Abstract
Alveolar type II (ATII) cells remain differentiated and express surfactant proteins when cultured at an air-liquid (A/L) interface. When cultured under submerged conditions, ATII cells dedifferentiate and change their gene expression profile. We have previously shown that gene expression under submerged conditions is regulated by hypoxia inducible factor (HIF) signaling due to focal hypoxia resulting from ATII cell metabolism. Herein, we sought to further define gene expression changes in ATII cells cultured under submerged conditions. We performed a genome wide microarray on RNA extracted from rat ATII cells cultured under submerged conditions for 24–48 h after switching from an A/L interface. We found significant alterations in gene expression, including upregulation of the HIF target genes stanniocalcin-1 (STC1), tyrosine hydroxylase (Th), enolase (Eno) 2, and matrix metalloproteinase (MMP) 13, and we verified upregulation of these genes by RT-PCR. Because STC1, a highly evolutionarily conserved glycoprotein with anti-inflammatory, anti-apoptotic, anti-oxidant, and wound healing properties, is widely expressed in the lung, we further explored the potential functions of STC1 in the alveolar epithelium. We found that STC1 was induced by hypoxia and HIF in rat ATII cells, and this induction occurred rapidly and reversibly. We also showed that recombinant human STC1 (rhSTC1) enhanced cell motility with extended lamellipodia formation in alveolar epithelial cell (AEC) monolayers but did not inhibit the oxidative damage induced by LPS. We also confirmed that STC1 was upregulated by hypoxia and HIF in human lung epithelial cells. In this study, we have found that several HIF target genes including STC1 are upregulated in AECs by a submerged condition, that STC1 is regulated by hypoxia and HIF, that this regulation is rapidly and reversibly, and that STC1 enhances wound healing moderately in AEC monolayers. However, STC1 did not inhibit oxidative damage in rat AECs stimulated by LPS in vitro. Therefore, alterations in gene expression by ATII cells under submerged conditions including STC1 were largely induced by hypoxia and HIF, which may be relevant to our understanding of the pathogenesis of various lung diseases in which the alveolar epithelium is exposed to relative hypoxia.
Keywords: HIF, STC1, Gene expression, Alveolar epithelial cells
Introduction
The alveolar epithelium is composed of two main cell types, alveolar type I (ATI) cells and alveolar type II (ATII) cells. ATI cells are large squamous cells that cover over 95% of the alveolar surface and are responsible for gas exchange. Cuboidal ATII cells make up the remaining 5% of the alveolar surface. They secrete surfactant, play an important role in ion transport, and contribute to the regulation of alveolar fluid and epithelial repair after lung injury [1]. It is well-established that ATII cells remain differentiated when cultured in air-liquid (A/L) interface but transdifferentiate when cultured under submerged conditions [2,3]. We have previously shown that the downregulation of surfactant protein expression under submerged conditions is induced by local hypoxia created by ATII cell metabolism and mediated by hypoxia inducible factor (HIF) [4]. Herein, we sought to further elucidate gene expression changes in ATII cells cultured under submerged conditions, as these gene expression changes might provide insight into the pathogenesis of diseases in which the alveolar epithelium is exposed to hypoxia.
Stanniocalcin-1 (STC1) is a hormone originally discovered in the corpuscles of Stannius, which are endocrine glands in the kidneys of bony fishes [5,6]. The mammalian STC1 is expressed in various tissues including the heart, lung, liver, adrenal, kidney, prostate, and ovary [7,8]. The STC1 gene is modulated in numerous developmental, physiological and pathological processes, including cancer, pregnancy, lactation, angiogenesis and organogenesis [9,10]. STC1 has been identified as one of the genes regulated by HIF-1 [11]. STC1 expression induces ischemia tolerance in mouse brains and hearts, suggesting its possible role in cell survival under hypoxic conditions [12,13]. STC1 also reduces the number of apoptotic lung cancer epithelial cells following hypoxia [14], which suggests that the induction of STC1 in a hypoxic microenvironment plays an important role in tumor progression [9,11,15]. Additionally, new functions of STC1 have recently been reported, including the regulation of re-epithelialization in human keratinocytes [16], suppression of inflammatory responses [17] and reduction of oxidative damage and subsequent apoptosis [17,18,19]. However, there has been no study of STC1 in AECs. Because a gene array in ATII cells cultured under submerged conditions revealed significant upregulation of STC1, we accessed the regulation and potential anti-oxidant and wound healing functions of STC1.
Materials and Methods
Rat ATII cell isolation and culture
ATII cells were isolated from pathogen-free adult male Sprague-Dawley rats, as previously described [4]. This research was approved by the Institutional Animal Care and Use Committee (IACUC) at National Jewish Health.
Culture of rat ATII cells and Affymetrix microarray experiments
Switch experiment from an A/L interface to a submerged condition
The rat ATII cells were cultured in DMEM containing 5% rat serum (RS), glutamine, antibiotics, and 10 ng/ml keratinocyte growth factor (KGF) for 6 days under A/L interface conditions. On day 7, the A/L interface conditions were continued or switched to submerged conditions for an additional 24–48 h. At 24 or 48 h, total RNA was extracted and purified using an RNeasy kit (QIAGEN, Valencia, CA). The samples were run on an Affymetrix Rat Gene 1.1 ST 24-sample Array (Affymetrix, Santa Clara, CA). Data from the array were analyzed using Partek Genomics Suite (Partek, St. Louis, MO) and Ingenuity Pathway Analysis (Ingenuity Systems). See the Supplementary Materials for additional details.
Switch experiment from a submerged condition to an A/L interface condition
The rat ATII cells were cultured for 6 days under submerged conditions. On day 7, the submerged conditions were continued or switched to A/L interface conditions for an additional 24–48 h.
Experiments with dimethyloxalyl glycine (DMOG)
The rat ATII cells were cultured with the HIF-α prolyl hydroxylase inhibitor, DMOG (Cayman Chemical Company, Ann Arbor, MI), to stabilize HIF. The cells were cultured with RS and KGF for 6 days under either A/L interface, submerged, or A/L interface with 1 mM DMOG conditions.
HIF adenovirus
Rat ATII cells were transduced with Ad.mutHIF1α [20] or Ad.GFP at a multiplicity of infection of 20 pfu/cell, as previously described [21].
Wound closure assay in rat AEC monolayers with recombinant human STC1 (rhSTC1)
Rat ATII cells were plated on collagen I (5 μg/cm2) (BD Biosciences)-coated 24-well cell-culture plates in DMEM with 10% fetal bovine serum (FBS). On day 4, crisscross scratch wounds were made with p10 pipette tips in the AEC monolayers and wound closure was compared with or without rhSTC1 (BioVendor, Brno, Czech Republic) at 24 h, as previously described [22].
Real time-PCR (RT-PCR)
The expression levels of genes were expressed as a ratio to the expression of the constitutive probe cyclophilin B (CyB) (in house). The specific primers and probes for tyrosine hydroxylase (Th), STC1, enolase 2 (Eno2), matrix metalloproteinase 13 (MMP13), glucose transporter 1 (Glut1) and uncoupling protein 2 (UCP2) were purchased from Applied Biosystems (Foster City, CA).
Western Blotting
Protein loading was normalized to β-actin. The primary antibody was anti-STC1 antibody (R&D systems, Minneapolis, MN).
Immunofluorescence-staining and measurement of lamellipodia area
Rat AECs were fixed at 1 h after wounding and stained with phalloidin–tetramethylrhodamine B isothiocyanate (Sigma) and DAPI (Vector Laboratories Inc., Burlingame, CA). The lamellipodia areas were analyzed from the leading edge at the four corners of crisscross wounds, as previously described [22].
5-bromo-2-deoxyuridine (BrdU) staining
The rat AECs were labelled with BrdU, and then BrdU incorporated into the cellular DNA was detected by immunostaining using mouse monoclonal BrdU antibody (GE Healthcare Life Science, Pittsburgh, PA) at 24 or 48 h after wounding. The labeling index of at least 500 epithelial cells at the wound edge was determined from three independent experiments.
Assessment of protein carbonyls in rat AECs stimulated by LPS
Rat ATII cells were plated on 12-well plates, on day 4 cells were stimulated with 10 μg/ml LPS (Sigma) with and without 500 ng/ml rhSTC1, and cell lysates were harvested at 24 h. Proteins from AEC lysates was derivatised with dinitrophenyl (DNP)-hydrazone using an OxyBlot Protein Oxidation Detection kit (Millipore, Billerica, MA). See the Supplementary Materials for details.
Statistical analysis
All data are presented as means +/− standard error of the mean (SEM). One-way ANOVA was used to compare differences between two or more groups. The post hoc Bonferroni/Dunn test was used for multiple comparisons. Statistical significance was set at p<0.05.
Results
STC1 and other HIF regulated genes are increased under submerged condition or treatment with DMOG
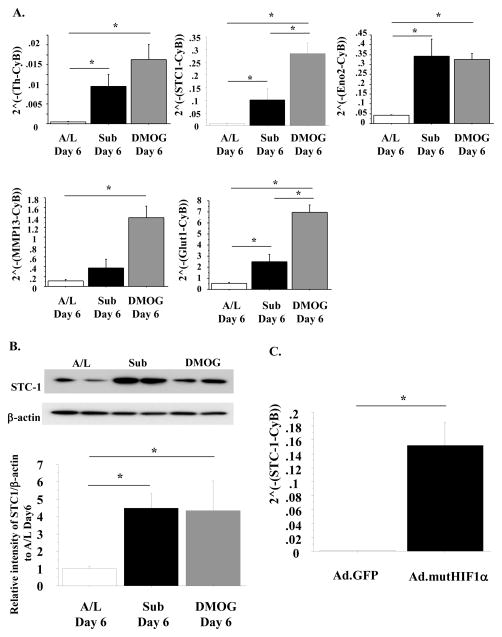
To evaluate changes in gene expression due to submersion, we conducted two switch experiments. After 6 days of culture under the A/L interface conditions, switching to the submerged condition for 24–48 h resulted in a dramatic up-regulation of many genes, including the HIF-regulated genes Th, STC1, Eno2, and MMP13 (Table 1A and 1B). We confirmed that these four genes were up-regulated at 6 days of culture under submerged conditions or A/L interface conditions with DMOG in additional independent experiments (Fig. 1A). Expression of Glut1, a well-known HIF-regulatory gene, is shown as a positive control. We also observed that the protein level of STC1 was up-regulated at 6 days of culture under submerged conditions or under A/L interface conditions with DMOG (Fig. 1B). Transduction of rat ATII cells with an adenovirus expressing a mutant, constitutively active form of HIF1α also resulted in an increase in STC1 mRNA (Fig. 1C).
Table 1.
The genes with the top 10 most highly increased or decreased expression levels under submerged conditions when compared to A/L interface conditions at 24 h (A) or 48 h (B) after switching from culturing under A/L interface conditions. n=3 independent experiments.
| A. | |||
|---|---|---|---|
| Gene Symbol | RefSeq | p-value (sub 24 hr vs. AL 24 hr) |
Fold-Change (sub 24 hr vs. AL 24 hr) |
| Increased in Submerged at 24 h | |||
| Fam70b | NM_001106094 | 1.08E-11 | 75.0466 |
| Th | NM_012740 | 3.88E-10 | 27.6918 |
| Dock6 | NM_001108997 | 4.73E-09 | 21.2716 |
| Osap | NM_001109556 | 7.10E-08 | 14.3145 |
| Stc1 | NM_031123 | 9.23E-08 | 12.5598 |
| Tmem213 | ENSRNOT00000018331 | 9.69E-06 | 12.3558 |
| Atp6v0a4 | NM_001106591 | 3.46E-06 | 11.1214 |
| Car9 | NM_001107956 | 3.40E-06 | 10.7408 |
| Hcrtr1 | NM_013064 | 8.59E-11 | 9.60985 |
| Eno2 | NM_139325 | 1.24E-06 | 9.15644 |
| Decreased in Submerged at 24 h | |||
| Lgi3 | NM_001107277 | 1.96E-05 | −7.87171 |
| Aqp5 | NM_012779 | 0.00285439 | −7.08992 |
| Angptl1 | NM_001109383 | 1.27E-06 | −6.77331 |
| Retnla | NM_053333 | 1.47E-06 | −6.01563 |
| Kcne2 | NM_133603 | 6.57E-05 | −5.96204 |
| Akr1c14 | NM_138547 | 4.54E-05 | −5.94269 |
| Slc43a3 | NM_001107743 | 0.00027989 | −5.53856 |
| Brms1l | NM_001106731 | 7.21E-07 | −5.19932 |
| Accn2 | NM_024154 | 0.00033784 | −4.89753 |
| Akr1cl1 | NM_001109900 | 0.00469004 | −4.58958 |
| B. | |||
| Gene Symbol | RefSeq | p-value (sub 48 hr vs. AL 48 hr) |
Fold-Change (sub 48 hr vs. AL 48 hr) |
| Increased in Submerged at 48 h | |||
| Fam70b | NM_001106094 | 2.33E-10 | 33.8273 |
| Th | NM_012740 | 1.06E-08 | 14.0543 |
| Osap | NM_001109556 | 1.89E-07 | 11.883 |
| Car9 | NM_001107956 | 4.14E-06 | 10.3389 |
| Stc1 | NM_031123 | 3.41E-07 | 9.92379 |
| Dock6 | NM_001108997 | 2.93E-07 | 9.65169 |
| Fxyd4 | NM_022388 | 2.60E-06 | 9.30901 |
| Ankrd34c | NM_001106845 | 1.98E-07 | 7.48247 |
| Eno2 | NM_139325 | 5.54E-06 | 7.10802 |
| Mmp13 | NM_133530 | 2.54E-06 | 6.94723 |
| Decreased in Submerged at 48 h | |||
| Retnla | NM_053333 | 4.83E-11 | −42.222 |
| Lgi3 | NM_001107277 | 1.83E-06 | −12.4042 |
| Slc43a3 | NM_001107743 | 4.28E-06 | −12.3659 |
| Aqp5 | NM_012779 | 0.000536135 | −11.0382 |
| Angptl1 | NM_001109383 | 1.59E-07 | −9.4264 |
| Akr1c14 | NM_138547 | 2.11E-05 | −6.75691 |
| Cyp2f4 | NM_019303 | 0.00153741 | −6.44771 |
| Brms1l | NM_001106731 | 1.83E-07 | −6.23451 |
| Accn2 | NM_024154 | 0.000104286 | −5.99548 |
| Pde7b | NM_080894 | 0.00452271 | −5.21055 |
Fig. 1. Submerged condition and HIF induce Th, STC1, Eno2 and MMP13 expression in rat ATII cells.
A. Rat ATII cells were cultured under either A/L interface, submerged, or A/L interface with 1 mM DMOG conditions for 6 days. The mRNA levels of Th, STC1, Eno2, MMP13 and Glut1 were measured by RT-PCR. These levels were normalized to CyB. n=3; B. The protein levels of STC1 in rat ATII cells cultured under either A/L interface, submerged, or A/L interface with 1 mM DMOG conditions for 6 days were measured and normalized to β-actin by western blotting. Upper: This blot is representative of one of three independent but reproducible experiments. Lower: Relative intensity of STC1 by western blotting analyzed by Image J software from three independent experiments (The intensity of STC1/β-actin of A/L condition on day 6 is designated as 1.00); C. The mRNA levels of STC1 in rat ATII cells with HIF1α-overexpression by adenovirus were measured by RT-PCR. These levels were normalized to CyB. n=4;
Values are means +/− SEM; *: p<0.05.
The culture condition-associated changes in STC1 expression were rapid and reversible
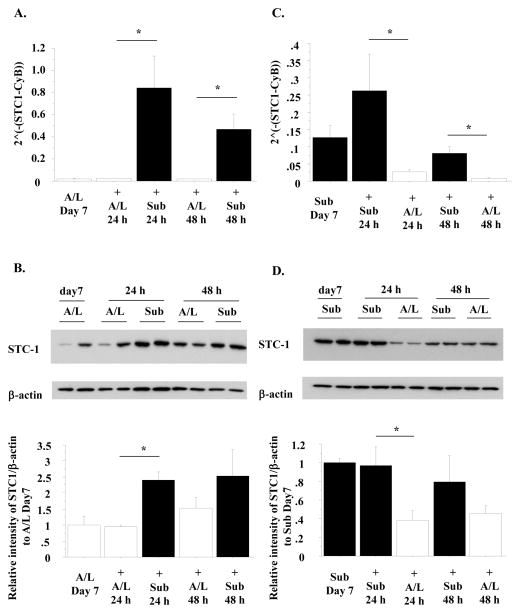
To evaluate whether the change in STC1 expression was reversible, we performed two switch experiments. In the first experiment, after 6 days of culture under A/L interface, switching to the submerged condition for an additional 24–48 h resulted in a dramatic increase in STC1 mRNA and protein levels (Fig. 2A, 2B). In the second experiment, after 6 days of culture under the submerged conditions, STC1 mRNA and protein levels were decreased by a subsequent 24 h of culture under A/L interface conditions (Fig. 2C, 2D). Hence, the effect of submersion was rapid and reversible.
Fig. 2. The mRNA and protein levels of STC1 are changed rapidly due to culture conditions and the changes are reversible.
A, B. Rat ATII cells were cultured under either A/L interface or submerged conditions for 24–48 h after culturing for 6 days under A/L interface conditions. The mRNA levels of STC1 were measured by RT-PCR. These levels were normalized to CyB (A). The protein levels of STC1 were measured and normalized to β-actin by western blotting (B). Upper: This blot is representative of one of three independent but reproducible experiments. Lower: Relative intensity of STC1 by western blotting analyzed by Image J software from three independent experiments (The intensity of STC1/β-actin of A/L condition on day 7 is designated as 1.00). C, D. Rat ATII cells were cultured under either A/L interface or submerged conditions for 24–48 h after 6 days under submerged conditions. The mRNA levels of STC1 (C) and the protein levels of STC1 (D) were measured. Upper: This blot is representative of one of three independent but reproducible experiments. Lower: Relative intensity of STC1 by western blotting analyzed by Image J software from three independent experiments (The intensity of STC1/β-actin of submerged condition on day 7 is designated as 1.00).
Values are means +/− SEM. *: p<0.05.
STC1 enhances wound closure in the rat AEC monolayers
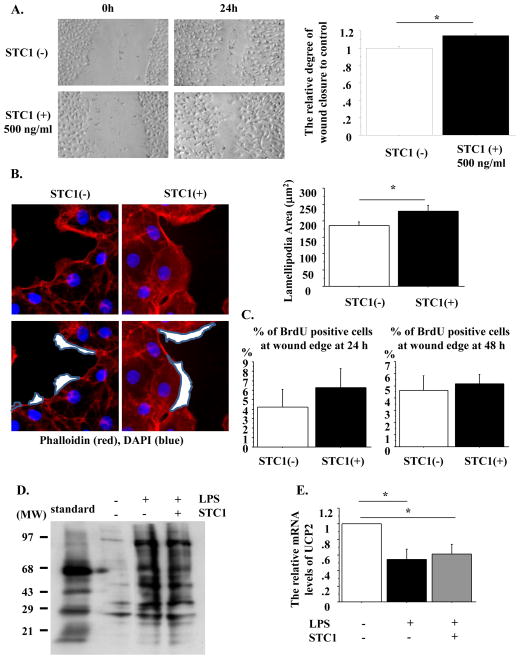
Acute respiratory distress syndrome (ARDS) results in the alveolar edema, where ATII cells are under focal alveolar hypoxia [23] and the re-epithelialization of the alveolar epithelial initially occurs by ATII cells [1,23]. STC1 is reported to regulate re-epithelialization in human keratinocytes and human A549 lung cancer epithelial cells [16,24]. Therefore, to determine whether rhSTC1 promoted re-etpithelialization in primary rat AEC monolayers, wound closure was compared with and without 500 ng/ml rhSTC1 at 24 h after wounding. Wound closure was promoted by rhSTC1 (Fig. 3A), with extended lamellipodia formation (Fig. 3B). However, rhSTC1 did not stimulate cell proliferation at 24–48 h after wounding (Fig. 3C).
Fig. 3. rhSTC1 promotes wound closure with extended lamellipodia formation but does not inhibit oxidative damage in primary rat AECs.
A. Left: Phase contrast pictures were taken from wound areas at 0 and 24 h after wounding to compare the degree of wound closure with and without rhSTC1. Right: The relative degree of wound closure was analyzed at 24 h after wounding. Each condition has 8 different marked wound areas in each well (The degree of wound closure in the rat AEC monolayer without STC1 is designated as 1.00). n=4; B. Left: Rat AEC monolayers with wounds were cultured with or without rhSTC1. Immunostaining of the scratched AEC monolayer with phalloidin (red) and DAPI (blue) at 1 h after wounding. The white area encircled with gray lines indicates the lamellipodia area. Magnification: x50. Right: The area of lamellipodia at 1 h after wounding was analyzed with Slidebook 5.0 software. The area was measured from a total of 70 lamellipodia for each condition, n=3/each condition. *: p<0.05. C. Percentage of BrdU-positive cells at wound edges at 24 (left) and 48 h (right) after wounding, Over 200 cells/each condition from each experiment were counted, n=3/each condition. D. The carbonyl protein levels of the control, LPS-stimulated and LPS-stimulated with rhSTC1 rat AEC monolayer were detected by the immunoblotting of carbonyl groups at 24 h. This blot is representative of one of three independent but reproducible experiments. E. Rat AEC monolayers were stimulated by LPS with or without rhSTC1 for 24 h. The mRNA levels of UCP2 were measured by RT-PCR. These levels were normalized to CyB (The mRNA levels of UCP2 without both LPS and STC1 were designated as 1.00). n=3;
Values are means +/− SEM. *: p<0.05.
rhSTC1 does not protect rat AECs from LPS-induced oxidative damage
A previous study reported that STC1 suppressed superoxide generation and protein carbonyls with induction of uncoupling protein 2 (UCP2) [17,19]. Therefore, we wondered whether STC1 would decrease oxidative damage with upregulation of UCP2 in rat AECs. To examine this issue, rat AEC monolayers were stimulated by LPS with or without 500 ng/ml STC1 and the levels of oxidatively modified protein, carbonyl proteins, and the expression of UCP2 were assessed. However, contrast to the effect of STC1 on LPS-induced oxidative stress in macrophage or the murine lungs where STC1 increases UCP2 and subsequent decreases oxidative stress [17,19,25,26], in AECs STC1 does not reduce oxidative stress and upregulate UCP2 levels (Fig. 3D and 3E).
STC1 is up-regulated in human lung epithelial cells
To determine whether STC1 would be up-regulated by hypoxia and HIF in human lung epithelial cells, cells of the human bronchial epithelial cell line 16HBE were exposed to a hypoxic condition (1% oxygen) for 24 h and primary human ATII cells were cultured under A/L interface conditions with DMOG for 6 days. STC1 mRNA levels were increased in both 16HBE cells exposed to hypoxia and human ATII cells cultured in the A/L interface with DMOG (Supplementary Fig. 1).
Discussion
We demonstrated that STC1 was induced due to focal hypoxia, the activation of HIF and under submerged conditions in rat AECs, and that the expression of STC1 was changed rapidly and reversibly by a change in culture conditions. We also found that wound closure in the rat AEC monolayer was enhanced by rhSTC1 with extended lamellipodia formation.
We previously reported that surfactant proteins were down-regulated under submerged conditions, which was regulated by oxygen tension through HIF signaling [4]. In a variety of clinical settings, such as ARDS, pneumonia, pulmonary fibrosis, or severe small airway obstruction, there is focal alveolar hypoxia. Therefore, to evaluate which genes were changed due to oxygen tension, we conducted a genechip analysis using rat ATII cells from an experiment employing a switch from A/L to submerged conditions. We then examined the 10 genes that showed the greatest increase or decrease in expression under submerged conditions when compared to A/L interface conditions at 24 or 48 h after switching from A/L interface conditions. Among the top 10 genes showing higher expression under submerged conditions, there were four known HIF-regulated genes, Th, STC1, Eno2 and MMP13 [11,27,28,29,30]. The present study is the first to report that HIF and hypoxic conditions regulated these genes in alveolar epithelial cells.
Wound healing is vital for the maintenance of alveolar integrity after injury. ATII cells are thought to be the main progenitor cells involved in restoring the alveolar epithelium after injury [1]. The repair of epithelial monolayers following mechanical disruption is dependent on an intercellular calcium wave propagated from the site of disruption to adjacent cells [31,32,33], and STC1 was originally described as an endocrine regulator of calcium and phosphate homeostasis in fish [5]. Pretreatment of the A549 monolayer with rhSTC1 dramatically enhances extracellular ATP-induced calcium wave propagation following scratch wounding [24]. Recently, STC1 overexpression was reported to promote lamellipodia formation and cell migration in a human epidermal keratinocyte cell line (HaCaT) [16]. As shown in Fig. 3A and 3B in the present study, the effect of recombinant STC1 on wound closure was modest, but we speculate that many potential molecules, including STC1, could have a synergetic effect on the enhancement of wound closure in AECs. Our laboratory has previously demonstrated the critical role of KGF and HGF in repair of the alveolar epithelium [22,34]. Further studies are necessary to elucidate the interaction of diverse growth factors, including STC1, in initiating AEC wound healing responses.
STC1 has also been shown to suppress oxidative damage, cell apoptosis and the proinflammatory pathway such as NFkB activation and induction of TNFα and CXCL2 [14,35,36] through induction of UCP2 [17,26]. Many lung diseases, including ARDS, emphysema, and many forms of interstitial lung disease are inflammatory diseases, involving apoptosis of the endothelium and alveolar epithelium and oxidative damage [23,37]. Thus, we wondered whether STC1 would protect LPS-stimulated rat AECs from oxidative damage through UCP2. Consistent with previous reports, we found that LPS induced oxidative damage (carbonyl protein) [19,38] and led to down-regulation of UCP2 [17,19,25]. However, we did not observe inhibition of oxidative damage or up-regulation of UCP2 by rhSTC1 in our model. Interestingly, Tang et al. recently demonstrated that STC1 ameliorates LPS-induced pulmonary oxidative stress, inflammation and apoptosis in mice [19]. However, they did not consider which type of lung cells are targeted by STC1. In addition, although current evidence supports the local action of STCs in Ca2+ and Pi transport [39], at present, information about the sequence, expression and distribution of the STC receptor(s) is lacking.
In summary, we have demonstrated that STC1 is regulated by hypoxic conditions and HIF in AECs and enhances wound healing moderately in rat AEC monolayers. However, STC1 did not inhibit oxidative damage in rat AECs stimulated by LPS in vitro. Previous reports and our results suggest that STC1 has a potential role in the resolution of various lung diseases. However, further investigations are required to determine which types of cells are target of STC1, and the identification of STC1 receptors in lungs.
Supplementary Material
Highlights.
We examined genes in rat alveolar type II cells regulated by culture conditions.
STC1 was up-regulated by the hypoxic condition and hypoxia-inducible factor.
STC1 expression was changed rapidly and reversibly by changes in culture conditions.
STC1 enhances wound closure and lamellipodia formation in rat alveolar epithelial monolayer.
STC1 does not suppress protein oxidation in LPS-stimulated rat alveolar epithelial cells.
Acknowledgments
This work was supported by NIH R21 HL106112-01A1 and the NCI Cancer Center Support Grant (P30CA046934). We thank Karen E. Edeen and Teneke M. Warren for assistance with the rat ATII cell isolations and manuscript preparation, respectively.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mason RJ. Biology of alveolar type II cells. Respirology. 2006;11(Suppl):S12–15. doi: 10.1111/j.1440-1843.2006.00800.x. [DOI] [PubMed] [Google Scholar]
- 2.Dobbs LG, Pian MS, Maglio M, Dumars S, Allen L. Maintenance of the differentiated type II cell phenotype by culture with an apical air surface. Am J Physiol. 1997;273:L347–354. doi: 10.1152/ajplung.1997.273.2.L347. [DOI] [PubMed] [Google Scholar]
- 3.Alcorn JL, Smith ME, Smith JF, Margraf LR, Mendelson CR. Primary cell culture of human type II pneumonocytes: maintenance of a differentiated phenotype and transfection with recombinant adenoviruses. Am J Respir Cell Mol Biol. 1997;17:672–682. doi: 10.1165/ajrcmb.17.6.2858. [DOI] [PubMed] [Google Scholar]
- 4.Ito Y, Ahmad A, Kewley E, Mason RJ. Hypoxia-inducible factor regulates expression of surfactant protein in alveolar type II cells in vitro. Am J Respir Cell Mol Biol. 2011;45:938–945. doi: 10.1165/rcmb.2011-0052OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagner GF, Hampong M, Park CM, Copp DH. Purification, characterization, and bioassay of teleocalcin, a glycoprotein from salmon corpuscles of Stannius. Gen Comp Endocrinol. 1986;63:481–491. doi: 10.1016/0016-6480(86)90149-8. [DOI] [PubMed] [Google Scholar]
- 6.De Niu P, Radman DP, Jaworski EM, Deol H, Gentz R, Su J, Olsen HS, Wagner GF. Development of a human stanniocalcin radioimmunoassay: serum and tissue hormone levels and pharmacokinetics in the rat. Mol Cell Endocrinol. 2000;162:131–144. doi: 10.1016/s0303-7207(00)00199-4. [DOI] [PubMed] [Google Scholar]
- 7.Varghese R, Wong CK, Deol H, Wagner GF, DiMattia GE. Comparative analysis of mammalian stanniocalcin genes. Endocrinology. 1998;139:4714–4725. doi: 10.1210/endo.139.11.6313. [DOI] [PubMed] [Google Scholar]
- 8.Chang AC, Janosi J, Hulsbeek M, de Jong D, Jeffrey KJ, Noble JR, Reddel RR. A novel human cDNA highly homologous to the fish hormone stanniocalcin. Mol Cell Endocrinol. 1995;112:241–247. doi: 10.1016/0303-7207(95)03601-3. [DOI] [PubMed] [Google Scholar]
- 9.Chang AC, Jellinek DA, Reddel RR. Mammalian stanniocalcins and cancer. Endocr Relat Cancer. 2003;10:359–373. doi: 10.1677/erc.0.0100359. [DOI] [PubMed] [Google Scholar]
- 10.Ishibashi K, Imai M. Prospect of a stanniocalcin endocrine/paracrine system in mammals. Am J Physiol Renal Physiol. 2002;282:F367–375. doi: 10.1152/ajprenal.00364.2000. [DOI] [PubMed] [Google Scholar]
- 11.Yeung HY, Lai KP, Chan HY, Mak NK, Wagner GF, Wong CK. Hypoxia-inducible factor-1-mediated activation of stanniocalcin-1 in human cancer cells. Endocrinology. 2005;146:4951–4960. doi: 10.1210/en.2005-0365. [DOI] [PubMed] [Google Scholar]
- 12.Westberg JA, Serlachius M, Lankila P, Penkowa M, Hidalgo J, Andersson LC. Hypoxic preconditioning induces neuroprotective stanniocalcin-1 in brain via IL-6 signaling. Stroke. 2007;38:1025–1030. doi: 10.1161/01.STR.0000258113.67252.fa. [DOI] [PubMed] [Google Scholar]
- 13.Westberg JA, Serlachius M, Lankila P, Andersson LC. Hypoxic preconditioning induces elevated expression of stanniocalcin-1 in the heart. Am J Physiol Heart Circ Physiol. 2007;293:H1766–1771. doi: 10.1152/ajpheart.00017.2007. [DOI] [PubMed] [Google Scholar]
- 14.Block GJ, Ohkouchi S, Fung F, Frenkel J, Gregory C, Pochampally R, DiMattia G, Sullivan DE, Prockop DJ. Multipotent stromal cells are activated to reduce apoptosis in part by upregulation and secretion of stanniocalcin-1. Stem Cells. 2009;27:670–681. doi: 10.1002/stem.20080742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu G, Yang G, Chang B, Mercado-Uribe I, Huang M, Zheng J, Bast RC, Lin SH, Liu J. Stanniocalcin 1 and ovarian tumorigenesis. J Natl Cancer Inst. 2010;102:812–827. doi: 10.1093/jnci/djq127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeung BH, Wong CK. Stanniocalcin-1 regulates re-epithelialization in human keratinocytes. PLoS One. 2011;6:e27094. doi: 10.1371/journal.pone.0027094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Huang L, Abdelrahim M, Cai Q, Truong A, Bick R, Poindexter B, Sheikh-Hamad D. Stanniocalcin-1 suppresses superoxide generation in macrophages through induction of mitochondrial UCP2. J Leukoc Biol. 2009;86:981–988. doi: 10.1189/jlb.0708454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SJ, Ko JH, Yun JH, Kim JA, Kim TE, Lee HJ, Kim SH, Park KH, Oh JY. Stanniocalcin-1 protects retinal ganglion cells by inhibiting apoptosis and oxidative damage. PLoS One. 2013;8:e63749. doi: 10.1371/journal.pone.0063749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang SE, Wu CP, Wu SY, Peng CK, Perng WC, Kang BH, Chu SJ, Huang KL. Stanniocalcin-1 ameliorates lipopolysaccharide-induced pulmonary oxidative stress, inflammation, and apoptosis in mice. Free Radic Biol Med. 2014 doi: 10.1016/j.freeradbiomed.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 20.Ahmad A, Ahmad S, Glover L, Miller SM, Shannon JM, Guo X, Franklin WA, Bridges JP, Schaack JB, Colgan SP, White CW. Adenosine A2A receptor is a unique angiogenic target of HIF-2alpha in pulmonary endothelial cells. Proc Natl Acad Sci U S A. 2009;106:10684–10689. doi: 10.1073/pnas.0901326106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang Y, Edeen K, Lu X, De Leon M, Mason RJ. Keratinocyte growth factor induces lipogenesis in alveolar type II cells through a sterol regulatory element binding protein-1c-dependent pathway. Am J Respir Cell Mol Biol. 2006;35:268–274. doi: 10.1165/rcmb.2006-0037OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito Y, Correll K, Schiel JA, Finigan JH, Prekeris R, Mason RJ. Lung fibroblasts accelerate wound closure in human alveolar epithelial cells through hepatocyte growth factor/c-Met signaling. Am J Physiol Lung Cell Mol Physiol. 2014;307:L94–105. doi: 10.1152/ajplung.00233.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122:2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Block GJ, DiMattia GD, Prockop DJ. Stanniocalcin-1 regulates extracellular ATP-induced calcium waves in human epithelial cancer cells by stimulating ATP release from bystander cells. PLoS One. 2010;5:e10237. doi: 10.1371/journal.pone.0010237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emre Y, Hurtaud C, Nubel T, Criscuolo F, Ricquier D, Cassard-Doulcier AM. Mitochondria contribute to LPS-induced MAPK activation via uncoupling protein UCP2 in macrophages. Biochem J. 2007;402:271–278. doi: 10.1042/BJ20061430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheikh-Hamad D. Mammalian stanniocalcin-1 activates mitochondrial antioxidant pathways: new paradigms for regulation of macrophages and endothelium. Am J Physiol Renal Physiol. 2010;298:F248–254. doi: 10.1152/ajprenal.00260.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powell FL, Fu Z. HIF-1 and ventilatory acclimatization to chronic hypoxia. Respir Physiol Neurobiol. 2008;164:282–287. doi: 10.1016/j.resp.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Law AY, Ching LY, Lai KP, Wong CK. Identification and characterization of the hypoxia-responsive element in human stanniocalcin-1 gene. Mol Cell Endocrinol. 2010;314:118–127. doi: 10.1016/j.mce.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Zhang T, Niu X, Liao L, Cho EA, Yang H. The contributions of HIF-target genes to tumor growth in RCC. PLoS One. 2013;8:e80544. doi: 10.1371/journal.pone.0080544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang S, Kim J, Ryu JH, Oh H, Chun CH, Kim BJ, Min BH, Chun JS. Hypoxia-inducible factor-2alpha is a catabolic regulator of osteoarthritic cartilage destruction. Nat Med. 2010;16:687–693. doi: 10.1038/nm.2153. [DOI] [PubMed] [Google Scholar]
- 31.Dignass AU, Becker A, Spiegler S, Goebell H. Adenine nucleotides modulate epithelial wound healing in vitro. Eur J Clin Invest. 1998;28:554–561. doi: 10.1046/j.1365-2362.1998.00330.x. [DOI] [PubMed] [Google Scholar]
- 32.Aihara E, Hentz CL, Korman AM, Perry NP, Prasad V, Shull GE, Montrose MH. In vivo epithelial wound repair requires mobilization of endogenous intracellular and extracellular calcium. J Biol Chem. 2013;288:33585–33597. doi: 10.1074/jbc.M113.488098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ehring GR, Szabo IL, Jones MK, Sarfeh IJ, Tarnawski AS. ATP-induced CA2+-signaling enhances rat gastric microvascular endothelial cell migration. J Physiol Pharmacol. 2000;51:799–811. [PubMed] [Google Scholar]
- 34.Yano T, Mason RJ, Pan T, Deterding RR, Nielsen LD, Shannon JM. KGF regulates pulmonary epithelial proliferation and surfactant protein gene expression in adult rat lung. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1146–1158. doi: 10.1152/ajplung.2000.279.6.L1146. [DOI] [PubMed] [Google Scholar]
- 35.Huang L, Garcia G, Lou Y, Zhou Q, Truong LD, DiMattia G, Lan XR, Lan HY, Wang Y, Sheikh-Hamad D. Anti-inflammatory and renal protective actions of stanniocalcin-1 in a model of anti-glomerular basement membrane glomerulonephritis. Am J Pathol. 2009;174:1368–1378. doi: 10.2353/ajpath.2009.080476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang L, Belousova T, Chen M, DiMattia G, Liu D, Sheikh-Hamad D. Overexpression of stanniocalcin-1 inhibits reactive oxygen species and renal ischemia/reperfusion injury in mice. Kidney Int. 2012;82:867–877. doi: 10.1038/ki.2012.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tuder RM, Petrache I. Pathogenesis of chronic obstructive pulmonary disease. J Clin Invest. 2012;122:2749–2755. doi: 10.1172/JCI60324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshida T, Nagai K, Inomata T, Ito Y, Betsuyaku T, Nishimura M. Relationship between neutrophil influx and oxidative stress in alveolar space in lipopolysaccharide-induced lung injury. Respir Physiol Neurobiol. 2014;191:75–83. doi: 10.1016/j.resp.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 39.Yeung BH, Law AY, Wong CK. Evolution and roles of stanniocalcin. Mol Cell Endocrinol. 2012;349:272–280. doi: 10.1016/j.mce.2011.11.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.