SUMMARY
The origin and developmental pathway of intestinal T cell receptor αβ+ CD4−CD8β− intraepithelial lymphocytes (unconventional iIELs), a major population of innate-like resident cytolytic T cells, have remained elusive. By cloning and expressing several TCRs isolated from unconventional iIELs, we identified immature CD4loCD8lo(DPlo)CD69hiPD-1hi thymocytes as the earliest postsignaling precursors for these cells. Although these precursors displayed multiple signs of elevated TCR signaling, a sizeable fraction of them escaped deletion to selectively engage unconventional iIEL differentiation. Conversely, TCRs cloned from DPloCD69hiPD-1hi thymocytes, a population enriched in autoreactive thymocytes, selectively gave rise to unconventional iIELs upon transgenic expression. Thus, the unconventional iIEL precursor overlaps with the DPlo population undergoing negative selection, indicating that, concomitant with the downregulation of both CD4 and CD8 coreceptors, a balance between apoptosis and survival signals results in outcomes as divergent as clonal deletion and differentiation to the unconventional iIEL lineage.
INTRODUCTION
In addition to conventional TCRαβ+ CD4+ or CD8+ resident effector cells, whose origin and antigen specificity are well understood, the intestinal epithelium harbors unique and abundant innate-like cytolytic lymphocytes that include not only TCRγδ+CD4−CD8β− cells but also a prominent population of TCRαβ+CD4−CD8β− lymphocytes called unconventional iIELs (Cheroutre et al., 2011). Together with the recently identified ILC1 subset, these innate-like populations represent long-lived resident lineages that express a conspicuously similar program dominated by the expression of the transcription factor Tbet, expression of natural killer (NK) cell receptors, and interleukin-15 (IL-15)-regulated homeostasis or function (Fuchs et al., 2013). Although there is no complete understanding of the individual capabilities of each iIEL subset, their similar gene expression programs suggest largely overlapping functions that include homeostatic crosstalk with intestinal epithelial cells through the expression of the herpesvirus entry mediator (HVEM)-receptor CD160, and with microbiota and diet through the expression of the aryl hydrocarbon receptor (Ahr) (Li et al., 2011; Shui et al., 2012). They can be rapidly activated in various microbial infections, where they are thought to function independently of MHC-peptide ligands through various cytolytic stress-specific NK-lineage receptors (Guy-Grand et al., 1996). They can also promote repair and regeneration of the epithelium through the secretion of various growth factors, and they can directly kill intestinal bacteria through the release of antimicrobial peptides (Boismenu and Havran, 1994; Ismail et al., 2011). Although these striking properties of mucosal host defense have been well described, the origin and development of these innate-like iIEL lineages have remained elusive.
This is particularly vexing in the case of the TCRαβ+ “unconventional” iIELs, because the origin and development of other TCRαβ+ T cells in general, with progression from CD4−CD8− (DN) to CD4+CD8+ DP and the required signaling events after TCRαβ expression, have been largely elucidated. How mature DN TCRαβ+ cells are selected and the sequence of their developmental process, e.g., whether they bypass or transit through a DP stage, have not been directly elucidated.
Some studies have suggested extrathymic origins based on the presence of unconventional iIELs in nude mice, and their expression of “forbidden” TCRβ chains that are usually removed by mouse mammary tumor virus-encoded superantigen mediated clonal deletion in the thymus (Guy-Grand et al., 1992; Poussier and Julius, 1994; Rocha et al., 1991). Further, transfer of lineage-negative cells from cryptopatches of nude mice into irradiated SCID mice generated unconventional iIELs but not splenic T cells (Saito et al., 1998). However, the thymus clearly plays a role because nude mice have drastically reduced numbers of unconventional iIELs.
Other studies have searched for putative thymic precursors by cell transfers into congenic recipients, but have come to different conclusions. In one study, DN2 and DN3 thymocytes intravenously transferred into thymectomized Rag2−/−Il2rg−/− mice gave rise to unconventional iIELs suggesting that the iIEL precursor might leave the thymus at the DN stage (Lambolez et al., 2006). In other studies, transfer of TCRαβ−DPhi thymocytes that stained with thymus leukemia antigen (TL)-tetramers specific for CD8αα homodimers (termed triple-positive [TP]) into irradiated recipients selectively generated unconventional iIELs (Gangadharan et al., 2006). Because these results rely on cell transfers into lymphopenic recipients, it is unclear whether these pathways are functional in a lymphoreplete mouse. A more recent study transferred thoracic duct lymphocytes from Rag-GFP mice into unirradiated recipients and revealed the presence of unconventional iIEL precursors with a naive CD62Lhi DN TCRαβ+ phenotype, therefore implying that some unconventional iIELs are primed and acquire effector phenotype in the periphery (Guy-Grand et al., 2013). These different conclusions could be reconciled if there existed different pathways that are selectively recapitulated by the different experimental approaches. Alternatively, low-efficiency cell transfers may not be a reliable method for identifying rare iIEL precursors.
Several studies have suggested that the unconventional iIEL lineage might be induced, at least in part, through encounter of agonist antigens in the thymic environment (Leishman et al., 2002; Levelt et al., 1999; Pobezinsky et al., 2012; Rocha et al., 1992; Yamagata et al., 2004). Such agonist signaling has been firmly demonstrated for other lineages such as natural killer T (NKT) cells and T regulatory (Treg) cells through direct studies of TCR signaling in thymic developmental intermediates (Moran et al., 2011; Seiler et al., 2012). In the case of unconventional iIELs, agonist signaling was inferred from examples of TCR transgenic systems where transgenic expression or exogenous addition of the agonist ligand resulted in the differentiation of DN cells with an activated cytolytic effector phenotype and innate properties similar to that of unconventional iIELs. However, the relevance of these studies was challenged on two grounds. First, the TCRs examined were not cloned from unconventional iIELs but from peripheral CD4+ or CD8+ T cells. Second, premature expression of TCRαβ, which is observed in most transgenic systems, invariably leads to the differentiation of a population of “lineage-confused” γδ-like DN cells, which would be at least partially unresponsive to agonist antigen due to the absence of coreceptor expression. These lineage-confused cells could be activated in the thymus or in the periphery and home to the gut. For example, premature expression of the HY TCR in transgenic male mice generated abundant unconventional iIEL-like cells, but delayed expression of the HY TCR until the DP stage of development decreased their number 50- to 100-fold (Baldwin et al., 2005). Even in the absence of agonist ligand, most TCR transgenic mice with premature expression of TCRα contain populations of gut-seeking DN T cells, which are capable of upregulating CD8αα homodimers in some conditions (Bruno et al., 1996; Fritsch et al., 1998; Terrence et al., 2000). This experimental artifact could be readily corrected by delaying TCRα chain expression to the DP stage, using a Cd4-driven Cre-lox system (Baldwin et al., 2005). In fact, two elegant approaches using the same Cd4-driven Cre-lox system to delete Rag or to fate-map iIELs have independently supported the conclusion that TCRα rearrangement in unconventional IEL precursors occurred after CD4 gene expression and that all unconventional iIELs, but not, for example, their TCRγδ+ counterparts, have gone through a CD4-expressing stage (Eberl and Littman, 2004; Hendricks and Fink, 2009).
The nature of the thymic ligands of unconventional iIELs is unknown, although TCRs specific for both classical and nonclassical MHC class I have been suggested by studies of mice lacking both H-2Kb and Db, or lacking β2-microglobulin, respectively, which displayed increasing defects in iIEL frequencies (Das and Janeway, 1999; Gapin et al., 1999; Park et al., 1999). Class II ligands have also been inferred from studies of mice expressing mouse mammary tumor retroviral superantigens (Pobezinsky et al., 2012). In these mice, “forbidden” superantigen-reactive Vβ-expressing clones accumulated among unconventional iIELs, a process increased by deletion of B7 ligands or their CD28 receptor, suggesting that this ligand-receptor pair increased thymic deletion of superantigen-reactive thymocytes and implying that unconventional iIELs might include the progeny of thymocytes recognizing mouse mammary tumor virus (MMTV)-encoded superantigens presented by cells lacking costimulation.
In contrast with previous reports, we examined the fate of thymocytes expressing TCR of natural unconventional iIELs. We utilized TCR expression methods that allowed for proper TCR expression kinetics. Further, we made use of mixed bone marrow chimeras where transgenic cells were diluted with wild-type cells to minimize the spurious development of unconventional iIELs in monoclonal environments. We found that TCRs cloned from unconventional iIELs consistently and selectively gave rise to unconventional iIELs. We identified their thymic developmental pathway, going from a classical DPhiTCRαβ− precursor to a peculiar DPloTCRαβ+ cell expressing high amounts of CD69, PD-1, Egr2, and Nur77, the hallmarks of thymocytes undergoing elevated TCR signaling. Furthermore, a large fraction of these signaled cells underwent Bim- and caspase 3-mediated deletion, whereas those that survived acquired key aspects of unconventional iIEL lineage differentiation first in the thymus, then in the periphery. Because the DPloPD-1hi phenotype has been previously associated with conventional DP thymocytes undergoing negative selection, we cloned TCRαβ directly from the accumulated population of DPloPD-1hi thymocytes in Bcl-xL transgenic mice and found that they consistently induced the unconventional iIELs lineage differentiation when transgenically expressed in thymocytes. Thus, we conclude that unconventional iIEL arise from the general population of thymocytes undergoing negative selection and clonal deletion by self-ligands expressed in the thymus.
RESULTS
TCR Specificity Directs Unconventional iIEL Development
To facilitate cloning of iIEL TCRs, we fixed the TCRβ chain by using one of two different TCRβ transgenes, an approach that facilitates TCR repertoire analysis by restricting TCR diversity to the TCRα chain, while preserving the frequency and function of unconventional iIELs and the diversity of their TCRα repertoire (Figures 1A–1C). Note that, as reported, each mouse harbors one or two expanded iIEL clones that can mask the underlying diversity of the TCR repertoire (Regnault et al., 1994). Using this approach, we cloned multiple Vα2+ TCR from unconventional iIELs and, as a control, conventional iIELs, and forced their expression in wild-type thymocytes using several different approaches (Table 1).
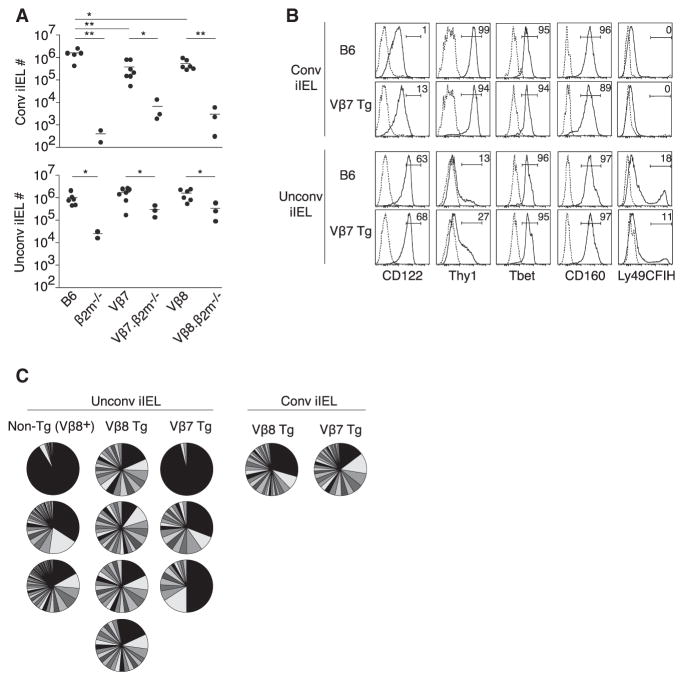
Figure 1. TCRβ Transgenic Mice Express Normal Populations of Conventional and Unconventional iIELs with Diverse TCRα Chains.
(A) Absolute numbers of conventional and unconventional TCRαβ+ iIELs in individual mice of indicated genotypes, including two different Vβ transgenic strains.
(B) Phenotype of conventional and unconventional iIELs in representative mice as indicated. Data representative of two experiments.
(C) Pie charts representing Vα2+ TCR diversity in conventional and unconventional TCRαβ+ iIELs sorted from wild-type Vβ8+ iIELs or from Tg Vβ7+ or Tg Vβ8+ iIELs. A pie represents an individual mouse, where each slice corresponds to a different TCR Vα2 sequence and the size of the slice is proportional to the relative frequency of the sequence. The large expanded clones in individual mice expressed different TCRs. Data combined from six experiments.
Table 1.
Description of TCRs Used
| TCR | Vβ | Vα | Jα | Junction Sequence | Expression Method | |||
|---|---|---|---|---|---|---|---|---|
| Tg | Tgcond | RVcond1 | RVcond2 | |||||
| Conventional | ||||||||
| C1 | 7 | TRAV14D-3/DV8 | 52 | CAVPNTGANTGKLTF | + | − | − | − |
| C2 | 7 | TRAV14D-3/DV8 | 37 | CAAPGNTGKLIF | + | − | − | − |
| C3 | 7 | TRAV14-2 | 45 | CAASDKTEGADRLTF | + | − | − | − |
| C4 | 7 | TRAV14-2*02 | 22 | CAVASSGSWQLIF | + | + | − | − |
| C5 | 7 | TRAV14D-1 | 32 | CAASEIYGSSGNKLIF | + | + | − | − |
| C6 | 8 | TRAV14-1 | 50 | CAAGASSSFSKLVF | − | − | + | − |
| Unconventional | ||||||||
| U1 | 7 | TRAV14D-1 | 23 | CAASEGYNQGKLIF | + | + | − | − |
| U2 | 7 | TRAV14D-1 | 30 | CAARAYKVIF | + | − | − | − |
| U3 | 7 | TRAV14D-1 | 43 | CAANWNNNAPRF | + | − | − | − |
| U4 | 7 | TRAV14-1 | 49 | CAASAGSYCTGYQNFYF | + | + | − | − |
| U5 | 8 | TRAV14D-3/DV8 | 26 | CAASYNYAQGLTF | − | − | + | − |
| U6 | 8 | TRAV14D-1 | 52 | CAASGGTGANTGKLTF | − | − | + | − |
| Negative Selection | ||||||||
| NS1 | 7 | TRAV14D-3/DV8 | 56 | CAASSCRGGNNKLTF | − | − | + | − |
| NS2 | 7 | TRAV14-2 | 23 | CAGGRNYNQGKLIF | − | − | + | − |
| NS3 | 7 | TRAV14-2 | 48 | CAASAGGCGNEKITF | − | − | + | − |
| NS4 | 7 | TRAV14D-3/DV8 | 30 | CAASGCTNAYKVIF | − | − | − | + |
| NS5 | 7 | TRAV14D-3/DV8 | 11 | CAASEPGYNKLTF | − | − | − | + |
| NS6 | 7 | TRAV14D-3/DV8 | 9 | CAAPNMGYKLTF | − | − | − | + |
| NS7 | 8 | TRAV14-1 | 7 | CAASSYSNNRLTL | − | − | − | + |
| NS8 | 8 | TRAV14-1 | 15 | CAASRGGRALIF | − | − | − | + |
| NS9 | 8 | TRAV14-1 | 49 | CAANTGYQNFYF | − | − | − | + |
Information on each TCR used includes the Vβ transgenic strain from which it was isolated, the Vα2 (TRAV14) subfamily, the Jα gene, the sequence of the junction between Vα and Jα, and the expression method. C-series TCRs are from conventional CD8αβ+TCRαβ+ iIELs; U-series TCRs are from unconventional CD4−CD8β−TCRαβ+ iIELs; NS-series TCRs are from DPloTCRαβhiPD-1hi thymocytes of Bcl-xL transgenic mice. RVcond1 has an IRES-tailless human CD4 and RVcond2 has an IRES-Thy1.1.
First, we found that, under conditions of premature TCRα transgenic expression, both conventional (C-series) and unconventional (U-series) iIEL TCRs generated substantial populations of CD4−CD8−TCRαβ+ DN thymocytes, splenocytes, and iIELs (Figure 2A, C2-Tg and U1-Tg; Figure S1 available online). These findings confirm a recent report and underlie why past TCR transgenic approaches were not appropriate to study iIEL development (Baldwin et al., 2005). Despite this “DN artifact,” however, we could already discern that the two classes of TCRs were associated with radically different developmental pathways. Unconventional iIEL TCRs induced a DPlo population in the thymus, without generating classical DPhi cells or any CD4+ or CD8+ SP cells. In contrast, conventional iIEL TCRs generated DPhi but not DPlo thymocytes and a “conventional-like” population of CD8αβ T cells in thymus, spleen, and (to a variable extent) in the intestinal epithelium (Figure 2A, compare C2-Tg and U1-Tg).
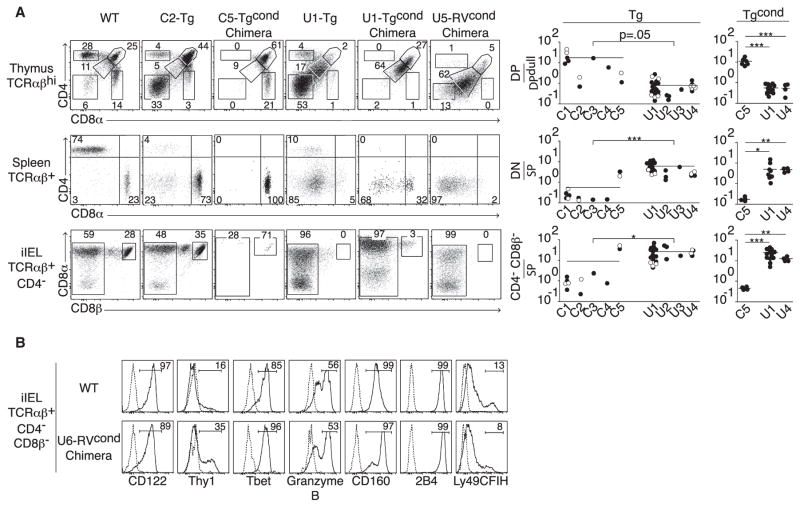
Figure 2. Distinct TCRs Direct Unconventional iIEL Development.
(A) Left: Flow cytometry of thymic, splenic, and intestinal lymphocyte populations, gated as indicated, from WT littermate and various TCR Tg, TCR Tgcond, and TCR RVcond mice. Conditional (cond) expression of Tg- or RV-encoded TCRα chain was controlled by Cd4-Cre. C-series and U-series TCRs were isolated from conventional and unconventional TCRαβ+ iIELs, respectively. In chimeras, the FACS profiles are further gated on cells expressing the Tg- or RV-encoded TCR. Right: Summary of lymphocyte populations in thymus, spleen, and iIELs as labeled to the left induced by various C-series and U-series TCRs generated with a Tg expression vector (prematurely expressed) or with a conditional vector activated by Cd4-Cre, Tgcond. Open circles denote individual mice from a second founder line for a given TCR. SP denotes CD4+ or CD8+ single-positive cells. p values were calculated by Student’s t test.
(B) Comparison of unconventional iIELs from WT and iIELs RVcond mice. Dotted line is unstained or isotype control. Data are representative of 33 experiments involving 12 different TCRs (6 conventional and 6 unconventional).
See also Figure S1.
To avoid premature TCR expression, we used conditional transgenic (Tgcond) or retroviral (RVcond) systems to express the TCRα chain under the control of a Cd4-driven Cre-lox system. Furthermore, to avoid artifacts associated with monoclonal T cell populations, we examined mixed bone marrow chimeras in which cells expressing the iIEL TCRs were competing with congenically marked wild-type (non-Tg non-RV) cells. Under these stringent conditions, TCRs from conventional CD8αβ iIELs exclusively induced the maturation of naive CD8αβ T cells in thymus and spleen, without CD4−CD8β− cells (Figure 2A, C5-Tgcond chimera; Figure S1). In addition, they often induced a small population of conventional CD8αβ iIELs, consistent with the notion that these cells originate from naive CD8 T cells and might encounter antigen in the gut environment (Sheridan and Lefrançois, 2011). In contrast, unconventional iIEL TCRs exclusively induced a DPlo phenotype in the thymus and led primarily to the generation of CD4−CD8β−/lo cells found in spleen and intestinal epithelium (Figure 2A, U1-Tgcond chimera and U5-RVcond chimera; Figure S1). These cells had all the characteristics of unconventional iIELs, including expression of high amounts of the IL-2 receptor β (IL2Rβ) CD122, NK-lineage markers such as 2B4 and Ly49, CD160, granzyme B, and the transcription factor Tbet, as well as a partial downregulation of the surface receptor Thy1 (Figure 2B). They also variably expressed CD8α, but with low or undetectable CD8β. These results were confirmed more broadly by examining 12 different TCR cloned from either conventional or unconventional iIELs, demonstrating the exquisite contribution of TCR specificity in directing unconventional iIEL development (Figure 2A and data not shown).
Thymic Precursors of Unconventional iIELs Undergo Strong TCR Signaling and Massive Apoptosis
The DPlo phenotype associated with unconventional iIEL TCRs was reminiscent of that of autoreactive DP thymocytes undergoing Bim-mediated negative selection as described in multiple systems (Bouillet et al., 2002; Kreslavsky et al., 2013; McCaughtry et al., 2008; Stritesky et al., 2013). Indeed, we found that DP thymocytes expressing unconventional iIEL TCRs exhibited hallmarks of elevated TCR signaling, including increased amounts of CD69, CD5, PD-1, Egr2, Nur77, and Bim as compared with those expressing conventional iIEL TCRs or nontransgenic TCRs (Figure 3A). Consistent with elevated Bim expression, they also had increased active caspase-3 and increased staining with annexin-V, suggesting ongoing apoptosis (Figure 3B). Using continuous BrdU labeling in vivo, we measured a turnover time of 3 days for the population of U1-TgcondTCR+CD69+ “signaled” thymocytes, which, combined with their absolute number of 9 × 106, allowed us to measure a turnover rate of 3 × 106 cells per day, contrasting with 0.1 × 106 thymic emigrants enumerated by examining all peripheral tissues, including gut, 24 hr after intrathymic injection of biotin (Figure 3C). Wild-type signaled thymocytes had a turnover rate of 2 × 106 cells per day and a thymic emigration rate of 0.8 × 106 cells per day. These calculations suggest a massive thymic deletion rate of 97% after signaling by unconventional iIEL TCRs, contrasting with little deletion for conventional TCRs. Furthermore, the DPlo cells failed to incorporate BrdU after a short pulse, indicating that apoptosis occurred in the absence of cell division.
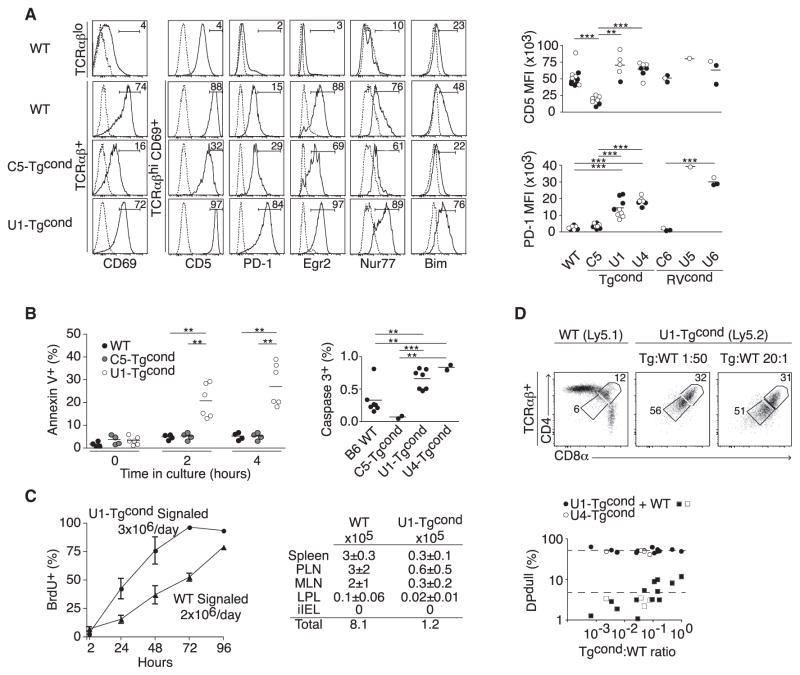
Figure 3. Thymic Precursors of Unconventional iIELs Undergo Strong TCR Signaling and Massive Apoptosis.
(A) Left: Analysis of markers associated with TCR signal strength in TCRαβlo and TCRαβhi thymocytes of WT littermate and Tgcond mice expressing a conventional (C5) or unconventional (U1) TCR. Data are representative of at least five independent experiments. Right: Summary plots of replicate experiments in different TCR Tg or RV mice, chimeras, or mixed chimeras showing the mean fluorescence intensity (MFI) of surface CD5 and PD-1 on TCRαβ+CD69+ thymocytes from mice of indicated genotypes. For WT and Tgcond experiments, filled circles denote data derived from mixed chimeras and open circles denote data from WT or Tgcond mice. For RVcond experiments, filled circles denote data from mixed chimeras and open circles denote data from straight chimeras. p values were calculated by Student’s t test.
(B) The percentage of Annexin V+ cells after different times of culture and the percentage of active caspase 3+ cells directly ex vivo among TCRαβ+ thymocytes are shown for indicated strains, with each dot representing an individual mouse, assayed in two to four independent experiments.
(C) BrdU incorporation among signaled (TCR+CD69+) thymocytes from U1-Tgcond or WT littermate under continuous administration of BrdU. Calculated turnover rates are shown. Thymic emigrant numbers (mean ± SEM) in different organs of U1-Tgcond or WT littermate measured 24 hr after intrathymic injection of biotin. Abbreviations are as follows: PLN, peripheral lymph nodes; MLN, mesenteric lymph nodes; LPL, small intestine lamina propria lymphocytes. Data pooled from two to three independent experiments.
(D) DPlo induction by unconventional U1 and U4 iIEL TCRs at low Tg cell frequency. Top: Representative dot plots in mixed chimeras with various U1:WT ratios. Bottom: Summary plots with dotted lines representing the average for WT or iIEL TCRs.
The thymic attrition rate after TCR signaling could be due to a cell-intrinsic mechanism or to a cell-extrinsic requirement for a limited amount of survival factors or “niche” space. In bone marrow chimeras where thymocytes expressing the unconventional iIEL TCRαβ were mixed at low frequencies with wild-type cells, the deletional phenotype and the generation of unconventional iIELs were fully preserved, even at a low ratio of 1:1,000 (Figure 3D), confirming the cell-intrinsic nature of this pathway and ruling out, for example, the type of cell-extrinsic competition effects reported for the generation of regulatory T cells (Bautista et al., 2009; Leung et al., 2009; Malchow et al., 2013).
Clonal Deletion Limits the Maturation and Export of iIEL Precursors
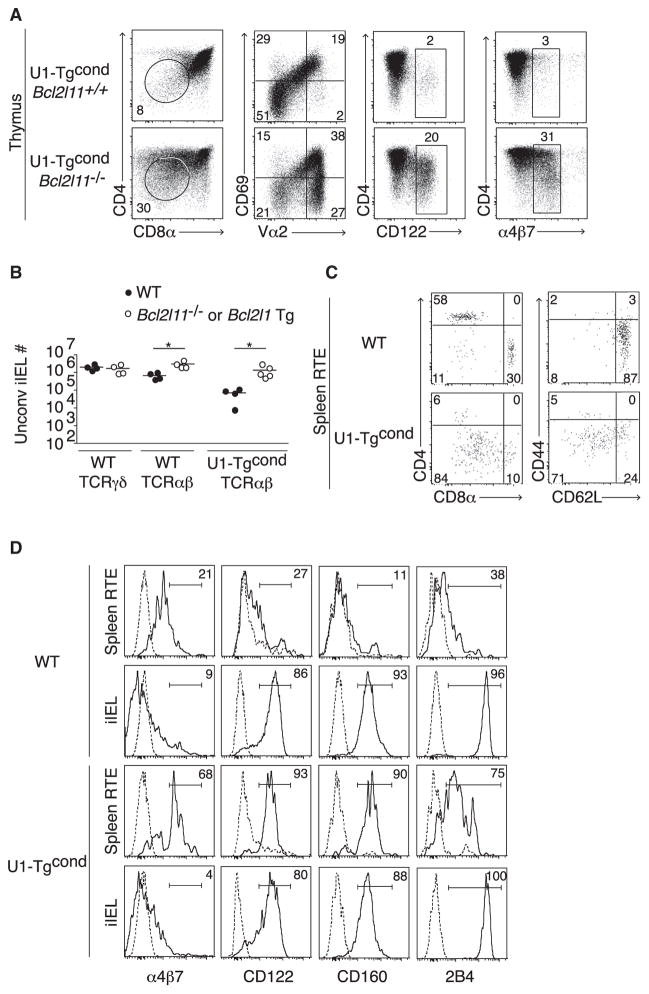
To further demonstrate that intrathymic apoptosis was limiting export of unconventional iIEL precursors, we reduced the rate of apoptosis by introducing a Bcl-xL transgene or Bim mutated alleles, which increased the frequency of mature thymocytes expressing CD122 and the intestinal homing integrin α4β7 (Figure 4A) as well as increased over 20-fold the numbers of unconventional iIELs (Figure 4B). Importantly, non-TCR transgenic mice expressing a Bcl2l1 (Bcl-xL) transgene or Bcl2l11 (Bim) mutated alleles also had increased TCRαβ+ unconventional iIELs, although the number of TCRγδ+ iIELs was notably unaffected (Figure 4B).
Figure 4. Clonal Deletion Limits the Maturation and Export of iIEL Precursors.
(A) Bim mutation rescues iIEL DN thymocytes (circled) with a CD122hiα4β7hi phenotype.
(B) Number of TCRγδ+ or TCRαβ+ unconventional iIELs from WT littermate or Bcl-xL transgenic or Bim-deficient mice.
(C and D) Phenotype of recent thymic emigrants (RTE) in the spleen (C) or spleen and iIEL (D) of WT littermate and U1-Tgcond mice. Dotted line is isotype control.
Representative of two (A, C, D) or four (B) independent experiments.
To directly characterize the rare cells emerging from the massive thymic deletion, we injected biotin intrathymically and analyzed the phenotype of streptavidin-bound recent thymic emigrants 24 hr later. Most were found in the spleen, where they expressed a CD4−CD8β− phenotype, had largely downregulated CD62L, and had increased expression of the intestinal homing integrin α4β7, CD122, CD160, and 2B4 (Figures 4C and 4D). Of note, once in the iIEL compartment, 2B4 was expressed in higher amounts while α4β7 was downregulated compared with the splenic RTE, suggesting that whereas thymic selection drives the acquisition of a portion of the effector phenotype of unconventional iIELs, further maturation unfolds or occurs in a stepwise manner in the periphery. In summary, we conclude that, like autoreactive thymocytes undergoing deletion, thymocytes expressing unconventional iIEL TCRs exhibited the hallmarks of elevated signaling and massive apoptotic cell death in the absence of cell division. A small proportion of these cells, however, were consistently rescued and survived to acquire a typical CD4−CD8β− iIEL program and selectively populate the intestinal epithelium.
Recognition of MHC Class I or Class II Ligands in the Presence of CD8 or CD4 Cause Thymic Deletion
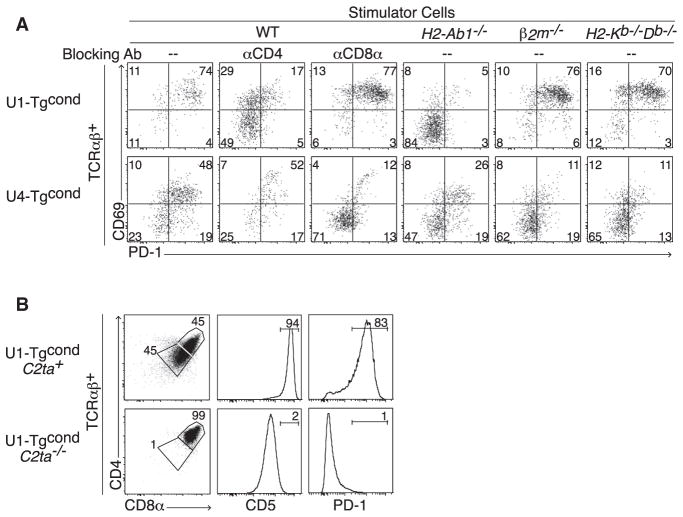
To test whether thymic deletion was a consequence of interaction with MHC ligands, we used in vitro and in vivo assays. In vitro, we incubated DPhi thymocytes, purified based on their “unsignaled” TCRβ−CD69−PD-1− phenotype, with spleen cells from wild-type, H2-Ab1−/−, B2 m−/−, or H2-Kb−/−Db−/− mice. Figure 5A shows that U1-Tgcond thymocytes adopted a CD69hiPD-1hi phenotype in the presence of WT and MHC class I-deficient spleen cells but not in the presence of MHC class II-deficient cells. Conversely, in vivo, the DPloCD69hiPD-1hi phenotype of U1-Tgcond thymocytes was specifically lost in the absence of their corresponding MHC II ligand and reverted to a DPhiCD69−PD-1− phenotype typical of “unsignaled” thymocytes, without generation of single-positive cells (Figure 5B). Thus, we conclude that the U1 TCR exhibits autoreactivity to MHC class II-restricted ligands. In contrast, in the case of the U4-Tgcond DPhi thymocytes cultured with wild-type and MHC-deficient spleen cells, we found autoreactivity specifically to classical MHC class I ligands (Figure 5A). Furthermore, autoreactivity to MHC class II or class I ligands was inhibited by anti-CD4 or anti-CD8α antibodies, respectively (Figure 5A). Thus, the DPloCD69hiPD-1hi phenotype could be induced after corecognition of either MHC class I or class II ligands by unconventional iIEL TCRs in the presence of corresponding CD8 or CD4 coreceptors.
Figure 5. Recognition of MHC Class I or Class II Ligands Cause Thymic Deletion.
(A) U1 and U4 TCR signaling after culture of purified TCRαβ−PD-1− thymocytes for 48 hr in the presence of splenocytes from WT or various MHC-deficient mice, or with blocking anti-CD4 or CD8α antibodies, as indicated.
(B) Frequency of DPlo and intensity of TCR signaling (estimated by CD5 and PD-1) in U1-Tgcond thymocytes in WT versus C2ta−/− background. Representative of four independent experiments.
TCRs Isolated from Thymocytes Undergoing Negative Selection Induce Unconventional iIEL Development
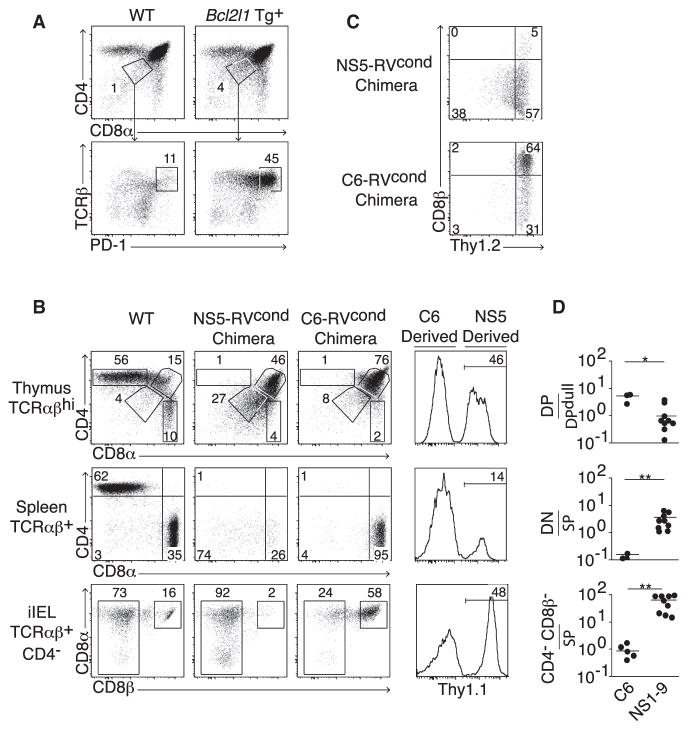
We next asked whether the induction of unconventional CD4−CD8β− iIEL differentiation was limited to a select group of autoreactive TCRs or was a property of most autoreactive TCRs. To examine natural autoreactive TCRs, we sorted DPloPD-1hi thymocytes from Bcl-xL transgenic mice where this population is markedly increased due to delayed apoptosis (Figure 6A; Chao et al., 1995). After cloning of individual TCRs and generation of conditional retroviral chimeras, we observed that nine out of nine TCRs (termed NS1–NS9, see Table 1) induced a DPlo phenotype with the hallmarks of elevated TCR signaling in the thymus of wild-type mice (Figures 6B and 6D). Detailed in vitro analysis of NS7 and NS9 demonstrated that the DPlo phenotype for these TCRs was dependent on recognition of MHC class I (data not shown). When the selecting MHC was present, a fraction of T cells expressing these “deletional” TCRs survived and differentiated in sufficient numbers to fully and selectively repopulate the pool of unconventional iIELs with characteristic downregulation of the T cell marker Thy1 (Figures 6B–6D). In contrast, T cells expressing a conventional iIEL TCR selectively repopulated the CD8αβ T cell pool when examined side-by-side with deletional T cells in competitive bone marrow chimeras. Altogether, these results demonstrate that unconventional iIELs are derived from cells that escaped negative selection.
Figure 6. NS-TCR Isolated from Thymocytes Undergoing Negative Selection Induce the Development of Unconventional iIELs.
(A) The strategy for isolation of NS-TCR from thymocytes undergoing clonal deletion is based on the sorting of DPloTCRαβhiPD-1hi cells that are rescued in the Bcl-xL Tg thymus.
(B) Head-to-head comparison of the thymic, splenic, and iIEL populations induced by a deletional thymocyte TCR (NS5) and a conventional iIEL TCR (C6) within the same mixed (NS5-RVcond:C6-RVcond) chimera. A WT mouse is shown for comparison. The rightmost column shows the fraction of TCR+ cells in each tissue that are derived from the NS5 versus C6 TCR.
(C) Selective downregulation of Thy1 in NS5-derived iIELs.
(D) Summary of thymic, splenic, and iIEL populations (labeled to the left of B) induced by negatively selecting TCR NS1 through NS9.
Data are representative of two (A and C) or nine (B) independent experiments. p values by Student’s t test.
DISCUSSION
This study reveals that, in addition to clonal deletion, an unexpected but consistent outcome of negative selection for DP thymocytes expressing a wide range of MHC class I- or MHC class II-autoreactive TCRαβ consists of differentiation into unconventional CD4−CD8β−iIELs. Our results demonstrate that the precursors to unconventional iIELs correspond largely to a population of TCRαβ+ thymocytes defined by a DPlo phenotype and displaying signs of elevated TCR signaling, including high amounts of CD69, CD5, PD-1, Egr2, and Nur77, as well as increased expression of the proapoptotic factors, Bim, and activated caspase 3. This population originates from TCRαβ− DPhi thymocytes and immediately follows TCR expression and engagement by agonist self-ligands presented by MHC class I or class II molecules in a coreceptor-dependent manner.
At a single-cell clonal level, the outcome of self-antigen encounter in the thymus seems to play out in a probabilistic manner, with a greater likelihood of undergoing deletion, but also with a significant opportunity to survive and differentiate into unconventional iIELs. In fact, given that Bim is induced downstream of TCR signaling and that its deletion in Bim-deficient thymocytes was sufficient to considerably increase the rate of survival of thymocytes expressing an individual autoreactive TCR as well as their conversion into iIELs, the decision between deletion or iIEL differentiation may be related in part to the amount of Bim reached in a particular cell and perhaps therefore to the intensity of TCR signaling.
The relationship between this signaled precursor and earlier TCRαβ− DP thymocytes expressing CD8αα homodimers (so-called TP) is unclear (Gangadharan et al., 2006). TP cells do not express a surface TCR but they have previously been suggested to selectively generate iIELs upon intrathymic transfer, perhaps because CD8αα homodimers would modify TCR signaling in some way by binding to TL ligands (Gangadharan et al., 2006). However, neither TL-deficient mice nor CD8α-deficient mice (data not shown) exhibited obvious alterations of their unconventional iIEL population (Olivares-Villagómez et al., 2008). Therefore, although our results do not address the previous suggestion of an unsignaled TP precursor, they unambiguously identify the committed unconventional iIEL precursor to be the DPloPD-1hi thymocyte that expresses an autoreactive TCR and has been signaled by the corresponding agonist thymic self-ligands. Our results are in agreement with previous work that identified a population of TCRαβ+ DN thymocytes sensitive to CD28-mediated negative selection that were able to selectively generate unconventional iIELs upon transfer (Pobezinsky et al., 2012). However, that study did not directly demonstrate that precise TCR sequences taken from the rescued DN thymocytes were found in the unconventional iIELs of WT mice and it did not test the prediction that forced expression of B7 ligands in the thymus should remove all unconventional iIELs. Nevertheless, the availability of B7:CD28 costimulation for a given antigen encounter on select thymic cells is an attractive candidate mechanism that might modulate the rate of negative selection versus diversion for a particular autoreactive thymocyte.
Our results help to explain a number of findings reported previously. For example, despite their innate-like properties, unconventional iIELs fail to display a conserved semi-invariant TCR, as in other innate TCRαβ+ lymphocytes such as NKT cells or mucosal-associated invariant T (MAIT) cells, but in fact express a broad polyclonal repertoire, which is masked in part by clonal expansions linked to microbiota exposure in the intestinal environment (Regnault et al., 1996; Treiner and Lantz, 2006). This large TCR repertoire is consistent with the iIEL origin from the broad pool of DPloPD-1hi autoreactive thymocytes which is reactive to a range of MHC ligands, including MHC class II and both classical and nonclassical class I. Our results are also consistent with studies suggesting that thymocytes bearing transgenic TCR cloned from conventional T cells and exposed to agonist ligands added as transgenes in vivo or exogenous peptides in vitro could in some cases differentiate into unconventional iIEL-like cells (Leishman et al., 2002; Levelt et al., 1999; Yamagata et al., 2004), although, as demonstrated by others and confirmed in this study, these studies were systematically confounded by premature expression of TCRα chain, which in and by itself, in the absence of agonist ligand, is sufficient to drive the CD4−CD8β− lineage. The finding that agonist signaling drives unconventional iIEL development is also consistent with the report that mice lacking store-operated calcium flux due to loss of stromal interaction molecule 1 (STIM1) and STIM2 show defective NKT cells and Treg cells but also unconventional iIELs (Oh-Hora et al., 2013). Although each of these agonist pathways operate between the classical positive and negative selection schemes, they rely on distinct components of TCR signaling. For example, Egr2 and nerve growth factor IB (Nur77) are highly expressed in all DPlo precursors but iIELs do not upregulate the corresponding target genes Plzf and forkhead box P3 (Foxp3) (not shown), which characterize the NKT and Treg cell lineages, respectively. Nur77 is also highly expressed in NKT precursors without induction of Foxp3 (Lee et al., 2013). These rapidly emerging observations suggest that additional aspects of signaling are intricately but differentially involved in the generation of distinct agonist-selected lineages. Furthermore, little negative selection and considerable cell division are observed after NKT cell precursor signaling whereas iIEL precursors exhibit a high rate of apoptosis without cell division (Bendelac et al., 2007).
The identification of a DPloPD-1hi precursor, itself originating from TCRαβ−DPhi thymocytes, is also consistent with genetic studies demonstrating that unconventional iIELs develop through a stage where the Cd4 promoter is active. Our results do not, however, rule out other mechanisms of iIEL development, although the consistent outcomes observed from the random sampling of a total of 21 different TCRs of different intra-epithelial or intrathymic origin isolated from 2 different TCRβ transgenic lines suggest that these alternative pathways must be rare. For example, a recent study analyzed the cells found in thoracic duct lymph and found that naive CD62Lhi T cells exiting the thymus recirculated through blood and lymphatics before they acquired the effector program in the intestinal environment (Guy-Grand et al., 2013).
There is a striking overlap in phenotype between TCRγδ+ and TCRαβ+ unconventional iIELs, which has led some to suggest that they share common developmental themes (Denning et al., 2007; Pennington et al., 2003). However, these population-level analyses did not focus on the most prevalent TCRγδ+ iIEL subset, which expresses Vγ7+ TCRs; this has led to several confusing and contradictory results. Specifically, one group claimed the effector program of TCRγδ+ iIELs was acquired in the thymus while another suggested it happened in the periphery (Lin et al., 1999; Pennington et al., 2003). The first study analyzed bulk populations whereas the second study used irrelevant TCRs such as the G8 TCRγδ that was derived from an alloreactive clone isolated from the peripheral lymph node of a nude mouse (Bluestone et al., 1988). A careful study focusing on iIEL-relevant Vγ7+ TCRs revealed that TCRγδ+ iIEL precursors exited the thymus as naive before being primed in the Peyer’s patch (Guy-Grand et al., 2013). Here, we observed that the acquisition of the effector phenotype of unconventional TCRαβ+ iIEL precursors began in the thymus. Further, we demonstrated that the absolute number of TCRγδ+ iIELs was unaffected by overexpression of Bcl-xL or deletion of Bim whereas unconventional TCRαβ+ iIELs expanded. Thus, although the functional programs of mature TCRαβ+ and TCRγδ+ iIELs appear very similar, the developmental pathways and requirements may differ significantly.
It is significant that both CD4 and CD8 coreceptors are down-regulated in the developmental process, thereby reducing the avidity of interaction with self-ligands in the periphery, as directly shown here. Furthermore, other studies have shown that, after maturation, unconventional iIELs responded poorly to TCRαβ stimulation but strongly to cytokines such as IL-12, IL-18, and IL-15 and to NK receptor ligands (Guy-Grand et al., 1996; Wencker et al., 2014). These striking properties suggest that, although TCR autoreactivity is required to drive lineage differentiation of unconventional iIELs in the thymus, a radical change ensues whereby the mature progeny shifts to an innate mode of recognition by expressing the set of NK receptors and cytokine receptors that characteristically activate unconventional iIELs in the intestinal environment, allowing this unusual lineage to become an integral component of the crosstalk between microbes, diet, and intestinal epithelium.
EXPERIMENTAL PROCEDURES
Mice
WT or mutant mice were purchased from Jackson Laboratories or Taconic. TCR Vβ7 transgenic mice were previously described and maintained in our colony (Savage et al., 2011). See also Supplemental Experimental Procedures.
TCR Transgenic Constructs
TCRα and TCRβ transgenic mice were generated using previously described constructs (Kouskoff et al., 1995). Conditional transgenic mice were generated using a construct from Hogquist et al. (Baldwin et al., 2005). The construct for generating conditional retrovirus was previously described (Turner et al., 2010). See also Supplemental Experimental Procedures.
Antibodies and Flow Cytometry
Fluorochrome- or biotin-conjugated monoclonal antibodies from commercial vendors were used before analyzing samples on an LSRII (Becton Dickinson) or sorting on a FACSAria (Becton Dickinson). Data were analyzed using FlowJo (Tree Star). See also Supplemental Experimental Procedures.
Retrovirus Production, Infection, and Chimera Generation
Conditional retrovirus was produced using PLAT-E cells after cloning TCRα to a previously described construct (Morita et al., 2000; Turner et al., 2010). TCR Vβ7 or Vβ8 transgenic Cd4-cre mice (usually also Tcra−/−) were injected with 5-Fluorouracil (APP Pharmaceuticals) 3 days prior to bone marrow harvest. After harvest, bone marrow was cultured for 2 days in X-Vivo 10 (Lonza) supplemented with 15% FCS, 1% penicillin/streptomycin, mouse SCF, mouse IL-3, and human IL-6 (all from Biolegend). Stimulated cells were infected with retrovirus in the presence of polybrene (EMD Millipore) by spinfection. After 24 hr of additional culture, bone marrow cells were stained with antibodies against human CD4 or mouse Thy1.1, MACS-enriched (Miltenyi Biotec), and injected into lethally irradiated recipient mice. See also Supplemental Experimental Procedures.
TCR Sequencing
Relevant populations were sorted and TCR were sequenced as previously described and analyzed using IMGT (Lefranc et al., 2009; Savage et al., 2011). See also Supplemental Experimental Procedures.
Generation of Mixed Bone Marrow Chimeras
CD45.1 mice were lethally irradiated with 1,000 Rads from a gamma cell 40 irradiator with a cesium source. Irradiated mice were injected i.v. with a mixture of CD3ε-depleted bone marrow cells isolated from Tgcond and CD45.1 congenic mice at various ratios. Mice were analyzed at least 5 weeks after reconstitution.
Cell Proliferation and Turnover
For BrdU pulse, 1 mg of BrdU (Sigma-Aldrich) was injected i.v. 2 hr prior to analysis. For turnover studies, BrdU was administrated in drinking water at 0.8 mg ml−1 with 2% sucrose and changed daily. Cells were analyzed using the APC BrdU Flow kit (BD Biosciences).
Thymic Emigration Assays
One or two lobes of the thymus were injected with 10 μl of a 0.5 mg ml−1 solution of Sulfo-NHC-LC-Biotin (Thermo Fisher Scientific). See also Supplemental Experimental Procedures.
In Vitro Thymocyte Culture
For stimulation cultures, thymocytes were depleted of CD3ε+ (or TCRαβ+) and PD-1+ cells by AutoMACS to enrich for unsignaled DP thymocytes, which were cultured with splenocytes from WT or from different MHC-deficient mice at a responder/stimulator ratio of 1 × 105/5 × 105 for 48 hr prior to analysis. For Annexin V assays, thymocytes were isolated and cultured for 0, 2, or 4 hr in RPMI-10% FCS at 37°C.
Statistical Analysis
Statistical analysis was performed in Prism (Graph Pad Software) using the unpaired t test. If the groups that were compared had significantly different variances (p < 0.05 by F test), Welch’s correction was applied. *p < 0.05; **p < 0.01; ***p < 0.001.
Supplementary Material
Acknowledgments
We thank P. Savage for discussion and advice; P. Verhoef for assistance with intrathymic injection; R. Brink, D. Mathis, and K. Hogquist for providing plasmids for TCR expression; R. Locksley for an IRES-thy1.1 plasmid; D. Leclerc, J. Cao, M. Olsen, and R. Duggan for cell sorting; and L. Degenstein for production of mice. B.D.M. and J.J.B. were supported by an NIH Medical Scientist Training Program grant T32GM007281. This work was supported by NIH grant R01AI038339 and by HHMI (A.B.), by NIH grant RO1 DK067180 (B.J.), and by Digestive Diseases Research Center of Excellence P30DK42086.
Footnotes
Supplemental Information includes one figure and Supplemental Experimental Procedures and can be found with this article online at http://dx.doi.org/10.1016/j.immuni.2014.07.008.
AUTHOR CONTRIBUTIONS
B.D.M. designed research, performed experiments, and analyzed data. J.J.B. and I.E.I. helped perform experiments. B.J. and A.B. supervised the research. B.D.M. and A.B. wrote the paper.
References
- Baldwin TA, Sandau MM, Jameson SC, Hogquist KA. The timing of TCR alpha expression critically influences T cell development and selection. J Exp Med. 2005;202:111–121. doi: 10.1084/jem.20050359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista JL, Lio CW, Lathrop SK, Forbush K, Liang Y, Luo J, Rudensky AY, Hsieh CS. Intraclonal competition limits the fate determination of regulatory T cells in the thymus. Nat Immunol. 2009;10:610–617. doi: 10.1038/ni.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- Bluestone JA, Cron RQ, Cotterman M, Houlden BA, Matis LA. Structure and specificity of T cell receptor gamma/delta on major histocompatibility complex antigen-specific CD3+, CD4−, CD8− T lymphocytes. J Exp Med. 1988;168:1899–1916. doi: 10.1084/jem.168.5.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boismenu R, Havran WL. Modulation of epithelial cell growth by intraepithelial gamma delta T cells. Science. 1994;266:1253–1255. doi: 10.1126/science.7973709. [DOI] [PubMed] [Google Scholar]
- Bouillet P, Purton JF, Godfrey DI, Zhang LC, Coultas L, Puthalakath H, Pellegrini M, Cory S, Adams JM, Strasser A. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415:922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- Bruno L, Fehling HJ, von Boehmer H. The alpha beta T cell receptor can replace the gamma delta receptor in the development of gamma delta lineage cells. Immunity. 1996;5:343–352. doi: 10.1016/s1074-7613(00)80260-5. [DOI] [PubMed] [Google Scholar]
- Chao DT, Linette GP, Boise LH, White LS, Thompson CB, Korsmeyer SJ. Bcl-XL and Bcl-2 repress a common pathway of cell death. J Exp Med. 1995;182:821–828. doi: 10.1084/jem.182.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheroutre H, Lambolez F, Mucida D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat Rev Immunol. 2011;11:445–456. doi: 10.1038/nri3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das G, Janeway CA., Jr Development of CD8alpha/alpha and CD8alpha/beta T cells in major histocompatibility complex class I-deficient mice. J Exp Med. 1999;190:881–884. doi: 10.1084/jem.190.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning TL, Granger SW, Mucida D, Graddy R, Leclercq G, Zhang W, Honey K, Rasmussen JP, Cheroutre H, Rudensky AY, Kronenberg M. Mouse TCRalphabeta+CD8alphaalpha intraepithelial lymphocytes express genes that down-regulate their antigen reactivity and suppress immune responses. J Immunol. 2007;178:4230–4239. doi: 10.4049/jimmunol.178.7.4230. [DOI] [PubMed] [Google Scholar]
- Eberl G, Littman DR. Thymic origin of intestinal alphabeta T cells revealed by fate mapping of RORgammat+ cells. Science. 2004;305:248–251. doi: 10.1126/science.1096472. [DOI] [PubMed] [Google Scholar]
- Fritsch M, Andersson A, Petersson K, Ivars F. A TCR alpha chain transgene induces maturation of CD4− CD8− alpha beta+ T cells from gamma delta T cell precursors. Eur J Immunol. 1998;28:828–837. doi: 10.1002/(SICI)1521-4141(199803)28:03<828::AID-IMMU828>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Fuchs A, Vermi W, Lee JS, Lonardi S, Gilfillan S, Newberry RD, Cella M, Colonna M. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-γ-producing cells. Immunity. 2013;38:769–781. doi: 10.1016/j.immuni.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangadharan D, Lambolez F, Attinger A, Wang-Zhu Y, Sullivan BA, Cheroutre H. Identification of pre- and postselection TCRalphabeta+ intraepithelial lymphocyte precursors in the thymus. Immunity. 2006;25:631–641. doi: 10.1016/j.immuni.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Gapin L, Cheroutre H, Kronenberg M. Cutting edge: TCR alpha beta+ CD8 alpha alpha+ T cells are found in intestinal intraepithelial lymphocytes of mice that lack classical MHC class I molecules. J Immunol. 1999;163:4100–4104. [PubMed] [Google Scholar]
- Guy-Grand D, Vanden Broecke C, Briottet C, Malassis-Seris M, Selz F, Vassalli P. Different expression of the recombination activity gene RAG-1 in various populations of thymocytes, peripheral T cells and gut thymus-independent intraepithelial lymphocytes suggests two pathways of T cell receptor rearrangement. Eur J Immunol. 1992;22:505–510. doi: 10.1002/eji.1830220232. [DOI] [PubMed] [Google Scholar]
- Guy-Grand D, Cuénod-Jabri B, Malassis-Seris M, Selz F, Vassalli P. Complexity of the mouse gut T cell immune system: identification of two distinct natural killer T cell intraepithelial lineages. Eur J Immunol. 1996;26:2248–2256. doi: 10.1002/eji.1830260942. [DOI] [PubMed] [Google Scholar]
- Guy-Grand D, Vassalli P, Eberl G, Pereira P, Burlen-Defranoux O, Lemaitre F, Di Santo JP, Freitas AA, Cumano A, Bandeira A. Origin, trafficking, and intraepithelial fate of gut-tropic T cells. J Exp Med. 2013;210:1839–1854. doi: 10.1084/jem.20122588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks DW, Fink PJ. Uneven colonization of the lymphoid periphery by T cells that undergo early TCRalpha rearrangements. J Immunol. 2009;182:4267–4274. doi: 10.4049/jimmunol.0804180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail AS, Severson KM, Vaishnava S, Behrendt CL, Yu X, Benjamin JL, Ruhn KA, Hou B, DeFranco AL, Yarovinsky F, Hooper LV. Gammadelta intraepithelial lymphocytes are essential mediators of host-microbial homeostasis at the intestinal mucosal surface. Proc Natl Acad Sci USA. 2011;108:8743–8748. doi: 10.1073/pnas.1019574108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouskoff V, Signorelli K, Benoist C, Mathis D. Cassette vectors directing expression of T cell receptor genes in transgenic mice. J Immunol Methods. 1995;180:273–280. doi: 10.1016/0022-1759(95)00002-r. [DOI] [PubMed] [Google Scholar]
- Kreslavsky T, Kim HJ, Koralov SB, Ghitza D, Buch T, Cantor H, Rajewsky K, von Boehmer H. Negative selection, not receptor editing, is a physiological response of autoreactive thymocytes. J Exp Med. 2013;210:1911–1918. doi: 10.1084/jem.20130876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambolez F, Arcangeli ML, Joret AM, Pasqualetto V, Cordier C, Di Santo JP, Rocha B, Ezine S. The thymus exports long-lived fully committed T cell precursors that can colonize primary lymphoid organs. Nat Immunol. 2006;7:76–82. doi: 10.1038/ni1293. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Holzapfel KL, Zhu J, Jameson SC, Hogquist KA. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat Immunol. 2013;14:1146–1154. doi: 10.1038/ni.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefranc MP, Giudicelli V, Ginestoux C, Jabado-Michaloud J, Folch G, Bellahcene F, Wu Y, Gemrot E, Brochet X, Lane J, et al. IMGT, the international ImMunoGeneTics information system. Nucleic Acids Res. 2009;37(Database issue):D1006–D1012. doi: 10.1093/nar/gkn838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leishman AJ, Gapin L, Capone M, Palmer E, MacDonald HR, Kronenberg M, Cheroutre H. Precursors of functional MHC class I- or class II-restricted CD8alphaalpha(+) T cells are positively selected in the thymus by agonist self-peptides. Immunity. 2002;16:355–364. doi: 10.1016/s1074-7613(02)00284-4. [DOI] [PubMed] [Google Scholar]
- Leung MW, Shen S, Lafaille JJ. TCR-dependent differentiation of thymic Foxp3+ cells is limited to small clonal sizes. J Exp Med. 2009;206:2121–2130. doi: 10.1084/jem.20091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levelt CN, de Jong YP, Mizoguchi E, O’Farrelly C, Bhan AK, Tonegawa S, Terhorst C, Simpson SJ. High- and low-affinity single-peptide/MHC ligands have distinct effects on the development of mucosal CD8alphaalpha and CD8alphabeta T lymphocytes. Proc Natl Acad Sci USA. 1999;96:5628–5633. doi: 10.1073/pnas.96.10.5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Innocentin S, Withers DR, Roberts NA, Gallagher AR, Grigorieva EF, Wilhelm C, Veldhoen M. Exogenous stimuli maintain intra-epithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 2011;147:629–640. doi: 10.1016/j.cell.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Lin T, Yoshida H, Matsuzaki G, Guehler SR, Nomoto K, Barrett TA, Green DR. Autospecific gammadelta thymocytes that escape negative selection find sanctuary in the intestine. J Clin Invest. 1999;104:1297–1305. doi: 10.1172/JCI7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malchow S, Leventhal DS, Nishi S, Fischer BI, Shen L, Paner GP, Amit AS, Kang C, Geddes JE, Allison JP, et al. Aire-dependent thymic development of tumor-associated regulatory T cells. Science. 2013;339:1219–1224. doi: 10.1126/science.1233913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaughtry TM, Baldwin TA, Wilken MS, Hogquist KA. Clonal deletion of thymocytes can occur in the cortex with no involvement of the medulla. J Exp Med. 2008;205:2575–2584. doi: 10.1084/jem.20080866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita S, Kojima T, Kitamura T. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 2000;7:1063–1066. doi: 10.1038/sj.gt.3301206. [DOI] [PubMed] [Google Scholar]
- Oh-Hora M, Komatsu N, Pishyareh M, Feske S, Hori S, Taniguchi M, Rao A, Takayanagi H. Agonist-selected T cell development requires strong T cell receptor signaling and store-operated calcium entry. Immunity. 2013;38:881–895. doi: 10.1016/j.immuni.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares-Villagómez D, Mendez-Fernandez YV, Parekh VV, Lalani S, Vincent TL, Cheroutre H, Van Kaer L. Thymus leukemia antigen controls intraepithelial lymphocyte function and inflammatory bowel disease. Proc Natl Acad Sci USA. 2008;105:17931–17936. doi: 10.1073/pnas.0808242105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Guy-Grand D, Lemonnier FA, Wang CR, Bendelac A, Jabri B. Selection and expansion of CD8alpha/alpha(1) T cell receptor alpha/beta(1) intestinal intraepithelial lymphocytes in the absence of both classical major histocompatibility complex class I and nonclassical CD1 molecules. J Exp Med. 1999;190:885–890. doi: 10.1084/jem.190.6.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington DJ, Silva-Santos B, Shires J, Theodoridis E, Pollitt C, Wise EL, Tigelaar RE, Owen MJ, Hayday AC. The inter-relatedness and interdependence of mouse T cell receptor gammadelta+ and alphabeta+ cells. Nat Immunol. 2003;4:991–998. doi: 10.1038/ni979. [DOI] [PubMed] [Google Scholar]
- Pobezinsky LA, Angelov GS, Tai X, Jeurling S, Van Laethem F, Feigenbaum L, Park JH, Singer A. Clonal deletion and the fate of autoreactive thymocytes that survive negative selection. Nat Immunol. 2012;13:569–578. doi: 10.1038/ni.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poussier P, Julius M. Thymus independent T cell development and selection in the intestinal epithelium. Annu Rev Immunol. 1994;12:521–553. doi: 10.1146/annurev.iy.12.040194.002513. [DOI] [PubMed] [Google Scholar]
- Regnault A, Cumano A, Vassalli P, Guy-Grand D, Kourilsky P. Oligoclonal repertoire of the CD8 alpha alpha and the CD8 alpha beta TCR-alpha/beta murine intestinal intraepithelial T lymphocytes: evidence for the random emergence of T cells. J Exp Med. 1994;180:1345–1358. doi: 10.1084/jem.180.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnault A, Levraud JP, Lim A, Six A, Moreau C, Cumano A, Kourilsky P. The expansion and selection of T cell receptor alpha beta intestinal intraepithelial T cell clones. Eur J Immunol. 1996;26:914–921. doi: 10.1002/eji.1830260429. [DOI] [PubMed] [Google Scholar]
- Rocha B, Vassalli P, Guy-Grand D. The V beta repertoire of mouse gut homodimeric alpha CD8+ intraepithelial T cell receptor alpha/beta + lymphocytes reveals a major extrathymic pathway of T cell differentiation. J Exp Med. 1991;173:483–486. doi: 10.1084/jem.173.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha B, von Boehmer H, Guy-Grand D. Selection of intraepithelial lymphocytes with CD8 alpha/alpha co-receptors by self-antigen in the murine gut. Proc Natl Acad Sci USA. 1992;89:5336–5340. doi: 10.1073/pnas.89.12.5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H, Kanamori Y, Takemori T, Nariuchi H, Kubota E, Takahashi-Iwanaga H, Iwanaga T, Ishikawa H. Generation of intestinal T cells from progenitors residing in gut cryptopatches. Science. 1998;280:275–278. doi: 10.1126/science.280.5361.275. [DOI] [PubMed] [Google Scholar]
- Savage AK, Constantinides MG, Bendelac A. Promyelocytic leukemia zinc finger turns on the effector T cell program without requirement for agonist TCR signaling. J Immunol. 2011;186:5801–5806. doi: 10.4049/jimmunol.1100119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler MP, Mathew R, Liszewski MK, Spooner CJ, Barr K, Meng F, Singh H, Bendelac A. Elevated and sustained expression of the transcription factors Egr1 and Egr2 controls NKT lineage differentiation in response to TCR signaling. Nat Immunol. 2012;13:264–271. doi: 10.1038/ni.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan BS, Lefrançois L. Regional and mucosal memory T cells. Nat Immunol. 2011;12:485–491. doi: 10.1038/ni.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shui JW, Larange A, Kim G, Vela JL, Zahner S, Cheroutre H, Kronenberg M. HVEM signalling at mucosal barriers provides host defence against pathogenic bacteria. Nature. 2012;488:222–225. doi: 10.1038/nature11242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stritesky GL, Xing Y, Erickson JR, Kalekar LA, Wang X, Mueller DL, Jameson SC, Hogquist KA. Murine thymic selection quantified using a unique method to capture deleted T cells. Proc Natl Acad Sci USA. 2013;110:4679–4684. doi: 10.1073/pnas.1217532110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrence K, Pavlovich CP, Matechak EO, Fowlkes BJ. Premature expression of T cell receptor (TCR)alphabeta suppresses TCRgammadelta gene rearrangement but permits development of gamma-delta lineage T cells. J Exp Med. 2000;192:537–548. doi: 10.1084/jem.192.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treiner E, Lantz O. CD1d- and MR1-restricted invariant T cells: of mice and men. Curr Opin Immunol. 2006;18:519–526. doi: 10.1016/j.coi.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Turner VM, Gardam S, Brink R. Lineage-specific transgene expression in hematopoietic cells using a Cre-regulated retroviral vector. J Immunol Methods. 2010;360:162–166. doi: 10.1016/j.jim.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Wencker M, Turchinovich G, Di Marco Barros R, Deban L, Jandke A, Cope A, Hayday AC. Innate-like T cells straddle innate and adaptive immunity by altering antigen-receptor responsiveness. Nat Immunol. 2014;15:80–87. doi: 10.1038/ni.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata T, Mathis D, Benoist C. Self-reactivity in thymic double-positive cells commits cells to a CD8 alpha alpha lineage with characteristics of innate immune cells. Nat Immunol. 2004;5:597–605. doi: 10.1038/ni1070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.