Abstract
Mutations in CEP290 are the most common cause of Leber congenital amaurosis (LCA), a severe inherited retinal degenerative disease for which there is currently no cure. Autosomal recessive CEP290-associated LCA is a good candidate for gene-replacement therapy, and cells derived from affected individuals give researchers the ability to study human disease and therapeutic gene correction in vitro. Here we report the development of lentiviral vectors carrying full-length CEP290 for the purpose of correcting the CEP290 disease-specific phenotype in human cells. A lentiviral vector containing CMV-driven human full-length CEP290 was constructed. Following transduction of patient-specific, iPSC-derived, photoreceptor precursor cells, rt-PCR analysis and western blotting revealed vector-derived expression. Because CEP290 is important in ciliogenesis, the ability of fibroblast cultures from CEP290-associated LCA patients to form cilia was investigated. In cultures derived from these patients, fewer cells formed cilia compared to unaffected controls. Cilia that were formed were shorter in patient derived cells than in cells from unaffected individuals. Importantly, lentiviral delivery of CEP290 rescued the ciliogenesis defect. The successful construction and viral transfer of full-length CEP290 brings us closer to the goal of providing gene- and cell- based therapies for patients affected with this common form of LCA.
Keywords: Gene transfer, Patient specific stem cells, LCA
Introduction
Leber congenital amaurosis (LCA) is a term used to refer to a group of severe inherited retinal disorders characterized by poor vision within the first year of life, nystagmus, and a non-recordable electroretinogram1. LCA is almost always inherited in an autosomal recessive fashion and about 30% of LCA patients harbor mutations in the CEP290 gene making it the most common contributor2,3. CEP290 is a centrosomal protein that is localized to the connecting cilium of the photoreceptors4,5 and is involved in both ciliogenesis and ciliary trafficking6-9. Patients with CEP290-associated LCA retain some cone nuclei in the central cone-rich foveal region. However, these cone photoreceptors have abnormal inner and outer segments resulting in severe vision loss in most patients10,11. MRI studies have demonstrated that the anatomy of CEP290-associated LCA patients’ intracranial visual pathways is normal, suggesting that gene and/or cell-based treatments could restore vision10 if the therapy could be delivered at an early enough age that the thalamic and cortical centers were still capable of meaningful interpretation of the information being transmitted from the retinal ganglion cells.
Very encouraging in the latter regard is the visual improvement that has been observed in a number of subjects enrolled in clinical trials of adeno-associated virus (AAV)-mediated gene therapy for RPE65-associated LCA12-14. Although it will undoubtedly be possible to devise some type of effective gene transfer approach for the treatment of CEP290-associated LCA, the size of CEP290 precludes the use of the AAV vector system for packaging the full-length gene. Thus employing lentivirus (which has a larger packaging limit – 8-10 kb versus 4.7 kb15) will be advantageous as it can accommodate the full-length CEP290 cDNA (7972 nt). Moreover, lentiviral vectors can transduce multiple cell types in the eye, including photoreceptors16,17, which are the retinal cells most affected by CEP290 mutations.
Induced pluripotent stem cell (iPSC)-based technologies are now providing researchers with the ability to model and study human diseases and to evaluate various therapeutic modalities in vitro. Recently, we were able to demonstrate that transplantation of iPSC-derived photoreceptor precursor cells generated from normal mice restored retinal structure and function in a mouse model of a retinal degenerative disease18. We have also used patient-specific iPSC-derived retinal progenitor cells to investigate the pathophysiology of autosomal recessive retinitis pigmentosa (RP) caused by mutations in MAK and USH2A19,20. Together, these studies illustrate the utility of iPSC technology for the investigation of pathophysiologic mechanisms involved in inherited retinal diseases and lay the groundwork for the in vitro investigation of gene replacement strategies for treating these disorders.
Here we describe the development of a lentiviral vector expressing full-length human CEP290 and demonstrate its ability to rescue the ciliogenesis defect observed in patient-derived fibroblasts. Furthermore, we report the generation and characterization of iPSCs from mice and humans affected with CEP290-associated LCA and the subsequent differentiation and characterization of photoreceptor precursors. Finally, we demonstrate gene transfer to iPSC-derived photoreceptor precursor cells using this vector.
Results
Full-length human CEP290 is packaged into a lentiviral vector
The CEP290 CDS is too large (~8kb) to package into the AAV system that was successfully used to treat RPE65-associated LCA12,13,21. However, lentivirus has a much larger packaging limit (8-10 kb) and is capable of efficient transduction of post-mitotic retinal cells15. Therefore, we generated a lentiviral transgene cassette plasmid carrying the CEP290 coding sequence driven by the cytomegalovirus (CMV) promoter (Fig. 1A). When packaged (LV-CMV-hCEP290), the titer was determined to be at least 1 × 108 transducing units per milliliter (TU/ml). Using a similar construct with the elongation factor 1 alpha (EF1a) promoter element (1190 bp versus 585 bp) we were unable to obtain titers higher than 5 × 106 TU/ml -- too low to employ in subsequent gene transfer experiments. These findings are in agreement with previously published studies suggesting that for lentiviruses as plasmid size increases, the efficiency of viral packaging decreases 22. Thus, the full-length wild type CEP290 coding sequence combined with the CMV promoter appears to be at the size limit for efficient lentiviral packaging.
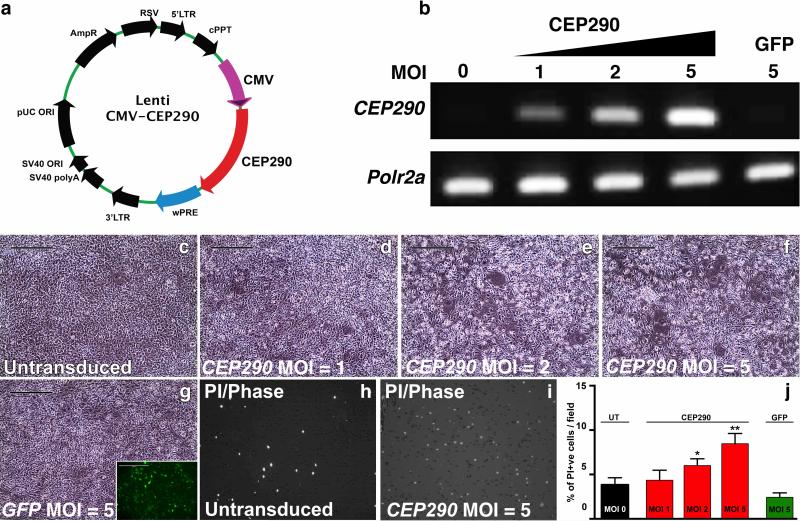
Figure 1. Lentiviral packaging and expression of full-length CEP290.
(a) Lentiviral transgene cassette. LTR – long terminal repeat; cPPT – central polypurine tract; CMV – cytomegalovirus promoter; wPRE – woodchuck posttranscriptional regulatory element. (b) RT-PCR in LV-hCEP290-transduced JK1 cells reveals a dose-dependent increase in transgene expression. (c–g) Phase contrast images of JK1 cells either untransduced (c) or transduced with LV-CEP290 at an MOI of 1 (d), 2 (e), and 5 (f) or LV-GFP at an MOI of 5 (g, inlay showing GFP expression). (h-i) Propidium iodide (PI) cell viability assay of untransduced cells (h) and cells transduced with LV-CEP290 at an MOI of 5 (i). (j) histogram depicting cell viability. Scale bar = 400 uM.
To demonstrate vector-mediated CEP290 expression, we first transduced a murine cell line, JK1, at increasing multiplicities of infection (MOI). A dose-dependent increase in human CEP290 transcript, as determined by rt-PCR was observed (Fig. 1B). At 5 days post-transduction, a noticeable drop in cell viability was evident for cultures transduced at an MOI of 5: clumping, morphological changes and death were detected (Figs. 1C-F). As clumping did not occur in cultures transduced with equal amounts of lentiviral vector expressing GFP (Fig. 1G), we hypothesized that overexpression of the CEP290 gene product is cytotoxic. To more accurately evaluate transduction induced cytotoxicity, cell viability assays were performed (Figs. 1H and I). At 5 days post-transduction, a slight increase in the number of propidium iodide-positive cells was detected in cultures transduced with full length CEP290 at an MOI of 2, a further statistically significant increase was detected in cultures transduced at an MOI of 5, compared to both untransduced and GFP (MOI of 5) transduced controls (Fig. 1J). No significant increase in cell death was detected in cultures transduced at an MOI of 1. Therefore, subsequent experiments were performed such that the predicted dosage of CEP290 would be below the estimated level of cytotoxicity. Additional control transductions with an identical lentiviral vector expressing unrelated proteins (the multicistronic transcription factors OCT4, SOX2, KLF4, and cMYC) yielded no difference in cell viability at an MOI of 5 compared to untransduced cells (Supplementary Fig. S1). Collectively, these data indicate that although we were able to successfully package and express full-length CEP290 via the lentiviral vector system, over expression of this gene is cytotoxic.
A lentiviral vector expressing human CEP290 transduces iPSC-derived photoreceptor precursors
To test the ability of the above described lentiviral gene transfer vector to transduce cell types relevant to the in vivo treatment of CEP290-associated LCA, we first needed to produce patient-specific pluripotent stem cells and use these to derive patient-specific photoreceptor precursors. To begin, dermal fibroblasts isolated from mouse (BXD24/TyJ-Cep290rd16/J mouse4) and human patients with known disease-causing mutations in CEP290 were targeted for iPSC generation via forced expression of the transcription factors OCT4 (POU5F1), SOX2, KLF4, and cMYC23. Approximately three weeks after transduction, densely packed colonies of cells with a large nucleus-to-cytoplasm ratio (typical of iPSCs) were identified in both murine (Fig. 2A) and human cultures (Fig. 2C). Following expansion, expression of the pluripotency transcripts NANOG, OCT4, KLF4, LIN28A, and DNMT1 was confirmed via rt-PCR (Figs. 2B and D).
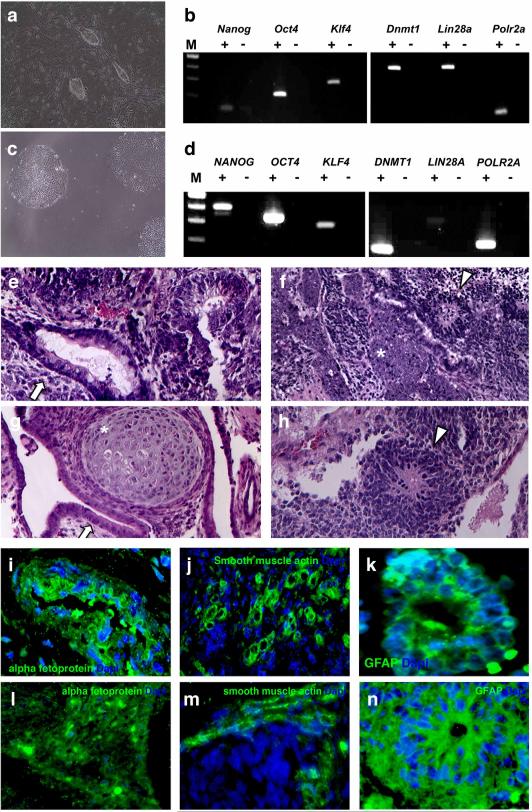
Figure 2. iPSC generation.
(a, b) Brightfield images (10x) of rd16 (a) and patient B294 (b) iPSC colonies. (c, d) RT-PCR analysis of pluripotency marker expression in mouse (c) and B294 (d) iPSCs. (e-h) H & E images (10X) of rd16 (e and f) and B294 (g and h) iPSC-derived teratomas containing endoderm (arrow), mesoderm (asterisk), and ectoderm (arrowhead). (i-n) Immunohistochemistry in murine (i-k) and human (l-n) iPSC-derived teratomas: endoderm (i and l), mesoderm (j and m), and ectoderm (k and n). iPSC – induced pluripotent stem cell, GFAP – glial fibrilary acidic protein. Images collected with 10x objective.
To assess the ability of the murine and patient iPSCs to differentiate into tissues specific to each of the three embryonic germ layers, we employed teratoma formation assays in immunocompromised mice. At four weeks post-injection of murine iPSCs and eight weeks post-injection of patient-specific iPSCs, teratomas were resected and evaluated histologically. Hemotoxylin and eosin staining of paraffin sections revealed the presence of tissues specific to each of the three embryonic germ layers in murine (Figs. 2E and F) and patient-specific (Fig. 2G and H, patient B294) iPSC-derived teratomas. Similarly, immunostaining with antibodies targeted against the endodermal marker alpha fetoprotein (Figs. 2I and L, mouse and human, respectively), the mesodermal marker smooth muscle actin (Figs. 2J and M), and ectodermal marker glial fibrilary acidic protein (Figs. 2K and N) indicate the formation of tissues specific to each of the three embryonic germ layers in both murine and human iPSC-derived teratomas.
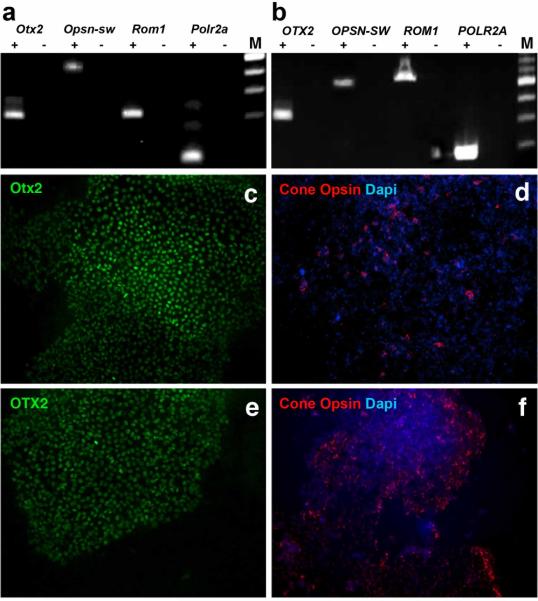
To produce photoreceptor precursor cells (PRPCs), we employed our previously reported step-wise differentiation protocol19,24. After 33 days (mouse) or 90 days (human) in culture we evaluated retinal cell marker transcripts via rt-PCR. Both murine and human differentiated cultures expressed CRX, RHO, OPSN1SW, RCVRN, and ROM1 transcripts (Figs. 3A and B, mouse and human, respectively). Moreover, the retinal cell markers OTX2 and OPSN1SW were expressed in both murine (Figs. 3C and 3D) and human (Figs. 3E and F) differentiated cultures as indicated by immunofluorescence. To further demonstrate that differentiated human CEP290 patient-specific cells adopt a neural ectoderm/photoreceptor cell fate, immunocytochemical analysis targeting the photoreceptor cell marker recoverin, mesodermal markers Smooth Muscle Actin (α-SMA) and myosin and the endodermal marker α-Fetoprotein (endodermal marker) was performed. While areas of neural differentitation (i.e. neural rosettes) were intensively positive for recoverin (Supplementary Fig. S2A), neither α-SMA (Supplementary Fig. S2B), Myosin (Supplementary Fig. S2C), nor α-Fetoprotein (Supplementary Fig. S2C) could be detected.
Figure 3. iPSC-derived photoreceptor precursor generation.
(a-b) RT-PCR in rd16 (a) and B294 (b) iPSC-derived photoreceptor precursors 31 and 90 days, respectively, post-differentiation. (c-f) Immunocytochemical analysis of 31 day rd16 cultures (c and d) and 90 day B294 cultures (e and f) shows expression of retinal markers OTX2 (c and d) and cone opsin (d and f).
To demonstrate vector-mediated CEP290 expression in a disease-relevant cell type, we first transduced iPSC-derived PRPCs from the rd16 mouse with LV-CMV-hCEP290. One week after transduction, we evaluated the presence of vector-derived CEP290 transcript by amplifying a portion of the transcript encoding the deleted in rd16 domain (DRD)25. The DRD transcript was undetectable in untreated cultures, but was amplified from cells transduced with lentiviral vector (Fig. 4A) demonstrating that CEP290 transgene expression could be accomplished in iPSC-derived PRPCs from the rd16 mouse model.
Figure 4. Lentivirus expressing full-length CEP290 transduces photoreceptor precursor cultures.

(a) RT-PCR (in duplicate) of LV-hCEP290-transduced rd16 photoreceptor precursor cultures at a multiplicity of infection (MOI) of 1 and 2. (b) RT-PCR in photoreceptor precursor cells from B294 and B062 transduced with LV-CEP290. (c) Western blot of photoreceptor precursor cells from B294 transduced with LV-CEP290. Untx – untransduced; G – GFP lentiviral transduced/negative control; LV – CEP290 lentiviral transduced; 1 & 2 - biological replicates; NT - no template; P – plasmid/positive control; M – marker.
Next we sought to verify lentiviral transduction and wild type CEP290 expression in iPSC-derived PRPCs from human patients with molecularly confirmed CEP290-associated LCA. Cultures from two patients were transduced at day 90 of the differentiation protocol with LV-CMV-hCEP290. One week following transduction, we evaluated transgene expression via rt-PCR. To differentiate between endogenous and vector-derived transcript, we used primers complimentary to the 3’ end of the transcript and the downstream WPRE element that is not present in the endogenous human transcriptome. As with the murine cells, we were able to detect vector-mediated transcript in cultures transduced with LV-CMV-hCEP290 but not in the untransduced control cultures (Fig. 4B). Western blots probed with anti-CEP290 antibody demonstrated that in contrast to untransduced cultures, LV-CMV-hCEP290-transduced PRPCs expressed full- length CEP290 protein (indicated by the presence of a 290 kDa band in both retinal and transduced cell lysates, Fig. 4C). Taken together, these results indicate the feasibility of packaging the large CEP290 transgene into lentiviral vectors and using these vectors to transduce iPSC-derived photoreceptor precursor cells affected with CEP290-associated LCA.
Lentiviral-mediated CEP290 gene addition rescues the ciliogenesis defect in LCA patient derived dermal fibroblasts
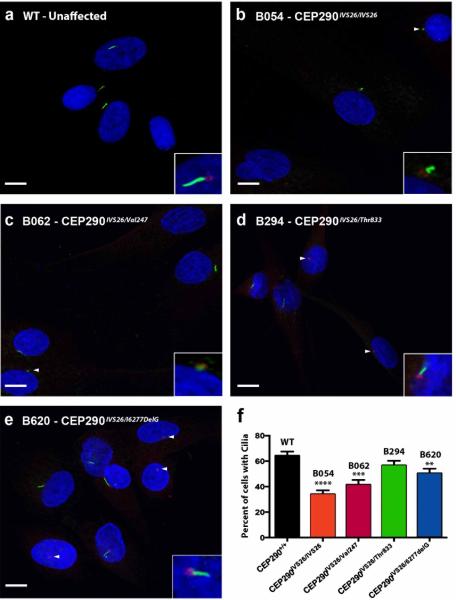
CEP290 plays a critical role in primary cilium formation and ciliary protein transport6,7,26. Knockdown experiments in hTERT-RPE cells showed that loss of CEP290 inhibited primary cilium formation26. To investigate the ciliogenesis phenotype in LCA patient-specific cells, fibroblasts from four patients with different CEP290 genotypes and an unaffected control individual were cultured under serum-free conditions. Primary cilia and basal bodies were detected via immunocytochemical staining with antibodies targeted against acetylated tubulin (which labels the axonemes of primary cilia) and gamma tubulin (which labels the basal bodies of primary cilia), respectively (Figs 5A-E). Counting cells from confocal images revealed significantly fewer cells with cilia in LCA patient cultures B054 (34.1% ± 3.0%), B062 (41.6% ± 3.7%), and B620 (50.5% ± 3.6%) when compared to those of an unaffected control individual (WT - 64.4% ± 3.2%) (Fig. 5F). Although cells isolated from patient B294 produced fewer cilia (56.7% ± 3.6%) than those isolated from an unaffected individual, this difference did not reach statistical significance. Gamma tubulin positive basal bodies with no axoneme or stunted/dismorphic axonemes were often identified in patient specific cells (Figs. 5B-E, arrowheads and high magnification inlays).
Figure 5. CEP290 mutations induce a ciliogenesis defect.
(a-e) Immunocytochemical analysis of human fibroblast cultures isolated from an unaffected control individual (a) and 4 patients with CEP290-associated LCA (be). Basal bodies were detected with anti-gamma tubulin (red),primary cilia with anti-acetylated tubulin (green; insets), and nuclei with DAPI (blue). Images were collected with 100X high performance objective. (f) Bars represent the percentage of cells that were positive for acetylated tubulin. Error bars represent standard error (n ≥ 6 cultures). ** p < 0.01, *** p < 0.001, **** p < 0.0001. WT-unaffected control individual, B054 – patient with homozygous IVS26 mutations, B062 – patient with IVS26 mutation and Val247del1gT frame shift mutation, B294 patients with IVS26 mutation and Thr833 del2acAG frame shift mutation, B620 – patient with IVS26 mutation and 6277delG frame shift mutation.
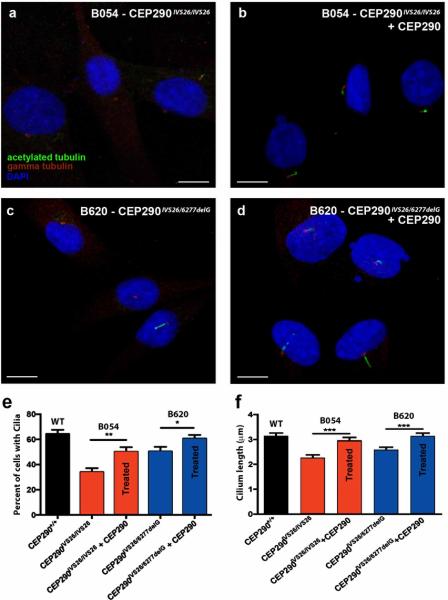
To determine if CEP290 gene replacement would rescue the disease-specific ciliogenesis phenotype, fibroblast cultures B054, B062 and B620 (the samples with statistically significant ciliogenesis defects), were targeted. Fibroblast cultures were transduced with LV-CMV-hCEP290. As above, the transduced cultures were subjected to serum-starvation to induce primary cilium formation. The percentage of ciliated cells in transduced cultures B054 (Figs. 6A and B, 34.1% ± 3.0% and 50.2% ± 3.6%, respectively) and B620 (Figs. 6C and D, 50.5% ± 3.6% and 60.7% ± 2.8%, respectively) significantly increased with respect to untransduced cells from the same individuals (Fig. 6E, p < 0.01 and p < 0.05, respectively). Moreover, the percentage of cells forming cilia in transduced B620 cultures was no longer significantly different from those of the unaffected control. Although, transduction of B062 increased the percentage of cells forming a cilium compared to untransduced B062 controls (48.7% ± 3.4% versus 41.6% ± 3.7%, respectively), the difference was not statistically significant (data not shown).
Figure 6. CEP290 gene addition rescues ciliogenesis defect.
(a-d) Immunocytochemical analysis of human fibroblast cultures isolated from patient B054 (a, b) and patient B620 (c, d) either untreated (a and c) or transduced with a lentiviral vector expressing full-length CEP290 at an MOI of 2 (b and d). Basal bodies were detected with anti-gamma tubulin (red) and primary cilia were detected with anti-acetylated tubulin (green). Nuclei were stained with DAPI (blue). Images were collected with 100X high performance objective. (e) Graphical representation of percentage of cells with a cilium in each culture. Error bars represent standard error (n ≥ 6 cultures). (f) Graphical representation of cilium length in each culture (n ≥ 100 cells). * p < 0.05, ** p < 0.01, *** p < 0.001. MOI – multiplicity of infection. DAPI – 4’,6-diamidino-2-phenylindole.
To further test the efficacy of our gene transfer strategy, the length of the primary cilia, both pre- and post-transduction of lines B054 and B620, was performed (primary cilia were measured from the base, at the basal body, to the tip of the axoneme). As shown in Fig. 6F, when cilia lengths were measured, transduced cultures demonstrated significantly longer cilia than their untransduced counterparts. Furthermore, the length of the primary cilia post-transduction was not significantly different from that of the unaffected control. Taken together, these results demonstrate that lentiviral-mediated addition of full-length CEP290 is able to rescue the abnormal ciliogenesis phenotype in cells from patients with CEP290-associated LCA.
Discussion
The restoration of vision in patients affected with RPE65-associated Leber congenital amaurosis via gene replacement therapy12,13,21 was an encouraging milestone for all patients affected with an inherited retinal disease. However, many heritable retinal disorders present two additional challenges: 1) a gene that is too large to package into AAV and 2) a gene product encoding a structural protein that must be expressed in appropriate proportion to other components to function properly. CEP290-associated LCA is such a disease. The CEP290 coding sequence is ~8 kb in length, which precludes AAV as a treatment vehicle for the full-length cDNA. In addition, CEP290 is known to physically interact with numerous components within the transition zone of primary cilia6,25-27. Thus, one might reasonably expect to find – as we did in this study -- that a favorable response to CEP290 gene replacement would only occur within a fairly narrow range of gene expression.
We took advantage of the relatively large packaging capacity of lentivirus [8-10 kb15], as well as its ability to stably transduce post-mitotic retinal cells, to develop a viral vector capable of delivering the full-length CEP290 coding sequence to both patient-specific fibroblasts and iPSC-derived PRPCs. Although we were able to demonstrate vector-mediated expression of CEP290 mRNA in JK1 cells transduced with high concentrations of the vector, these cells exhibited poor viability compared to those transduced with an equal MOI of GFP-expressing vector, suggesting that cell death occurred as a result of CEP290 overexpression. Of note, we have also observed toxicity in mice in which another cilia-associated protein, BBS1, was overexpressed28. Our observations agree with previous studies that found photoreceptors to be sensitive to the level of transgene expression. For example, in oxygen-induced retinopathy, overexpression of claudin transmembrane proteins results in mislocalization to the cytosolic compartment and neovascularization29. Similarly, a five-fold overexpression of the human rhodopsin allele in mice causes photoreceptor degeneration similar to that found in retinitis pigmentosa30. Tan and colleagues also reported a rhodopsin-dose-dependent degenerative phenotype in transgenic mice31.
Kim and colleagues reported that hTERT-RPE1 cells failed to form cilia when CEP290 was depleted via siRNA26. Furthermore, the rd16 mouse model for CEP290-associated LCA is deficient in primary cilia formation7. Thus, we hypothesized that patients with mutations in CEP290 would exhibit a ciliogenesis defect. Indeed, serum-starved fibroblasts from three of four LCA patients formed significantly fewer and shorter primary cilia than those from an unaffected control individual. Importantly, when we treated these cells with LV-CMV-hCEP290, we rescued both the number of cells forming a cilium and the length of the cilia in two of three patients tested, highlighting the fact that different genotypes and genetic backgrounds can result in different levels of cellular pathology. At present, the mechanism by which various disease allele combinations contribute to LCA phenotype(s) is incompletely understood. However, Drivas and colleagues recently identified functional domains in CEP290 that contribute to membrane and microtubule binding as well as ciliogenesis7. One of the alleles carried by patient B062 expresses a truncation mutation in the N-terminal domain. The resulting protein is missing the C-terminal microtubule binding domain [amino acids 1695-19667] and is associated with failure of cilia formation in these cells in vitro. These observations are in agreement with previous results from the rd16 mouse model implicating the requirement of this domain for the formation and maintenance of primary cilia in photoreceptors4,7. Although delivery of full-length CEP290 trended towards restoration of ciliogenesis in this patient, the number of cilia did not increase significantly in treated cells. Perhaps other factors play a role in the pathogenesis of the disease. Indeed, variations in the MKKS and BBS genes have been reported to modify the CEP290 phenotype32,33. Nonetheless, demonstrating rescue of ciliogenesis is an important step in treating these and other patients with CEP290-associated LCA. When culturing photoreceptor precursor cells in vitro, cilia formation is difficult to appreciate. Acetylated tubulin-positive structures can be identified in densely-packed neural rosettes20, however tracing these structures back to cell of origin is problematic. Thus, transplantation of corrected photoreceptor precursor cells will be an important future study to determine functional rescue in these cells as full maturation and cilia formation does occur post-transplantation.
Although careful titration of LV-CMV-hCEP290 allowed us to avoid the adverse effects of transgene overexpression in vitro, the manipulation of viral titer alone may not be practical for clinical applications. It will likely be necessary to deliver a very specific amount of CEP290 protein to a specific cell type, such as the photoreceptors. This may be achieved by using the endogenous CEP290 promoter to drive expression of CEP290 cDNA. However, for many genes, like CEP290, the endogenous promoter elements have not yet been identified. Thus, the development of a gene transfer approach in which the transgene is driven under control of its native promoter will require further investigation. An alternative approach that would allow gene transfer-based treatment of a wide variety of retinal degenerative diseases would be to develop a library of synthetic promoters which would each be capable of a very specific level of transgene expression in a specific retinal cell type.
We predict that the effectiveness of any gene therapy approach for the treatment of CEP290-associated LCA will also be highly dependent upon both the severity of the patients’ genotypes and the resultant degree of photoreceptor cell loss at the time treatment is undertaken. In some patients, foveal cones are spared well into adulthood10,34 and these cones are sufficient to subserve very useful vision. For others, these cells never seem to function even though they can be seen by optical coherence tomography (e.g., patient 110). For the latter individuals, delivery of full length CEP290 directly to foveal cones may be useful.
Although lentivirus is an effective vector system for packaging cDNAs as large as 8 kb, for genes larger than CEP290 a different vector system will be required. However, it may be possible to adapt the transgene cassette design of the current study to other vector systems to allow the efficient construction of constructs that exceed lentivirus and AAV packaging limits with a wide variety of promoters. For example, the herpes simplex virus vector system can accommodate inserts up to ~150 kb35 and can easily transduce dividing and post-mitotic cell types without eliciting an immunological response36,37.
Lentivirus integrates into the genomic DNA of transduced cells and thus has the potential for insertional mutagenesis38. This is somewhat less of a concern for retinal therapy than it would be for treating cells of other organs or the hematopoietic system because the retina can be readily inspected at microscopic resolution after treatment. If any transduced cells are found to exhibit abnormal growth, they can be destroyed with laser photocoagulation or cryotherapy without harm to the remainder of the eye. Moreover, the continued development of deep-sequencing technologies will enable the safety of specific constructs to be assessed by surveying the genome-wide distribution of vector integration39-41.
The difficulties associated with large cargo size, the need for precise gene expression, and the concerns associated with insertional mutagenesis may all be overcome if genome-editing technologies can be perfected. Transcription activator-like effector nucleases (TALENs)42,43 and Clustered Regularly Interspersed Short Palindromic Repeats (CRISPRs)44,45 can be engineered to direct gene correction at specific sites in the genome and may prove useful for in vivo gene correction as well as ex vivo correction of iPSCs46-48 prior to transplantation into diseased tissues.
In summary, we have shown that a lentiviral construct is capable of transducing both mouse and human cells with full-length CEP290 cDNA and that this transduction results in expression of full-length transcript and functional rescue of the ciliogenesis defect in patient cells. Although this work brings us one step closer to gene- and cell-based treatments of CEP290-associated LCA, strategies for ensuring the proper level of CEP290 expression in treated cells must still be devised before extending this work to human patients.
Materials and Methods
Patients
All patients provided written, informed consent for this study, which was approved by the Institutional Review Board of the University of Iowa (project approval #200202022) and adhered to the tenets set forth in the Declaration of Helsinki. Patient B054 carries two alleles with the most common mutation, IVS26 c.2991 + 1655 A>G (IVS26). Patient B062 carries IVS26 c.2991 + 1655 A > G on one allele and a one-base pair deletion in the coding sequence at amino acid position 247 (Val247 del1gT) on the other allele. Patient B294 also harbors two heterozygous mutations at the CEP290 locus. One allele carries the IVS26 mutation, the other allele carries a two-base pair deletion in the coding sequence at amino acid position 835 (Thr835 del2acAG). Patient B620 carries the IVS26 mutation on one CEP290 allele and an intronic deletion at nucleotide 6277 (6277delG).
Cell culture
We chose to test vector toxicity on the easily-propagated, fibroblast-like JK1 cell line because patient fibroblasts are in limited supply. Furthermore, differentiating photoreceptor precursor cell cultures exhibit differentiation-induced apoptosis, making cell death assays difficult to perform in a convincing fashion. JK1 cells (Cell Biolabs) and patient fibroblasts were cultured in complete medium [MEMα (Life Technologies) containing 10% Fetal Bovine Serum (Life Technologies) and 0.2% Primocin™ (Invivogen)].
Lentiviral transduction
JK1 cells (Cell Biolabs, San Diego, CA) were transduced with lentivirus expressing human CEP290 at a multiplicity of infection of 1, 2, and 5 in the presence of polybrene (Sigma-Aldrich, St. Louis, MO) for six hours in MEMα (Life Technologies). Differentiated photoreceptor precursor cultures were transduced with 1 × 105 or 2 × 105 transducing units. Following transduction, cells were cultured in complete medium for five days.
Characterization of CEP290 expression
rt-PCR
Total RNA was isolated and amplified as described above using the forward primer GCAATGAGCGACTTTTCATCAGAC and the reverse primer ACAACACCACGGAATTGTCAGTGC. POLR2A transcripts were amplified as an internal control (Tables S1 and S2).
Immunoblotting
Human retina was obtained from the Iowa Lions Eye Bank after informed consent of the donors’ next of kin and was prepared for immunoblotting as described previously19. Differentiated human photoreceptor precursor cell cultures from patient B294 at day 60 were transduced with 2 × 105 TU LV-CMV-CEP290. Control cultures were left untransduced. One week post-transduction, cells were treated with 0.25% Trypsin-EDTA (Gibco), homogenized in lysis buffer [50 mM Tris-HCl, pH 7.6, 150 mM NaCl, 10mM CaCl2, 1% triton X-100, 0.02% NaN3, (Sigma Aldrich)] and centrifuged. Supernatant protein concentrations were determined via BCA according to manufacturer's instructions (Pierce, Rockford, IL). Fifty micrograms each were subjected to SDS-PAGE (4-20% acrylamide), transferred to PVDF, and probed with anti-human CEP290 (Abcam, Cambridge, England; 1:1000). Blots were visualized with ECL reagents (GE Healthcare Life Sciences, Pittsburgh, PA) and exposed to X-ray film (Fisher Scientific, Pittsburgh, PA).
Quantification of cell death
JK1 cells were plated at a density of 2.5 × 105 cells per well. Sixteen hours after plating, cells were transduced with VSVG-LV-CMV-hCEP290 vector at MOIs of 1, 2, or 5. Control cultures were transduced at an MOI of 5 with VSVG-LV-CMVGFP or left untreated. Five days after transduction, cells were dissociated with 0.05% Trypsin-EDTA (Life Technologies) and cell death was quantified using the Tali® Apoptosis Kit – Annexin V Alexa Fluor® 488 and Propidium Iodide (Life Technologies) and the Tali® Image-based Cytometer (Life Technologies) according to manufacturer's instructions.
iPSC generation
Animals used in these studies were cared for in accordance with the Institutional Animal Care and Use Committee (University of Iowa, Iowa City, IA; approval #130815). Murine dermal fibroblasts from the BXD24/TyJ-Cep290rd16/J mouse4 (The Jackson Laboratory, Bar Harbor, ME) and human dermal fibroblasts from LCA patients with mutations in CEP290 were reprogrammed via viral transduction of the transcription factors OCT4, SOX2, KLF4, and cMYC as previously described 19,24. Rd16-derived iPSC colonies were cultured in pluripotency medium [(DMEM/F12; Life Technologies), 15% heat-inactivated fetal bovine serum (Lifeblood Medical Inc., Adelphia, NJ), 0.0008% β-mercaptoethanol (Sigma-Aldrich), 1X nonessential amino acids (NEAA; Life Technologies), 1 × 106 units/L leukemia inhibitory factor (LIF; Millipore, Billerica, MA), 0.2% Primocin™ (Invivogen)] on Matrigel™-coated plates (BD Biosciences, Franklin Lakes, NJ). Human LCA iPSCs were cultured in mTser1 media on Corning® Synthemax™-coated plates (Corning, Inc.).
iPSC characterization
rt-PCR
Total RNA was isolated from passage 10 iPSCs using the RNeasy Mini kit (Qiagen, Germantown, MD) according to manufacturer's instructions. One-hundred nanograms of RNA template was amplified in one-step rt-PCR reactions using the Superscript III One-Step RT-PCR System (Life Technologies) with primers hybridizing to NANOG, OCT4, KLF4, DNMT1, and LIN82A transcripts from mouse (Table S1) or human (Table S2).
Teratoma formation
Severe combined immunodeficiency mice (SCID; Jackson Laboratories) were used to assess the ability of iPSCs to form teratomas containing tissues of all three germ layers. Approximately 2 × 106 cells were resuspended in 100 μl Hank's Buffered Saline Solution (HBSS; Life Technologies) and injected intramuscularly into the rear limb of SCID hosts. Six to 8 weeks following injection, tumors were resected and analyzed for the presence of tissues of ectodermal, mesodermal and endodermal origin.
Histology
Teratomas were fixed in 10% formalin, embedded in paraffin, sectioned, and counterstained with hemotoxylin and eosin using standard protocols.
Immunocytochemistry
Teratoma sections were de-paraffinized, fixed in 4% paraformaldehyde, and stained with antibodies targeted against alpha fetoprotein (R & D Systems, Minneapolis, MN; 1:100), glial fibrillary acidic protein (GFAP; Life Technologies; 1:100), and smooth muscle actin (Abcam; 1:100). Slides were coverslipped as described above and imaged on an EVOS fluorescent microscope (Life Technologies).
iPSC differentiation
Rd16 and LCA patient-specific iPSCs were cultured on ultra low-binding plates (Corning Life Sciences, Tewksbury, MA) in embryoid body formation medium [DMEM F-12 (Life Technologies), 10% knockout serum replacement (Life Technologies), 2% B27 supplement (Life technologies), 1% N2 supplement (Life Technologies), 1% L-glutamine (Life Technologies), 1X NEAA (Life Technologies), 0.2% Primocin™ (Invivogen), 1 ng/ml Dkk-1 (R&D Systems), 1 ng/ml IGF-1 (R&D Systems), 1 ng/ml Noggin (R&D Systems) and 0.5 ng/ml bFGF (R&D Systems)] for 4-5 days. Embryoid bodies (200-300/well) were plated on 6-well plates (Corning) coated with collagen (BD Bioscience, San Jose, CA; 25 ug/ml), laminin (Life Technologies; 50 ug/ml), and fibronectin (Sigma-Aldrich; 100 ug/ml) and cultured in differentiation medium one [DMEM F-12 (Life Technologies), 2% B27 supplement (Life Technologies), 1% N2 supplement (Life Technologies), 1% L-Glutamine (Life Technologies), 1X NEAA (Life Technologies), 0.2% Primocin™ (Invivogen), 10 ng/ml Dkk-1 (R&D Systems), 10 ng/ml IGF-1 (R&D Systems), 10 ng/ml Noggin (R&D Systems) and 5 ng/ml bFGF (R&D Systems)]. Embryoid bodies were differentiated for ten days followed by six days in differentiation medium two [(differentiation media one plus 10 uM DAPT (Calbiochem, Gibbstown, NJ)] and an additional 12 days of culture in differentiation medium three [(differentiation media two plus 2 ng/ml aFGF (R & D Systems)]. Human photoreceptor precursors were cultured an additional 60 days in differentiation medium four [DMEM F-12 (Life Technologies), 2% B27 supplement (Life Technologies), 1% N2 supplement (Life Technologies), 1% LGlutamine (Life Technologies), 1X NEAA (Life Technologies), 0.2% Primocin™ (Invivogen)].
Differentiation characterization
rt-PCR
Total RNA was isolated and amplified as described above using primers hybridizing to OTX2, OPSN1SW, and ROM1 transcripts (Table S3).
Immunocytochemistry
iPSC-derived photoreceptor precursor cells from the rd16 mouse model were fixed, stained, and imaged as described above with antibodies targeted against Otx2 (R & D Systems; 1:100) and Opsn1sw (EMD Millipore, Billerca, MA; 1:100). Cells from LCA patients were stained with antibodies targeted against OTX2 (EMD Millipore; 1:100) and OPSN1SW (EMD Millipore; 1:100).
Ciliogenesis in patient fibroblast cultures
Fibroblasts from four patients (B054, B062, B294, and B620) and an unaffected control individual were cultured on collagen-coated chamber slides in serum-free conditions [MEMα (Life Technologies), 2% v/v Primocin (Life Technologies)] for 72 hours. Cells were fixed in 4% paraformaldehyde and methanol and stained with antibodies targeted against acetylated tubulin (1:200, Sigma-Aldrich) and gamma tubulin (Sigma-Aldrich; 1:1000). Slides were coverslipped with Poly(vinyl alcohol) (PVA)-based mounting medium containing 1,4-Diazabicyclo[2.2.2]octane (DABCO) (100 μg/ml PVA, Sigma-Aldrich; 25% v/v glycerol, Sigma-Aldrich; 0.1M Tris-HCl, pH 8-8.5, 25 μg/ml DABCO, Sigma-Aldrich) and 4’,6-Diamidino-2-phenylindole dihydrochloride (DAPI) (Sigma-Aldrich; 1:10,000).
Ciliogenesis rescue in patient fibroblast cultures
Fibroblasts from patients B054, B062, B294, and B620 were transduced with lentivirus expressing CEP290 at a multiplicity of infection of 2 in the presence of polybrene (Sigma-Aldrich) for six hours in MEMα (Life Technologies) and cultured in complete medium for six days. Five thousand transduced cells were passaged into each well of collagen-coated 16-well chamber slides. The following day, cells were cultured in serum-free conditions [MEMα (Life Technologies), 0.2% Primocin™ (Life Technologies)] for 72 hours. Cells were fixed and stained for acetylated tubulin and gamma tubulin as indicated above.
Confocal microscopy analysis of ciliogenesis
Following ciliogenesis and antibody labeling of patient fibroblasts, the slides were coded to eliminate any bias in the experiment. Cells and cilia were imaged and then counted by two individuals, both of whom were masked to the identity of the treatment groups. Cilia were imaged using a Leica DM 2500 SPE confocal microscope (Leica Microsystems, Wetzlar, Germany). Fields of cells were found using DAPI followed by imaging acetylated and gamma tubulin channels. Primary cilia were counted as elongated or punctate acetylated tubulin-positive structures localized to nuclei or immediately perinuclear.
Quantification of Cilia Length
Cilia were imaged via confocal microscopy as described above using a 63X high-performance objective. Cilia were measured using Leica LAS-AF, Version 3.2.0 software (Leica Microsystems). Briefly, images were magnified and cilia (n ≥ 100) were measured using the scale bar tool specified for the 63X objective. Cilia displaying clear labeling of gamma tubulin (basal body) at the base and acetylated tubulin (axoneme) extending from the basal body were measured.
Statistical analysis
Pairwise significance was determined using two-tailed t-test in GraphPad Prism software. P ≤ 0.05 was considered significant. Data points laying outside of 1.5 times the interquartile range were excluded from analysis.
Supplementary Material
Acknowledgments
We are grateful for the assistance of The University of Iowa Gene Transfer Vector Core in production of the lentiviral vector. We thank members of the Iowa Eye Interest Group for helpful discussions. We are grateful for financial support from the following: Directors New Innovator Award 1-DP2-OD007483-01; NEI EY017451; 1F32-EY022834-01; HHMI; Foundation Fighting Blindness; Stephen A. Wynn Foundation; Grousbeck Family Foundation; Leo, Jacques & Marion Hauser Family Vision Restoration Fund. The authors declare no conflicts of interest with the submission of this work.
Footnotes
Supplementary information is available at Gene Therapy's website.
References
- 1.Coppieters F, Lefever S, Leroy BP, De Baere E. CEP290, a gene with many faces: mutation overview and presentation of CEP290base. Hum Mutat. 2010;31:1097–108. doi: 10.1002/humu.21337. [DOI] [PubMed] [Google Scholar]
- 2.Stone EM. Leber congenital amaurosis - a model for efficient genetic testing of heterogeneous disorders: LXIV Edward Jackson Memorial Lecture. Am. J. Ophthalmol. 2007;144:791–811. doi: 10.1016/j.ajo.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 3.Perrault I, Delphin N, Hanein S, Gerber S, Dufier J-L, Roche O, et al. Spectrum of NPHP6/CEP290 mutations in Leber congenital amaurosis and delineation of the associated phenotype. Hum Mutat. 2007;28:416. doi: 10.1002/humu.9485. [DOI] [PubMed] [Google Scholar]
- 4.Chang B, Khanna H, Hawes N, Jimeno D, He S, Lillo C, et al. In-frame deletion in a novel centrosomal/ciliary protein CEP290/NPHP6 perturbs its interaction with RPGR and results in early-onset retinal degeneration in the rd16 mouse. Hum Mol Genet. 2006;15:1847–57. doi: 10.1093/hmg/ddl107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sayer JA, Otto EA, O'Toole JF, Nurnberg G, Kennedy MA, Becker C, et al. The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat Genet. 2006;38:674–81. doi: 10.1038/ng1786. [DOI] [PubMed] [Google Scholar]
- 6.Craige B, Tsao C-C, Diener DR, Hou Y, Lechtreck K-F, Rosenbaum JL, et al. CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. J Cell Biol. 2010;190:927–40. doi: 10.1083/jcb.201006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drivas TG, Holzbaur ELF, Bennett J. Disruption of CEP290 microtubule/membrane-binding domains causes retinal degeneration. J Clin Invest. 2013;123:4525–39. doi: 10.1172/JCI69448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsang WY, Bossard C, Khanna H, Peränen J, Swaroop A, Malhotra V, et al. CP110 suppresses primary cilia formation through its interaction with CEP290, a protein deficient in human ciliary disease. Dev Cell. 2008;15:187–97. doi: 10.1016/j.devcel.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McEwen DP, Koenekoop RK, Khanna H, Jenkins PM, Lopez I, Swaroop A, et al. Hypomorphic CEP290/NPHP6 mutations result in anosmia caused by the selective loss of G proteins in cilia of olfactory sensory neurons. Proc Natl Acad Sci USA. 2007;104:15917–22. doi: 10.1073/pnas.0704140104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cideciyan AV, Aleman TS, Jacobson SG, Khanna H, Sumaroka A, Aguirre GK, et al. Centrosomal-ciliary gene CEP290/NPHP6 mutations result in blindness with unexpected sparing of photoreceptors and visual brain: implications for therapy of Leber congenital amaurosis. Hum Mutat. 2007;28:1074–83. doi: 10.1002/humu.20565. [DOI] [PubMed] [Google Scholar]
- 11.Cideciyan AV, Rachel RA, Aleman TS, Swider M, Schwartz SB, Sumaroka A, et al. Cone photoreceptors are the main targets for gene therapy of NPHP5 (IQCB1) or NPHP6 (CEP290) blindness: generation of an all-cone Nphp6 hypomorph mouse that mimics the human retinal ciliopathy. Hum Mol Genet. 2011;20:1411–23. doi: 10.1093/hmg/ddr022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bainbridge JWB, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358:2231–9. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 13.Hauswirth WW, Aleman TS, Kaushal S, Cideciyan AV, Schwartz SB, Wang L, et al. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther. 2008;19:979–90. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maguire AM, High KA, Auricchio A, Wright JF, Pierce EA, Testa F, et al. Age-dependent effects of RPE65 gene therapy for Leber's congenital amaurosis: a phase 1 dose-escalation trial. Lancet. 2009;374:1597–605. doi: 10.1016/S0140-6736(09)61836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balaggan KS, Ali RR. Ocular gene delivery using lentiviral vectors. Gene Ther. 2012;19:145–53. doi: 10.1038/gt.2011.153. [DOI] [PubMed] [Google Scholar]
- 16.Kong J, Kim S-R, Binley K, Pata I, Doi K, Mannik J, et al. Correction of the disease phenotype in the mouse model of Stargardt disease by lentiviral gene therapy. Gene Ther. 2008;15:1311–20. doi: 10.1038/gt.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verrier JD, Madorsky I, Coggin WE, Geesey M, Hochman M, Walling E, et al. Bicistronic lentiviruses containing a viral 2A cleavage sequence reliably co-express two proteins and restore vision to an animal model of LCA1. PLoS ONE. 2011;6:e20553. doi: 10.1371/journal.pone.0020553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tucker BA, Park I-H, Qi SD, Klassen HJ, Jiang C, Yao J, et al. Transplantation of adult mouse iPS cell-derived photoreceptor precursors restores retinal structure and function in degenerative mice. PLoS ONE. 2011;6:e18992. doi: 10.1371/journal.pone.0018992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tucker BA, Scheetz TE, Mullins RF, DeLuca AP, Hoffmann JM, Johnston RM, et al. Exome sequencing and analysis of induced pluripotent stem cells identify the cilia-related gene male germ cell-associated kinase (MAK) as a cause of retinitis pigmentosa. Proc Natl Acad Sci USA. 2011;108:E569–76. doi: 10.1073/pnas.1108918108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tucker BA, Mullins RF, Streb LM, Anfinson K, Eyestone ME, Kaalberg E, et al. Patient-specific iPSC-derived photoreceptor precursor cells as a means to investigate retinitis pigmentosa. eLife. 2013;2:e00824. doi: 10.7554/eLife.00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maguire AM, Simonelli F, Pierce EA, Pugh EN, Mingozzi F, Bennicelli J, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240–8. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar M, Keller B, Makalou N, Sutton RE. Systematic determination of the packaging limit of lentiviral vectors. Hum Gene Ther. 2001;12:1893–905. doi: 10.1089/104303401753153947. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 24.Tucker BA, Anfinson KR, Mullins RF, Stone EM, Young MJ. Use of a synthetic xeno-free culture substrate for induced pluripotent stem cell induction and retinal differentiation. Stem Cells Transl Med. 2013;2:16–24. doi: 10.5966/sctm.2012-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murga-Zamalloa CA, Ghosh AK, Patil SB, Reed NA, Chan LS, Davuluri S, et al. Accumulation of the Raf-1 kinase inhibitory protein (Rkip) is associated with Cep290-mediated photoreceptor degeneration in ciliopathies. J Biol Chem. 2011;286:28276–86. doi: 10.1074/jbc.M111.237560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim J, Krishnaswami SR, Gleeson JG. CEP290 interacts with the centriolar satellite component PCM-1 and is required for Rab8 localization to the primary cilium. Hum Mol Genet. 2008;17:3796–805. doi: 10.1093/hmg/ddn277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rachel RA, Li T, Swaroop A. Photoreceptor sensory cilia and ciliopathies: focus on CEP290, RPGR and their interacting proteins. Cilia. 2012;1:22. doi: 10.1186/2046-2530-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seo S, Mulins RF, Dumitrescu AV, Bhattarai S, Gratie D, Wang K, et al. Subretinal gene therapy of mice with Bardet-Biedl Syndrome type 1. Invest Ophthalmol Vis Sci. doi: 10.1167/iovs.13-11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo Y, Xiao W, Zhu X, Mao Y, Liu X, Chen X, et al. Differential expression of claudins in retinas during normal development and the angiogenesis of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2011;52:7556–64. doi: 10.1167/iovs.11-7185. [DOI] [PubMed] [Google Scholar]
- 30.Olsson JE, Gordon JW, Pawlyk BS, Roof D, Hayes A, Molday RS, et al. Transgenic mice with a rhodopsin mutation (Pro23His): a mouse model of autosomal dominant retinitis pigmentosa. Neuron. 1992;9:815–30. doi: 10.1016/0896-6273(92)90236-7. [DOI] [PubMed] [Google Scholar]
- 31.Tan E, Wang Q, Quiambao AB, Xu X, Qtaishat NM, Peachey NS, et al. The relationship between opsin overexpression and photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2001;42:589–600. [PubMed] [Google Scholar]
- 32.Rachel RA, May-Simera HL, Veleri S, Gotoh N, Choi BY, Murga-Zamalloa C, et al. Combining Cep290 and Mkks ciliopathy alleles in mice rescues sensory defects and restores ciliogenesis. J Clin Invest. 2012;122:1233–45. doi: 10.1172/JCI60981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Seo S, Bhattarai S, Bugge K, Searby CC, Zhang Q, et al. BBS mutations modify phenotypic expression of CEP290-related ciliopathies. Hum Mol Genet. 2013 doi: 10.1093/hmg/ddt394. doi:10.1093/hmg/ddt394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pasadhika S, Fishman GA, Stone EM, Lindeman M, Zelkha R, Lopez I, et al. Differential macular morphology in patients with RPE65-, CEP290-, GUCY2D-, and AIPL1-related Leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2010;51:2608–14. doi: 10.1167/iovs.09-3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fraefel C, Marconi P, Epstein AL. Herpes simplex virus type 1-derived recombinant and amplicon vectors. Methods Mol Biol. 2011;737:303–43. doi: 10.1007/978-1-61779-095-9_13. [DOI] [PubMed] [Google Scholar]
- 36.Manservigi R, Argnani R, Marconi P. HSV Recombinant Vectors for Gene Therapy. Open Virol J. 2010;4:123–56. doi: 10.2174/1874357901004010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neve RL. Overview of gene delivery into cells using HSV-1-based vectors. Curr Protoc Neurosci. 2012 doi: 10.1002/0471142301.ns0412s61. Chapter 4: Unit4.12. [DOI] [PubMed] [Google Scholar]
- 38.Hacein-Bey-Abina S, Kalle Von C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–9. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 39.Ronen K, Negre O, Roth S, Colomb C, Malani N, Denaro M, et al. Distribution of lentiviral vector integration sites in mice following therapeutic gene transfer to treat β-thalassemia. Mol Ther. 2011;19:1273–86. doi: 10.1038/mt.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bartholomae CC, Glimm H, Kalle von C, Schmidt M. Insertion site pattern: global approach by linear amplification-mediated PCR and mass sequencing. Methods Mol Biol. 2012;859:255–65. doi: 10.1007/978-1-61779-603-6_15. [DOI] [PubMed] [Google Scholar]
- 41.Arens A, Appelt J-U, Bartholomae CC, Gabriel R, Paruzynski A, Gustafson D, et al. Bioinformatic clonality analysis of next-generation sequencing-derived viral vector integration sites. Hum Gene Ther Methods. 2012;23:111–8. doi: 10.1089/hgtb.2011.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moscou MJ, Bogdanove AJ. A simple cipher governs DNA recognition by TAL effectors. Science. 2009;326:1501. doi: 10.1126/science.1178817. [DOI] [PubMed] [Google Scholar]
- 43.Cermak T, Doyle EL, Christian M, Wang L, Zhang Y, Schmidt C, et al. Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res. 2011;39:e82. doi: 10.1093/nar/gkr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–23. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, et al. RNA-guided human genome engineering via Cas9. Science. 2013;339:823–6. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hockemeyer D, Wang H, Kiani S, Lai CS, Gao Q, Cassady JP, et al. Genetic engineering of human pluripotent cells using TALE nucleases. Nat Biotechnol. 2011;29:731–4. doi: 10.1038/nbt.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ding Q, Lee Y-K, Schaefer EAK, Peters DT, Veres A, Kim K, et al. A TALEN genome-editing system for generating human stem cell-based disease models. Cell Stem Cell. 2013;12:238–51. doi: 10.1016/j.stem.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;153:910–8. doi: 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.