Abstract
Background
Approximately 45% of nongonococcal urethritis cases have no identified etiology. Novel bacteria recently associated with bacterial vaginosis (BV) in women may be involved. We evaluated the association of idiopathic nongonococcal urethritis and 5 newly described BV-associated bacteria (BVAB).
Methods
Heterosexual men 16 years or older attending a sexually transmitted disease clinic in Seattle, Washington, from May 2007 to July 2011 and negative for Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis, Mycoplasma genitalium, and Ureaplasma urealyticum–biovar2 were eligible. Cases had urethral discharge or 5 or more polymorphonuclear leukocytes per high-power field in urethral exudates. Controls had no urethral discharge and less than 5 polymorphonuclear leukocytes per high-power field. Urine was tested for Atopobium spp., BVAB-2, BVAB-3, Megasphaera spp., and Leptotrichia/Sneathia spp. using quantitative taxon-directed polymerase chain reaction.
Results
Cases (n = 157) and controls (n = 102) were of similar age, education, and income, and most were white. Leptotrichia/Sneathia spp. was significantly associated with urethritis (24/157 [15.3%] vs. 6/102 [5.9%], P = 0.03). BVAB-2 was more common in cases than in controls (7/157 [4.5%] vs. 1/102 [1.0%], P = 0.15), and BVAB-3 (n = 2) and Megasphaera spp. (n = 1) were only detected in men with urethritis, but these bacteria were found only in men who also had Leptotrichia/Sneathia spp. Atopobium spp. was not associated with urethritis. The quantity of bacteria did not differ between cases and controls. Among treated cases, doxycycline was more effective than azithromycin for clinical cure of men with Leptotrichia/Sneathia spp. (9/10 vs. 7/12, P = 0.16) and BVAB-2 (3/3 vs. 0/3, P = 0.10).
Conclusions
Leptotrichia/Sneathia spp. may be urethral pathogens or contribute to a pathogenic microbiota that can also include BVAB-2, BVAB-3, and Megasphaera spp. Doxycycline may be more effective than azithromycin against these newly identified bacteria.
Urethritis is the most common male reproductive tract syndrome and is caused primarily by Neisseria gonorrhoeae (GC) and Chlamydia trachomatis (CT). However, up to 50% of cases have neither GC nor CT detected1 and are referred to simply as nonchlamydial nongonococcal urethritis (NGU). Clinically defined as symptoms of urethral irritation (dysuria, pruritis) or visible urethral discharge and 5 or more polymorphonuclear leukocytes per high-power field (PMNs/HPF) in urethral exudates, nonchlamydial NGU has been associated with a number of known pathogens; Mycoplasma genitalium (MG) in 15% to 25%, Trichomonas vaginalis (TV) in 5% to 15%, and, less commonly, herpes simplex virus (HSV) and adenovirus in 2% to 4%.1 The differentiated Ureaplasma urealyticum (UU) has been associated with urethritis in some2–4 but not all studies.4,5 However, in a recent case-control study, 45% of NGU cases had no known pathogen,4 even after testing for CT, MG, TV, and UU. Although noninfectious causes of male urethritis exist, most cases are likely caused by sexually transmitted pathogens. Therefore, bacteria involved in female reproductive tract disease are reasonable candidates for involvement in NGU.
Bacterial vaginosis (BV) is the most prevalent genital tract syndrome in women, affecting 29% of reproductive-aged women,6 and has been associated with increased risk for perinatal complications,7 pelvic inflammatory disease,8 and HIV acquisition9 and transmission.10 Although there is debate as to whether BV can be sexually transmitted, the protective effect of condoms11 and the concordance of infection in lesbian couples12 support this concept. The syndrome is characterized by a shift in the vaginal microbiota from a predominance of lactobacilli to a predominance of anaerobic bacteria13 and does not seem to be caused by a single organism, but rather represents a change in the vaginal microbiota from one with several predominant Lactobacillus species to one marked by increased species richness and diversity.14–16 Several recently identified bacteria have been highly predictive of and specific for BV, including Leptotrichia/Sneathia spp., Atopobium spp., Megasphaera spp. and BVAB-1, BVAB-2, and BVAB-3 (all Clostridiales order bacteria)17 Most of these organisms, with the exception of BVAB-1, were also more common in women with than in those without cervicitis in a pilot study we conducted (unpublished data), suggesting that their presence in men may be associated with similar inflammation in the male urethra.
To assess the role of these newly described bacteria in male urethritis, we conducted a case-control study of men with and without idiopathic NGU. All men were tested for 5 recently identified bacteria associated with BV in women, and we estimated the risk of urethritis associated with detection of each bacterial species.
Methods
Study Population and Recruitment
Participants were heterosexual men with and without idiopathic urethritis, originally recruited into a case-control study to assess the association of the differentiated Ureaplasma species (UU-biovar 2 and Ureaplasma parvum) with NGU.4,18 Eligible men were English speaking, 16 years or older, and attending the Public Health–Seattle & King County (PHSKC) sexually transmitted disease (STD) clinic in Seattle, Washington. Men with positive test results for CT, GC, TV, MG, and UU-biovar 2 were excluded, as were those who reported any male partners in the past 12 months. Because the original study was nested within a randomized trial of antibiotic therapy,18 men who had received antibiotics in the past month or had allergies to azithromycin or doxycycline were also excluded.
Providers in the clinic referred men to the study clinician who assessed eligibility and obtained written informed consent, including permission to use collected specimens for other studies. Participants completed a brief computer-assisted self-interview and underwent a routine STD examination, during which they provided 25 mL of first void urine and a urethral swab for Gram staining. Cases were defined as men with either visible urethral discharge on examination or 5 or more PMNs/HPF in urethral exudates. Controls were defined as men with no visible urethral discharge and less than 5 PMNs/HPF. Men with urethritis were given a randomized treatment packet containing either active doxycycline (100 milligram capsules twice daily for 7 days) plus placebo azithromycin, or active azithromycin (1 gram single dose) plus placebo doxycycline, and were scheduled for a follow-up visit 3 weeks later for a test of cure. Control men underwent no further study procedures.
Although cases provided urethral swab and urine specimens, only the urine specimen from controls was retained. Therefore, urine was tested for CT and GC using the APTIMA Combo 2 transcription mediated amplification assay and for TV on the same platform using analyte-specific reagents (GenProbe, Inc, San Diego, CA). M. genitalium was assessed by in-house polymerase chain reaction (PCR)19; Ureaplasma spp. were detected in broth urine culture followed by species-specific PCR.3,20 Atopobium spp., BVAB-2, BVAB-3, Megasphaera spp. and Leptotrichia/Sneathia spp. were detected using quantitative species-specific PCR (qPCR) assays in conjunction with an inhibition control as previously described.17,21 These assays, previously developed for use with vaginal swab specimens, were adapted for use in male urine specimens and validated against urethral swab specimens. All study procedures and analyses were approved by the University of Washington Human Subjects Division.
We used Pearson χ2 test to assess the associations of categorical variables and Student t tests assuming unequal variance to examine differences in continuous variables between cases and controls. Fisher's exact test was used to determine the association between prevalence of bacteria in cases compared with controls and the association between study drug and clinical cure. Logistic regression with robust standard errors was used to estimate the odds ratio (OR) and 95% confidence interval (CI) of the association between the presence of each of the bacteria and urethritis.
Results
Study Population
A total of 575 men with NGU and 191 control men were recruited from the Public Health–Seattle & King County STD clinic between May 2007 and July 2011. Of the 575 cases, 323 (56.2%) had pathogens detected, 82 (14.3%) reported either no sexual intercourse or sex with a male partner in the past year, and 13 (2.3%) had insufficient sample volume for testing, resulting in 157 evaluable cases. Of the eligible controls, 39 (20.4%) had pathogens detected and 50 (26.2%) reported no sex or sex with a male partner in the past year, resulting in 102 evaluable controls, all with sufficient sample remaining for testing.
Cases and controls were similar with respect to age, education, and income. Mean (standard deviation [SD]) age was 34.7 (9.9), and most men were white, although cases were more likely to be black and less likely to be Hispanic than controls (Table 1). Cases were also significantly more likely to have a history of gonorrhea, chlamydia, or NGU. In contrast, sexual behavior in the past 2 months was similar between cases and controls. Nearly all men reported having engaged in vaginal sex, and approximately 75% reported receiving oral sex from their female partner(s).
Table 1. Demographic, Clinical, and Behavioral Characteristics of Cases and Controls (n = 259)*.
| Characteristic | Cases (n = 157) | Controls (n = 102) | Total (n = 259) | P† |
|---|---|---|---|---|
| Age, mean (SD), years | 35.4 (9.9) | 33.5 (9.8) | 34.7 (9.9) | 0.14 |
| Race | ||||
| White | 86 (57.0) | 73 (76.0) | 159 (64.4) | <0.001 |
| Black | 55 (36.4) | 12 (12.5) | 67 (27.1) | |
| Other | 10 (6.6) | 11 (11.5) | 21 (8.5) | |
| Hispanic | 4 (2.6) | 8 (8.0) | 12 (4.7) | 0.05 |
| Education | ||||
| ≤High school | 68 (43.3) | 37 (36.6) | 105 (40.7) | 0.29 |
| >High school | 89 (56.7) | 64 (63.4) | 153 (59.3) | |
| Income | ||||
| <$10,000 | 55 (35.5) | 33 (32.7) | 88 (34.4) | 0.65 |
| $10,000–$29,999 | 49 (31.6) | 29 (28.7) | 78 (30.5) | |
| ≥$30,000 | 51 (32.9) | 39 (38.6) | 90 (35.2) | |
| History of STD | ||||
| Gonorrhea | 32 (21.1) | 5 (4.9) | 37 (14.6) | <0.001 |
| Chlamydia | 59 (37.8) | 14 (13.7) | 73 (28.3) | <0.001 |
| NGU | 47 (30.9) | 9 (8.8) | 56 (22.1) | <0.001 |
| HSV | 20 (13.3) | 14 (14.1) | 34 (13.6) | 0.84 |
| Genital warts | 20 (13.1) | 17 (16.7) | 37 (14.5) | 0.43 |
| Syphilis | 3 (2.0) | 0 (0.0) | 3 (1.2) | 0.16 |
| Other STD | 9 (5.7) | 6 (5.9) | 15 (5.8) | 0.96 |
| Sexual history, past 2 mo | ||||
| No. FSP, mean (SD) | 1.7 (1.2) | 1.6 (1.5) | 1.6 (1.3) | 0.88 |
| No. new FSP, mean (SD) | 1.1 (1.4) | 1.4 (1.5) | 1.2 (1.4) | 0.16 |
| Vaginal sex with FSP | 140 (95.9) | 86 (95.6) | 226 (95.8) | 0.90 |
| Anal sex with FSP | 13 (8.9) | 8 (8.8) | 21 (8.9) | 0.98 |
| Oral sex with FSP | 108 (74.0) | 71 (78.9) | 179 (75.9) | 0.39 |
Values are presented as n (%), unless otherwise indicated. Bold denotes significance at 0.05 level.
Column totals may not sum because of missing values.
Pearson χ2 test for categorical variables and t test assuming unequal variance for continuous variables.
FSP indicates female sex partner.
Prevalence of Bacteria and Associated Sociodemographic Characteristics
Leptotrichia/Sneathia spp. were the most common bacteria, detected in 30 (11.6%) men. Atopobium spp. was detected in 21 (8.1%) men. Bacterial vaginosisYassociated bacteriium 2 was infrequently detected, found in 8 (3.1%) men. BVAB-3 and Megasphaera spp. were rarely found, identified in 2 (0.8%) and 1 (0.4%) men, respectively. Only Leptotrichia/Sneathia spp. (16/30 positive men) and Atopobium spp. (9/21 positive men) were found alone. BVAB-2, BVAB-3 and Megasphaera spp. were all found only in men who also had Leptotrichia/Sneathia spp., and 7 (77%) of these men also had Atopobium spp detected (Fig. 1). Approximately 82% of cases had none of these specific bacteria detected.
Figure 1.
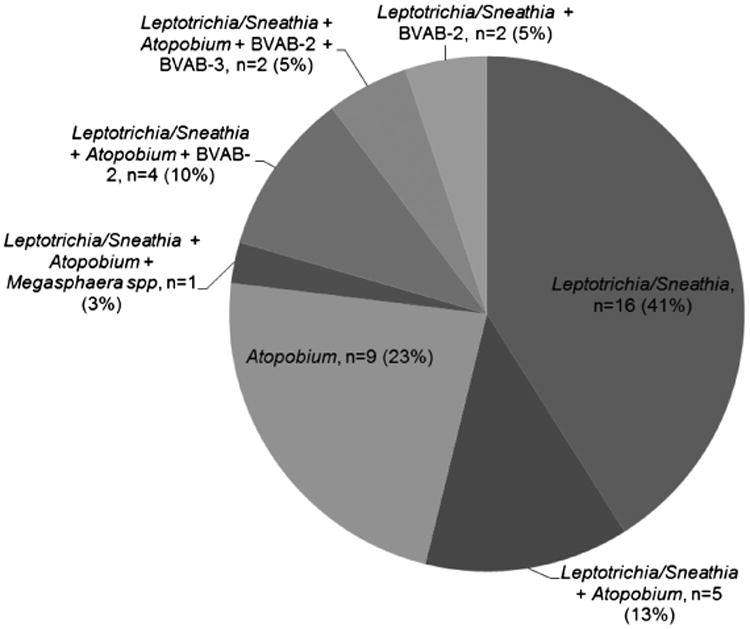
Coinfections among men with candidate pathogens detected (n = 39).
Atopobium spp. was associated with low income but not with any other characteristics suggestive of sexually transmitted pathogens (Table 2). In contrast, BVAB-2, BVAB-3, and Leptotrichia/Sneathia spp. were all associated with a history of NGU, and BVAB-2 and Leptotrichia/Sneathia spp. were associated with previous infection with GC and/or CT. Overall, sexual practices were not significantly associated with any of these bacteria, with the exception of BVAB-2, which was less common among men reporting receiving oral sex from a female partner in the past 2 months (38% vs. 70%, P = 0.02).
Table 2. Characteristics of Men Infected With Candidate Pathogens (n = 259)*.
| Characteristic | Atopobium spp. | BVAB-2 | BVAB-3 | Leptotrichia/Sneathia spp. | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Positive (n = 21) | Negative (n = 238) | Positive (n = 8) | Negative (n = 251) | Positive (n = 2) | Negative (n = 257) | Positive (n = 30) | Negative (n = 229) | |
| Age, mean (SD), years | 32.4 (11.5) | 34.9 (9.7) | 38.3 (10.8) | 34.6 (9.8) | 39.2 (12.8) | 34.6 (9.9) | 36.5 (10.2) | 34.4 (9.8) |
| Race | ||||||||
| White | 11 (55) | 148 (65) | 3 (38) | 156 (65) | 1 (50) | 158 (64) | 14 (47) | 145 (67) |
| Black | 7 (35) | 60 (26) | 4 (50) | 63 (26) | 1 (50) | 66 (27) | 13 (43) | 54 (25) |
| Other | 2 (10) | 19 (8) | 1 (12) | 20 (8) | 0 (0) | 21 (9) | 3 (10) | 18 (8) |
| Hispanic | 1 (5) | 11 (5) | 0 (0) | 12 (5) | 0 (0) | 12 (5) | 0 (0) | 12 (5) |
| Education | ||||||||
| ≤High school | 12 (57) | 93 (39) | 7 (88) | 98 (39) | 1 (50) | 104 (41) | 17 (57) | 88 (39) |
| >High school | 9 (43) | 144 (61) | 1 (12) | 152 (61) | 1 (50) | 152 (59) | 13 (43) | 140 (61) |
| Income | ||||||||
| <$10,000 | 12 (60) | 76 (32) | 5 (71) | 83 (33) | 1 (100) | 87 (34) | 12 (41) | 76 (33) |
| $10,000– $29,999 | 5 (25) | 73 (31) | 1 (14) | 77 (31) | 0 (0) | 78 (31) | 9 (31) | 69 (30) |
| ≥$30,000 | 3 (15) | 87 (37) | 1 (14) | 89 (36) | 0 (0) | 90 (35) | 8 (28) | 82 (36) |
| History of STD | ||||||||
| Gonorrhea | 3 (14) | 34 (15) | 3 (38) | 34 (14) | 1 (50) | 36 (14) | 9 (30) | 28 (12) |
| Chlamydia | 6 (29) | 67 (28) | 5 (63) | 68 (27) | 2 (100) | 71 (28) | 16 (53) | 57 (25) |
| NGU | 7 (33) | 49 (21) | 5 (63) | 51 (21) | 2 (100) | 54 (21) | 13 (43) | 43 (19) |
| HSV | 1 (5) | 33 (14) | 1 (12) | 33 (14) | 0 (0) | 34 (14) | 5 (17) | 29 (13) |
| Genital warts | 4 (19) | 33 (14) | 2 (25) | 35 (14) | 1 (50) | 36 (14) | 5 (17) | 32 (14) |
| Syphilis | 1 (5) | 2 (1) | 1 (12) | 2 (1) | 0 (0) | 3 (1) | 1 (3) | 2 (1) |
| Other STD | 1 (5) | 14 (6) | 1 (12) | 14 (6) | 0 (0) | 15 (6) | 1 (3) | 14 (6) |
| Sexual history, past 2 mo | ||||||||
| No. FSP, mean (SD) | 1.6 (1.1) | 1.7 (1.3) | 1.5 (0.8) | 1.7 (1.3) | 1.0 (0) | 1.7 (1.3) | 1.7 (1.1) | 1.6 (1.3) |
| No. new FSP, mean (SD) | 1.5 (2.7) | 1.1 (1.3) | 2.1 (4.0) | 1.1 (1.3) | 6.5 (7.8) | 1.1 (1.2) | 1.1 (2.3) | 1.2 (1.3) |
| Vaginal sex with FSP | 20 (95) | 206 (87) | 8 (100) | 218 (87) | 2 (100) | 224 (87) | 28 (93) | 198 (86) |
| Anal sex with FSP | 2 (10) | 19 (8) | 0 (0) | 21 (8) | 0 (0) | 21 (8) | 1 (3) | 20 (9) |
| Oral sex with FSP | 14 (67) | 165 (69) | 3 (38) | 176 (70) | 0 (0) | 179 (70) | 21 (70) | 158 (69) |
Values are presented as n (%), unless otherwise indicated. Bold: P < 0.05; bold italic: P < 0.01.
Megasphaera only detected in 1 man and not included.
FSP indicates female sex partner
Clinical Characteristics
Only detection of Leptotrichia/Sneathia spp. was significantly associated with urethritis. These bacteria were detected in 24 (15.3%) of 157 cases, but in only 6 (5.9%) of 102 controls (P = 0.03; Table 3), resulting in approximately a 3-fold increased risk of urethritis among men with these bacteria (OR, 2.9; 95% CI, 1.1–7.4). This relationship was unchanged when the case group was limited to men who met a stricter definition of NGU consisting of urethral symptoms or visible urethral discharge on examination plus 5 or more PMNs/HPF (OR, 3.1; 95% CI, 1.2–8.2). BVAB-2 was also found more often in cases (7/157, or 4.5%) than controls (1/102, or 1.0%), but this difference was not statistically significant (OR, 4.7; 95% CI, 0.6–39.1; P = 0.15). Although BVAB-3 and Megasphaera spp. were uncommon, they were only detected in men with urethritis and not among controls.
Table 3. Prevalence of BVAB in Men, Overall and by Case/Control Status.
| Bacterium | Total (n = 259), n (%) | Case (n = 157), n (%) | Control (n = 102), n (%) | P* | OR (95% CI) |
|---|---|---|---|---|---|
| Atopobium spp. | 21 (8.1) | 13 (8.3) | 8 (7.8) | 1.00 | 1.1 (0.42–2.66) |
| BVAB-2 | 8 (3.1) | 7 (4.5) | 1 (1.0) | 0.15 | 4.7 (0.57–39.05) |
| BVAB-3 | 2 (0.8) | 2 (1.3) | 0 (0.0) | 0.52 | — |
| Leptotrichia/Sneathia spp. | 30 (11.6) | 24 (15.3) | 6 (5.9) | 0.03 | 2.9 (1.13–7.35) |
| Megasphaera spp. | 1 (0.4) | 1 (0.6) | 0 (0.0) | 1.00 | — |
Fisher's exact test.
Among men in whom these bacteria were detected, the mean (SD) quantity of bacteria ranged from 4.2 (SD not applicable) to 7.7 (SD not applicable) log gene copies per milliliter of urine for Megasphaera spp. and BVAB-3, respectively. Despite this range, there were no significant differences between cases and controls with respect to quantity of bacteria.
Among the 53 urethritis cases in whom these bacteria were detected, 70% or greater had visible urethral discharge noted on examination (Table 4). Discharge was rarely profuse and most often cloudy, although 20% to 30% of men with Atopobium spp., BVAB-2, and Leptotrichia/Sneathia spp. had clear discharge. Most men had 10 or more PMNs/HPF, although lower PMN counts were also observed in men with Atopbium spp. and Leptotrichia/Sneathia spp.
Table 4. Clinical Signs Among Cases With BVAB Detected at Enrollment (n = 157).
| Characteristic | Atopobium spp. (n = 13), n (%) | BVAB-2 (n = 7), n (%) | BVAB-3 (n = 2), n (%) | Megasphaera spp. (n = 1), n (%) | Leptotrichia/Sneathia spp. (n = 24), n (%) | No Candidate Pathogens (n = 129), n (%) |
|---|---|---|---|---|---|---|
| Urethral discharge | ||||||
| Yes | 9 (69.2) | 5 (71.4) | 2 (100) | 1 (100) | 18 (75.0) | 112 (86.8) |
| No | 4 (30.8) | 2 (28.6) | 0 (0.0) | 0 (0.0) | 6 (25.0) | 17 (13.2) |
| Discharge amount* | ||||||
| Small | 7 (77.8) | 2 (40.0) | 2 (100) | 1 (100) | 11 (61.1) | 81 (72.3) |
| Moderate | 2 (22.2) | 2 (40.0) | 0 (0.0) | 0 (0.0) | 6 (33.3) | 27 (24.1) |
| Large | 0 (0.0) | 1 (20.0) | 0 (0.0) | 0 (0.0) | 1 (5.6) | 4 (3.6) |
| Discharge character*† | ||||||
| Clear | 2 (22.2) | 1 (20.0) | 0 (0.0) | 0 (0.0) | 5 (29.4) | 60 (56.1) |
| Cloudy | 7 (77.8) | 4 (80.0) | 2 (100) | 1 (100) | 12 (70.6) | 45 (42.1) |
| Purulent | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (1.9) |
| PMNs/HPF | ||||||
| 0–4 | 0 (0.0) | 1 (14.3) | 0 (0.0) | 0 (0.0) | 3 (12.5) | 22 (17.1) |
| 5–9 | 2 (15.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 6 (25.0) | 36 (27.9) |
| ≥10 | 11 (84.6) | 6 (85.7) | 2 (100) | 1 (100) | 15 (62.5) | 71 (55.0) |
Denominator for proportions is the number of men with discharge present on examination.
Missing values for 1 man with Leptotrichia/Sneathia spp. and 5 men with no candidate pathogens.
Response to Antimicrobial Therapy
Of the 157 cases, 132 (84.1%) returned for a follow-up visit and had data on clinical response to treatment (control men were not asked to return). Eighty (51%) of the returning cases originally received azithromycin, and 77 (49%) received doxycycline. Clinical cure was defined as less than 5 PMNs/HPF (with or without urethral symptoms) and absence of urethral discharge. Although most men initially infected with Leptotrichia/Sneathia spp. who received doxycycline (9/10) experienced clinical cure, this was true for only 58% (7/12) of men who received azithromycin (P = 0.16). Azithromycin was also less effective than doxycycline in achieving clinical cure among men with Atopobium spp. (2/5 [40%] vs. 6/6 [100%], P = 0.06), and BVAB-2 (0/3 [0%] vs. 3/3 [100%], P = 0.10). The number of men with BVAB-3 and Megasphaera spp. was too small for meaningful comparisons of response to therapy (data not shown).
Discussion
In this population of heterosexual men, Leptotrichia/Sneathia spp. was found in 15% of men with idiopathic urethritis and significantly associated with NGU. Other recently identified bacteria that have been significantly associated with BVin women (BVAB-2, BVAB-3, Megasphaera) were less commonly found but, when detected, were most often identified in men with urethritis and rarely or never in men without urethritis. BVAB-2, BVAB-3, and Leptotrichia/Sneathia spp. were also associated with reported prior episodes of NGU and previously detected sexually transmitted pathogens, supporting the possibility of sexual transmission between men and their female partners. Among men with urethritis, azithromycin was less effective in eradicating any of these bacteria than doxycycline, although differences were not statistically significant (possibly because of small sample sizes).
Detection of Leptotrichia/Sneathia spp. was first reported in 1995, when Leptotrichia sanguinegens (subsequently classified as Sneathia sanguinegens) was detected in women with postpartum bacteremia.22 The related Leptotrichia amnionii was subsequently isolated from a woman with second trimester fetal death in 2002.23 More recent 16S rDNA phylogenetic analyses suggested that L. amnionii was more appropriately classified in the genus Sneathia24 and the type strain was recently isolated, sequenced, characterized, and renamed Sneathia amnii sp. nov. (S. amnii Sn35).25 Among women enrolled in the Vaginal Human Microbiome Project, S. amnii was the most commonly detected organism, found in ∼40% of 736 women. In that same population, S. amnii and S. sanguinigens often co-occurred; 70% of women with 1 Sneathia species also had the other.25
Although infrequently studied in men, these bacteria seem to be highly prevalent in women. Leptotrichia/Sneathia spp. were detected in 62% of women with pelvic inflammatory disease,26 and L. amnionii was present in 78% of women with and without BV attending an urban STD clinic,14 although much more common in women with BV than in women without BV. Notably, women having sex with women were more likely to acquire BV when Leptotrichia/Sneathia spp. were detected in the anus,27 highlighting their potential role in incident BV. Treatment of women with BV with metronidazole was followed by significant reductions in bacterial concentrations of these bacteria,28 and the type strain was sensitive to vancomycin but resistant to tetracycline (>50 μg/mL) and ciprofloxacin (>25 μg/mL).25 This latter observation is in sharp contrast to the high clinical efficacy of doxycycline that we observed among these men with NGU and may be caused by differences in antimicrobial susceptibility of individual strains.
Leptotrichia/Sneathia spp., BVAB-2, BVAB-3, and Megasphaera spp were all associated with other sexually transmitted infections (history of NGU, CT, or GC), suggesting that they may be similarly sexually transmitted. Notably, Atopobium spp. were not associated with a history of STI and, unlike the other bacteria, were evenly distributed between men who did and did not have urethritis. Although Leptotrichia/Sneathia spp. were the only bacteria significantly associated with urethritis; BVAB-2, BVAB-3, and Megasphaera spp were predominantly if not exclusively identified in men with urethritis, despite being rarely detected. Furthermore, BVAB-2, BVAB-3, and Megasphaera spp. were only found in men who also had Leptotrichia/Sneathia spp. This gives rise to three potential hypotheses: (a) all 4 of the bacteria may be individual pathogens; (b) all 4 of the bacteria may be part of a “pathogenic microbiota”; or (c) Leptotrichia/Sneathia spp. may be a sole pathogenic organism, and the microenvironment that favors these bacteria may also be conducive to BVAB-2, BVAB-3, and Megasphaera spp. Larger studies specifically designed to evaluate this will be required to determine which of these hypotheses, if any, are true.
Despite using sensitive qPCR assays for 5 different bacteria, 82% of these men with idiopathic NGU remained without an identified organism, and one of the bacteria we tested for was unrelated to NGU. Other uncultivated organisms and/or characteristic microbiota may be responsible for urethral inflammation in men, and further analyses of this population using broad-range PCR combined with pyrosequencing to evaluate this are ongoing. Nelson and colleagues29 used this approach among asymptomatic men attending an STD clinic and found that Sneathia spp. were exclusively detected in men who were also infected with GC and/or CT. However, men with and without urethritis were not compared. An earlier study using 16S rRNA gene PCR followed by restriction fragment length polymorphism assessed men with and without NGU attending an STD clinic but did not detect either Leptotrichia/Sneathia spp. or any other putative pathogens.30 However, restriction fragment length polymorphism is an older technology and may not have been sufficiently sensitive and specific to identify all the bacterial taxa present.
We defined NGU using the long-held standard of 5 or less PMNs/HPF, yet a recent evaluation suggested that a lower cutpoint (≤2 PMNs/HPF) may be more sensitive for CT infection.31 Current clinical criteria for urethritis were based on the pathogenesis of gonorrhea and may be less useful as we explore the role of the microbiome in disease. As this field of study progresses, continuous measures of inflammation may provide better distinction between normal and pathogenic microflora.
This study is characterized by a number of strengths, including a clear and consistent clinical definition of NGU, exclusion of men with known pathogens, and the use of sensitive and specific qPCR assays to detect the target bacteria. However, there were also limitations. Polymerase chain reaction assays are sometimes less sensitive when applied to urine than to urethral swab specimens, and some bacteria may not have been detected. We did not test for viral pathogens, and some of these men may have had HSV, adenovirus, or other viruses. Our population consisted of men with idiopathic NGU, and therefore, we could not determine the extent to which the target bacteria are found with other known sexually transmitted pathogens. The urethral microbiota of men who have sex with men is likely influenced by exposure to different microflora; thus, our results among men with exclusively female partners may not be generalizable to men who have sex with men. We observed intriguing trends, some of which were not statistically significant, likely a reflection of our relatively small bacterium-specific sample sizes. Larger studies with more statistical power will be required to confirm these findings. Because we evaluated 5 different bacteria and assessed associations with demographic, sexual behavior, and clinical characteristics, we performed a large number of statistical tests and some of the relationships we detected may have been caused by chance. Finally, with the exception of our analyses of the response to therapy among cases who returned for follow-up, this was a cross-sectional assessment, and we cannot attribute causality to the observed association between Leptotrichia/Sneathia spp. and urethritis. Longitudinal studies will be required to determine whether acquisition of Leptotrichia/Sneathia spp. and/or characteristic microbial communities actually precede the development of urethritis and whether clinical symptoms disappear after eradication of the bacteria, or change in the composition of the urethral microbiota.
In summary, Leptotrichia/Sneathia spp. were significantly associated with idiopathic NGU and characterized by a history of STI in these men, suggesting that they may be sexually transmitted urethral pathogens. Several other BVAB less commonly identified in men (BVAB2, BVAB3, Megasphaera spp.) were found predominantly among NGU cases, and they may also cause urethritis or contribute to a pathogenic microbiota. However, the absence of any of these bacteria in most men suggests that there are other additional uncultivated pathogens or characteristic microbiota that we have not yet identified. Longitudinal studies of the acquisition and subsequent clinical consequences of these bacterial infections in men, as well as the rates of transmission to female partners, will be essential to determine their role in male and female reproductive tract disease. Broad-range 16S rRNA gene PCR and pyrosequencing among men with and without urethritis may uncover additional uncultivated urethral pathogens and/or pathogenic microbiota.
Acknowledgments
This work was supported by the National Institutes of Health/National Institute of Allergy and Infectious Diseases (R21 AI1091970, U19 AI31448, and R01 AI072728).
Footnotes
Conflict of interest: None declared.
References
- 1.Holmes KK, et al., editors. Sexually Transmitted Diseases. New York: McGraw Hill; 2008. Martin DH Urethritis in males; pp. 1107–1126. [Google Scholar]
- 2.Deguchi T, Yoshida T, Miyazawa T, et al. Association of Ureaplasma urealyticum (biovar 2) with nongonococcal urethritis. Sex Transm Dis. 2004;31:192–195. doi: 10.1097/01.olq.0000114653.26951.71. [DOI] [PubMed] [Google Scholar]
- 3.Ondondo RO, Whittington WL, Astete SG, et al. Differential association of ureaplasma species with non-gonococcal urethritis in heterosexual men. Sex Transm Infect. 2010;86:271–275. doi: 10.1136/sti.2009.040394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wetmore CM, Manhart LE, Lowens MS, et al. Ureaplasma urealyticum is associated with nongonococcal urethritis among men with fewer lifetime sexual partners: A case-control study. J Infect Dis. 2011;204:1274–1282. doi: 10.1093/infdis/jir517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradshaw CS, Tabrizi SN, Read TRH, et al. Etiologies of nongonococcal urethritis: Bacteria, viruses, and the association with orogenital exposure. J Infect Dis. 2006;193:336–345. doi: 10.1086/499434. [DOI] [PubMed] [Google Scholar]
- 6.Koumans EH, Sternberg M, Bruce C, et al. The prevalence of bacterial vaginosis in the United States, 2001–2004: Associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis. 2007;34:864–869. doi: 10.1097/OLQ.0b013e318074e565. [DOI] [PubMed] [Google Scholar]
- 7.Minkoff H, Grunebaum AN, Schwarz RH, et al. Risk factors for prematurity and premature rupture of membranes: A prospective study of the vaginal flora in pregnancy. Am J Obstet Gynecol. 1984;150:965–972. doi: 10.1016/0002-9378(84)90392-2. [DOI] [PubMed] [Google Scholar]
- 8.Paavonen J, Teisala K, Heinonen PK, et al. Microbiological and histopathological findings in acute pelvic inflammatory disease. Br J Obstet Gynaecol. 1987;94:454–460. doi: 10.1111/j.1471-0528.1987.tb03125.x. [DOI] [PubMed] [Google Scholar]
- 9.Taha TE, Hoover DR, Dallabetta GA, et al. Bacterial vaginosis and disturbances of vaginal flora: Association with increased acquisition of HIV. AIDS. 1998;12:1699–1706. doi: 10.1097/00002030-199813000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Cohen CR, Lingappa JR, Baeten JM, et al. Bacterial vaginosis associated with increased risk of female-to-male HIV-1 transmission: A prospective cohort analysis among African couples. PLoS Med. 2012;9:e1001251. doi: 10.1371/journal.pmed.1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanchez S, Garcia PJ, Thomas KK, et al. Intravaginal metronidazole gel versus metronidazole plus nystatin ovules for bacterial vaginosis: A randomized controlled trial. Am J Obstet Gynecol. 2004;191:1898–1906. doi: 10.1016/j.ajog.2004.06.089. [DOI] [PubMed] [Google Scholar]
- 12.Marrazzo JM, Koutsky LA, Eschenbach DA, et al. Characterization of vaginal flora and bacterial vaginosis in women who have sex with women. J Infect Dis. 2002;185:1307–1313. doi: 10.1086/339884. [DOI] [PubMed] [Google Scholar]
- 13.Hillier SL, Marrazzo JM, Holmes KK. Bacterial vaginosis. In: Holmes KK, et al., editors. Sexually Transmitted Diseases. New York: McGraw-Hill; 2008. pp. 737–768. [Google Scholar]
- 14.Srinivasan S, Hoffman NG, Morgan MT, et al. Bacterial communities in women with bacterial vaginosis: High resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS One. 2012;7:e37818. doi: 10.1371/journal.pone.0037818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verstraelen H, Verhelst R, Claeys G, et al. Culture-independent analysis of vaginal microflora: The unrecognized association of Atopobium vaginae with bacterial vaginosis. Am J Obstet Gynecol. 2004;191:1130–1132. doi: 10.1016/j.ajog.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 16.Zozaya-Hinchliffe M, Martin DH, Ferris MJ. Prevalence and abundance of uncultivated Megasphaera-like bacteria in the human vaginal environment. Appl Environ Microbiol. 2008;74:1656–1659. doi: 10.1128/AEM.02127-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med. 2005;353:1899–1911. doi: 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- 18.Manhart LE, Gillespie CW, Lowens MS, et al. Standard treatment regimens for nongonococcal urethritis have similar but declining cure rates: A randomized controlled trial. Clin Infect Dis. 2012 doi: 10.1093/cid/cis1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dutro SM, Hebb JK, Garin CA, et al. Development and performance of a microwell-plate-based polymerase chain reaction assay for Mycoplasma genitalium. Sex Transm Dis. 2003;30:756–763. doi: 10.1097/01.OLQ.0000078821.27933.88. [DOI] [PubMed] [Google Scholar]
- 20.Kenny GE. Mycoplasmata. In: Lenette E, et al., editors. Manual of Clinical Microbiology. Washington, DC: American Society for Microbiology; 1980. pp. 365–370. [Google Scholar]
- 21.Fredricks DN, Fiedler TL, Thomas KK, et al. Targeted PCR for detection of vaginal bacteria associated with bacterial vaginosis. J Clin Microbiol. 2007;45:3270–3276. doi: 10.1128/JCM.01272-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanff PA, Rosol-Donoghue JA, Spiegel CA, et al. Leptotrichia sanguinegens sp. nov., a new agent of postpartum and neonatal bacteremia. Clin Infect Dis. 1995;20(suppl 2):S237–239. doi: 10.1093/clinids/20.supplement_2.s237. [DOI] [PubMed] [Google Scholar]
- 23.Shukla SK, Meier PR, Mitchell PD, et al. Leptotrichia amnionii sp. nov., a novel bacterium isolated from the amniotic fluid of a woman after intrauterine fetal demise. J Clin Microbiol. 2002;40:3346–3349. doi: 10.1128/JCM.40.9.3346-3349.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eribe ER, Paster BJ, Caugant DA, et al. Genetic diversity of Leptotrichia and description of Leptotrichia goodfellowii sp. nov., Leptotrichia hofstadii sp. nov., Leptotrichia shahii sp. nov. and Leptotrichia wadei sp. nov. Int J Syst Evol Microbiol. 2004;54(pt 2):583–592. doi: 10.1099/ijs.0.02819-0. [DOI] [PubMed] [Google Scholar]
- 25.Harwich M, Serrano MG, Fettweis JM, et al. Genomic sequence analysis and characterization of Sneathia amnii sp. nov. BMC Genom. 2012;13(suppl 8):S4. doi: 10.1186/1471-2164-13-S8-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haggerty CL, Totten PA, Ferris M, et al. Clinical characteristics of bacterial vaginosis among women testing positive for fastidious bacteria. Sex Transm Infect. 2009;85:242–248. doi: 10.1136/sti.2008.032821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marrazzo JM, Fiedler TL, Srinivasan S, et al. Extravaginal reservoirs of vaginal bacteria as risk factors for incident bacterial vaginosis. J Infect Dis. 2012;205:1580–1588. doi: 10.1093/infdis/jis242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fredricks DN, Fiedler TL, Thomas KK, et al. Changes in vaginal bacterial concentrations with intravaginal metronidazole therapy for bacterial vaginosis as assessed by quantitative PCR. J Clin Microbiol. 2009;47:721–726. doi: 10.1128/JCM.01384-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelson DE, Van Der Pol B, Dong Q, et al. Characteristic male urine microbiomes associate with asymptomatic sexually transmitted infection. PLoS One. 2010;5:e14116. doi: 10.1371/journal.pone.0014116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riemersma WA, Van Der Shee CJ, Van Der Meijden WI, et al. Microbial population diversity in the urethras of healthy males and males suffering from nonchlamydial, nongonococcal urethritis. J Clin Microbiol. 2003;41:1977–1986. doi: 10.1128/JCM.41.5.1977-1986.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rietmeijer CA, Mettenbrink CJ. Recalibrating the Gram stain diagnosis of male urethritis in the era of nucleic acid amplification testing. Sex Transm Dis. 2012;39:18–20. doi: 10.1097/OLQ.0b013e3182354da3. [DOI] [PubMed] [Google Scholar]


