Abstract
Background
ST2 is involved in cardioprotective signaling in the myocardium and has been identified as a potentially promising biomarker in HF. We evaluated ST2 levels and their association with functional capacity and long-term clinical outcomes in a cohort of ambulatory heart failure (HF) patients enrolled in the HF-ACTION study—a multicenter, randomized study of exercise training in HF.
Methods and Results
HF-ACTION randomized 2331 patients with left ventricular ejection fraction <0.35 and New York Heart Association class II–IV HF to either exercise training or usual care. ST2 was analyzed in a subset of 910 patients with evaluable plasma samples. Correlations and Cox models were used to assess the relationship among ST2, functional capacity, and long-term outcomes.
The median baseline ST2 level was 23.7 ng/mL (interquartile range, 18.6–31.8). ST2 was modestly associated with measures of functional capacity. In univariable analysis, ST2 was significantly associated with death or hospitalization (hazard ratio [HR], 1.48; p<0.0001), cardiovascular death or HF hospitalization (HR, 2.14; p<0.0001), and all-cause mortality (HR, 2.33; p<0.0001)(all HRs for log-base2 ng/mL). In multivariable models, ST2 remained independently associated with outcomes after adjustment for clinical variables and amino-terminal pro–B-type natriuretic peptide. However, ST2 did not add significantly to reclassification of risk as assessed by changes in the C statistic, net reclassification improvement, and integrated discrimination improvement.
Conclusions
ST2 was modestly associated with functional capacity and was significantly associated with outcomes in a well-treated cohort of ambulatory HF patients, although it did not significantly affect reclassification of risk.
Keywords: heart failure, biomarker, prognosis
Introduction
Chronic heart failure is a major and growing public health issue, affecting more than 5 million people in the United States. Although there has been substantial progress in heart failure therapy, overall prognosis remains poor, with 5-year mortality approaching 50% in symptomatic patients1, 2. At the same time, the available treatment options have proliferated and include many expensive and complex treatments such as cardiac resynchronization therapy, left ventricular assist devices, and invasive hemodynamic monitors. Risk stratification is therefore increasingly critical in order to apply costly and invasive therapies to those patients most likely to benefit.
Biomarkers, in particular the natriuretic peptides, play an increasingly important role in the risk stratification of heart failure patients3. A variety of biomarkers have emerged as potentially useful clinical tools for heart failure, including ST24, galectin-35, growth-differentiation factor 156, copeptin7, and midregional pro-hormone adrenomedullin8 among many others. There remains a need to validate these newer biomarkers in diverse populations with careful covariate adjustment in order to clarify their role in heart failure management.
ST2 has been identified as the ligand for interleukin-33 (IL-33) and exists in both a trans-membrane and soluble form. IL-33/ST2 signaling in the myocardium appears to be a cardioprotective mechanism that limits hypertrophy and myocardial fibrosis9. Circulating ST2 appears to function as a “decoy receptor,” modulating the effects of excessive IL-33 signaling10. The soluble form of ST2 has recently been identified as a promising biomarker in patients with both acute coronary syndromes11 and heart failure4, 12. Accordingly, we sought to evaluate how circulating ST2 levels are associated with other measures of heart failure status and with long-term clinical outcomes in a cohort of ambulatory heart failure patients enrolled in the Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training(HF-ACTION) study—a large, multicenter, randomized study of exercise training in heart failure.
Methods
The primary results, design and rationale of the HF-ACTION study have been previously published13, 14. Briefly, HF-ACTION (ClinicalTrials.gov identifier: NCT00047437) was a randomized clinical study evaluating the effects of exercise training vs. usual care on long-term morbidity and mortality in patients with chronic heart failure due to left ventricular systolic dysfunction (New York Heart Association [NYHA] class II–IV, left ventricular ejection fraction [LVEF]<0.35). The primary endpoint of HF-ACTION was a composite of all-cause mortality and all-cause hospitalization. Randomization to exercise training in the HF-ACTION study was associated with modest reductions in the primary endpoint and other cardiovascular endpoints. An independent clinical events committee adjudicated all deaths and first cardiovascular hospitalizations. HF-ACTION was approved by local institutional review boards, and all enrolled patients provided written informed consent.
Biomarker assays
A subset of patients enrolled in the HF-ACTION study who agreed to participate in the biomarker substudy underwent plasma collection at baseline, 3 months, and 12 months. Baseline blood samples were obtained on the same day as baseline cardiopulmonary exercise (CPX) testing but prior to exercise. Samples were collected via peripheral vein into EDTA-containing tubes, centrifuged immediately, and then stored at −70°C for subsequent analysis. Soluble ST2 levels were assessed on baseline samples using a highly sensitive sandwich monoclonal immunoassay (Presage® ST2 Assay, Critical Diagnostics, New York, NY), with a lower limit of detection of 2 ng/mL, an upper limit of detection of 200 ng/mL, an intra-assay coefficient of variation <2.5%, and an interassay coefficient of variation of <4.6%15. Amino-terminal pro–B-type natriuretic peptide (NT-proBNP) was assessed at a central core lab using a clinically available assay (Roche Elecys®, Roche Diagnostics, Indianapolis, IN). The core laboratories were blinded to all clinical data.
Statistical analysis
Baseline characteristics were described using medians and interquartile ranges or proportions. The outcome variables of interest were 1) time to all-cause hospitalization or all-cause mortality (the primary outcome of the HF-ACTION study), 2) time to cardiovascular death or heart failure hospitalization, 3) time to all-cause mortality, and 4) change in peak VO2 from baseline to 3 months.
ST2 was a continuous variable in all models but was log transformed for analysis because it was not normally distributed. Hazard ratios (HRs) were calculated for the log base 2, such that the reported HR represented the risk per doubling of ST2. Although our primary analysis was focused on ST2 as a continuous variable, for descriptive purposes we also examined ST2 as a dichotomous variable above and below the clinical cutpoint of 35 ng/mL (based on receiver operating characteristic curve analysis and U.S. Food and Drug Administration labeling)4.
The relationships among ST2 levels and other baseline variables of interest were analyzed using simple correlations. For the clinical outcomes of interest, we analyzed the relationships using a series of Cox proportional hazards models, including adjusting for demographics alone (age, sex, race)as well as additional adjustment for the more comprehensive set of predictors (“final clinical model”) that were identified in the adjustment model developed for the overall HF-ACTION cohort for each endpoint as previously described16.(see Appendix for a list of covariates). For the relationship between baseline ST2 and change in peak VO2 at 3 months, inverse probability weighting was used to adjust for missingness of exercise parameters during follow-up (16% of patients with available ST2 data were missing peak VO2 at 3 months). The relationship between ST2 at baseline and change peak VO2 at 3 months was then analyzed using a linear regression model that included change in peak VO2 at 3 months (transformed to achieve normality) as the response variable and ST2 (log 2 ST2) along with potential confounders as explanatory variables.
Although NT-proBNP was not part of the modeling process for the overall HF-ACTION study because it was not available in the entire cohort, we additionally examined each model with and without NT-proBNP, given its known strong association with outcomes in chronic heart failure17. Because the final adjustment model from the overall HF-ACTION dataset included variables from CPX testing, which is not routinely available in some clinical settings, we examined each adjusted model with and without CPX variables.
We examined ST2’s added value to existing models by comparing model discrimination and risk prediction between models with and without log 2 ST2. The specific measures that we considered were the C statistic, the integrated discrimination improvement (IDI), and the net reclassification improvement (NRI). For each model comparison, the summary measures were reported along with 95% bootstrap confidence intervals based on 100 replications. We calculated NRI using the Kaplan-Meier estimates of event probabilities at the end of study follow-up. Because there are no widely accepted categories for the absolute risk of HF, we calculated the NRI without categories.
Finally, to further explore the potential relationship between NT-proBNP and ST2, we evaluated the risk in 4 groups defined by dichotomizing each variable (ST2 as above or below 35 ng/mL cutpoint and NT-proBNP at the median = 857 ng/ml). A p-value ≤0.05 was considered statistically significant for all analyses. All analyses were completed using SAS 9.2 (Cary, NC).
Results
Evaluable baseline plasma samples were available for 910 patients. Baseline characteristics for the entire HF-ACTION cohort and the subset with ST2 data available, further stratified by ST2 above and below 35 ng/mL, are shown in Table 1. The median age of the study cohort was 59 years; 64% were white, and 71% were male. The median NT-proBNP level was 857 pg/mL, median LVEF was 24%, and median serum creatinine was 1.2 mg/dL. Utilization of guideline-based medical therapy for systolic heart failure was high, with 95% receiving beta-blockers and 74% receiving angiotensin-converting enzyme (ACE) inhibitors. The subset of patients with available plasma samples for analysis (n=910) was broadly similar to the HF-ACTION cohort as a whole (n=2331, see Table 1), except that the biomarker substudy was limited to patients enrolled in North America. The median ST2 level was 23.7 ng/mL (IQR, 18.6–31.8), and 19.6% (n=179 of 910) of patients had ST2 levels above the proposed prognostic cutpoint of 35 ng/mL. Patients with elevated ST2 levels were more likely to be men and to have markers of more advanced disease, including higher NYHA class, higher NT-proBNP, and worse renal function (p<0.001 for all).
Table 1.
Baseline Characteristics by ST2 Levels
| Variable | Total Population (N=2331) | Substudy Popluiation (N=912) | ST2 ≤ 35ng/mL (N=731) | ST2 > 35ng/mL (N=179) | P-Value |
|---|---|---|---|---|---|
| Randomized treatment (exercise) | 1159/2331 (49.7%) | 453/912 (49.7%) | 364/731 (49.8%) | 87/179 (48.6%) | 0.775 |
| Age, y | 59.3 (51.1,68.0) | 59.2 (50.6,68.4) | 58.5 (50.0,67.5) | 63.3 (55.1,71.9) | <.001 |
| Sex, % woman | 661/2331 (28.4%) | 264/910 (29.0%) | 235/731 (32.1%) | 29/179 (16.2%) | <.001 |
| Race (%Caucasian) | 1426/2296 (62.1%) | 578/898 (64.4%) | 458/721 (63.5%) | 120/177 (67.8%) | 0.525 |
| History of diabetes | 748/2331 (32.1%) | 299/910 (32.9%) | 230/731 (31.5%) | 69/179 (38.5%) | 0.071 |
| History of MI | 979/2331 (42.0%) | 375/910 (41.2%) | 284/731 (38.9%) | 91/179 (50.8%) | 0.003 |
| History of hypertension | 1388/2318 (59.9%) | 578/906 (63.8%) | 464/728 (63.7%) | 114/178 (64.0%) | 0.939 |
| Current Smoker | 388/2320 (16.7%) | 147/905 (16.2%) | 122/726 (16.8%) | 25/179 (14.0%) | 0.352 |
| Ischemic HF Etiology | 0.010 | ||||
| Ischemic | 1197/2331 (51.4%) | 460/910 (50.5%) | 354/731 (48.4%) | 106/179 (59.2%) | |
| Non-Ischemic | 1134/2331 (48.6%) | 450/910 (49.5%) | 377/731 (51.6%) | 73/179 (40.8%) | |
| NYHA class at random assignment | <.001 | ||||
| II | 1477/2331 (63.4%) | 570/910 (62.6%) | 489/731 (66.9%) | 81/179 (45.3%) | |
| III | 831/2331 (35.6%) | 328/910 (36.0%) | 238/731 (32.6%) | 90/179 (50.3%) | |
| IV | 23/2331 (1.0%) | 12/910 (1.3%) | 4/731 (0.5%) | 8/179 (4.5%) | |
| Systolic BP, mmHg | 111.0 (100.0,126.0) | 112.0 (100.5,126.5) | 114.0 (102.0,128.0) | 110.0 (100.0,126.0) | 0.153 |
| Resting HR, bpm | 70.0 (63.0,79.0) | 71.0 (63.0,80.0) | 70.0 (63.0,79.0) | 73.0 (66.0,81.0) | 0.007 |
| LVEF, % | 24.7 (20.0,30.1) | 24.4 (19.4,30.0) | 24.8 (19.5,30.1) | 23.2 (18.2,28.4) | 0.051 |
| Creatinine, mg/dL | 1.2 (1.0,1.5) | 1.2 (1.0,1.5) | 1.2 (1.0,1.4) | 1.3 (1.1,1.8) | <.001 |
| Blood urea nitrogen, mg/dL | 20.0 (15.0,28.0) | 21.0 (15.0,28.0) | 19.0 (14.0,26.0) | 25.0 (18.0,35.3) | <.001 |
| Hemoglobin, g/dL | 13.5 (12.3,14.6) | 13.3 (12.2,14.5) | 13.3 (12.3,14.6) | 13.2 (11.9,14.2) | 0.019 |
| NTproBNP (Baseline) | 815.0 (340.5,1805) | 857.0 (359.3,1955) | 671.8 (311.6,1473) | 2212 (976.0,4995) | <.001 |
| Exercise duration, CPX test, min | 9.6 (6.9,12.0) | 9.5 (7.0,12.0) | 10.0 (7.7,12.4) | 7.4 (5.3,9.5) | <.001 |
| Peak V02 in mL/kg/min CPX test at baseline | 14.4 (11.5,17.7) | 14.5 (11.6,17.4) | 15.2 (12.2,18.0) | 12.1 (9.8,14.5) | <.001 |
| 6-Minute walk distance, m | 370.6 (298.7,435.0) | 368.5 (297.2,432.8) | 378.3 (313.9,442.0) | 315.2 (259.1,388.6) | <.001 |
| Loop diuretic dose, mg/day | 40.0 (20.0,80.0) | 40.0 (20.0,80.0) | 40.0 (10.0,80.0) | 60.0 (40.0,120.0) | <.001 |
| Mitral Regurgitation, moderate or severe | 256/2135 (12.0%) | 98/840 (11.7%) | 65/679 (9.6%) | 33/161 (20.5%) | <.001 |
| VE/VCO2 slope | 32.6 (28.1,38.5) | 32.4 (28.0,38.0) | 31.3 (27.6,36.3) | 36.9 (32.4,44.8) | <.001 |
| KCCQ symptom stability score | 50.0 (50.0,50.0) | 50.0 (50.0,50.0) | 50.0 (50.0,50.0) | 50.0 (50.0,50.0) | 0.323 |
| Rest ECG ventricular conduction | <.001 | ||||
| Normal | 979/2271 (43.1%) | 390/891 (43.8%) | 331/714 (46.4%) | 59/177 (33.3%) | |
| LBBB | 379/2271 (16.7%) | 135/891 (15.2%) | 116/714 (16.2%) | 19/177 (10.7%) | |
| RBBB | 85/2271 (3.7%) | 30/891 (3.4%) | 22/714 (3.1%) | 8/177 (4.5%) | |
| IVCD | 292/2271 (12.9%) | 97/891 (10.9%) | 74/714 (10.4%) | 23/177 (13.0%) | |
| Paced | 536/2271 (23.6%) | 239/891 (26.8%) | 171/714 (23.9%) | 68/177 (38.4%) | |
| Region: US v Non-US | 0.012 | ||||
| 0 | 2068/2331 (88.7%) | 851/910 (93.5%) | 691/731 (94.5%) | 160/179 (89.4%) | |
| 1 | 263/2331 (11.3%) | 59/910 (6.5%) | 40/731 (5.5%) | 19/179 (10.6%) | |
| BB Dose at baseline | 25.0 (13.0,50.0) | 25.0 (13.0,50.0) | 37.5 (13.0,50.0) | 25.0 (13.0,50.0) | 0.228 |
| BMI | 29.9 (26.0,35.1) | 30.4 (26.4,35.7) | 30.9 (26.5,35.9) | 29.2 (25.1,35.1) | 0.018 |
| Canadian Angina Class | 200/2328 (8.6%) | 92/909 (10.1%) | 76/730 (10.4%) | 16/179 (8.9%) | 0.744 |
| HF Hosp in prior 6 months | 610/2311 (26.4%) | 243/903 (26.9%) | 196/725 (27.0%) | 47/178 (26.4%) | 0.865 |
Data are presented as medians (Q1, Q3) or percentages.
BP = blood pressure; BUN = blood urea nitrogen; CPX = cardiopulmonary exercise; HF = heart failure; LVEF; left ventricular ejection fraction; MI = myocardial infarction; NYHA = New York Heart Association; peak VO2 = maximal oxygen consumption; NT-proBNP = amino-terminal pro–B-type natriuretic peptide.
ST2, functional capacity, and quality of life
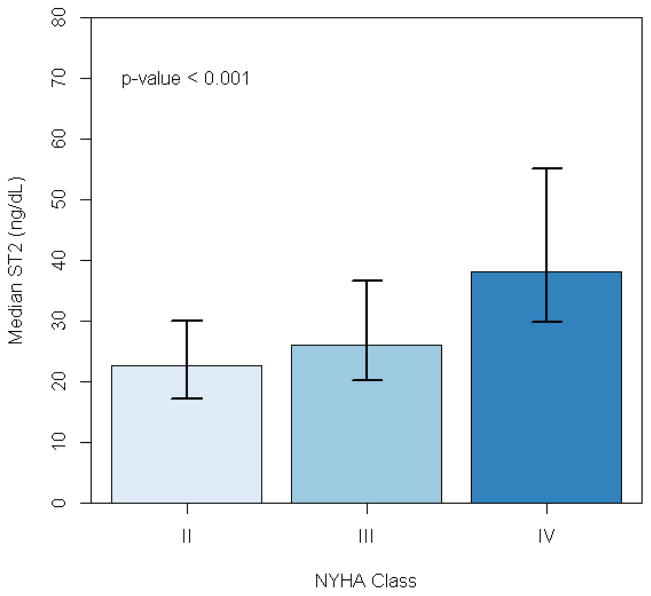
We assessed the relationships between ST2 and physician-assessed functional capacity (NYHA class), objective measures of maximal functional capacity (maximal oxygen consumption [peak VO2]) and submaximal functional capacity (6-minute walk distance), and patient-reported quality of life (Kansas City Cardiomyopathy Questionnaire [KCCQ] summary score). ST2 levels progressively increased with worsening NYHA class (Figure 1). In general, higher log ST2 levels were significantly but modestly correlated with poorer baseline functional capacity, including peak VO2 on CPX testing (r= −0.30; p<0.001)and 6-minute walk distance (r= −0.22; p<0.001). Higher levels of ST2 were modestly associated with lower quality of life summary scores for the KCCQ (r = 0.15; p<0.001). When ST2 was considered as a dichotomous variable, an ST2 level >35 ng/mL was associated with significantly worse peak VO2, CPX exercise duration, and 6-minute walk distance (p<0.001 for all).
Figure 1.
Median ST2 levels by NYHA class. Error bars represent intraquartile range.
ST2 and long-term outcomes
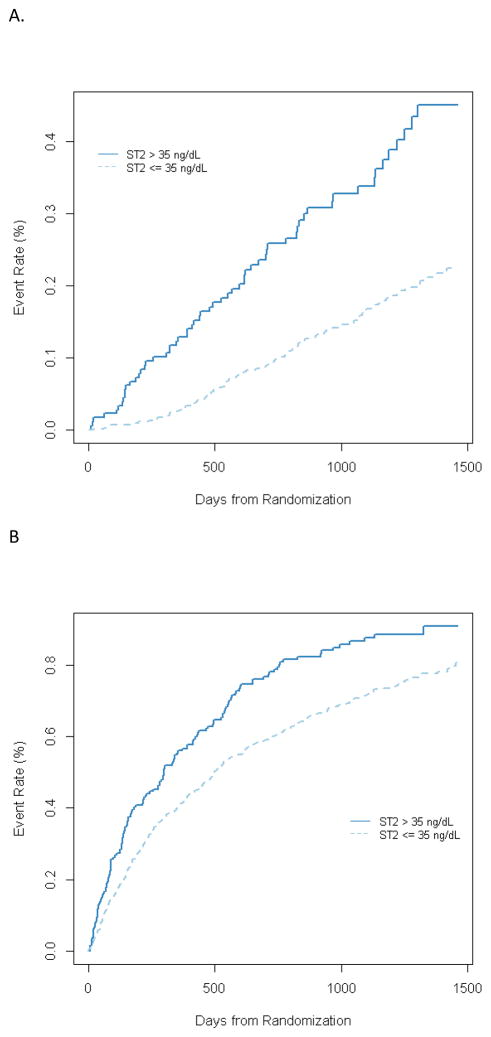
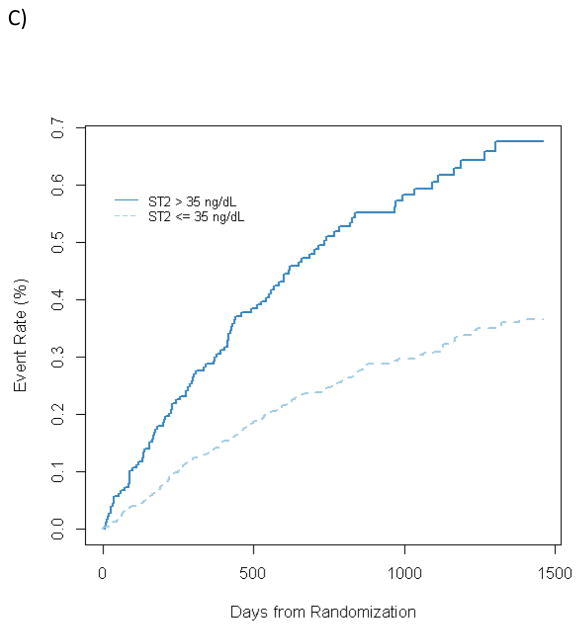
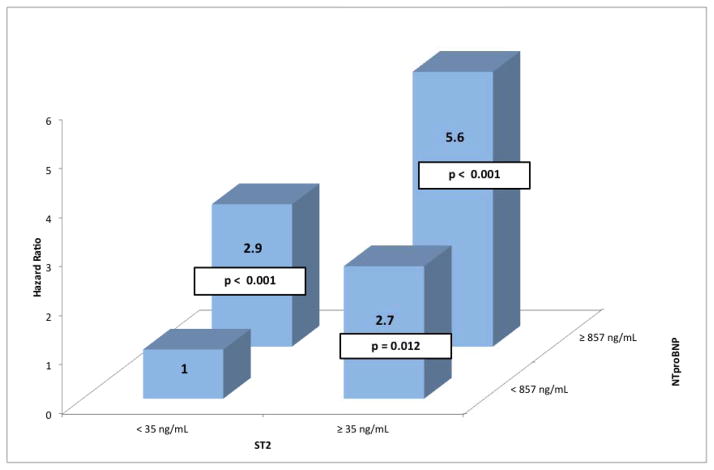
The study cohort was followed for 32 months, during which the raw events rates were 171 deaths (19%), 646 all-cause deaths or hospitalizations (71%), and 312 cardiovascular deaths or heart failure hospitalizations (34%). In univariable analysis, ST2 was a significantly associated with all 3 long-term outcomes. Specifically, doubling of the baseline ST2 level was associated with a significantly increased risk of all-cause death or hospitalization (HR, 1.48; p<0.0001), cardiovascular death or heart failure hospitalization (HR, 2.14; p<0.0001), and all-cause mortality (HR, 2.33; p<0.0001) (Figure 2). When considered along with NT-proBNP as 4 groups based on NT-proBNP levels above or below the median and ST2 levels above or below the 35 ng/mL cutpoint, patients with both markers elevated had a 5.6-fold increased risk of death during the course of long-term follow up compared to those with neither marker elevated. (Figure 3). There was no statistical interaction between ST2 and NTproBNP (p = 0.44).
Figure 2.
Kaplan Meier plost for ST2 above or below 35 ng/mL for (A) All cause mortality, (B) all-cause mortality+ all cause hospitalization, and C) CV death + Heart failure hospitalization
Figure 3.
Hazard for all-cause mortality based on groups defined by NT-proBNP (above or below median of 857 pg/mL) and ST2 (above or below 35 ng/mL), compared with a reference group with low NT-proBNP and low ST2. P values are for change relative to reference group. Interaction between NTproBNP and ST2 was not signficant (p = 0.44).
To adjust for how other covariates may affect the association between ST2 and outcome, we constructed a series of multivariable models (Table 2). Models were progressively adjusted for demographics alone, demographics + clinical variables, and demographics + clinical variables + CPX variables. Finally, each step was assessed with and without the inclusion of NT-proBNP. For each endpoint, ST2 remained independently associated with long-term outcomes after adjusting for demographics and NT-proBNP. For the endpoint of cardiovascular death + heart failure hospitalization, ST2 remained statistically associated(adjusted HR, 1.32 per doubling of ST2; p=0.0129) even after adjusting for demographics, NT-proBNP, and clinical and CPX variables. For all-cause mortality, the final adjusted HR was similar (1.26), though it did not reach nominal statistical significance (p=0.0877). ST2 was not significantly associated with the composite of all-cause mortality + rehospitalization (adjusted HR, 1.08; p=0.3605) after final adjustment for demographics, NT-proBNP, and the final clinical model, including CPX variables.
Table 2.
Multivariable Models for ST2 and Risk for Long Term Outcomes
| All-Cause Death/Hospitalization | CV Death/HF Hospitalization | All-Cause Death | CV Death | HF Hospitalization | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | |
| ST2 univariable | 1.48 | 1.32–1.66 | <0.0001 | 2.14 | 1.84–2.49 | <0.0001 | 2.33 | 1.93–2.81 | <0.0001 | 2.53 | 2.04–3.14 | <0.0001 | 2.00 | 1.69–2.36 | <0.0001 |
| Demographics | |||||||||||||||
| + age, sex, race | 1.42 | 1.26–1.61 | <0.0001 | 2.07 | 1.78–2.42 | <0.0001 | 1.99 | 1.63–2.43 | <0.0001 | 2.30 | 1.82–2.90 | <0.0001 | 1.98 | 1.67–2.34 | <0.0001 |
| + age, sex, race, NT-proBNP | 1.17 | 1.02–1.34 | 0.0210 | 1.47 | 1.22–1.77 | <0.0001 | 1.52 | 1.20–1.93 | 0.0006 | 1.68 | 1.26–2.24 | 0.0004 | 1.44 | 1.18–1.77 | 0.0005 |
| Final clinical model without CPX variables | |||||||||||||||
| + age, sex, race | 1.29 | 1.13–1.50 | 0.0003 | 1.68 | 1.40–2.02 | <0.0001 | 1.74 | 1.40–2.16 | <0.0001 | 1.98 | 1.50–2.60 | <0.0001 | 1.52 | 1.25–1.86 | <0.0001 |
| + age, sex, race, NT-proBNP | 1.13 | 0.97–1.33 | 0.1213 | 1.37 | 1.11–1.69 | 0.0037 | 1.42 | 1.10–1.82 | 0.0067 | 1.60 | 1.15–2.21 | 0.0047 | 1.32 | 1.05–1.65 | 0.0174 |
| Final clinical model with CPX variables | |||||||||||||||
| + age, sex, race | 1.21 | 1.05–1.40 | 0.0072 | 1.50 | 1.24–1.82 | <0.0001 | 1.47 | 1.18–1.85 | 0.0008 | 1.70 | 1.27–2.27 | 0.0004 | 1.35 | 1.10–1.66 | 0.0047 |
| + age, sex, race, NT-proBNP | 1.08 | 0.92–1.27 | 0.3605 | 1.32 | 1.06–1.63 | 0.0129 | 1.26 | 0.97–1.63 | 0.0877 | 1.54 | 1.10–2.14 | 0.0114 | 1.22 | 0.97–1.54 | 0.0967 |
HR = Hazard ratio, CI = confidence interval, NT-proBNP = amino-terminal-pro-b-type natriuretic peptide, CPX = cardiopulmonary exercise test
Because CPX testing is infrequently used or is unavailable at some centers, we examined the predictive value of ST2 when adjusted for clinical variables and NT-proBNP but excluded CPX variables (peak V02, exercise duration, and VE-VCO2 slope). In this analysis, ST2 was significantly associated with all-cause mortality (adjusted HR, 1.42; p=0.0067) and cardiovascular death or heart failure hospitalization (adjusted HR, 1.37; p=0.0037), but it was not with all-cause mortality or hospitalization (adjusted HR, 1.13; p=0.1213). To evaluate the incremental value of ST2, we examined several measures of model performance, including the change in the C statistic, IDI and category free NRI. The addition of ST2 did not substantially change the C-statistic, IDI, or NRI for any of the clinical endpoints (Table 3). This suggests that while ST2 is significantly associated with outcomes, adding ST2 to models containing the clinical risk factors and NT-proBNP does not incrementally improve discrimination or risk prediction.
Table 3.
Added Value of ST2 to Final Clinical Model and NT-proBNP
| All-Cause Death/Hospitalization | CV Death/HF Hospitalization | All-Cause Death | CV Death | HF Hospitalization | |
|---|---|---|---|---|---|
| C-Statistics | |||||
| Without CPX variables + age, race, NT-proBNP | 0.641 | 0.759 | 0.751 | 0.778 | 0.762 |
| Without CPX variables + age, race, NT-proBNP + ST2 | 0.644 | 0.768 | 0.758 | 0.789 | 0.771 |
| 95% CI for Change in C-Statistic | (0.0003, 0.0048) | (0.0084, 0.0155) | (0.0043, 0.0125) | (0.0055, 0.0145) | (0.0079, 0.0149) |
| IDI | 0.0002 | 0.0065 | 0.0078 | 0.0123 | 0.0043 |
| 95% CI for IDI | (−0.044, 0.0027) | (−0.0293, 0.0181) | (−0.0282, 0.0597) | (−0.0059, 0.0264) | (−0.0056, 0.0123) |
| NRI | 0.025 | 0.015 | 0.049 | 0.0472 | 0.0638 |
| 95% CI for NRI | (−0.1142, 0.187) | (−0.2123, 0.2468) | (−0.2222, 0.1492) | (−0.3260, 0.1812) | (−0.0985, 0.2763) |
CI = confidence interval, CV = cardiovascular, IDI = integrated discrimination improvement, NRI = net reclassification improvement (without categories)
ST2 and response to exercise training
Given that HF-ACTION was a randomized, controlled study of a unique intervention (exercise training), we tested for an interaction between baseline ST2 levels and the treatment effect of exercise training with regard to each of the clinical outcomes of interest. Of the endpoints tested, there was a statistical interaction between exercise training and the outcome of all-cause mortality, such that patients with lower ST2 levels were more likely to have a benefit of exercise training than were patients with higher levels (p=0.016 for ST2 * treatment interaction), and a similar interaction for cardiovascular mortality (p = 0.032 for ST2* treatment interaction). There was not a similar statistical interaction between ST2 levels and treatment effects with regard to the composite endpoints of all-cause death or hospitalization (p=0.620) or cardiovascular death or heart failure hospitalization (p=0.224).
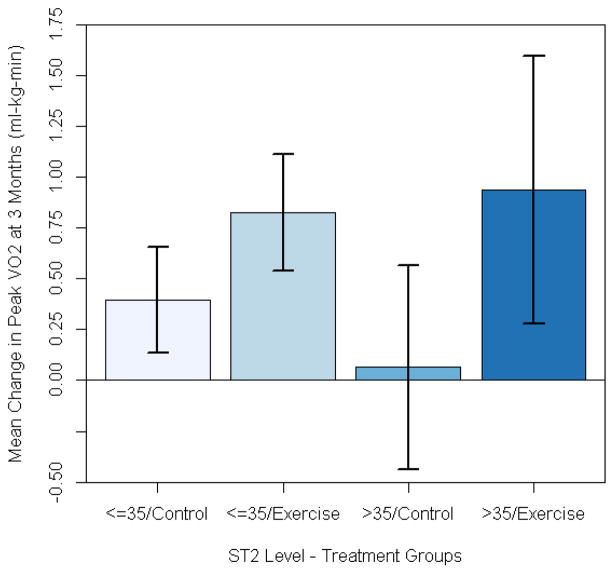
We also assessed the relationship between baseline ST2 levels and the change in peak VO2 between baseline and 3 months. Higher baseline ST2 levels were associated with a greater training effect (i.e., the increase in peak VO2 from baseline to 3 months (adjusted p value = 0.034)). There was no significant interaction between baseline ST2 levels and treatment assignment(exercise vs. control) (Figure 4).
Figure 4.
Mean change from baseline to 3 months in peak VO2 stratfied by treatment assignment and ST2 levels. Baseline ST2 level was siginficant predictor of change in peak VO2 (adjusted p = 0.034). There was no signficant interaction between treatment assignment and ST2 levels with regard to predicting improvement in peak V02 (p = 0.67 for treatment*ST2 interaction). Error bars represent standard deviations.
Discussion
Soluble ST2 is a novel biomarker that reflects a variety of important pathologic processes in heart failure, including myocyte stretch and inflammation. ST2/IL-33 signaling is induced by myocyte stretch and modulates hypertrophic responses and fibrosis in the myocardium18. Intact ST2/IL-33 signaling is believed to be a mechanically activated cardioprotective paracrine signaling system that shields the myocardium from the adverse effects of overload. Because ST2 elevations reflect activation of distinct biologic systems compared with the natriuretic peptides, there is significant interest in whether ST2 can add additional prognostic information. In this analysis of the HF-ACTION study, ST2 was significantly associated with long-term outcome even after a adjustment for clinical covariates. Addition of NT-proBNP attenuated the association with the primary outcome of all-cause death and hospitalizations. To our knowledge, this is the most robust test of this novel biomarker to date with regard to covariate adjustment in a large, multicenter cohort of ambulatory heart failure patients. Although the association between ST2 and outcomes was consistent across all outcomes, the addition of ST2 to a model adjusted for known risk factors did not lead to improved discrimination and risk prediction based on reclassification analysis. The overall effect sizes were modest (all adjusted HR were < 1.5 per doubling of ST2). These data demonstrate the limitations of multivariable modeling alone as an assessment of the clinical value of new biomarkers.
We believe these findings are of interest for several reasons. To our knowledge, this is the first study to have comprehensively examined the relationship between ST2, commonly used measures of functional capacity, and response to exercise training in heart failure patients. We found that ST2 levels were significantly associated with both physician assessed (NYHA class) and objective measures of functional capacity (6-minute walk distance and peak VO2). Additionally, baseline levels of ST2 were predictive of training effect (the change in peak VO2 over 3 months). We also identified an interaction between ST2 levels and exercise training, such that patients with lower ST2 levels were more likely to have beneficial effects of exercise training with regard to the endpoint of all-cause mortality. We did not observe a similar interaction for the composite endpoints, and it is uncertain whether this observation represents a true effect or is a due to chance. Prior data have suggested a relationship between extracellular matrix turnover and changes in exercise capacity over time19. Weir et al showed that high ST2 levels are associated with greater response to eplerenone therapy after acute myocardial infarction20. Recent data regarding galectin-3, another marker thought to reflect myocardial fibrosis and remodeling, have suggested a similar differential effect (greater efficacy of statin therapy in patients with lower levels of the biomarker)21. Further analyses in other randomized trial datasets will be needed to confirm or refute this initial signal regarding the interaction between ST2 levels and treatment response in heart failure.
Our study extends the results of other studies that have examined the relationship between ST2 and outcomes in chronic heart failure4, 22, 23. Most notably, our data complement the previous analysis from Ky et al from the Penn Heart Failure Study (PHFS), which showed an association between ST2 levels and outcomes in an observational cohort from 3 tertiary care referral centers4. In comparison, our study cohort was more broadly representative of the systolic heart failure population because we included both a variety of academic- and community-based clinical sites and had a more representative proportion of patients with ischemic etiology (51% for HF-ACTION vs. 31% for PHFS). Additionally, our study had blinded independent adjudication of clinical outcomes, allowing for the robust distinction between disease-specific and all-cause endpoints. We found that the relationship between ST2 and outcomes may be stronger for disease-specific outcomes (such as HF hospitalization and CV morality) compared with all-cause outcomes. HF-ACTION routinely collected detailed data on functional capacity using CPX testing and 6-minute walk distance, allowing for both an assessment of the relationship between ST2 and functional capacity as well as more detailed covariate adjustment in multivariable models. Finally, because HF-ACTION was an intervention study, we were able to assess interactions between the intervention of exercise training (which has putative immunomodulatory effects)24 and ST2 levels with regard to outcomes.
Limitations
The study population for this analysis was derived from the HF-ACTION study, and as such, is susceptible to the limitations inherent in clinical trial populations. Although more broadly generalizable than other cohorts in which ST2 has been examined, our population was still relatively young (median age 59) compared with the broader heart failure population. HF-ACTION was a randomized study and the study intervention (exercise training) did demonstrate a treatment effect, a fact that may have potentially influenced our results and could limit the generalizability to other settings. Our study population consisted only of patients with impaired ejection fraction (LVEF <0.35), so our results cannot be extrapolated to the population of patients with heart failure and preserved ejection fraction.
Conclusions
In this analysis of a large clinical study cohort of well-treated ambulatory heart failure patients, elevation of ST2 was significantly associated with long-term outcomes, especially disease-specific outcomes. These associations were relatively robust for disease specific endpoints (cardiovascular death and heart failure hospitalization) in traditional multivariable modeling, persisting even after comprehensive covariate adjustment, including demographics, clinical variables, and NT-proBNP. Despite this robust relationship in traditional multivariable modeling, ST2 did not add significantly to discrimination or risk prediction. Ongoing research will further clarify the role of ST2 in risk stratification in chronic heart failure.
Acknowledgments
Funding Source: The HF-ACTION study was funded by the National Heart, Lung, and Blood Institute. The HF-ACTION Biobank was funded independently by the Duke Clinical Research Institute. This analysis was funded by Critical Diagnostics.
Abbreviations
- ACE
angiotensin-converting enzyme
- CPX
cardiopulmonary exercise test
- HR
hazard ratio
- IL-33
interleukin-33
- KCCQ
Kansas City Cardiomyopathy Questionnaire
- LVEF
left ventricular ejection fraction
- NT-proBNP
amino-terminal pro–B-type natriuretic peptide
- NYHA
New York Heart Association
- Peak VO2
maximal oxygen consumption
Appendix
Final covariates used in adjusted models for each endpoint:
All-cause death or all-cause hospitalization (primary endpoint): peak VO2 (as Weber class), sex, region (United States vs. non-United States), mitral regurgitation severity, ventricular conduction on electrocardiogram, blood urea nitrogen, ejection fraction, beta-blocker dose (truncated at 50 mg/day carvedilol equivalent), and Kansas City Cardiomyopathy Questionnaire symptom stability score.
Cardiovascular death or heart failure hospitalization: sex, race, age (truncated above 62 years), loop diuretic dose (using furosemide equivalent, truncated above 100 mg/day), left ventricular ejection fraction, mitral regurgitation severity, blood urea nitrogen (truncated above 39 mg/dL), VE/VC02 slope (truncated above 80), Kansas City Cardiomyopathy Questionnaire symptom stability score, ventricular conduction on electrocardiogram, baseline maximal oxygen consumption, truncated above 28 mL/kg/min.
All-cause mortality:sex, body mass index (truncated above 25), loop diuretic dose (truncated above 100mg furosemide daily), Canadian Angina Class, ventricular conduction on electrocardiogram, left ventricular ejection fraction, exercise duration on cardiopulmonary exercise test, serum creatinine (truncated above 2.3).
Footnotes
Disclosures:
Drs. Felker, Fiuzat, and O’Connor have received research funding from BG Medicine Inc, Critical Diagnostics, and Roche Diagnostics. Drs. Felker and O’Connor have served as consultants for BG Medicine, and Roche Diagnostics. Dr. Zannad has received research funding from BG Medicine and Roche Diagnostics and has served as a consultant for BG Medicine. None of the other authors report any conflicts.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics—2012 update: A report from the american heart association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community-based population. Jama. 2004;292:344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 3.Braunwald E. Biomarkers in heart failure. New England Journal of Medicine. 2008;358:2148–2159. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- 4.Ky B, French B, McCloskey K, Rame JE, McIntosh E, Shahi P, Dries DL, Tang WHW, Wu AHB, Fang JC, Boxer R, Sweitzer NK, Levy WC, Goldberg LR, Jessup M, Cappola TP. High-sensitivity st2 for prediction of adverse outcomes in chronic heart failure / clinical perspective. Circulation: Heart Failure. 2011;4:180–187. doi: 10.1161/CIRCHEARTFAILURE.110.958223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Felker GM, Fiuzat M, Shaw LK, Clare R, Whellan DJ, Bettari L, Shirolkar SC, Donahue M, Kitzman DW, Zannad F, Pina IL, O’Connor CM. Galectin-3 in ambulatory patients with heart failure: Results from the hf-action study. Circ Heart Fail. 2012;5:72–78. doi: 10.1161/CIRCHEARTFAILURE.111.963637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anand IS, Kempf T, Rector TS, Tapken H, Allhoff T, Jantzen F, Kuskowski M, Cohn JN, Drexler H, Wollert KC. Serial measurement of growth-differentiation factor-15 in heart failure / clinical perspective. Circulation. 2010;122:1387–1395. doi: 10.1161/CIRCULATIONAHA.109.928846. [DOI] [PubMed] [Google Scholar]
- 7.Maisel A, Xue Y, Shah K, Mueller C, Nowak R, Peacock WF, Ponikowski P, Mockel M, Hogan C, Wu AH, Richards M, Clopton P, Filippatos GS, Di Somma S, Anand IS, Ng L, Daniels LB, Neath SX, Christenson R, Potocki M, McCord J, Terracciano G, Kremastinos D, Hartmann O, von Haehling S, Bergmann A, Morgenthaler NG, Anker SD. Increased 90-day mortality in patients with acute heart failure with elevated copeptin: Secondary results from the biomarkers in acute heart failure (bach) study. Circ Heart Fail. 2011;4:613–620. doi: 10.1161/CIRCHEARTFAILURE.110.960096. [DOI] [PubMed] [Google Scholar]
- 8.Maisel A, Mueller C, Nowak RM, Peacock WF, Ponikowski P, Mockel M, Hogan C, Wu AH, Richards M, Clopton P, Filippatos GS, Di Somma S, Anand I, Ng LL, Daniels LB, Neath SX, Christenson R, Potocki M, McCord J, Hartmann O, Morgenthaler NG, Anker SD. Midregion prohormone adrenomedullin and prognosis in patients presenting with acute dyspnea results from the bach (biomarkers in acute heart failure) trial. J Am Coll Cardiol. 2011;58:1057–1067. doi: 10.1016/j.jacc.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Seki K, Sanada S, Kudinova AY, Steinhauser ML, Handa V, Gannon J, Lee RT. Interleukin-33 prevents apoptosis and improves survival after experimental myocardial infarction through st2 signaling. Circ Heart Fail. 2009;2:684–691. doi: 10.1161/CIRCHEARTFAILURE.109.873240. [DOI] [PubMed] [Google Scholar]
- 10.Miller AM, Liew FY. The il-33/st2 pathway - a new therapeutic target in cardiovascular disease. Pharmacol Ther. 2011;131:179–186. doi: 10.1016/j.pharmthera.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Sabatine MS, Morrow DA, Higgins LJ, MacGillivray C, Guo W, Bode C, Rifai N, Cannon CP, Gerszten RE, Lee RT. Complementary roles for biomarkers of biomechanical strain st2 and n-terminal prohormone b-type natriuretic peptide in patients with st-elevation myocardial infarction. Circulation. 2008;117:1936–1944. doi: 10.1161/CIRCULATIONAHA.107.728022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Januzzi JL, Jr, Peacock WF, Maisel AS, Chae CU, Jesse RL, Baggish AL, O’Donoghue M, Sakhuja R, Chen AA, van Kimmenade RRJ, Lewandrowski KB, Lloyd-Jones DM, Wu AHB. Measurement of the interleukin family member st2 in patients with acute dyspnea: Results from the pride (pro-brain natriuretic peptide investigation of dyspnea in the emergency department) study. J Am Coll Cardiol. 2007;50:607–613. doi: 10.1016/j.jacc.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 13.O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Pina IL. Efficacy and safety of exercise training in patients with chronic heart failure: Hf-action randomized controlled trial. Jama. 2009;301:1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whellan DJ, O’Connor CM, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Houston-Miller N, Fleg JL, Schulman KA, Pina IL. Heart failure and a controlled trial investigating outcomes of exercise training (hf-action): Design and rationale. Am Heart J. 2007;153:201–211. doi: 10.1016/j.ahj.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Lu J, Snider JV, Grenache DG. Establishment of reference intervals for soluble st2 from a united states population. Clin Chim Acta. 2010;411:1825–1826. doi: 10.1016/j.cca.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 16.O’Connor CM, Whellan DJ, Wojdyla D, Leifer E, Clare RM, Ellis SJ, Fine LJ, Fleg JL, Zannad F, Keteyian SJ, Kitzman DW, Kraus WE, Rendall D, Pina IL, Cooper LS, Fiuzat M, Lee KL. Factors related to morbidity and mortality in patients with chronic heart failure with systolic dysfunction: The hf-action predictive risk score model. Circ Heart Fail. 2012;5:63–71. doi: 10.1161/CIRCHEARTFAILURE.111.963462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masson S, Latini R, Anand IS, Vago T, Angelici L, Barlera S, Missov ED, Clerico A, Tognoni G, Cohn JN on behalf of the Val-HeFT Investigators. Direct comparison of b-type natriuretic peptide (bnp) and amino-terminal probnp in a large population of patients with chronic and symptomatic heart failure: The valsartan heart failure (val-heft) data. Clin Chem. 2006;52:1528–1538. doi: 10.1373/clinchem.2006.069575. [DOI] [PubMed] [Google Scholar]
- 18.Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. Il-33 and st2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. 2007;117:1538–1549. doi: 10.1172/JCI30634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radauceanu A, Ducki C, Virion JM, Rossignol P, Mallat Z, McMurray J, Van Veldhuisen DJ, Tavazzi L, Mann DL, Capiaumont-Vin J, Li M, Hanriot D, Zannad F. Extracellular matrix turnover and inflammatory markers independently predict functional status and outcome in chronic heart failure. J Card Fail. 2008;14:467–474. doi: 10.1016/j.cardfail.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 20.Weir RA, Miller AM, Murphy GE, Clements S, Steedman T, Connell JM, McInnes IB, Dargie HJ, McMurray JJ. Serum soluble st2: A potential novel mediator in left ventricular and infarct remodeling after acute myocardial infarction. J Am Coll Cardiol. 2010;55:243–250. doi: 10.1016/j.jacc.2009.08.047. [DOI] [PubMed] [Google Scholar]
- 21.Gullestad L, Ueland T, Kjekshus J, Nymo SH, Hulthe J, Muntendam P, Adourian A, Bohm M, van Veldhuisen DJ, Komajda M, Cleland JG, Wikstrand J, McMurray JJ, Aukrust P. Galectin-3 predicts response to statin therapy in the controlled rosuvastatin multinational trial in heart failure (corona) Eur Heart J. 2012;33:2290–2296. doi: 10.1093/eurheartj/ehs077. [DOI] [PubMed] [Google Scholar]
- 22.Pascual-Figal DA, Ordonez-Llanos J, Tornel PL, Vazquez R, Puig T, Valdes M, Cinca J, de Luna AB, Bayes-Genis A. Soluble st2 for predicting sudden cardiac death in patients with chronic heart failure and left ventricular systolic dysfunction. J Am Coll Cardiol. 2009;54:2174–2179. doi: 10.1016/j.jacc.2009.07.041. [DOI] [PubMed] [Google Scholar]
- 23.Weinberg EO, Shimpo M, Hurwitz S, Tominaga S, Rouleau JL, Lee RT. Identification of serum soluble st2 receptor as a novel heart failure biomarker. Circulation. 2003;107:721–726. doi: 10.1161/01.cir.0000047274.66749.fe. [DOI] [PubMed] [Google Scholar]
- 24.Abramson JL, Vaccarino V. Relationship between physical activity and inflammation among apparently healthy middle age and older us adults. Arch Intern Med. 2002;162:1286–1292. doi: 10.1001/archinte.162.11.1286. [DOI] [PubMed] [Google Scholar]