Abstract
Pseudomonas aeruginosa mannose sensitive hamemagglutination strain (PA-MSHA) is a kind of peritrichous P. aeruginosa strain with MSHA fimbriae and has been shown to activate kinds of immunocytes. Dendritic cells (DCs) are specialized antigen-presenting cells required for the stimulating and priming CD4+ T cells toward the T helper cell type 1 (Th1), Th2 and other different phenotypes. PA-MSHA effecting on Th1 remains an important missing link. Here we demonstrated that PA-MSHA augmented monocytes derived-dendritic cells (Mo-DCs) expression of HLA-DR, co-stimulatory and adhesion molecules, and induced Th1-promoting interleukin-12 and tumor necrosis factor α secretion, in addition, PA-MSHA treated Mo-DCs displayed lesser endocytic capacity. Furthermore, in mixed lymphocyte reactions, allostimulatory capacity of Mo-DCs was enhanced by PA-MSHA, CD4+ T cells stimulated by PA-MSHA -activated Mo-DCs showed a Th1-polarized cytokine production, increasing secretion of IFN-γ and decreasing secretion of IL-10 and IL-4. Our findings identified PA-MSHA as an important exogenous factor that induced DCs maturation toward a Th1-promoting phenotype.
Keywords: PA-MSHA strain, Differentiation of Th cell, Dendritic cells, Immunomodulatory capacity
Introduction
The Pseudomonas aeruginosa mannose sensitive hamemagglutination (PA-MSHA) strain is a kind of peritrichous P. aeruginosa strain with MSHA fimbriae established by Professor Xi-ya Mu, a Chinese microbiologist. He adopted biological engineering technology to make the non-MSHA heat-inactivated P. aeruginosa strain have many tenuous and upright MSHA fimbriae around the mycelium, which is widely used for anti-infection and anti-inflammation purposes and even in anti-tumor therapies [1]. Recently, it was reported that PA-MSHA made Th2 differentiation index decreased, and shift Th1 cell increased in spleen cells of IgA nephropathy mouse model [2]. Although, it is known that PA-MSHA can induce Th1-mediated immune responses, it is unknown whether it induces Th1-mediated responses by dendritic cells (DCs).
Dendritic cells were the most efficient antigen-presenting cells in priming naïve T cells toward the Th1, Th2 or other types and were considered promising targets for immunotherapy [3]. Many studies had demonstrated that the Th1/Th2 balance was closely correlated with the outcome of many diseases [4], Many studies had demonstrated that the Th1/Th2 balance was closely correlated with the outcome of many diseases [4]. Such as, Leishmania major [5], Schistosoma japonicum [6], tuberculosis [7], head and neck cancer [8] and multiple myeloma [9]. In addition, the direction of T cells polarization determined the prognosis of many infectious diseases and cancers. In cancer patients with high expression of the Th1 cells had a prolonged disease-free survival [10], while with high expression of the Th2 cells, the patients had a poor progressive [11]. It was reported that Th1 immunity was compromised in infections, while enhancing Th1 responses improved the anti-inflammatory effect [12, 13]. These finds suggest a better understanding of the role of DCs in Th1 cells polarization is crucial for combating with infections and tumors [14], moreover, the status of DCs plays a pivotal role in initiating and guiding the immune response [15]. Therefore, it is important to identify reagents for promoting DCs maturation and inducing towards a Th1-polarizing phenotype. In this study, we investigated whether PA-MSHA can promote the maturation of human monocyte derived immature DCs (Mo-DCs) and induce its function and differentiation towards a Th1-polarizing phenotype.
Materials and Methods
Culture Medium, Reagents and Monoclonal Antibodies
RPMI 1640, fetal bovine serum and carboxyfluorescein succinimidyl ester (CFSE) molecular probes were purchased from Invitrogen (Grand Island, NY). Ficoll/Isopaque LymphoprepTM was purchased from Axis-shield (Axis-shield, Norway). Recombinant human IL-4 and recombinant human granulocyte-macrophage colony-stimulation factor (GM-CSF) were purchased from protech (Rehovot, Israel). CD14 MicroBeads, CD4 MicroBeads and monoclonal antibodies (mAbs) for flow cytometry, toward the following antigens were purchased from Becton–Dickinson (San Diego, CA): anti-CD14-FITC, anti-CD4-FITC, anti-CD80-PE, anti-CD11c-APC, anti-CD40-FITC and anti-HLADR-PEcy5. PA-MSHA (each piece is 1 ml, containing inactivated PA-MSHA strain 1.8 × 109) was purchased from Beijing wanteer bio-pharmacetical Co. Ltd. (Beijing, China). Fluorescein isothiocyanate (FITC)-dextran (40 kDa) and lipopolysaccharide (LPS) were purchased from Sigma (St. Louis, MO). Enzyme-linked immunosorbent assay (ELISA) kits of IL-4, IL-10, INF-γ, TNF-α and IL-12 were purchased from Dakewe Biotech Company (Guangzhou, China).
Generation of Mo-DCs
Peripheral blood mononuclear cells (PBMC) were first isolated from buffy coats obtained from normal healthy donors. Blood was loaded in a 1:1 (vol/vol) ratio on Ficoll and centrifuged without braking for 25 min. The PBMC were washed four times with phosphate-buffered saline (PBS). Monocytes were purified from PBMC by positive selection with human CD14+ microbeads, to increase purity, the cells were passed over a second CD14 microbead column, the purity was more than 95 %. Monocytes were cultured in RPMI 1640 culture medium supplemented with 10 % fetal bovine serum (FBS), and were induced by IL-4 (1000 U ml−1) and GM-CSF (500 U ml−1). The culture lasted 6 days at 37 °C in a 5 %CO2 incubator. At day 6, immature Mo-DCs were harvested, and were stimulated with PA-MASH at the concentration of (1.8 × 107 ml−1, 7.2 × 107 ml−1, 2.88 × 108 ml−1 and 1.152 × 109 ml−1) at 37 °C for 48 h respectively. Positive controls were added LPS (1 μg ml−1) and negative controls were added PBS at the same volume. All subsequent tests were performed after harvesting the cells on day 8 and after removing the above factors by extensive washing.
Mo-DCs Viability
On day 8, cells viability of PA-MSHA, LPS and PBS stimulated Mo-DCs was determined by Cell Counting Kit-8 (CCK-8) as follows: 10 μl of CCK-8 was added per well, cells were incubated for an additional 4 h, and absorbance at 450 nm was recorded by a 96-well plate reader. Cell viability % = OD value of the adds medicine cell group/OD value of negative control group × 100 %.
Mo-DCs Endocytic Activity
Monocytes derived-dendritic cells were collected for endocytosis assay on day 8. Briefly, 50 μl of Mo-DCs (2 × 105 cells) in 2 % FBS-PBS were added into triplicate wells of two 96-well bottom plates before adding FITC-dextran for a final concentration of 1 mg ml−1. The one plate was incubated at 37 °C and the another one was incubated at 4 °C for 1 h, respectively. Both plates were gently tapped every 10 min to ensure adequate mixing. After incubation, plates were washed with 2 % FBS-PBS twice to remove excessive FITC-dextran, followed by cell surface markers staining and flow cytometry analysis. The mean fluorescence intensity (MFI) difference between 37 and 4 °C was considered as the result of antigen uptake.
Flow Cytometric Analysis for Surface Markers
The expression of surface molecules on Mo-DCs was analyzed by flow cytometry on day 8, stimulated Mo-DCs were stained with an anti-CD80-PE, anti-CD11c-APC, anti-CD40- FITC and anti-HLADR-PEcy5 for 15 min at 4 °C. FACS analysis was performed using a FACScan cytofluorometer (BD Biosciences, Franklin Lakes, NJ). Mo-DCs surface marker expression was analyzed by the CellQuest program, dead cells being excluded.
Mixed Lymphocyte Reactions (MLRs)
T cells for MLRs were enriched (purity >95 %) by immunomagnetic negative-depletion with CD4+ MicroBeads according to the manufacturer’s instructions. CD4+ T cells were incubated with 1 μM CFSE at 2 × 107 cells ml−1 for 15 min at room temperature in the dark. CFSE was quenched for 3 min at room temperature with five volumes of ice-cold culture media, and cells were washed twice. Matured Mo-DCs were then treated with mytomycin C (25 μg ml−1, 1 h) and adequate washing, then co-cultured with CD4+ T cells which were stained with CFSE, in triplicate. DCs at 104 cells/200 μl per well in 96-well flat-bottom plates were cultured with 105 allogenic T cells (DCs: CD4+T = 1:10). After 6 days,culture supernatants were stored for cytokine detection, proliferation of CD4+ T cells were detected by FACS analysis which was performed using a FACScan cytofluorometer (BD Biosciences, Franklin Lakes, NJ).
Cytokine Levels in Supernatants of Mo-DCs and MLRs
The cell free supernatants from Mo-DCs and MLRs were harvested at the indicated time points and stored at −80 °C until measurement. Culture supernatants from stimulated Mo-DCs were subjected to IL-12, IL-10 and TNF-α detection. IFN-γ, IL-4 and IL-10 secretions were detected in supernatants from MLRs. Cytokines were quantified by ELISA according to the manufacturer’s instructions and measured at an extinction of 450 nm (MR5000 ELISA-reader and Bio-Linx Software; Dynatech, Chantilly, VA).
Statistics
Statistical analysis of the results was performed by one-way ANOVA. Differences were considered statistically significant when p values were less than 0.05.
Results
PA-MSHA Influenced Mo-DCs Viability
To further exclude the possibility that PA-MSHA has a cytotoxic effect, we assessed the effect of PA-MSHA on cultured Mo-DCs by CCK-8 assays. Our findings showed in PA-MSHA groups at the concentrations of 1.8 × 107 ml−1 and 7.2 × 107 ml−1, the viability of cells was >97 % as that in PBS groups and LPS groups. However, at concentration of 2.88 × 108 ml−1 groups, the viability of cells was 79.46 %, when the concentration up to 1.152 × 109 ml−1, Mo-DCs were damaged more than 50 %, and cell morphology was abnormal, so PA-MSHA at the concentration of 1.152 × 109 ml−1 was not used in the next study.
PA-MSHA Influenced Immune Phenotype of Mo-DCs
To investigate the effects of PA-MSHA on the immune phenotypic Mo-DCs, PA-MSHA was added into immature Mo-DCs culture. After 48 h, FACS analyses showed that CD80, CD11c, CD40 and HLA-DR expression levels on PA-MSHA-primed Mo-DCs were enhanced in a dose-dependent manner when the concentration of PA-MSHA under 7.2 × 107 ml−1. As the concentration up to 2.88 × 108 ml−1, the surface expression descended. The expression patterns of HLA-DR, CD40, CD80 and CD11c in the PA-MSHA (7.2 × 107 ml−1)-treated groups were very similar to those in LPS groups as positive control groups (Fig. 1).
Fig. 1.
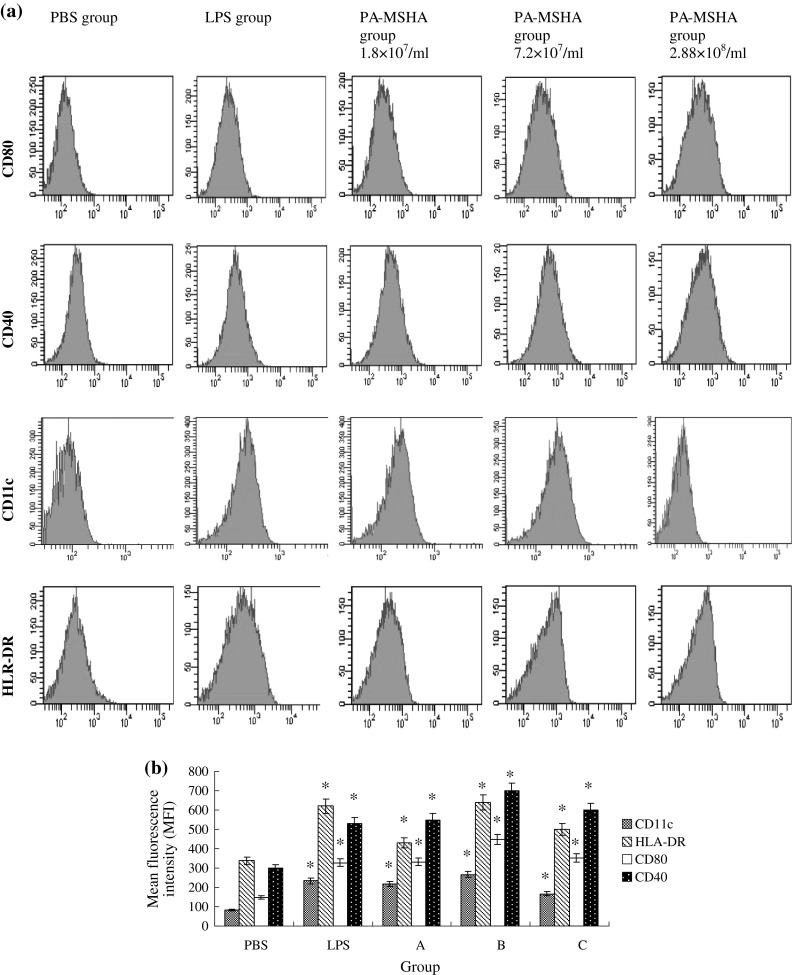
PA-MSHA induces differentiation of Mo-DCs with up-regulated expression of co-stimulatory, adhesion and MHC II molecules. Mo-DCs derived from peripheral blood monocytes were untreated or stimulated with PA-MSHA, or 1 μg ml−1 LPS on day 5 and cultured for another 48 h. Cells were stained with antibodies against CD80, CD40, CD11c and HLA-DR to determine the phenotype of Mo-DCs by flow cytometry. a FACS analysis of 1 representative of 5 experiments is shown. b The result is shown as the mean fluorescence intensity (MFI) ± SD. *P < 0.05 compared to negative control (PBS group). (Note PBS:PBS group; LPS:LPS group; A:PA-MSHA group 1.8 × 107 ml−1; B:PA-MSHA group 7.2 × 107 ml−1; C:PA-MSHA group 2.88 × 108 ml−1)
PA-MSHA Inhibits Endocytotic Capacity of Mo-DCs
To determine whether mechanisms of Ag capture could also be modulated by PA-MSHA, The endocytic activity was measured in PA-MSHA-, PBS- or LPS-primed Mo-DCs. FITC-dextran uptake mediated by PA-MSHA and LPS-primed Mo-DCs was significantly lower than immature Mo-DCs (Fig. 2). PA-MSHA groups at different three concentrations were identical with LPS group on the FITC-dextran uptake by Mo-DCs. These results suggested that Mo-DCs differentiated by PA-MSHA and LPS down-regulated their endocytic capacity (Fig. 2).
Fig. 2.
PA-MSHA reduces the endocytosis of FITC-dextran by Mo-DCs. PA-MSHA was pre-incubated with Mo-DCs for 48 h before endocytosis analysis. Endocytosis was evaluated as uptake of 1 mg ml−1 FITC-dextran by flow cytometry. The result is shown as the solution of mean fluorescence intensity (MFI) at 37 °C subtracting MFI at 4 °C. The data are representative of three independent experiments. The result is shown as the mean fluorescence intensity (MFI) ± SD. *P < 0.05 compared to negative control (PBS group). (Note PBS:PBS group; LPS:LPS group; A:PA-MSHA group 1.8 × 107 ml−1; B:PA-MSHA group 7.2 × 107 ml−1; C:PA-MSHA group 2.88 × 108 ml−1)
PA-MSHA Stimulates the Secretion of IL-12p70 and TNF-α from Mo-DCs
Dentritic Cells play the key role for shaping T cell–mediated immune responses through their secretion of specific cytokine patterns, we went on to determine the effect of PA-MSHA on the cytokine release of DCs. In PA-MSHA- or LPS-treated group, TNF-α and IL-12p70 was induced to a higher expression level compared to that in the PBS group, while the production of IL-10 was down-regulated by PA-MSHA or LPS. However, the concentrations of PA-MSHA up to 2.88 × 108 ml−1, the expression of IL-12 p70, IL-10 and TNF-α descended, which may be the result of PA-MSHA harmful effect on Mo-DCs. These findings gave strong evidence that PA-MSHA affected T-cell–mediated responses by provoking Mo-DCs to release Th1-promoting cytokines (Fig. 3).
Fig. 3.
PA-MSHA induces the secretion of IL-12 p70, TNF-α and IL-10 by Mo-DCs. On day 5, immature Mo-DCs were cultured with PBS, LPS and different concentrations of PA-MSHA for 48 h. Supernatants were collected and the production of IL-12 p70, TNF-α (a) and IL-10 (b) were detected by ELASA kits. Experiments were performed five times. *P < 0.05 compared to negative control (PBS group). (Note PBS:PBS group; LPS:LPS group; A:PA-MSHA group 1.8 × 107 ml−1; B:PA-MSHA group 7.2 × 107 ml−1; C:PA-MSHA group 2.88 × 108 ml−1)
PA-MSHA Enhanced the Ability of Mo-DCs to Stimulate CD4+ T cell Proliferation
The ability to induce allogeneic T cell proliferation is a functional hallmark of DCs in vitro [9]. We have demonstrated that PA-MSHA can induce Mo-DCs maturation and stimulate the Th1-promoting cytokines secretion. Hence, we went on to study whether PA-MSHA could enhance the immunostimulatory capacity of Mo-DCs. We compared the abilities of unstimulated immature DCs, PA-MSHA- or LPS-treated Mo-DCs to stimulate allogeneic T cells in MLRs. Mature Mo-DCs stimulated by PA-MSHA or LPS exhibited higher immunostimulatory capacity than unstimulated immature Mo-DCs, no difference was found at three different concentrations of PA-MSHA groups. These results suggest that PA-MSHA can functionally induce DCs maturation and enhance the immunostimulatory capacity of Mo-DCs (Fig. 4).
Fig. 4.
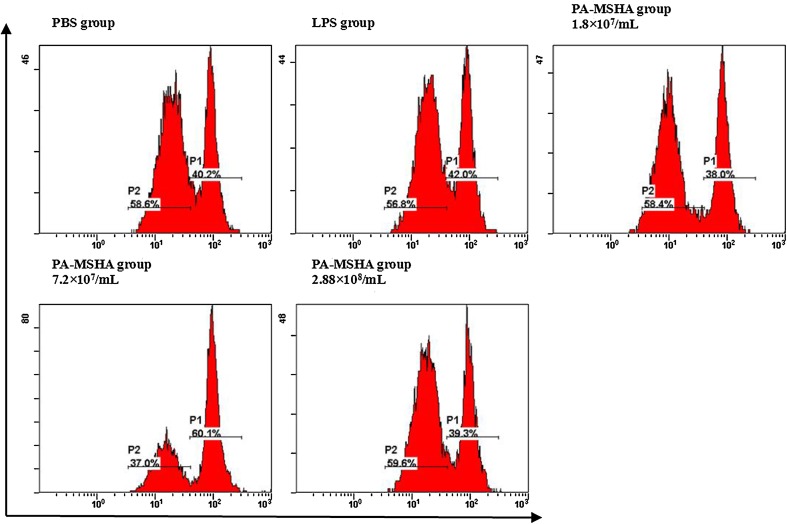
PA-MSHA-treated Mo-DCs exhibit enhanced T cell allostimulatory capacity. CD4+ T stained by CSFE were co-cultured with graded doses of PA-MSHA, PBS or LPS-primed Mo-DCs for 6 days, the proliferation was analyzed by flow cytometry. The analysis of 1 representative of 3 similar experiments is shown
PA-MSHA-Activated Mo-DCs Polarize Naive T Cells Toward a Th1 Phenotype
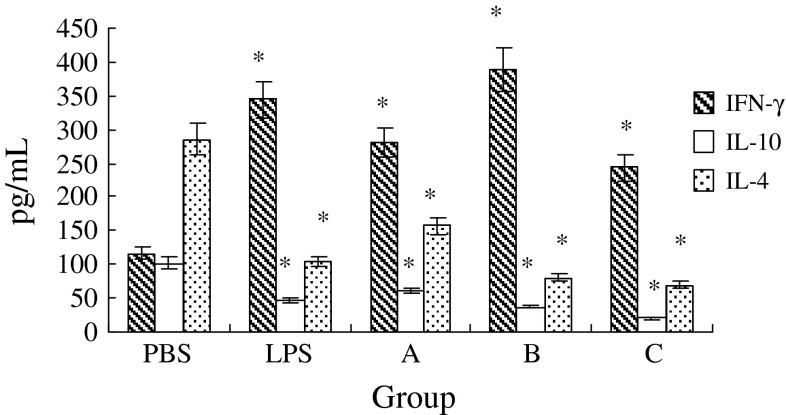
It is the unique ability of DCs to induce a primary immune response through activation and polarization of naive T cells. In addition to the ability of PA-MSHA-treated Mo-DCs to induce T cell proliferation, we next analyzed the state of Th cell polarization. Therefore, IFN-γ, IL-10 and IL-4 were measured to evaluate Th-polarizing cytokines. We found that Th cells induced the increasing secretion of IFN-γ by PA-MSHA-stimulated Mo-DCs. PA-MSHA groups in concentrations of 7.2 × 107 ml−1 were highest than other PA-MSHA groups, while the secretion of IL-10 and IL-4 were reduced. There finds provide strong evidence that Th cells were polarized toward a Th1 phenotype (Fig. 5).
Fig. 5.
PA-MSHA -activated Mo-DCs polarize naive T cells toward a Th1 phenotype. MLR was performed with immature DCs, PA-MSHA-treated DCs or LPS-treated DCs and allogeneic T cells. Supernatants were obtained after 6 days of co-culture and analyzed for the content of IFN-γ, IL-4 and IL-10. Data are given the mean pg ml−1 ± SD of five independent experiments. *P < 0.05 compared to negative control (PBS group). (PBS:PBS group; LPS:LPS group; A:PA-MSHA group 1.8 × 107 ml−1; B:PA-MSHA group 7.2 × 107 ml−1; C:PA-MSHA group 2.88 × 108 ml−1)
Discussion
In the present study, we demonstrated that PA-MSHA as an exogenous factor had a potential to induce Th1 cells polarization by promoting human DCs maturation. Specifically, we observed that incubation of immature Mo-DCs with PA-MSHA promoted secretion of IL-12p70 and TNF-α, and upregulated the expressions of HLA-DR, CD80, CD11c, and CD40. Furthermore, we analyzed the influence of PA-MSHA treated Mo-DCs on CD4+ T cells, and demonstrated that PA-MSHA treated Mo-DCs induced Th cells to Th1 cells with high proliferative capacity. These results are quite similar to the Mo-DCs treated with anti-tumor or anti-infection pharmacological agents to induce Th1 [10].
Dentritic Cells have a critical impact on the adaptive immune response. As maturation, DCs lose their endocytic ability, up-regulate MHC class II and costimulatory molecules, and present antigen to T cells for the initiation of immune responses [11]. DCs influence T cell proliferation and effector function by providing costimulation and establishing the cytokine environment at the time of T cell priming [12, 13]. Cytokines, secreted by DCs at the time of initial T cell stimulation, play an important role in the subsequent differentiation of native T cells. IL-12 and TNF-α produced by DCs were the critical Th1-polarizing cytokine for Th cells, while that failed to produce IL-12p70 were assumed to promote the generation of a Th2 response [14–17], and IL-10 modulated DCs in a tolerogenic manner [18]. In this study, PA-MSHA decreased Mo-DCs endocytic ability, up-regulated expression of HLA-DR, CD80, CD11c, and CD40, then induced the secretion of IL-12 and decreased the secretion of IL-10 by DCs, which demonstrated that PA-MSHA induced Mo-DCs maturation, matured Mo-DCs had potency to prime Th1 immune response.
Dentritic Cells influence Th cells proliferation, which most commonly polarize to Th1 or Th2 cells [19]. IL-12 excreted by DCs stimulates the secretion of IFN-γ by Th cells [20]. In general, type 1 responses are dominated by T cells producing primarily IFN-γ, which plays a pivotal role in enhancing cellular immunity and exerting a powerful anti-tumor or anti-infection effect. In contrast, type 2 responses are dominated by T cells producing IL-4, IL-5, and IL-10 [21], which is involved in progression of tumors and infections [5, 22]. PA-MSHA-primed Mo-DCs directed T cell differentiation and primed Th cell to Th1 by elevated secretion of IFN-γ and decreased secretion of IL-10 and IL-4.
In addition, Our data demonstrated the immunoenhancement in low concentrations of PA-MSHA, inducing Th1 immune response, nevertheless, when the concentrations up to (1.15 × 109 ml−1) more than 50 % Mo-DCs lost activity. As the whole bacterial PA-MSHA constitutes many potential immunogenic components, further studies are needed to identify the exact components and mechanisms that regulate Mo-DCs.
In conclusion, we have characterized a variety of effects exerted by PA-MSHA on DCs and found PA-MSHA is an exogenesis factor, through its ability to activate DCs, furthermore, matured DCs drive Th cells toward Th1 type in vitro. Utilizing the characteristics of PA-MSHA effect on DCs may be helpful in controlling and achieving the specific Th1 immune response desired.
References
- 1.Liu ZB, Hou YF, Min-Dong, Di GH, Wu J, Shen ZZ, Shao ZM. PA-MSHA inhibits proliferation and induces apoptosis through the up-regulation and activation of caspases in the human breast cancer cell lines. J Cell Biochem. 2009;108:195–206. doi: 10.1002/jcb.22241. [DOI] [PubMed] [Google Scholar]
- 2.Jia L, Wang C, Kong H, Yang J, Li F, Lv S, Xu G. Effect of PA-MSHA vaccine on plasma phospholipids metabolic profiling and the ratio of Th2/Th1 cells within immune organ of mouse IgA nephropathy. J Pharm Biomed Anal. 2007;43:646. doi: 10.1016/j.jpba.2006.07.040. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau J, Steiman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 4.D’elios M, Del Prete G. Th1/Th2 balance in human disease. Transplant Proc. 1998;30:2373–2377. doi: 10.1016/S0041-1345(98)00659-9. [DOI] [PubMed] [Google Scholar]
- 5.Suzue K, Kobayashi S, Takeuchi T, Suzuki M, Koyasu S. Critical role of dendritic cells in determining the Th1/Th2 balance upon Leishmania major infection. Int Immunol. 2008;20(3):337–343. doi: 10.1093/intimm/dxm147. [DOI] [PubMed] [Google Scholar]
- 6.Xu X, Wen X, Chi Y, He L, Zhou S, Wang X, Zhao J, Liu F, Su C. Activation-induced T helper cell death contributes to Th1/Th2 polarization following murine Schistosoma japonicum infection. J Biomed Biotechnol. 2010;2010:202397. doi: 10.1155/2010/202397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawakami K. Factors for the onset of and the exacerbation of tuberculosis. 2. Host factors promoting the occurrence and exacerbation of tuberculosis: significance of Th1–Th2 cytokine balance. Kekkaku. 1999;74:735–740. [PubMed] [Google Scholar]
- 8.Jebreel A, Mistry D, Loke D, Dunn G, Hough V, Oliver K, Stafford N, Greenman J. Investigation of interleukin 10, 12 and 18 levels in patients with head and neck cancer. J Laryngol Otol. 2007;121:246–252. doi: 10.1017/S0022215106002428. [DOI] [PubMed] [Google Scholar]
- 9.Hong S, Qian J, Yang J, Li H, Kwak LW, Yi Q. Roles of idiotype-specific T cells in myeloma cell growth and survival: Th1 and CTL cells are tumoricidal while Th2 cells promote tumor growth. Cancer Res. 2008;68:8456–8464. doi: 10.1158/0008-5472.CAN-08-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang M, Ma C, Sun J, Shao Q, Gao W, Zhang Y, Li Z, Xie Q, Dong Z, Qu X. Fucoidan stimulation induces a functional maturation of human monocyte-derived dendritic cells. Int Immunopharmacol. 2008;8:1754–1760. doi: 10.1016/j.intimp.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Benvenuti F, Lagaudrière-Gesbert C, Grandjean I, Jancic C, Hivroz C, Trautmann A, Lantz O, Amigorena S. Dendritic cell maturation controls adhesion, synapse formation, and the duration of the interactions with naive T lymphocytes. J Immunol. 2004;172:292–301. doi: 10.4049/jimmunol.172.1.292. [DOI] [PubMed] [Google Scholar]
- 12.O’Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8:275–283. doi: 10.1016/S1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- 13.Weaver D, Poligone B, Bui T, Abdel-Motal U, Baldwin A, Tisch R. Dendritic cells from nonobese diabetic mice exhibit a defect in NF-κB regulation due to a hyperactive IκB kinase. J Immunol. 2001;167:1461–1468. doi: 10.4049/jimmunol.167.3.1461. [DOI] [PubMed] [Google Scholar]
- 14.Ueno H, Klechevsky E, Morita R, Aspord C, Cao T, Matsui T, Di Pucchio T, Connolly J, Fay JW, Pascual V, Palucka AK, Banchereau J. Dendritic cell subsets in health and disease. Immunol Rev. 2007;219:118–142. doi: 10.1111/j.1600-065X.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- 15.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 16.Heufler C, Koch F, Stanzl U, Topar G, Wysocka M, Trinchieri G, Enk A, Steinman RM, Romani N, Schuler G. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-gamma production by T helper 1 cells. Eur J Immunol. 1996;26:659–668. doi: 10.1002/eji.1830260323. [DOI] [PubMed] [Google Scholar]
- 17.Merad Miriam, Manz Markus G. Dendritic cell homeostasis. Blood. 2009;113:3418–3427. doi: 10.1182/blood-2008-12-180646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mark Wallet A, Pradip Sen, Roland Tisch Immunoregulation of dendritic cells. Clin Med Res. 2005;3:166–175. doi: 10.3121/cmr.3.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feili-Hariri M, Falkner DH, Morel Polarization of naive T cells into Th1 or Th2 by distinct cytokine-driven murine dendritic cell populations: implications for immunotherapy. J Leukoc Biol. 2005;78:656–664. doi: 10.1189/jlb.1104631. [DOI] [PubMed] [Google Scholar]
- 20.Trinchieri G. The two faces of interleukin 12: a pro-inflammatory cytokine and a key immunoregulatory molecule produced by antigen-presenting cells. Ciba Found Symp. 1995;195:203–214. doi: 10.1002/9780470514849.ch14. [DOI] [PubMed] [Google Scholar]
- 21.Morel PA, Oriss TB. Cross regulation between Th1 and Th2 cells. Crit Rev Immunol. 1998;18:275–303. doi: 10.1615/CritRevImmunol.v18.i4.10. [DOI] [PubMed] [Google Scholar]
- 22.Kanazawa M, Yoshihara K, Abe H, Iwadate M, Watanabe K, Suzuki S, Endoh Y, Takita K, Sekikawa K, Takenoshita S, Ogata T, Ohto H. Effects of PSK on T and dendritic cells differentiation in gastric or colorectal cancer patients. Anticancer Res. 2005;25:443–449. [PubMed] [Google Scholar]