Abstract
Human brain bacterial meningitis is a life-threatening disease mainly caused by Neisseria meningitidis, lead to several complications including damage of brain or even death. The present available methods for diagnosis of meningitis have one or more limitations. A rmpM gene based genosensor was fabricated by immobilizing 5′-amino modified 19-mer single stranded DNA probe onto carbon-mercaptooctadecane/carboxylated multi-walled carbon nanotubes composite electrode and hybridized with 2.5–40 ng/6 μL of single stranded genomic DNA (ssG-DNA) of N. meningitidis from cerebrospinal fluid (CSF) of the suspected meningitis patients. The electrochemical response was measured by using cyclic voltammetry and differential pulse voltammetry (DPV) using 1 mM methylene blue as redox indicator in 30 min (including a response time of 1 min) at 25 °C. The sensitivity of the genosensor was 3.762 (μA/cm2)/ng and limit of detection was 2 ng of ssG-DNA of N. meningitidis with DPV. The genosensor has specificity only to N. meningitidis and does not hybridize with the genomic DNA of any other possible pathogen in human CSF. The immobilization of the probe and hybridization of the ssG-DNA were characterized by using electrochemical impedance in presence of 5 mM potassium ferricyanide and scanning electron microscopy. The genosensor loses only 12 % of its original DPV current on storage at 4 °C for 6 months. Carbon composite based electrochemical array can be constructed to detect multiple bacterial meningitis suspected patient CSF samples during an outbreak of the disease.
Electronic supplementary material
The online version of this article (doi:10.1007/s12088-013-0435-7) contains supplementary material, which is available to authorized users.
Keywords: Bacterial meningitis, Genosensor, Meningitis sensor, Multi-walled carbon nanotubes
Introduction
Meningitis is a life-threatening inflammation of the meninges of human brain and spinal cord which can lead to lethargy, seizures, loss of consciousness, visual abnormalities, hearing loss and some time to coma or death [1, 2]. Bacterial meningitis is a contagious infection which spreads rapidly among people and a quick diagnosis is necessary for initiation of early treatment and to prevent the outbreak of the disease. The detection of bacterial meningitis using culture of bacteria followed by latex agglutination (LA) test or coagglutination assay (COAG) may lead to uncertain results and are inefficient for detection of N. meningitidis [3]. Gram staining and other biochemical methods are preliminary and non specific methods which can be misleading in case of prior antibiotic treated patients [4]. PCR [5], RT-PCR [6] and microarray [7] involve culture of N. meningitidis, isolation of genomic DNA (G-DNA) and PCR amplification of partial sequence. The Dot-blot ELISA test is expensive and sometimes may give false results therefore, is not preferred for diagnosis of bacterial meningitis [8].
Nowadays, DNA sensors are slowly replacing the ancient methods of diagnosis because of their sensitivity, simplicity and low cost [9]. The DNA sensors reported by Patel et al. [9, 10] detects chemically synthesized complementary ssDNA and PCR ampilicon or isolated G-DNA from N. meningitidis culture, respectively. These are time consuming, less sensitive and clinically insignificant. The Omp85 genosensor reported earlier by our laboratory was with lower sensitivity and with higher limit of detection (LOD) since it was based on gold electrode as transducer [11]. Nanomaterial based genosensor can be a better choice for diagnosis of the disease in order to increase the sensitivity and decrease the LOD by increasing the efficiency of immobilization of the probe onto the transducers [12].
Carbon electrodes are preferable for developing electrochemical sensors because of their good electron transfer kinetics and biocompatibility [13]. Carbon nanotubes (CNT) have flexible surface chemistry, high surface to volume ratio and good conductivity therefore, preferably used in development of genosensors [14]. Mercaptooctadecane (MOD) can act as an interlinking layer between MWCNT and carbon [15]. The virulent gene specific single stranded DNA (ssDNA) probe from a pathogen can be immobilized onto CNT for the development of highly sensitive and specific genosensors against the corresponding pathogen [16]. Methylene blue (MB) and potassium ferricyanide (K3[Fe(CN)6]) are routinely used as redox indicators in electrochemical genosensors [17].
There are five classes of outer membrane proteins (OMPs) present in N. meningitidis of which the class 4 proteins are termed as reduction-modifiable protein M (rmpM) due to its characteristic electrophoresis behavior after reduction. rmpM proteins have two interlinking domains one of which bind with peptidoglycan layer of bacteria while the other binds to OMPs and act as stabilizers of the receptor complexes [2]. rmpM is a unique virulent gene conserved in all the strains of N. meningitidis and the NCBI BLAST result shows that this gene sequence does not show homology with any other gene sequence of possible pathogens of the human cerebrospinal fluid (CSF). Therefore, rmpM gene was chosen for development a specific genosensor to detect all the strains of N. meningitidis. A rmpM genosensor was developed for quick detection of human brain meningitis by immobilization of 5′-amino modified 19-mer ssDNA probe onto carbon composite electrode and followed by hybridization of the immobilized probe with the single stranded G-DNA of N. meningitidis directly from the patient CSF.
Materials and Methods
Chemicals and Patient Samples
MWCNT (outer diameter: 7–15 nm; length: 0.5–10 μm; purity: >99 %), MB, MOD, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and N-hydroxysuccinimide (NHS) were purchased from Sigma Aldrich, USA. 2-(N-morpholino) ethanesulfonic acid (MES) and dimethylformamide (DMF) were procured from Sisco Research Laboratories, India. All other chemicals purchased were of analytical grade. The 5′-amino modified 19-mer ssDNA probe (5′-GCTCGCTTCCGGCACTGCT-3′) was synthesized from Innovision Medichem, India. Screen printed carbon electrode (SPCE) was procured from DropSens and modified in our lab.
Streptococcus pneumoniae and Haemophilus influenzae type cultures were purchased from MTCC, Chandigarh, India to check the specificity of the genosensor. All other bacterial samples were obtained from National Centre for Disease Control (NCDC), Delhi, India. Meningitis patient CSF samples (0.5 mL) were obtained from NCDC and centrifuged at 16,000×g for 1 min. The pellet was suspended in minimal volume of TE (10 mM Tris and 1 mM EDTA) buffer, pH 8 and heated at 95 °C for 5 min for lysis of the cell wall. The heated solution was further centrifuged at 16,000×g for 1 min and the supernatant containing G-DNA of N. meningitidis was used for hybridization with the immobilized probe on carbon composite electrode.
Construction of Genosensor
The working carbon electrode (0.126 cm2) of SPCE (carbon: working; platinum: counter; silver: reference) was treated with MOD saturated ethanol for 30 min and then washed with absolute ethanol to remove the extra amount of MOD and dried at 25 °C. The MWCNT was dispersed in H2SO4:HNO3 (3:1, v/v) to obtain a concentration of 1.2 mg/mL and the MWCNT suspension was sonicated for 12 h using an Ultrasonic cleaner at 40 kHz, 220 ± 10 V, 120 W (Ningbo Scientz Biotech Co. Ltd., China) for functionalization of carboxyl (COO−) groups at the side walls [18]. The COO− functionalized MWCNT (c-MWCNT) was repeatedly washed with autoclaved double distilled water to neutralize the pH and finally dispersed in DMF by sonicating for 5 min. The suspension of c-MWCNT was diluted two-folds with autoclaved double distilled water to obtain a final concentration of 0.6 mg/mL. 5 μL of c-MWCNT (0.6 mg/mL) and 5 μL of 10 mM EDC in PBS (50 mM sodium phosphate buffer; 0.9 % NaCl) were mixed (1:1, v/v) and placed onto the carbon-MOD surface for 3 h at 25 °C. The excess of c-MWCNT was removed by washing with DMF:H2O (1:1, v/v) and dried at 25 °C. The EDC acts as dehydrating agent to induce thioester (S–C) bond between thiol (SH) group of MOD and COO− group of c-MWCNT. The carbon composite transducer was characterized by using scanning electron microscopy (SEM) (Carl Zeiss EVO 40 SEM, Germany).
Probe Immobilization
The 308 bp partial cds of rmpM gene of N. meningitidis was amplified by using specific forward (5′-GCTCGCTTCCGGCACTGCT-3′) and reverse primers (5′-TGAGCTTCGGCGCGCAATGA-3′) using G-DNA isolated from Indian patient CSF following the procedure in our laboratory [2]. The PCR amplicon was purified using GFX column and sequenced twice at The Centre of Genomic Application, Delhi [2]. The final interpreted sequence was compared with the already reported sequence in NCBI using ClustalW and subsequently the partial rmpM gene sequence (308 bp) was submitted to NCBI GenBank with an accession number of HQ712170. A 19-mer ssDNA probe (5′-GCTCGCTTCCGGCACTGCT-3′) was designed complementary to the partial cds HQ712170 in order to avoid any mutation or change in the gene sequence. The carbon composite electrode surface was treated with 10 μL of a mixture of 10 mM EDC and 10 mM NHS (1:1, v/v) in PBS, pH 7 for 4 h to activate the COO− groups present on the side wall of c-MWCNT. The carbon composite electrode was washed 3–4 times with PBS, pH 7 followed by 3–4 times with 0.1 M MES buffer, pH 4.5 to remove the unused EDC and NHS and finally dried at 25 °C. A rmpM genosensor was constructed by immobilizing 5 μL of 1 μM 5′-NH2 modified 19-mer ssDNA probe of rmpM gene of N. meningitidis in 0.1 M of MES buffer, pH 4.5 onto carbon composite electrode for 12 h at 25 °C through EDC-NHS chemistry [19]. The electrode was washed 4–5 times with 0.1 M MES buffer, pH 4.5, 4–5 times with autoclaved double distilled water, consecutively 2–3 times with PBS pH 7 to remove the unbound probe and finally dried at 25 °C before electrochemical measurement.
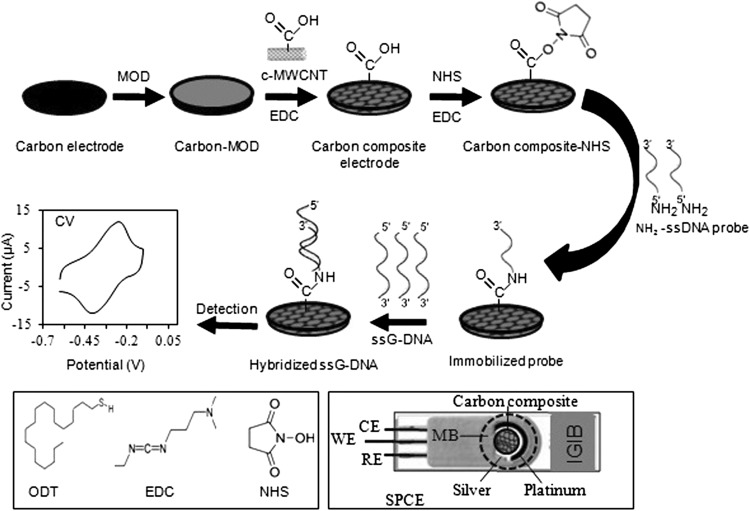
G-DNA Hybridization
The G-DNA isolated from patient CSF was denatured by heating at 95 °C for 5 min to make ssG-DNA. The ssG-DNA (2.5, 5.0, 10, 20 and 40 ng in 6 μL TE) was treated onto the immobilized probe on the carbon composite electrode for 10 min at 25 °C for hybridization with the immobilized probe. The hybridized electrode was washed 4–5 times with TE buffer, pH 8 followed by PBS, pH 7 to remove the unhybridized ssG-DNA and finally dried at 25 °C before electrochemical measurement. The fabrication of carbon composite electrode, immobilization of the ssDNA probe onto the composite electrode, hybridization of the immobilized probe with the ssG-DNA and electrochemical detection of the genosensor is shown in Fig. 1. The Genosensor was detected by using cyclic voltammetry (CV) and differential pulse voltammetry (DPV) in a potentiostat/galvanostat (FRA2 μAutolab type iii, Metrohm, India) in 30 min (including a response time of 1 min). The immobilization of the probe onto carbon composite electrode and hybridization of the immobilized probe with the ssG-DNA of N. meningitidis from patient CSF were characterized by using electrochemical impedance (EI) and SEM. 1 mM of MB in 50 mM PBS, pH 7 and 5 mM of K3[Fe(CN)6] in 50 mM PBS, pH 7 was used as redox indicator for the CV/DPV and EI studies, respectively.
Fig. 1.
Schematic representation of the fabrication of carbon composite electrode, immobilization of ssDNA probe onto the carbon composite, hybridization of immobilized probe with ssG-DNA of N. meningitidis and electrochemical detection
Results and Discussion
Cyclic Voltammetry
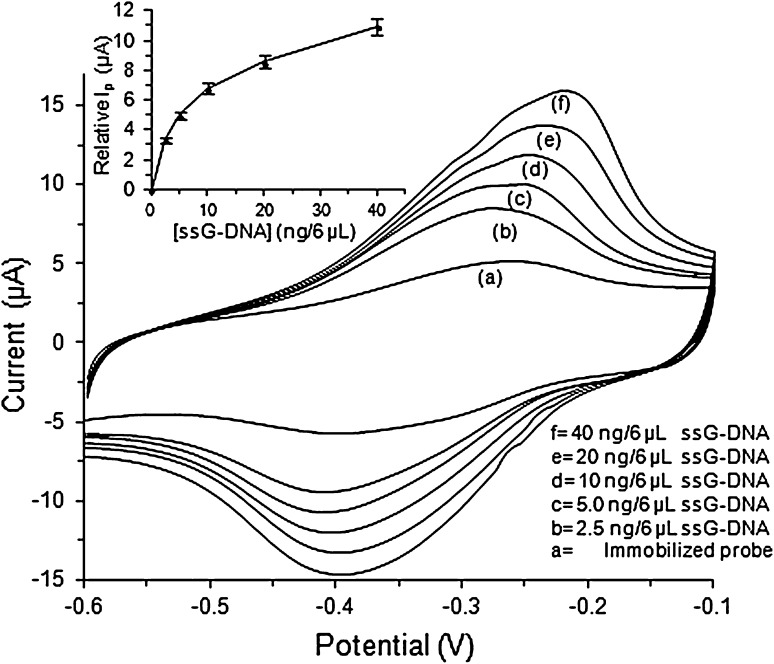
Cyclic voltammogram of the immobilized probe on carbon composite electrode and hybridization of the immobilized probe with ssG-DNA of N. meningitidis from the patient CSF using 1 mM MB in PBS, pH 7 is shown in Fig. 2. The oxidation peak current (Ip) for CV of carbon composite electrode was found higher than the carbon electrode (figure not shown) due to the higher conductivity and stronger affinity of MWCNT towards MB [20]. The CV current of immobilized probe on carbon composite electrode (5.12 μA, curve a) was found higher than that of simple carbon composite electrode may be due to the increase in binding of the MB molecules to the ssDNA probe that has led to increased supply of MB probe for redox reaction at the surface. The Ip for CV of the immobilized probe electrode after hybridization with 2.5, 5.0, 10, 20 and 40 ng/6 μL of ssG-DNA was found as 8.46 μA (curve b), 10.10 μA (curve c), 11.92 μA (curve d), 13.71 μA (curve e) and 16.14 μA (curve f), respectively. The oxidation peak current of hybridized G-DNA was found higher than that of immobilized probe electrode and increased further with increase in the concentrations of hybridizing ssG-DNA from N. meningitidis. This may be attributed to the higher affinity of dsDNA to MB as compared to ssDNA and the affinity kept on increasing with increase in the amount of hybridizing dsG-DNA on the electrode surface that has led to increase in the binding of MB to the working surface (available for oxidation on the surface) and an increase in the current [21]. The plot between relative Ip with respect to the immobilized probe and the concentrations of ssG-DNA used for hybridization was found hyperbolic (Fig. 2, inset) and linear in the range of 0–5 ng/6 μL. The above experiment was repeated three times and the average mean value of each reading is given in the figure with their error values. The sensitivity (S) of the fabricated genosensor was 7.810 (μA/cm2)/ng with regression coefficient (R2) as 0.9869 was calculated using the formula S = m/A, where, m is slope of the linear equation; A is area of the carbon composite electrode surface. The LOD of the carbon composite based genosensor was calculated as 1 ng with CV (Fig. S1a) using the formula LOD = 3(σ/S), where, σ is standard deviation and S is sensitivity [22, 23].
Fig. 2.
Cyclic voltammetry of a the immobilized probe and b–f after hybridization with 2.5, 5.0, 10, 20 and 40 ng/6 μL of N. meningitidis ssG-DNA at 50 mVs−1 using 1 mM MB in 50 mM PBS, pH 7. The inset shows hyperbolic curve between relative I p with respect to the immobilized probe and increasing concentrations of the hybridizing ssG-DNA of N. meningitidis from patient CSF
Differential Pulse Voltammetry
The Ip for DPV of bare carbon was lower than that of carbon composite electrode as discussed earlier for CV analysis (not shown in figure) and the Ip value increased further after immobilization of ssDNA probe onto the carbon composite electrode (16.66 μA, curve a). The Ip for DPV of hybridized G-DNA was found higher than that of immobilized probe and increased further with increase in the concentrations of hybridizing ssG-DNA as 18.02 μA (2.5 ng/6 μL, curve b), 19.03 μA (5.0 ng/6 μL, curve c), 21.15 μA (10 ng/6 μL, curve d), 22.51 μA (20 ng/6 μL, curve e) and 23.91 μA (40 ng/6 μL, curve f), (Fig. 3). The trend of variation of the relative Ip with respect to the immobilized probe at different concentrations of hybridizing ssG-DNA of N. meningitidis was found similar to that of CV studies (Fig. 3, inset). The experiment was repeated three times and the average mean value of each data are given in the figure with their error values. The S and R2 of the carbon composite based genosensor was calculated as 3.762 (μA/cm2)/ng and 0.9928, respectively with the similar formulas used in case of CV analysis (Fig. S1b). The LOD of the carbon composite based genosensor was also calculated as discussed in CV studies and found as 2 ng of ssG-DNA of N. meningitidis from patient CSF.
Fig. 3.
Differential pulse voltammetry of a the immobilized probe and b–f after hybridization with 2.5, 5.0, 10, 20 and 40 ng/6 μL of N. meningitidis ssG-DNA at amplitude potential 25 mV using 1 mM MB in 50 mM PBS, pH 7. The inset shows hyperbolic curve between relative I p with respect to the immobilized probe and increasing concentrations of hybridizing ssG-DNA of N. meningitidis from patient CSF
Electrochemical Impedance
THE EI spectra (Nyquist plot) for immobilization of ssDNA probe onto the carbon composite electrode and hybridization of the immobilized probe with different concentrations of ssG-DNA of N. meningitidis from patient CSF is shown in Fig. 4. The diameter of the semicircle in the Nyquist plot corresponds to the charge transfer resistance (Rct) and the semicircle at higher frequency corresponds to electron-transfer-limited process [24]. The Rct of the carbon composite electrode (figure not shown) was lower than that of bare carbon may be because of the fabricated c-MWCNT has increased the electron transfer kinetics by increasing the conductivity of the working surface which resulted in decrease in the impedance [25]. The Rct of the immobilized probe electrode was found as 2.62 kΩ (curve a) while after hybridization with 2.5, 5.0, 10, 20 and 40 ng/6 μL ssG-DNA of N. meningitidis was found as 5.03 kΩ (curve b), 6.99 kΩ (curve c), 9.17 kΩ (curve d), 10.12 kΩ (curve e) and 11.37 kΩ (curve f), respectively (Fig. 4). The Rct of the immobilized probe electrode was found higher than the simple carbon composite electrode may be due to the negatively charged phosphate (PO2−) groups of the ssDNA probe of the immobilized probe electrode has prevented [Fe(CN)6]3−/4− ions to reach the electrode surface leading to increase in the Rct value. The Rct value of the G-DNA hybridized electrode was found higher than the immobilized probe electrode and increased further with increase in the concentrations of hybridizing ssG-DNA. This may be due to the increase in the ssG-DNA of N. meningitidis has led to increase in the negative charged PO2− groups on the electrode surface and an increase in the surface thickness which prevented the binding of [Fe(CN)6]3−/4− ions to the working surface resulting in increase in impedance. The plot between relative Rct and different concentrations of hybridizing ssG-DNA was also hyperbolic (Fig. 4, inset a) and linear in the range of 0–5 ng/6 μL (Fig. 4, inset b) as in the case of CV and DPV studies. The experiment was repeated three times and the average mean value of each point is given in the figure with their error values. The result of the EI studies supported the results obtained with CV and DPV studies.
Fig. 4.
Electrochemical Impedance spectra of a the immobilized probe and b–f after hybridization with 2.5, 5.0, 10, 20 and 40 ng/6 μL of N. meningitidis ssG-DNA in a frequency range of 10−2–105 and an applied potential of +0.18 (± 0.01) V using 5 mM [K3FeCN6]3−/4− in 50 mM PBS, pH 7. The inset A and inset B shows the hyperbolic curve and linear range (0–5 ng/6 μL) of the hyperbolic curve, respectively between relative R ct with respect to the immobilized probe and increasing concentrations of the hybridizing ssG-DNA of N. meningitidis from patient CSF
Scanning Electron Microscopy
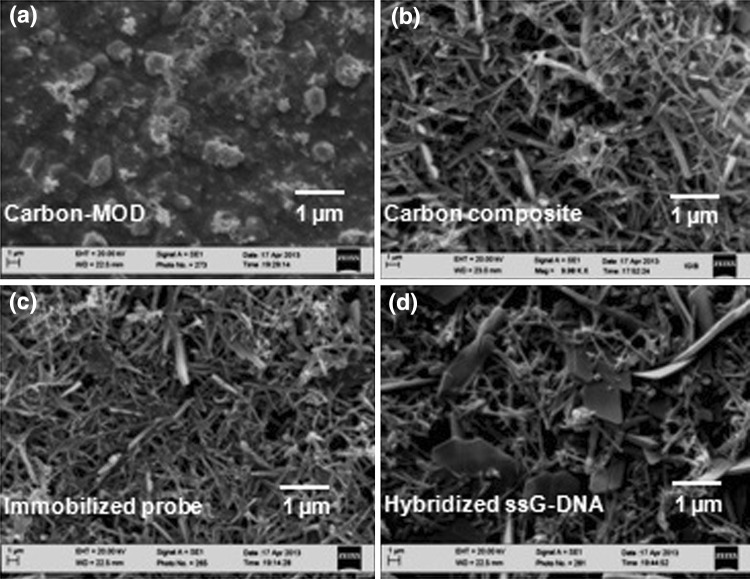
The change in surface morphology of the carbon composite transducer after immobilization of ssDNA probe onto it and followed by hybridization of the immobilized probe electrode with the ssG-DNA from N. meningitidis was studied by SEM (Fig. 5). The SEM image of the bare carbon showed a uniform smooth black surface (figure not shown). The modified carbon with MOD layer showed a deposition of granular layer on the carbon surface (Fig. 5a). A porous tubular inter-crossed net like structure was seen in the case of carbon composite c-MWCNT electrode (Fig. 5b) [26]. After immobilization of ssDNA probe onto the carbon composite electrode, a thin film appeared on the network of c-MWCNT due to the covalent (amide bond) immobilization of ssDNA probe (Fig. 5c) [27] which was changed into a dense surface morphology after hybridization with ssG-DNA of N. meningitidis from patient CSF (Fig. 5d) [28].
Fig. 5.
Scanning electron micrograph of a carbon-MOD, b carbon-MOD/c-MWCNT composite c immobilized probe onto carbon composite electrode and d after hybridization of the immobilized probe on carbon composite electrode with ssG-DNA of N. meningitidis
Specificity and Stability of Genosensor
The specificity of the genosensor was tested by hybridizing the immobilized probe electrode with the control sample (6 μL of CSF without bacteria) and 10 ng/6 μL of ssG-DNA from Escherichia coli (DH5α), Mycobacterium tuberculosis (smegmatis), S. pyogenes (M140), H. influenzae, S. pneumoniae and N. meningitidis (patient CSF). The DPV current of hybridization with 10 ng/6 μL of ssG-DNA from other pathogens was compared with the DPV current of immobilized probe electrode. The DPV current of hybridization with lower concentration (2.5 ng/6 μL) of N. meningitidis ssG-DNA was compared with the DPV current of hybridization with 10 ng/6 μL of ssG-DNA from other pathogens.
The DPV peak current of the genosensor after hybridization with 10 ng/6 μL of ssG-DNA from other pathogens was found almost same as the DPV current of immobilized probe except with N. meningitidis (Fig. 6). The current increased only in case of hybridization with N. meningitidis ssG-DNA 2.5 and 10 ng/6 μL which confirms the specificity of the genosensor only to N. meningitidis.
Fig. 6.
Specificity of the genosensor with N. meningitidis G-DNA. The percentage of relative I p for DPV of the immobilized probe, after hybridization with control (6 μL of CSF), N. meningitidis (2.5 and 10 ng/6 μL) and with other pathogens (10 ng/6 μL)
The self-life of the genosensor electrode was calculated by measuring the DPV current of the immobilized probe electrode at a regular interval of every 30 days for 180 days (6 months) and comparing with the original DPV current obtained at day one of probe immobilization. The genosensor showed a loss of about 12 % of the original DPV current after 180 days when stored at 4 °C (Fig. 7).
Fig. 7.
The self-life of rmpM genosensor electrode stored at 4 °C by by measuring the change (%) in original DPV current for 180 days at a regular interval of every 30 days. Each value is an average of three readings at same storage conditions
Conclusion
The carbon composite based genosensor is better than the earlier reported meningitis sensors due to lower LOD (2 ng) of ssG-DNA and higher specificity with rmpM gene of N. meningitidis. The sensitivity of the genosensor for the detection of life-threatening human brain bacterial meningitis was 3.762 (μA/cm2)/ng using DPV in 30 min. The CV and DPV were used as amperometric methods for detection of human brain bacterial meningitis while EI (impedimetric method) was used to support the results of CV and DPV studies. The sensitivity of the genosensor with CV was found 7.810 (μA/cm2)/ng with LOD as 1 ng while with DPV the sensitivity was 3.762 (μA/cm2)/ng with LOD as 2 ng. Carbon composite electrode arrays can be constructed using the principles of the rmpM genosensor for simultaneous detection of multiple patient CSF samples during an outbreak of the disease.
Electronic supplementary material
Acknowledgments
This work was financially supported by Department of Science and Technology (Project No. DST/TSG/ME/2008/37), India.
References
- 1.Dash SK, Sharma M, Kumar A. Diagnostic techniques and treatment of meningitis. In: Houllis G, Karachalios M, editors. Meningitis: causes, diagnosis and treatment. 1. New York: Nova Science Publishers; 2012. pp. 203–223. [Google Scholar]
- 2.Dash SK, Sharma M, Khare S, Kumar A. RmpM gene as a genetic marker for human bacterial meningitis. Cell Mol Biol. 2012;58:26–30. [PubMed] [Google Scholar]
- 3.Negi SS, Grover SS, Rautela SS, Rawat DS, Gupta S, Khare S, Lal S, Rai A. Direct detection and serogroup characterization of Neisseria meningitidis from outbreak of meningococcal meningitis in Delhi. Iran J Microbiol. 2010;2:73–79. [PMC free article] [PubMed] [Google Scholar]
- 4.Chanteau S, Dartevelle S, Mahamane AE, Djibo S, Boisier P, Nato F. New rapid diagnostic tests for Neisseria meningitidis serogroups A, W135, C and Y. PLoS Med. 2006;3:1579–1586. doi: 10.1371/journal.pmed.0030337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dash SK, Sharma M, Khare S, Kumar A. Quick diagnosis of human brain meningitis using Omp85 gene amplicon as a genetic marker. Ind J Microbiol. 2013;53:238–240. doi: 10.1007/s12088-013-0371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu HM, Cordeiro SM, Harcourt BH, Carvalho MGS, Azevedo J, Oliveira TQ, Leite MC, Salgado K, Reis MG, Plikytis BD, Clark TA, Mayer LW, Ko AI, Martin SW, Reis JN. Accuracy of real-time PCR, Gram stain and culture for Streptococcus pneumoniae, Neisseria meningitidis and Haemophilus influenzae meningitis diagnosis. BMC Infect Dis. 2013;13:1–10. doi: 10.1186/1471-2334-13-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patnaik S, Dash SK, Sethi D, Kumar A, Gupta KC, Kumar P. Engineered polymer-supported synthesis of 3′-carboxyalkyl-modified oligonucleotides and their applications in the construction of biochips for diagnosis of the diseases. Bioconjug Chem. 2012;23:664–670. doi: 10.1021/bc200610u. [DOI] [PubMed] [Google Scholar]
- 8.Ferraz AS, Belo EFT, Coutinho LMCC, Oliveira AP, Carmo AMS, Franco DL, Ferreira T, Yto AY, Machado MSF, Scola MSG, de Gaspari E. Storage and stability of IgG and IgM monoclonal antibodies dried on filter paper and utility in Neisseria meningitidis serotyping by dot-blot ELISA. BMC Infect Dis. 2008;8:1–8. doi: 10.1186/1471-2334-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel MK, Solanki PR, Seth S, Gupta S, Khare S, Malhotra BD, Kumar A. CtrA gene based electrochemical DNA sensor for detection of meningitis. Electrochem Commun. 2009;11:969–973. doi: 10.1016/j.elecom.2009.02.037. [DOI] [Google Scholar]
- 10.Patel MK, Solanki PR, Kumar A, Khare S, Gupta S, Malhotra BD. Electrochemical DNA sensor for Neisseria meningitidis detection. Biosens Bioelectron. 2010;25:2586–2591. doi: 10.1016/j.bios.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 11.Dash SK, Sharma M, Khare S, Kumar A. Omp85 genosensor for detection of human brain bacterial meningitis. Biotechnol Lett. 2013;35:929–935. doi: 10.1007/s10529-013-1161-2. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh N, Gupta G, Boopathi M, Pal V, Singh AK, Gopalan N, Goel AK. Surface plasmon resonance biosensor for detection of Bacillus anthracis, the causative agent of anthrax from soil samples targeting protective antigen. Ind J Microbiol. 2013;53:48–55. doi: 10.1007/s12088-012-0334-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Wu Y, Hu X. Metal-organic framework modified carbon paste electrode for lead sensor. Sens Actuators B. 2013;177:1161–1166. doi: 10.1016/j.snb.2012.12.048. [DOI] [Google Scholar]
- 14.Park S, Vosguerichian M, Bao Z. A review of fabrication and applications of carbon nanotube film-based flexible electronics. Nanoscale. 2013;5:1727–1752. doi: 10.1039/c3nr33560g. [DOI] [PubMed] [Google Scholar]
- 15.DiBenedetto SA, Facchetti A, Ratner MA, Marks TJ. Molecular self-assembled monolayers and multilayers for organic and unconventional inorganic thin-film transistor applications. Adv Mater. 2009;21:1407–1433. doi: 10.1002/adma.200803267. [DOI] [Google Scholar]
- 16.Chen RJ, Bangasaruntip SO, Drouvalakis KA, Kam NWS, Shim M, Li Y, Kim W, Utz PZ, Dai H (2003) Noncovalent functionalization of carbon nanotubes for highly specific electronic biosensors. Proc Natl Acad Sci 100:4984–4989 [DOI] [PMC free article] [PubMed]
- 17.Kumar A, Dash SK, Sharma DP, Suman . DNA based biosensors for detection of pathogens. In: Singh HP, Chowdappa P, Chakroborty BN, Podie AR, editors. Molecular approaches for plant fungal disease management. 1. Delhi: Westville Publishers; 2012. pp. 31–35. [Google Scholar]
- 18.Saleh TA. The influence of treatment of temperature on the acidity of MWCNT oxidized by HNO3 or a mixture of HNO3/H2SO4. Appl Surf Sci. 2011;257:7746–7751. doi: 10.1016/j.apsusc.2011.04.020. [DOI] [Google Scholar]
- 19.Rahman MM, Shiddiky MJ, Rahman MA, Shim YB. A lactate biosensor based on lactate dehydrogenase/nictotinamide adenine dinucleotide (oxidized form) immobilized on a conducting polymer/multi-walled carbon nanotube composite film. Anal Biochem. 2009;384:159–165. doi: 10.1016/j.ab.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 20.Shahryari Z, Goharrizi AS, Azadi M. Experimental study of methylene blue adsorption from aqueous solutions onto carbon nanotubes. Int J Water Res Environ Eng. 2010;2:16–28. [Google Scholar]
- 21.Zhang Z, Wang X, Wang Y, Yang X. Distinction of single base mismatches in duplex DNA using methylene blue as optical indicator. Analyst. 2010;135:2960–2964. doi: 10.1039/c0an00359j. [DOI] [PubMed] [Google Scholar]
- 22.Jindal K, Tomar M, Gupta V. CuO thin film based uric acid biosensor with enhanced response characteristics. Biosens Bioelectron. 2012;38:11–18. doi: 10.1016/j.bios.2012.03.043. [DOI] [PubMed] [Google Scholar]
- 23.Wu NY, Gao W, He XH, Chang Z, Xu MT. Direct electrochemical sensor for label-free DNA detection based on zero current potentiometry. Biosens Bioelectron. 2013;39:210–214. doi: 10.1016/j.bios.2012.07.038. [DOI] [PubMed] [Google Scholar]
- 24.Zhang W, Zhong P, Zheng X, Wang L. An enhanced sensing platform for ultrasensitive impedimetric detection of target genes based on ordered FePt nanoparticles decorated carbon nanotubes. Biosens Bioelectron. 2013;42:481–485. doi: 10.1016/j.bios.2012.10.052. [DOI] [PubMed] [Google Scholar]
- 25.Hayat A, Barthelmebs L, Marty JL. Electrochemical impedimetric immunosensor for the detection of okadaic acid in mussel sample. Sens Actuators B. 2012;171–172:810–815. doi: 10.1016/j.snb.2012.05.075. [DOI] [Google Scholar]
- 26.Muleja AA, Mbianda XY, Krause RW, Pillay K. Synthesis, characterization and thermal decomposition behavior of triphenylphosphine-linked multi-walled carbon nanotubes. Carbon. 2012;50:2741–2751. doi: 10.1016/j.carbon.2012.02.033. [DOI] [Google Scholar]
- 27.Kyaw BM, Champakalakshmi R, Sakharkar MK, Lim CS, Sakharkar KR. Biodegradation of low density polythene (LDPE) by pseudomonas species. Ind J Microbiol. 2012;52:411–419. doi: 10.1007/s12088-012-0250-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pati PK, Sharma M, Salar RK, Sharma A, Gupta AP, Singh B. Studies on leaf spot disease of Withania somnifera and its impact on secondary metabolites. Ind J Microbiol. 2008;48:432–437. doi: 10.1007/s12088-008-0053-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.