Abstract
A case of fulminant myocarditis associated with the H1N1 influenza virus. This case report describes the patient's clinical course and emphasizes the importance of bedside echocardiography as an aid in the early diagnosis and management of children with severe myocardial dysfunction. It also discusses aspects relevant to the treatment and prognosis of fulminant myocarditis. The patient was a female, 4 years and 8 months old, previously healthy and with a history of flu symptoms in the past two weeks. The patient was admitted to the emergency room with signs of hemodynamic instability, requiring ventilatory support and vasoactive drugs. The laboratory tests, chest X-ray and echocardiogram suggested the presence of myocarditis. The test for H1N1 in nasopharyngeal secretions was positive. The patient evolved to refractory cardiogenic shock despite the clinical measures applied and died 48 hours after admission to the intensive care unit. The H1N1 influenza virus is an etiological agent associated with acute myocarditis, but there are few reported cases of fulminant myocarditis caused by the H1N1 virus. The identification of signs and symptoms suggestive of fulminant progression should be immediate, and bedside echocardiography is a useful tool for the early detection of myocardial dysfunction and for therapeutic guidance. The use of immunosuppressive therapy and antiviral therapy in acute myocarditis of viral etiology is controversial; hence, the treatment is based on hemodynamic and ventilatory support. The use of hemodynamic support by extracorporeal membrane oxygenation emerges as a promising treatment.
Keywords: Myocarditis; Influenza, human; Echocardiography; Child; Case reports
Abstract
Caso de miocardite fulminante associada ao vírus influenza H1N1, em que foi descrita a evolução clínica do paciente e enfatizada a importância do ecocardiograma à beira do leito como auxílio no diagnóstico precoce e manejo de crianças com disfunção miocárdica grave, além de terem sido discutidos aspectos relevantes relacionados à terapêutica e ao prognóstico da miocardite fulminante. Trata-se de paciente do sexo feminino, 4 anos e 8 meses, previamente hígida, com história de quadro gripal há 2 semanas. Admitida no pronto-socorro com sinais de instabilidade hemodinâmica, necessitando de suporte ventilatório e drogas vasoativas. Exames laboratoriais, radiografia de tórax e ecocardiograma sugestivos de miocardite. Pesquisa positiva para H1N1 em secreção de nasofaringe. Evoluiu com choque cardiogênico refratário a despeito das medidas clínicas, indo a óbito em 48 horas após admissão na unidade de terapia intensiva. O vírus influenza H1N1 é agente etiológico associado a quadros de miocardite aguda, porém poucos são os casos relatados de miocardite fulminante pelo vírus H1N1. A identificação de sinais e sintomas sugestivos de evolução fulminante deve ser imediata e o ecocardiograma à beira do leito é uma ferramenta útil para detecção precoce de disfunção miocárdica e orientação terapêutica. O uso de terapia imunossupressora, em casos de miocardite fulminante de etiologia viral, é controverso, bem como o de terapia antiviral, de tal forma que o tratamento baseia-se em suporte hemodinâmico e ventilatório. O uso de suporte hemodinâmico, por meio de oxigenação por membrana extracorpórea, aparece como terapia promissora.
INTRODUCTION
Acute myocarditis exhibits a broad spectrum of clinical presentations. Fulminant acute myocarditis presents with sudden onset of cardiac symptoms, which typically manifest after nonspecific flu-like symptoms and rapidly progress to severe hemodynamic deterioration with severe heart failure, cardiogenic shock and potentially fatal arrhythmias. In pediatric patients, fulminant myocarditis corresponds to 30-40% of cases of myocarditis and has a mortality rate of up to 48%, as reported in a recent publication by Saji et al.(1) The prevalence of myocardial involvement in infections by the influenza virus ranges from 0 to 11%, depending on the criteria used to define myocarditis.(2) Influenza A virus subtype H1N1, which is responsible for the 2009 pandemic and which has since been the focus of attention of the World Health Organization,(3) plays a role in the pathogenesis of acute myocarditis.(4) However, there are few cases of fulminant myocarditis due to H1N1 influenza reported in the literature, especially in children.(2)
We report a case of fulminant myocarditis associated with the H1N1 influenza virus to emphasize the importance of this virus as an etiological agent, describe the clinical course, emphasize the importance of bedside echocardiography, and discuss aspects relevant to the treatment and prognosis of fulminant myocarditis.
CASE REPORT
Female patient, 4 years and 8 months old, previously healthy and with normal weight, without prior vaccination for influenza virus. History of cough and rhinorrhea in the last two weeks, with unmeasured fever. Two days prior, she presented with abdominal pain, vomiting and poor general condition.
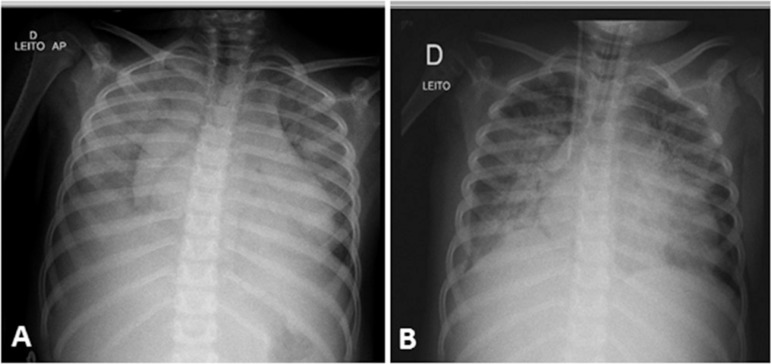
She was admitted to the emergency room in poor general condition, severely dehydrated, normotensive, moaning, tachypneic and tachycardic (Table 1). Initially diagnosed with septic shock, she received fluid resuscitation with 70mL/kg crystalloid, oxygen therapy and empiric antibiotic therapy (ceftriaxone 100mg/kg/day). Laboratory tests showed anemia, metabolic acidosis and elevated C-reactive protein concentration (Table 2). Still in the emergency room, the patient evolved with worsening of the tachydyspnea, hypotension and moaning, in addition to hepatomegaly, anasarca and presence of heart murmur. Electrocardiogram showed sinus tachycardia (155 beats per minute), and chest X-ray showed an enlarged cardiac area, mild right pleural effusion and diffuse pulmonary infiltrates, suggesting alveolar congestion (Figure 1). At that moment, a diagnosis of congestive heart failure secondary to myocarditis or cardiomyopathy associated with sepsis was established. Twelve hours after admission to the emergency room, due to hemodynamic deterioration and signs of pulmonary congestion, diuretic and inotropic therapies (milrinone) were initiated, with some improvement of the hemodynamic parameters (Table 2).
Table 1.
Test results throughout the clinical course
| Tests | Emergency room | ICU | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 6 hours | 18 hours | 0 hour | 12 hours | 18 hours | 24 hours | 36 hours | 40 hours | 48 hours | |
| Hemoglobin (g/dL) | 8.3 | 7.7 | 11 | 9.3 | 9.6 | 9.4 | |||
| Hematocrit (%) | 25.9 | 25 | 34 | 29 | 30 | 29 | |||
| Urea (mg/dL) | 33 | 31 | 32 | 44 | 39 | 49 | 58 | ||
| Creatinine (mg/dL) | 0.4 | 0.33 | 0.42 | 0.78 | 0.61 | 0.99 | 1.37 | ||
| Troponin (ng/dL) | 0.109 | 0.151 | 0.259 | ||||||
| CPK (U/L) | 162 | 191 | |||||||
| CK-MB (ng/mL) | 7.5 | 8.8 | |||||||
| Myoglobin (ng/mL) | 2429 | ||||||||
| pH/Bic (mEq/L) | 7.36/19.2 | 7.28/15.8 | 7.36/17.7 | 7.32/19.4 | 7.37/19.8 | 7.26/17.3 | 7.18/17.0 | 7.19/16.3 | 7.25/17.2 |
| Lactate (mg/dL) | 14 | 12 | 11 | 7 | 10 | ||||
| C-reactive protein (mg/L) | 158 | 90.88 | 94.2 | 84.29 | 103.83 | ||||
| SvO2 (%) | 83.3 | 78 | 52.4 | 73.1 | |||||
ICU - intensive care unit; CPK - creatinine phosphokinase; CK-MB - creatine kinase MB isoenzyme; Bic - bicarbonate; SvO2 - central venous oxygen saturation. Reference values: CK-MB <4.97ng/mL; CPK 26-192U/L; creatinine 0.31-0.47mg/dL; lactate 4.5-14mg/dL; myoglobin 25-58ng/dL; C-reactive protein <5mg/L; Troponin <0.014ng/ml; urea 11-38g mg/dL.
Table 2.
Vasoactive drugs and ventilatory and hemodynamic parameters
| Emergency room | ICU | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 6 hours | 18 hours | 0 hour | 6 hours | 12 hours | 18 hours | 24 hours | 36 hours | 42 hours | 48 hours | |
| Milrinone (mcg/kg/min) | 0.25 | 0.25 | 0.25 | 0.5 | 0.5 | 0.5 | 0.5 | 0.75 | 1 | |
| Dobutamine (mcg/kg/min) | 10 | 15 | 15 | 15 | ||||||
| Norepinephrine (mcg/kg/min) | 0.5 | 0.6 | 1.2 | 1.6 | ||||||
| Epinephrine (mcg/kg/min) | 0.3 | 0.1 | 0.1 | |||||||
| Ventilatory support (cmH2O) | Venturi | Venturi | NIV | FiO2 100 | FiO2 55 | FiO2 55 | FiO2 55 | FiO2 100 | FiO2 100 | FiO2 100 |
| 50% | 50% | FiO2 60% | inspP34 | inspP27 | inspP27 | inspP27 | inspP30 | inspP32 | inspP32 | |
| IPAP16 EPAP8 | PEEP14 Fr26 | PEEP12 Fr22 | PEEP12 Fr22 | PEEP12 Fr22 | PEEP12 Fr22 | PEEP10 Fr25 | Peep10 F25 | |||
| SAP (mmHg) | 86x52 | 100x50 | 85x46 (57) | 80x46 (61) | 78x41 (58) | 87x56 (67) | 74x41 (52) | 74x43 (59) | 61x41 (49) | |
| HR (bpm) | 166 | 145 | 140 | 155 | 155 | 145 | 160 | 173 | 179 | |
| Urine output (ml/kg/hour) | 3.5 | 1.53 | 0.7 | 0.5 | 0.5 | 0.5 | 0.44 | 0.4 | 0.2 | |
| CVP (mmHg) | 27 | 22 | 18 | 21 | 21 | |||||
ICU - intensive care unit; NIV - noninvasive ventilation; FiO2 - fraction of inspired oxygen (expressed in percentage); inspP - inspiratory pressure (expressed in cmH2O); IPAP - Inspiratory pressure in NIV (expressed in cmH2O); PEEP - end-expiratory pressure (expressed in cm H2O); EPAP - Expiratory pressure in NIV (in cmH2O); SAP - systemic arterial pressure; HR - heart rate; CVP - central venous pressure.
Figure 1.
(A) Chest X-ray after admission showing bilateral alveolar infiltrates. (B) Chest X-ray after admission to the intensive care unit with worsening alveolar infiltration and the presence of invasive devices (tracheal tube and central venous catheter).
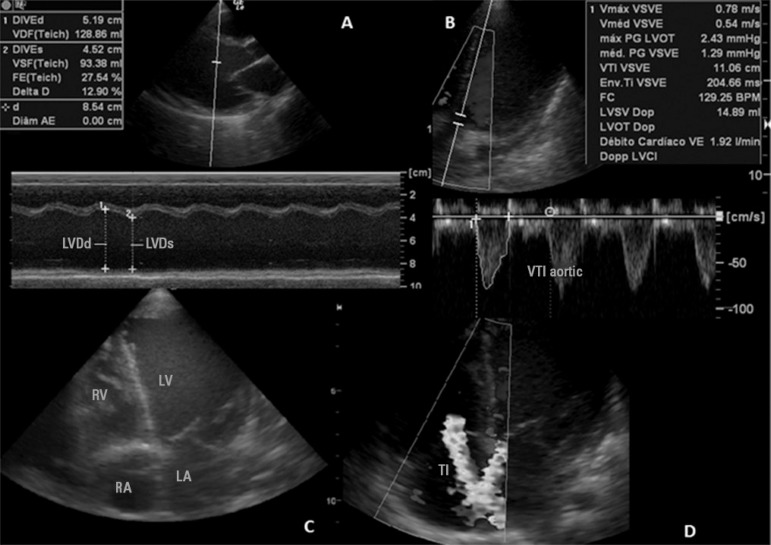
Twenty-four hours after admission to the emergency room, the patient was transferred to the intensive care unit (ICU), where she presented progressive worsening of respiratory pattern, without improvement with noninvasive ventilation and requiring tracheal intubation (six hours after ICU admission). A central venous catheter was inserted into the right internal jugular vein (central venous pressure of 27mmHg), and the inotropic and diuretic therapies were intensified without satisfactory clinical response (Table 1). An echocardiogram was performed by an ICU physician and confirmed by the echocardiographer. The exam showed the following results: ejection fraction of 27% (reference value - RV: >60%), cardiac inde of 2.1L/min/m2 (RV: 3.3 and 6.0L/min/m2) and left ventricular diastolic diameter of 50mm (RV: <20 mm), with the presence of diffuse left ventricular hypokinesia and moderate insufficiency of the tricuspid and mitral valves (Figure 2). Dobutamine infusion, for the treatment of severe cardiac dysfunction, and norepinephrine, due to the hypotension, were added to the therapy. The antibiotic therapy was also broadened with the inclusion of oxacillin and clarithromycin. Tests to identify the etiologic agent (search for adenovirus, enterovirus serology, mycoplasma and identification of H1N1 in nasopharyngeal secretions by polymerase chain reaction) were performed. Laboratory tests showed elevated myocardial necrosis and inflammatory markers (Table 1). All cultures remained negative, and C-reactive protein (CRP) was positive for H1N1. Despite clinical treatment, the patient did not show improvement of her hemodynamic profile, staying in refractory shock. After the introduction of adrenaline and an increase of the dose of the other vasoactive drugs, the patient showed no clinical improvement. The patient died 48 hours after ICU admission.
Figure 2.
(A) Measurement of ejection fraction by M-mode; (B) measurement of cardiac index; (C) apical 4-chamber image showing significant dilation of the left ventricle; (D) presence of significant tricuspid insufficiency by color Doppler.
LVDd - left ventricular end-diastolic diameter; LVDs - left ventricular end-systolic diameter; VTI - velocity-time integral; RV - right ventricle; LV - left ventricle; RA - right atrium; LA - left atrium; TI - tricuspid insufficiency.
Macroscopic histopathological analysis showed minor ascites, thickened mitral valve with yellowish exudate deposition in valve leaflets and endocardium, signs of pulmonary congestion and dilation of the left cardiac chambers (Figure 3). Microscopically, the endocardium presented areas of necrosis associated with the fibrin-leukocyte crust, and lymphocytic inflammatory infiltrate was present in the myocardium with degeneration of some myocytes (Figure 4). The pulmonary parenchyma exhibited signs of congestion, with alveoli filled with hyaline membranous content (hyaline membrane) and extravasation of red blood cells (Figure 4). The hepatic parenchyma was congested, with areas of ischemia in the central vein region.
Figure 3.
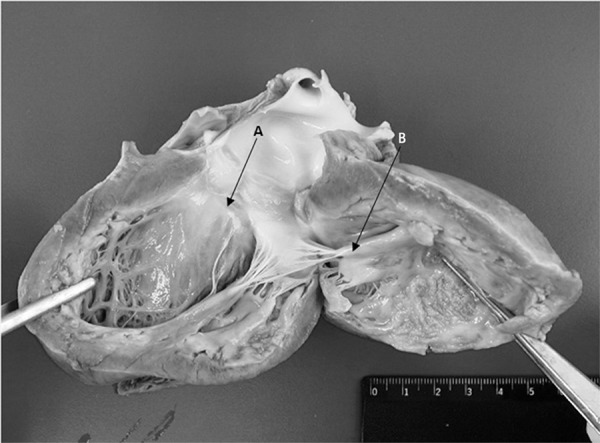
Macroscopy of the left ventricle. (A) Thickening of the mitral valve. (B) Deposition of yellowish material in the endocardium.
Figure 4.
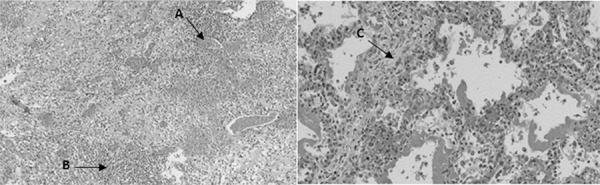
(A) Lymphocytic inflammatory infiltrate in the myocardium with degeneration of some myocytes; (B) interalveolar septum with signs of congestion; (C) alveoli filled with hyaline membranous content.
DISCUSSION
The clinical presentation of fulminant myocarditis of viral etiology is characterized by the presence of nonspecific symptoms suggestive of viral infection, such as fever, cough, runny nose, nausea, vomiting, abdominal pain and diarrhea, accompanied by dyspnea, arrhythmia, syncope, heart failure and cardiogenic shock. Upon cardiac examination, tachycardia, muffled first heart sound and third and fourth heart sound gallop may be present. Patients with fulminant presentation, when compared to patients with acute non-fulminant myocarditis, have higher heart rate, lower blood pressure and higher elevation of myocardial necrosis markers and CRP, data that support the greater severity and worse clinical outcome of these patients.(1) The diagnosis of fulminant viral myocarditis is based on clinical and laboratory data, such as electrocardiogram, imaging tests (echocardiography, myocardial scintigraphy and MRI) and endomyocardial biopsy for histopathological analysis, as previously described.(1,5,6) The patient had a clinical history, physical examination, laboratory tests (significant elevation of CRP and markers of myocardial necrosis) and echocardiographic alterations consistent with fulminant myocarditis. The clinical picture was preceded by flu-like symptoms, which directed the etiological investigation to viral research, with subsequent confirmation of H1N1 influenza in the nasopharyngeal secretions.
Echocardiography is an important tool in the diagnosis of myocardial dysfunction and is able to exclude other causes of heart failure, such as secondary to congenital heart disease and valvular or pericardial diseases, and helps to identify the fulminant course of the disease. Classical findings include global hypokinesia, with or without pericardial effusion, variable degree of myocardial dilation and atrioventricular regurgitation.(7) In this report, the patient presented severe left ventricular systolic dysfunction, and the initial echocardiographic evaluation was performed by a pediatric ICU physician, which anticipated the detection of myocardial dysfunction and directed the early therapy to fluid resuscitation and vasoactive drugs. Other studies in the literature also report the advantages of medical emergency physicians being trained to perform echocardiograms, specifically echocardiograms used for the evaluation of ventricular systolic function and blood volume.(8)
Histological examination of the myocardium, although considered the gold standard by certain experts, presents risks related to the procedure, which may make it prohibitive in critically ill patients. Moreover, this procedure has limited utility due to sampling errors, variations in interpretation, and undefined sensitivity for the detection of viral genomes.(9) Immunohistochemical analysis for specific markers, such as CD3 T lymphocytes, macrophages and leukocyte antigens, may be further used for diagnosis together with coronary angiography and MRI.(6,9,10) The patient did not undergo endomyocardial biopsy, MRI or myocardial scintigraphy due to hemodynamic instability. The post-mortem pathological examination showed deposition of yellowish exudate in leaflets, associated with areas of necrosis in the endocardium with inflammatory infiltrate and degeneration of myocytes, which reinforces the hypothesis of cardiogenic shock due to fulminant myocarditis.
Regarding the treatment of acute viral myocarditis in the pediatric population, there are reports of a slight improvement in left ventricular function and increased survival with the use of high-dose intravenous immunoglobulin, but there is no consensus in the literature.(11,12) Immunosuppressive therapy was beneficial in the subgroup of patients with giant cell acute myocarditis and myocarditis associated with autoimmune disease, although no similar results were observed in patients with acute viral myocarditis.(13) There is some evidence that antiviral and antibacterial therapies show therapeutic benefit, but there has been no randomized controlled study to determine the role of these drugs in the course of myocarditis.(13) In the reported case, the patient did not receive immunosuppressive, immunoglobulin or antiviral therapy, and the treatment was based on intensive hemodynamic and ventilatory support.
Invasive mechanical ventilation is often required in cases of fulminant myocarditis with cardiogenic shock. Oxygen-induced toxicity and high levels of positive end-expiratory pressure should be avoided. Mild to moderate hyperventilation may help to correct acidemia.(14) There are no controlled studies that demonstrate better clinical outcomes with the use of a given ventilation mode in this group of patients. It is known that methods that allow spontaneous respiratory activity are associated with lower hemodynamic repercussions because they tend to produce lower levels of mean airway pressure and pleural pressure. However, patients with severe cardiogenic shock may benefit from controlled ventilatory support, which is associated with reduced oxygen consumption. The choice of ventilatory parameters depends on the experience of the ICU physician and the type of ventilation equipment available in the unit; these parameters should be adjusted individually and dynamically throughout the clinical course to ensure effective minute-volume ventilation, optimize the work of breathing and minimize adverse hemodynamic effects.(15) The ventilatory parameters used are shown in table 2.
The hemodynamic support is based on the use of inotropic drugs, and the need for vasopressor therapy in combination with inotropic therapy is common. Dobutamine is a potent beta-1 agonist with a lower beta-2 and alpha agonist ability; it increases myocardial contractility, reduces systemic vascular resistance and decreases the pressure in the pulmonary capillaries, but it also has an arrhythmogenic effect. Milrinone is a phosphodiesterase-3 inhibitor with inodilatory and lusitropic action. This drug increases systolic volume and cardiac output, reduces the pressure in the pulmonary vasculature and induces arrhythmia less frequently than dobutamine, but its vasodilator effect limits its use in hypotensive patients, and the associated use of vasopressor drugs may be necessary. The arterial vasoconstrictor norepinephrine is useful in cases of hypotension; however, it increases cardiac oxygen consumption with no increase in cardiac output. Epinephrine exerts a similar action to norepinephrine, but with an inotropic effect, particularly when used in low doses. In cases of pulmonary congestion, the use of intravenous diuretics should be considered.(16) In the reported case, the diagnosis of myocardial dysfunction without hypotension guided the choice of milrinone as an initial vasoactive drug. The lack of improvement in systolic left ventricular function (ejection fraction of 27%) and development of hypotension led to the addition of dobutamine and norepinephrine, respectively (Table 2).
Despite intensive treatment, the mortality associated with fulminant myocarditis is high. Ukimura et al. reported a mortality rate of 39% for patients with fulminant myocarditis associated with the H1N1 influenza virus,(17) while Saji et al. reported a mortality rate of 83% in patients with fulminant myocarditis who failed to respond to initial medical treatment and who did not undergo mechanical circulatory support.(1) The use of extracorporeal membrane oxygenation in patients with rapidly progressive heart failure and cardiogenic shock, unresponsive to medical treatment, may reduce mortality from fulminant myocarditis to less than 20%.(18,19) The patient in this case report presented clinical indication for the use of extracorporeal hemodynamic support, but we do not offer this treatment in our service.
Patients who survive acute fulminant myocarditis exhibit a favorable long-term prognosis, with good recovery of cardiac function, which reinforces the importance of adequate hemodynamic support in the acute phase of major cardiovascular instability.(20)
CONCLUSION
The H1N1 influenza virus should be considered an etiologic agent of myocarditis, and features such as seasonality, endemic status and vaccination coverage should be considered. In cases of fulminant progression, the identification of signs and symptoms suggesting greater severity should be immediate, and bedside echocardiography may be useful for the early detection of myocardial dysfunction and for therapeutic guidance. The use of immunosuppressive or antiviral therapy for fulminant myocarditis of viral etiology is controversial; hence, the treatment is based on hemodynamic and ventilatory support. The use of extracorporeal membrane oxygenation therapy appears promising but has not yet been routinely implemented in underdeveloped countries.
ACKNOWLEDGMENTS
We thank the physicians Eduarda Damasceno Bittencourt and Arthur Ferreira Neto, both from the Department of Pathology, for providing the images of the histopathological examination.
Footnotes
Conflict of interests: None.
Responsible editor: Jefferson Piva
REFERENCES
- 1.Saji T, Matsuura H, Hasegawa K, Nishikawa T, Yamamoto E, Ohki H, et al. Comparison of the clinical presentation, treatment, and outcome of fulminant and acute myocarditis in children. Circ J. 2012;76(5):1222–1228. doi: 10.1253/circj.cj-11-1032. [DOI] [PubMed] [Google Scholar]
- 2.Mamas MA, Fraser D, Neyses L. Cardiovascular manifestations associated with influenza virus infection. Int J Cardiol. 2008;130(3):304–309. doi: 10.1016/j.ijcard.2008.04.044. [DOI] [PubMed] [Google Scholar]
- 3.Fineberg HV. Pandemic preparedness and response-lessons from the H1N1 influenza of 2009. N Engl J Med. 2014;370(14):1335–1342. doi: 10.1056/NEJMra1208802. [DOI] [PubMed] [Google Scholar]
- 4.Bratincsák A, El-Said HG, Bradley JS, Shayan K, Grossfeld PD, Cannavino CR. Fulminant myocarditis associated with pandemic H1N1 influenza A virus in children. J Am Coll Cardiol. 2010;55(9):928–929. doi: 10.1016/j.jacc.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Feldman AM, McNamara D. Myocarditis. N Engl J Med. 2000;343(19):1388–1398. doi: 10.1056/NEJM200011093431908. [DOI] [PubMed] [Google Scholar]
- 6.Levi D, Alejos J. Diagnosis and treatment of pediatric viral myocarditis. Curr Opin Cardiol. 2001;16(2):77–83. doi: 10.1097/00001573-200103000-00001. Review. [DOI] [PubMed] [Google Scholar]
- 7.Felker GM, Boehmer JP, Hruban RH, Hutchins GM, Kasper EK, Baughman KL, et al. Echocardiographic findings in fulminant and acute myocarditis. J Am Coll Cardiol. 2000;36(1):227–232. doi: 10.1016/s0735-1097(00)00690-2. [DOI] [PubMed] [Google Scholar]
- 8.Longjohn M, Wan J, Joshi V, Pershad J. Point-of-care echocardiography by pediatric emergency physicians. Pediatr Emerg Care. 2011;27(8):693–696. doi: 10.1097/PEC.0b013e318226c7c7. [DOI] [PubMed] [Google Scholar]
- 9.Baughman KL. Diagnosis of myocarditis: death of Dallas criteria. Circulation. 2006;113(4):593–595. doi: 10.1161/CIRCULATIONAHA.105.589663. [DOI] [PubMed] [Google Scholar]
- 10.Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel-Aty H, Gutberlet M, Prasad S, Aletras A, Laissy JP, Paterson I, Filipchuk NG, Kumar A, Pauschinger M, Liu P, International Consensus Group on Cardiovascular Magnetic Resonance in Myocarditis International Consensus Group on Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol. 2009;53(17):1475–1487. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson J, Hartling L, Vandermeer B, Crumley E, Klassen TP. Intravenous immunoglobulin for presumed viral myocarditis in children and adults. Cochrane Database Syst Rev. 2005;(1): doi: 10.1002/14651858.CD004370.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Camargo PR, Snitcowsky R, da Luz PL, Mazzieri R, Higuchi ML, Rati M, et al. Favorable effects of immunosuppressive therapy in children with dilated cardiomyopathy and active myocarditis. Pediatr Cardiol. 1995;16(2):61–68. doi: 10.1007/BF00796819. [DOI] [PubMed] [Google Scholar]
- 13.Kindermann I, Barth C, Mahfoud F, Ukena C, Lenski M, Yilmaz A, et al. Update on myocarditis. J Am Coll Cardiol. 2012;59(9):779–792. doi: 10.1016/j.jacc.2011.09.074. [DOI] [PubMed] [Google Scholar]
- 14.Cooper HA, Panza JA. Cardiogenic shock. Cardiol Clin. 2013;31(4):567-80, viii. doi: 10.1016/j.ccl.2013.07.009. Review. [DOI] [PubMed] [Google Scholar]
- 15.Corredor C, Jaggar SI. Ventilator management in the cardiac intensive care unit. Cardiol Clin. 2013;31(4):619-36, ix. doi: 10.1016/j.ccl.2013.07.002. Review. [DOI] [PubMed] [Google Scholar]
- 16.Ginsberg F, Parrillo JE. Fulminant myocarditis. Crit Care Clin. 2013;29(3):465–483. doi: 10.1016/j.ccc.2013.03.004. Review. [DOI] [PubMed] [Google Scholar]
- 17.Ukimura A, Izumi T, Matsumori A, Clinical Research Committee on Myocarditis Associated with 2009 Influenza A (H1N1) Pandemic in Japan organized by Japanese Circulation Society A national survey on myocarditis associated with the 2009 influenza A (H1N1) pandemic in Japan. Circ J. 2010;74(10):2193–2199. doi: 10.1253/circj.cj-10-0452. [DOI] [PubMed] [Google Scholar]
- 18.Duncan BW, Bohn DJ, Atz AM, French JW, Laussen PC, Wessel DL. Mechanical circulatory support for the treatment of children with acute fulminant myocarditis. J Thorac Cardiovasc Surg. 2001;122(3):440–448. doi: 10.1067/mtc.2001.115243. [DOI] [PubMed] [Google Scholar]
- 19.Bohn D, Macrae D, Chang AC. Acute viral myocarditis: mechanical circulatory support. Pediatr Crit Care Med. 2006;7(6):S21–S24. [Google Scholar]
- 20.Schultz JC, Hilliard AA, Cooper LT, Jr, Rihal CS. Diagnosis and treatment of viral myocarditis. Mayo Clin Proc. 2009;84(11):1001–1009. doi: 10.1016/S0025-6196(11)60670-8. [DOI] [PMC free article] [PubMed] [Google Scholar]