Abstract
Background
Although left ventricle ejection fraction (LVEF) is the primary determinant for sudden cardiac death (SCD) risk stratification, in isolation, LVEF is a sub-optimal risk stratifier. We assessed whether a multi-marker strategy would provide more robust SCD risk stratification than LVEF alone.
Methods
We collected patient-level data (n=3355) from 6 studies assessing the prognostic utility of microvolt T-wave alternans (MTWA) testing. Two-thirds of the group was used for derivation (n=2242) and one-third for validation (n=1113). The discriminative capacity of the multivariable model was assessed using the area under the receiver-operating characteristic (ROC) curve (c-index). The primary endpoint was SCD at 24 months.
Results
In the derivation cohort, 59 patients experienced SCD by 24 months. Stepwise selection suggested that a model based on 3 parameters (LVEF, coronary artery disease [CAD] and MTWA status) provided optimal SCD risk prediction. In the derivation cohort, the c-index of the model was 0.817, which was significantly better than LVEF used as a single variable (0.637, p < 0.001). In the validation cohort, 36 patients experienced SCD by 24 months. The c-index of the model for predicting the primary endpoint was again significantly better than LVEF alone (0.774 vs. 0.671, p = 0.020).
Conclusions
A multivariable model based on presence of CAD, LVEF and MTWA status provides significantly more robust SCD risk prediction than LVEF as a single risk marker. These findings suggest that multi-marker strategies based on different aspects of the electro-anatomic substrate may be capable of improving primary prevention ICD treatment algorithms.
Introduction
Although improved pharmacologic therapies for coronary artery disease (CAD) and congestive heart failure (CHF) have a favorable impact on the incidence of sudden cardiac death (SCD), the implantable cardioverter-defibrillator (ICD) has emerged as a mainstay of SCD prevention and seminal clinical trials have demonstrated significant reduction in all-cause mortality among patients at heightened risk for SCD but without a history of ventricular arrhythmias (i.e. “primary prevention” ICDs) 1, 2.
Currently both New York Heart Association (NYHA) class and left ventricle ejection fraction (LVEF) are recommended in guiding ICD implantation for primary prevention 3. Unfortunately, given the dynamic nature of NYHA class and the notorious limitations of its subjective assessment 4, LVEF has emerged as the primary determinant of eligibility for primary prevention ICD therapy 3. However, as highlighted in the recent National Heart, Lung and Blood Institute and Heart Rhythm Society (HLBI/HRS) report on SCD prediction and prevention 5, there is widespread recognition that LVEF reflects only one aspect of the complex electro-anatomic substrate that gives rise to ventricular arrhythmias and in isolation, LVEF is a suboptimal risk stratification tool. Specifically, among patients who are currently candidates for primary prevention ICD therapy (i.e. LVEF ≤ 35%), only a small percentage of patients (~ 2-5% per year) will suffer a ventricular arrhythmia resulting in SCD 5, 6, demonstrating that the positive predictive value and specificity of low LVEF for predicting SCD is quite limited. Conversely, the majority of SCD events occur in patients with only mildly impaired or even preserved LV systolic function 7, 8, thus highlighting the limited negative predictive value and low sensitivity of impaired LVEF for determining SCD risk.
At least part of the limitation of using LVEF cut points for SCD risk stratification is that although patients with impaired LVEF are at heightened risk for SCD, they are also at increased risk for other causes of death, such as progressive heart failure 6, in which case ICD therapy is not expected to be beneficial. In order to optimize ICD utilization and reduce the burden of SCD, more robust risk stratification tools are necessary which better reflect the complex electro-anatomic substrate that gives rise to malignant arrhythmias and sudden death. Although numerous invasive and non-invasive markers have been tested for ventricular arrhythmia and SCD risk prediction 5, currently available metrics remain suboptimal for determining which patients are most or least likely to benefit from ICD therapy.
In order to test the hypothesis that a multi-marker strategy reflecting different aspects of the electro-anatomic substrate is capable of providing better SCD risk prediction than LVEF alone, we have developed and validated a model based on three easily accessible clinical parameters to predict the risk of SCD across a wide range of LVEF.
Methods
Derivation and validation cohorts
We performed a Pubmed® literature search for all studies with “alternans” in the title published between 1998 and 2010. We chose 1998 as the beginning for the literature search because the first version of the MTWA–specific exercise protocol with the Cambridge Heart® testing system was released in September 2000. However, certain studies were performed using the new protocol prior to its official release and in an effort to capture all studies performed with the MTWA-specific protocol, we extended the search back to 1998. We identified prospective clinical trials involving at least 100 patients in which MTWA testing using the spectral analytic method was used to predict the risk of SCD with at least 12 month follow-up. In order to minimize the impact of ICD therapy on study endpoints, we excluded studies where ≥15% of the patients had ICDs implanted at baseline or ≥15% of the total arrhythmic outcome events were due to “appropriate” ICD therapy 9. Additionally, in order to further minimize the impact of ICD therapy, patients with ICDs from the included studies were excluded from the final pooled cohort analysis. We also excluded studies where MTWA testing was performed soon after (i.e. ≤ 4 weeks) acute myocardial infarction.
The initial search identified 17 studies of >100 patients in which MTWA was used to predict SCD. Seven studies were excluded because ≥15% of the patients had ICDs or ≥15% of the arrhythmic outcome events were due to “appropriate” ICD therapy 10-16 and 2 studies were excluded because they used an older version of the Cambridge Heart® system which did not include MTWA-specific exercise protocols and did not require sub-maximal exercise 17, 18. One study was excluded because MTWA testing was performed early after myocardial infarction (mean 8.1 ± 2.4 days) 19. Two studies did not include a SCD endpoint in the original publication 20, 21. The authors of both studies were contacted to find out if data on SCD was available: one study was excluded because data on SCD was not available 20 whereas the authors of the other study were able to provide data on SCD and therefore, that study was included in our cohort 21. Ultimately, six studies met the inclusion criteria and were included in the final cohort 21-26. Of note, although there were a significant number of patients with ICDs reported in the paper by Chan et al.22, the ICD and non-ICD cohorts were prospectively followed and described separately and therefore, we included the non-ICD cohort in the pooled analysis.
To minimize heterogeneity across studies, we obtained patient level data from the authors of the six studies included in this pooled cohort. After exclusion of 556 patients with ICDs, the final study cohort included 3355 patients. The baseline characteristics of the 6 studies included in our cohort have been published previously and are summarized in Table 1. Two-thirds of the patients from each of the 6 studies were randomly selected and merged to form the derivation cohort (n=2242) and the remaining one-third of patients from each of the 6 studies was merged to form the validation cohort (n=1113).
Table 1. Baseline characteristics stratified by study of origin.
| Bloomfield et al. 21 n=472 | Klingenheben et al. 26 n=107 | ALPHA 25 n=415 | Ikeda et al. 23 n=834 | Ikeda et al. 24 n=1041 | Chan et al. 22 n=486 | |
|---|---|---|---|---|---|---|
| Age (years) | 55.3 ± 12.9 | 54.2 ± 10.1 | 58.6 ± 12.6 | 62.8 ± 10.8 | 63.9 ± 9.0 | 65.9 ± 12.5 |
| Male gender (n) | 328 (70%) | 86 (80%) | 349 (78%) | 696 (83%) | 824 (79%) | 366 (75%) |
| Ischemic cardiomyopathy (n) | 215 (46%) | 67 (63%) | 0 | 834 (100%) | 1041 (100%) | 375 (77%) |
| LV ejection fraction (%) | 25.2 ± 8.5 | 28.2 ± 7.5 | 29.5 ± 7.1 | 50.3 ± 13.4 | 54.6 ± 10.4 | 27.8 ± 6.4 |
| Beta blocker therapy at enrollment (n) | 367 (78%) | 45 (42%) | 357 (80%) | 232 (28%) | 216 (21%) | 399 (82%) |
| MTWA results (n) | ||||||
| positive | 140 | 52 | 175 | 302 | 176 | 151 |
| negative | 163 | 33 | 151 | 4343 | 773 | 236 |
| indeterminate | 169 | 22 | 89 | 98 | 92 | 99 |
| Duration of follow-up (months) | 20.3 ± 5.9 | 14.6 ± 9.6 | 31.2 ± 16.2 | 24.7 ± 13.3 | 31.8 ± 14.0 | 36.9 ± 18.5 |
| Definition of SCD endpoint | Unwitnessed deaths, witnessed instantaneous deaths nonsudden deaths due to incessant tachycardia, deaths considered to be sequelae of cardiac arrest | Sudden death, resuscitated VF or sustained VT | Unwitnessed deaths, witnessed instantaneous deaths nonsudden deaths due to incessant tachycardia, deaths considered to be sequelae of cardiac arrest | Instantaneous, unexpected death or death within one hour of symptom on-set not related to circulatory failure or resuscitated VF | Instantaneous, unexpected death or death within one hour of symptom on-set not related to circulatory failure or resuscitated VF | Unwitnessed death (if observed to be stable within 24 hrs before death), witnessed instantaneous death or sequelae of cardiac arrest |
| HR for SCD endpoint | Not reported | ∞** | 4.01* | 11.4** | 23.5** | Not reported |
Data are presented as mean ± standard deviation or n (%).
LV=left ventricle; MTWA=microvolt T-wave alternans; MI=myocardial infarction; SCD=sudden cardiac death; VF=ventricular fibrillation; VT=ventricular tachycardia; HR=hazard ratio.
for non-negative MTWA (positive and indeterminate) vs. negative MTWA
for positive MTWA vs. negative MTWA
Microvolt T-wave alternans testing
All 6 of the pooled studies utilized MTWA testing with the spectral method 27 (CH 2000 system, Cambridge Heart, Bedford, MA, USA) and the results of each MTWA test (positive, negative or indeterminate) were classified by the investigators within each study based on established criteria 28. In brief, MTWA studies were classified as positive if there was sustained alternans > 1.9 μV for at least 1 minute with an alternans ratio (k score) > 3.0 with an onset heart rate (HR) <110 beats/min. Studies were classified as negative if criteria for positive were not met in an artifact-free period of data collection with a HR of at least 105 beats/min for at least 1 minute. All remaining studies not meeting criteria for either positive or negative were classified as indeterminate.
Statistical analysis
The primary endpoint for this study was SCD/arrhythmic mortality at 24 months. All arrhythmic events and mortality endpoints were adjudicated by the study investigators based on the specific definitions used within each study protocol 21-26. Clinical covariates available for all patients included age, gender, LVEF, presence of CAD, beta adrenergic blocker use at the time of study enrollment and MTWA status. Logistic regression models were used to identify univariate and multivariate predictors of the primary endpoint.
A parsimonious set of covariates was selected with the use of stepwise selection to define a multivariable model to predict the risk of SCD at 24 months. Three main effects were selected for inclusion in the final model (LVEF, presence of CAD and MTWA status). Additionally, we also analyzed interactions among the three main effects and found a significant interaction between LVEF and MTWA status and therefore, an LVEF*MTWA interaction term was also included in the final model.
The discriminative capacity of the multivariable model was compared to other predictors by assessing the area under the receiver-operating characteristic (ROC) curve as an index of model performance (c-index). The time course of the primary endpoint, stratified by predicted SCD risk, was estimated by Kaplan-Meier time to first event curves. The association between predicted SCD risk and observed SCD events was assessed by Kaplan-Meier product-limit estimates and tested with the log-rank test. A two-sided p value of <0.05 was considered statistically significant.
All analyses were performed with SAS (SAS Institute Inc., Cary, NC).
Results
Predictors of sudden cardiac death in the derivation cohort
Baseline characteristics of the derivation cohort are included in Table 2. Results of MTWA testing were positive in 29%, negative in 53% and indeterminate in 18%. Sudden cardiac death occurred in 59 patients in the derivation cohort during 24 months of follow-up. In univariate analysis, LVEF, presence of CAD and MTWA status were significantly associated with the primary endpoint (Table 3). The odds ratio (OR) for SCD at 24 months was 0.969 per point increase in LV ejection fraction (95% confidence internal [CI] 0.952-0.986, p < 0.001), consistent with a reduction in SCD risk as LVEF increases. For MTWA status, relative to a negative test result, both a positive MTWA test (OR 9.0760, 95% CI 3.992-20.634, p < 0.001) and an indeterminate MTWA result (OR 8.612, 95% CI 3.593-20.643, p < 0.001) were significantly associated with an increased risk of SCD.
Table 2. Derivation and validation cohorts.
| Characteristic | Derivation cohort (n=2242) | Validation cohort (n=1113) |
|---|---|---|
| Age (years) | 61.7±11.5 | 61.7±11.7 |
| Male gender (n) | 1747 (78%) | 885 (80%) |
| Coronary artery disease (n) | 1687 (75%) | 845 (76%) |
| Left ventricle ejection fraction (%) | 41.6±16.1 | 41.3±16.5 |
| β-blocker use (n) | 1067 (48%) | 531 (48%) |
| Microvolt T-wave alternans result (n) | ||
| Positive | 651 (29%) | 345 (31%) |
| Negative | 1197 (53%) | 593 (53%) |
| Indeterminate | 394 (18%) | 175 (16%) |
| Duration of follow-up (months) | 20.4±6.0 | 20.4±6.3 |
Data are presented as mean±standard deviation or n (%).
Table 3. Univariate predictors of sudden cardiac death at 24 months.
| Univariate model
|
|||
|---|---|---|---|
| Risk marker | Odds Ratio | 95% Wald Confidence Limit | p value |
| Male gender | 0.908 | 0.986-1.033 | 0.439 |
| Coronary Artery Disease | 2.489 | 1.124-5.512 | 0.025 |
| Left ventricle ejection fraction | 0.969 | 0.952-0.986 | <0.001 |
| β-blocker use | 0.927 | 0.552-1.558 | 0.776 |
| Age | 1.009 | 0.986-1.033 | 0.439 |
| Microvolt T-wave alternans | |||
| Indeterminate | 8.612 | 3.593-20.643 | <0.001 |
| Positive | 9.08 | 3.992-20.634 | <0.001 |
LVEF, reported per 1% increase in ejection fraction; age reported per year increase; MTWA status reported relative to negative test result.
The results of multivariate analysis are presented in table 4. In addition to the three significant parameters identified in univariate models (LVEF, MTWA status, CAD), the presence of a significant interaction between MTWA status and LVEF was also identified in multivariate models. In order to better define the interaction between LVEF and MTWA status, in Figure 1 we plot the probability of SCD at 24 months based on LVEF and MTWA status. As expected, the risk of SCD remains relatively low among patients with negative MTWA tests across the entire range of LVEF, although there is a rise at the lowest end of the LVEF spectrum (i.e. LVEF ≤ 20%). In contrast, compared to the negative MTWA cohort, patients with a positive MTWA test result demonstrate a significantly heightened risk of SCD across the entire LVEF spectrum. The positive MTWA cohort demonstrates a relatively less marked rise in SCD risk as LVEF decreases but the absolute risk remains higher than among the negative MTWA patients. Lastly, patients with an indeterminate MTWA test result demonstrate a substantial difference in SCD risk based on the underlying LVEF, with an inflection point visible at an LVEF of approximately 30%. Above this inflection point, patients with an indeterminate MTWA test demonstrate a relatively low risk of SCD that roughly approximates the risk in the negative MTWA cohort. In contrast, below the inflection point the risk of SCD rises sharply in the indeterminate cohort and exceeds even that among the patients with positive MTWA test results.
Table 4. Multivariate model parameters.
| Multivariate model
|
|||
|---|---|---|---|
| Risk marker | Likelihood estimate | Standard error | p value |
| Intercept | -2.287 | ||
| β coefficients | |||
| Coronary Artery Disease | 0.839 | 0.211 | <0.001 |
| Left ventricle ejection fraction | -0.053 | 0.013 | <0.001 |
| Positive MTWA | -0.718 | 0.522 | 0.169 |
| Indeterminate MTWA | 1.226 | 0.593 | 0.039 |
| LVEF*MTWA positive interaction | 0.043 | 0.014 | 0.003 |
| LVEF*MTWA indeterminate interaction | -0.023 | 0.019 | 0.226 |
Left ventricle ejection fraction (LVEF) presented per point increase in ejection fraction. Microvolt T-wave alternans (MTWA)
Figure 1.
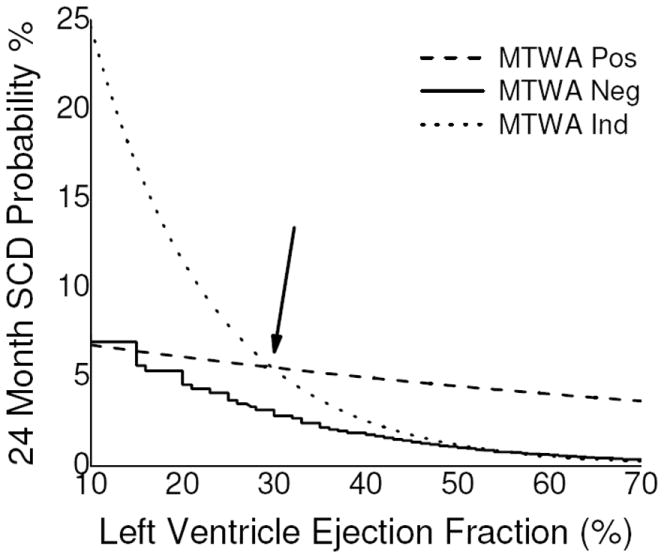
Predicted 24 month probability of sudden cardiac death (SCD) based on left ventricle ejection fraction and microvolt T-wave alternans status. The inflection point in the risk of SCD associated with an indeterminate MTWA test is highlighted with the arrow.
In order to further highlight the important interaction between LVEF and MTWA status in predicting SCD risk, in Table 5 the risk of SCD at 24 months using sample patient risk profiles is shown. Again, we see that compared to patients with a negative MTWA test result, the increased risk of SCD in the positive MTWA cohort is relatively smaller at the lower range of LVEF and increases significantly as LVEF rises, suggesting that although the absolute risk of SCD among patients with preserved LVEF may be small, the relative risk of SCD associated with a positive MTWA test at the higher range of LVEF is significantly increased. In contrast, compared to the negative MTWA cohort, the relative risk of SCD for patients with an indeterminate test result is increased at the lower range of LVEF and decreases significantly at higher LVEFs.
Table 5. Estimated odds ratio for sudden cardiac death based on hypothetical risk profiles.
| Left ventricle ejection fraction (%) | Microvolt T-wave alternans status | Odds ratio | 95% confidence interval |
|---|---|---|---|
| 10 | Indeterminate vs. Negative | 2.72 | 1.18 - 6.25 |
| Positive vs. Negative | 0.75 | 0.35 - 1.62 | |
| 20 | Indeterminate vs. Negative | 2.16 | 1.24 - 3.77 |
| Positive vs. Negative | 1.15 | 0.66 - 1.98 | |
| 30 | Indeterminate vs. Negative | 1.73 | 1.11 - 2.68 |
| Positive vs. Negative | 1.76 | 1.17 - 2.65 | |
| 40 | Indeterminate vs. Negative | 1.38 | 0.76 - 2.48 |
| Positive vs. Negative | 2.69 | 1.73 - 4.19 | |
| 50 | Indeterminate vs. Negative | 1.10 | 0.46 - 2.64 |
| Positive vs. Negative | 4.13 | 2.23 - 7.65 | |
| 60 | Indeterminate vs. Negative | 0.87 | 0.26 - 2.93 |
| Positive vs. Negative | 6.33 | 2.70 - 14.85 |
Odds ratios are presented relative to patients with same left ventricle ejection fraction and negative microvolt T-wave alternans status.
Multivariable risk profiling
We derived a multivariable model including three clinical parameters (LVEF, MTWA status and presence of CAD) and an interaction term (between LVEF and MTWA status) to predict the primary endpoint (Table 4). In the derivation cohort, the c-index for the model was 0.817 (95% confidence interval [CI] 0.770-0.863), suggesting a robust capacity to predict SCD at 24 months. Because LVEF has emerged as the primary clinical tool for gauging SCD risk and determining eligibility for ICD therapy, we compared the ability of the model to predict SCD risk to the ability of LVEF as a single variable to predict SCD risk. Although in clinical practice, LVEF is most commonly used as a dichotomous variable (i.e. LVEF > or ≤ 35%), we chose to use LVEF as a continuous variable in order to directly compare ROC curves. In the derivation cohort, the use of LVEF as a lone variable resulted in a c-index of 0.637 (95% CI 0.565-0.708) for predicting the primary endpoint, which was significantly less than the c-index for the multivariable model (0.817, p < 0.001) (Figure 2), demonstrating a significant improvement in SCD risk prediction when using a multi-marker strategy. We also compared the performance of the multivariable model to MTWA as a lone variable (c-index 0.716, 95% CI 0.665-0.767) and again demonstrated a significant improvement in SCD risk prediction with a multi-marker strategy (p < 0.001).
Figure 2.
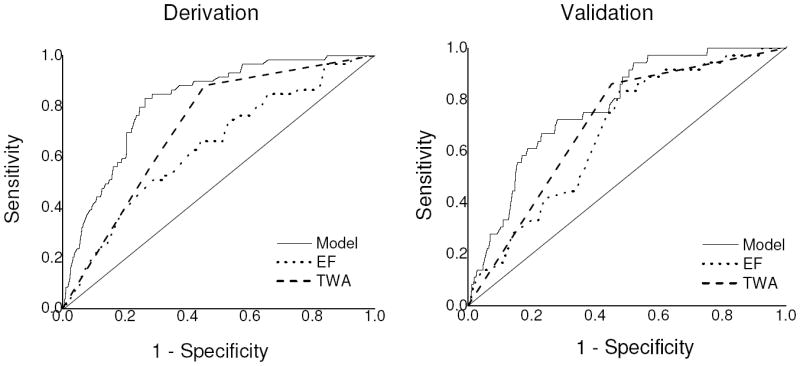
Receiver-operating characteristic (ROC) curves for predicting sudden cardiac death at 24 months for the multivariable model based on three clinical parameters (presence of coronary artery disease, left ventricle ejection fraction [LVEF] & microvolt T-wave alternans status) versus either LVEF or MTWA as single variables. In the derivation cohort, the area under the ROC curve (c-index) for the multivariate model (0.817) is significantly greater than both LVEF (0.637) and MTWA (0.716) (p < 0.001 for both comparisons). In the validation cohort, the c-index of the model (0.774) is also significantly better than LVEF (0.671, p = 0.020), and non-significantly greater than MTWA as a lone variable (0.729, p = 0.170).
Validation cohort
The baseline characteristics of the validation cohort are presented in Table I. In the validation cohort, the multivariable model again demonstrated significantly better ability to predict SCD at 24 months compared to LVEF as a lone variable (c-index 0.774 [95% CI 0.709-0.840] vs. 0.671 [95% CI 0.594-0.747], p = 0.020). Although the c-index of the multivariable model in the validation cohort was also higher than MTWA as a lone variable (c-index 0.729, 95% CI 0.660-0.7980), the difference was not significant (p = 0.170).
Lastly, in order to highlight the benefits of using a multi-marker strategy to more specifically gauge SCD risk, in Figure 3 we compared the predicted and observed SCD free survival for patients with LVEF ≤ or > 35%. Using the multivariable model, patients in the validation cohort were stratified into three groups based on the predicted risk of SCD at 24 months: <1%, 1-6% and >6%. For patients in each of the predicted risk groups, the observed rate of SCD was then plotted using Kaplan-Meier estimates. Although the specific threshold of arrhythmic risk at which ICD therapy is likely to be beneficial has not been specifically defined, based on extrapolation from the Sudden Cardiac Death in Heart Failure (SCD-HeFT) trial, it has been suggested that patients with an annual SCD risk of as low as 3% may benefit from ICDs 29, 30. Therefore, the 6% SCD rate at 24 months was chosen for this analysis.
Figure 3.
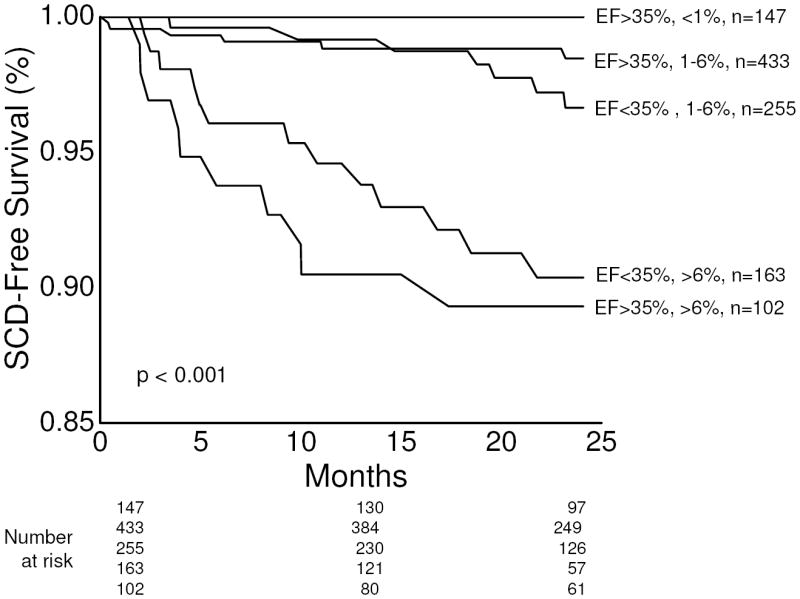
Kaplan-Meier event-free survival curves for the primary endpoint of sudden cardiac death, stratified by predicted SCD risk based on the multivariable model. Using the multivariate model, patients in the validation cohort with left ventricle ejection fraction (LVEF) ≤ or > 35% were further stratified into one of three groups based on predicted SCD risk at 24 months: <1%, 1-6% and >6%. The survival curves demonstrate that even among patients stratified by LVEF, there is still significant heterogeneity in SCD risk, which can be accurately predicted by the multivariable model. The number of patients (n) in each predicted risk group is listed. Of note, there were no patients in the LVEF ≤ 35% cohort with predicted SCD risk of <1% at 24 months, and therefore, only 5 curves are plotted. The p value by log-rank test is <0.001, suggesting a significant difference in survival across subgroups.
From Figure 3, it is evident that the risk of SCD is not homogeneous for patients stratified by LVEF and important overlap exists between patients above and below the LVEF 35% threshold. Specifically, among patients with LVEF ≤ 35%, 61% (255 out of 418) are predicted to have a 24 month SCD risk of 1-6% and in this cohort, the observed event rate is approximately 3.4% at 24 months. The remaining patients with LVEF ≤ 35% (163 out of 418) are predicted to have to a 24 month SCD risk of >6% and the observed event rate in this subgroup is approximately 10%. These observations highlight the fact that even among patients with impaired LV systolic function, the use of multivariable risk profiling can be used to identify subgroups with heterogeneous levels of SCD risk and may be able to identify a group of patients whose SCD risk is below the threshold at which primary prevention ICD therapy may be beneficial. In contrast, among patients with LVEF > 35%, approximately 15% (102 out of 682) are predicted to have a 24 month SCD risk >6%. In this subgroup, the observed SCD event rate exceeds 10%, clearly identifying a group with relatively preserved LVEF but whose SCD risk exceeds that among many patients with LVEF ≤ 35%. This subgroup of patients at high risk of SCD would not have been identified using LVEF criterion alone.
Discussion
With regard to SCD risk prediction, our data demonstrate that important and complex interactions exist between individual SCD risk predictors and that those interactions need to be accounted for when attempting to estimate the risk of SCD for any individual patient. Furthermore, a multivariable model based on three easily accessible clinical parameters (presence of coronary disease, MTWA status and LVEF) is capable of predicting 24 month SCD risk with significantly better sensitivity and specificity than either LVEF or MTWA alone.
Numerous non-invasive markers of SCD risk have been evaluated 5 but in isolation, none of these markers has been demonstrated to significantly improve beyond LVEF in refining primary prevention ICD treatment algorithms. However, there is widespread recognition that LVEF represents only one aspect of the complex electro-anatomic substrate that gives rise to lethal arrhythmias and SCD. In contrast to coronary/cardiovascular disease 31, 32 or congestive heart failure 33, 34, where multi-marker risk models have been developed and validated, the use of multi-marker strategies for SCD risk prediction has lagged behind.
Several prior studies have assessed the incremental utility of multi-marker strategies for SCD risk prediction. Sub-group analyses from both the Multicenter Unsustained Tachycardia Trial (MUSTT) 35 and the Multicenter Automatic Defibrillator Implantation Trial II (MADIT II) 36 have been used to develop multi-marker risk scores for SCD risk prediction. Of note, both studies were limited to patients with impaired LVEF (≤40% in MUSTT and ≤30% in MADIT-II). Although patients with impaired LVEF are at increased risk of SCD, the majority of SCD events at the population level occur in patients with only mildly impaired or preserved LV systolic function 7, 8, demonstrating an important need to develop risk stratification tools which can be applied across the entire spectrum of SCD risk. Atwater et al. developed a multivariate model for SCD risk prediction derived from the Duke Databank for Cardiovascular disease 37 and similar to our study, included patients across a broader range of LV ejection fraction. However, all three of the aforementioned studies included only patients with underlying coronary artery disease. Patients with non-ischemic cardiomyopathy are also at risk of SCD and benefit from primary prevention ICD therapy when appropriately selected 2, highlighting the need to extend risk stratification efforts to patients without coronary disease. Our multivariable model has the particular advantage of being applicable across a wide range of risk, regardless of whether an ischemic substrate is present or not.
More recently, there has also been significant interest in the use of imaging modalities such as cardiac magnetic resonance imaging 38 and single-photon emission computed tomography (SPECT) with agents such as 123-iodine metaiodobenzylguanidine (123-I MIBG) 39 to improve SCD risk stratification and predict response to ICD therapy. Although such imaging modalities may prove to be useful in the context of multi-marker SCD risk profiling strategies, none has yet been validated in large cohorts for this purpose.
The potential benefit of primary prevention ICD therapy is likely determined by a balance between competing risks of arrhythmic and non-arrhythmic mortality. A lower than expected rate of sudden death 40, or a higher than expected rate of non-arrhythmic death 41, have been suggested as explanations for why certain trials of primary prevention ICD therapy have failed to show benefit. Gauging the risk of non-arrhythmic death is likely to be highly patient specific and dependent on clinical context. However, developing systematic algorithms to predict the risk of arrhythmic death is likely to improve ICD allocation. The data in Figure 3 demonstrate that among patients with LVEF ≤ 35%, a subgroup can be identified with a relatively low risk of SCD (<6% at 24 months), such that ICD therapy may not be beneficial, particularly in light of competing non-arrhythmic risk. The ability to safely withhold ICD therapy from these patients based on multivariable risk profiling holds promise for developing patient-specific treatment plans. In contrast, among those patients with LVEF > 35% who are predicted to be at heightened SCD risk (>6% at 24 months), the benefits of pharmacologic and device therapies should be assessed systematically. The purpose of our study is not necessarily to define a specific SCD risk threshold at which primary prevention ICD therapy is likely to be beneficial but rather, to move from a binary decision making process (based predominantly on an LVEF cut point), to a more granular and patient-specific risk profile. If confirmed in prospective studies, the use of multivariable SCD risk profiling has the potential to significantly improve primary prevention ICD therapy algorithms.
Several limitations of our study should be noted. First, because our derivation cohort was pooled from six individual studies, we were limited in the number of variables for which complete data was available on all patients. It is conceivable that the inclusion of more covariates may have significantly improved the final model. Second, the definitions used for identifying cases of sudden cardiac death were slightly different according to the individual protocols for each of the studies included in the pooled cohort. These differences may have introduced some bias in pooling endpoints; however, there is widespread recognition that clinical definitions of SCD are quite limited 42 and this represents a challenge to the entire field of SCD risk prediction. Third, in contrast to patients with CAD where the full spectrum of LVEF was included, our cohort only included patients with non-ischemic cardiomyopathy who had an LVEF ≤ 40%. This may limit the applicability of our findings for SCD risk prediction in patients without CAD who have mildly impaired or preserved LV systolic function. Fortunately, at the population level, patients with relatively preserved systolic function without an ischemic substrate are at particularly low risk of SCD. Lastly, given the widespread acknowledgement that “appropriate” ICD therapies are a poor surrogate for aborted SCD 43, we chose to exclude studies where a significant percentage of patients were implanted with ICDs (i.e. ≥ 15%) 9 in order to minimize the potential confounding effect that ICD therapies may introduce in clinical endpoints. In the current era of primary prevention ICD implantation based on MADIT-II/SCD-HeFT criteria 3, excluding studies with a high percentage of implanted ICDs would introduce another form of bias by excluding the highest risk primary prevention ICD candidates. However, the studies in our pooled cohort enrolled patients prior to the widespread uptake of contemporary primary prevention ICD criteria and therefore, the exclusion of studies with a high percentage of implanted ICDs would not necessarily exclude high risk primary prevention ICD candidates. Although we acknowledge the limitations of this approach, we believe exclusion of studies with high percentages of ICDs provides one important approach to studying the natural history of SCD risk markers without the confounding impact of ICD therapies.
Conclusion
Our findings suggest that a simple model based on three clinical parameters (presence of CAD, LVEF and MTWA status) is capable of predicting risk of SCD over the following 24 months with robust sensitivity and specificity. In contrast to prior attempts at multi-marker SCD risk stratification, this model can be applied to patients with and without coronary artery disease and across a broad range of LVEF. These findings may have important implications for improving current primary prevention ICD treatment algorithms.
Acknowledgments
Funding sources
Dr. Armoundas was supported by a Scientist Development Grant (#0635127N) and by NIA grant 1R21AG035128. This work was also supported by a Fellowship and a Science Award from the Center for Integration of Medicine and Innovative Technology (CIMIT), the Deane Institute for Integrative Research in Atrial Fibrillation and Stroke and the Cardiovascular Research Society. Dr. Chan was supported by Grant Number K23HL102224 from the National Heart, Lung, And Blood Institute. This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (NIH Award #UL1 RR 025758 and financial contributions from Harvard University and its affiliated academic health care centers). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Center for Research Resources, or the National Institutes of Health.
The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
References
- 1.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. The New England journal of medicine. 2002;346(12):877–83. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 2.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. The New England journal of medicine. 2005;352(3):225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 3.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, 3rd, Freedman RA, Gettes LS, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation. 2008;117(21):e350–408. doi: 10.1161/CIRCUALTIONAHA.108.189742. [DOI] [PubMed] [Google Scholar]
- 4.Raphael C, Briscoe C, Davies J, Ian Whinnett Z, Manisty C, Sutton R, et al. Limitations of the New York Heart Association functional classification system and self-reported walking distances in chronic heart failure. Heart. 2007;93(4):476–82. doi: 10.1136/hrt.2006.089656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fishman GI, Chugh SS, Dimarco JP, Albert CM, Anderson ME, Bonow RO, et al. Sudden cardiac death prediction and prevention: report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop. Circulation. 2010;122(22):2335–48. doi: 10.1161/CIRCULATIONAHA.110.976092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buxton AE, Ellison KE, Lorvidhaya P, Ziv O. Left ventricular ejection fraction for sudden death risk stratification and guiding implantable cardioverter-defibrillators implantation. Journal of cardiovascular pharmacology. 2010;55(5):450–5. [PubMed] [Google Scholar]
- 7.Stecker EC, Vickers C, Waltz J, Socoteanu C, John BT, Mariani R et al. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two-year findings from the Oregon Sudden Unexpected Death Study. Journal of the American College of Cardiology. 2006;47(6):1161–6. doi: 10.1016/j.jacc.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 8.Gorgels AP, Gijsbers C, de Vreede-Swagemakers J, Lousberg A, Wellens HJ. Out-of-hospital cardiac arrest--the relevance of heart failure. The Maastricht Circulatory Arrest Registry. Eur Heart J. 2003;24(13):1204–9. doi: 10.1016/s0195-668x(03)00191-x. [DOI] [PubMed] [Google Scholar]
- 9.Hohnloser SH, Ikeda T, Cohen RJ. Evidence regarding clinical use of microvolt T-wave alternans. Heart rhythm : the official journal of the Heart Rhythm Society. 2009;6(3 Suppl):S36–44. doi: 10.1016/j.hrthm.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Gold MR, Bloomfield DM, Anderson JM, El-Sherif N, Wilber D, Groh WJ, et al. A comparison of T-wave alternans, signal averaged electrocardiography and programmed ventricular stimulation for arrhythmia risk stratification. Journal of the American College of Cardiology. 2000;36(7):2247–53. doi: 10.1016/s0735-1097(00)01017-2. [DOI] [PubMed] [Google Scholar]
- 11.Hohnloser SH, Klingenheben T, Bloomfield D, Dabbous O, Cohen RJ. Usefulness of microvolt T-wave alternans for prediction of ventricular tachyarrhythmic events in patients with dilated cardiomyopathy: results from a prospective observational study. Journal of the American College of Cardiology. 2003;41(12):2220–4. doi: 10.1016/s0735-1097(03)00467-4. [DOI] [PubMed] [Google Scholar]
- 12.Rashba EJ, Osman AF, Macmurdy K, Kirk MM, Sarang SE, Peters RW, et al. Enhanced detection of arrhythmia vulnerability using T wave alternans, left ventricular ejection fraction, and programmed ventricular stimulation: a prospective study in subjects with chronic ischemic heart disease. Journal of cardiovascular electrophysiology. 2004;15(2):170–6. doi: 10.1046/j.1540-8167.2004.03428.x. [DOI] [PubMed] [Google Scholar]
- 13.Cantillon DJ, Stein KM, Markowitz SM, Mittal S, Shah BK, Morin DP, et al. Predictive value of microvolt T-wave alternans in patients with left ventricular dysfunction. Journal of the American College of Cardiology. 2007;50(2):166–73. doi: 10.1016/j.jacc.2007.02.069. [DOI] [PubMed] [Google Scholar]
- 14.Gold MR, Ip JH, Costantini O, Poole JE, McNulty S, Mark DB, et al. Role of microvolt T-wave alternans in assessment of arrhythmia vulnerability among patients with heart failure and systolic dysfunction: primary results from the T-wave alternans sudden cardiac death in heart failure trial substudy. Circulation. 2008;118(20):2022–8. doi: 10.1161/CIRCULATIONAHA.107.748962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chow T, Kereiakes DJ, Onufer J, Woelfel A, Gursoy S, Peterson BJ, et al. Does microvolt T-wave alternans testing predict ventricular tachyarrhythmias in patients with ischemic cardiomyopathy and prophylactic defibrillators? The MASTER (Microvolt T Wave Alternans Testing for Risk Stratification of Post-Myocardial Infarction Patients) trial. Journal of the American College of Cardiology. 2008;52(20):1607–15. doi: 10.1016/j.jacc.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 16.Costantini O, Hohnloser SH, Kirk MM, Lerman BB, Baker JH, 2nd, Sethuraman B, et al. The ABCD (Alternans Before Cardioverter Defibrillator) Trial: strategies using T-wave alternans to improve efficiency of sudden cardiac death prevention. Journal of the American College of Cardiology. 2009;53(6):471–9. doi: 10.1016/j.jacc.2008.08.077. [DOI] [PubMed] [Google Scholar]
- 17.Kitamura H, Ohnishi Y, Okajima K, Ishida A, Galeano E, Adachi K, et al. Onset heart rate of microvolt-level T-wave alternans provides clinical and prognostic value in nonischemic dilated cardiomyopathy. Journal of the American College of Cardiology. 2002;39(2):295–300. doi: 10.1016/s0735-1097(01)01718-1. [DOI] [PubMed] [Google Scholar]
- 18.Grimm W, Christ M, Bach J, Muller HH, Maisch B. Noninvasive arrhythmia risk stratification in idiopathic dilated cardiomyopathy: results of the Marburg Cardiomyopathy Study. Circulation. 2003;108(23):2883–91. doi: 10.1161/01.CIR.0000100721.52503.85. [DOI] [PubMed] [Google Scholar]
- 19.Tapanainen JM, Still AM, Airaksinen KEJ, Huikuri HV. Prognostic significance of risk stratifiers of mortality, including T wave alternans, afer acute myocardial infarction: Results of a propsective follow-up study. Journal of cardiovascular electrophysiology. 2001;12(6):645–52. doi: 10.1046/j.1540-8167.2001.00645.x. [DOI] [PubMed] [Google Scholar]
- 20.Gorodeski EZ, Cantillon DJ, Goel SS, Kaufman ES, Martin DO, Hsich EM, et al. Microvolt T-wave alternans, peak oxygen consumption, and outcome in patients with severely impaired left ventricular systolic function. J Heart Lung Transplant. 2009;28(7):689–96. doi: 10.1016/j.healun.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bloomfield DM, Bigger JT, Steinman RC, Namerow PB, Parides MK, Curtis AB, et al. Microvolt T-wave alternans and the risk of death or sustained ventricular arrhythmias in patients with left ventricular dysfunction. Journal of the American College of Cardiology. 2006;47(2):456–63. doi: 10.1016/j.jacc.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 22.Chan PS, Nallamothu BK, Spertus JA, Masoudi FA, Bartone C, Kereiakes DJ, et al. Impact of age and medical comorbidity on the effectiveness of implantable cardioverter-defibrillators for primary prevention. Circ Cardiovasc Qual Outcomes. 2009;2(1):16–24. doi: 10.1161/CIRCOUTCOMES.108.807123. [DOI] [PubMed] [Google Scholar]
- 23.Ikeda T, Saito H, Tanno K, Shimizu H, Watanabe J, Ohnishi Y, et al. T-wave alternans as a predictor for sudden cardiac death after myocardial infarction. The American journal of cardiology. 2002;89(1):79–82. doi: 10.1016/s0002-9149(01)02171-3. [DOI] [PubMed] [Google Scholar]
- 24.Ikeda T, Yoshino H, Sugi K, Tanno K, Shimizu H, Watanabe J, et al. Predictive value of microvolt T-wave alternans for sudden cardiac death in patients with preserved cardiac function after acute myocardial infarction: results of a collaborative cohort study. Journal of the American College of Cardiology. 2006;48(11):2268–74. doi: 10.1016/j.jacc.2006.06.075. [DOI] [PubMed] [Google Scholar]
- 25.Salerno-Uriarte JA, De Ferrari GM, Klersy C, Pedretti RF, Tritto M, Sallusti L, et al. Prognostic value of T-wave alternans in patients with heart failure due to nonischemic cardiomyopathy: results of the ALPHA Study. Journal of the American College of Cardiology. 2007;50(19):1896–904. doi: 10.1016/j.jacc.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Klingenheben T, Zabel M, D’Agostino RB, Cohen RJ, Hohnloser SH. Predictive value of T-wave alternans for arrhythmic events in patients with congestive heart failure [letter] Lancet. 2000;356(9230):651–2. doi: 10.1016/s0140-6736(00)02609-x. [DOI] [PubMed] [Google Scholar]
- 27.Rosenbaum DS, Jackson LE, Smith JM, Garan H, Ruskin JN, Cohen RJ. Electrical alternans and vulnerability to ventricular arrhythmias. The New England journal of medicine. 1994;330(4):235–41. doi: 10.1056/NEJM199401273300402. [DOI] [PubMed] [Google Scholar]
- 28.Bloomfield DM, Hohnloser SH, Cohen RJ. Interpretation and classification of microvolt T wave alternans tests. Journal of cardiovascular electrophysiology. 2002;13(5):502–12. doi: 10.1046/j.1540-8167.2002.00502.x. [DOI] [PubMed] [Google Scholar]
- 29.Packer DL, Prutkin JM, Hellkamp AS, Mitchell LB, Bernstein RC, Wood F, et al. Impact of implantable cardioverter-defibrillator, amiodarone, and placebo on the mode of death in stable patients with heart failure: analysis from the sudden cardiac death in heart failure trial. Circulation. 2009;120(22):2170–6. doi: 10.1161/CIRCULATIONAHA.109.853689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta A, Hoang DD, Karliner L, Tice JA, Heidenreich P, Wang PJ, et al. Ability of microvolt T-wave alternans to modify risk assessment of ventricular tachyarrhythmic events: a meta-analysis. Am Heart J. 2012;163(3):354–64. doi: 10.1016/j.ahj.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 31.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 32.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA the journal of the American Medical Association. 2007;297(6):611–9. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 33.Velagaleti RS, Gona P, Larson MG, Wang TJ, Levy D, Benjamin EJ, et al. Multimarker approach for the prediction of heart failure incidence in the community. Circulation. 2010;122(17):1700–6. doi: 10.1161/CIRCULATIONAHA.109.929661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kannel WB, D’Agostino RB, Silbershatz H, Belanger AJ, Wilson PW, Levy D. Profile for estimating risk of heart failure. Archives of internal medicine. 1999;159(11):1197–204. doi: 10.1001/archinte.159.11.1197. [DOI] [PubMed] [Google Scholar]
- 35.Buxton AE, Lee KL, Hafley GE, Pires LA, Fisher JD, Gold MR, et al. Limitations of ejection fraction for prediction of sudden death risk in patients with coronary artery disease: lessons from the MUSTT study. J Am Coll Cardiol. 2007;50(12):1150–7. doi: 10.1016/j.jacc.2007.04.095. [DOI] [PubMed] [Google Scholar]
- 36.Goldenberg I, Vyas AK, Hall WJ, Moss AJ, Wang H, He H, et al. Risk stratification for primary implantation of a cardioverter-defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol. 2008;51(3):288–96. doi: 10.1016/j.jacc.2007.08.058. [DOI] [PubMed] [Google Scholar]
- 37.Atwater BD, Thompson VP, Vest RN, 3rd, Shaw LK, Mazzei WR, Jr, Al-Khatib SM, et al. Usefulness of the Duke Sudden Cardiac Death risk score for predicting sudden cardiac death in patients with angiographic (>75% narrowing) coronary artery disease. Am J Cardiol. 2009;104(12):1624–30. doi: 10.1016/j.amjcard.2009.07.042. [DOI] [PubMed] [Google Scholar]
- 38.Scott PA, Rosengarten JA, Curzen NP, Morgan JM. Late gadolinium enhancement cardiac magnetic resonance imaging for the prediction of ventricular tachyarrhythmic events: a meta-analysis. European journal of heart failure. 2013 doi: 10.1093/eurjhf/hft053. [DOI] [PubMed] [Google Scholar]
- 39.Boogers MJ, Borleffs CJ, Henneman MM, van Bommel RJ, van Ramshorst J, Boersma E, et al. Cardiac sympathetic denervation assessed with 123-iodine metaiodobenzylguanidine imaging predicts ventricular arrhythmias in implantable cardioverter-defibrillator patients. Journal of the American College of Cardiology. 2010;55(24):2769–77. doi: 10.1016/j.jacc.2009.12.066. [DOI] [PubMed] [Google Scholar]
- 40.Kadish A, Dyer A, Daubert JP, Quigg R, Estes NA, Anderson KP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350(21):2151–8. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 41.Pouleur AC, Barkoudah E, Uno H, Skali H, Finn PV, Zelenkofske SL, et al. Pathogenesis of sudden unexpected death in a clinical trial of patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. Circulation. 2010;122(6):597–602. doi: 10.1161/CIRCULATIONAHA.110.940619. [DOI] [PubMed] [Google Scholar]
- 42.Chugh SS, Jui J, Gunson K, Stecker EC, John BT, Thompson B, et al. Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate-based review in a large U.S. community. J Am Coll Cardiol. 2004;44(6):1268–75. doi: 10.1016/j.jacc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 43.Ellenbogen KA, Levine JH, Berger RD, Daubert JP, Winters SL, Greenstein E, et al. Are implantable cardioverter defibrillator shocks a surrogate for sudden cardiac death in patients with nonischemic cardiomyopathy? Circulation. 2006;113(6):776–82. doi: 10.1161/CIRCULATIONAHA.105.561571. [DOI] [PubMed] [Google Scholar]
- 44.Moss AJ, Schuger C, Beck CA, Brown MW, Cannom DS, Daubert JP, et al. Reduction in inappropriate therapy and mortality through ICD programming. The New England journal of medicine. 2012;367(24):2275–83. doi: 10.1056/NEJMoa1211107. [DOI] [PubMed] [Google Scholar]


