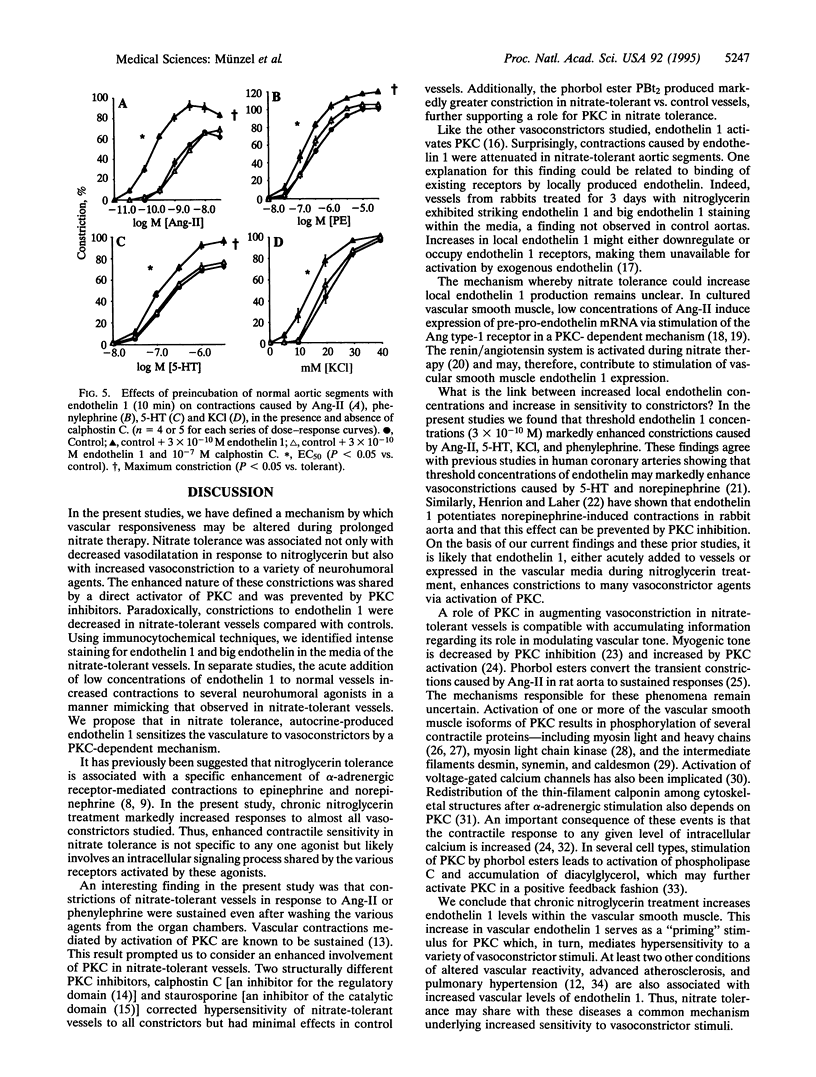
Abstract
We sought to examine mechanisms responsible for increased vasoconstriction that occurs during development of nitroglycerin tolerance. Rabbits were treated for 3 days with nitroglycerin patches (0.4 mg/hr), and their aortic segments were studied in organ chambers. This treatment resulted in attenuated in vitro relaxations to nitroglycerin and increased contractile sensitivity to angiotensin II, serotonin, phenylephrine, KCl, and a direct activator of protein kinase C, the phorbol ester phorbol 12,13-dibutyrate. The protein kinase C antagonists calphostin C (100 nM) and staurosporine (10 nM) corrected the hypersensitivity to constrictors in tolerant vessels, yet had minimal effects on constrictions in control vessels. Paradoxically, constrictions caused by endothelin 1 were decreased in nitrate-tolerant vessels. Immunocytochemical analysis revealed intense endothelin 1-like and big endothelin 1-like immunoreactivity in the media of nitroglycerin-tolerant but not of control aortas. The enhanced vasoconstriction to angiotensin II, serotonin, KCl, and phenylephrine could be mimicked in normal vessels by addition of subthreshold concentrations of endothelin 1, and this effect was prevented by calphostin C. We propose that increased autocrine production of endothelin 1 in nitrate tolerance sensitizes vascular smooth muscle to a variety of vasoconstrictors through a protein kinase C-mediated mechanism.
Full text
PDF

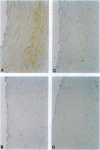
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrea J. E., Walsh M. P. Protein kinase C of smooth muscle. Hypertension. 1992 Nov;20(5):585–595. doi: 10.1161/01.hyp.20.5.585. [DOI] [PubMed] [Google Scholar]
- Bruns R. F., Miller F. D., Merriman R. L., Howbert J. J., Heath W. F., Kobayashi E., Takahashi I., Tamaoki T., Nakano H. Inhibition of protein kinase C by calphostin C is light-dependent. Biochem Biophys Res Commun. 1991 Apr 15;176(1):288–293. doi: 10.1016/0006-291x(91)90922-t. [DOI] [PubMed] [Google Scholar]
- Clozel M., Löffler B. M., Breu V., Hilfiger L., Maire J. P., Butscha B. Downregulation of endothelin receptors by autocrine production of endothelin-1. Am J Physiol. 1993 Jul;265(1 Pt 1):C188–C192. doi: 10.1152/ajpcell.1993.265.1.C188. [DOI] [PubMed] [Google Scholar]
- Collins E. M., Walsh M. P., Morgan K. G. Contraction of single vascular smooth muscle cells by phenylephrine at constant [Ca2+]i. Am J Physiol. 1992 Mar;262(3 Pt 2):H754–H762. doi: 10.1152/ajpheart.1992.262.3.H754. [DOI] [PubMed] [Google Scholar]
- Danthuluri N. R., Brock T. A. Endothelin receptor-coupling mechanisms in vascular smooth muscle: a role for protein kinase C. J Pharmacol Exp Ther. 1990 Aug;254(2):393–399. [PubMed] [Google Scholar]
- Danthuluri N. R., Deth R. C. Acute desensitization to angiotensin II: evidence for a requirement of agonist-induced diacylglycerol production during tonic contraction of rat aorta. Eur J Pharmacol. 1986 Jul 15;126(1-2):135–139. doi: 10.1016/0014-2999(86)90749-1. [DOI] [PubMed] [Google Scholar]
- Elkayam U. Tolerance to organic nitrates: evidence, mechanisms, clinical relevance, and strategies for prevention. Ann Intern Med. 1991 Apr 15;114(8):667–677. doi: 10.7326/0003-4819-114-8-667. [DOI] [PubMed] [Google Scholar]
- Feelisch M., Kelm M. Biotransformation of organic nitrates to nitric oxide by vascular smooth muscle and endothelial cells. Biochem Biophys Res Commun. 1991 Oct 15;180(1):286–293. doi: 10.1016/s0006-291x(05)81290-2. [DOI] [PubMed] [Google Scholar]
- Figueras J., Lidon R., Cortadellas J. Rebound myocardial ischaemia following abrupt interruption of intravenous nitroglycerin infusion in patients with unstable angina at rest. Eur Heart J. 1991 Mar;12(3):405–411. doi: 10.1093/oxfordjournals.eurheartj.a059909. [DOI] [PubMed] [Google Scholar]
- Giaid A., Yanagisawa M., Langleben D., Michel R. P., Levy R., Shennib H., Kimura S., Masaki T., Duguid W. P., Stewart D. J. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med. 1993 Jun 17;328(24):1732–1739. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
- Hahn A. W., Resink T. J., Scott-Burden T., Powell J., Dohi Y., Bühler F. R. Stimulation of endothelin mRNA and secretion in rat vascular smooth muscle cells: a novel autocrine function. Cell Regul. 1990 Aug;1(9):649–659. doi: 10.1091/mbc.1.9.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrion D., Laher I. Effects of staurosporine and calphostin C, two structurally unrelated inhibitors of protein kinase C, on vascular tone. Can J Physiol Pharmacol. 1993 Jul;71(7):521–524. doi: 10.1139/y93-076. [DOI] [PubMed] [Google Scholar]
- Henrion D., Laher I. Potentiation of norepinephrine-induced contractions by endothelin-1 in the rabbit aorta. Hypertension. 1993 Jul;22(1):78–83. doi: 10.1161/01.hyp.22.1.78. [DOI] [PubMed] [Google Scholar]
- Huang C. F., Cabot M. C. Phorbol diesters stimulate the accumulation of phosphatidate, phosphatidylethanol, and diacylglycerol in three cell types. Evidence for the indirect formation of phosphatidylcholine-derived diacylglycerol by a phospholipase D pathway and direct formation of diacylglycerol by a phospholipase C pathway. J Biol Chem. 1990 Sep 5;265(25):14858–14863. [PubMed] [Google Scholar]
- Ikebe M., Hartshorne D. J., Elzinga M. Identification, phosphorylation, and dephosphorylation of a second site for myosin light chain kinase on the 20,000-dalton light chain of smooth muscle myosin. J Biol Chem. 1986 Jan 5;261(1):36–39. [PubMed] [Google Scholar]
- Kobayashi E., Nakano H., Morimoto M., Tamaoki T. Calphostin C (UCN-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1989 Mar 15;159(2):548–553. doi: 10.1016/0006-291x(89)90028-4. [DOI] [PubMed] [Google Scholar]
- Kuźnicki J. Phosphorylation of myosin in non-muscle and smooth muscle cells. Possible rules and evolutionary trends. FEBS Lett. 1986 Aug 18;204(2):169–176. doi: 10.1016/0014-5793(86)80806-7. [DOI] [PubMed] [Google Scholar]
- Laporte R., Haeberle J. R., Laher I. Phorbol ester-induced potentiation of myogenic tone is not associated with increases in Ca2+ influx, myoplasmic free Ca2+ concentration, or 20-kDa myosin light chain phosphorylation. J Mol Cell Cardiol. 1994 Mar;26(3):297–302. doi: 10.1006/jmcc.1994.1038. [DOI] [PubMed] [Google Scholar]
- Lerman A., Edwards B. S., Hallett J. W., Heublein D. M., Sandberg S. M., Burnett J. C., Jr Circulating and tissue endothelin immunoreactivity in advanced atherosclerosis. N Engl J Med. 1991 Oct 3;325(14):997–1001. doi: 10.1056/NEJM199110033251404. [DOI] [PubMed] [Google Scholar]
- Molina C. R., Andresen J. W., Rapoport R. M., Waldman S., Murad F. Effect of in vivo nitroglycerin therapy on endothelium-dependent and independent vascular relaxation and cyclic GMP accumulation in rat aorta. J Cardiovasc Pharmacol. 1987 Oct;10(4):371–378. doi: 10.1097/00005344-198710000-00001. [DOI] [PubMed] [Google Scholar]
- Münzel T., Sayegh H., Freeman B. A., Tarpey M. M., Harrison D. G. Evidence for enhanced vascular superoxide anion production in nitrate tolerance. A novel mechanism underlying tolerance and cross-tolerance. J Clin Invest. 1995 Jan;95(1):187–194. doi: 10.1172/JCI117637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa M., Shirakawa S., Adelstein R. S. Phosphorylation of smooth muscle myosin light chain kinase by protein kinase C. Comparative study of the phosphorylated sites. J Biol Chem. 1985 Jul 25;260(15):8978–8983. [PubMed] [Google Scholar]
- Packer M., Lee W. H., Kessler P. D., Gottlieb S. S., Medina N., Yushak M. Prevention and reversal of nitrate tolerance in patients with congestive heart failure. N Engl J Med. 1987 Sep 24;317(13):799–804. doi: 10.1056/NEJM198709243171304. [DOI] [PubMed] [Google Scholar]
- Park S., Rasmussen H. Carbachol-induced protein phosphorylation changes in bovine tracheal smooth muscle. J Biol Chem. 1986 Nov 25;261(33):15734–15739. [PubMed] [Google Scholar]
- Parker C. A., Takahashi K., Tao T., Morgan K. G. Agonist-induced redistribution of calponin in contractile vascular smooth muscle cells. Am J Physiol. 1994 Nov;267(5 Pt 1):C1262–C1270. doi: 10.1152/ajpcell.1994.267.5.C1262. [DOI] [PubMed] [Google Scholar]
- Parker J. D., Farrell B., Fenton T., Cohanim M., Parker J. O. Counter-regulatory responses to continuous and intermittent therapy with nitroglycerin. Circulation. 1991 Dec;84(6):2336–2345. doi: 10.1161/01.cir.84.6.2336. [DOI] [PubMed] [Google Scholar]
- Parker J. O. Intermittent transdermal nitroglycerin therapy in the treatment of chronic stable angina. J Am Coll Cardiol. 1989 Mar 15;13(4):794–795. doi: 10.1016/0735-1097(89)90217-9. [DOI] [PubMed] [Google Scholar]
- Parker J. O. Nitrate tolerance in angina pectoris. Cardiovasc Drugs Ther. 1989 Jan;2(6):823–829. doi: 10.1007/BF00133214. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Waldman S. A., Ginsburg R., Molina C. R., Murad F. Effects of glyceryl trinitrate on endothelium-dependent and -independent relaxation and cyclic GMP levels in rat aorta and human coronary artery. J Cardiovasc Pharmacol. 1987 Jul;10(1):82–89. doi: 10.1097/00005344-198707000-00012. [DOI] [PubMed] [Google Scholar]
- Rydell E. L., Axelsson K. L. Adrenaline toxicity in mice: sensitization of alpha 1 adrenoreceptors by nitroglycerin. Acta Pharmacol Toxicol (Copenh) 1984 Jul;55(1):73–77. doi: 10.1111/j.1600-0773.1984.tb01964.x. [DOI] [PubMed] [Google Scholar]
- Sung C. P., Arleth A. J., Storer B. L., Ohlstein E. H. Angiotensin type 1 receptors mediate smooth muscle proliferation and endothelin biosynthesis in rat vascular smooth muscle. J Pharmacol Exp Ther. 1994 Oct;271(1):429–437. [PubMed] [Google Scholar]
- Tamaoki T., Nomoto H., Takahashi I., Kato Y., Morimoto M., Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem Biophys Res Commun. 1986 Mar 13;135(2):397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Yang Z. H., Richard V., von Segesser L., Bauer E., Stulz P., Turina M., Lüscher T. F. Threshold concentrations of endothelin-1 potentiate contractions to norepinephrine and serotonin in human arteries. A new mechanism of vasospasm? Circulation. 1990 Jul;82(1):188–195. doi: 10.1161/01.cir.82.1.188. [DOI] [PubMed] [Google Scholar]