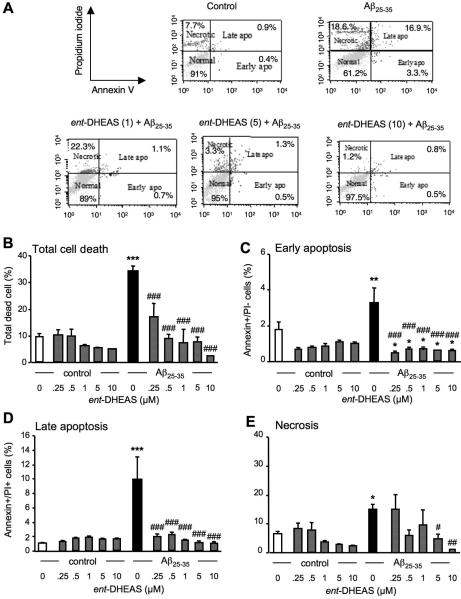
ent-DHEAS prevents Aβ25-35-induced apoptotic and necrotic death in B104 neuroblastoma cells
The percentage of early- and late apoptotic cells, as well as secondary necrotic cells was analyzed by flow cytometry in untreated control B104 cell cultures and after treatment by Aβ25-35 without or with ent-DHEAS, as illustrated in a typical experiment (Fig. 7A). Aβ25-35 significantly increased the percentage of total dead cells by three-fold (p < 0.001). ent-DHEAS prevented in a concentration-dependent manner Aβ25-35-induced death and this effect was significant at the 0.25-10 μM concentration range (p < 0.001, Fig. 7B). The significant increase in the percentage of cells in early apoptotic phase after Aβ25-35 treatment (p < 0.01) was strongly and significantly reduced by ent-DHEAS whatever the concentration (p < 0.001, Fig. 7C). The percentage of cells in late apoptosis was also highly significantly increased by the Aβ25-35 treatment (p < 0.001). The ent-DHEAS co-treatment significantly prevented this increase in a concentration-dependant manner from 0.25 μM to 10 μM (p < 0.001; Fig. 7D). Aβ25-35 significantly enhanced the percentage of necrotic cells (p < 0.05). This effect was prevented by ent-DHEAS only at the highest concentrations of 5 μM (p < 0.05) and 10 μM (p < 0.01) (Fig. 7E).
Figure 7.
Cytometric analysis of the prevention by ent-DHEAS (0.25-10 μM) of Aβ25-35-induced B104 cell death: (A) representative Annexin-V-Alexa 488/propidium iodide (PI) double stainings of B104 cells treated with Aβ25-35 peptide ± ent-DHEAS (1, 5, 10 μM) followed by Aβ25-35 peptide. The percentages of cells analyzed by flow cytometry in each quadrant under each condition are indicated: lower left, Alexa 488−/PI−, normal intact cells; lower right, Alexa 488+/PI−, early apoptototic cells; upper left, Alexa 488−/PI+, necrotic cells; and upper right, Alexa 488+/PI+, late apoptotic cells. Graphs show the quantification of the percentages of dead cells (B), early apoptotic cells (C), late apoptotic cells (D), and necrotic cells (E). Oneway ANOVA: F(11,27) = 6.75, p < 0.001 in (B); F(11,27) = 4.07, p <0.001 in (C); F(11,27) = 5.49, p < 0.001 in (D); F(11,27) = 2.27, p < 0.04 in (E). * p < 0.05, ** p < 0.01, *** p < 0.001 vs. control cells; # p < 0.05, ## p < 0.01, ### p < 0.001 vs. Aβ25-35-treated cells; Fisher's PLSD test.
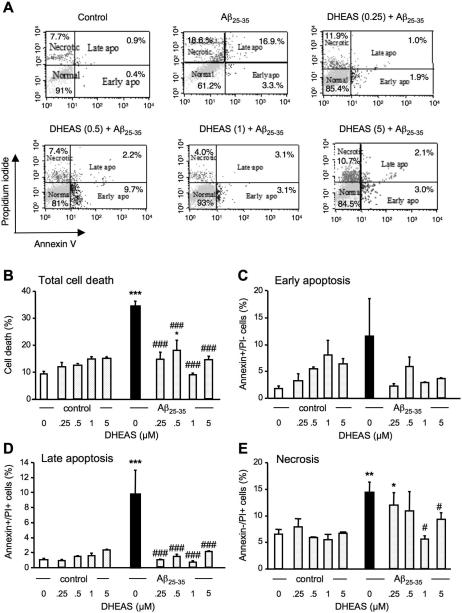
DHEAS prevents Aβ25-35 peptide induced late apoptotic and necrotic death in B104 neuroblastoma cells
Figure 8A illustrates a typical experiment showing the percentage of cells in early or late apoptosis, and necrotic cells determined by flow cytometry in untreated control B104 cell cultures and after treatment by Aβ25-35 without or with DHEAS. Aβ25-35 significantly increased the percentage of total dead cells compared to control (p < 0.001, Fig. 8B). DHEAS dose-dependently prevented the death induced by Aβ25-35 and this effect was significant for all concentrations tested, in the 0.25-5 μM concentration-range. The percentage of cells in the early apoptotic phase was not significantly modified whatever the treatment. A highly significant effect of the treatments was measured on late apopoptic cells (Fig. 8D). The percentage of cells in late apoptosis was significantly increased by Aβ25-35 (p < 0.001). It was significantly lowered by DHEAS at all concentrations tested ((p < 0.001). The percentage of necrotic cells was significantly increased by Aβ25-35 (p < 0.05, Fig. 8E). Pre-treatment with DHEAS attenuated the peptide effect significantly at 1 μM and 5.0 μM (Fig. 5E).
Figure 8.
Cytometric analysis of the prevention by DHEAS (0.25-5 μM) of Aβ25-35-induced B104 cell death: (A) representative Annexin-V-Alexa 488/propidium iodide (PI) double stainings of B104 cells treated with Aβ25-35 peptide alone and cells pre-treated with DHEAS followed by Aβ25-35 peptide. The percentages of cells analyzed by flow cytometry in each quadrant under each condition are indicated: lower left, Alexa 488−/PI−, normal intact cells; lower right, Alexa 488+/PI−, early apoptototic cells; upper left, Alexa 488−/PI+, necrotic cells; and upper right, Alexa 488+/PI+, late apoptotic cells. Graphs show the quantifications of the percentages of dead cells (B), early apoptotic cells (C), late apoptotic cells (D), and necrotic cells (E). Oneway ANOVA: F(9,23) = 6.36, p < 0.001 in (B); F(9,23) = 1.28, p > 0.05 in (C); F(9,23) = 4.01, p < 0.01 in (D); F(9,23) = 2.62, p < 0.05 in (E). * p < 0.05, ** p < 0.01, *** p < 0.001 vs. control cells; # p < 0.05, ## p < 0.01, ### p < 0.001 vs. Aβ25-35-treated cells; Fisher's PLSD test.
Neurotrophic effects of ent-PREGS and PREGS in B104 neuroblastoma cells
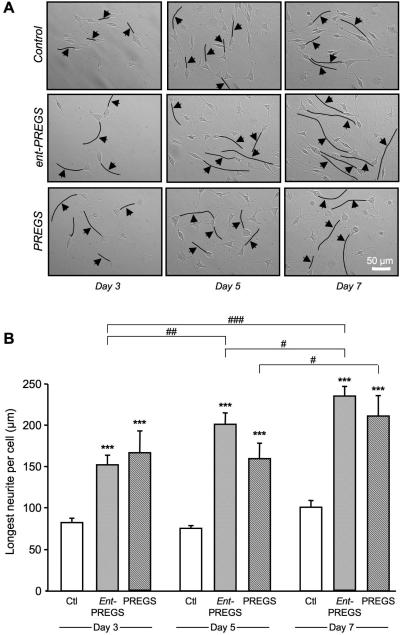
The effects of ent-PREGS and PREGS on B104 cell morphology was investigated by analysing neurite outgrowth. Cells were plated at low density and incubated in the absence (control) or presence of ent-PREGS or PREGS for 3, 5 and 7 days. Figure 9A shows representative phase-contrast photomicrographs of cells. Over time exposure to ent-PREGS or PREGS lead to striking differences in neurite length in steroid treated cells as compared to controls. The length of the longest neurite per cell was significantly increased by ent-PREGS and by PREGS as compared to control, at days 3, 5 and 7 (Fig. 9B). The ent-PREGS treatment increased neurite length over time. Significant differences were observed between day 3 and day 7, day 5 and day 7, and day 3 and 5 (Fig. 9B). The PREGS treatment increased the length of the longest neurite significantly only between day 5 and day 7 (Fig. 9B).
Figure 9.
Effects of ent-PREGS and PREGS on neurite outgrowth in B104 cultured cells: (A) representative photomicrographs of control and steroid-treated cells at day 3, 5 or 7. The longest neurite per cell was marked by a black line and indicated by an arrow (magnification 10×). Scale bar = 50 μm. (B) Histogramms of the length of the longest neurite per cell corresponding to the pool of 3-4 independent experiments. An average of 30-40 longest neurites was counted per treatment group and per day. Two-way ANOVA: F(2,317) = 79.7, p < 0.001 for the steroid treatment; F(2,317) = 11.7, p < 0.001 for the day; F(4,317) = 2.02, p > 0.05 for the treatment × day interaction. *** p < 0.001 vs. control (ctl); # p < 0.05, ## p < 0.01, ### p < 0.001 for pairwise comparisons of steroid treatment between days; Fisher's PLSD test.
Neurotrophic effects of ent-DHEAS and DHEAS in B104 neuroblastoma cells
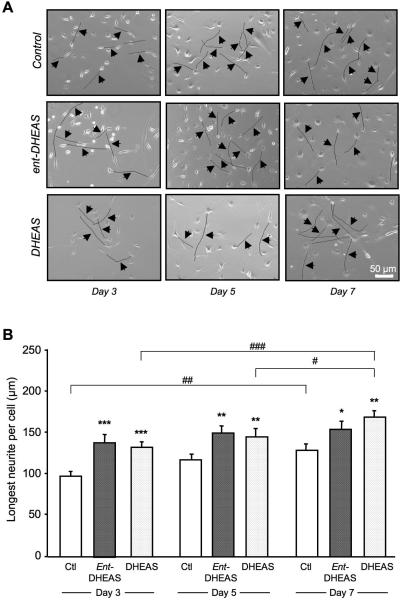
The effects of ent-DHEAS and DHEAS on B104 neurite outgrowth were determined. Cells plated at low density were incubated in the absence (control) or presence of ent-DHEAS or DHEAS for 3, 5 and 7 days. Representative phase-contrast photomicrographs of cells are shown in Figure 10A. The length of the longest neurite per cell was significantly increased by ent-DHEAS as compared to control, at day 3, 5 and 7 (Fig. 10B). It was also significantly increased by DHEAS as compared to control, at day 3, day 5 and 7 (Fig. 10B). The ent-DHEAS treatment slightly, but not significantly increased neurite length over time. In contrast, significant differences were observed with DHEAS treatment between day 3 and day 7 and, day 5 and day 7 (Fig. 10B).
Figure 10.
Effects of ent-DHEAS and DHEAS on neurite outgrowth in B104 cultured cells: (A) representative photomicrographs of control and steroid-treated cells at day 3, 5 or 7. The longest neurite per cell was marked by a black line and indicated by an arrow (magnification 10×). Scale bar = 50 μm. (B) Histograms of the length of the longest neurite per cell corresponding to the pool of 3 independent experiments. An average of 30-35 longest neurites was counted per treatment group and per day. Two-way ANOVA: F(2,290) = 16,3, p < 0.001 for the steroid treatment; F(2,290) = 8,19, p < 0.001 for the day; F(4,290) = 0.64, p > 0.05 for the treatment × day interaction. * p < 0.05, ** p < 0.01, *** p < 0.001 vs. control (ctl); # p < 0.05, ## p < 0.01, ### p < 0.001 for pairwise comparisons of steroid treatment between days; Fisher's PLSD test.
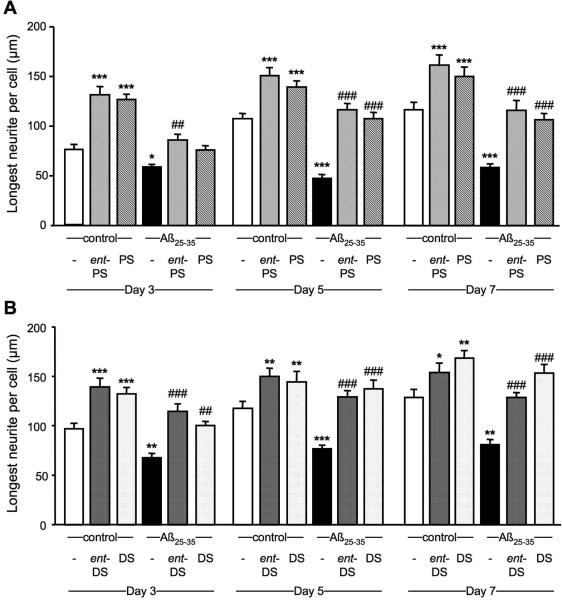
ent-PREGS and PREGS prevents Aβ25-35 peptide induced decrease in neurite outgrowth in B104 neuroblastoma cells
Cells were untreated or treated with Aβ25-35 alone or together with ent-PREGS or PREGS for 3, 5 and 7 days. Exposure to Aβ25-35 peptide significantly decreased the length of the longest neurite at each day tested, as compared with untreated control cells (Fig. 11A) Pretreatment with ent-PREGS significantly attenuated the Aβ25-35-induced decrease in neurite length at days 3, 5 and 7 (Fig. 11A). Pretreatment with PREGS also significantly diminished the Aβ25-35-induced decrease in neurite length at days 5 and 7 but not at day 3 (Fig. 11A).
Figure 11.
Neuroprotective effects of steroids against Aβ25-35-induced decrease on neurite outgrowth in B104 cultured cells. Histograms of the length of the longest neurite per cell correspond to the pool of 3-4 independent experiments. An average of 30-40 longest neurites was counted per treatment group and per day. (A) Effects of ent-PREGS (ent-PS, 5 μM) and PREGS (PS, 5 μM) alone or in presence of Aβ25-35 (20 μM). Two-way ANOVA: F(5,664) = 79.5, p < 0.001 for the steroid treatment; F(2,664) = 26.1, p < 0.001 for the day; F(10,664) = 2.1, p > 0.05 for the treatment × day interaction. (B) Effects of ent-DHEAS (ent-DS, 5 μM) and DHEAS (DS, 5 μM) alone or in the presence of of Aβ25-35 (20 μM). Two-way ANOVA: F(5,585) = 40.3, p < 0.001 for the steroid treatment; F(2,585) = 20.8, p < 0.001 for the day; F(10,664) = 1,4, p > 0.05 for the treatment × day interaction. * p < 0.05, *** p < 0.001 vs. control; ## p < 0.01, ### p < 0.001 vs. Aβ25-35-treated cells. Fisher's PLSD test.
ent-DHEAS and DHEAS prevents Aβ25-35 peptide induced decrease in neurite outgrowth in B104 neuroblastoma cells
Cells were untreated or treated with Aβ25-35 alone or together with ent-DHEAS or DHEAS for 3, 5 and 7 days. Treatment with Aβ25-35 peptide significantly decreased the length of the longest neurite at each day tested, as compared with untreated control cells (Fig. 11B). Pretreatment with ent-DHEAS significantly attenuated the Aβ25-35-induced decrease in neurite length at days 3, 5 and 7 (Fig. 11B). Pretreatment with DHEAS also significantly diminished the Aβ25-35-induced decrease in neurite length at day 3, 5 and 7 (Fig. 11B).
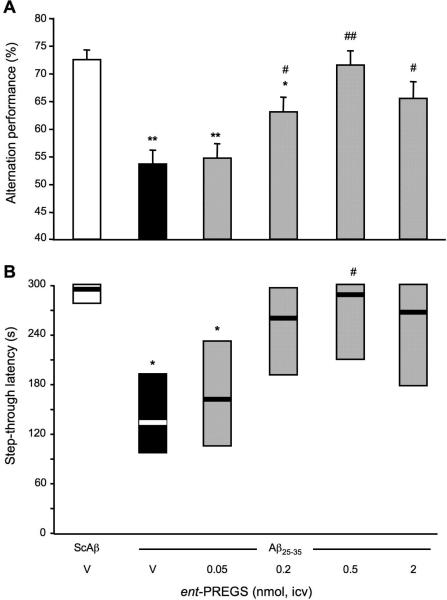
Protective effects of ent-PREGS against Aβ25-35-induced memory deficits
In order to analyze in vivo the steroid enantiomer effects, ent-PREGS, or vehicle, was co-administered i.c.v. with Aβ25-35 in mice at day 0 and the learning performances of the mice were analyzed after one week. Mice were first tested for their spontaneous alternation performance in the Y-maze, a spatial working memory test. Aβ25-35 treatment resulted in a significant decrease in alternation performance as compared to the (ScAβ+V)-treated group (Fig. 12A). The pre-treatment with ent-PREGS led to a dose-dependent attenuation of Aβ25-35-induced deficits, with significant effects at doses higher than 0.2 nmol. Mice performances were then examined using the step-through passive avoidance test (Fig. 12B). The administration of Aβ25-35 peptide significantly reduced the latency in comparison with the controls. The Aβ25-35 peptide induced-impairment was attenuated by ent-PREGS, significantly at 0.5 nmol of ent-PREGS only (Fig. 12B). Of note, the performances of the groups treated with the two higest doses (0.5 and 2 nmol) of ent-PREGS in both tests were similar to that of the (ScAβ+V)-treated control group, showing a complete blockade of the Aβ25-35 peptide induced deficits.
Figure 12.
Protective effects of ent-PREGS against the Aβ25-35-induced memory deficits in mice: (A) spontaneous alternation performances; and (B) step-through passive avoidance. Mice were administered i.c.v. with distilled water (V) or ent-PREGS (0.05-2 nmol) simultaneously with Aβ25-35 peptide (9 nmol). The i.c.v. injection of ScAβ (9 nmol) was used as control. Spontaneous alternation performances in the Y-maze were measured on day 7. Passive avoidance training was carried out on day 8 and retention on day 9. F(5,51) = 7.92, p < 0.0001, n = 6-10 per group, in (A); H = 11.4, p < 0.05, n = 6-10, in (B). * p < 0.05, ** p < 0.01 vs. the (ScAβ+V)-treated group; # p < 0.05 ## p < 0.01 vs. the (Aβ25-35+V)-treated group; Dunnett's test in (A); Dunn's test in (B).
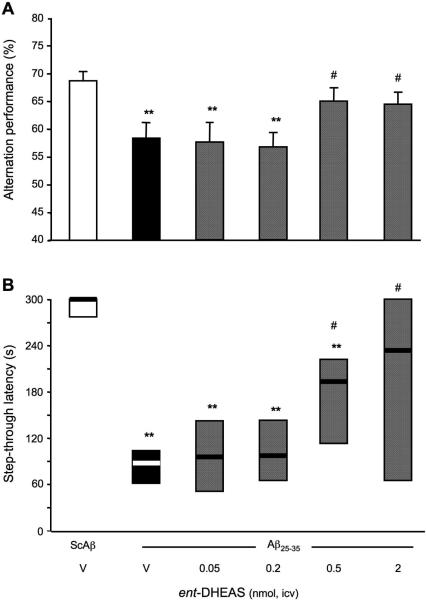
Protective effects of ent-DHEAS against the Aβ25-35-induced memory deficits
We extended the in vivo studies by testing ent-DHEAS. ent-DHEAS was also co-administered i.c.v. in the 0.05-2 nmol dose range with Aβ25-35 peptide and behavioral performances were analyzed one week later. Aβ25-35 resulted in a significant decrease in alternation performance as compared to (ScAβ+V)-treated groups (Fig. 13A). ent-DHEAS significantly attenuated Aβ25-35 peptide-induced deficits, with significant effects at the two highest doses tested. In the passive avoidance test, Aβ25-35 decreased significantly the step-through latency as compared to (ScAβ+Veh)-treated mice (Fig. 13B). ent-DHEAS pre-treatment (0.5 and 2 nmol) significantly prevented the diminution in latency induced by Aβ25-35 (p < 0.05; Fig. 13B). At the highest dose tested, the latency was not significantly different from the control (ScAβ+V)-treated group data, showing a complete prevention of Aβ25-35-induced deficits.
Figure 13.
Protective effects of ent-DHEAS against the Aβ25-35 peptide-induced memory deficits in mice: (A) spontaneous alternation performances; and (B) step-through passive avoidance. Mice were administered i.c.v. with distilled water (V) or ent-DHEAS (0.05-2 nmol) simultaneously with Aβ25-35 peptide (9 nmol). The i.c.v. injection of ScAβ (9 nmol) was used as control. Spontaneous alternation performances in the Y-maze were measured on day 7. Passive avoidance training was carried out on day 8 and retention on day 9. F(5,94) = 3.86, p < 0.01, n = 10, in (A), H = 15.3, p < 0.01, n = 12, in (B). ** p < 0.01 vs. the (ScAβ+V)-treated group; # p < 0.05 vs. the (Aβ25-35+V)-treated group; Dunnett's test in (A), Dunn's test in (B).
Long-term effects of ent-PREGS and ent-DHEAS pre-injected 6 or 12 h before the peptide
Since we considered that the steroid enantiomers are unlikely to be substrates for enzymes involved in steroid biosynthesis in the brain, thereby causing them to remain unchanged longer than natural steroids, we pre-injected the enantiomers at their most active dose (0.5 nmol) at different time-points before the Aβ25-35 peptide and checked the resulting protection in terms of behavioral deficits after 7 days (spontaneous alternation) or 8-9 days (passive avoidance). Results are summarized in Fig. 14. Aβ25-35 peptide-induced alternation deficits are prevented when ent-PREGS or ent-DHEAS is injected 6 h but not 12 h before the peptide on day 0 (Fig. 14A, B). Under these administration schedules, neither PREGS, or DHEAS appeared active. Similarly, in the passive avoidance, a significant prevention of Aβ25-35-induced decrease in step-through latency was observed when ent-PREGS or ent-DHEAS is injected 6 h before the peptide (Fig. 14A), but not 12 h before (Fig. 14D).
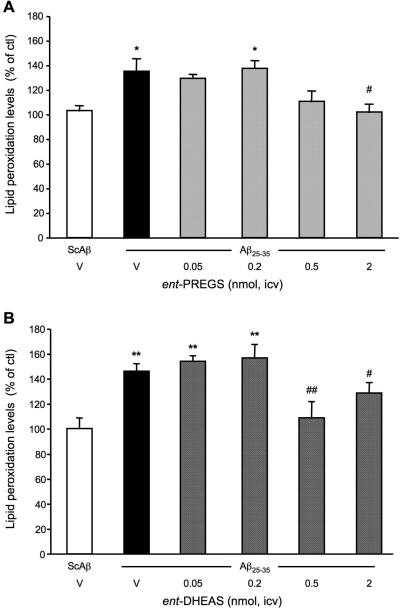
Effects of ent-PREGS and ent-DHEAS on the levels of hippocampal lipid peroxidation
To validate that ent-PREGS and ent-DHEAS protected against Aβ25-35 peptide toxicity, we analyzed the levels of lipid peroxidation in the hippocampus 7 days after i.c.v. injection of the peptide (Fig. 15). Aβ25-35 induced a significant 31-38 % increase in the level of peroxidized lipid, as compared with the control (ScAβ+V)-treatment data (Fig. 15A, B; black columns). ent-PREGS significantly prevented this increase at the dose of 2 nmol (p < 0.05; Fig. 15A). ent-DHEAS also significantly prevented this increase at the doses of 0.5 nmol (p < 0.01) and 2 nmol (p < 0.05; Fig. 15B).
Figure 15.
Neuroprotective effects of ent-PREGS and ent-DHEAS against the Aβ25-35 peptide-induced oxidative stress in the hippocampus of mice: measure of lipid peroxidation levels. Mice were administered i.c.v. with distilled water (V) or ent-PREGS (0.05-2 nmol) in (A) or ent-DHEAS (0.05-2 nmol) in (B), simultaneously with Aβ25-35 peptide (9 nmol). The i.c.v. injection of ScAβ (9 nmol) was used as control. Lipid peroxidation levels were measured on day 9. F(5,30) = 4.69, p < 0.01, n = 6 per group, in (A), F(5,69) = 7.54, p < 0.0001, n = 10-11 per group, in (B). * p < 0.05, ** p < 0.01 vs. the (ScAβ+V)-treated group; ## p < 0.01 vs. the (Aβ25-35+V)-treated group; Dunnett's test.
DISCUSSION
In the present study, we provide the first evidence that synthetic enantiomers of steroids efficiently protect against Aβ peptide toxicity in vitro and in vivo. In vitro, both PREGS and ent-PREGS did not affect the viability of B104 neuroblastoma cells at concentrations up to 20 μM, indicating that they are not detrimental to the cells, nor do they induce their proliferation, under the conditions used. It is clear that the effect of PREGS on cell viability depends on its concentration and the cell type. In rat primary hippocampal cell cultures, PREGS was also devoid of any effect on cell viability, even at 100 μM (Weaver et al. 1998). In rat PC12 cell cultures, however, the cell viability profile under PREGS treatment was bell-shaped with a maximal response at 1 μM (Akan et al. 2009). How ent-PREGS affects the viability of other cell types remains to be determined.
Administration of Aβ25-35 peptide decreased the viability of B104 neuroblastoma cells in a dose-dependent manner. A powerful and significant toxicity was observed in the 5-20 μM range, with the highest concentration showing a relatively high reduction (about 30%) of cell survival, showing toxicity in this concentration range, as previously described in other cell lines including mouse hippocampal HT-22 cells (Gursoy et al. 2001) or SKN-SH human neuroblastoma cells (Gridley et al. 1997).
The neuroprotective effects of steroids were compared in terms of effectiveness (which refers to the ability of the steroid to produce a beneficial effect), efficacy (which refers to the maximum response achievable) and potency (which refers to the amount required to produce an effect of given intensity). Both ent-PREGS and PREGS were effective as they significantly and dose-dependently prevented the decreased of cell viability induced by Aβ25-35. Steroid application for 24 h prior to the peptide resulted in different profiles of cytoprotection. The magnitude of protection by PREGS was higher than that of ent-PREGS at the same concentrations, 0.25-5 μM, indicating that the natural steroid may be more efficient than its synthetic analogue in counteracting the toxic effect of the peptide. In addition, PREGS was more potent than its enantiomer as the minimally active concentration of PREGS was 0.25 μM as compared to 5 μM for ent-PREGS. Complete protection of Aβ-induced toxicity was observed at 5 μM for PREGS and 10 μM for ent-PREGS. These results revealed that ent-PREGS and PREGS have different pharmacological activities in terms of intensity and active dose. Whether or not this enantioselectivity is indicative of different mechanisms of actions for the natural and enantiomeric steroids remains to be determined, but this possibility is intriguing. ent-DHEAS and DHEAS were also found to be neuroprotective by preventing the decrease of cell viability induced by Aβ25-35. The actions of both steroids were similar and they were highly efficient and highly potent. At the lowest steroid concentration, a complete protection of Aβ-induced toxicity was reached. Since PREGS neuroprotection is enantioselective and DHEAS was not enantioselective, this finding may also imply differences in the mechanism of action for PREGS and DHEAS.
It is established that cell death induced by amyloid peptides, including Aβ25-35, involves apoptotic processes (Forloni et al. 1996; Ekinci et al. 2000) and necrosis (Behl 1997), differ according to the cell type. In the present work, the combination of annexin-V and PI in flow cytometric analyses allowed us to distinguish between living, necrotic and apoptotic cells in early- or late-phase. Exposure of B104 cells to Aβ25-25 for 24 h led to significant increases of the percentage of cells in late- not early- apoptosis and necrosis. The increased cell death in late apoptotic phase may be the result of serum withdrawal before steroid and peptide applications. Solovyan et al (Solovyan et al. 1998) have shown evidence of late phase apoptosis occurring in NB2a neuroblastoma cells after serum deprivation. ent-PREGS and PREGS exert protective effects against Aβ toxicity by both preventing late-phase apoptotic toxicity, but with different potencies (ent-PREGS action was significant at the high 5-10 μM concentration while PREGS was already efficient at the lowest 0.25 μM concentration).
One major event of late apoptosis is the potentiation of DNA damages in the cell nucleus. One can then speculate that Aβ25-35-dependent DNA damages may be reduced under steroid treatment. The fact that PREGS also inhibit Aβ-induced necrotic cell death might be one explanation for its higher potency and efficacy in decreasing cell viability as compared to ent-PREGS. Both steroids had no effect on early-phase apoptosis, suggesting that they may not intervene in the initiation of the apoptotic cascades that involves cell membrane and organelle damage without nuclear alterations. With regards to ent-DHEAS and DHEAS, they both prevented Aβ toxicity by reducing late apoptotic and necrotic cells with similar potencies and efficacy.
In addition to their protective properties, ent-PREGS and PREGS highly promoted neurite process growth over time, with the same efficacy. ent-DHEAS and DHEAS also enhanced neurite outgrowth with similar efficacy. Since these trophic effects were not enantioselective, we do not have any evidence that they are the result of steroids directly binding to a receptor. This raises the possibility that the trophic effects might be indirect (e.g., receptors responding differently due to changes in the surrounding membrane caused by the steroids).
We then analyzed the neuroprotective potential of both ent-PREGS and ent-DHEAS in vivo in Aβ25-35-teated mice. ent-Steroids were administered at the same time as Aβ25-35, i.e., 1 week before the behavioural and biochemical analyses. Aβ25-35 induces delayed deficits in spontaneous alternation and passive avoidance 1 week after injection with an active i.c.v. dose of 9 nmol, an effect consistent with our earlier reports (Maurice et al. 1998; Villard et al. 2009). ent-PREGS induced a bell-shaped but significant prevention of Aβ-induced amnesia. The dose of 0.5 nmol i.c.v. was the most effective on both alternation and avoidance responses. Interestingly, equal potency was observed for the pro-mnesiant effect of ent-PREGS in mice tested in the two-trial arm-recognition task (Akwa et al. 2001). Coadministration of ent-DHEAS with Aβ also prevented Aβ-induced memory deficits, with similar potency as ent-PREGS. In contrast to ent-PREGS, the behavioural deficits were reversed with increasing doses of ent-DHEAS, the most active being the highest dose tested (2 nmol). We can thus conclude that both steroid enantiomers protect from the deficits of the short- and long-term memory induced by Aβ25-35, with ent-PREGS being more potent than ent-DHEAS. Using the same behavioural paradigms, we previously showed that systemic administration of PREGS or DHEAS can attenuate with the same potency (20 mg/kg) Aβ25-35 induced memory deficits 1 week after Aβ25-35 icv injection (Maurice et al. 1998). After daily chronic systemic injections for 7 days of PREGS (20 mg/kg), an improvement of Aβ25-35-impaired spatial memory can be observed in the Morris water maze test (Yang et al. 2012).
Results from pre-administration studies clearly suggested that ent-PREGS and ent-DHEAS maintain a longer duration of action that their natural counterparts, since their effect could be detectable in a pretreatment timeframe of at least 6 h. Because of their opposite absolute configuration, ent-PREGS and ent-DHEAS are not expected to be substrates for the biosynthetic enzyme that convert PREGS and DHEAS to other steroids. A high production of PREG and DHEA (identified by gas chromatography-mass spectrometry) could be observed in the rat brain, 5 min after i.c.v injections of PREGS or DHEAS, respectively, while ent-PREGS and ent-DHEAS were not converted into the respective unsulfated ent-steroids (Akwa, unpublished data). These experiments suggested that both steroid enantiomers were not substrates of the brain 3β-hydroxysteroid sulfatase, and were probably less susceptible to further metabolism for this reason. PREGS however can form pregnenolone (PREG) in the brain in rodents (Zwain and Yen 1999; Compagnone and Mellon 2000), which can protect against Aβ25-35 induced cell death with the same efficacy (at low concentration of 0.5 μM), for example in mouse hippocampal (HT-22) (Gursoy et al. 2001) and PC12 (Gursoy et al. 2001) cell lines.
The neuroprotective effects of ent-PREGS and ent-DHEAS were also tested on lipid peroxidation which is an important and early biochemical mechanism of the oxidative stress in AD and Aβ-induced neurotoxicity (Butterfield et al. 2001; Butterfield et al. 2002b). Evidence is presented here for the antioxidant effects of ent-PREGS and ent-DHEAS as pre-treatment of both enantiomers strongly prevented Aβ25-35-induced lipid peroxidation in the hippocampus. This is consistent with the neuroprotective effect of the synthetic enantiomer of 17β-E2 against H2O2 toxicity in human neuroblastoma SH-N-SY cells (Wang et al. 2006).
In conclusion, we report the first demonstration of active and powerful neuroprotection by the synthetic enantiomers of PREGS and DHEAS against Aβ25-35 peptide-induced cell death, amnesia or oxidative stress. The higher efficacy and longer duration of action of ent-PREGS and ent-DHEAS in improving memory deficits, as compared to their natural counterparts, may provide better therapeutic benefits in early stages of AD.
AKNOWLEDGEMENTS
We thank Dr A. Meiniel for generously providing us with B104 neuroblastoma cells. We thank the Flow cytometry core facility at King Faisal Specialist Hospital & Research Center (Riyadh) for help in cytometry experiments. This work is supported in part by external ressources of the Institut National de la Santé et de la Recherche Médicale (INSERM, Paris) and the University of Montpellier 2 (Montpellier), and by the United States National Institutes of Health grant GM 47969 (DFC).
Footnotes
AUTHOR CONTRIBUTIONS
F.E.B. carried out research, analyzed data and implemented the manuscript. J.M., V.V. and M.A. performed research. K.K and D.F.C. provided drug and participated in the design of the study. T.M. and Y.A. designed experiments, carried out research, analyzed data and wrote the manuscript.
CONFLICTS OF INTEREST
J. M. and V. V. are now employees of Amylgen (Montpellier). T. M. is Scientific Director of Amylgen and scientific board adviser of Anavex Life Sciences (Hoboken, NJ, USA). D. F. C. holds equity in Sage Therapeutics Inc. The companies were not involved, scientifically or financially, in the present experiments. The authors declare that they have no other conflict of interest.
REFERENCES
- Akan P, Kizildag S, Ormen M, Genc S, Oktem MA, Fadiloglu M. Pregnenolone protects the PC-12 cell line against amyloid β peptide toxicity but its sulfate ester does not. Chem Biol Interact. 2009;177:65–70. doi: 10.1016/j.cbi.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Akwa Y, Ladurelle N, Covey DF, Baulieu EE. The synthetic enantiomer of pregnenolone sulfate is very active on memory in rats and mice, even more so than its physiological neurosteroid counterpart: distinct mechanisms? Proc Natl Acad Sci USA. 2001;98:14033–7. doi: 10.1073/pnas.241503698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behl C, Davis JB, Klier FG, Schubert D. Amyloid β peptide induces necrosis rather than apoptosis. Brain Research. 1994;645:253–64. doi: 10.1016/0006-8993(94)91659-4. [DOI] [PubMed] [Google Scholar]
- Behl C. Amyloid β-protein toxicity and oxidative stress in Alzheimer's disease. Cell Tissue Res. 1997;290:471–80. doi: 10.1007/s004410050955. [DOI] [PubMed] [Google Scholar]
- Blennow K, de Leon MJ, Zetterberg H. Alzheimer's disease. Lancet. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Drake J, Pocernich C, Castegna A. Evidence of oxidative damage in Alzheimer's disease brain: central role for amyloid β-peptide. Trends Mol Med. 2001;7:548–54. doi: 10.1016/s1471-4914(01)02173-6. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Castegna A, Lauderback CM, Drake J. Evidence that amyloid β-peptide-induced lipid peroxidation and its sequelae in Alzheimer's disease brain contribute to neuronal death. Neurobiol Aging. 2002a;23:655–64. doi: 10.1016/s0197-4580(01)00340-2. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Griffin S, Munch G, Pasinetti GM. Amyloid β-peptide and amyloid pathology are central to the oxidative stress and inflammatory cascades under which Alzheimer's disease brain exists. J Alzheimers Dis. 2002b;4:193–201. doi: 10.3233/jad-2002-4309. [DOI] [PubMed] [Google Scholar]
- Compagnone NA, Mellon SH. Dehydroepiandrosterone: a potential signalling molecule for neocortical organization during development. Proc Natl Acad Sci U S A. 1998;95:4678–83. doi: 10.1073/pnas.95.8.4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compagnone NA, Mellon SH. Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol. 2000;21:1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- Covey DF. ent-Steroids: novel tools for studies of signaling pathways. Steroids. 2009;74:577–85. doi: 10.1016/j.steroids.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey DF, Evers AS, Mennerick S, Zorumski CF, Purdy RH. Recent developments in structure-activity relationships for steroid modulators of GABAA receptors. Brain Res Brain Res Rev. 2001;37:91–7. doi: 10.1016/s0165-0173(01)00126-6. [DOI] [PubMed] [Google Scholar]
- Deshpande A, Mina E, Glabe C, Busciglio J. Different conformations of amyloid beta induce neurotoxicity by distinct mechanisms in human cortical neurons. J Neurosci. 2006;26:6011–8. doi: 10.1523/JNEUROSCI.1189-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekinci FJ, Linsley MD, Shea TB. β-amyloid-induced calcium influx induces apoptosis in culture by oxidative stress rather than tau phosphorylation. Brain Res Mol Brain Res. 2000;76:389–95. doi: 10.1016/s0169-328x(00)00025-5. [DOI] [PubMed] [Google Scholar]
- El Bitar F, Dastugue B, Meiniel A. Neuroblastoma B104 cell line as a model for analysis of neurite outgrowth and neuronal aggregation induced by Reissner's fiber material. Cell Tissue Res. 1999;298:233–42. doi: 10.1007/s004419900081. [DOI] [PubMed] [Google Scholar]
- Farr SA, Banks WA, Uezu K, Gaskin FS, Morley JE. DHEAS improves learning and memory in aged SAMP8 mice but not in diabetic mice. Life Sci. 2004;75:2775–85. doi: 10.1016/j.lfs.2004.05.026. [DOI] [PubMed] [Google Scholar]
- Flood JF, Smith GE, Roberts E. Dehydroepiandrosterone and its sulfate enhance memory retention in mice. Brain Res. 1988;447:269–78. doi: 10.1016/0006-8993(88)91129-8. [DOI] [PubMed] [Google Scholar]
- Forloni G, Bugiani O, Tagliavini F, Salmona M. Apoptosis-mediated neurotoxicity induced by β-amyloid and PrP fragments. Mol Chem Neuropathol. 1996;28:163–171. doi: 10.1007/BF02815218. [DOI] [PubMed] [Google Scholar]
- Gridley KE, Green PS, Simpkins JW. Low concentrations of estradiol reduce β-amyloid25-35-induced toxicity, lipid peroxidation and glucose utilization in human SKN-SH neuroblastoma cells. Brain Res. 1997;778:158–65. doi: 10.1016/s0006-8993(97)01056-1. [DOI] [PubMed] [Google Scholar]
- Gursoy E, Cardounel A, Kalimi M. Pregnenolone protects mouse hippocampal (HT-22) cells against glutamate and amyloid-β protein toxicity. Neurochem Res. 2001;26:15–21. doi: 10.1023/a:1007668213330. [DOI] [PubMed] [Google Scholar]
- Hales TG, Tyndale RF. Few cell lines with GABAA mRNAs have functional receptors. J Neurosci. 1994;14:5429–5436. doi: 10.1523/JNEUROSCI.14-09-05429.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Gwinn-Hardy K. Genetic classification of primary neurodegenerative disease. Science. 1998;282:1075–9. doi: 10.1126/science.282.5391.1075. [DOI] [PubMed] [Google Scholar]
- Harkany T, Hortobagyi T, Sasvari M, Konya C, Penke B, Luiten PG, Nyakas C. Neuroprotective approaches in experimental models of β-amyloid neurotoxicity: relevance to Alzheimer's disease. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:963–1008. doi: 10.1016/s0278-5846(99)00058-5. [DOI] [PubMed] [Google Scholar]
- Hermes-Lima M, Willmore WG, Storey KB. Quantification of lipid peroxidation in tissue extracts based on Fe(III)xylenol orange complex formation. Free Radical Biology and Medicine. 1995;19:271–280. doi: 10.1016/0891-5849(95)00020-x. [DOI] [PubMed] [Google Scholar]
- Holscher C, Gengler S, Gault VA, Harriott P, Mallot HA. Soluble β-amyloid[25-35] reversibly impairs hippocampal synaptic plasticity and spatial learning. Eur J Pharmacol. 2007;561:85–90. doi: 10.1016/j.ejphar.2007.01.040. [DOI] [PubMed] [Google Scholar]
- Kaminsky YG, Marlatt MW, Smith MA, Kosenko EA. Subcellular and metabolic examination of amyloid-β peptides in Alzheimer disease pathogenesis: Evidence for Aβ25-35. Experimental Neurology. 2010;221:26–37. doi: 10.1016/j.expneurol.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Loo DT, Copani A, Pike CJ, Whittemore ER, Walencewicz AJ, Cotman CW. Apoptosis is induced by β-amyloid in cultured central nervous system neurons. Proc Natl Acad Sci USA. 1993;90:7951–5. doi: 10.1073/pnas.90.17.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malouf AT. Effect of β-amyloid peptides on neurons in hippocampal slice cultures. Neurobiol Aging. 1992;13:543–51. doi: 10.1016/0197-4580(92)90054-2. [DOI] [PubMed] [Google Scholar]
- Mark RJ, Blanc EM, Mattson MP. Amyloid β-peptide and oxidative cellular injury in Alzheimer's disease. Mol Neurobiol. 1996;12:211–24. doi: 10.1007/BF02755589. [DOI] [PubMed] [Google Scholar]
- Markowski M, Ungeheuer M, Bitran D, Locurto C. Memory-enhancing effects of DHEAS in aged mice on a win-shift water escape task. Physiol Behav. 2001;72:521–5. doi: 10.1016/s0031-9384(00)00446-7. [DOI] [PubMed] [Google Scholar]
- Mathis C, Vogel E, Cagniard B, Criscuolo F, Ungerer A. The neurosteroid pregnenolone sulfate blocks deficits induced by a competitive NMDA antagonist in active avoidance and lever-press learning tasks in mice. Neuropharmacology. 1996;35:1057–64. doi: 10.1016/s0028-3908(96)00041-x. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Cheng B, Davis D, Bryant K, Lieberburg I, Rydel RE. β-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J Neurosci. 1992;12:376–89. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice T, Lockhart BP, Privat A. Amnesia induced in mice by centrally administered β-amyloid peptides involves cholinergic dysfunction. Brain Res. 1996;706:181–193. doi: 10.1016/0006-8993(95)01032-7. [DOI] [PubMed] [Google Scholar]
- Maurice T, Gregoire C, Espallergues J. Neuro(active)steroids actions at the neuromodulatory sigma1 (σ1) receptor: biochemical and physiological evidences, consequences in neuroprotection. Pharmacol Biochem Behav. 2006;84:581–97. doi: 10.1016/j.pbb.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Maurice T, Junien JL, Privat A. Dehydroepiandrosterone sulfate attenuates dizocilpine-induced learning impairment in mice via sigma 1-receptors. Behav Brain Res. 1997;83:159–64. doi: 10.1016/s0166-4328(97)86061-5. [DOI] [PubMed] [Google Scholar]
- Maurice T, Su TP, Privat A. Sigma1 (σ1) receptor agonists and neurosteroids attenuate β25-35-amyloid peptide-induced amnesia in mice through a common mechanism. Neuroscience. 1998;83:413–28. doi: 10.1016/s0306-4522(97)00405-3. [DOI] [PubMed] [Google Scholar]
- Meunier J, Ieni J, Maurice T. The anti-amnesic and neuroprotective effects of donepezil against amyloid β25-35 peptide-induced toxicity in mice involve an interaction with the sigma1 receptor. Br J Pharmacol. 2006;149:998–1012. doi: 10.1038/sj.bjp.0706927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda S, Opazo C, Larrondo LF, Munoz FJ, Ruiz F, Leighton F. The role of oxidative stress in the toxicity induced by amyloid β-peptide in Alzheimer's disease. Prog Neurobiol. 2000;62:633–48. doi: 10.1016/s0301-0082(00)00015-0. [DOI] [PubMed] [Google Scholar]
- Nilsson KR, Zorumski CF, Covey DF. Neurosteroid analogues. 6. The synthesis and GABAA receptor pharmacology of enantiomers of dehydroepiandrosterone sulfate, pregnenolone sulfate, and (3α,5β)-3-hydroxypregnan-20-one sulfate. J Med Chem. 1998;41:2604–13. doi: 10.1021/jm980148h. [DOI] [PubMed] [Google Scholar]
- Petit GH, Tobin C, Krishnan K, Moricard Y, Covey DF, Rondi-Reig L, Akwa Y. Pregnenolone sulfate and its enantiomer: differential modulation of memory in a spatial discrimination task using forebrain NMDA receptor deficient mice. Eur Neuropsychopharmacol. 2011;21:211–5. doi: 10.1016/j.euroneuro.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike CJ, Walencewicz-Wasserman AJ, Kosmoski J, Cribbs DH, Glabe CG, Cotman CW. Structure-activity analyses of β-amyloid peptides: contributions of the β25-35 region to aggregation and neurotoxicity. J Neurochem. 1995;64:253–65. doi: 10.1046/j.1471-4159.1995.64010253.x. [DOI] [PubMed] [Google Scholar]
- Sayre LM, Zelasko DA, Harris PL, Perry G, Salomon RG, Smith MA. 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer's disease. J Neurochem. 1997;68:2092–7. doi: 10.1046/j.1471-4159.1997.68052092.x. [DOI] [PubMed] [Google Scholar]
- Schubert D, Brass B, Dumas JP. Protein complexity of central nervous system cell lines. J Neurosci. 1986;6:2829–2836. doi: 10.1523/JNEUROSCI.06-10-02829.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D, Heinemann S, Carlisle W, Tarikas H, Kimes B, Patrick J, Steinbach JH, Culp W, Brandt BL. Clonal cell lines from the rat central nervous system. Nature. 1974;249:224–7. doi: 10.1038/249224a0. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease: genotypes, phenotypes, and treatments. Science. 1997;275:630–1. doi: 10.1126/science.275.5300.630. [DOI] [PubMed] [Google Scholar]
- Solovyan V, Bezvenyuk Z, Huotari V, Tapiola T, Suuronen T, Salminen A. Distinct mode of apoptosis induced by genotoxic agent etoposide and serum withdrawal in neuroblastoma cells. Molecular Brain Research. 1998;62:43–55. doi: 10.1016/s0169-328x(98)00234-4. [DOI] [PubMed] [Google Scholar]
- Stepanichev MY, Moiseeva YV, Lazareva NA, Onufriev MV, Gulyaeva NV. Single intracerebroventricular administration of amyloid-β25-35 peptide induces impairment in short-term rather than long-term memory in rats. Brain Res Bull. 2003;61:197–205. doi: 10.1016/s0361-9230(03)00118-7. [DOI] [PubMed] [Google Scholar]
- Tyndale RF, Hales TG, Olsen RW, Tobin AJ. Distinctive patterns of GABAA receptor subunit mRNAs in 13 cell lines. J Neurosci. 1994;14:5417–5428. doi: 10.1523/JNEUROSCI.14-09-05417.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallée M, Mayo W, Le Moal M. Role of pregnenolone, dehydroepiandrosterone and their sulfate esters on learning and memory in cognitive aging. Brain Res Brain Res Rev. 2001a;37:301–12. doi: 10.1016/s0165-0173(01)00135-7. [DOI] [PubMed] [Google Scholar]
- Vallée M, Shen W, Heinrichs SC, Zorumski CF, Covey DF, Koob GF, Purdy RH. Steroid structure and pharmacological properties determine the anti- amnesic effects of pregnenolone sulphate in the passive avoidance task in rats. Eur J Neurosci. 2001b;14:2003–10. doi: 10.1046/j.0953-816x.2001.01817.x. [DOI] [PubMed] [Google Scholar]
- Villard V, Espallergues J, Keller E, Alkam T, Nitta A, Yamada K, Nabeshima T, Vamvakides A, Maurice T. Antiamnesic and neuroprotective effects of the aminotetrahydrofuran derivative ANAVEX1-41 against amyloid β25-35-induced toxicity in mice. Neuropsychopharmacology. 2009;34:1552–66. doi: 10.1038/npp.2008.212. [DOI] [PubMed] [Google Scholar]
- Wang X, Dykens JA, Perez E, Liu R, Yang S, Covey DF, Simpkins JW. Neuroprotective Effects of 17β-Estradiol and Nonfeminizing Estrogens against H2O2 Toxicity in Human Neuroblastoma SK-N-SH Cells. Molecular Pharmacology. 2006;70:395–404. doi: 10.1124/mol.106.022384. [DOI] [PubMed] [Google Scholar]
- Weaver CE, Jr., Wu FS, Gibbs TT, Farb DH. Pregnenolone sulfate exacerbates NMDA-induced death of hippocampal neurons. Brain Res. 1998;803:129–36. doi: 10.1016/s0006-8993(98)00640-4. [DOI] [PubMed] [Google Scholar]
- Weill-Engerer S, David JP, Sazdovitch V, Liere P, Eychenne B, Pianos A, Schumacher M, Delacourte A, Baulieu EE, Akwa Y. Neurosteroid Quantification in Human Brain Regions: Comparison between Alzheimer's and Nondemented Patients. J Clin Endocrinol Metab. 2002;87:5138–43. doi: 10.1210/jc.2002-020878. [DOI] [PubMed] [Google Scholar]
- Xu B, Yang R, Chang F, Chen L, Xie G, Sokabe M. Neurosteroid PREGS protects neurite growth and survival of newborn neurons in the hippocampal dentate gyrus of APPswe/PS1dE9 mice. Curr Alzheimer Res. 2012;9:361–72. doi: 10.2174/156720512800107591. [DOI] [PubMed] [Google Scholar]
- Yang R, Chen L, Wang H, Xu B, Tomimoto H. Anti-amnesic effect of neurosteroid PREGS in Aβ25-35-injected mice through σ1 receptor- and α7nAChR-mediated neuroprotection. Neuropharmacology. 2012;63:1042–50. doi: 10.1016/j.neuropharm.2012.07.035. [DOI] [PubMed] [Google Scholar]
- Yankner BA, Duffy LK, Kirschner DA. Neurotrophic and neurotoxic effects of amyloid β protein: reversal by tachykinin neuropeptides. Science. 1990;250:279–82. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]
- Zussy C, Brureau A, Delair B, Marchal S, Keller E, Ixart G, Naert G, Meunier J, Chevallier N, Maurice T, Givalois L. Time-course and regional analyses of the physiopathological changes induced after cerebral injection of an amyloid-β fragment in rats. Am J Pathol. 2011;179:315–34. doi: 10.1016/j.ajpath.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwain IH, Yen SS. Neurosteroidogenesis in astrocytes, oligodendrocytes, and neurons of cerebral cortex of rat brain. Endocrinology. 1999;140:3843–52. doi: 10.1210/endo.140.8.6907. [DOI] [PubMed] [Google Scholar]