Abstract
Although the incidence rate of acute lymphoblastic leukemia (ALL) is slightly higher in older than in younger adults, response rates to induction chemotherapy and survival rates are poorer. The contribution of disease-related versus treatment-related factors remains unclear. We analysed 100 older patients (age-range 55–65) treated on the UKALLXII/ECOG2993 trial compared with 1814 younger patients (age-range 14–54). We compared baseline characteristics, induction chemotherapy course, infections, drug reductions and survival outcomes. There were more Philadelphia-positive (Ph+) patients in the older group (28% vs. 17%, p=0.02), and a trend in higher combined cytogenetic risk score (46% vs. 35%, p=0.07). The complete remission rate was worse (73% vs. 93%, p<0.0001) as was five-year overall survival (21% vs. 41%, p<0.0001) and event-free survival (EFS) (19% vs. 37%, p<0.0001). Older patients had more infections during induction (81% vs. 70%, p=0.05), and drug reductions (46% vs. 28%, p=0.0009). Among older patients, Ph+ and cytogenetic risk category as well as infection during induction predicted for worse EFS. Poorer outcomes in these patients are partly due to cytogenetic risk, but there is significant morbidity and mortality with induction chemotherapy with frequent delays and drug reductions. New approaches including better risk stratification and use of targeted therapies could improve treatment for these patients.
Introduction
Acute lymphoblastic leukemia (ALL) is often seen as a disease of the young, but the age-specific annual incidence for individuals over 60 years is 0.9–1.6 per 100,000, compared to 0.4–0.6 per 100,000 in those between 25 and 50 years (Larson 2005). Estimates for the proportion of new cases that present in older patients range from 16 to 31% (Taylor et al, 1992; Pagano et al, 2004).
The outcomes for older patients have consistently been found to be worse, both in response to induction chemotherapy, and in long term survival. Furthermore based on an analysis of SEER data from the United States, in contrast to younger patients there has been no significant improvement in outcomes for this group over the last 25 years (Pulte et al, 2009). Despite these differences there are few large cohorts of older patients described, and conclusions about their management are largely extrapolated from younger patients. All of the major cooperative groups are heavily biased towards trials for those under 55 years, with very few patients over the age of 70 included.
Disease-based differences have been shown in older patients, with a higher proportion of B-lineage immunophenotype reported by some groups (Larson 2005; Gökbuget et al, 2000; Robak et al, 2004), as well as cytogenetic differences in particular a higher proportion of Philadelphia chromosome positivity (Ph+) (33%–54%) (Gökbuget et al, 2000; Groupe Français de Cytogénétique Hématologique 1996; Wetzler et al, 2000, Appelbaum et al, 2005; Moorman et al, 2007). However, most treatment protocols include an intensive induction phase with significant toxicities, (often based on pediatric regimens (Huguet et al, 2003)), and the extent to which treatment-based toxicity contributes to poorer outcomes remains unclear.
An increased understanding of the relative importance of disease-related versus treatment-related factors could make a considerable difference when planning optimal treatment strategies. We describe here the characteristics and outcomes of those patients aged ≥55 years enrolled on the MRC UKALL XII / ECOG 2993 trial. This constitutes one of the largest cohorts of older ALL patients treated prospectively on a standard protocol. Analysis provides insight into the reasons for their poorer outcomes, and suggests future strategies to mitigate these.
Methods
Patients
The study was conducted jointly by the Medical Research Council (MRC) of the United Kingdom, and the Eastern Cooperative Oncology Group (ECOG) of the United States. Patients with newly diagnosed, untreated ALL and no prior malignancy were recruited between 1993 and 2006. The Ethics Committee or Institutional Review Board of each participating center approved the study. Informed consent was obtained from all subjects in accordance with the Declaration of Helsinki. There were no exclusion criteria for abnormal renal or hepatic function, or poor performance status at diagnosis. Beginning in 2003 (MRC) or 2004 (ECOG), patients with Ph+ disease were entered into an Imatinib sub-study, and these patients have been excluded from this analysis. The initial age range for MRC patients was 15–55 years, whereas it was 15–59 for ECOG patients. When the Imatinib sub-study began, the upper age limit for all ECOG and Ph+ MRC patients was subsequently increased to 64, but remained at 55 for MRC patients with Ph− disease. Two Ph− MRC patients aged 56–59, were however entered into the trial.
Diagnosis, Treatment and Response Assessment
Diagnosis of ALL was established by documenting more than 25% marrow lymphoblasts. Confirmation of the diagnosis by central morphology review was recommended as well as submission of blood or marrow samples for cytogenetic analysis and immunophenotyping. A combined cytogenetic risk score was subsequently calculated on all patients, with high risk cytogenetics, defined as t(9;22), t(4;11), t(8;14), low hypodiploidy / near-triploidy (HoTr) or complex karyotype.10 CNS involvement was assessed by the presence of lymphoblasts in CSF.
All patients were treated according to the protocol as previously described (Table S1) (Rowe et al, 2005; Goldstone et al, 2008), receiving the identical two stage induction therapy irrespective of risk assessment, including CNS prophylaxis and treatment of CNS disease if present at diagnosis (Figure S1). Antifungal prophylaxis was recommended, but participating centres adhered to local policies. Pneumocystis jiroveci pneumonia (PCP) prophylaxis was recommended with co-trimoxazole, or inhaled pentamidine if the WBC would not tolerate this.
Patients were evaluated for response by bone marrow aspirate at the end of each of the 2 phases of induction. Those who achieved complete remission (CR) went on to the intensification and post-remission consolidation parts of the study. Younger patients achieving CR with a matched sibling donor were to receive an allogeneic matched sibling donor transplant (or a matched unrelated donor for those with Philadelphia positive disease). Patients aged 50 or more (55 or more from 2003) and those with no suitable donor, were eligible for randomization between autologous transplantation and consolidation / maintenance chemotherapy. Patients not achieving CR were taken off protocol, but followed for survival.
Randomization, Data Collection and Statistical Analysis
All patients were centrally registered at either the Clinical Trial Service Unit (CTSU) for MRC patients, or the ECOG Coordinating Center for ECOG patients. These centers were also responsible for randomization and collection of follow-up information.
χ2 tests (for categorical variables) and the Mann-Whitney U test (for continuous variables) were used for comparing age groups by initial characteristics and for remission rates by age group. The primary outcome measure was event-free survival (EFS), defined as the time to relapse or death. Other outcomes analyzed were overall survival (OS), relapse-free survival (RFS) defined as time to relapse excluding patients who never entered remission and censoring at death in remission, and death in remission, excluding non-remitters and censoring at relapse. Patients who did not relapse or die within the follow-up period were censored at the earlier of (1) the date of last contact or (2) October 31, 2010. All event times were measured from the time of diagnosis. Kaplan-Meier curves were used for survival analyses, and univariate comparisons were made by the log-rank method. Odds ratios (ORs) were calculated and are given with their 95% confidence intervals (CIs). Unless otherwise indicated, an OR of less than 1.0 indicates a worse prognosis in the second group compared with the first.
Results
Patient Characteristics
1914 patients were recruited from 1993 up to the study’s closure in 2006, of whom 100 were aged 55 years or more (median 56, range 55–65) and 1814 aged under 55 (median 30, 14–54). More older patients were enrolled into the trial from ECOG than MRC centers (80% vs. 20%), in contrast to the younger group (34% vs. 66%, p<0.0001), as the upper age limit for eligible MRC patients was 55 years but was 60 (later 65) for ECOG patients.
There was no significant difference between the age groups in terms of sex distribution, white cell count (WCC) or B vs. T immunophenotype. The proportion of those with enlarged lymph nodes was lower in the older age group (17% vs. 31%, p=0.004), as was splenomegaly (16% vs. 29%, p=0.005) and hepatomegaly (9% vs. 17%, p=0.04). There was no difference in the presence of anterior mediastinal mass or CNS involvement.
Restricting the analysis to only those patients enrolled before the addition of imatinib to the separate Ph+ substudy, a higher proportion of older patients were Ph+ (28% vs. 17%, p=0.02). No other individual cytogenetic abnormalities varied significantly between groups, although there was a trend towards a higher proportion within the cytogenetic high-risk group in the older patients (46% vs. 35%, p=0.07). (Table 1)
Table 1.
Patient Characteristics
| Characteristic | Age | p value | ||
|---|---|---|---|---|
| <55 | ≥55 | |||
| Number | 1814 | 100 | ||
| Median Age at Entry (Range) | 30 (14–54) | 56 (55–65) | ||
| Group | MRC | 1204 (66%) | 20 (20%) | p<0.0001 |
| ECOG | 610 (34%) | 80 (80%) | ||
| Sex | Male | 1113 (61%) | 53 (53%) | p=0.1 |
| Female | 701 (39%) | 47 (47%) | ||
| Lineage | B | 1401 (77%) | 81 (81%) | p=0.2 B vs. T |
| T | 350 (19%) | 14 (14%) | ||
| Other/Unknown | 63 (3%) | 5 (5%) | ||
| Disease Bulk | CNS disease | 91 (5%) | 5 (5%) | p=1.0 |
| Lymph nodes enlarged | 554 (31%) | 17 (17%) | p=0.004 | |
| Splenomegaly | 527 (29%) | 16 (16%) | p=0.005 | |
| Hepatomegaly | 305 (17%) | 9 (9%) | p=0.04 | |
| Anterior mediastinal mass | 158 (9%) | 4 (4%) | p=0.1 | |
| Comorbidities | Creatinine raised | 141 (8%) | 3 (3%) | p=0.08 |
| Bilirubin raised | 246 (14%) | 11 (11%) | p=0.5 | |
| AST raised | 493 (27%) | 30 (30%) | p=0.5 | |
| Presenting WCC | <50 × 109 /L | 1334 (74%) | 78 (78%) | p=0.2 (excluding missing) |
| ≥50 × 109 /L | 468 (26%) | 20 (20%) | ||
| Unknown | 12 (<1%) | 2 (2%) | ||
|
Ph t(9;22)* All Patients |
Positive | 247 (14%) | 20 (20%) | p=0.07 Ph+ vs. Ph−/unknown |
| Negative | 1370 (76%) | 67 (67%) | ||
| Unknown | 197 (11%) | 13 (13%) | ||
|
Ph t(9;22)* Pre-Imatinib sub-study |
Positive | 246 (17%) | 20 (28%) | p=0.02 Ph+ vs. Ph−/unknown |
| Negative | 1064 (73%) | 44 (62%) | ||
| Unknown | 141 (10%) | 7 (10%) | ||
| Other cytogenetics* | t(8;14) | 18/1258 (1%) | 1/60 (2%) | p=0.8 |
| t(4;11) | 74/1327 (6%) | 3/60 (5%) | p=0.8 | |
| t(1;19) | 28/1214 (2%) | 2/59 (3%) | p=0.6 | |
| Complex | 58/1213 (5%) | 3/59 (5%) | p=0.9 | |
| HeH* | 130/1226 (11%) | 6/59 (10%) | p=0.9 | |
| HoTr* | 39/1224 (3%) | 3/59 (5%) | p=0.4 | |
| Cytogenetic risk group* | Standard | 811 (65%) | 35 (54%) | p=0.07 |
| High | 437 (35%) | 30 (46%) | ||
HeH:High hyperdiploidy (51–65 chromosomes), HoTr: Low hypodiploidy / near-triploidy, High risk cytogenetics: t(9;22)(q34;q11), t(4;11)(q21;q23), t(8;14)(q24;q32), low hypodiploidy / near-triploidy (HoTr) or complex karyotype.
Genetic results were a mixture of cytogenetic, FISH and PCR results. No genetic data was available in 87 cases in the younger age group and 7 in the older. In cases where molecular data only was available, it was not possible to investigate the complete set of so the numbers analysed differ between abnormalities.
Outcomes
Median follow-up was 8.7 years. The CR rate for older patients was 73%, compared to 93% in the younger group (p<0.0001). The rate of death in induction was 18% in the older group compared to 4% within the younger group. 9% of the older group survived induction without attaining CR compared to 3% in the younger group. (Table 2).
Table 2.
Outcome by Age Group
| All Patients | ||||
|---|---|---|---|---|
| Age | p value | |||
| <55 | ≥ 55 | |||
| Number | 1814 | 100 | ||
| CR* | 1683 (93%) | 73 (73%) | p<0.0001 CR vs. No CR* |
|
| No CR | All | 131 (7%) | 27 (27%) | |
| Survived induction, but no CR | 61 (3%) | 9 (9%) | ||
| Died in Induction | 70 (4%) | 18 (18%) | ||
| 5 year Overall Survival (95% CI) | 41% (39–43%) | 21% (12–29%) | p<0.0001 | |
| 5 year Event Free Survival (95% CI) | 37% (34–39%) | 19% (11–27%) | p<0.0001 | |
| 5 year Relapse Free Survival (95% CI) | 50% (48–53%) | 40% (27–53%) | p=0.1 | |
| 5 year Deaths in Remission (95% CI) | 79% (76–81%) | 65% (49–81%) | p=0.07 | |
| 5 year Overall Survival (95% CI) in those who achieved CR ** | 44% (42–47%) | 30% (18–41%) | p=0.03 | |
Those with undocumented date of CR but who continued as per protocol treatment (42 aged <55 and three aged ≥55) are assumed to have achieved remission
Excludes those who died in induction and those who never achieved remission
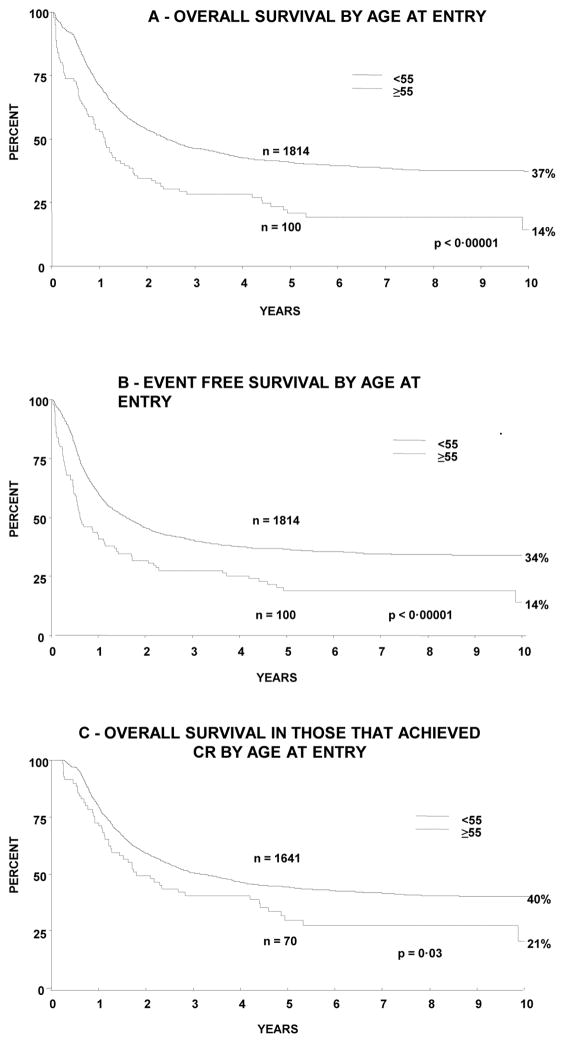
Overall outcomes were worse in the older group compared to the younger: 5 year OS 21% (95% CI 12–29%) vs. 41% (39–43%) (p<0.0001) and 5 year EFS 19% (11–27%) vs. 37% (34–39%) (p<0.0001). 5 year OS among those who achieved CR was also significantly worse in the older group: 30% (18–41%) vs. 44% (42–47% (p=0.03). There was a non-significant difference in RFS in the older compared to the younger group - 40% vs. 50% (p=0.1), as well as the proportion of deaths in remission at 5 years: 65% v 79% (p=0.07). Survival curves are shown in Figure 1.
Figure 1.
Survival of patients by age at entry to study showing (A) overall survival and (B) event free survival in all patients and (C) overall survival in just those who received chemotherapy.
Causes of death in induction without ever achieving remission were similar to those in younger patients. Infection contributed to death in approximately 50% in the older group, compared to around 60% in younger patients (p=0.2). Of the 17 older patients who achieved remission but died without further relapse infection was a contributing cause in ten patients. Only 26 of the older group underwent randomization after achieving CR of which 13 were randomized to chemotherapy and 13 to autograft. These numbers were too small for meaningful log rank comparison.
Infections During Induction
Infection rates during induction chemotherapy varied significantly between the two age groups. As shown in Table S2, the rate of reported infections was higher in the older group in phase 1 (67% vs. 45%, p<0.0001) but similar in phase 2 (59% vs. 55%, p=0.6).
Bacterial infections were the commonest infections reported, with the difference between the two age groups was much more pronounced in phase 1 (50% vs. 33%, p=0.0009). Viral and fungal infections were reported for all patients in both phases, while all except 2 cases of pneumocystis pneumonia (PCP) occurred in phase 2, with none at all reported in the older group.
Drug Reductions During Induction
There were significantly more drug reductions, omissions or delays in the older group in phase 1 (30% vs. 15%, p=0.0001), phase 2 (30% vs. 19%, p=0.02) and either phase 1 or 2 (46% vs. 28%, p=0.0009). In phase 1, the commonest recorded reason for drug reductions in both age groups was hepatotoxicity, but again the rates were significantly higher in the older age group (14% vs. 5%, p=0.0001). (Table S3) Review of the data sheets for the older age group, showed that the asparaginase was the drug most commonly omitted (20 of 66 cases where a drug was specified), with liver toxicity the reason given in most cases.
Prognostic Features within Older Group
Among disease-related factors, presenting WCC >50 × 109 /L predicted for a significantly worse outcome (5 year EFS 0% vs. 23%, p=0.0005), while immunophenotype had no effect. Ph+ was associated with a worse outcome (5 year EFS 0% vs. 22%, p=0.008) as was high vs. standard cytogenetic risk group (5 year EFS 7% vs. 25%, p=0.02). (Table 3)
Table 3.
Prognostic features within older patient group
| Patient factors | ||||
|---|---|---|---|---|
| Variable | 5 year EFS (95% CI) | Odds ratio (95% CI) | p value | |
| Sex | Male | 20% (8–31%) | 1.25 (0.80–1.94) | p=0.3 |
| Female | 18% (6–29%) | |||
| WBC | <50 × 109 /L | 23% (13–33%) | 3.96 (1.96–7.99), | p=0.0005 |
| ≥50 × 109 /L | 0% | |||
| Immunophenotype | B lineage | 17% (8–26%) | 0.69 (0.39–1.24) | p=0.8 |
| T lineage | 36% (11–61%) | |||
| Ph (pre Imatinib study) | Positive | 0% | 0.38 (0.20–0.75), | p=0.008 |
| Negative | 22% (10–34%) | |||
| Cytogenetic risk * | Standard | 25% (10–41%) | 2.03 (1.15–3.59) | p=0.02 |
| High | 7% (0–16%) | |||
| Treatment factors | ||||
|---|---|---|---|---|
| Variable | 5 year EFS (95% CI) | Odds ratio (95% CI) | p value | |
| Full dose induction phase 1 | No | 22% (5–38%) | 0.98 (0.59–1.62) | p=0.9 |
| Yes | 20% (10–30%) | |||
| Full dose induction phase 2 | No | 15% (0–31%) | 0.59 (0.31–1.13), | p=0.1 |
| Yes | 32% (18–46%) | |||
| Full dose induction phase 1 and 2 | Neither | 25% (0–55%) | 0.86 (0.57–1.28), | p=0.4 |
| Phase 1 or 2 | 21% (4–38%) | |||
| Both | 31% (15–47%) | |||
| Significant infection induction phase 1 | No | 34% (16–51%) | 1.76 (1.10–2.80) | p=0.02 |
| Yes | 14% (5–23%) | |||
| Significant infection induction phase 2 | No | 40% (21–59%) | 2.00 (1.14–3.50) | p=0.01 |
| Yes | 17% (5–28%) | |||
| Significant infection induction phase 1 and 2 | Neither | 35% (6–63%) | 1.70 (1.14–2.53) | p=0.008 |
| Phase 1 or 2 | 40% (22–59%) | |||
| Both | 8% (0–18%) | |||
High risk cytogenetics: t(9;22)(q34;q11), t(4;11)(q21;q23), t(8;14)(q24;q32), low hypodiploidy/near-triploidy (HoTr) or complex karyotype
Significant infections during induction chemotherapy were associated with a worse outcome, especially those patients who had infections during both phase 1 and 2 compared to neither phase or one phase only (5 year EFS 8% vs. 39%, p=0.002).
Comparison of outcomes based on the whether significant drug reductions took place do not show a clear trend when patients who died in induction are excluded. There was no difference in EFS in those who had full dose chemotherapy compared to those who had reductions in phase 1 (5 year EFS 23% vs. 29%, p=0.6), phase 2 (33% vs. 15%, p=0.1) or both phases (32% vs. 21% phase 1 or 2 reductions vs. 25% both phase 1 and 2 reductions, p=0.4).
Discussion
The MRC UKALL XII / ECOG E2993 trial included 100 patients between the ages of 55 and 65 representing one of the largest single cohorts of older ALL patients treated prospectively according a standard protocol.
The CR rate in the older group was 70%, which while significantly worse than for the younger patients treated on this protocol (91%), is similar to or better than other cohorts of this age group. The OS overall and EFS are also significantly reduced in the older age group – a common finding in other cohorts, although direct comparisons are made more difficult by differences in follow-up, age-group categories and censoring definitions for disease or event-free survival (Larson 2005; Kantarjian et al, 2000; Petersdorf et al, 2001; Annino et al, 2002; Sancho et al, 2007; Pullarkat et al, 2008; Goekbuget et al, 2008; O’Brien et al, 2008). (Table 4)
Table 4.
Comparative prospective ALL trials looking at older patients
| Group and Trial | Age group | Number | CR rate (%) | OS (%) | |||
|---|---|---|---|---|---|---|---|
| 2yr | 3yr | 5yr | 8yr | ||||
| MRC/ECOG UKALL12/E2993 | 55–65 | 100 | 70 | 35 | 28 | 21 | 19 |
| CALGB (Cumulative*) (Larson 2005) | >60 | 129 | 57 | 12 | |||
| MD Anderson (Kantarjian et al, 2000) | >60 | 44 | 79 | 17 | |||
| SWOG 8419 (Petersdorf et al, 2001) | 50–84 | 85 | 41 | - | |||
| GIMEMA 0288 (Annino et al, 2002) | 50–60 | 121 | 68 | 15 | |||
| PETHEMA ALL96 (Sancho et al, 2007) | 56–67 | 33 | 58 | 39 | |||
| SWOG 9400 (Pullarkat et al, 2008) | 50–65 | 43 | 63 | 23 | |||
| EWALL (Goekbuget et al, 2008) | 56–73 | 40 | 85 | - | |||
CALGB 8811, 9111, 9311, 9511, and 19802
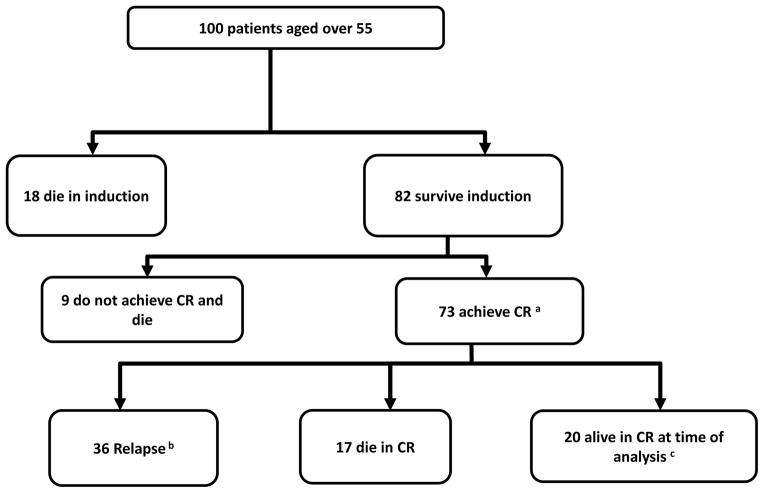
The key issues addressed in this study are the reasons for the difference between younger and older patients, and whether changes in treatment strategy could improve outcomes. As shown in Figure 2, a numeric analysis of the outcomes of the patients in this group illustrates the extent of the problem. Of the 100 older patients in this trial only 20 were alive and in CR at analysis. Of the remaining 80, nine never achieved CR and 36 did, but subsequently relapsed; all except one of these patients had died at the time of analysis. The remaining 35 patients died either during induction chemotherapy (18) or in CR (17). This population therefore is at significant risk of dying due to both a highly aggressive disease and a highly toxic treatment strategy.
Figure 2.
Patient flow diagram showing outcomes following induction in all patients aged 55 years and over.
aIncludes 3 without CR status recorded – presumed to be CR.
bIncludes 1 alive 4 years post-autograft, then lost to follow-up
cIncludes 2 patients lost to follow-up at 2.4 and 8.2 years
At least some of the difference in outcomes is likely to be due to biological differences in the leukemia itself. The incidence of Ph positivity was significantly higher in the older group (28% vs. 17%, p=0.02), a finding well established in other cohorts (Gökbuget et al, 2000; Groupe Français de Cytogénétique Hématologique 1996; Wetzler et al, 2000; Moorman et al 2010; Thomas et al, 2001). Although the combined cytogenetic risk score showed a trend higher risk in the older group, none of the other individual cytogenetic abnormalities showed a significant difference by age. This partly reflects the difficulty in collecting sufficient data for rarely occurring individual abnormalities, even in a large dataset. In contrast to other groups, we found no significant difference in the presenting WCC or immunophenotype between age groups.
The poor remission rates and longer-term outcomes are also related to issues related to toxicity of treatment. The first major point to note is the higher incidence of significant infections reported during induction in the older group. As the infection rate is a reflection of the treatment toxicity rather than underlying disease, this supports the contention that in this age group, the treatment itself contributes to the poor outcomes. Most ALL induction regimens consist of high doses of steroids (prednisolone or dexamethasone), vincristine, daunorubicin and asparaginase, with later exposure to cyclophosphamide and cytarabine. The HyperCVAD regimen does not include asparaginase (Kantarjian et al, 2000), and appears to have similar results in CR rates, but has not been shown to be superior to more traditional protocols.
The combination of myelosuppresssion with high dose steroids probably increases the infection risk. Over 80% of those ≥55 years are recorded as having a significant infection in induction, and this figure is 70% even in the younger group. By comparison, the major infection rate in AML induction has been estimated at 29–35% (Gardner et al, 2008). Within the older group, infection rates during induction had a clear prognostic correlation with EFS, even when those patients who died in induction were excluded (data not shown). Although part of the explanation for this could be the identification of those less fit patients who would be predicted to do worse, we believe that at least part of the explanation is that treatment-induced infections themselves have an impact on longer-term survival, in an already vulnerable patient group.
The second area that we examined in detail was drug reductions, delays and omissions. Here the correlation with EFS within the older groups is less clear, and a causative argument is less obvious. However the proportion of patients who had drug reductions was substantially higher in the older patient group throughout both induction phases, with 46% of the older group having some reduction in phase 1 or 2 compared to 28% in the younger group. This indicates that the induction protocol for this trial, which is fairly typical for ALL induction regimens, is too intensive for many older patients. Hepatotoxicity - a common complication in adult ALL patients undergoing induction - was an important cause of this attenuation of therapy, and the data suggest that asparaginase was poorly tolerated. Although this drug is critical to outcome, its use in the older age group requires re-evaluation
The long recruitment time required for a trial of this size looking at a relatively uncommon disease, means that some features of the trial protocol have been superseded by the time of analysis. With regards to drug toxicity, patients on this trial were treated with daily asparaginase, which has now been effectively replaced by pegylated-asparaginase. There is less toxicity data in adults using the pegylated form of the drug, but it appears that liver toxicity remains an issue with elevated liver enzymes in 52% of cases in a recent series (Rytting 2010). The Ph+ patients reported here exclude those who were treated from 2003 (MRC) or 2004 (ECOG) on a separate substudy utilizing imatinib (Fielding et al, 2010). Given the efficacy and tolerability of imatinib, this drug would certainly be used in treatment protocols for older Ph+ patients.
In our analysis we defined older patients as those between 55 and 65 years, and within this group the median age was 56 years. This demonstrates the difficulty in providing a substantial evidence base for the treatment of genuinely old patients. The conclusions that we have drawn here regarding toxicity and intolerance of standard chemotherapy, must be assumed to hold even more so for an older population. Many older patients off-protocol are treated with a less intensive regimens consisting of steroids and vincristine with or without targeted agents. These patients are presently poorly represented in the medical literature, but they almost certainly outnumber those treated with intensive approaches.
In conclusion, the poor outcomes of older patients with ALL are only partly due to differences in leukemia biology, and an inability to tolerate intensive induction chemotherapy regimens plays a large part in the poorer outcomes. Better risk stratification based not only on age may help to identify those fitter patients who are able to manage standard therapy, and those who are less fit and should be treated less aggressively. There may be benefit in early consolidation with reduced-intensity conditioning allografts in selected patients and the use of MRD monitoring early on to make decisions on further treatment. Combinations of less intensive protocols with targeted therapies such as rituximab in CD20 positive disease (Thomas et al, 2010), nelarabine in T cell lineages (DeAngelo et al, 2007) and dasatinib in Ph+ disease (Rousselot et al, 2010) should play a larger part in these patients’ management. In addition, newer agents such as Blinatumomab appear to have the potential to eliminate MRD, with limited toxicity (Topp et al, 2011). Some of these questions are being addressed in prospective trials, and the coming years should allow a more nuanced approach to treatment of ALL, as a disease of the old as well as the young.
Supplementary Material
Acknowledgments
The authors thank all participating centers, physicians, and patients.
Footnotes
Authorship Contributions
J.I.S. wrote the paper. All authors contributed to the manuscript, checked the final version, and participated in data collection, study design, and coordination. G.B. and S.M.R. analyzed the data. A.H.G. and J.M.R. were study chairs in the United Kingdom and United States, respectively.
Disclosure of Conflicts of Interest
The authors declare no competing financial interests.
References
- Annino L, Vegna ML, Camera A, et al. Treatment of adult acute lymphoblastic leukemia (ALL): long-term follow-up of the GIMEMA ALL 0288 randomized study. Blood. 2002 Feb 1;99(3):863–71. doi: 10.1182/blood.v99.3.863. [DOI] [PubMed] [Google Scholar]
- Appelbaum FR. Impact of age on the biology of acute leukemia. In: Perry MC, editor. American Society of Clinical Oncology Educational Book. 2005. pp. 528–532. [Google Scholar]
- DeAngelo DJ, Yu D, Johnson JL, et al. Nelarabine induces complete remissions in adults with relapsed or refractory T-lineage acute lymphoblastic leukemia or lymphoblastic lymphoma: Cancer and Leukemia Group B study 19801. Blood. 2007 Jun 15;109(12):5136–42. doi: 10.1182/blood-2006-11-056754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding A, Buck G, Lazarus HM, et al. Imatinib Significantly Enhances Long-Term Outcomes In Philadelphia Positive Acute Lymphoblastic Leukaemia; Final Results of the UKALLXII/ECOG2993 Trial. ASH Abstract. 2010:169. [Google Scholar]
- Gardner A, Mattiuzzi G, Faderl S. Randomized comparison of cooked and noncooked diets in patients undergoing remission induction therapy for acute myeloid leukemia. J Clin Oncol. 2008;26(35):5684–8. doi: 10.1200/JCO.2008.16.4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goekbuget N. First European Chemotherapy Schedule for Elderly Patients with Acute Lymphoblastic Leukemia: Promising Remission Rate and Feasible Moderate Dose Intensity Consolidation. Blood (ASH Annual Meeting Abstracts) 2008;112:304. [Google Scholar]
- Gökbuget N, Hoelzer D, Arnold R, et al. Treatment of Adult ALL according to protocols of the German Multicenter Study Group for Adult ALL (GMALL) Hematol Oncol Clin North Am. 2000 Dec;14(6):1307–25. doi: 10.1016/s0889-8588(05)70188-x. [DOI] [PubMed] [Google Scholar]
- Goldstone AH, Richards SM, Lazarus HM, et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993) Blood. 2008;111:1827–1833. doi: 10.1182/blood-2007-10-116582. [DOI] [PubMed] [Google Scholar]
- Groupe Français de Cytogénétique Hématologique. Cytogenetic abnormalities in adult acute lymphoblastic leukemia: correlations with hematologic findings and outcome. Blood. 1996;87:3135–3142. [PubMed] [Google Scholar]
- Huguet F, Leguay T, Raffoux E, et al. Pediatric-inspired therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: the GRAALL-2003 study. J Clin Oncol. 2009;27(6):911–8. doi: 10.1200/JCO.2008.18.6916. [DOI] [PubMed] [Google Scholar]
- Kantarjian HM, O’Brien S, Smith TL, et al. Results of treatment with hyper-CVAD, a dose-intensive regimen, for adult acute lymphoblastic leukemia. J Clin Oncol. 2000;18:547–561. doi: 10.1200/JCO.2000.18.3.547. [DOI] [PubMed] [Google Scholar]
- Larson RA. Acute lymphoblastic leukemia: older patients and newer drugs. Hematology Am Soc Hematol Educ Program. 2005:131–136. doi: 10.1182/asheducation-2005.1.131. [DOI] [PubMed] [Google Scholar]
- Moorman AV, Harrison CJ, Buck GA, et al. Adult Leukaemia Working Party, Medical Research Council/National Cancer Research Institute. Karyotype is an independent prognostic factor in adult acute lymphoblastic leukemia (ALL): analysis of cytogenetic data from patients treated on the Medical Research Council (MRC) UKALLXII/Eastern Cooperative Oncology Group (ECOG) 2993 trial. Blood. 2007;109(8):3189–97. doi: 10.1182/blood-2006-10-051912. [DOI] [PubMed] [Google Scholar]
- Moorman AV, Chilton L, Wilkinson J, Ensor HM, Bown N, Proctor SJ. A population-based cytogenetic study of adults with acute lymphoblastic leukemia. Blood. 2010;115(2):206–14. doi: 10.1182/blood-2009-07-232124. [DOI] [PubMed] [Google Scholar]
- O’Brien S, Thomas DA, Ravandi F, Faderl S, Pierce S, Kantarjian H. Results of the hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone regimen in elderly patients with acute lymphocytic leukemia. Cancer. 2008 Oct 15;113(8):2097–101. doi: 10.1002/cncr.23819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano L, Mele L, Trapè G, Leone G. The treatment of acute lymphoblastic leukaemia in the elderly. Leuk Lymphoma. 2004 Jan;45(1):117–2. doi: 10.1080/1042819031000139648. [DOI] [PubMed] [Google Scholar]
- Petersdorf SH, Kopecky KJ, Head DR, et al. Comparison of the L10M consolidation regimen to an alternative regimen including escalating methotrexate/L-asparaginase for adult acute lymphoblastic leukemia: a Southwest Oncology Group Study. Leukemia. 2001 Feb;15(2):208–16. doi: 10.1038/sj.leu.2402006. [DOI] [PubMed] [Google Scholar]
- Pullarkat V, Slovak ML, Kopecky KJ, Forman SJ, Appelbaum FR. Impact of cytogenetics on the outcome of adult acute lymphoblastic leukemia: results of Southwest Oncology Group 9400 study. Blood. 2008 Mar 1;111(5):2563–72. doi: 10.1182/blood-2007-10-116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulte D, Gondos A, Brenner H. Improvement in survival in younger patients with acute lymphoblastic leukemia from the 1980s to the early 21st century. Blood. 2009 Feb 12;113(7):1408–11. doi: 10.1182/blood-2008-06-164863. [DOI] [PubMed] [Google Scholar]
- Robak T, Szmigielska-Kaplon A, Wrzesien-Kus A, et al. Acute lymphoblastic leukemia in elderly: the Polish Adult Leukemia Group experience. Ann Hematol. 2004;83:225–231. doi: 10.1007/s00277-003-0808-9. [DOI] [PubMed] [Google Scholar]
- Rousselot P, Cayuela JM, Hayette S, et al. Dasatinib and Low Intensity Chemotherapy for First-Line Treatment In Elderly Patients with De Novo Philadelphia Positive ALL (EWALL-PH-01): Kinetic of Response, Resistance and Prognostic Significance. Blood (ASH Annual Meeting Abstracts), Nov. 2010;116:172. [Google Scholar]
- Rowe JM, Buck G, Burnett AK, et al. Induction therapy for adults with acute lymphoblastic leukemia: results of more than 1500 patients from the international ALL trial: MRC UKALL XII/ECOG E2993. Blood. 2005;106(12):3760–7. doi: 10.1182/blood-2005-04-1623. [DOI] [PubMed] [Google Scholar]
- Rytting M. Peg-asparaginase for acute lymphoblastic leukemia. Expert Opin Biol Ther. 2010 May;10(5):833–9. doi: 10.1517/14712591003769808. [DOI] [PubMed] [Google Scholar]
- Sancho JM, Ribera JM, Xicoy B, et al. Results of the PETHEMA ALL-96 trial in elderly patients with Philadelphia chromosome-negative acute lymphoblastic leukemia. Eur J Haematol. 2007 Feb;78(2):102–10. doi: 10.1111/j.1600-0609.2006.00778.x. [DOI] [PubMed] [Google Scholar]
- Taylor PR, Reid MM, Bown N, Hamilton PJ, Proctor SJ. Acute lymphoblastic leukemia in patients aged 60 years and over: a population-based study of incidence and outcome. Blood. 1992 Oct 1;80(7):1813–7. [PubMed] [Google Scholar]
- Thomas DA, O’Brien S, Faderl S, et al. Chemoimmunotherapy with a modified hyper-CVAD and rituximab regimen improves outcome in de novo Philadelphia chromosome-negative precursor B-lineage acute lymphoblastic leukemia. J Clin Oncol. 2010 Aug 20;28(24):3880–9. doi: 10.1200/JCO.2009.26.9456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas X, Olteanu N, Charrin C, et al. Acute lymphoblastic leukemia in the elderly: the Edouard Herriot Hospital experience. Am J Hematol. 2001;67:73–83. doi: 10.1002/ajh.1083. [DOI] [PubMed] [Google Scholar]
- Topp MS, Kufer P, Gökbuget N. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011 Jun 20;29(18):2493–8. doi: 10.1200/JCO.2010.32.7270. [DOI] [PubMed] [Google Scholar]
- Wetzler M, Dodge RK, Mrozek K, et al. Prospective karyotype analysis in adult acute lymphoblastic Leukemia. The Cancer and Leukemia Group B experience. Blood. 1999;93:3983–3993. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.