Abstract
Ecobiotechnological approach is an attractive and economical strategy to enrich beneficial microbes on waste biomass for production of Polyhydroxyalkanoate (PHA). Here, six strains of Bacillus spp. were used to produce co-polymers of PHA from pea-shells. Of the 57 mixed bacterial cultures (BCs) screened, two of the BCs, designated as 5BC1 and 5BC2, each containing 5 strains could produce PHA co-polymer at the rate of 505–560 mg/l from feed consisting of pea-shell slurry (PSS, 2 % total solids) and 1 % glucose (w/v). Co-polymer production was enhanced from 65–560 mg/l on untreated PSS to 1,610–1,645 mg/l from PSS treated with defined hydrolytic bacteria and 1 % glucose. Supplementation of the PSS hydrolysate with sodium propionate enabled 5BC1 to produce co-polymer P(3HB-co-3HV) with a 3HV content up to 13 % and a concomitant 1.46-fold enhancement in PHA yield. Using the principles of ecobiotechnology, this is the first demonstration of PHA co-polymer production by defined co-cultures of Bacillus from biowaste as feed under non-axenic conditions.
Electronic supplementary material
The online version of this article (doi:10.1007/s12088-014-0457-9) contains supplementary material, which is available to authorized users.
Keywords: Bacillus, Biowaste, Co-polymer, Defined mixed culture, Volatile fatty acid
Introduction
Petroleum-based plastics are a constant burden on the ever dwindling fossil fuel reserves and being non-biodegradable, these add to environmental pollution [1, 2]. The commonly accepted alternative to this issue is the production of biodegradable plastics such as polyhydroxyalknoates (PHAs). A large number of microbes have been reported to produce PHAs under stressed physiological conditions [3, 4]. However, the process is still uneconomical as more than 45 % of the production cost is incurred on feed material and recovery process [5]. Another limiting factor is the brittle nature of most widely produced homopolymer—polyhydroxybutyrate (PHB). In order to circumvent the issues of production cost and the quality of the polymer, quite a few efforts have been made. It has been realized that co-polymers of PHA such as P(3HB-co-3HV) have better physical properties and a broad range of applications. The production of PHA and its co-polymers have been reported from cheap and renewable sources like biowastes such as olive oil mill pomace, dairy wastes, oily waste, industrial waste, agricultural waste, sugar, molasses, cannery and paper mill wastes, biodiesel production waste, etc. [4, 6–8]. The major apprehension in employing a single bacterial strain for driving a biotechnological process, especially if biowastes are being used as feed, is the presence of unwanted microflora accompanying it. The process is likely to be highly fluctuative. Use of well-defined feed stocks and aseptic condition results in higher production costs. Here, it is desirable to exploit the principles of ecobiotechnology, where natural selection and competition are the prime factors. It engineers the ecosystem rather than the PHA producing organism [9, 10]. Unlike the use of undefined mixed culture for the enrichment of a desirable bacteria, which may be in the minority or may not even exist, the use of defined high PHA producing organisms as mixed bacterial culture is likely to drive the process successfully. Here, Bacillus could be a potent contender as a robust bacterium. Bacillus is an established organism which can withstand adverse conditions, by undergoing sporulation, compete well with other organisms by producing antibiotics and antibacterials such as acylhomoserine lactonases, biosurfactants, hydrolytic enzymes [1, 11–13]. Its candidature is further supported by its status as an organism, which is generally regarded as safe (GRAS) by the Food and Drug Administration [4, 12]. In addition, the most important feature is that Bacillus spp. are among those few organisms, which can produce homopolymer and co-polymer of PHA from pure substrates as well as biological wastes [4, 14, 15]. Thus, defined co-cultures consisting of exclusively Bacillus spp. are likely to succeed in utilizing biowastes to produce co-polymers of PHA. In this study, it has been shown that co-polymers of PHA using pea-shells (PS) as feed can be produced by integrating various approaches: (i) use co-culture of bacteria to hydrolyze biowaste, (ii) use well defined co-culture of Bacillus strains to produce PHA, (iii) use precursors as a supplement for co-polymer production from biowastes.
Materials and Methods
Organisms and Growth Conditions
Bacterial strains, isolated in our laboratory, were used for preparing: (a) mixed hydrolytic culture (MHC2) using high hydrolytic bacteria—Bacillus sphaericus EGU542; Bacillusthuringiensis EGU378; Bacillus sp. EGU85, Bacillus sp. EGU367, Bacillus sp. EGU447, and Proteus mirabilis EGU30 [16, 17], and (b) PHA producing co-cultures using—Bacillus cereus EGU3, EGU43, EGU44 and EGU520; B. thuringiensis EGU45 and Bacillus sp. EGU75 [5]. Bacterial strains were grown on nutrient broth_ 13 g/l (HiMedia, Mumbai, India) and incubated at 37 °C at 200 rev/min. The actively growing bacterial cultures were centrifuged at 5,600 g for 20 min and protein content was estimated by Lowry’s method [16].
Preparation of Co-cultures for PHA Production
A total fifty seven combinations of Bacillus co-cultures (BCs) were prepared using 6 Bacillus strains, where each contained 2-6 strains and were designated as 2BC1-15, 3BC1-20, 4BC1-15, 5BC1-6 and 6BC1 (Table S1). In each BC, all isolates were mixed in equal proportions to achieve a final cell protein concentration of 1 μg/ml in the case of GM2 medium and 10 μg/ml in the case of the PSS [16].
PHA Production and Analysis
PHB Production on GM2 Medium
Screening of 57 BCs for their ability to produce PHB was done by inoculating them at the rate of 1 μg cell protein/ml into GM2 medium containing (g/l): Yeast extract, 1.0; K2HPO4, 1.0; MgSO4.7H2O, 0.5; Glucose, 10; pH 7.2. For each BC, 200 ml of the medium was taken in 1 l conical flasks and incubated at 37 °C at 200 rev/min for 48 h. Nine BCs showing high PHB producing abilities were selected for further experimental work. These 9 BCs were analyzed for PHA production at 24, 48 and 72 h after incubation.
PHA Production on Hydrolyzed pea-Shell Slurry
Batch-culture digestion (250 ml) of PS slurry (PSS) consisting of 2 % total solids (TS) was prepared in distilled water by inoculating it with MHC2 and processed as described previously [16]. Uninoculated PSS served as control. PSS hydrolysate (200 ml) supplemented with 1 % glucose (w/v) was inoculated with 9 high PHA producing BCs at the rate of 10 μg cell protein/ml. PHA production was monitored for 24 and 48 h of incubation at 37 °C and 200 rev/min. The effect of hydrolysis by MHC2 on PHA production was estimated by comparing it with the control. High PHA producing mixed culture 5BC1 and B. cereus EGU44 were used for the production of co-polymer from PSS hydrolysate supplemented with precursor substrates: sodium propionate (SP) and valeric acid (VA) at concentrations of 0.5, 1 and 2 % (v/v) at time zero (i) as the C source, (ii) along with 0.5 % glucose, and (iii) after 24 h of growth on glucose. The cultures were incubated at 37 °C, 200 rev/min and analyzed for PHA at 48 h after supplementation with precursor.
PHA Analysis
Aliquots (200 ml) were used to estimate the dry cell mass (DCM) and PHA production as described previously [16]. PHA content was analyzed by GC fitted with dimethylpolysiloxane capillary column DB-1 (30 m × 0.25 mm × 0.25 μm) and flame ionization detector (FID). PHB and P(3HB-co-3HV) (Sigma-Aldrich, USA) were used as standards and benzoic acid as the internal standard.
Volatile Fatty Acid Analysis
Organic acids (1 ml) were sampled after accumulation stage, centrifuged at 10,000 rpm, 4 °C and filtered through a syringe filter (0.45 μm). 1 μl of samples were injected to GC equipped with Chromosorb 101 column (2 m × 0.317 cm × 0.3 mm) with FID. 1 % aqueous solution of SP and VA (GC grade, Sigma-Aldrich, USA) were used as standard [16].
Results
PHB Production on GM2 Medium
Six Bacillus strains used here as defined co-cultures, have been reported to produce homopolymers–PHB [5]. Their PHB contents were found to vary from 190–435 mg/l with a PHB yield of 31–62 % w/w on GM2 medium containing 1 % glucose as C source [5]. A major limitation associated with this bioprocess driven by a single bacterial strain is the risk of getting outcompeted by microbial flora which may accompany biowaste, in case it is to be used as feed. Hence, we used these 6 bacterial cultures in various combinations. It is expected to improve the chances of at least one of them getting enriched, depending upon the type of waste being employed. Fifty seven different co-cultures involving 2–6 bacterial strains were found to produce PHB (mg/l) as follows: (i) 150 with 6BC1, (ii) 115–230 with 5BC1–5BC6, (iii) 5–250 with 4BC1–4BC15, (iv) 10–185 with 3BC1–3BC20, and (v) 120–230 with 2BC1–2BC15 (Table S1).
Nine BCs representing combinations of 2–6 bacteria, each producing PHB in the range of 150–230 mg/l were selected for further processing (Table 1). All these 57 combinations tested on GM2 medium containing 1 % glucose (w/v) were found to produce only PHB. In comparison to the initial screening of 57 BCs tested for PHB production for 48 h, the best 9 BCs were evaluated at 24, 48 and 72 h after incubation. Since no improvement in the PHB yield was recorded by extending the incubation period beyond 48 h, we selected this period for subsequent analysis.
Table 1.
Polyhydroxybutyrate (PHB) producing abilities of mixed cultures of Bacillus spp. on GM2 mediuma
| Bacillus co-culture (BC)b | Incubation period (h) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 24 | 48 | 72 | ||||||||
| DCMc (mg/l) | PHB | DCM (mg/l) | PHB | DCM (mg/l) | PHB | |||||
| mg/l | % DCM | mg/l | % DCM | mg/l | % DCM | |||||
| 6BC1 | 265 | 40 | 15.1 | 315 | 150 | 47.6 | 320 | 140 | 43.8 | |
| 5BC1 | 300 | 100 | 33.3 | 430 | 230 | 53.5 | 485 | 225 | 46.4 | |
| 5BC2 | 410 | 105 | 25.6 | 685 | 230 | 33.6 | 710 | 235 | 33.1 | |
| 4BC1 | 385 | 110 | 28.6 | 530 | 250 | 47.2 | 560 | 240 | 42.9 | |
| 4BC2 | 250 | 95 | 38.0 | 410 | 205 | 50.0 | 435 | 195 | 44.8 | |
| 4BC3 | 220 | 75 | 34.1 | 445 | 190 | 42.7 | 475 | 200 | 42.1 | |
| 3BC1 | 235 | 90 | 38.3 | 430 | 185 | 43.0 | 490 | 190 | 38.8 | |
| 2BC1 | 210 | 120 | 57.1 | 380 | 230 | 60.5 | 430 | 235 | 54.7 | |
| 2BC2 | 265 | 105 | 39.6 | 445 | 220 | 49.4 | 485 | 210 | 43.3 | |
Values are based on three sets of experiments. The standard deviation was less than 5 %
aTotal volume of feed: 200 ml of GM2 media supplemented with 1 % (w/v) glucose
b Bacillus co-culture for PHA production (See Table S1 for details)
cDry cell mass
PHA Production from Biowaste
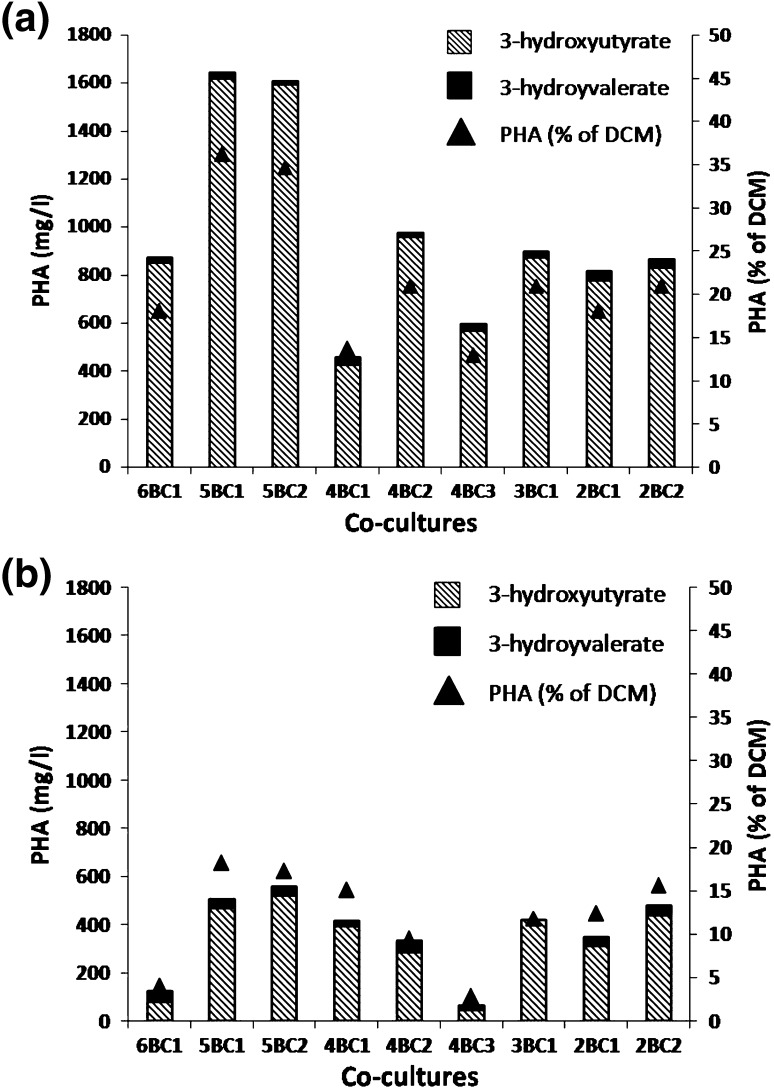
Biowaste hydrolysis leads to release of various simple organic compounds including volatile fatty acids (VFAs). These VFAs may be utilized by bacteria as C source and serve as precursor substrate for PHA production [18–21]. PHA production from PSS supplemented with 1 % glucose (w/v) by 9 selected BCs was found to vary from 65–560 mg/l with a yield of 2.8–18.2 % of DCM. This is in clear contrast with the capacities of BCs on GM2 medium, where they could store up to 60.5 % of DCM as PHB. The interesting observations recorded with PSS were (i) the high DCM in the range of 2,385–3,580 mg/l, and (ii) production of co-polymers P(3HB-co-3HV) (Table 2). At this stage, bacteria known for hydrolyzing PSS efficiently were combined and used as inoculum to solubilize PSS. This treatment of PSS with hydrolytic bacterial co-culture designated as MHC2 [16], lead to three advantages to the PHA producing co-cultures over untreated PSS: i) DCM increased up to 4,850 mg/l, ii) PHA content was enhanced up to 1,645 mg/l and (iii) co-polymer production was improved largely due to higher 3HB content (Fig. 1). Among the 9 BCs, 5BC1 and 5BC2 proved to be the best combinations with unhydrolyzed and hydrolyzed PSS, where they yielded PHA in the range of 505–560 and 1,610–1,645 mg/l, respectively (Table 2). In addition, hydrolysis of PSS resulted in (i) 1.43–1.62-fold higher DCM, (ii) 2.87–3.25-fold improvement in PHA content. Although BCs could produce co-polymers of PHA from PSS, however, hydrolysis of PSS resulted in lowering HV content from 6 to 1 mol%. Efforts were thus made to improve the quality of P(3HB-co-3HV) by supplementing the hydrolyzed PSS with PHA precursor molecules.
Table 2.
Polyhydroxyalkanoate producing abilities of mixed cultures of Bacillus spp. on pea-shell slurry
| Bacillus co-culture (BC)a | Hydrolyzed PSS (MHC2)b | Control PSS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DCM | Polyhydroxyalkanoate | DCM | Polyhydroxyalkanoate | |||||||
| 3HB | 3HV | % DCM | HV mol% | 3HB | 3HV | % DCM | HV mol% | |||
| mg/l | mg/l | mg/l | mg/l | mg/l | mg/l | |||||
| 6BC1 | 4,850 | 855 | 20 | 18.1 | 2 | 3,005 | 80 | 45 | 4.1 | 32 |
| 5BC1 | 4,510 | 1,620 | 25 | 36.2 | 1 | 2,770 | 470 | 35 | 18.2 | 6 |
| 5BC2 | 4,640 | 1,595 | 15 | 34.7 | 1 | 3,230 | 520 | 40 | 17.3 | 6 |
| 4BC1 | 3,330 | 430 | 30 | 13.8 | 6 | 2,745 | 395 | 20 | 15.1 | 4 |
| 4BC2 | 4,670 | 960 | 20 | 21.0 | 2 | 3,525 | 285 | 50 | 9.5 | 13 |
| 4BC3 | 4,590 | 570 | 30 | 13.0 | 4 | 2,385 | 45 | 20 | 2.8 | 28 |
| 3BC1 | 4,390 | 875 | 25 | 21.0 | 2 | 3,580 | 420 | ndc | 11.8 | 0 |
| 2BC1 | 4,540 | 780 | 40 | 18.1 | 4 | 2,815 | 310 | 40 | 12.4 | 10 |
| 2BC2 | 4,205 | 835 | 35 | 21.0 | 4 | 3,080 | 440 | 40 | 15.6 | 7 |
Values are based on three sets of experiments. Standard deviation was less than 5 %
DCM dry cell mass, 3HB 3-hydroxybutyrate, 3HV 3-hydroxyvalerate, nd not detectable
a Bacillus co-culture for PHA production (See Table S1 for details)
bPea-shell slurry, PSS (2 % TS) pretreated for 2 days with MHC2 and supplemented with 1 % w/v glucose
cNot detectable
Fig. 1.
Polyhydroxyalkanoate (PHA) production by different co-cultures of Bacillus spp. (BCs) on pea-shell slurry: a treated with mixed hydrolytic bacteria (MHC2) and b control; Values are based on three sets of experiments. Standard deviation was less than 5 %
Effect of Precursor Substrates on PHA Co-polymer
An analysis of fatty acid profile of PSS hydrolyzed with MHC2 had revealed the production of acetate and butyrate only, the precursors for PHB [16]. Since no other VFAs including VA were detectable in the PSS hydrolysate, it was opined that the addition of SP and VA, the precursors for 3HV, may prove helpful in improving the 3HV content of the co-polymer. Between 5BC1 and 5BC2, the 3HV content was higher with 5BC1 and was thus considered for precursor supplementation studies. Although glucose is a preferred source of C over other substrates, especially for growth and PHB production, it is desirable to minimize the use of glucose as it adds to the cost of production. Secondly, since SP and VA were used as additional sources of odd chain length hydroxyacid monomers, glucose concentration was reduced from 1 to 0.5 % in these experiments. SP as supplement proved effective in enhancing DCM yield from 3,460 in the control to 3,850 mg/l (Table 3). In contrast, addition of VA had a negative effect on DCM of 5BC1. Similar observations were also recorded for PHA contents, which improved from 650 to 1,050 mg/l in feed supplemented with SP, whereas VA was not helpful in this respect. The most interesting feature of the supplemented SP was the significant improvement in the quality of P(3HB-co-3HV): from homopolymer (PHB) in control to co-polymers containing 7–13 % 3HV. Once more VA addition was slightly less effective than SP, however, compared to control 3HV content was improved from 0 to 10 % (Table 3). It may be remarked further that 5BC1 in combination with hydrolyzed PSS supplemented with 3HV precursors could produce up to 84 mg/l 3HV with SP and up to 64 mg/l 3HV in comparison to 25 mg/l of 3HV recorded with hydrolysed PSS. The effect of 5BC1, a combination of 5 Bacillus strains was thus quite evident compared to a single Bacillus strain EGU44 as PHA producing bacteria. With B. cereus EGU44, although DCM was quite similar, PHA content was lower than that recorded with PSS but 3HV contents at/of 10–17 mol% were slightly better than those with PSS.
Table 3.
Effect of precursor substrates on polyhydroxyalkanoates production by Bacillus spp. from pea-shell slurrya
| Precursor substrate added to feed | Substrate (%) | DCM (mg/l) | Polyhydroxyalkanoates | |||
|---|---|---|---|---|---|---|
| mg/l | % DCM | 3HB | 3HV | |||
| mol% | ||||||
| Bacillus co-culture 5BC1 | ||||||
| Controlb | 0.0 | 3,460 | 650 | 18.8 | 100 | 0 |
| Sodium propionate | 0.5 | 3,850 | 650 | 16.9 | 87 | 13 |
| 1.0 | 3,800 | 1,050 | 27.6 | 93 | 7 | |
| 2.0 | 3,600 | 900 | 25.0 | 93 | 7 | |
| Valeric acid | 0.5 | 2,760 | 640 | 23.2 | 90 | 10 |
| 1.0 | 2,340 | 520 | 22.2 | 93 | 7 | |
| 2.0 | 1,810 | 300 | 16.6 | 93 | 7 | |
| Bacillus cereus EGU44 | ||||||
| Control | 0.0 | 4,000 | 1,200 | 30.0 | 100 | 0 |
| Sodium propionate | 0.5 | 3,950 | 855 | 21.6 | 89 | 11 |
| 1.0 | 4,000 | 660 | 16.5 | 84 | 16 | |
| 2.0 | 3,650 | 610 | 16.7 | 85 | 15 | |
| Valeric acid | 0.5 | 3,135 | 525 | 16.7 | 83 | 17 |
| 1.0 | 3,080 | 720 | 23.4 | 90 | 10 | |
| 2.0 | 2,860 | 720 | 24.5 | 90 | 10 | |
Values are based on three sets of experiments. Standard deviation was less than 5 %
DCM dry cell mass, 3HB 3-hydroxybutyrate, 3HV 3-hydroxyvalerate
aPea-shell slurry (2 % TS) pretreated for 2 days with MHC2 and supplemented with 0.5 % (w/v) glucose
bNo precursor substrate added
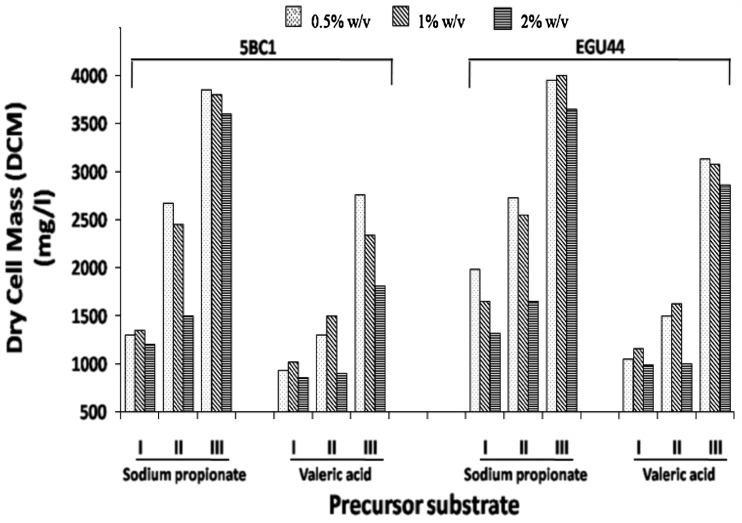
In addition to the impact on PHA accumulation, supplementation of precursors also influenced the DCM. With hydrolysed PSS supplemented with SP or VA, the DCM of 5BC1 was in the range of 1,200–1,300 and 600–800 mg/l, respectively. However, the simultaneous addition of glucose (0.5 % w/v) and precursors (0.5–1.0 % w/v) resulted in higher DCM: 2,500–2,700 mg/l with SP and 1,200–1,400 mg/l with the VA (Fig. 2). A substantial enhancement in DCM was recorded when precursors were added 24 h after the initial incubation: (i) 1.42–2.37-fold with SP (0.5–2.0 % v/v) and (ii) 1.47–2.13-fold with VA (0.5–2.0 % v/v). It may be concluded that the addition of 0.5 % v/v of the precursors after 24 h of incubation is sufficient to get high DCM content. Similar trends in DCM were also recorded with B. cereus EGU44.
Fig. 2.
Bacterial biomass production with co-culture (5BC1) and Bacillus cereus EGU44 using hydrolyzed pea-shell slurry supplemented with different precursor substrates at different time periods: I 0 h, without glucose, II 0 h, with glucose (0.5 %), and III after 24 h growth on glucose (0.5 %); Values are based on three sets of experiments. Standard deviation was less than 5 %
Discussion
Biological processes driven by a single bacterial strain are always at the risk of getting contaminated and destabilized. Ecobiotechnological approach engineers the ecosystem rather than the bacterial strain. It can be achieved through the use of defined cultures. Undefined and mixed cultures have been used quite frequently to produce PHA [22–26], while no defined co-cultures of Bacillus to produce co-polymers of PHA have been reported so far [10]. Here, bacteria may shift their metabolism from the production of homopolymer to co-polymers, especially on supplementation with precursor molecules [1, 27, 28]. B. cereus EGU44 strain, on an individual basis, yielded 62.5 g PHA/kg TS fed [16], which could be enhanced to 71 g PHA/kg TS fed with a mixture of 5 strains (5BC1). Another interesting feature is that pure Bacillus strain could produce only homopolymer–PHB, whereas the use of mixed defined culture lead to the production of co-polymer of PHA (3HB-co-3HV). This improvement in PHA characteristic was seen with shift in physiological conditions as follows: biowaste, hydrolysed biowaste and finally the addition of precursors, leading to changes in co-polymer contents (3HB:3HV, mol%): 94:6, 99:1 and 93:7, respectively (Tables 2 and 3). We could find only one report of Bacillus with an ability to produce PHA co-polymer having 3HV content up to 10 mol% from Madhuca sp. flower extract [29]. Using undefined mixed cultures, P(3HB-co-3HV) having a 3HV content up to 69 % could be achieved on substrates like fermented cannery wastewater sludge, paper mill wastewater or sugar cane molasses [30–32]. More recent efforts using undefined mixed microbial cultures from activated sludge consisting predominantly of Firmicutes and Proteobacteria could yield up to 8 mol% of 3HV on sludge waste water as substrate [23]. In the present study, the two major strategic changes: (i) defined co-cultures to alleviate the risks of failures generally associated with biodegradation of biowastes and (ii) precursors supplementation helped to enhance PHA characteristics and yield. In addition, during co-polymer production, the availability of acetic acid and butyric acid in the hydrolysate of PS proved beneficial in reducing the amount of glucose by half in comparison to 1.0 % (W/v), which was used in the previous study [16]. In fact, the usage of a mixture of VFAs results in the formation of acetyl-CoA and propionyl-CoA as precursors for PHA producers, consequently yielding a co-polymer containing HB and HV monomers [19–21].
This study provides a promising strategy to: (i) exploit the co-polymer producing abilities of Bacillus strains, (ii) use defined combination of Bacillus strains as PHA producers, (iii) use PSS as feed material, (iv) reduce the usage of glucose as C source and (v) enhance the DCM content with biowaste. Since these Bacillus spp. are also known to produce H2 from pea-shells [16], an integrated approach will certainly help to improve the economics of the process.
Electronic supplementary material
Acknowledgments
The authors wish to thank the Director of CSIR-Institute of Genomics and Integrative Biology, Delhi, India, CSIR-WUM (ESC0108) and Department of Biotechnology (DBT-BT/PR-11517/BCE/08/709/2008) Government of India for providing the necessary funds, facilities and moral support. P. Kumar and S. Mehariya are thankful to CSIR and DBT for Senior Research Fellowship, respectively. This research was also supported by the 2013 KU Brain Pool of Konkuk University.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Kumar P, Patel SKS, Lee JK, Kalia VC. Extending the limits of Bacillus for novel biotechnological applications. Biotechnol Adv. 2013;31:1543–1561. doi: 10.1016/j.biotechadv.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Reddy CSK, Ghai R, Rashmi, Kalia VC. Polyhydroxyalkanoates: an overview. Bioresour Technol. 2003;87:137–146. doi: 10.1016/S0960-8524(02)00212-2. [DOI] [PubMed] [Google Scholar]
- 3.Kalia VC, Chauhan A, Bhattacharyya G, Rashmi Genomic databases yield novel bioplastic producers. Nat Biotechnol. 2003;21:845–846. doi: 10.1038/nbt0803-845. [DOI] [PubMed] [Google Scholar]
- 4.Singh M, Patel SKS, Kalia VC. Bacillus subtilis as potential producer for polyhydroxyalkanoates. Microb Cell Fact. 2009;8:38. doi: 10.1186/1475-2859-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar T, Singh M, Purohit HJ, Kalia VC. Potential of Bacillus sp. to produce polyhydroxybutyrate from biowaste. J Appl Microbiol. 2009;106:2017–2023. doi: 10.1111/j.1365-2672.2009.04160.x. [DOI] [PubMed] [Google Scholar]
- 6.Koller M, Bona R, Braunegg G, Hermann C, Horvat P, Kroutil M, Martinz J, Neto J, Pereira L, Varila P. Production of polyhydroxyalkanoates from agricultural waste and surplus materials. Biomacromolecules. 2005;6:561–565. doi: 10.1021/bm049478b. [DOI] [PubMed] [Google Scholar]
- 7.Marshall CW, LaBelle EV, May HD. Production of fuels and chemicals from waste by microbiomes. Curr Opin Biotechnol. 2013;24:391–397. doi: 10.1016/j.copbio.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Tanadchangsaeng N, Yu J. Microbial synthesis of polyhydroxybutyrate from glycerol: gluconeogenesis, molecular weight and material properties of biopolyester. Biotechnol Bioeng. 2012;109:2808–2818. doi: 10.1002/bit.24546. [DOI] [PubMed] [Google Scholar]
- 9.Johnson K, Jiang Y, Kleerebezem R, Muyzer G, van Loosdrecht MCM. Enrichment of a mixed bacterial culture with a high polyhydroxyalkanoate storage capacity. Biomacromolecules. 2009;10:670–676. doi: 10.1021/bm8013796. [DOI] [PubMed] [Google Scholar]
- 10.Kleerebezem R, van Loosdrecht MCM. Mixed culture biotechnology for bioenergy production. Curr Opin Biotechnol. 2007;18:207–212. doi: 10.1016/j.copbio.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Huma N, Shankar P, Kushwah J, Bhushan A, Joshi J, Mukherjee T, Raju SC, Purohit HJ, Kalia VC. Diversity and polymorphism in AHL-lactonase gene (aiiA) of Bacillus. J Microbiol Biotechnol. 2011;21:1001–1011. doi: 10.4014/jmb.1105.05056. [DOI] [PubMed] [Google Scholar]
- 12.Kalia VC, Purohit HJ. Quenching the quorum sensing system: potential antibacterial drug targets. Crit Rev Microbiol. 2011;37:121–140. doi: 10.3109/1040841X.2010.532479. [DOI] [PubMed] [Google Scholar]
- 13.Sen R. Biotechnology in petroleum recovery: the microbial EOR. Prog Energy Combust Sci. 2008;34:714–724. doi: 10.1016/j.pecs.2008.05.001. [DOI] [Google Scholar]
- 14.Reddy SV, Thirumala M, Mahmood SK. A novel Bacillus sp. accumulating poly (3-hydroxybutyrate-co-3-hydroxyvalerate) from a single carbon substrate. J Ind Microbiol Biotechnol. 2009;36:837–843. doi: 10.1007/s10295-009-0561-8. [DOI] [PubMed] [Google Scholar]
- 15.Thirumala M, Reddy SV, Mahmood SK. Production and characterization of PHB from two novel strains of Bacillus spp. isolated from soil and activated sludge. J Ind Microbiol Biotechnol. 2010;37:271–278. doi: 10.1007/s10295-009-0670-4. [DOI] [PubMed] [Google Scholar]
- 16.Patel SKS, Singh M, Kumar P, Purohit HJ, Kalia VC. Exploitation of defined bacterial cultures for production of hydrogen and polyhydroxybutyrate from pea-shells. Biomass Bioenergy. 2012;36:218–225. doi: 10.1016/j.biombioe.2011.10.027. [DOI] [Google Scholar]
- 17.Porwal S, Kumar T, Lal S, Rani A, Kumar S, Cheema S, Purohit HJ, Sharma R, Patel SKS, Kalia VC. Hydrogen and polyhydroxybutyrate producing abilities of microbes from diverse habitats by dark fermentative process. Bioresour Technol. 2008;99:5444–5451. doi: 10.1016/j.biortech.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 18.Chang H-F, Chang W-C, Tsai C-Y. Synthesis of poly(3-hydroxybutyrate/3-hydroxyvalerate) from propionate-fed activated sludge under various carbon sources. Bioresour Technol. 2012;113:51–57. doi: 10.1016/j.biortech.2011.12.138. [DOI] [PubMed] [Google Scholar]
- 19.Albuquerque MGE, Eiroa M, Torres C, Nunes BR, Reis MAM. Strategies for the development of a side stream process for polyhydroxyalkanoate (PHA) production from sugar cane molasses. J Biotechnol. 2007;130:411–421. doi: 10.1016/j.jbiotec.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Dias JM, Lemos PC, Serafim LS, Oliveira C, Eiroa M, Albuquerque MGE, Ramos AM, Oliveira R, Reis MAM. Recent advances in polyhydroxyalkanoate production by mixed aerobic cultures: from the substrate to the final product. Macromol Biosci. 2006;6:885–906. doi: 10.1002/mabi.200600112. [DOI] [PubMed] [Google Scholar]
- 21.Serafim LS, Lemos PC, Torres C, Reis MAM, Ramos AM. The influence of process parameters on the characteristics of polyhydroxyalkanoates produced by mixed cultures. Macromol Biosci. 2008;8:355–366. doi: 10.1002/mabi.200700200. [DOI] [PubMed] [Google Scholar]
- 22.Jiang Y, Marang L, Kleerebezem R, Muyzer G, van Loosdrecht MCM. Polyhydroxybutyrate production from lactate using a mixed microbial culture. Biotechnol Bioeng. 2011;108:2022–2035. doi: 10.1002/bit.23148. [DOI] [PubMed] [Google Scholar]
- 23.Reddy MV, Venkata Mohan S. Effect of substrate load and nutrients concentration on the polyhydroxyalkanoates (PHA) production using mixed consortia through wastewater treatment. Bioresour Technol. 2012;114:573–582. doi: 10.1016/j.biortech.2012.02.127. [DOI] [PubMed] [Google Scholar]
- 24.Serafim LS, Lemos PC, Oliveira R, Reis MA. Optimization of polyhydroxybutyrate production by mixed cultures submitted to aerobic dynamic feeding conditions. Biotechnol Bioeng. 2004;87:145–160. doi: 10.1002/bit.20085. [DOI] [PubMed] [Google Scholar]
- 25.Villano M, Valentino F, Barbetta A, Martino L, Scandola M, Majone M. Polyhydroxyalkanoates production with mixed microbial cultures: from culture selection to polymer recovery in a high-rate continuous process. N Biotechnol. 2013 doi: 10.1016/j.nbt.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Waller JL, Green PG, Loge FJ. Mixed culture polyhydroxyalkanoate production from olive oil mill pomace. Bioresour Technol. 2012;120:285–289. doi: 10.1016/j.biortech.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 27.Yu ST, Lin CC, Too JR. PHBV production by Ralstonia eutropha in continuous stirred tank reactor. Process Biochem. 2005;40:2729–2734. doi: 10.1016/j.procbio.2004.12.023. [DOI] [Google Scholar]
- 28.Singh M, Kumar P, Patel SKS, Kalia VC. Production of polyhydroxyalkanoate co-polymer by Bacillus thuringiensis. Indian J Microbiol. 2013;53:77–83. doi: 10.1007/s12088-012-0294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anil-Kumar PK, Shamla TR, Kshama L, Prakash MH, Joshi GJ, Chandrashekar A, Kumari KSL, Divyashree MS. Bacterial synthesis of poly(hydroxybutyrate-co-hydroxyvalerate) using carbohydrate-rich mahua (Madhuca sp.) flowers. J Appl Microbiol. 2007;103:204–209. doi: 10.1111/j.1365-2672.2006.03221.x. [DOI] [PubMed] [Google Scholar]
- 30.Albuquerque MGE, Torres CA, Reis MAM. Polyhydroxyalkanoate (PHA) production by a mixed microbial culture using sugar molasses: effect of the influent substrate concentration on culture selection. Water Res. 2010;44:3419–3433. doi: 10.1016/j.watres.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 31.Arcos-Hernandez MV, Gurieff N, Pratt S, Magnusson P, Werker A, Vargas A, Lant P. Rapid quantification of intracellular PHA using infrared spectroscopy: an application in mixed cultures. J Biotechnol. 2010;150:372–379. doi: 10.1016/j.jbiotec.2010.09.939. [DOI] [PubMed] [Google Scholar]
- 32.Bengtsson S, Werker A, Christensson M, Welander T. Production of polyhydroxyalkanoates by activated sludge treating a paper mill wastewater. Bioresour Technol. 2008;99:519–526. doi: 10.1016/j.biortech.2007.01.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.