Abstract
Vibrio parahaemolyticus, a halophilic gram-negative bacterium, is a food-borne pathogen that largely inhabits marine and estuarine environments, and poses a serious threat to human and animal health all over the world. The hollow “needle” channel, a specific assemble of T3SS which exists in most of gram-negative bacteria, plays a key role in the transition of virulence effectors to host cells. In this study, needle protein VP1694 was successfully expressed and purified, and the fusion protein Trx-VP1694 was used to immunize Balb/c mice. Subsequently, a phage single-chain fragment variable antibody (scFv) library was constructed, and a specific scFv against VP1694 named scFv-FA7 was screened by phage display panning. To further identify the characters of scFv, the soluble expression vector pACYC-scFv-skp was constructed and the soluble scFv was purified by Ni2+ affinity chromatography. ELISA analysis showed that the scFv-FA7 was specific to VP1694 antigen, and its affinity constant was 1.07 × 108 L/mol. These results offer a molecular basis to prevent and cure diseases by scFv, and also provide a new strategy for further research on virulence mechanism of T3SS in V. parahaemolyticus by scFv.
Keywords: Vibrio parahaemolyticus, T3SS, Needle, scFv, Biopanning
Introduction
Vibrio parahaemolyticus is a gram-negative, halophilic bacterium that occasionally infects humans via wounds or consumption of contaminated seafood [1], and often leads to acute gastroenteritis and other serious diseases including septicemia in aquatic species [2, 3]. The harmful outcomes caused by this bacterium are increasingly becoming serious, especially in coastal waters and fish farms [4]. V. parahaemolyticus infection has gradually become a major health and economic issue in several coastal cities. Given the harmful effects and prevalence of V. parahaemolyticus, it is very important to develop an effective drug or antibody for improving this situation. However, no effective antibodies are available until now.
Like other gram-negative pathogenic bacteria, V. parahaemolyticus also contains a contact-dependent type III secretion system (T3SS) that delivers several effectors into the cytosol of the infected host cells, leading to the death of cells [5–10]. The needle complex is the main component of T3SS, and the translocon is a pore-forming complex of T3SS that directly inserts into the cytoplasm membrane of host cells and links the needle complex to the host cell membrane [10–13]. T3SS also contains two other important accessories, one is the regulator that ensures the normal function of T3SS by regulating the refolding of the target proteins [14, 15], and another is the effector (several virulence proteins) that can induce the lysis and death of cells [16–19]. In V. parahaemolyticus, the needle complex is formed by a needle subunit protein (VP1694) with 88-residues, and its function only relies on a single polymerized protein [20–22]. The above depiction shows that the needle subunit might be a useful target protein for screening an effective antibody or inhibitor that can prevent the formation of needle complex.
To study the virulence mechanism of T3SS, some small molecular inhibitors were used to block the assembly of the T3SS. However, these studies demonstrated that blocking of T3SS assembly was incomplete [23–26]. Hence, it is required to develop an effective antibody or inhibitor to enhance the blocking efficiency. Single-chain variable-fragment antibody (scFv) generation is a versatile technology for getting antibody that is specific for a given antigen [27]. scFv plays a critical role in several human diseases, and may in fact also be developed into a potential diagnostic and/or therapeutic agent. Furthermore, scFv has strong penetrability in the host tissue and has special neutralization against specific target [28, 29]. Combining scFv generation with biopanning strategy provides a useful tool that allows the selection of antibody against specific antigen. In the present study, the needle subunit protein was successfully expressed and a specific scFv-FA7 was obtained for the first time by phage display technology and the skp co-expressed scFv-FA7 was specific to VP1694.
Materials and Methods
Material
Vibrio parahaemolyticus ATCC 17802 and other E. coli strains were from our laboratory. Balb/c mice were purchased from Shanghai Laboratory Animal Center (China), and animal experiments were performed according to relevant national and international guidelines. All other reagents were of analytical reagent grade.
Expression and Purification of VP1694
The needle subunit gene (VP1694 gene) was amplified from V. parahaemolyticus ATCC 17802 genome with primers VP1694-F(5′-CCCCGAATTCATGTCATTTTACG-3′) and VP1694-R (5′-TTACTCGAGCACCTTCTGCAGGA-3′), and the VP1694 gene was designed for cloning into the pET32a (+) and pGEX-6p-1 vectors. The constructed vectors were transformed into E. coli BL21 by electroporation, and a single colony from the selection plate was inoculated into 5 mL LB liquid media containing 100 μg/mL ampicillin for the expression of VP1694. The expressed protein was purified using Ni2+ or GST affinity chromatography .
Immunization and Anti-Serum Titer Assay
Six-week-old female Balb/c mice were immunized with a mixture of 100 μg purified fusion protein and equal volume of complete Freund’s adjuvant by s.c route as the first immunization. After 3 weeks, mice were given first booster dose using incomplete Freund’s adjuvant [27]. After second booster dose, the anti-serum titer was detected by indirect enzyme-linked immunosorbent assay (ELISA) [30].
Construction of Phage-Antibody Library Against VP1694
Total mRNA was extracted from isolated spleens by Trizol method. First-strand cDNA was synthesized by reverse transcription-PCR (RT-PCR) with reverse transcriptase and random hexadeoxyribonucleotide primers. The variable regions of the heavy chain (VH) and light chain (VL) were amplified by PCR using cDNA as a template. Then the assembled scFv gene was amplified using the products derived from first-step PCR as a template. Subsequently, these amplified scFv fragments were cloned into pCANTAB-5E vector, and transformed into E. coli TG1 competent cells by electroporation. To count colonies, 100 μL of diluted transformed cells or untransformed negative-control cells was plated onto SOB-AG plates and incubated at 30 °C overnight.
Biopanning
All the transformants were used for phage rescue with M13KO7 helper phage, and recombinant phages were collected by centrifugation for further biopanning. To obtain the specific scFv antibody, the phage display and phage-ELISA were used. A 96-well micro-plate was coated with diluted GST-VP1694 antigen (2.5 μg/mL) in PBS buffer, and incubated at 4°C overnight. After washing and blocking, the recombinant phage was then diluted and added to a pretreated plate (100 μL/well). After incubation at 37 °C for 2 h, the plate was washed 20 times with PBS and then 20 times with PBST (PBS containing 0.05 %Tween 20) to remove unbound phage. Phage which reacted with VP1694 was eluted with 10 mL triethylamine followed by 10 mL of Tris–HCl (pH7.4) to neutralize the reaction. Eluted phage was used to infect log-phase E. coli TG1 cells, and 10 μL of infected E. coli cells were plated onto SOB-AG (SOB medium containing 100 μg/mL ampicillin and 2 % glucose) plates for screening of individual colonies.
Screening of Specific Antibody Against VP1694
After six rounds of biopanning, 100 single clones were individually picked out and cultured in a tube with M13KO7 for rescue. A 96-well micro-plate was coated with purified GST-VP1694 antigen at 5 μg/mL, followed by blocking with PBSM (PBS solution containing 5 % non-fat milk) and subsequent washing. The binding activity of bound phage was detected with an anti-M13 monoclonal antibody and mouse monoclonal antibody conjugated with horseradish peroxidase (HRP). TMB was used as substrate, and the reaction was stopped by 2 M H2SO4. Absorbance was determined at 450 nm by microplate reader. The wells showing higher binding activity were marked and chosen for further verification.
Identification of the Specific scFv Clone
The enriched clones were identified by PCR and restriction enzyme digestion. Once the positive clone was obtained, DNA sequence was performed for further identification. The sequence of scFv gene was blasted with known murine genes for homology analysis from the Genbank/EMBL database [31], and IMGT/V-Quest database of mouse was also used for the homology analysis of scFv [32].
Specificity Analysis of Positive scFv Against VP1694
To test the specificity of positive scFv-FA7 against VP1694, phage-ELISA was carried out in this study. Briefly, associated V. parahaemolyticus proteins (TDH, TLH, VopQ, VP1694), Homologous protein HrpA, and PBS were used to coat the 96-well micro-plate and incubated at 4 °C overnight. After blocking with PBSM, precipitated phage particles were added into the reaction wells and incubated at 37 °C for 2 h. The specificity of anti-VP1694 scFv-FA7 was detected with an anti-M13 monoclonal antibody and mouse monoclonal antibody conjugated with HRP, and the following steps were done in the same way as above.
Soluble Expression and Purification
To express the soluble scFv protein, primers scFv-f (5′-TTGACGAATTCCCAGGTCCAACTG CA-3′) and scFv-R(5′-CCCCCCAAGCTTCCGTTTTATTTCCA) with EcoR I and Hind III restriction sites respectively were designed according to the sequence of scFv-FA7 and used for cloning scFv-FA7 gene into the co-expressed vector pACYC-Duet-1-skp, which was containing skp chaperone for helping scFv soluble expression. After sequencing, the scFv-FA7 protein was expressed in E. coli BL21 (DE3) strain by adding 1 mM IPTG and purified by Ni2+ affinity chromatography.
Western Blot
To further investigate the interaction between scFv-FA7 and VP1694, western blot was performed as described [27]. First, the purified GST-VP1694 antigen was transferred from SDS-PAGE gel onto a polyvinylidene difluoride membrane, and the membrane was treated with the purified anti-VP1694 scFv and anti-His tag IgG. After washing and blocking, the membrane was subsequently incubated with HRP-conjugated anti-mouse IgG. Signals were visualized by enhanced chemiluminescence.
Affinity Determination
Indirect ELISA was used to determine the affinity of scFv-FA7 as described by Wang et al. [26]. Serially diluted GST-VP1694 antigens (5, 2.5, 1.25 and 0.625 μg/mL) was used to coat the 96-well micro-plate and incubated at 4 °C overnight. After blocking with PBSM, different concentrations of the purified anti-VP1694 antibody were added into well accordingly and incubated at 37 °C for 2 h, and the following steps was same as described for phage-ELISA. The affinity constant of scFv-FA7 (Kaff) were calculated using the equation Kaff = (n − 1)/(n[Ab2] − [Ab1])[33].
Results
Expression and Purification of VP1694
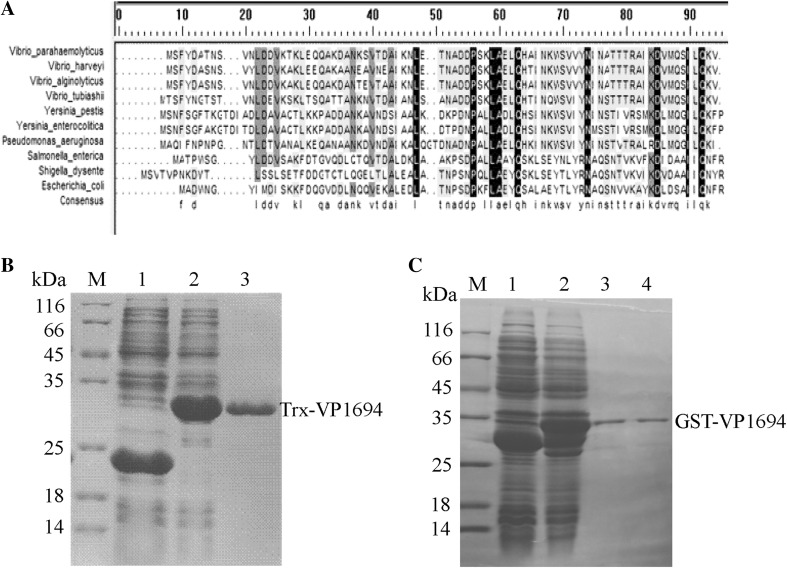
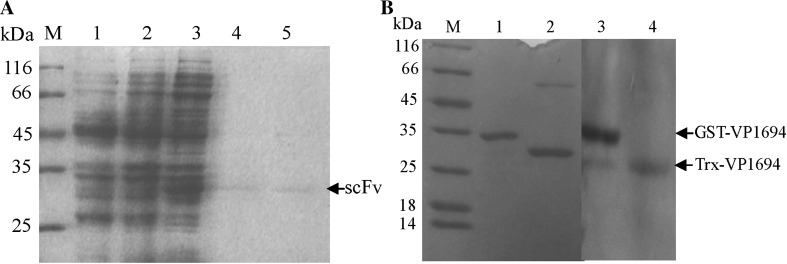
After sequencing, the similarity of VP1694 was identified through BLAST. The BLAST results of protein sequence identity VP1694 with its counterparts including YscF, PscF, MixH and EscF are shown in Fig. 1a, and the VP1694 has high similarity (about 43–56 %) with needle genes in other gram-negative bacterium. The results of protein expression and purification were analyzed by SDS-PAGE. As shown in Fig. 1b, c, GST-VP1694 and Trx-VP1694 were successfully expressed and purified, respectively.
Fig. 1.
Expression and purification of VP1694. a Homology analysis of the needle gene among several gram-negative bacteria. b Expression and purification of Trx-VP1694 fusion protein. M molecular weight of marker proteins, Lanes 1 negative control (pET32a), Lane 2 the expressed products of pET32a-scFv, Lane 3 the purified protein of Trx-VP1694. c Expression and purification of GST-VP1694 fusion protein. M molecular weight of marker proteins, Lanes 1 negative control (pGEX-6p-1), Lane 2 the expressed products of pGEX-6p-scFv, Lane 3 the purified protein of GST-VP1694
Construction of Phage-Antibody Library Against VP1694
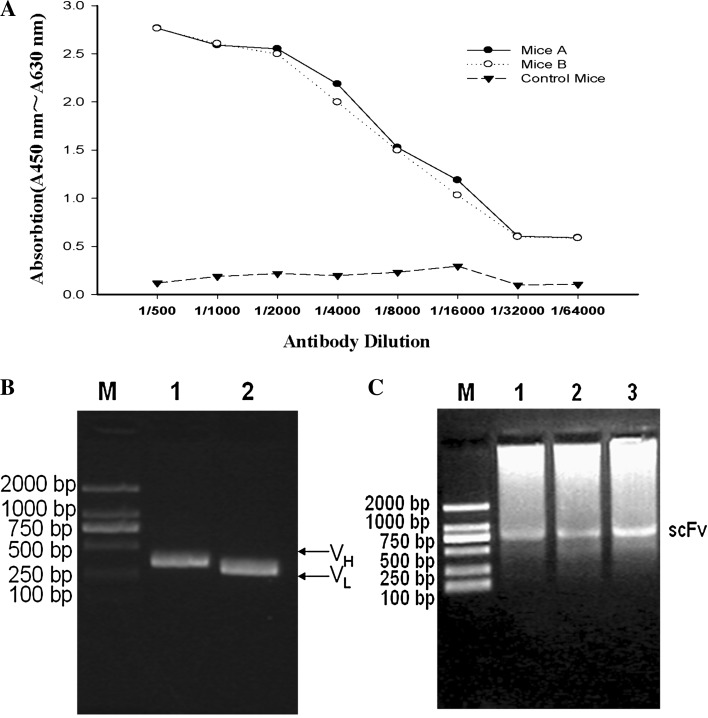
In this study, Trx-VP1694 antigen was used to immunize the mouse, while GST-VP1694 fusion protein was chosen to detect the anti-serum titer via indirect ELISA. As shown in Fig. 2a, both mouse A and mouse B generated a similar anti-VP1694 serum titer, and the serum titer reached to 1: 16000 (Fig. 2a), indicating that both mice had a positive and high titer of serum antibody against VP1694.
Fig. 2.
Construction of phage-scFv library against VP1694. a Anti-serum titer assay by indirect ELISA. b PCR analysis of VH and VL gene. Lane 1 the PCR product of VH gene, Lane 2 the product of VL gene, Lane M the marker DL-2000. c the amplified fragment of scFv gene. Lane M DL-2000 Marker, Lane 1–3: The amplified fragment of scFv gene
The VH and VL gene encoding the scFv fragments were amplified by PCR using the cDNA as template (Fig. 2b), and the scFv fragment was assembled by joining the VH and VL together with a linker DNA (Fig. 2c). Primers with Sfi I and Not I restriction enzyme sites were used for cloning of the scFv genes into the pCANTAB-5E vector. An initial phage-scFv library was constructed, and its capability was up to 1.4 × 107 CFU/mL.
Screening and Identification of Specific Antibody Against VP1694
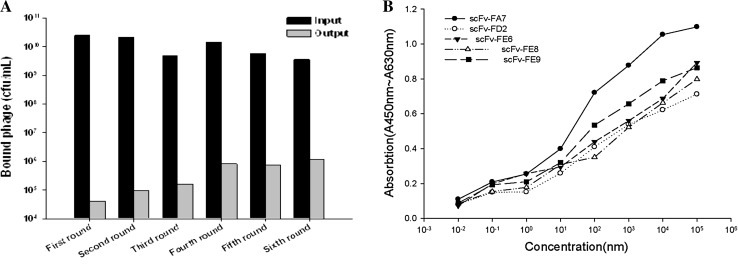
After six rounds of biopanning, the capability of eluted phage-scFv library was approximately up to 1 × 106 and a stable capability was maintained (Fig. 3a). At last, five high capability positive clones were selected. One clone showed the highest activity and much better binding to VP1694 than the others, and was named scFv-FA7. It was selected for further analysis (Fig. 3b).
Fig. 3.
Screening and identification of specific antibody against VP1694. a Biopanning of specific antibody against VP1694. b The binding activity analysis of five scFv clones
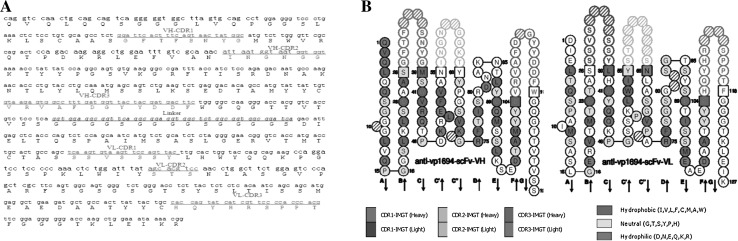
Bioinformatics Analysis of scFv-FA7
The length of scFv-FA7 gene was 729 nucleotides and encoded 245 amino acids, containing the VH, VL and Linker DNA sequence. The IMGT/V-QUEST results showed that scFv-FA7 had high similarity to other reported mouse scFv, and contained the complementary determining regions of VH-CDR1, VH-CDR2, VH-CDR3, and VL-CDR1, VL-CDR2, VL-CDR3 (Fig. 4a). The homology analysis classified VH gene as IGHV5, IGHJ2 and IGHD2 while VL gene was classified as IGKV4 and IGKJ2. Also, the graphical two-dimensional representation of scFv-FA7 was obtained by IMGT/Collier-de-Perles (Fig. 4b), and a result similar to the IMGT/V-QUEST was obtained.
Fig. 4.
Bioinformatics analysis of scFv-A7. a IMGT/V-QUEST analysis of scFv-FA7. b IMGT/Collier-de-Perles analysis of scFv-FA7
Co-expression and Purification of scFv-FA7
As shown in Fig. 5a the target protein was expressed and purified successfully, and that the apparent molecular weight of the target scFv-FA7 was consistent with its corresponding theoretical molecular weight (about 29 kDa). Western blot was performed to test the interaction between scFv-FA7 and VP1694. As shown in Fig. 5b, a clear band was revealed from western blotting (Fig. 5b, lane 3), demonstrating that the GST-VP1694 band at 35 kDa could be recognized by scFv-FA7.
Fig. 5.
Co-expression and purification of scFv-FA7 antibody. a SDS-PAGE analysis of co-expressed scFv-FA7 antibody. b Western blot analysis of scFv-FA7. Left SDS-PAGE results for the purified protein of GST-VP1694 and Trx-VP1694. Right Western blotting results
Specificity Analysis and Affinity Determination
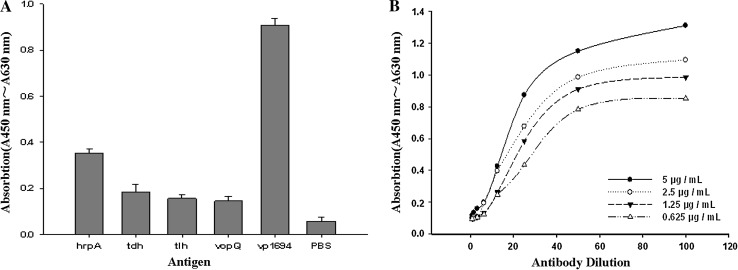
As shown in Fig. 6a, the scFv-FA7 antibody reaction with VP1694 was significant (P < 0.01), but not with HrpA, TDH, TLH, and VopQ. These results indicated that the scFv-FA7 antibody is a specific antibody against VP1694 antigen. Besides, the indirect ELISA results showed that its affinity constant to VP1694 was 1.07 × 108 M−1 (Fig. 6b).
Fig. 6.
Identification of scFv-FA7 antibody. a Specificity analysis of scFv-FA7 by phage-ELISA. b Binding of scFv-FA7 to different concentrations of VP1694 antigen as determined by ELISA. The four VP1694 concentrations were 5, 2.5, 1.25 and 0.625 μg/mL respectively
Discussion
In the past decades, antibody has been widely utilized in pharmaceutical and clinical applications. However, its high molecular weight is not beneficial for application in therapy, and its yield is limited since there is no effective means of production until now. Single chain variable fragment (scFv) as drugs are being rapidly developed in various clinical areas because they have many advantages. Firstly, they have effective penetrability as they are composed of VH, VL, so it can easily be manipulated for immunological application. Secondly, they can be easily produced at large scales using engineered cloning vectors in bacterial hosts [34, 35].
Compared to traditional methods of hybridoma development, phage display technology has many benefits. Most importantly, phage display technology is a fast screening method that takes the place of the tedious and time-consuming hybridoma development. In addition, the biopanning process can be repeated several times to enrich the population of phages with highest affinity and specificity of the scFv for specific antigen. So phage display has become a more widely used method than bacterial surface display, ribosomal display, puromy display and yeast surface display [28, 36].
During the process of preparing anti-VP1694 scFv, there were some questions we confronted. We were able to solve these problems by designing the experiments carefully. To avoid the false positive antibody generation, two prokaryotic recombinant vectors were constructed to express GST-VP1694 and Trx-VP1694, which were used for immunization and biopanning respectively. The second challenge was to obtain the high affinity antibody, so six rounds of biopanning were carried out and the clone showing highest binding activity (scFv-FA7) was successfully obtained. What’s more, the yield of functional scFv antibody is a limiting factor in its clinical application. To solve this problem, the co-expression vector of pACYC-Duet-1-skp was used to help the expression of scFv in cytoplasm of E. coli cells [27, 37].
In conclusion, a specific and high affinity scFv antibody against VP1694 (needle subunit) was obtained for the first time by phage display technology, and a soluble scFv antibody was easily expressed at low cost in E. coli cells. But the reaction with needle subunit during the assembly in T3SS is not known for anti-VP1694 scFv. Therefore, further studies are required to identify the effect of this scFv in inhibiting assembly of T3SS.
Acknowledgments
This work was supported by the Program for New Century Excellent Talents in University (Grant NCET-10-0010), the Fujian Fund for Distinguished Young Scientists (Grant 2009J06008), the National Agricultural Achievements Transformation Fund (Grant 2011GB2C400012), and Agricultural Five-new Engineering Projects of Fujian Development and Reform Commission.
Contributor Information
Rongzhi Wang, Email: wrz0629@126.com.
Shihua Wang, Phone: +086-591-87984471, FAX: +086-591-87984471, Email: wshyyl@sina.com.
References
- 1.Shimohata T, Takahashi A. Diarrhea induced by infection of Vibrio parahaemolyticus. J Med Invest. 2010;57:179–182. doi: 10.2152/jmi.57.179. [DOI] [PubMed] [Google Scholar]
- 2.McLaughlin JB, DePaole A, Bopp CA, et al. Outbreak of Vibrio parahaemolyticus gastroent-eritis associated with Alaskan oysters. N Engl J Med. 2005;353:1463–1470. doi: 10.1056/NEJMoa051594. [DOI] [PubMed] [Google Scholar]
- 3.Wang RZ, Huang JD, Zhang W, et al. Detection and identification of Vibrio parahaemolyticus bymultiplex PCR andDNA–DNA hybridization on a microarray. J Genet Genomics. 2011;38:129–135. doi: 10.1016/j.jgg.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Hardy WG, Klontz KC. The epidemiology of Vibrio infections in Florida, 1981-1993. Infect Dis. 1996;173:1176–1183. doi: 10.1093/infdis/173.5.1176. [DOI] [PubMed] [Google Scholar]
- 5.Makino K, Oshima K, Kurokawa K, et al. Genome sequence of Vibrio parahaemolyticus: a pathogenic mechanism distinct from that of V cholerae. Lancet. 2003;361:743–749. doi: 10.1016/S0140-6736(03)12659-1. [DOI] [PubMed] [Google Scholar]
- 6.Park KS, Ono T, Rokuda M, et al. Functional characterization of two III secretion systems of Vibrio parahaemolyticus. Infect Immun. 2004;72:6659–6665. doi: 10.1128/IAI.72.11.6659-6665.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hueck CJ. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev. 1998;62:379–433. doi: 10.1128/mmbr.62.2.379-433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mota LJ, Sorg I, Cornelis GR. Type III secretion: the bacteria-eukaryotic cell express. FEMS Microbiol Lett. 2005;252:1–10. doi: 10.1016/j.femsle.2005.08.036. [DOI] [PubMed] [Google Scholar]
- 9.Worrall LJ, Lameignere E, Strynadka NC. Structural overview of the bacterial injectisome. Curr Opin Microbiol. 2011;14:3–8. doi: 10.1016/j.mib.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Marlovits TC, Kubori T, Sukhan A, et al. Structural insights into the assembly of the type III secretion Needle complex. Science. 2004;306:1040–1042. doi: 10.1126/science.1102610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deane JE, Abrusci P, Johnson S, Lea SM. Timing is everything: the regulation of type III secretion. Cell Mol Life Sci. 2010;67:1065–1075. doi: 10.1007/s00018-009-0230-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veenendaal AK, Hodagkinson JL, Schwarzer L, et al. The type III secretion system needle tip complex mediates host cell sensing and translocon insertion. Mol Microbiol. 2007;63:1719–1730. doi: 10.1111/j.1365-2958.2007.05620.x. [DOI] [PubMed] [Google Scholar]
- 13.Matteï PJ, Faudry E, Job V, et al. Membrane targeting and pore formation by the type III secretion system translocon. FEBS J. 2011;278:414–426. doi: 10.1111/j.1742-4658.2010.07974.x. [DOI] [PubMed] [Google Scholar]
- 14.Quinaud M, Plé S, Job V, et al. Structure of the heterotrimeric complex that regulates type III secretion needle formation. Proc Natl Acad Sci USA. 2007;104:7803–7805. doi: 10.1073/pnas.0610098104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun P, Tropea JE, Austin BP, Cherry S, Waugh DS. Structural characterization of the Yersinia pestis type III secretion needle protein YscF in complex with its heterodimeric chaperone YscE/YscG. J Mol Biol. 2008;377:819–830. doi: 10.1016/j.jmb.2007.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liverman ADB, Cheng HC, Trosky JE, et al. Arp2/3-independent assembly of actin by Vibrio type III effector VopL. Proc Natl Acad Sci USA. 2007;104:17117–17122. doi: 10.1073/pnas.0703196104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burdette DL, Seemann J, Orth K. Vibrio VopQ induces P13-kinase-independent autophagy and antagonizes phagocytosis. Mol Microbiol. 2009;73:639–649. doi: 10.1111/j.1365-2958.2009.06798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trosky JE, Li Y, Mukherjee S, et al. VopA inhibits ATP binding by acetylating the catalytic loop of MAPK kinases. J Biol Chem. 2007;282:34299–34305. doi: 10.1074/jbc.M706970200. [DOI] [PubMed] [Google Scholar]
- 19.Bhattacharjee RN, Park KS, Kumagai Y, et al. VP1686, a Vibrio type III secretion protein, induces toll-like receptor-independent apoptosis in macrophage through NF-κB inhibition. J Biol Chem. 2006;281:36897–36904. doi: 10.1074/jbc.M605493200. [DOI] [PubMed] [Google Scholar]
- 20.Tamano K. Supra molecular structure of the Shigella type III secretion machinery: the needle part is changeable in length and essential for delivery of effectors. EMBO J. 2000;19:3876–3887. doi: 10.1093/emboj/19.15.3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blocker AJ, Deane JE, Veenendaal AK, et al. What’s the point of the type III secretion system needle? Proc Natl Acad Sci USA. 2008;105:6507–6513. doi: 10.1073/pnas.0708344105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis AJ, Mecsas J. Mutations in the Yersinia pseudotuberculosis type III secretion system needle protein, YscF, that specifically abrogate effector translocation into host cells. J Bacteriol. 2007;189:83–97. doi: 10.1128/JB.01396-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andreas KJ, Charlotta S, Ariel J. Small molecule type III secretion system inhibitors block assembly of the shigella type III secretion. J Bacteriol. 2009;191:563–570. doi: 10.1128/JB.01004-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gauthier A, Robertson ML, Lowden M, et al. Transcriptional inhibitor of virulence factors in enteropathogenic Escherichia coli. Antimicrob Agents Ch. 2005;49:4101–4109. doi: 10.1128/AAC.49.10.4101-4109.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan N, Lee C, Goguen J. High throughput screening for small molecule inhibitors of type III secretion in Yersinia pestis. Adv Exp Med Biol. 2007;603:367–375. doi: 10.1007/978-0-387-72124-8_34. [DOI] [PubMed] [Google Scholar]
- 26.Lzoré T, Job V, Dessen A. Biogenesis, regulation, and targeting of the type III SZECRETION system. Structure. 2011;19:603–612. doi: 10.1016/j.str.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 27.Wang RZ, Fang S, Wu DL, et al. Screening of a ScFv antibody that can neutralize effectively the cytotoxicity of Vibrio parahaemolyticus TLH. Appl Environ Microbiol. 2012;78:4967–4975. doi: 10.1128/AEM.00435-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hagemeyer CE, Schwarz M, Peter K. Single-chain antibodies as new antithrombotic drugs. Semin Thromb Hemost. 2007;33:185–195. doi: 10.1055/s-2007-969033. [DOI] [PubMed] [Google Scholar]
- 29.Hagemeyer CE, Von Zur Muhlen C, et al. Single-chain antibodies as diagnostic tools and therapeutic agents. J Thromb Haemost. 2009;101:1012–1019. [PubMed] [Google Scholar]
- 30.Wang SH, Zhang JB, Zhang ZP, et al. Construction of single chain variable fragment (ScFv) and biscFv-alkaline phosphatase fusion protein for detection of Bacillus anthracis. Anal Chem. 2006;78:997–1004. doi: 10.1021/ac0512352. [DOI] [PubMed] [Google Scholar]
- 31.Kabat EA, Wu TT, Gottesman KS, Foeller C. Sequences of proteins of immunological interest. Darby: Diane Publishing Company; 1991. [Google Scholar]
- 32.Singh PK, Agrawal R, Kamboj DV, et al. Construction of a single chain variable fragment antibody against the superantigen staphylococcal enterotoxin B. Appl Environ Microbiol. 2010;76:8184–8191. doi: 10.1128/AEM.01441-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dai HP, Gao H, Qiao P. Construction and characterization of a novel recombinant single-chain variable fragment antibody against white spot syndrome virus from shrimp. J Immun Method. 2003;279:267–275. doi: 10.1016/S0022-1759(03)00182-0. [DOI] [PubMed] [Google Scholar]
- 34.Huston JS, Levinson D, Mudgett-Hunter M, et al. Protein engineering of antibody binding sites: recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli. Proc Natl Acad Sci USA. 1988;85:5879–5883. doi: 10.1073/pnas.85.16.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skerra A, Pluckthun A. Assembly of a functional immunoglobulin Fv fragment in Escbericbia coli. Science. 1988;240:1038–1041. doi: 10.1126/science.3285470. [DOI] [PubMed] [Google Scholar]
- 36.Topping KP, Hough VC, Monson JR, Greenman J. Isolation of human colorectal tumor reactive antibodies using phage display technology. Int J Oncol. 2000;16:187–195. doi: 10.3892/ijo.16.1.187. [DOI] [PubMed] [Google Scholar]
- 37.Chadd HE, Chamow SM. Therapeutic antibody expression technology. Curr Opin Biotech. 2001;12:188–194. doi: 10.1016/S0958-1669(00)00198-1. [DOI] [PubMed] [Google Scholar]