Abstract
The serotonergic system plays a key modulatory role in the brain and is the target for many drug treatments for brain disorders either through reuptake blockade or via interactions at the 14 subtypes of 5-HT receptors. This review provides the history and current status of radioligands used for positron emission tomography (PET) and single photon emission computerized tomography (SPECT) imaging of human brain serotonin (5-HT) receptors, the 5-HT transporter (SERT), and 5-HT synthesis rate. Currently available radioligands for in vivo brain imaging of the 5-HT system in humans include antagonists for the 5-HT1A, 5-HT1B, 5-HT2A, and 5-HT4 receptors, and for SERT. Here we describe the evolution of these radioligands, along with the attempts made to develop radioligands for additional serotonergic targets. We describe the properties needed for a radioligand to become successful and the main caveats. The success of a PET or SPECT radioligand can ultimately be assessed by its frequency of use, its utility in humans, and the number of research sites using it relative to its invention date, and so these aspects are also covered. In conclusion, the development of PET and SPECT radioligands to image serotonergic targets is of high interest, and successful evaluation in humans is leading to invaluable insight into normal and abnormal brain function, emphasizing the need for continued development of both SPECT and PET radioligands for human brain imaging.
Keywords: 5-HT, PET, SPECT, radioligand
1. INTRODUCTION
The serotonergic (5-HT) system plays a key modulatory role in many central nervous system (CNS) functions. Multiple serotonergic receptor subtypes and region-specific innervations from the dorsal and medial raphe nuclei within the brainstem result in a complex pattern of modulatory control over various physiological, emotional, and cognitive processes. These processes include control of mood and sleep, regulation of cognitive performance, learning and memory, modulation of ingestive behavior, and influence on reward circuits that mediate, for example, motivation.1–4 Serotonergic dysfunction has been implicated in the etiology of many psychiatric disorders including depression,5 anxiety,6 schizophrenia,7 and other neurological disorders such as Alzheimer’s disease,8,9 and epilepsy.10 Although our basic understandings of the serotonergic system are derived from animal models, the development of noninvasive brain imaging techniques, such as positron emission tomography (PET) and single positron emission computerized tomography (SPECT), increasingly allows the study of the serotonergic system in the human brain.11
This review covers the history and current status of the development of PET and SPECT radioligands for imaging serotonergic targets within the brain. First, we briefly introduce the serotonergic system and the uses of PET and SPECT imaging in general. A more detailed description of the different radioligands follows.
A. 5-HT Targets for PET and SPECT
The 5-HT receptors are among the most diverse group of neurotransmitter receptors in the human genome and the 5-HT system is also one of the phylogenetically oldest systems. Currently, 14 structurally and pharmacologically distinct mammalian 5-HT receptor subtypes have been described. Based on their structure, affinity for different ligands, and second messenger pathway, they are assigned to one of the seven families, 5-HT1–7.12 All 5-HT receptors, except the 5-HT3 receptor, are G-protein coupled seven transmembrane spanning receptors (GPCRs). The 5-HT3 receptor is a ligand-gated sodium ion channel. In addition, the 5-HT transporter (SERT) responsible for 5-HT reuptake and 5-HT synthetic enzymes, especially tryptophan hydroxylase, are also targets for tracer development. Even though much still needs to be elucidated, it is known that each of these targets has its own distinct pattern of distribution and function in the brain.1,13–15 The 5-HT targets and their characteristics are summarized in Table I.
Table I.
5-HT Receptor Properties and Available Selective Radioligands
| Target | Nomenclature (transduction) | Highest Bmax fmol/mg protein (region) | Selective ligands | Selective radioligand | PET radioligand | |
|---|---|---|---|---|---|---|
| GPCRs | 5-HT1A | (Gi/o) | 860–3,140* (hipp)63 300–1,9103 (ctx) |
✔ | ✔ | ✔ |
| 5-HT1B | (Gi/o) | 400–500* (g pall)141 3963 (occ ctx)141 |
✔ | ✔ | ✔ | |
| 5-HT1D | (Gi/o) | 226 (g pall)144 | ✔ | (✔) | – | |
| 5-ht1e | (Gi/o) | 224 (put)144 | – | – | – | |
| 5-HT1F | (Gi/o) | 79 (rat wb)145 | ✔ | ✔ | – | |
| 5-HT2A | (Gq/11) | 570 (ctx)149 | ✔ | ✔ | ✔ | |
| 5-HT2B | (Gq/11) | ND | ✔ | – | – | |
| 5-HT2C | (Gq/11) | 688 (pig ch plx)264 | ✔ | ✔ | – | |
| 5-HT4 | (Gs) | 223 (stri)287 | ✔ | ✔ | ✔ | |
| 5-ht5a | (?) | ND | ✔ | – | – | |
| 5-ht5b | (?) | ND | – | – | – | |
| 5-HT6 | (Gs) | 215 (stri)303 | ✔ | ✔ | ✔ | |
| 5-HT7 | (Gs) | 68 (thal)310 | ✔ | ✔ | – | |
| Ion channel | 5-HT3 | – | 465 (pig ctx)271 | ✔ | ✔ | – |
| Transporter | SERT | – | 587 (ctx)322 682 (thal)320 |
✔ | ✔ | ✔ |
Receptor nomenclature is agreed by IUPHAR (International Union of Basic and Clinical Pharmacology). The term “receptor” is only applied to entities for which operational, structural, and signal transduction information is available; thus 5-ht1E, 5-ht5A, and 5-ht5B have lower case letters indicating that no function has yet been attributed to them. Bmax values are taken from the literature (references provided as numerical superscript) and are from human brain unless otherwise stated. Units are equivalent fmol/mg protein; * denotes a simple conversion from fmol/mg tissue assuming that10% of tissue weight is protein. N.B. Bmax values vary between tracers, species, and laboratories but only one such example is provided here. Hipp, hippocampus; ctx, cortex; occ, occipital; g pall, globus pallidus; stri, striatum; put, putamen; wb, whole brain; ch plx, choroid plexus; thal, thalamus; ND, not determined. ✔ indicates that a ligand is available. -- indicates that no ligand is available. (✔) Ligands also have 5-HT1B affinity. ✔ in PET probe column indicates a PET tracer is available that shows promise in human subjects.
2. IN VIVO BRAIN IMAGING
In vivo brain imaging is an important and widely used tool for the study of the living brain. For in vivo pharmacology, PET and SPECT are most often used for quantification of brain receptor concentrations, and since the first PET study was published in 1983,16 this neuro-receptor mapping technique has been used to study various neurotransmitter systems in health and disease.17–20 In brain, PET has also been widely used to study glucose metabolism,21–24 blood–brain barrier transport,25–28 and neurotransmitter release.29,30 PET and SPECT imaging are also useful for tracking pharmacokinetic and pharmacodynamic properties of drugs, and can be used to determine the occupancy of therapeutic drugs, which ultimately can be used to estimate the optimal doses to be used in Phase II studies. For example, it has been demonstrated that between 60 and 80% occupancy of D2 receptors is required for anti-psychotic medication efficacy and that beyond this level, side effects are likely to occur.31 Further, treatment with clinically effective doses of the selective serotonin reuptake inhibitors (SSRIs) paroxetine or citalopram is associated with approximately 80% occupancy of SERT.32 For that reason, the pharmaceutical industry has increasingly taken advantage of these techniques to determine doses of new agents. SPECT is a more widely accessible functional imaging tool than PET, due to its lower costs and use of radioligands with longer half-lives. In spite of its lower sensitivity and resolution as compared to PET, SPECT is widely used for brain imaging, and radioligands suitable for this technique are in great demand.
Significant discoveries within the 5-HT system in human brain have been made following the development of selective PET and SPECT radioligands, for example, that the 5-HT1A receptor is reduced in social anxiety and panic disorder33,34 whereas the 5-HT2A receptor may be increased in depressed individuals following treatment.35 However, the full potential of 5-HT-related treatments cannot be realized before appropriate radioligands selective for all target receptors and enzymes have been developed and validated, including those that will allow endogenous 5-HT release and synaptic levels to be measured.30
A. The Principles of PET and SPECT
Radioisotopes that are typically used include 11C, 13N, 15O, 18F, and 76Br in PET and 99mTc and 123I in SPECT. Permissive of the molecular structure, these can be incorporated into potential tracers of interest. Radioligands for imaging brain neurotransmitter receptors and transporters are based structurally on receptor antagonists or agonists. Since the radioligands are targeted to work when administered in tracer doses (typically below 5 μg per injection and with receptor occupancies of less than 5%), they should rarely elicit pharmacological effects.
The vast majority of PET and SPECT radioligands available today are antagonists; there are many more molecules and chemical series of antagonists available from pharmaceutical drug discovery programs and they tend to be easier to develop. For GPCRs, antagonist radiotracers label the whole population of receptors with the same affinity, thus providing a good indication of total receptor number and higher Bmax, whereas agonist radioligands preferentially label the high-affinity states of the receptor that are capable of eliciting signalling events. Compared with the total number of receptors, the distribution of receptors in the high-affinity state is predicted to be a more precise outcome in studies with a functional aim36 or studies attempting to image the release or depletion of endogenous agonist neu-rotransmitter.37,38 Agonist radioligands should also provide a better measure of occupancy for new agonist pharmacological agents or any agent at the “functional” proportion of the receptor. Because of these considerations, the interest in agonist radioligands is increasing.
B. Overview of Successful Radioligand Properties
In order to generate quantifiable images of cerebral binding, radioligands must possess certain qualities. Radioligands suitable for in vitro quantification by competition, saturation, or autoradiographical methodologies cannot necessarily be used in vivo. This is mainly because of the need to penetrate the blood–brain barrier and not be excluded by pumps such as P-glycoprotein (P-gp), but also due to problems arising from high non-specific binding or issues with radiosynthesis, metabolism, or pharmacokinetics. The main properties necessary for a radioligand to be useful for in vivo imaging are as follows.
Radiosynthesis should be as simple and fast as possible, especially with short-lived 11C, giving a high specific radioactivity to avoid any significant pharmacological blockade of the receptor in question with “cold” compound from the synthesis.
The radioligand must possess high affinity toward the target receptor, normally in the nanomolar range, to maximize the target to background ratio. On the other hand, to achieve suitable reversible receptor kinetics, the affinity must not be too high. The association rate should be high, and the dissociation rate should be long enough to be measured, but short enough for the washout phase to be appreciable within the time frame of scanning.
The radioligand must bind selectively to the target of interest. To obtain meaningful data for target distribution studies, the binding potential measured by PET or SPECT using the radioligand must be obtained from binding to the desired target only. Since the binding potential is the product of the number of receptors available for binding (Bavail) and the affinity (1/KD), these two factors must be considered when determining the selectivity of a radioligand. Often the affinity of a radioligand, as 1/Ki, toward the desired target is determined in a competition assay against a well-known ligand. This is then compared with the affinities of the ligand toward a wide spectrum of other receptors and binding sites. Ki values toward other targets that are more than 20–100 times higher than for the desired target are normally acceptable. Acceptability, however, depends on Bavail for the binding sites in question. If Bavail for the desired target is much higher than Bavail for non-targets, then the binding potential will still selectively reflect the wanted target, and vice versa if a non-target binding site is very abundant, since then even low affinity to this non-target will influence the total signal. In order to address the selectivity of a radioligand one can perform an in vivo blocking study, where the radioligand is co-administered with a known selective antagonist that binds only to the target.
Generally, the radioligand should be moderately lipophilic to achieve adequate penetration of the blood–brain barrier without incurring excessive non-specific binding to brain tissue or very slow brain clearance. Moderate lipophilicity does not, however, guarantee brain entry. For example, some moderately lipophilic compounds are substrates for efflux transporters at the blood–brain barrier, such as P-gp, and these compounds are effectively excluded from entry into brain.39
Plasma clearance rate of radioligand should also be considered. Rapid clearance results in difficulties with accurate determination of the input curve, particularly at late time points and this complicates the subsequent mathematical modelling with an arterial input function.
The metabolites produced should either be polar, so excluded from the brain, or unlabelled, so as not to interfere with the signal obtained in brain.39 Interference from lipophilic radiometabolites can, however, be taken into account if it is possible to establish a bolus–infusion design (e.g.,40) or if the radiometabolites do not have specific binding in the brain.
One thing that must be considered when designing and synthesizing potential PET radioligands is the likelihood of being able to label them successfully without interfering with their pharmacology. 11C-labelling is usually feasible because of the presence of carbon atoms in all organic compounds. The introduction of the small electronegative 18F atom in place of hydrogen or hydroxyl can be unpredictably beneficial or detrimental to the pharmacological properties of the ligand in terms of its affinity and selectivity. Introduction of a bulky123I atom in place of hydrogen generally affects pharmacology and will increase ligand lipophilicity. The half-life of the radionuclide is an important consideration. The relatively short half-life of 11C (t1/2 = 20.4 min) is suitable for following quite rapid pharmacokinetics, and may permit more than one study session in the same subject in a single day. However, the necessity to produce 11C-labelled radioligands on-site is a logistical disadvantage. Longer-lived 18F (t1/2 = 109.8 min) for PET and 123I (t1/2 = 13 hr) for SPECT do not require on-site production and are suitable for following slower pharmacokinetics. Alternative labels are being investigated, such as the SPECT label 99mTc (t1/2 = 6 hr), because of its relative safety and availability.
3. CURRENT RADIOLIGANDS FOR IMAGING THE 5-HT SYSTEM
A multitude of radioligands exist for in vitro studies of serotonergic targets, and over the last decade, we have seen an impressive increase in the number of useful PET and SPECT radioligands. With this review, we describe the PET and SPECT radioligands published to date for imaging the serotonergic system. Table I summarizes the 5-HT targets, their brain distribution, and density and availability of selective ligands. Table II summarizes the development history of all published PET and SPECT ligands for those targets where tracer development has been undertaken. In addition, since the relative success of a radioligand can be estimated through its frequency of use and publication rate, these data are given in Table III.
Table II.
Development of PET and SPECT Radioligands for the 5-HT System Designed for Use in Human Subjects
| Target | Type | Radioligand | Rodent | Nonhuman primate | Human | Ongoing studies | Reason for failure | Known problems |
|---|---|---|---|---|---|---|---|---|
| 5-HT1A | SPECT | [123I]p-MPPI | ✔ | ✔ | × | – | No specific 5-HT1A binding Rapid metabolism Possible brain exclusion via efflux transporter | – |
| PET | [11C]WAY-100635 | ✔ | ✔ | ✔ | ✔ | – | Fast systemic metabolism; difficult kinetic modelling with arterial input function, reference tissue models complicated by radiometabolites in plasma | |
| [11C]CPC-222 | ✔ | ND | ✔ | ND | Lower signal-to-background ratio than [11C]WAY-100635 | |||
| [11C](R)-RWAY | × | ✔ | × | – | Possible influx of lipophilic radiometabolite | P-gp substrate in rodent | ||
| [11C]DWAY | ✔ | ✔ | ✔ | ND | Unreliable radiolabelling technique; low radioactive yield | |||
| [18F]6FPWAY | ND | ✔ | × | – | Moderate uptake | – | ||
| [18F]MPPF | ✔ | ✔ | ✔ | ✔ | – | P-gp substrate; fast brain clearance and low uptake | ||
| [18F]FCWAY | ✔ | ✔ | ✔ | ✔ | – | Defluorination of parent compound; sub-optimal imaging | ||
| [18F]MefWAY | ND | ✔ | ND | – | ||||
| [11C]NAD-299 | ND | ✔ | ND | – | ||||
| [11C]CUMI-101 | ✔ | ✔ | ✔ | – | Partial, not full agonist | |||
| 5-HT1B | PET | [11C]AZ10419369 | ND | ✔ | ✔ | – | ||
| [11C]P943 | ND | ✔ | ✔ | ✔ | – | |||
| 5-HT2A | SPECT | [123I]DOI | × | × | – | – | Low target-to-background ratio | Non-selective (5-HT2C) |
| [123I]MSP | ✔ | ND | ND | ND | ||||
| [123I]-R91150 | ✔ | ✔ | ✔ | ✔ | – | Lower target-to-background ratio than equivalent PET tracers | ||
| [123I]-3-I-CO | × | ND | – | – | – | Low target-to-background ratio, possible P-gp substrate | ||
| PET | [11C]Ketanserin | ND | ND | × | – | Low target-to-background ratio, fast metabolism | Non-selective (α1, H1, 5-HT2C) | |
| [11C]NMSP | ND | ND | ✔ | – | – | Non-selective (D2) | ||
| [11C]MBL | ND | ✔ | ✔ | – | Non-selective (some D2, α1, 5-HT1 and 5-HT2C) | |||
| [18F]setoperone | ✔ | ✔ | ✔ | ✔ | – | Non-selective (D2) | ||
| [18F]altanserin | ✔ | ✔ | ✔ | ✔ | – | Need for bolus-infusion due to radiometabolites requiring complex modelling using other methods. Possible mixed pharmacology | ||
| [18F]deuteroaltanserin | ND | ✔ | ✔ | ✔ | – | |||
| [18F]RP62203 | ✔ | ND | – | – | Multi-step radiosynthesis | |||
| [11C]MDL100907 | ✔ | ✔ | ✔ | ✔ | – | Arterial input function needed | ||
| [18F]MH.MZ | × | ND | ND | – | Extensive first-pass metabolism, slow washout | - | ||
| (R)-[18F]MH.MZ | ✔ | ND | ND | |||||
| [11C]Cimbi-5 and 36 | ✔ | ✔ (pig) | ND | ND | ||||
| 5-HT3 | PET | [11C]MDL 72222 | × | × | – | – | Lack of specific binding, high lipophilicity | Low 5-HT3 brain density |
| [11C]YM060, [11C]Y-25130 | × | – | – | – | Low brain uptake, low lipophilicity, ionisation of tertiary amide | |||
| [11C]KF17643 | × | – | – | – | No specific binding | |||
| [11C]S21007 | × | × | – | – | No specific binding | |||
| [18F]MR18445 | × | × | – | – | No specific binding | |||
| [11C]NMQ | × | × | – | – | Rapid kinetics, high non-specific binding | |||
| 5-HT4 | SPECT | [123I]SB207710 | × | ✔ | ND | – | – | |
| PET | [11C]SB207145 | ✔ | ✔ (pig) | ✔ | ✔ | – | Slow kinetics | |
| 5-HT6 | PET | [18F]12ST05 | × | – | – | – | No specific binding | – |
| [11C]GSK215083 | ND | ✔ (pig) | ✔ | |||||
| [11C]GSK224558 | ND | (pig only) | ND | – | Inferior ligand compared with [11C]GSK215083 | |||
| 5-HT7 | PET | [11C]DR4446 | ND | × | – | – | Minimal specific binding | – |
| SERT | SPECT | β-[123I]CIT, nor-β-[123I]CIT | ✔ | ✔ | ✔ | ✔ | – | Non-selective (DAT, NET) |
| [123I]ADAM | ✔ | ✔ | ✔ | ✔ | – | Lower resolution than equivalent PET tracers. Ratio method of quantification over-estimates specific binding | ||
| PET | [11C]McN5652 | ✔ | ✔ | ✔ | ✔ | – | Slow brain uptake, irreversible kinetics complicate quantification | |
| [11C]DASB | ✔ | ✔ | ✔ | ✔ | – | |||
| [11C]MADAM | ND | ✔ | ✔ | ✔ | – | |||
| [18F]ADAM | ✔ | ✔ | ND | – | – | |||
| 5-HT synthesis | [11C]-AMT | ✔ | ✔ | ✔ | ✔ | – | Low target-to-background ratio, analysis complicated by possible blood-brain barrier exchange and additional metabolic pathways | |
| [11C]-HTP | ✔ | ✔ | ✔ | ✔ | – | Lack of correlation with [11C]-AMT in direct comparison suggests further validation is necessary |
✔ indicates that ex vivo, PET, or SPECT studies were performed and the ligand was considered successful in the species identified. In certain instances, radioligands were tested in pig brain either in addition to, or instead of nonhuman primate. × indicates that ex vivo, PET, or SPECT studies were performed but the ligand was not considered successful in the species indicated. “Ongoing studies” indicate that the ligand is still being used for research or clinical purposes in humans. ND indicates not yet determined, i.e. experiments have not been published in the species indicated. Missing data are either not applicable (−) or not available/not known (blank cells).
Table III.
Successful and Promising PET and SPECT Radioligands for Serotonergic Targets in Humans
| Target | Radioligand | First in man | Animal studies | Human studies | Research institutions |
|---|---|---|---|---|---|
| 5-HT1A | [11C]WAY-100635 | 1995 | 10 | 80 | 12 |
| [18F]MPPF | 2000 | 27 | 21 | 6 | |
| [18F]FCWAY | 2000 | 7 | 11 | 1 | |
| [11C]CUMI-101 | 2008a | 2 | 1a | 1 | |
| 5-HT1B | [11C]AZ10419369 | 2008 | 3 | 1 | 1 |
| [11C]P943 | 2009 | 1 | 3 | 1 | |
| 5-HT2A | [123I]-5-I-R91150 | 1997 | 9 | 19 | 6 |
| [18F]setoperoneb | 1990 | 3 | 36 | 7 | |
| [18F]altanserin | 1994 | 5 | 51 | 10 | |
| [18F]deuteroaltanserin | 1998 | 2a | 5a | 1 | |
| [11C]MDL100907 | 1998 | 5 | 6 | 5 | |
| 5-HT4 | [11C]SB207145 | 2008 | 3a | 3a | 2 |
| 5-HT6 | [11C]GSK215083 | 2008 | 1 | 2 | 1 |
| SERT | Beta-[123I]CITb | 1993 | 20 | 87 | 18 |
| [123I]ADAM | 2005 | 11 | 28 | 12 | |
| [11C]DASB | 2000 | 17 | 48 | 16 | |
| [11C]MADAM | 2005 | 1 | 7 | 2 | |
| 5-HT synthesis | [11C]-AMT | 1997 | 2c | 17d | 2 |
| [11C]-HTP | 1991 | 4 | 10 | 3 |
“First in man” indicates the year when the tracer was first tested inhuman subjects as determined from publication of full papers or conference proceedings. “Animal studies” indicate the number of published manuscripts that include data from any animal species other than humans; these include ex vivo, SPECT, and/or PET studies in rat, cat, pig, dog, or nonhuman primate. “Human studies” indicate the number of PET or SPECT published manuscripts.
Publications may also include conference proceedings. “Research institutions” indicates the number of centers using the tracer in human subjects. The number of publications and research institutions indicated are correct at the time of writing (July 2010).
Tracer has multiple binding sites but only those reporting on the serotonergic target of interest are included.
Many animal studies have been performed with the carbon-14 equivalent of this tracer but these are not listed here.
Majority of studies are measuring kynurenine metabolism in tumors rather than 5-HTsynthesis per se.
A. 5-HT1A Receptor Radioligands
The 5-HT1A receptor is one of the best characterized receptors in the serotonergic family. This is especially due to its role as an inhibitory autoreceptor in the raphe nuclei and the possible implications of this role for the treatment of depression and anxiety with serotonin reuptake inhibitors and the potential for the treatment of learning and memory deficits.4,41,42
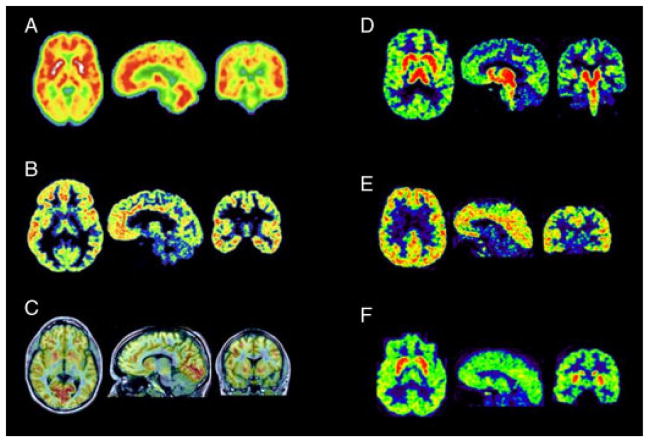
A number of reviews have detailed PET radioligands available for the study of 5-HT1A receptors,43,44 and many have been summarized quite recently.45 Therefore, not all radioligands will be described in detail here. Many of the 5-HT1A radioligands were based on WAY-100635 (N-[2-[4-(2-methoxyphenyl)piperazin-1-yl]ethyl]-N-pyridin-2-ylcyclohexane-carboxamide), which in its carbonyl-11C-labelled form is widely used for 5-HT1A receptor imaging. In total, 39 potential PET 5-HT1A radioligands are described in Kumar and Mann,45 but to date, only two of these ligands are in frequent use for determining 5-HT1A receptor densities; [carbonyl-11C]WAY-100635 and [18F]MPPF (4-[18F]fluoranyl-N-[2-[4-(2-methoxyphenyl)piper-azin-1-yl]ethyl]-N-pyridin-2-ylbenzamide). [18F]MefWAY (4-([18F]fluoranylmethyl)-N-[2-[4-(2-methoxyphenyl)piperazin-1-yl]ethyl]-N-pyridin-2-ylcyclohexane-1-carboxamide) and agonist ligands [11C]CUMI-101 ([O-methyl-11C]2-(4-(4-(2-methoxyphenyl)-6-piperazin-1-yl)butyl)-4-methyl-1,2,4-triazine-3,5(2H,4H)dione) and [18F]F15599 (3-chloro-4-[18F]fluorophenyl-(4-fluoro-4{[(5-methyl-pyrimidin-2-ylmethyl)-amino]-methyl}-piperidin-1-yl)-methanone) seem to be promising future radioligands.46–48 The agonists are of growing interest for this target because of the functional differences between pre- and post-synaptic 5-HT1A receptors, which may translate into differences in binding. Here we have chosen to describe these radioligands and a few others that have been important for 5-HT1A receptor radioligand development. A PET image of 5-HT1A brain receptors obtained in healthy volunteers using [11C]CUMI-101 is shown in Figure 1.
Figure 1.
Six PET radioligand brain images in healthy control individuals, taken with different serotonergic markers. Transverse, sagittal, and coronal sections are shown. (A) 5-HTsynthesis. Normalized [11C]AMT Trapping (K*), average image from 60 healthy controls. Image courtesy of P. Gravel, M. Leyton, M. Diksic and C. Benkelfat, McGill University Health Center (MUHC), Montreal, Canada. (B) 5-HT1A receptor. HRRTPET image of [11C]CUMI-101brain distribution, from Center for Integrated Molecular Brain Imaging (Cimbi), Copenhagen, Denmark. (C) 5-HT1B receptor. Fused MR and PET images of [11C]AZ10419369 brain distribution. Average images from 3 to 93 min after injection. Image courtesy of K. Varnäs, C. Halldin and L. Farde, Karolinska Institutet, Dept of Clinical Neuroscience, Stockholm, Sweden. (D) 5-HT transporter (SERT). HRRT PET image of [11C]DASB brain distribution, from Center for Integrated Molecular Brain Imaging (Cimbi), Copenhagen, Denmark. (E) 5-HT2A receptor. HRRT PET image of [18F]altanserin brain distribution, from Center for Integrated Molecular Brain Imaging (Cimbi),Copenhagen, Denmark. (F) 5-HT4 receptor. HRRTPET image of [11C]SB207145 brain distribution, from Center for Integrated Molecular Brain Imaging (Cimbi),Copenhagen, Denmark.
B. 5-HT1A SPECT Radioligands
1. [123I]p-MPPI
[123I]p-MPPI (4-[123I]iodo-N-[2-[4-(2-methoxyphenyl)piperazin-1-yl]ethyl]-N-pyridin-2-ylbenzamide) was initially a promising 5-HT1A SPECT radioligand. In the rat, brain uptake was rapid and high, the maximum ratio of hippocampus to cerebellum was 3.3 and specific binding was blocked by the 5-HT1A receptor ligands, 8-OH-DPAT and WAY-100635.49 A similar profile was seen in the nonhuman primate brain, suggesting [123I]p-MPPI could prove useful as a SPECT radioligand. Specific binding could be blocked by pretreatment with 8-OH-DPAT or WAY-100635, as is seen both in ex vivo and in vitro autoradiographic studies.50 By contrast, [123I]p-MPPI did not display specific localization to 5-HT1A receptor-rich brain areas in humans. This may be due to rapid in vivo metabolism causing breakdown of the amide bond, precluding its use in humans.51 Another perhaps more likely possibility is that this radioligand was excluded from human brain by an efflux transporter, such as P-gp, since close structural analogues, such as the fluoro-analogue MPPF, are known to be P-gp substrates.52 This experience illustrates the difficulties imposed by translating findings in other species to humans.
2. 99mTc-Labeled Radioligands
Several attempts to develop a 99mTc-labelled 5-HT1A SPECT radioligand have also been reported. Progress was initially slow because of low brain uptake for many early candidates, but more recent publications suggest that further development could result in a successful radioligand for use in humans.53–58
C. 5-HT1A Antagonist PET Radioligands
1. [11C]WAY-100635
WAY-100635 (N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexane-carboxamide) is a selective and high-affinity 5-HT1A receptor antagonist (KD = 0.2–0.4 nM). It was originally found to have very low affinity for all other tested binding sites,59 but recent evidence has suggested that it is also a potent D4 agonist (KD = 2.4 nM60). The latter property is unproblematic for PET and SPECT imaging because D4 receptors exist in brain at very low density relative to 5-HT1A receptors. The binding of tritiated WAY-100635 to postmortem rodent and human brain has been mapped autoradiographically61–63 and the binding of 11C-labelled WAY-100635 to living nonhuman primate and human brain with PET.64,65 Regional binding densities obtained from [11C]WAY-100635 PET studies have been compared with agonist and antagonist binding in human postmortem tissues62 and good correlations between the 5-HT1A receptor distribution were found for autoradiography versus in vivo imaging.
The first radioligand tested with PET in human subjects was [O-methyl-11C]WAY-10063565 which, unfortunately, after systemic injection produced a lipophilic radiometabolite, [O-methyl-11C]WAY-100634, that crossed the blood–brain barrier.66 To avoid this problem, the position of the 11C- label was placed in the carbonyl position of WAY-100635 instead of the methyl position, to give a new radioligand, [carbonyl-11C]WAY-100635. The new label position improved the target to background radioactivity ratio in both nonhuman primate and human PET studies,67 so this radiotracer is currently the most utilized for in vivo imaging of cerebral 5-HT1A receptors (Table I). A database of 5-HT1A binding with PET in normal volunteers was published in 2002.68 As reviewed by Kumar and Mann,45 [11C]WAY-100635 binding has been investigated in numerous studies of patients with psychiatric or neurological disorders. Since 2007 more than 25 further PET studies using [11C]WAY-100635 have been published to date, extending the list of human disorders susceptible to changes in 5-HT1A receptor binding to panic disorder,34 bipolar depression,69 and anorexia nervosa70 among others.
One drawback with [11C]WAY-100635 is that it undergoes a fast systemic metabolism, which makes kinetic modelling difficult, because the arterial input function is less well determined at later time points.71 To circumvent this problem, reference tissue methods have been used for quantification of [11C]WAY-100635 data. Receptor-poor cerebellar white matter may serve as a reference region.72,73 However, this approach may also be associated with some inaccuracy, since the rapid appearance of radiometabolites in plasma violates the fundamental assumption that both the reference region and the pool of free tracer in the binding regions continue to exchange significant quantities of tracer with the blood pool.74
2. [11C]CPC-222
CPC-222 (N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridinyl)-2-bicyclo-[2,2,2] octanecarboxamide) is a WAY-100635 derivative developed in an attempt to improve its metabolic profile, and in particular its resistance to amide bond hydrolysis. Initial PET studies were promising, demonstrating high hippocampal to cerebellar radioactivity ratios in rats and humans.75,76 As predicted, the metabolism of [11C]CPC-222 is slower than that of [11C]WAY-100635. However, [11C]CPC-222 gives lower signal than [11C]WAY-100635 and there are no further studies published with this radioligand.
3. [11C](R)-RWAY
The R-enantiomer of RWAY ((2R)-1-(azepan-1-yl)-4-[4-(2-methoxyphenyl)piperazin-1-yl]-2-phenylbutan-1-one) was designed in another attempt to provide a radioligand more resistant than WAY-100635 to amide bond hydrolysis. (R)-RWAY is a high-affinity 5-HT1A receptor antagonist. This ligand is structurally quite similar to WAY-100635, but the direction of its amide group is reversed. Because of this feature, RWAY is less susceptible to amide hydrolysis in vivo. In PET studies of nonhuman primate studies, [11C](R)-RWAY cerebral binding in 5-HT1A receptor-rich regions was high and could be blocked by pretreatment with WAY-100635.77,78 In humans, although target to background ratios were acceptable (up to 3), determinations of distribution volumes were unstable, possibly due to the greater presence of a lipophilic radiometabolite in human plasma compared with that in primate.79 This limits the usefulness of [11C](R)-RWAY for human brain imaging.
4. [11C]DWAY
[11C]Desmethyl-WAY-100635 ([11C]DWAY; [carbonyl-11C]N-[2-[4-(2-hydroxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexanecarboxamide) is a putative low level radio-metabolite of [11C]WAY-100635.67 It has been shown that DWAY has very similar pharmacology to WAY, and that [carbonyl-11C]DWAY exhibits favorable PET characteristics. These include, higher brain uptake than [11C]WAY-100635 at equipotent doses in human brain, suitable pharmacokinetics, and a radioactive signal comparable to that of [11C]WAY-100635 in rat and monkey.80,81 However, further human studies utilizing this ligand have not yet been forthcoming, perhaps due to the difficulty of setting up the radiolabelling process. Despite attempts to improve the production methodology, radiochemical yield has continued to be low.82
5. [18F]6FPWAY
In an attempt to design an alternative 5-HT1A receptor PET ligand with a better metabolic profile and a longer radioactive half-life, bromo- and fluoro-analogues of WAY-100635 were produced: 6FPWAY (N-(2-(1-(4-(2-methoxyphenyl)-piperazinyl)ethyl))-N-(2-(6-fluoropyridinyl))-cyclohexanecarboxamide) and 6BPWAY (N-(2-(1-(4-(2-methoxyphenyl)-piperazinyl)ethyl))-N-(2-(6-bromopyridinyl))cyclohexanecarboxamide).83 The introduction of the halogen groups was predicted to reduce the rate of metabolism and to provide opportunities for labelling with 18F or 76Br. Both halogenated ligands were initially labelled with 11C in their carbonyl positions for preliminary PET studies in nonhuman primates and were found promising. Satisfactory radioactive yields were obtained. Both radioligands and especially [11C]6FPWAY accumulated in brain areas according to 5-HT1A receptor density and gave high target to background ratios. Metabolism of [11C]6BPWAY was reduced, as intended. While the metabolism of [11C]6FPWAY was comparable with that of [11C]WAY-100635, the radiometabolites were quite polar; therefore, 6FPWAY was subsequently labelled with 18F84 and tested in monkey PET studies. [18F]6FPWAY demonstrated resistance to defluorination, but only moderately high uptake in 5-HT1A receptor-rich regions.85 Therefore, this radio-ligand has not seen use in human subjects.
6. [18F]MPPF
[18F]MPPF is another successful 5-HT1A PET ligand.86 Its use in animal and human PET studies, specifically in relation to its potential for measuring changes in endogenous 5-HT, has recently been reviewed by Aznavour and Zimmer.87 Similar to WAY-100635, MPPF acts as a reversible, competitive antagonist at 5-HT1A receptors, and it has high 5-HT1A receptor affinity (KD = 0.3 nM) and selectivity.86,88 Optimization of production methods has resulted in a simple one-step procedure giving high yield.89 [11C]MPPF displays low nonspecific binding in the human brain and its distribution matches that of postmortem human brain 5-HT1A receptor distribution.90,91 [18F]MPPF is a P-gp substrate in rats,52,92 and although it is still unknown whether this applies in humans, it has not detracted from its clinical application. A number of [18F]MPPF studies in patients have now been published, including in epilepsy,93 cognitive impairment, and Alzheimer’s disease,94 migraine,95,96 and depression.97 A database of PET normative data with age- and gender-related binding variables is also available,98 as well as test–retest data.99
Although [18F]MPPF binding to 5-HT1A autoreceptors in the raphe nuclei seems sensitive to endogenous 5-HT levels at least in some studies,100 it is still questionable if such sensitivity applies elsewhere in the brain. Studies in healthy volunteers and depressive patients in remission have concluded that [18F]MPPF binding is not sensitive to reductions in 5-HT after tryptophan depletion.101,102 One study, however, reported an increase in several brain regions in [18F]MPPF binding potential following sleep, which is presumed to reduce 5-HT release.103 In a recent review, it was concluded that currently available 5-HT1A receptor radioligands do not appear to be sensitive to endogenous 5-HT.30
In order to improve brain uptake, the desmethylated analogue [18F]DMPPF was synthesized through a two-step radiochemical procedure. Brain uptake was indeed found to be higher, and the compound showed better signal-to-noise ratio and a slower clearance in rats compared to [18F]MPPF.104 This radioligand has to our knowledge not yet been tested in vivo in humans.
7. [18F]FCWAY
A series of radioligands based on fluorocyclohexyl analogues of WAY-100635 were developed by Lang et al.105 Following evidence of high target to background ratios, several of these radioligands were further developed, and in particular two proved to be potentially useful as PET ligands. [18F]FCWAY ([18F]trans-4-fluoro-N-2-[4-(2-methoxyphenyl)piperazin-1-yl]ethyl]-N-(2-pyridyl)cyclohexanecarboxamide) was demonstrated to possess high 5-HT1A affinity (Ki = 0.25 nM) and a high hippocampal to cerebellar binding ratio and thus had potential for imaging 5-HT1A receptor density. [3-cis-18F]FCWAY, which has a lower affinity (Ki = 1.2 nM) and consequently faster pharmacokinetics, was proposed to be more useful for measuring dynamic changes in receptors, e.g., competition with endogenous 5-HT.106,107
[18F]FCWAY has been used to image 5-HT1A receptors in epilepsy,108–111 panic disorder,112 and post-traumatic stress disorder.113 However, defluorination of the parent compound leads to high bone uptake of radioactivity, which interferes with optimal imaging of superficial brain areas. Radiodefluorination in human subjects can be abolished by pre-administration of disulfiram, resulting in enhanced receptor visualization.114,115 However, the major defluorination issue with this radioligand may be the reason for its use not expanding beyond a single PET centre.
So far, despite its slower defluorination, [3-cis-18F]FCWAY has not yet been utilized in further PET studies, possibly due to its lower affinity.
8. [18F]MefWAY
[18F]MefWAY was developed in an attempt to produce an 18F-labelled ligand that would be stable to defluorination in vivo.116 As an analogue of WAY-100635, [18F]MefWAY has very comparable affinity and gives a higher target to background radioactivity ratio than many other 5-HT1A radioligands, including [18F]MPPF. In nonhuman primates, the signal is comparable with that obtained with [carbonyl-11C]WAY-100635 and superior to [18F]MPPF.46 [18F]MefWAY appeared stable to radiodefluorination in monkey. Although this radioligand seems very promising for further evaluation in human subjects, none have yet been published.
9. [11C]NAD-299
NAD-299 ([R]-3-N,N-dicyclobutylamino-8-fluoro-3,4-dihydro-2H-1-benzopyran-5-carbox-amide) has several promising characteristics: subnanomolar affinity (KD = 0.17 nM), favorable lipophilicity, high selectivity in rat brain,117 and high specific binding in postmortem human brain.118 In the nonhuman primate, [11C]NAD-299 displayed good PET characteristics. These include rapid accumulation in brain and high uptake in 5-HT1A-rich brain areas like frontal cortex (ratio of 3 to cerebellum) and raphe nuclei that was displaceable by WAY-100635. The radiometabolites were more polar than the parent radioligand, and slower to accumulate than those of WAY-100635.119 However, the study of this radioligand in humans has not been reported.
D. 5-HT1A Agonist PET Radioligands
The development of a successful 5-HT1A agonist PET radioligand has proved difficult. There are several examples based on the agonist 8-OH-DPAT,45 but only a couple have been reasonably successful in vivo. [11C]MPT ([O-methyl-11C]2-(4-[4-(7-methoxynaphthalen-1-yl)-piperazin-1-yl]butyl)-4-methyl-2H-[1,2,4]triazine-3,5-dione) gave a good signal in the baboon brain, but slow washout and immeasurable plasma-free fraction limit its utility,120 and [11C]MMT ([O-methyl-11C]2-(4-[4-(3-methoxyphenyl)piperazin-1-yl]-butyl)-4-methyl-2H-[1,2,4]-triazine-3,5-dione) had impressive in vitro characteristics, but PET studies in baboon failed because of low specific binding and fast clearance.121
1. [11C]CUMI-101
Further optimization of structure–activity relationship in MPT analogues produced [11C]MMP (now known as [11C]CUMI-101) ([O-methyl-11C]2-(4-(4-(2-methoxyphenyl)-piperazin-1-yl)butyl)-4-methyl-1,2,4-triazine-3,5(2H,4H)dione), a high affinity partial agonist compound (Ki = 0.15 nM) with satisfactory radiochemical yield, and which appeared superior to both [11C]MPT and [11C]MMT in terms of binding ratios and wash out times.122 PET studies in baboons confirmed good 5-HT1A selectivity with high specific binding that was displaceable by WAY-100635 and 8-OH-DPAT. [11C]CUMI-101 has polar radio-metabolites that do not cross the blood–brain barrier, and in vivo modelling data in baboons have been published.48 Test–retest studies in the baboon were satisfactory (8–13%) and it was suggested that this radioligand may be sensitive to endogenous changes in 5-HT,123 although studies in rodents do not support this.124 In humans, [11C]CUMI-101 has promising features and may preferentially label the high affinity site (Fig. 1125), but it remains to be seen whether it can be used to image endogenous 5-HT release in humans or whether it will make a good in vivo radioligand in clinical populations.
2. Other 5-HT1A Agonist Radioligands
The development of other 5-HT1A agonists is ongoing and several new leads have been investigated. F15599 is an agonist with a Ki of 2.2 nM, and over 1,000-fold selectivity with respect to a wide range of other receptors, transporters, ion channels, and enzymes.126 It seems that F15599 preferentially activates postsynaptic 5-HT1A receptors in rat frontal cortex.127 It contains fluorine in a way that is compatible with 18F labelling, and the radiosynthesis and validation of [18F]F15599 as a potential PET radioligand were recently reported.47 Using autoradiography, this study reported similar receptor distributions using [18F]F15599 and [18F]MPPF in rat and cat brain. In vivo microPET showed a rapid accumulation of the radioligand in rat brain but with a cortex to cerebellum ratio of only 1.6. Similar results were obtained in the cat brain. The low target to background ratio augurs poorly for the usefulness of [18F]F15599 as a radioligand for human 5-HT1A receptor PET studies.
S14506 (1-[2-(4-fluorobenzoylamino)ethyl]-4-(7-methoxy-naphthyl)piperazine) is another high-affinity ligand (Kd = 0.79 nM) with reasonable selectivity demonstrated in in vitro and ex vivo studies. It was recently labelled to produce [11C]S14506 and [18F]S14506 and tested for its suitability as an in vivo tracer in rat and monkey PET studies.128 Unfortunately, due to low uptake and low signal-to-background ratio neither tracer was deemed suitable for in vivo imaging.
E. 5-HT1A Radioligands: Conclusions
In conclusion, there are three radioligands in current use for PET studies of the 5-HT1A receptor in human subjects: [carbonyl-11C]WAY-100635, [18F]MPPF, and [18F]FCWAY. A further two promising radioligands are emerging: [18F]MefWAY and [11C]CUMI-101. [carbonyl-11C]WAY-100635 is the most widely used 5-HT1A receptor radioligand. It has the advantage of a high target-to-background ratio and its distribution matches that of the 5-HT1A receptors. Its only disadvantage is its fast metabolism in plasma and consequently, there are difficulties with its accurate quantification. [18F]MPPF has the advantage of the longer lived 18F-label, and it also selectively labels the 5-HT1A receptors with a low non-specific binding. Its major disadvantage is its low brain uptake. [18F]FCWAY also benefits from being 18F-labelled, and displays affinity and selectivity comparable to its analogue WAY-100635 and also to [18F]MPPF. [18F]FCWAY is, however, prone to defluorination in vivo, which severely compromises its use for some brain regions, especially cortex. [18F]MefWAY is also analogous to WAY-100635, and its resistance to defluorination makes it a promising radioligand. [11C]CUMI-101 is a high affinity 5-HT1A (partial) agonist radio-ligand that displays high specific binding in the baboon and also appears suitable for imaging the high affinity site within human brain.
F. 5-HT1B Receptor Radioligands
The evolution of the study of 5-HT1B receptors has been complicated because 5-HT1B pharmacology was long confused with that of 5-HT1D receptors found in the rodent brain. Initially, 5-HT1B and 5-HT1D were considered to be species homologues of the same receptor, but it was later revealed that the human 5-HT1D receptor encompassed two receptor sub-types, 5-HT1Dα and 5-HT1Dβ, encoded by separate genes; 5-HT1Dα became what is now known as the 5-HT1D receptor and 5-HT1Dβ became the 5-HT1B receptor.15 Most studies suggest that in humans, the 5-HT1B receptor makes up the vast proportion of 5-HT1B/D receptors in the brain.129,130
Interest in the receptor was enhanced by the discovery of sumatriptan and related 5-HT1B/D agonist drugs for the treatment of migraine.131 The majority of 5-HT1B ligands have mixed 5-HT1B/1D pharmacology, but selective 5-HT1B ligands do exist and are now of interest due to their emerging utility in the study of depression, aggression, and drug reinforcement.132–134 Radioligands have been synthesized to facilitate in vitro studies of the 5-HT1B receptor and development of PET radioligands for the 5-HT1B receptor is ongoing. Recent interest has been sparked by the suggestion that even 5-HT1B antagonist ligands may be sensitive to displacement by endogenous 5-HT.135
G. 5-HT1B PET Radioligands
1. [11C]AZ10419369
[11C]AZ10419369 (5-methyl-8-(4-[11C]methyl-piperazin-1-yl)-4-oxo-4H-chromene-2-carboxylic acid(4-morpholin-4-yl-phenyl)-amide) has recently been reported to be a selective 5-HT1B antagonist suitable for PET studies in nonhuman primates136 and humans.137 [3H]AZ10419369 has high affinity (KD = 0.4 nM) and high specific binding to the human 5-HT1B receptor,138 although its selectivity remains to be documented. Initial [11C]AZ10419369 PET studies in nonhuman primates demonstrated a high brain uptake and a regional brain distribution in line with that previously reported for the 5-HT1B receptor, also correlating with autoradiographic images with [3H]AZ10419369. Other promising characteristics included a relatively high in vitro target-to-background ratio (cortex/cerebellum =2.3 in human tissue), displaceable binding by a 5-HT1B specific antagonist AR-A000002, a slow metabolism, and no lipophilic radiometabolites. This ligand may also demonstrate sensitivity to displacement by endogenous 5-HT in nonhuman primates.135 The first human PET study with [11C]AZ10419369 suggested similarly good in vivo characteristics.137 This radioligand displayed rapid uptake, a good target-to-background ratio, no evidence of the formation of radiometabolites, and a binding distribution that correlated with receptor autoradiographical studies and known 5-HT1B densities. In addition, the binding potentials obtained with reference tissue modelling correlated well with those arising from arterial input kinetic modelling. A PET image of 5-HT1B brain receptors obtained in healthy volunteers using [11C] AZ10419369 is shown in Figure 1.
2. [11C]P943
The development and validation of the 5-HT1B radioligand, [11C]P943 (R-1-[4-(2-methoxy-isopropyl)-phenyl]-3-[2-(4-methyl-piperazin-1-yl)benzyl]-pyrrolidin-2-one), has very recently been reported, including modelling approaches for its quantification in the human brain.139 P943 is a high affinity 5-HT1B antagonist (Ki = 1.2 nM), with affinities for most other serotonergic receptors being more than 100-fold lower. It has some affinity toward the 5-HT1D receptor (Ki = 12 nM), but only a very small fraction of [11C]P943 was expected to bind to the 5-HT1D receptors due to their low abundance. This has not yet been confirmed, since the only in vivo blocking study so far was done using the mixed 5-HT1B/1D receptor antagonist, GR127935.140 A comparison between autoradiography data and binding potentials measured in vivo by human PET, however, shows a fairly good correlation and suggests that the signal is indeed specific to the 5-HT1B receptor. Radiosynthesis of [11C]P943 is simple, and the substance is taken up slowly into the brain with a peak after 20 min. On the contrary, the washout appears to be relatively fast. Another promising characteristic is the slow metabolism of the radioligand with the production of polar radiometabolites only. Cerebellar gray matter can be used as a reference region, as this is devoid of 5-HT1B receptors,141 and reference tissue models could potentially be used.139 Even though pharmacological displacement of [11C]P943 has not yet been performed in humans to confirm the specificity of the radioligand, [11C]P943 appears to be a promising radioligand for the quantification of 5-HT1B receptor densities in the human brain in vivo. The first patient studies are now emerging; the first in alcohol dependence142 and another in depression.143
H. 5-HT1D, 5-ht1e, and 5-HT1F Radioligands
The distribution of 5-ht1e, 5-HT1D, and 5-HT1F receptors has been mapped in brain tissue.144,145 However, a lack of selective ligands for these receptors has hampered research into their function and potential for drug discovery. There are no reported selective ligands of any kind for the 5-ht1e receptor. 5-HT1D and 5-HT1F receptors are of interest in migraine treatment.146,147 There now exists a few selective ligands for the 5-HT1D receptor,147 and a selective radioligand for in vitro characterization is available for the 5-HT1F receptor.148 To date, no selective PET or SPECT radioligands for in vivo brain imaging of the 5-HT1D, or 5-HT1F receptor have been developed.
I. 5-HT2A Receptor Radioligands
5-HT2A receptors and their mRNAs have been extensively mapped in the brain by auto-radiography, in situ hybridization, and immunocytochemical techniques.149–152 There are several selective 5-HT2A ligands available, and a review of certain 5-HT2 ligands, their affinities, efficacies, and indications was published several years ago.153 5-HT2A receptors are of interest for many reasons: they are a primary target of psychedelic compounds, contribute to the efficacy of many antipsychotic medications, and are involved in the etiology or treatment of various psychiatric disorders.17,153,154
The selective radioligands available for studying 5-HT2A receptors include [3H]RP62203 (fananserin, (N-[3-[4-(4-fluorophenyl)piperazin-1-y1]propyl]-1,8-naphthalenesultam)), [3H]ke-tanserin (3-[2-[4-(4-fluorobenzoyl)piperidin-1-yl]ethyl]-1H-quinazoline-2,4-dione), [3H]MDL 100,907 ((R)-4-(l-hydroxy-1-(2,3-dimethoxyphenyl)methyl)-N-2-(4-fluorophenylethyl)piperidine), and [3H]altanserin (3-[2-[4-(4-fluorobenzoyl)piperidin-1-yl]ethyl]-2-sulfanylidene-1H-quinazolin-4-one). Attempts have been made to label these ligands for PET and/or SPECT imaging with mixed results, as described below. Only one successful SPECT ligand for imaging of 5-HT2A receptors has so far been produced: [123I]-R91150 (4-amino-N-[1-[3-(4-fluorophenox-y)propyl]-4-methyl-4-piperidinyl]-5-iodo-2-methoxybenzamide), also termed [123I]-5-I-R91150. [11C]MDL 100,907 and especially [18F]altanserin have both become successful PET ligands, among others that show promise. A PET image of 5-HT2A brain receptors obtained in healthy volunteers using [18F]altanserin is shown in Figure 1.
J. 5-HT2A SPECT Ligands
1. [123I]DOI
Scintigraphic images of 5-HT2 receptors in the human brain were obtained in the 1970s, well before the first PET or SPECT studies emerged. Sargent and colleagues demonstrated the preferential accumulation of radioactivity in brain after administration of radio-labelled DOI (1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane) and DOB (1-(2,5-di-methoxy-4-bromophenyl)-2-aminopropane).155,156 As these two drugs are routinely used to test 5-HT2 receptor function in vivo, there is a plethora of data on their pharmacology and kinetics.
[125I]DOI is a radiolabelled non-selective 5-HT2 agonist, often used to detect 5-HT2A/2C receptors in the brain with autoradiography.157,158 Very early imaging studies utilizing racemic DOI with a brominated label were not particularly successful.155 However, it was later revealed that the R-enantiomer displayed the highest affinity and selectivity,159 and therefore [123I]R-DOI was synthesized for in vivo testing as a potential SPECT ligand in rodents and baboon.160 [123I]R-DOI had good brain penetration, with high accumulation in 5-HT2A receptor-rich brain areas, but its low target-to-background ratio and its inability to be displaced by ketanserin or cold DOI suggested a high nonspecific uptake and made the ligand unsuitable for in vivo imaging by SPECT.
2. [123I]MSP
After the butyrophenones emerged as the foundation for 5-HT2A selective antagonist ligands, F[11C]MSP (8-[3-(4-fluorobenzoyl)propyl]-1-methyl-1,3,8-triazaspiro[4,5]decan-4-one) and [123I]MSP (8-[3-(4-iodobenzoyl)propyl]-1-methyl-1,3,8-triazaspiro[4,5]decan-4-one) were developed as potential PET and SPECT ligands, respectively.161 F[11C]MSP was quickly abandoned, due to problems with low specific radioactivity. Nevertheless, in vivo pharma-cokinetic and brain binding characterization of [123I]MSP in mice suggested sufficient uptake and retention in the brain, with regional accumulation corresponding to known 5-HT2A receptor distribution. Binding in frontal cortex (target-to-background ratio of 3.5) could be partly blocked by pre-treatment with ritanserin and IMSP but not by ketanserin. No further studies with [123I]MSP have been published.
3. [123I]-R91150
The SPECT radioligand [123I]-R91150 has several different names in the literature. Originally, it was called either [123I]-5-I-R91150162 or [123I]-R93274,163 but it is now often named [123I]-R91150, even though R91150 originally was used to designate the noniodinated analogue of 5-I-R91150. For simplicity, we will refer to it as [123I]-R91150.
125I-labelled R91150 was originally synthesized by Mertens et al.164 and further characterized by Terrière et al.165 It has high affinity (KD = 0.11 nM) and selectivity for 5-HT2A receptors in vitro, and preferential retention in rat frontal cortex in vivo with a target-to-background ratio of 10. Preliminary SPECT studies in baboons using [123I]-R91150 were also promising.163 Target-to-background ratios were lower than in rats (1.3–1.5 in cortical areas), and kinetics and metabolism were faster. Only polar radiometabolites were seen and binding could be blocked by administration of ketanserin (5-HT2 antagonist) but not by raclopride (D2 antagonist). In humans, frontal cortex-to-cerebellum ratios reached values of 1.4, and remained stable, probably due to a slow dissociation rate of [123I]-R91150.162
In early human studies, a single bolus injection of [123I]-R91150 was used to calculate the specific uptake ratio using the simple tissue ratio method at pseudoequilibrium. A later study validated this method against full kinetic compartmental analysis and found a good correlation between compartmental modelling with arterial plasma input and the tissue ratio method.166 Catafau et al.167 further evaluated the in vivo properties of [123I]-R91150 by conduction of a dose-dependent displacement of [123I]-R91150 binding with ketanserin.
Despite the lower signal-to-noise ratio of [123I]-R91150 compared with the available PET radioligands, the widespread availability of SPECT facilities made [123I]-R91150 quite popular. A string of publications appeared, based on [123I]-R91150-SPECT determined in vivo 5-HT2A receptor occupancy by various ligands168–170 and changes in 5-HT2A density involved in disease states including cognitive decline,171 suicidal behavior,172,173 and anorexia nervosa.174,175 The influence of age and gender on binding potential in healthy subjects has also been determined.176 Due to its success, R91150 was also labelled with 18F and evaluated as a potential PET radioligand. Ex vivo data from mice were promising, but the six-step radiosynthesis prevents a more widespread use of this radioligand.177
4. [123I]-3-I-CO
Fu et al.178 developed a series of halogenated novel compounds based on common structural features of altanserin and MDL 100,907 to provide a new series of 4′-substituted phenyl-4-piperidinylmethanol and benzoyl-4-piperidine derivatives. Affinity for the 5-HT2A receptor was determined and the compounds were further evaluated for selectivity for 5-HT2A versus 5-HT2C, 5-HT6, and 5-HT7, as well as dopamine D2 and adrenergic α1 and α2 receptors. Several promising compounds were reported, of which three have been labelled with 123I and evaluated as potential SPECT ligands179–181: [123I]-(4-fluorophenyl)(1-[2-(2-iodophenyl) ethyl]piperidin-4-yl)methanone, [123I]-(4-fluorophenyl)(1-[2-(4-iodophenyl)ethyl]piperidin-4-yl) methanone, and [123I]-(4-fluorophenyl)[1-(3-iodophenethyl)piperidin-4-yl]methanone ([123I]-3-I-CO). Of these [123I]-3-I-CO was the most promising ligand with its high affinity (Ki = 0.51 nM) and selectivity toward 5-HT2A receptors. In vivo, it readily entered the rat brain and its binding was displaceable by ketanserin. In addition, in rats no radiometabolites entered the brain. However, target-to-background ratio was low, and it is possible that [123I]-3-I-CO is a substrate for P-gp.182
K. 5-HT2A PET Radioligands
1. [11C]Ketanserin and [18F]FEK
The first mention of a PET study specifically aiming at imaging 5-HT2 receptors in the brain utilized [11C]ketanserin183 based on its known favorable in vitro and in vivo characteristics, although it also displays high histamine sub-type-1 (H1) receptor affinity and lower affinities for the α1-adrenoceptor and the 5-HT2C receptor. Here, preferential accumulation in frontal cortex relative to cerebellum was described, and the binding could be blocked by pretreatment with unlabeled chlorpromazine. However, the target-to-background ratio was low in the human brain, and the radiotracer metabolized fast.183 An analogue to ketanserin, [18F]fluoroethylketanserin ([18F]FEK) was subsequently developed. It had better PET properties184 at least in the baboon, where the frontal cortex-to-cerebellar ratio was 2.5, and pretreatment with ketanserin blocked the specific binding. Despite these initial promising observations, further [18F]FEK human studies have not been published.
2. [11C]NMSP
[11C]NMSP (N-methylspiperone, 8-[4-(4-fluorophenyl)-4-oxobutyl]-2-methyl-4-phenyl-2,4,8-triazaspiro[4.5]decan-1-one) is a dual D2/5-HT2 ligand. Although NMSP has high affinity for both receptors, the majority of the specific binding in neocortex is due to 5-HT2 receptor binding, since D2 receptors are only present at low density in cortex, thus justifying its use as a 5-HT2 radioligand in this area.185,186
[11C]NMSP has been used primarily as an imaging tool to visualize D2 receptor binding in striatum, but was also used in early PET studies to estimate changes in cortical 5-HT2 receptor binding in, for example, aging,187 schizophrenia,188 and 5-HT2 receptor occupancy by antipsychotic medications such as risperidone,189 clozapine,190,191 MDL 100,907,192 and flupentixol.193 At that time, the more selective PET radioligands, [18F]setoperone and [18F]altanserin, had yet to be fully characterized.
3. [11C]MBL
The radiolabelled LSD derivative, N1-([11C]-methyl)-2-Br-LSD ([11C]-MBL), has also been tested as a PET radioligand.194,195 MBL has high 5-HT2A receptor affinity in vitro (Ki = 0.5 nM), eight-fold lower affinity for D2 receptors, high affinity for 5-HT2C receptors,196 and weak α1-adrenoceptor and 5-HT1 receptor interactions.195 Nonetheless, initial studies in baboons suggested that [11C]-MBL selectively labelled cortical regions and was blocked by ketanserin, and human studies suggested the same; highest labelling in frontal, temporal, and parietal cortex (cortical-to-cerebellar ratios: 1.7–2.7) with lower levels observed in caudate and putamen and lowest in cerebellum. The lack of further PET imaging papers could be indicative of its nonselective pharmacological profile combined with the emergence of better radiotracers for imaging the 5-HT2A receptors.
4. [18F]Setoperone
The structure of setoperone is related to that of ketanserin. [18F]setoperone (6-[2-[4-(4-[18F]fluorobenzoyl)piperidin-1-yl]ethyl]-7-methyl-2,3-dihydro-[1,3]thiazolo[3,2-a]pyrimidin-5-one) in vivo showed high binding in baboon brain in areas known to be rich in 5-HT2A receptors such as cerebral cortex, but also high binding in striatum.197 The target-to-background ratio was 3 in cortex and binding could be blocked by pretreatment with spiperone and ketanserin. In striatum, radioligand binding was fully prevented by spiperone but only partly by ketanserin suggestive of a significant contribution from dopamine D2 receptors. In humans, pretreatment with ketanserin, sulpiride, and prazosin confirmed that the [18F]setoperone signal was due to 5-HT2 receptors in frontal cortex, to D2 receptors in striatum and that no significant α1-adrenoceptor binding was evident.198 In vivo, [18F]setoperone produced radiometabolites, but these were less lipophilic than the parent compound. Due to the differential localization of the 5-HT2A relative to D2 receptors, [18F]setoperone became a relatively successful 5-HT2A PET ligand despite its affinity for D2 receptors. It has been used to estimate the occupancy of 5-HT2A receptors by antipsychotics199–201 and to assess possible changes in 5-HT2A density in diseases such as Alzheimer’s disease, migraine, stroke, and depression, and in response to electro-convulsive shock therapy.202–207
5. [18F]Altanserin
Altanserin, like setoperone, is a fluorobenzoyl derivative structurally related to ketanserin. Its Ki for 5-HT2A receptors is 0.13 nM, so given its α1-adrenoceptor affinity of 5 nM and D2 affinity of 62 nM, the majority of the signal can be attributed to 5-HT2A receptor binding. The first [18F]altanserin study was published in 1991,208 and today, [18F]altanserin is the most frequently used 5-HT2A receptor PET radioligand (Table II and Fig. 1).
The potential of [18F]altanserin as a PET radioligand was first demonstrated in the rat brain where the frontal cortex-to-cerebellum ratio is 11, and its binding can be blocked by pretreatment with ritanserin, ketanserin, and setoperone, and only to a minor degree by D2 ligands.208 In a follow-up study in humans, the synthesis methodology was improved,209 ketanserin pretreatment blocked [18F]altanserin binding in the human brain,210 and displaced [18F]altanserin binding.40 Although [18F]altanserin produces radiometabolites, these were initially considered not to interfere with binding characteristics.211 Later, it was realized that the radiometabolites of [18F]altanserin contributed to the nonspecific binding, and thus quantification required complex kinetic modelling.212,213 To simplify the quantification and to take into account the lipophilic radiometabolite, a bolus–infusion approach was developed. Initially, 6 hr of infusion were required to obtain equilibrium measurements,214 but a later study showed that this time could be reduced to 2 hr, making infusion studies more feasible.40 The bolus–infusion paradigm with [18F]altanserin was later shown to have excellent test–retest reliability, particularly in large brain regions with high binding.215
Following the positive validation studies, [18F]altanserin was used to determine changes in 5-HT2A receptor density in relation to aging,216–219 depression,220,221 anorexia nervosa/bulimia,222,223 obsessive–compulsive disorder,224 cognitive decline,225,226 Tourette’s syndrome,227 risk or onset of schizophrenia,228–231 and in relation to the personality trait neuroticism.232,233 A database of 5-HT2A binding in healthy volunteers has been published,218 and it has been reported that binding in healthy subjects correlates with body mass index234 but does not vary with gender.235 Furthermore, twin studies have shown that [18F]altanserin binding has a strong genetic component.236
6. [18F]Deuteroaltanserin
The significant advantages of [18F]altanserin include its specific brain uptake, kinetics that allow for bolus–infusion schedules, a high target-to-background ratio, and a high reproducibility. Prior to the development of suitable bolus–infusion regimes, formation of lipophilic radiometabolites from [18F]altanserin was considered a major disadvantage because of the resulting complex quantification methods in data analysis. Development of a PET radioligand based on [18F]altanserin, which did not give rise to radiometabolites crossing the blood–brain barrier, led to the synthesis of [18F]deuteroaltanserin.237 The two deuterium atoms, which are present in place of hydrogen atoms, were hypothesized to retard metabolism. This was indeed the case, and [18F]deuteroaltanserin was reported to have better brain uptake in baboon and humans compared with [18F]altanserin, using a bolus–infusion paradigm. In humans, cortical-to-cerebellar ratio was increased by 26% above that observed previously for [18F]altanserin, suggesting it might be a superior PET radioligand.237 In a subsequent study by the same group, the test–retest reliability was also quite good.238
Staley et al.239 directly compared [18F]altanserin and [18F]deuteroaltanserin in baboons under equilibrium receptor binding conditions. They demonstrated 5-HT2A specificity of both tracers by injecting the 5-HT2A antagonists ketanserin and SB46349B, and they concluded that [18F]deuteroaltanserin was essentially equivalent to [18F]altanserin for 5-HT2A receptor imaging in the baboon.
Since then, only two further studies have been published using [18F]deuteroaltanserin in humans; one to demonstrate that oestrogen replacement therapy increases prefrontal 5-HT2A receptor density240 and the other suggesting that 5-HT2A receptors are reduced in cortical regions in Alzheimer’s disease.241 It remains to be seen whether this ligand will become as successful as its predecessor.
7. [18F]RP62203
Although [18F]setoperone and especially [18F]altanserin are both successful PET radio-ligands, they both display somewhat mixed pharmacology, as described above, which could limit their utility. [3H]RP62203 was shown to be a 5-HT2A antagonist with very high affinity (Ki = 50.0 pM) and a good selectivity profile in rat cerebral cortex,242 without any significant D2 and α1 receptor affinity. Subsequent in vivo binding characteristics of 18F-labelled RP62203 ([18F]RP62203) revealed specific binding that correlated with the known 5-HT2A receptor distribution in the rat brain. Binding was four times higher in cortical regions than cerebellar regions and was abolished by prior dosing with ritanserin. This suggested that [18F]RP62203 might make a good PET radioligand.243 Shortly after, this was confirmed in rat studies by a different group244 who found that [3H]RP62203 gave a cortex-to-cerebellum activity ratio in rats in vivo of 9. However, the radiosynthesis of [18F]RP62203 is multi-step and therefore probably too demanding for regular implementation. For this reason and perhaps also because of the subsequent emergence of [11C]MDL 100,907, no further reports on [18F]RP62203 have been seen.
8. [11C]MDL 100,907
MDL 100,907 is a reversible, highly selective 5-HT2A ligand with subnanomolar affinity (KD = 0.14–0.19 nM).245 Despite differences in their in vitro selectivity profiles, the binding of [18F]altanserin and [3H]MDL 100,907 to the 5-HT2A receptor is quite comparable.246 [11C]MDL 100,907 showed promise in nonhuman primates where it accumulated in 5-HT2A receptor-rich regions, with a neocortex-to-cerebellum ratio of 4.5 that was abolished after injection of ketanserin.247 Later, radioligand binding and autoradiography studies using [3H]MDL 100,907 and [11C]MDL 100,907 confirmed its selectivity and high specific binding in rat, nonhuman primate, and human brain,152,245,248,249 making it the first truly selective 5-HT2A receptor ligand. In parallel, favorable PET characteristics for [11C]MDL 100,907 were reported in humans.250 MDL 100,907 has moderate lipophilicity and the binding potential is 4–6 times higher in neocortex than cerebellum, where binding is low.
Further studies have validated the methodology for modelling [11C]MDL 100,907 binding in PET studies, and find that a two-tissue compartment model using arterial input is superior to reference tissue models.251,252 This somewhat complicates the use of [11C]MDL 100,907, since compartment modelling requires the metabolite-corrected arterial plasma radioactivity concentration to be determined during each scan. A recent study suggests that noninvasive graphical analysis (NIGA) is comparable with the two-tissue compartment model,253 which greatly enhances the applicability of [11C]MDL 100,907. [11C]MDL 100,907 has so far only been used in a limited number of clinical studies including determination of 5-HT2A receptor binding in patients recovered from depression35 and in patients with obsessive–compulsive disorder.254
9. [18F]MH.MZ and (R)-[18F]MH.MZ
The advantages of [11C]MDL 100,907 over [18F]altanserin include its lack of radio-metabolites, and its higher 5-HT2A receptor selectivity. Its disadvantages include the short half-life of the 11C label. To combine the favorable properties of each ligand, Herth et al.255 developed MH.MZ (FE1-MDL 100,907) ((3-fluoro-ethoxy-2-methoxyphenyl)-1-[2-(4-fluoro-phenyl)ethyl-4-piperidine-methanol), by replacing an O-methyl group within MDL 100,907 with a labelled fluoroethyl moiety. MH.MZ has a lower affinity for the 5-HT2A receptor than MDL 100,907 and altanserin (Ki = 3 nM), but its visualization by autoradiography was good, nonspecific binding was low, and competition studies suggested relatively high 5-HT2A selectivity. The high specificity was later confirmed, as it was shown that [18F]MH.MZ has very high Ki values for a whole range of other receptors including all other serotonergic receptors (the lowest being for 5-HT2C where Ki = 71 nM).256 Following this [18F]MH.MZ was evaluated in vivo and it was found that the radioligand readily entered rat brain and gave a cortex-to-cerebellum ratio of 2.7. It was observed that [18F]MH.MZ undergoes extensive first-pass metabolism, which significantly reduced its bioavailability. Further, its time–activity curves showed a very slow washout from the rat brain. One polar radiometabolite was found in ex vivo homogenates from rat brain, but this was considered to be contamination from blood still located in vessels in the brain rather than from brain gray matter tissue.256 To further improve selectivity and increase affinity, a new series of similar compounds was developed including the enantioselective derivative, (R)-[18F]MH.MZ.257 This compound demonstrated higher affinity (Ki = 0.72 nM), lower nonspecific binding, and higher target-to-background ratio (ratios of between 3.3 and 3.9 in cortex relative to cerebellum), as demonstrated by PET in rats.258 Whether [18F]MH.MZ or (R)-[18F]MH.MZ has better characteristics than the 5-HT2A radioligands already used in human PET still remains to be settled.
10. [11C]CIMBI Compounds
As mentioned above, agonist tracers could potentially enable imaging of the active, high-affinity state of receptors, which may provide a more meaningful assessment of membrane-bound receptors. The first radiolabelled high-affinity 5-HT2A receptor agonist was 2-(4-iodo-2,5-dimethoxyphenyl)-N-(2-[11C-O-methyl]methoxybenzyl)ethanamine ([11C]Cim-bi-5). In the pig brain, [11C]Cimbi-5 showed a cortex-to-cerebellum binding ratio in the same order of magnitude as [18F]altanserin, and it was displaceable by ketanserin in both rats and pigs.259 Subsequently, the in vivo validation of an additional nine novel 5-HT2A receptor agonist PET tracers in the pig brain has been presented,260 and of these, [11C]Cimbi-36 had the most favorable kinetics and the highest target-to-background ratio. This series of compounds seem to be the first promising radioligands for the investigation of 5-HT2A agonist binding in the living human brain.
L. 5-HT2A Radioligands: Conclusions
Five of the above described radioligands for the 5-HT2A receptor have successfully been used in human studies; the SPECT radioligand [123I]-R91150, and the PET radioligands [18F]setoperone, [18F]altanserin, [18F]deuteroaltanserin, and [11C]MDL 100,907.
[123I]-R91150 displays a lower signal-to-noise ratio compared to the available PET radioligands, but the widespread availability of SPECT facilities makes it a practical imaging tool for, e.g., drug occupancy studies, in spite of the lower resolution provided by SPECT.
Of the PET radioligands [18F]altanserin has continued to be most widely used, despite its lipophilic radiometabolite. This use is especially due to its longer lived 18F-label, which enables the application of a bolus/infusion paradigm. Imaging data obtained from [18F]altanserin binding in the human brain are highly reproducible and the large number of publications based on this radioligand provides a convenient reference for new findings. The radioligand [18F]setoperone is less selective than [18F]altanserin and is being used less and less. [11C]MDL 100,907 is a more selective 5-HT2A ligand than [18F]altanserin in vitro, but is much less widely used as a 5-HT2A radioligand for in vivo studies than [18F]altanserin (Table III). The reason for this could be that the modelling issues make [11C]MDL 100,907 less attractive and only time will tell whether the quantification of [11C]MDL 100,907 with NIGA will change this. Similarly, it will be interesting to see whether (and how) imaging 5-HT2A receptors with agonist radioligands differs from that of antagonist radioligands.
M. 5-HT2B Radioligands
The 5-HT2B receptor is predominantly expressed in peripheral tissues such as cardiac and intestinal tissue. 5-HT2B mRNA and protein has been detected in human brain, but only in a few discrete nuclei.261 Although only low levels of 5-HT2B receptors are present in the brain,262 certain selective compounds have started to emerge that may permit further investigation of their function.263 However, no selective radioligands have been developed so far.
N. 5-HT2C Radioligands
Very high levels of the 5-HT2C receptor are found in the epithelial cells of the choroid plexus, but they are also present in gray matter.264–266 Due to the lack of selective radioligands, this receptor is less well studied than its pharmacologically related 5-HT2A receptor. Selective nonlabelled ligands for the 5-HT2C receptor exist, but currently all the labelled 5-HT2C radioligands have shared pharmacology with other receptors (e.g. Hamedah et al.267), and none have been developed for SPECT or PET imaging. This is disappointing given the potential role of this receptor in feeding behavior and mood. Many atypical antipsychotics are potent 5-HT2C antagonists,268 which has been suggested to relate to their propensity to cause weight gain and the new antidepressant agomelatine is thought to act in part by blocking 5HT2C receptors so elevating noradrenaline and dopamine levels in cortex.269
O. 5-HT3 Receptor Radioligands
The 5-HT3 receptor is unique among 5-HT receptors in being a ligand-gated ion channel. Its structure consists of a pentamer of subunits that come together to form a cation channel. Five different subunits have been identified: 3A–E. The 3A subunits have been shown to form functional homomers, the others form hetero-oligomeric pentamers, with the inclusion of a 3A subunit being necessary for functional integrity.270 Located on both central and peripheral neurons, the highest brain levels are found in the dorsal vagal complex of the brain stem, with low levels found in the forebrain.271–274
Growth in the availability of selective ligands for the 5-HT3 receptor came about once the anti-emetic properties of 5-HT3 receptor antagonists were established and is still of interest as an emerging targets in irritable bowel syndrome and alcohol abuse.275,276 Several selective agonists and antagonists are available, of which some have been labelled with 3H or 125I for in vitro studies (e.g. Hewlett et al.277). In all, eight potential PET ligands have been tested in the attempt to create a successful radioligands for 5-HT3 receptors. Six were unsuccessful due to a lack of specific binding and two because of poor brain uptake.
P. 5-HT3 SPECT Radioligands
1. Zacopride Derivatives
Ebert and co-workers developed a series of zacopride derivatives as potential high-affinity 5-HT3 antagonists radiolabelled with 125I.277 Most of these failed due to various reasons such as low 5-HT3 receptor affinity or a mixed pharmacology. One promising analogue [125I]DAIZAC ((S)-5-chloro-3-iodo-2-methoxy-N-(1-azobicyclo-[2.2.2]oct-3-yl)benzamide) displayed high affinity and very high selectivity for the 5-HT3 receptor over a wide range of other CNS receptors tested. Zacopride is a well-known mixed 5-HT3 receptor antagonist and 5-HT4 agonist, but DAIZAC showed a selectivity for 5-HT3 over 5-HT4 receptors >120 times that of (S)-zacopride. Despite these promising features, the ligand has not yet been further developed for SPECT imaging.
Q. 5-HT3 PET Radioligands
1. [11C]MDL 72222
The first candidate PET ligand for imaging the 5-HT3 receptor was [11C]MDL 72222 ((8-methyl-8-azabicyclo[3.2.1]octan-3-yl)3,5-dichlorobenzoate), a selective 5-HT3 receptor antagonist. In the rat brain and baboon brain an initial study showed that [11C]MDL 72222 rapidly crossed the blood–brain barrier and was distributed throughout the brain. Prior treatment with unlabelled MDL 72222 did not displace [11C]MDL 72222 in brain areas with 5-HT3 receptors, suggesting a lack of specific binding, probably due to a combination of its high lipophilicity and the relatively low density of 5-HT3 receptors.278
2. [11C]YM060, [11C]Y-25130, and [11C]KF17643
Ishiwata et al.279,280 radiolabelled several specific 5-HT3 receptor antagonists: Y-25130 (azasetron): N-(1-azabicyclo[2.2.2]oct-3-yl)-6-chloro-4-methyl-3-oxo-3,4-dihydro-2H-1, 4-benzoxazine-8-carboxamide), YM060: (R)-5-[1-methyl-3-indolyl)carbonyl]-4,5,6,7-tetra-hydro-1H-benzimidazole, and KF17643: endo-8-methyl-8-azabicyclo[3.2.1]oct-3-yl 2-(n-propyloxy)-4-quinolinecarboxylate for PET imaging. [11C]Y-25130 and [11C]YM060 brain uptake in mice was low for both compounds, possibly due to low lipophilicity of [11C]Y-25130, or ionization of the tertiary amine or both. In contrast to [11C]Y-25130 and [11C]YM060, [11C]KF17643 exhibited high uptake in mice and appeared metabolically stable. However, no specific binding to the 5-HT3 receptor was detected in mice.
3. [11C]S21007
[11C]S21007 (5-(4-benzylpiperazin-1-yl)4Hpyrrolo[1,2-a]thieno[3,2-e]pyrazine), a 5-HT3 partial agonist with nanomolar affinity, has also been evaluated as a potential PET ligand in rat and baboon.281,282 [11C]S21007 failed to give a specific binding signal from the 5-HT3 receptor in the baboon since blocking with unlabelled S21007 was not associated with any changes in [11C]S21007 binding. Despite favorable kinetics, brain penetration, lack of contamination by labelled metabolites, high affinity, and selectivity [11C]S21007 failed in detecting the 5-HT3 receptors, and this points to the difficulty in measuring the rather low levels of 5-HT3 receptors in the brain.
4. [18F]MR18445
After the failure of the first arylpiperazine derivative, [11C]S21007, MR18445 (4-[4-(4-fluor-obenzyl)piperazino]-7-methoxypyrrolo[1,2-α]quinoxaline) was synthesized and examined as a PET radioligand for 5-HT3 receptor imaging, this time with an 18F label. [18F]MR18445 uptake in rat brain was rapid and high, and there was no evidence of radiometabolites. However, neither autoradiography in rats nor PET studies in baboons revealed any specific binding.283
5. [11C]NMQ
N-[methyl-11C]Methylquipazine ([11C]NMQ, (2-[1-(4-methyl)-piperazinyl]quinoline)), a derivative of quipazine, was examined for suitability as a PET radioligand in rat and nonhuman primate.284 In rat brain uptake and wash-out were very rapid. In the nonhuman primate brain kinetics were similar; initial rapid accumulation was followed by a slower decrease with all regions approaching that of nonspecific binding. Binding was high in 5-HT3 receptor-rich regions, and although prior treatment with cold quipazine decreased binding in the medulla compared to the cerebellum, nonspecific binding was also high. Overall, this suggests that if the specific binding signal could be further improved, an arylpiperazine derivative might become a better radioligand for PET studies of the 5-HT3 receptor than those previously reported.
R. 5-HT3 Radioligands: Conclusions
Despite a number of research centres undertaking a concerted effort to develop 5-HT3 selective PET and SPECT tracers, it seems that the very discrete localization and relatively low levels of 5-HT3 receptors in the brain makes it a very difficult target to image in vivo. Ligands with even higher affinity and/or specific activity and lower nonspecific binding are required if this is to happen. More recently, several new benzoxazole derivatives have been synthesized with a view to PET radioligand development,285 but these have yet to be tested in vivo.
S. 5-HT4 Receptor Tracers
5-HT4 receptors are involved in learning and memory and are potential targets for the treatment of Alzheimer’s disease.286 Positively coupled to adenylate cyclase, downstream functions include regulation of GABA-A currents in cortical neurones, and enhancement of neurotransmitter release (e.g. Ach, DA, 5-HT, GABA). Various radioligands have been used to map them in brain tissue, showing that they are predominantly found in the mesolimbic and nigrostriatal system with highest densities in the caudate nucleus, putamen, nucleus accumbens, globus pallidus, and substantia nigra.287–289 Potential PET ligands are beginning to emerge. So far, only one PET ligand, [11C]SB207145 (8-amino-7-chloro-(N-[11C]methyl-4-piperidylmethyl)-1,4-benzodioxan-5-carboxylate), has been successfully evaluated in humans.290,291 A PET image of 5-HT4 brain receptors obtained in healthy volunteers using [11C]SB207145 is shown in Figure 1.
T. 5-HT4 Radioligands
1. [123I]SB207710
In parallel with the development of the very high affinity 5-HT4 antagonist [3H]GR113808, Brown et al.292 reported the binding characteristics of another potent, selective 5-HT4 radioligand [125I]SB207710 ([125I](1-butylpiperidin-4-yl)methyl-8-amino-7-iodo-2,3-dihy-drobenzo[b][1,4]dioxine-5-carboxylate), and soon after its distribution was mapped by autoradiography in rat brain,293 confirming its potency, selectivity, and correlation with [3H]GR113808 labelled sites. Pike et al.294 characterized its efficacy as a radioligand in vivo, with the perspective of creating a SPECT ligand. In rats, [125I]SB207710 entered the brain but had a rapid clearance rate. Radioactive accumulation correlated with the known in vitro 5-HT4 receptor distribution, with a striatum-to-cerebellum ratio of 3.4. [123I]SB207710 was then examined by SPECT in nonhuman primates. The tracer readily accumulated in 5-HT4 receptor-rich areas, i.e., striatum with a target-to-background ratio of 4. The binding was displaceable by the antagonist SB204070, and cerebellum showed no specific binding. These results indicated that [123I]SB207710 might become a suitable candidate for SPECT studies in humans, but further studies have not yet been published.
2. [11C]SB207145
The 5-HT4 receptor antagonist SB207145 was radiolabelled with 11C by Gee et al.295 and evaluated for its potential as a PET radioligand for 5-HT4 imaging. The first preliminary in vivo data showed that the radioligand readily entered the brain and was distributed according to known 5-HT4 receptor distribution, and it was further shown that binding was sensitive to prior treatment with cold SB207145 or with the structurally dissimilar 5-HT4 antagonist SB207040. A later study confirmed the promising characteristics of the radioligand in the pig brain, and further explored radioligand metabolism and binding kinetics. [11C]SB207145 proved to have slow but reversible kinetics in high-binding brain regions, and using cerebellum as a reference region, the simplified reference tissue model was found to be the model of choice in the pig brain.296 [11C]SB207145 was subsequently successfully evaluated in the human brain290 (Fig. 1). Here a binding potential of 3.1 was reported in caudate nucleus and binding was displaceable by the selective 5-HT4 receptor inverse agonist piboserod (SB207266). In this study, a comprehensive quantification of the binding of [11C]SB207145 to cerebral 5-HT4 receptors in the human brain in vivo was further provided. Estimation of distribution volumes and binding potentials of [11C]SB207145 showed good test–retest reproducibility and time-stability. The blocking study with piboserod confirmed that the cerebellum is a suitable reference region devoid of specific binding, and that non-specific binding is constant across brain regions. Subsequently, it was shown that [11C]SB207145 is not displaceable by increased levels of endogenous 5-HT.291 Together this shows that [11C]SB207145 can be used for quantitative PET measurements of 5-HT4 receptors in the human brain.
U. 5-ht5 Radioligands
Two genes for the 5-ht5 receptor have been described: A and B. The 5-ht5a receptor is expressed in human brain, e.g. in the suprachiasmatic nucleus (SCN), but low levels of expression have made characterization very difficult. There are no selective agonists, but two selective antagonists have recently become available,297 one of which has been shown to alter circadian function in vivo.298 Only mRNA for the 5-ht5b receptor has so far been found in humans and it is thought that no functional protein is produced due to stop codons.299,300 There are no available radioligands for either of the 5-ht5 receptors.
V. 5-HT6 Radioligands
5-HT6 receptors are found exclusively in the CNS and are predominantly expressed in the striatum, limbic system, and cortex.301,302 They are of particular interest because of their involvement in learning and memory.4 Selective radioligands for in vitro studies are available, such as [125I]SB258585, but its development as an in vivo imaging tool was hampered due to poor brain penetration.303 More recently, radioligands for PET imaging of the 5-HT6 receptor have been developed by GSK, of which [11C]GSK215083 3-[(3-fluorophenyl)-sulfonyl]-8-(4-[11C]methyl-1-piperazinyl)quinoline is the most promising.
1. [18F]12ST05
Based on the selective 5-HT6 antagonist SB271046 a novel series of compounds was developed. The specificities of these compounds toward the 5-HT6 receptor were tested.304 This lead to the evaluation of [18F]12ST05 ([18F]-N-[2-(1-[(4-fluorophenyl)sulfonyl]-1H-indol-4-yloxy)ethyl]-N,N-dimethylamine) as the first potential 5-HT6 PET radioligand.305 Synthesis technique, specific activity, and uptake kinetics were all found to be satisfactory, with the radioligand showing excellent brain penetration in rat and cat brain. In vitro, it had high affinity, and ex vivo autoradiography demonstrated high labelling in regions known to contain 5-HT6 receptors, which to some extent was displaceable by unlabelled 12ST05 and SB258585. However, the initial evaluation in vivo in cat did not reveal any specific binding to the cerebral 5-HT6 receptors, and does not seem to have been further developed for PET.305
2. [11C]GSK215083
Recent conference proceedings identified two novel compounds that might be suitable for PET studies of the 5-HT6 receptor. Based on the 3-benzenesulfonyl-8-piperazine-1-yl-quinoline scaffold, [11C]GSK215083 and [11C]GSK224558 (8-(4-[11C]methyl-1-piperazinyl)-3-[(2-(trifluoromethoxy)phenyl)sulfonyl]quinoline) were both investigated in pig brain306,307 and [11C]GSK215083, the more superior ligand, subsequently showed promise in a human PET study.308 It has high affinity for 5-HT6 (in vitro Ki, 0.16 nM), but also has high 5-HT2A affinity (in vitro Ki, 0.79 nM). However, the differential localization of 5-HT2A and 5-HT6 receptors (predominantly cortical and striatal respectively) combined with the ~5-fold difference in affinity meant that it was still possible to discriminate between these two receptor types in vivo. A recent study in nonhuman primates demonstrated this; [11C]GSK215083 binding was preferentially blocked by unlabelled GSK215083 within striatum relative to cortex, suggesting an ~8-fold difference in affinity in vivo. Concerns of a possible mass dosing effect were also addressed. Although BP values are relatively low, [11C]GSK215083 represents the only available 5-HT6 PET radioligand to date.309
W. 5-HT7 Radioligands
5-HT7 receptors are found in several brain regions such as hippocampus, thalamus, and hypothalamus, especially the SCN, where they are thought to be involved in circadian rhythm, sleep, and mood regulation.310,311 Three different splice variants of the human 5-HT7 receptor have so far been found, but they do not seem to differ markedly in their pharmacological profile.312,313 Several potent and selective ligands for the 5-HT7 receptor have been developed, but so far only [11C]DR4446 has been prepared and evaluated in vivo as a potential 5-HT7 PET radioligand, which was unsuccessful. A recent attempt to produce a fluorinated radioligand also failed; the lead compound demonstrated very low specific binding in ex vivo autoradiography.314
1. [11C]DR4446
DR4446 (1-methyl-2a-[4-(4,5,6,7-tetrahydrothieno[3,2-c]pyridin-5-yl)butyl]-2a,3,4,5-tetrahydro-1H-benz[cd]indole-2-one) was shown to possess moderate affinity (Ki = 9.7 nM) and high selectivity for 5-HT7 receptors over other 5-HT receptors.315 [11C]DR4446 was therefore synthesized and tested in the nonhuman primate brain,316 revealing good brain penetrability, metabolic stability, but a minimal specific binding component. No reports on [11C]DR4446 in humans have been published.
X. SERT Tracers
SERT is the serotonin reuptake transporter and is one of three monoamine reuptake transporters in the brain, along with transporters for dopamine (DAT) and noradrenaline (NET).317,318
Because of the interest in SERT generated by the success of its inhibitors in the treatment of depression and anxiety disorders, successful imaging of SERT in living human brain was for a long time the topic of intensive investigation, and several PET and SPECT ligands have been developed for this purpose. A recent, very detailed review of SERT imaging by PET and SPECT can be found in Huang et al.319 so the whole spectrum of radioligands available will not be discussed at great length here.
Many of the ligands initially tested as PET and SPECT radioligands were labelled derivatives of successful antidepressant drugs like the SSRI paroxetine,320 the SNRI venla-faxine,321 and the tricyclics imipramine322 and cyanoimipramine.323 These radiotracers were, however, unsuccessful in vivo, mainly due to insufficient specific to nonspecific binding in the human brain.
Initially, better images of SERT in the human brain came from the cocaine derivative SPECT ligand [123I]β-CIT, and later with the selective but kinetically irreversible PET ligand [11C](+)McN5652. Today, the most successful line of SERT radioligands are those developed from diarylsulfides such as [123I]ADAM, and especially [11C]MADAM, and [11C]DASB. A PET image of 5-HT transporter binding obtained in healthy volunteers using [11C]DASB is shown in Figure 1.
Y. SPECT Radioligands for SERT
1. β-[123I]CIT and Nor-β-[123I]CIT
β-[123I]CIT (2-β-carbomethoxy-3-β-(4-iodophenyl)tropane) is a nonselective monoamine transporter SPECT ligand that has approximately equal affinity for all three transporters. It was developed to improve the metabolic instability of the PET ligand [11C]cocaine, and was evaluated as a SPECT ligand in both nonhuman primates324 and in humans.325 Despite its nonselectivity, β-[123I]CIT has been used for imaging both DAT and SERT, by taking advantage of the differential localization of these transporters (striatum and midbrain, respectively) and the different tracer kinetics in these regions. Various analogues of β-[123I]CIT have been made through modest modifications of the structure, such as labelling at different positions and creation of fluoroalkyl analogues to decrease the time to reach pseudoequilibrium,326 but these efforts were mainly directed at improving its effectiveness for imaging the dopamine reuptake transporter (DAT). The development of nor-β-[123I]CIT (2-β-carbomethoxy-3-β-(4-iodophenyl)nortropane)327,328 produced a radioligand with supposedly increased specific binding for SERT over DAT in humans, but it remains controversial whether nor-β-[123I]CIT is a better radioligand for SERT than β-[123I]CIT.329
Today, several hundred publications (Table II shows publications only related to SERT) using β-[123I]CIT demonstrate the level of success this radioligand has enjoyed especially for measuring the dopamine transporter. β-[123I]CIT and nor-β-[123I]CIT have been used to image SERT density in human midbrain in a number of conditions such as depression,330,331 generalized anxiety disorder,332 obsessive–compulsive disorder,333 panic disorder,334 and Parkinson’s disease,335 among others. The disadvantage of these SPECT ligands is their inability to selectively image SERT.
2. Diarylsulfides
The diarylsulfides are a class of potent SSRIs that so far have given rise to three potential SPECT ligands: [123I]IDAM,336–338 [123I]ODAM,339 and [123I]ADAM.340,341
3. [123I]ADAM
The most successful of the diarylsulfide SPECT radioligands listed above is [123I]ADAM (2-((2-((dimethylamino)methyl)phenyl)thio)-5-iodophenylamine), which has been shown to be potent, selective, and have a high target-to-background ratio in human studies.340–343 It has been used for estimating SERT occupancy of SSRIs344–348 and to demonstrate changes in SERT binding density in depression,349 migraine,350 borderline personality disorder,351 and night eating syndrome.352 Quantification of the SERT binding with [123I]ADAM SPECT is most often done with a ratio method, based on data acquired from 200 to 240 min. This has, however, been shown to overestimate the specific binding by about 10%. The most reliable outcome is based on a 0–120 min SPECT acquisition followed by Logan noninvasive modelling.343
Z. PET Radioligands for SERT
1. [11C]McN5652
The first selective PET radioligand for imaging SERT in the human brain was [11C](+) McN5652 (trans-1,2,3,5,6,10-β-hexahydro-6-[4-(methylthio)phenyl]pyrrolo-[2,1-a]isoquino-line).353 Its use has been limited by a relatively low target-to-background ratio in vivo, as well as a slow brain uptake and irreversible kinetics that complicate quantification in high binding regions.354,355 Despite this, [11C](+)McN5652 PET has been used to investigate SERT binding in ecstasy use,356 impulsive aggression,357 and depression.358 In a series of experiments, the fluoromethyl analogue of [11C](+)McN5652 was developed and evaluated as a 18F-labelled PET radioligand359 and it was concluded that [18F](+)-FMe-McN5652 has better features than [11C](+)McN5652 for SERT imaging with PET. No further studies using [18F](+)-FMe-McN5625 have been forthcoming, perhaps because of the subsequent success of the diarylsulfides.
2. Diarylsulfides
Many PET radioligands for measuring SERT have been developed from the diarylsulfide series including [11C]ADAM,360 [18F]ADAM,361,362 [11C]S-Me-ADAM,363 [11C]DAPA,364 [11C]AFM,360 [11C]AFA,365 [11C]EADAM,366 [11C]DAPP,367 [11C]AFE,368 [11C]MADAM,369 and [11C]DASB.370 All these ligands demonstrated selectivity for SERT, with varying target-to-background ratios and brain kinetics. Following a comparison between [11C]ADAM, [11C]DASB, [11C]DAPA, and [11C]AFM in reference to [11C]McN5652, [11C]DASB emerged as a favourite due to its ability to measure SERT binding within a short scan time.360 Today [11C]DASB and [11C]MADAM have become the most popular SERT PET radioligands.
3. [11C]DASB
[11C]DASB (3-amino-4-(2-dimethylamino-methyl-phenylsulfanyl)-benzonitrile) is one of a series of 11C-labelled arylthiobenzylamines developed by Wilson and Houle.371 [11C]DASB displayed good selectivity (negligible affinity for more than 40 other receptors) and high affinity. Ex vivo studies further demonstrated saturable binding in rats.370 PET studies of [11C]DASB in humans showed that the uptake distribution matched that expected for SERT and binding kinetics could be quantified with reference tissue models.367,372
It was quickly realized that [11C]DASB was a superior PET ligand to [11C](+)McN5652, both in baboons373 and in humans354 but also superior to several other promising new PET ligands.360 Furthermore, test–retest data showed high reproducibility and reliability.374 [11C]DASB PET has since been used to measure SERT occupancy at clinical doses of fluoxetine, citalopram, sertraline, duloxetine, and venlafaxine.32,375–377 These were followed by patient studies, investigating a multitude of conditions including MDMA use,356 depression,378–380 schizophrenia,381 obsessive–compulsive disorder,382 alcoholism,383 and bipolar disorder.384 [11C]DASB binding has further been investigated in healthy individuals in relation to personality traits,385 seasonal changes,386 and familial risk for mood disorders.387
4. [11C]MADAM
After demonstrating promising characteristics in nonhuman primate PET studies,369 [11C]MADAM (N,N-dimethyl-2-(2-amino-4-methylphenylthio)benzylamine) was tested in humans.388 The brain accumulation pattern was consistent with human postmortem SERT binding with [3H]MADAM389 as well as [3H]imipramine, [3H]paroxetine, and [3H]citalopram.390,391 In a test–retest study, [11C]MADAM had excellent reproducibility when done several weeks apart.392 Since then, [11C]MADAM has been used to estimate relative SERT occupancy of citalopram and escitalopram393 and to investigate the relationship between 5-HT1A and SERT binding in healthy young men and women.394–397
5. [18F]ADAM
The 18F-labelled form of ADAM, 4-[18F]ADAM (N,N-dimethyl-2-(2-amino-4-[18F]fluor-ophenylthio)benzylamine) has been synthesized and a synthesis methodology suitable for PET imaging has been reported.398,399 With microPET and autoradiography, [18F]ADAM has a rapid brain uptake and high target-to-background ratios and the specific signal was displaceable by paroxetine.400 Further studies in rat and monkey PET confirmed that bio-distribution, dosimetry, and toxicity data support its investigation in human subjects.401
AA. SERT Radioligands: Conclusions
Several SERT radioligands are available and among these the most successful are [123I]ADAM, [11C]DASB, and [11C]MADAM. The most widely used PET SERT radioligand is [11C]DASB because of its good selectivity, high reproducibility, and simple quantification. The primary reason why new radioligands are still being developed is that the low density of SERT binding sites in neocortex is challenging for accurate measurement. [11C]AFM with its high target-to-background ratio might show improved detection of cortical SERT density, and thus be a valuable addition to the existing SERT radioligands. It would also be valuable to have an 18F-labelled SERT radioligand, and [18F]ADAM shows promise in this respect.
BB. 5-HT Synthesis Radiotracers
Some effort has been devoted toward developing PET tracers for probing presynaptic 5-HT synthesis. 5-HT in the brain is synthesized from dietary tryptophan, and tracer candidates should thus be analogues of tryptophan and follow the 5-HT synthesizing pathway. α-[11C]-methyl-L-tryptophan ([11C]AMT or α[11C]MTrp) and 5-hydroxy-L-[β-11C]tryptophan ([11C]HTP) are substrates in the first and second enzymatic steps in the biosynthesis of 5-HT, and have both been tested for their ability to determine 5-HT synthesis rate in the human brain. A PET image of sites of 5-HT synthesis within brain using [11C]AMT in healthy volunteers is shown in Figure 1.
1. [11C]AMT
[11C]AMT is a 11C-labelled methyl analogue of tryptophan and a substrate for tryptophan hydroxylase. [11C]AMT is not used for protein synthesis and is not catabolized by the monoamine oxidases, and is thus trapped by the 5-HT synthesis pathway.402 The use of [11C]AMT to measure 5-HT synthesis rate was evaluated ex vivo403 and also in vivo in animals.404,405 The first study in humans showed that uptake values were stable within an individual, which was taken as an indication of the potential of [11C]AMT to serve as an index of individual 5-HT synthesis capacity.406 Furthermore, changes in the uptake with age and gender were found to be consistent with previously reported biochemical measurements of 5-HT in brain tissue.407 There are, however, several caveats with the use of [11C]AMT. The signal-to-noise ratio is rather low, there is a time delay between uptake and conversion of the radiotracer,405,408 and in some elegant nonhuman primate studies it has been found that [11C]AMT binding seems to be primarily driven by blood–brain barrier exchange, rather than 5-HT synthesis rate.409 Further, under pathologic conditions, [11C]AMT is metabolized by means of the kynurenine pathway and the measurements thus reflect both pathways.410
Clinically, [11C]AMT has been used to determine changes in the 5-HT synthesis rate during acute changes in mood411 and during antidepressant treatment,412 but its main use is now in the study of pathological conditions such as epilepsy and endocrine tumors where uptake can be marked so it assists in diagnoses.
2. [11C]HTP
Conversion of tryptophan to 5-hydroxytryptophan (5-HTP) is the rate-limiting step in 5-HT synthesis, but neither tryptophan hydroxylase nor amino acid decarboxylase is saturated in the process of 5-HT synthesis. Therefore, in principle, measurement of either enzymatic step may be used to determine the rate of 5-HT synthesis. Further, since the endogenous level of 5-HTP in the brain is normally very low, the rate of decarboxylation to 5-HT is most probably equal to its formation. This has lead to the use of [11C]HTP in PET as a measure of 5-HT synthesis rate.413 In vivo nonhuman primate brain studies showed that following injection of [11C]HTP, the accumulation of its radiometabolite decreases following co-injection with unlabelled 5-HTP414 and increases after co-administration of vitamin B6,415 a cofactor for the decarboxylase, indicating that the radiotracer is sensitive to pharmacological challenges. In humans, [11C]HTP utilization rates were shown to be region-dependent and could be altered by serotonergic pharmacological challenges,416 suggesting that data from PET studies using [11C]HTP might be interpreted as a measure of brain 5-HT turnover. A later study showed that human PET data from this tracer could be reliably modelled when using a model that included irreversible trapping of the tracer.417 As for [11C]AMT, the majority of studies using [11C]HTP are in diagnostic investigations in pathological conditions rather than investigating 5-HT synthesis per se.
[11C]AMT and [11C]HTP binding were found to correlate in some postmortem studies,417,418 but not in a head-to head comparison study in rhesus monkeys.419 More in vivo validation studies are required if firm conclusions are to be drawn about the suitability of these tracers for estimating 5-HT synthesis.
4. CONCLUSIONS
Currently, well-validated radiotracers for imaging the 5-HT system exist as antagonist radioligands for the 5-HT1A, 5-HT1B, 5-HT2A, and 5-HT4 receptors, and for SERT.
Three radioligands are currently used for PET studies of the 5-HT1A receptor: [11C]WAY100635, [18F]MPPF, and [18F]FCWAY. There are four suitable tracers for the 5-HT2A receptor: the SPECT tracer [123I]5-I-R91150, the nonselective PET tracer [18F]setoperone, and the selective tracers [18F]altanserin and [11C]MDL 100,907. Several SERT radioligands exist and among these the most successful are [123I]ADAM, [11C]DASB, and [11C]MADAM.
Several promising radioligands are currently emerging. Of these, the two 5-HT1B ligands [11C]AZ10419369 and [11C]P943, the 5-HT4 receptor radioligand [11C]SB207145, and the 5-HT6 receptor radioligand [11C]GSK215083 are the only ones where successful evaluations in humans have been published so far.
Unfortunately, despite significant investment and many failed attempts, there are currently no PET or SPECT radioligands for the study of 5-HT3 receptor in humans. In the past, a lack of selective ligands appears to be the reason for the lack of radioligand development programs for 5-HT1D/e/F, 5-HT2B/C, 5-HT5, and 5-HT7 receptors. However, there are now a number of selective ligands available for many of these targets, which should facilitate renewed effort to tracer development at these targets. Emerging functions for these receptors make them interesting targets for future study with in vivo brain imaging.
From the present review it is clear that development of radioligands for in vivo brain imaging in humans is a long process of trial and error. Despite substantial effort to predict the usefulness of a potential radioligand for in vivo brain studies, most radiotracers fail during tracer development. Among the main reasons for this are insufficient blood–brain barrier passage, too high nonspecific binding, inability to displace the binding of the radioligand with an unlabelled target-specific ligand, production of lipophilic radiometabolites, or kinetic properties that complicate quantification. Preclinical evaluations in both rats and larger species are useful for selecting the best candidates, but often species differences make it difficult to directly translate results from animals to the human situation.28 Therefore, one cannot firmly conclude on the suitability of a radioligand for human studies before a thorough evaluation in humans has been performed, including blocking, modelling, and test–retest studies.
It is also evident from this review that once a radioligand for imaging of a serotonergic target has been successfully evaluated, the interest in using the radioligand for studies of a whole range of human conditions and for establishing receptor occupancy from clinical doses of drug compounds is huge. In addition, the potential use of agonist tracers for imaging “functional” receptors in vivo may provide us with additional information that cannot be obtained using antagonist tracers. They may also be more susceptible to changes in endogenous neurotransmitter, a property that may provide new avenues to understanding disease. With this in mind we strongly encourage the continuing efforts to develop radioligands for imaging of serotonergic targets.
Acknowledgments
V.W.P. is supported by the Intramural Research Program of the National Institutes of Health (NIMH). The authors acknowledge the Lundbeck Foundation and H. Lundbeck A/S for providing the financial support that enabled this review to be written and the METPETS (Measuring Endogenous Neurotransmitters by PET and SPECT) consortium members for sharing their recent research. The METPETS consortium is a collaborative network of European neuroscientists who have come together with the aim of developing tracers to image neurotransmitter release in the human brain. See http://www.p1vital.com/consortium_metpets.html.
Biographies
Dr. Louise M. Paterson trained in Pharmacology at the University of Bristol, UK, where she obtained her B.Sc. (2000). She continued her studies at Bristol and was awarded a Ph.D. in Pharmacology (2004), characterizing the imidazoline-2 binding site and its relationship with monoamine oxidase. This was followed by a series of post-doctoral positions in translational pharmacology at the University of Bristol between 2004 and 2009. These projects combined pre-clinical laboratory work with healthy volunteer and patient studies, developing her expertise in sleep and depression and the involvement of the 5-HT system. She recently moved to Imperial College London, where she is pursuing her interest in combining translational medicine with human brain imaging. She has recently published a critical review of the use of PET and SPECT to measure the release of endogenous 5-HT.
Dr. Birgitte R. Kornum gained a B.Sc. degree in Chemical Engineering from the Danish Technical University (2000) and continued her training at the University of Copenhagen, where she gained an M.Sc. degree in Human Biology (2003) and a Ph.D. degree from the School of Neuroscience (2009). Her Ph.D. project focussed on using the pig as a model in brain research, and using this model she, among other things, performed in vitro and in vivo evaluations of new radiotracers for PET imaging. She now holds a post doc position at Stanford Center for Sleep Sciences.
Professor David J. Nutt (DM, FRCP, FRCPsych, FMedSci) received his undergraduate training in medicine at Cambridge and Guy’s Hospital, and continued training in neurology to MRCP. After completing psychiatric training in Oxford, he continued as a lecturer and later as a Wellcome Senior Fellow in psychiatry. He then spent two years as Chief of the Section of Clinical Science in the National Institute of Alcohol Abuse and Alcoholism in NIH, Bethesda. In 1988, he returned to the UK to set up the Psychopharmacology Unit at the University of Bristol, an interdisciplinary research group spanning Psychiatry and Pharmacology before moving to Imperial College London in 2008 where he is currently the Edmund J. Safra Professor of Neuropsychopharmacology and Director of the Neuropsychopharmacology Unit. His main research interests are in merging pharmacological approaches with brain imaging techniques, in particular PET imaging of brain disorders such as addiction and affective disorders.
Dr. Victor W. Pike trained as a chemist at the University of Birmingham (UK), where he gained his B.Sc. (1972) and Ph.D. degrees (1976). After a postdoctoral position, he entered a Staff Scientist position at the MRC Cyclotron Unit (Hammersmith Hospital/Imperial College, UK) in 1978 where he later became Head of its Chemistry and Engineering Section. In 2001 he moved to the National Institutes of Health (Bethesda, Maryland) to become Chief of the PET Radiopharmaceutical Sciences Section in the newly established Molecular Imaging Branch of the National Institute of Mental Health, where he has continued his primary interests in all aspects of PET radiotracer development, and especially radiotracers for brain imaging.
Professor Gitte M. Knudsen received her training as a neurologist at the University hospitals in Copenhagen, and obtained her D.M.Sc. degree in 1994 (Application of the double-indicator technique for measurement of blood-brain barrier permeability in humans). She subsequently conducted research within cerebrovascular regulation and since 1998 her main research interest has been within molecular brain imaging, in particular of the 5-HT system. She is the Director of the Neurobiology Research Unit (NRU) and of the Center for Integrated Molecular Brain Imaging (Cimbi) and is employed at Rigshospitalet and Professor at the University of Copenhagen.
Footnotes
This review was produced as part of the remit of the METPETS (Measuring endogenous neurotransmitters with PET and SPECT) consortium.
References
- 1.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 2.Monti JM. The role of dorsal raphe nucleus serotonergic and non-serotonergic neurons, and of their receptors, in regulating waking and rapid eye movement (REM) sleep. Sleep Med Rev. 2010;14:319–327. doi: 10.1016/j.smrv.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Schmitt JA, Wingen M, Ramaekers JG, Evers EA, Riedel WJ. Serotonin and human cognitive performance. Curr Pharm Des. 2006;12:2473–2486. doi: 10.2174/138161206777698909. [DOI] [PubMed] [Google Scholar]
- 4.King MV, Marsden CA, Fone KC. A role for the 5-HT(1A), 5-HT4 and 5-HT6 receptors in learning and memory. Trends Pharmacol Sci. 2008;29:482–492. doi: 10.1016/j.tips.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Millan MJ. Multi-target strategies for the improved treatment of depressive states: Conceptual foundations and neuronal substrates, drug discovery and therapeutic application. Pharmacol Ther. 2006;110:135–370. doi: 10.1016/j.pharmthera.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Stein DJ, Stahl S. Serotonin and anxiety: Current models. Int Clin Psychopharmacol. 2000;15:S1–S6. doi: 10.1097/00004850-200008002-00002. [DOI] [PubMed] [Google Scholar]
- 7.Remington G. Alterations of dopamine and serotonin transmission in schizophrenia. Prog Brain Res. 2008;172:117–140. doi: 10.1016/S0079-6123(08)00906-0. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Alloza M, Gil-Bea FJ, ez-Ariza M, Chen CP, Francis PT, Lasheras B, Ramirez MJ. Cholinergic-serotonergic imbalance contributes to cognitive and behavioral symptoms in Alzheimer’s disease. Neuropsychologia. 2005;43:442–449. doi: 10.1016/j.neuropsychologia.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Michelsen KA, Prickaerts J, Steinbusch HW. The dorsal raphe nucleus and serotonin: Implications for neuroplasticity linked to major depression and Alzheimer’s disease. Prog Brain Res. 2008;172:233–264. doi: 10.1016/S0079-6123(08)00912-6. [DOI] [PubMed] [Google Scholar]
- 10.Jobe PC, Dailey JW, Wernicke JF. A noradrenergic and serotonergic hypothesis of the linkage between epilepsy and affective disorders. Crit Rev Neurobiol. 1999;13:317–356. doi: 10.1615/critrevneurobiol.v13.i4.10. [DOI] [PubMed] [Google Scholar]
- 11.Veltman DJ, Ruhe HG, Booij J. Investigating serotonergic function using positron emission tomography: Overview and recent findings. Curr Pharm Des. 2010;16:1979–1989. doi: 10.2174/138161210791293033. [DOI] [PubMed] [Google Scholar]
- 12.Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP. International Union of Pharmacology classification of receptors for 5-hydroxy-tryptamine (serotonin) Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- 13.Bockaert J, Claeysen S, Becamel C, Dumuis A, Marin P. Neuronal 5-HT metabotropic receptors: Fine-tuning of their structure, signaling, and roles in synaptic modulation. Cell Tissue Res. 2006;326:553–572. doi: 10.1007/s00441-006-0286-1. [DOI] [PubMed] [Google Scholar]
- 14.Fink KB, Gothert M. 5-HT receptor regulation of neurotransmitter release. Pharmacol Rev. 2007;59:360–417. doi: 10.1124/pr.107.07103. [DOI] [PubMed] [Google Scholar]
- 15.Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behav Brain Res. 2008;195:198–213. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 16.Wagner HN, Jr, Burns HD, Dannals RF, Wong DF, Langstrom B, Duelfer T, Frost JJ, Ravert HT, Links JM, Rosenbloom SB, Lukas SE, Kramer AV, Kuhar MJ. Imaging dopamine receptors in the human brain by positron tomography. Science. 1983;221:1264–1266. doi: 10.1126/science.6604315. [DOI] [PubMed] [Google Scholar]
- 17.Kaye WH, Frank GK, Bailer UF, Henry SE, Meltzer CC, Price JC, Mathis CA, Wagner A. Serotonin alterations in anorexia and bulimia nervosa: New insights from imaging studies. Physiol Behav. 2005;85:73–81. doi: 10.1016/j.physbeh.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 18.Fowler JS, Ding YS, Volkow ND. Radiotracers for positron emission tomography imaging. Semin Nucl Med. 2003;33:14–27. doi: 10.1053/snuc.2003.127297. [DOI] [PubMed] [Google Scholar]
- 19.Volkow ND, Fowler JS, Wang GJ. Positron emission tomography and single-photon emission computed tomography in substance abuse research. Semin Nucl Med. 2003;33:114–128. doi: 10.1053/snuc.2003.127300. [DOI] [PubMed] [Google Scholar]
- 20.Smith DF, Jakobsen S. Molecular tools for assessing human depression by positron emission tomography. Eur Neuropsychopharmacol. 2009;19:611–628. doi: 10.1016/j.euroneuro.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Phelps ME, Huang SC, Hoffman EJ, Selin C, Sokoloff L, Kuhl DE. Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-D-glucose: Validation of method. Ann Neurol. 1979;6:371–388. doi: 10.1002/ana.410060502. [DOI] [PubMed] [Google Scholar]
- 22.Friedland RP, Budinger TF, Ganz E, Yano Y, Mathis CA, Koss B, Ober BA, Huesman RH, Derenzo SE. Regional cerebral metabolic alterations in dementia of the Alzheimer type: Positron emission tomography with [18F]fluorodeoxyglucose. J Comput Assist Tomogr. 1983;7:590–598. doi: 10.1097/00004728-198308000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Hasselbalch SG, Knudsen GM, Videbaek C, Pinborg LH, Schmidt JF, Holm S, Paulson OB. No effect of insulin on glucose blood-brain barrier transport and cerebral metabolism in humans. Diabetes. 1999;48:1915–1921. doi: 10.2337/diabetes.48.10.1915. [DOI] [PubMed] [Google Scholar]
- 24.Foster NL, Wang AY, Tasdizen T, Fletcher PT, Hoffman JM, Koeppe RA. Realizing the potential of positron emission tomography with 18F-fluorodeoxyglucose to improve the treatment of Alzheimer’s disease. Alzheimers Dement. 2008;4:S29–S36. doi: 10.1016/j.jalz.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Kessler RM, Goble JC, Bird JH, Girton ME, Doppman JL, Rapoport SI, Barranger JA. Measurement of blood-brain barrier permeability with positron emission tomography and [68Ga]EDTA. J Cereb Blood Flow Metab. 1984;4:323–328. doi: 10.1038/jcbfm.1984.48. [DOI] [PubMed] [Google Scholar]
- 26.Josserand V, Pelerin H, de Bruin B, Jego B, Kuhnast B, Hinnen F, Duconge F, Boisgard R, Beuvon F, Chassoux F, Dumas-Duport C, Ezan E, Dolle F, Mabondzo A, Tavitian B. Evaluation of drug penetration into the brain: A double study by in vivo imaging with positron emission tomography and using an in vitro model of the human blood-brain barrier. J Pharmacol Exp Ther. 2006;316:79–86. doi: 10.1124/jpet.105.089102. [DOI] [PubMed] [Google Scholar]
- 27.Bartels AL, Willemsen AT, Kortekaas R, de Jong BM, de Vries R, de Klerk O, Van Oostrom JC, Portman A, Leenders KL. Decreased blood-brain barrier P-glycoprotein function in the progression of Parkinson’s disease, PSP and MSA. J Neural Transm. 2008;115:1001–1009. doi: 10.1007/s00702-008-0030-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Syvänen S, Lindhe O, Palner M, Kornum BR, Rahman O, Langstrom B, Knudsen GM, Hammarlund-Udenaes M. Species differences in blood-brain barrier transport of three PET radioligands with emphasis on P-glycoprotein transport. Drug Metab Dispos. 2009;37:635–643. doi: 10.1124/dmd.108.024745. [DOI] [PubMed] [Google Scholar]
- 29.Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: A critical review. J Cereb Blood Flow Metab. 2000;20:423–451. doi: 10.1097/00004647-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Paterson LM, Tyacke RJ, Nutt DJ, Knudsen GM. Measuring endogenous 5-HT release by emission tomography: Promises and pitfalls. J Cereb Blood Flow Metab. 2010;30:1682–1706. doi: 10.1038/jcbfm.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farde L, Nordstrom AL, Wiesel FA, Pauli S, Halldin C, Sedvall G. Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Relation to extrapyramidal side effects. Arch Gen Psychiatry. 1992;49:538–544. doi: 10.1001/archpsyc.1992.01820070032005. [DOI] [PubMed] [Google Scholar]
- 32.Meyer JH, Wilson AA, Ginovart N, Goulding V, Hussey D, Hood K, Houle S. Occupancy of serotonin transporters by paroxetine and citalopram during treatment of depression: A [(11)C]DASB PET imaging study. Am J Psychiatry. 2001;158:1843–1849. doi: 10.1176/appi.ajp.158.11.1843. [DOI] [PubMed] [Google Scholar]
- 33.Lanzenberger RR, Mitterhauser M, Spindelegger C, Wadsak W, Klein N, Mien LK, Holik A, Attarbaschi T, Mossaheb N, Sacher J, Geiss-Granadia T, Kletter K, Kasper S, Tauscher J. Reduced serotonin-1A receptor binding in social anxiety disorder. Biol Psychiatry. 2007;61:1081–1089. doi: 10.1016/j.biopsych.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 34.Nash JR, Sargent PA, Rabiner EA, Hood SD, Argyropoulos SV, Potokar JP, Grasby PM, Nutt DJ. Serotonin 5-HT1A receptor binding in people with panic disorder: Positron emission tomography study. Br J Psychiatry. 2008;193:229–234. doi: 10.1192/bjp.bp.107.041186. [DOI] [PubMed] [Google Scholar]
- 35.Bhagwagar Z, Hinz R, Taylor M, Fancy S, Cowen P, Grasby P. Increased 5-HT(2A) receptor binding in euthymic, medication-free patients recovered from depression: A positron emission study with [(11)C]MDL 100,907. Am J Psychiatry. 2006;163:1580–1587. doi: 10.1176/ajp.2006.163.9.1580. [DOI] [PubMed] [Google Scholar]
- 36.Verdurand M, Berod A, Le Bars D, Zimmer L. Effects of amyloid-beta peptides on the serotoninergic 5-HT(1A) receptors in the rat hippocampus. Neurobiol Aging. 2011;32:103–114. doi: 10.1016/j.neurobiolaging.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Narendran R, Hwang DR, Slifstein M, Talbot PS, Erritzoe D, Huang Y, Cooper TB, Martinez D, Kegeles LS, Abi-Dargham A, Laruelle M. In vivo vulnerability to competition by endogenous dopamine: Comparison of the D2 receptor agonist radiotracer (−)-N-[11C]propyl-norapomorphine ([11C]NPA) with the D2 receptor antagonist radiotracer [11C]-raclopride. Synapse. 2004;52:188–208. doi: 10.1002/syn.20013. [DOI] [PubMed] [Google Scholar]
- 38.Narendran R, Mason NS, Laymon CM, Lopresti BJ, Velasquez ND, May MA, Kendro S, Martinez D, Mathis CA, Frankle WG. A comparative evaluation of the dopamine D(2/3) agonist radiotracer [11C](−)-N-propyl-norapomorphine and antagonist [11C]raclopride to measure amphetamine-induced dopamine release in the human striatum. J Pharmacol Exp Ther. 2010;333:533–539. doi: 10.1124/jpet.109.163501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pike VW. PET radiotracers: Crossing the blood-brain barrier and surviving metabolism. Trends Pharmacol Sci. 2009;30:431–440. doi: 10.1016/j.tips.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pinborg LH, Adams KH, Svarer C, Holm S, Hasselbalch SG, Haugbol S, Madsen J, Knudsen GM. Quantification of 5-HT2A receptors in the human brain using [18F]altanserin-PET and the bolus/infusion approach. J Cereb Blood Flow Metab. 2003;23:985–996. doi: 10.1097/01.WCB.0000074092.59115.23. [DOI] [PubMed] [Google Scholar]
- 41.Lanfumey L, Hamon M. 5-HT1 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3:1–10. doi: 10.2174/1568007043482570. [DOI] [PubMed] [Google Scholar]
- 42.Akimova E, Lanzenberger R, Kasper S. The serotonin-1A receptor in anxiety disorders. Biol Psychiatry. 2009;66:627–635. doi: 10.1016/j.biopsych.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 43.Pike VW, Halldin C, Wikstrom HV. Radioligands for the study of brain 5-HT1A receptors in vivo. Prog Med Chem. 2001;38:189–247. doi: 10.1016/s0079-6468(08)70094-8. [DOI] [PubMed] [Google Scholar]
- 44.Passchier J, van Waarde A. Visualisation of serotonin-1A (5-HT1A) receptors in the central nervous system. Eur J Nucl Med. 2001;28:113–129. doi: 10.1007/s002590000394. [DOI] [PubMed] [Google Scholar]
- 45.Kumar JS, Mann JJ. PET tracers for 5-HT(1A) receptors and uses thereof. Drug Discov Today. 2007;12:748–756. doi: 10.1016/j.drudis.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 46.Wooten D, Moraino J, Hillmer A, Engle J, Dejesus O, Murali D, Barnhart T, Nickles R, Davidson R, Schneider M, Mukherjee J, Christian B. In vivo kinetics of [F-18]mefway: A comparison with [C-11]WAY100635 and [F-18]MPPF in the nonhuman primate. Synapse. 2011;65:592–600. doi: 10.1002/syn.20878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lemoine L, Verdurand M, Vacher B, Blanc E, Le Bars D, Newman-Tancredi A, Zimmer L. [(18)F]F15599, a novel 5-HT(1A) receptor agonist, as a radioligand for PET neuroimaging. Eur J Nucl Med Mol Imaging. 2010;37:594–605. doi: 10.1007/s00259-009-1274-y. [DOI] [PubMed] [Google Scholar]
- 48.Milak MS, Severance AJ, Ogden RT, Prabhakaran J, Kumar JS, Majo VJ, Mann JJ, Parsey RV. Modeling considerations for 11C-CUMI-101, an agonist radiotracer for imaging serotonin 1A receptor in vivo with PET. J Nucl Med. 2008;49:587–596. doi: 10.2967/jnumed.107.046540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kung MP, Zhuang ZP, Frederick D, Kung HF. In vivo binding of [123I]4-(2′-methoxyphenyl)-1-[2′-(N-2″-pyridinyl)-p-iodobenzamido-]ethyl-piperazine, p-MPPI, to 5-HT1A receptors in rat brain. Synapse. 1994;18:359–366. doi: 10.1002/syn.890180412. [DOI] [PubMed] [Google Scholar]
- 50.Kung HF, Frederick D, Kim HJ, McElgin W, Kung MP, Mu M, Mozley PD, Vessotskie JM, Stevenson DA, Kushner SA, Zhuang ZP. In vivo SPECT imaging of 5-HT1A receptors with [123I]p-MPPI in nonhuman primates. Synapse. 1996;24:273–281. doi: 10.1002/(SICI)1098-2396(199611)24:3<273::AID-SYN10>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 51.Zhuang ZP, Kung MP, Mu M, Kung HF. Isoindol-1-one analogues of 4-(2′-methoxyphenyl)-1-[2′-[N-(2″-pyridyl)-p-iodobenzamido]ethyl]pipera zine (p-MPPI) as 5-HT1A receptor ligands. J Med Chem. 1998;41:157–166. doi: 10.1021/jm970296s. [DOI] [PubMed] [Google Scholar]
- 52.Passchier J, van Waarde A, Doze P, Elsinga PH, Vaalburg W. Influence of P-glycoprotein on brain uptake of [18F]MPPF in rats. Eur J Pharmacol. 2000;407:273–280. doi: 10.1016/s0014-2999(00)00752-4. [DOI] [PubMed] [Google Scholar]
- 53.Heimbold I, Drews A, Syhre R, Kretzschmar M, Pietzsch HJ, Johannsen B. A novel technetium-99m radioligand for the 5-HT(1A) receptor derived from desmethyl-WAY-100635 (DWAY) Eur J Nucl Med Mol Imaging. 2002;29:82–87. doi: 10.1007/s00259-001-0660-x. [DOI] [PubMed] [Google Scholar]
- 54.Park SH, Gwon HJ, Jang SH, Lee H. Highly efficient synthesis and imaging studies of an arylpiperazine derivative as a 5-HT1A receptor imaging agent. Chem Lett. 2006;35:1304–1305. [Google Scholar]
- 55.Zhang XZ, Zhou PW, Liu JJ, Huang Y, Lin Y, Chen YL, Gu T, Yang WJ, Wang XB. Preparation and biodistribution of Tc-99-tricarbonyl complex with 4-[(2-methoxyphenyl)piperazin-1-yl]-dithioformate as a potential 5-HT1A receptor imaging agent. Appl Radiat Isot. 2007;65:287–292. doi: 10.1016/j.apradiso.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 56.Wang FL, Wang XB, Yang SY, Liu J, Zhang XZ. N-[3-[4-(2-methoxyphenyl) piperaziny-1-yl]propyl]cyclam: Synthesized as a potential 5-HT1A receptor ligand and labelled with Tc-99m-nitrido core. J Labl Compd Radiopharm. 2008;51:347–351. [Google Scholar]
- 57.Choi KH, Pyun MS, Hong YD, Choi SJ. Novel Tc-99m(CO)(3) complexes with WAY-100635 moiety for the development of 5-HT1A receptor imaging agent. Bull Korean Chem Soc. 2009;30:1107–1112. [Google Scholar]
- 58.Fan WW, Lin Y, Zhang XZ, Pang Y, Ma C, Tang ZG, Zhang JB, Wang XB. Synthesis and biological evaluation of Tc-99m-HEDTA/HYNIC-MPP4 complex for 5-HT1A receptor imaging. Sci China Ser B Chem. 2009;52:590–598. [Google Scholar]
- 59.Fletcher A, Forster EA, Bill DJ, Brown G, Cliffe IA, Hartley JE, Jones DE, McLenachan A, Stanhope KJ, Critchley DJ, Childs KJ, Middlefell VC, Lanfumey L, Corradetti R, Laporte AM, Gozlan H, Hamon M, Dourish CT. Electrophysiological, biochemical, neurohormonal and behavioural studies with WAY-100635, a potent, selective and silent 5-HT1A receptor antagonist. Behav Brain Res. 1996;73:337–353. doi: 10.1016/0166-4328(96)00118-0. [DOI] [PubMed] [Google Scholar]
- 60.Chemel BR, Roth BL, Armbruster B, Watts VJ, Nichols DE. WAY-100635 is a potent dopamine D4 receptor agonist. Psychopharmacology. 2006;188:244–251. doi: 10.1007/s00213-006-0490-4. [DOI] [PubMed] [Google Scholar]
- 61.Laporte AM, Lima L, Gozlan H, Hamon M. Selective in vivo labelling of brain 5-HT1A receptors by [3H]WAY 100635 in the mouse. Eur J Pharmacol. 1994;271:505–514. doi: 10.1016/0014-2999(94)90812-5. [DOI] [PubMed] [Google Scholar]
- 62.Hall H, Lundkvist C, Halldin C, Farde L, Pike VW, McCarron JA, Fletcher A, Cliffe IA, Barf T, Wikstrom H, Sedvall G. Autoradiographic localization of 5-HT1A receptors in the post-mortem human brain using [3H]WAY-100635 and [11C]way-100635. Brain Res. 1997;745:96–108. doi: 10.1016/s0006-8993(96)01131-6. [DOI] [PubMed] [Google Scholar]
- 63.Burnet PW, Eastwood SL, Harrison PJ. [3H]WAY-100635 for 5-HT1A receptor autoradiography in human brain: A comparison with [3H]8-OH-DPAT and demonstration of increased binding in the frontal cortex in schizophrenia. Neurochem Int. 1997;30:565–574. doi: 10.1016/s0197-0186(96)00124-6. [DOI] [PubMed] [Google Scholar]
- 64.Mathis CA, Simpson NR, Mahmood K, Kinahan PE, Mintun MA. [11C]WAY 100635: A radioligand for imaging 5-HT1A receptors with positron emission tomography. Life Sci. 1994;55:PL403–PL407. doi: 10.1016/0024-3205(94)00324-6. [DOI] [PubMed] [Google Scholar]
- 65.Pike VW, McCarron JA, Lammerstma AA, Hume SP, Poole K, Grasby PM, Malizia A, Cliffe IA, Fletcher A, Bench CJ. First delineation of 5-HT1A receptors in human brain with PET and [11C]WAY-100635. Eur J Pharmacol. 1995;283:R1–R3. doi: 10.1016/0014-2999(95)00438-q. [DOI] [PubMed] [Google Scholar]
- 66.Osman S, Lundkvist C, Pike VW, Halldin C, McCarron JA, Swahn CG, Ginovart N, Luthra SK, Bench CJ, Grasby PM, Wikstrom H, Barf T, Cliffe IA, Fletcher A, Farde L. Characterization of the radioactive metabolites of the 5-HT1A receptor radioligand, [O-methyl-11C]WAY-100635, in monkey and human plasma by HPLC: Comparison of the behaviour of an identified radioactive metabolite with parent radioligand in monkey using PET. Nucl Med Biol. 1996;23:627–634. doi: 10.1016/0969-8051(96)00061-3. [DOI] [PubMed] [Google Scholar]
- 67.Osman S, Lundkvist C, Pike VW, Halldin C, McCarron JA, Swahn C-G, Farde L, Ginovart N, Luthra SK, Gunn RN, Bench CJ, Sargent PA, Grasby PM. Characterisation of the appearance of radioactive metabolites in monkey and human plasma from the 5-HT1A receptor radioligand, [carbonyl-11C]WAY-100635—Explanation of high signal contrast in PET and an aid to biomathematical modelling. Nucl Med Biol. 1998;25:215–223. doi: 10.1016/s0969-8051(97)00206-0. [DOI] [PubMed] [Google Scholar]
- 68.Rabiner EA, Messa C, Sargent PA, Husted-Kjaer K, Montgomery A, Lawrence AD, Bench CJ, Gunn RN, Cowen P, Grasby PM. A database of [(11)C]WAY-100635 binding to 5-HT(1A) receptors in normal male volunteers: Normative data and relationship to methodological, demographic, physiological, and behavioral variables. Neuroimage. 2002;15:620–632. doi: 10.1006/nimg.2001.0984. [DOI] [PubMed] [Google Scholar]
- 69.Sullivan GM, Ogden RT, Oquendo MA, Kumar JS, Simpson N, Huang YY, Mann JJ, Parsey RV. Positron emission tomography quantification of serotonin-1A receptor binding in medication-free bipolar depression. Biol Psychiatry. 2009;66:223–230. doi: 10.1016/j.biopsych.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bailer UF, Frank GK, Henry SE, Price JC, Meltzer CC, Mathis CA, Wagner A, Thornton L, Hoge J, Ziolko SK, Becker CR, McConaha CW, Kaye WH. Exaggerated 5-HT1A but normal 5-HT2A receptor activity in individuals ill with anorexia nervosa. Biol Psychiatry. 2007;61:1090–1099. doi: 10.1016/j.biopsych.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 71.Gunn RN, Sargent PA, Bench CJ, Rabiner EA, Osman S, Pike VW, Hume SP, Grasby PM, Lammertsma AA. Tracer kinetic modeling of the 5-HT1A receptor ligand [carbonyl-11C]WAY-100635 for PET. Neuroimage. 1998;8:426–440. doi: 10.1006/nimg.1998.0379. [DOI] [PubMed] [Google Scholar]
- 72.Parsey RV, Arango V, Olvet DM, Oquendo MA, Van Heertum RL, John Mann J. Regional heterogeneity of 5-HT1A receptors in human cerebellum as assessed by positron emission tomography. J Cereb Blood Flow Metab. 2005;25:785–793. doi: 10.1038/sj.jcbfm.9600072. [DOI] [PubMed] [Google Scholar]
- 73.Hirvonen J, Kajander J, Allonen T, Oikonen V, Nagren K, Hietala J. Measurement of serotonin 5-HT1A receptor binding using positron emission tomography and [carbonyl-(11)C]WAY-100635-considerations on the validity of cerebellum as a reference region. J Cereb Blood Flow Metab. 2007;27:185–195. doi: 10.1038/sj.jcbfm.9600326. [DOI] [PubMed] [Google Scholar]
- 74.Moller M, Rodell A, Gjedde A. Parametric mapping of 5HT1A receptor sites in the human brain with the hypotime method: Theory and normal values. J Nucl Med. 2009;50:1229–1236. doi: 10.2967/jnumed.108.053322. [DOI] [PubMed] [Google Scholar]
- 75.Houle S, Wilson AA, Inaba T, Fisher N, DaSilva JN. Imaging 5-HT1A receptors with positron emission tomography: Initial human studies with [11C]CPC-222. Nucl Med Commun. 1997;18:1130–1134. doi: 10.1097/00006231-199712000-00004. [DOI] [PubMed] [Google Scholar]
- 76.Houle S, DaSilva JN, Wilson AA. Imaging the 5-HT(1A) receptors with PET: WAY-100635 and analogues. Nucl Med Biol. 2000;27:463–466. doi: 10.1016/s0969-8051(00)00112-8. [DOI] [PubMed] [Google Scholar]
- 77.Yasuno F, Zoghbi SS, McCarron JA, Hong J, Ichise M, Brown AK, Gladding RL, Bacher JD, Pike VW, Innis RB. Quantification of serotonin 5-HT1A receptors in monkey brain with [11C](R)-(−)-RWAY. Synapse. 2006;60:510–520. doi: 10.1002/syn.20327. [DOI] [PubMed] [Google Scholar]
- 78.McCarron JA, Zoghbi SS, Shetty HU, Vermeulen ES, Wikstrom HV, Ichise M, Yasuno F, Halldin C, Innis RB, Pike VW. Synthesis and initial evaluation of [11C](R)-RWAY in monkey—A new, simply labeled antagonist radioligand for imaging brain 5-HT1A receptors with PET. Eur J Nucl Med Mol Imaging. 2007;34:1670–1682. doi: 10.1007/s00259-007-0460-z. [DOI] [PubMed] [Google Scholar]
- 79.Zhang XY, Yasuno F, Zoghbi SS, Liow JS, Hong J, McCarron JA, Pike VW, Innis RB. Quantification of serotonin 5-HT1A receptors in humans with [11C](R)-(−)-RWAY: Radio-metabolite(s) likely confound brain measurements. Synapse. 2007;61:469–477. doi: 10.1002/syn.20392. [DOI] [PubMed] [Google Scholar]
- 80.Pike VW, Halldin C, McCarron JA, Lundkvist C, Hirani E, Olsson H, Hume SP, Karlsson P, Osman S, Swahn CG, Hall H, Wikstrom H, Mensonidas M, Poole KG, Farde L. [carbonyl-11C]Desmethyl-WAY-100635 (DWAY) is a potent and selective radioligand for central 5-HT1A receptors in vitro and in vivo. Eur J Nucl Med. 1998;25:338–346. doi: 10.1007/s002590050230. [DOI] [PubMed] [Google Scholar]
- 81.Andree B, Halldin C, Pike VW, Gunn RN, Olsson H, Farde L. The PET radioligand [carbonyl-(11)C]desmethyl-WAY-100635 binds to 5-HT(1A) receptors and provides a higher radioactive signal than [carbonyl-(11)C]WAY-100635 in the human brain. J Nucl Med. 2002;43:292–303. [PubMed] [Google Scholar]
- 82.Maiti DK, Chakraborty PK, Chugani DC, Muzik O, Mangner TJ, Chugani HT. Synthesis procedure for routine production of [carbonyl-11C]desmethyl-WAY-100635. Appl Radiat Isot. 2005;62:721–727. doi: 10.1016/j.apradiso.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 83.Sandell J, Halldin C, Pike V, Chou Y, Varnas K, Hall H, Marchais S, Nowicki B, Wikstrom H, Swahn C, Farde L. New halogenated [11C]WAY analogues, [11C]6FPWAY and [11C]6BPWAY—Radiosynthesis and assessment as radioligands for the study of 5-HT1A receptors in living monkey. Nucl Med Biol. 2001;28:177–185. doi: 10.1016/s0969-8051(00)00181-5. [DOI] [PubMed] [Google Scholar]
- 84.Karramkam M, Hinnen F, Berrehouma M, Hlavacek C, Vaufrey F, Halldin C, McCarron JA, Pike VW, Dolle F. Synthesis of a [6-pyridinyl-18F]-labelled fluoro derivative of WAY-100635 as a candidate radioligand for brain 5-HT1A receptor imaging with PET. Bioorg Med Chem. 2003;11:2769–2782. doi: 10.1016/s0968-0896(03)00225-6. [DOI] [PubMed] [Google Scholar]
- 85.McCarron JA, Pike VW, Halldin C, Sandell J, Sovago J, Gulyas B, Cselenyi Z, Wikstrom HV, Marchais-Oberwinkler S, Nowicki B, Dolle F, Farde L. The pyridinyl-6 position of WAY-100635 as a site for radiofluorination—Effect on 5-HT1A receptor radioligand behavior in vivo. Mol Imaging Biol. 2004;6:17–26. doi: 10.1016/j.mibio.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 86.Shiue CY, Shiue GG, Mozley PD, Kung MP, Zhuang ZP, Kim HJ, Kung HF. P-[18F]-MPPF: A potential radioligand for PET studies of 5-HT1A receptors in humans. Synapse. 1997;25:147–154. doi: 10.1002/(SICI)1098-2396(199702)25:2<147::AID-SYN5>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 87.Aznavour N, Zimmer L. [18F]MPPF as a tool for the in vivo imaging of 5-HT1A receptors in animal and human brain. Neuropharmacology. 2007;52:695–707. doi: 10.1016/j.neuropharm.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 88.Kung HF, Stevenson DA, Zhuang ZP, Kung MP, Frederick D, Hurt SD. New 5-HT1A receptor antagonist: [3H]p-MPPF. Synapse. 1996;23:344–346. doi: 10.1002/(SICI)1098-2396(199608)23:4<344::AID-SYN13>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 89.Le Bars D, Lemaire C, Ginovart N, Plenevaux A, Aerts J, Brihaye C, Hassoun W, Leviel V, Mekhsian P, Weissmann D, Pujol JF, Luxen A, Comar D. High-yield radiosynthesis and preliminary in vivo evaluation of p-[18F]MPPF, a fluoro analog of WAY-100635. Nucl Med Biol. 1998;25:343–350. doi: 10.1016/s0969-8051(97)00229-1. [DOI] [PubMed] [Google Scholar]
- 90.Passchier J, van Waarde A, Pieterman RM, Elsinga PH, Pruim J, Hendrikse HN, Willemsen AT, Vaalburg W. In vivo delineation of 5-HT1A receptors in human brain with [18F]MPPF. J Nucl Med. 2000;41:1830–1835. [PubMed] [Google Scholar]
- 91.Passchier J, van Waarde A, Pieterman RM, Elsinga PH, Pruim J, Hendrikse HN, Willemsen AT, Vaalburg W. Quantitative imaging of 5-HT(1A) receptor binding in healthy volunteers with [(18)f]p-MPPF. Nucl Med Biol. 2000;27:473–476. doi: 10.1016/s0969-8051(00)00114-1. [DOI] [PubMed] [Google Scholar]
- 92.Lacan G, Plenevaux A, Rubins DJ, Way BM, Defraiteur C, Lemaire C, Aerts J, Luxen A, Cherry SR, Melega WP. Cyclosporine, a P-glycoprotein modulator, increases [18F]MPPF uptake in rat brain and peripheral tissues: MicroPET and ex vivo studies. Eur J Nucl Med Mol Imaging. 2008;35:2256–2266. doi: 10.1007/s00259-008-0832-z. [DOI] [PubMed] [Google Scholar]
- 93.Merlet I, Ostrowsky K, Costes N, Ryvlin P, Isnard J, Faillenot I, Lavenne F, Dufournel D, Le Bars D, Mauguiere F. 5-HT1A receptor binding and intracerebral activity in temporal lobe epilepsy: An [18F]MPPF-PET study. Brain. 2004;127:900–913. doi: 10.1093/brain/awh109. [DOI] [PubMed] [Google Scholar]
- 94.Truchot L, Costes SN, Zimmer L, Laurent B, Le Bars D, Thomas-Anterion C, Croisile B, Mercier B, Hermier M, Vighetto A, Krolak-Salmon P. Up-regulation of hippocampal serotonin metabolism in mild cognitive impairment. Neurology. 2007;69:1012–1017. doi: 10.1212/01.wnl.0000271377.52421.4a. [DOI] [PubMed] [Google Scholar]
- 95.Lothe A, Merlet I, Demarquay G, Costes N, Ryvlin P, Mauguiere F. Interictal brain 5-HT1A receptors binding in migraine without aura: A 18F-MPPF-PET study. Cephalalgia. 2008;28:1282–1291. doi: 10.1111/j.1468-2982.2008.01677.x. [DOI] [PubMed] [Google Scholar]
- 96.Demarquay G, Lothe A, Royet J, Costes N, Mick G, Mauguiere F, Ryvlin P. Brainstem changes in 5-HT1A receptor availability during migraine attack. Cephalalgia. 2011;31:84–94. doi: 10.1177/0333102410385581. [DOI] [PubMed] [Google Scholar]
- 97.Lothe A, Didelot A, Hammers A, Costes N, Saoud M, Gilliam F, Ryvlin P. Comorbidity between temporal lobe epilepsy and depression: A [18F]MPPF PET study. Brain. 2008;131:2765–2782. doi: 10.1093/brain/awn194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Costes N, Merlet I, Ostrowsky K, Faillenot I, Lavenne F, Zimmer L, Ryvlin P, Le Bars D. A 18F-MPPF PET normative database of 5-HT1A receptor binding in men and women over aging. J Nucl Med. 2005;46:1980–1989. [PubMed] [Google Scholar]
- 99.Costes N, Zimmer L, Reilhac A, Lavenne F, Ryvlin P, Le Bars D. Test-retest reproducibility of 18F-MPPF PET in healthy humans: A reliability study. J Nucl Med. 2007;48:1279–1288. doi: 10.2967/jnumed.107.041905. [DOI] [PubMed] [Google Scholar]
- 100.Sibon I, Benkelfat C, Gravel P, Aznavour N, Costes N, Mzengeza S, Booij L, Baker G, Soucy JP, Zimmer L, Descarries L. Decreased [(18)F]MPPF binding potential in the dorsal raphe nucleus after a single oral dose of fluoxetine: A positron-emission tomography study in healthy volunteers. Biol Psychiatry. 2008;63:1135–1140. doi: 10.1016/j.biopsych.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 101.Udo de Haes JI, Bosker FJ, Van Waarde A, Pruim J, Willemsen AT, Vaalburg W, Den Boer JA. 5-HT(1A) receptor imaging in the human brain: Effect of tryptophan depletion and infusion on [(18)F]MPPF binding. Synapse. 2002;46:108–115. doi: 10.1002/syn.10134. [DOI] [PubMed] [Google Scholar]
- 102.Praschak-Rieder N, Hussey D, Wilson AA, Carella A, Lee M, Dunn E, Willeit M, Bagby RM, Houle S, Meyer JH. Tryptophan depletion and serotonin loss in selective serotonin reuptake inhibitor-treated depression: An [(18)F] MPPF positron emission tomography study. Biol Psychiatry. 2004;56:587–591. doi: 10.1016/j.biopsych.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 103.Derry C, Benjamin C, Bladin P, le Bars D, Tochon-Danguy H, Berkovic SF, Zimmer L, Costes N, Mulligan R, Reutens D. Increased serotonin receptor availability in human sleep: Evidence from an [18F]MPPF PET study in narcolepsy. Neuroimage. 2006;30:341–348. doi: 10.1016/j.neuroimage.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 104.Defraiteur C, Lemaire C, Luxen A, Plenevaux A. Radiochemical synthesis and tissue distribution of p-[18F]DMPPF, a new 5-HT1A ligand for PET, in rats. Nucl Med Biol. 2006;33:667–675. doi: 10.1016/j.nucmedbio.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 105.Lang L, Jagoda E, Schmall B, Vuong BK, Adams HR, Nelson DL, Carson RE, Eckelman WC. Development of fluorine-18-labeled 5-HT1A antagonists. J Med Chem. 1999;42:1576–1586. doi: 10.1021/jm980456f. [DOI] [PubMed] [Google Scholar]
- 106.Carson RE, Lang L, Watabe H, Der MG, Adams HR, Jagoda E, Herscovitch P, Eckelman WC. PET evaluation of [(18)F]FCWAY, an analog of the 5-HT(1A) receptor antagonist, WAY-100635. Nucl Med Biol. 2000;27:493–497. doi: 10.1016/s0969-8051(00)00118-9. [DOI] [PubMed] [Google Scholar]
- 107.Lang L, Jagoda E, Ma Y, Sassaman MB, Eckelman WC. Synthesis and in vivo biodistribution of F-18 labeled 3-cis-, 3-trans-, 4-cis-, and 4-trans-fluorocyclohexane derivatives of WAY 100635. Bioorg Med Chem. 2006;14:3737–3748. doi: 10.1016/j.bmc.2006.01.064. [DOI] [PubMed] [Google Scholar]
- 108.Toczek MT, Carson RE, Lang L, Ma Y, Spanaki MV, Der MG, Fazilat S, Kopylev L, Herscovitch P, Eckelman WC, Theodore WH. PET imaging of 5-HT1A receptor binding in patients with temporal lobe epilepsy. Neurology. 2003;60:749–756. doi: 10.1212/01.wnl.0000049930.93113.20. [DOI] [PubMed] [Google Scholar]
- 109.Giovacchini G, Toczek MT, Bonwetsch R, Bagic A, Lang L, Fraser C, Reeves-Tyer P, Herscovitch P, Eckelman WC, Carson RE, Theodore WH. 5-HT 1A receptors are reduced in temporal lobe epilepsy after partial-volume correction. J Nucl Med. 2005;46:1128–1135. [PMC free article] [PubMed] [Google Scholar]
- 110.Hasler G, Bonwetsch R, Giovacchini G, Toczek MT, Bagic A, Luckenbaugh DA, Drevets WC, Theodore WH. 5-HT1A receptor binding in temporal lobe epilepsy patients with and without major depression. Biol Psychiatry. 2007;62:1258–1264. doi: 10.1016/j.biopsych.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liew CJ, Lim YM, Bonwetsch R, Shamim S, Sato S, Reeves-Tyer P, Herscovitch P, Dustin I, Bagic A, Giovacchini G, Theodore WH. 18F-FCWAY and 18F-FDG PET in MRI-negative temporal lobe epilepsy. Epilepsia. 2009;50:234–239. doi: 10.1111/j.1528-1167.2008.01789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Neumeister A, Bain E, Nugent AC, Carson RE, Bonne O, Luckenbaugh DA, Eckelman W, Herscovitch P, Charney DS, Drevets WC. Reduced serotonin type 1A receptor binding in panic disorder. J Neurosci. 2004;24:589–591. doi: 10.1523/JNEUROSCI.4921-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bonne O, Bain E, Neumeister A, Nugent AC, Vythilingam M, Carson RE, Luckenbaugh DA, Eckelman W, Herscovitch P, Drevets WC, Charney DS. No change in serotonin type 1A receptor binding in patients with posttraumatic stress disorder. Am J Psychiatry. 2005;162:383–385. doi: 10.1176/appi.ajp.162.2.383. [DOI] [PubMed] [Google Scholar]
- 114.Tipre DN, Zoghbi SS, Liow JS, Green MV, Seidel J, Ichise M, Innis RB, Pike VW. PET imaging of brain 5-HT1A receptors in rat in vivo with 18F-FCWAY and improvement by successful inhibition of radioligand defluorination with miconazole. J Nucl Med. 2006;47:345–353. [PubMed] [Google Scholar]
- 115.Ryu YH, Liow JS, Zoghbi S, Fujita M, Collins J, Tipre D, Sangare J, Hong J, Pike VW, Innis RB. Disulfiram inhibits defluorination of (18)F-FCWAY, reduces bone radioactivity, and enhances visualization of radioligand binding to serotonin 5-HT1A receptors in human brain. J Nucl Med. 2007;48:1154–1161. doi: 10.2967/jnumed.107.039933. [DOI] [PubMed] [Google Scholar]
- 116.Saigal N, Pichika R, Easwaramoorthy B, Collins D, Christian BT, Shi B, Narayanan TK, Potkin SG, Mukherjee J. Synthesis and biologic evaluation of a novel serotonin 5-HT1A receptor radioligand, 18F-labeled mefway, in rodents and imaging by PET in a nonhuman primate. J Nucl Med. 2006;47:1697–1706. [PubMed] [Google Scholar]
- 117.Jerning E, Svantesson GT, Mohell N. Receptor binding characteristics of [3H]NAD-299, a new selective 5-HT1A receptor antagonist. Eur J Pharmacol. 1998;360:219–225. doi: 10.1016/s0014-2999(98)00667-0. [DOI] [PubMed] [Google Scholar]
- 118.Sandell J, Halldin C, Hall H, Thorberg SO, Werner T, Sohn D, Sedvall G, Farde L. Radiosynthesis and autoradiographic evaluation of [11C]NAD-299, a radioligand for visualization of the 5-HT1A receptor. Nucl Med Biol. 1999;26:159–164. doi: 10.1016/s0969-8051(98)00091-2. [DOI] [PubMed] [Google Scholar]
- 119.Sandell J, Halldin C, Chou YH, Swahn CG, Thorberg SO, Farde L. PET-examination and metabolite evaluation in monkey of [(11)C]NAD-299, a radioligand for visualisation of the 5-HT(1A) receptor. Nucl Med Biol. 2002;29:39–45. doi: 10.1016/s0969-8051(01)00272-4. [DOI] [PubMed] [Google Scholar]
- 120.Kumar JS, Majo VJ, Hsiung SC, Millak MS, Liu KP, Tamir H, Prabhakaran J, Simpson NR, Van Heertum RL, Mann JJ, Parsey RV. Synthesis and in vivo validation of [O-methyl-11C]2-4-[4-(7-methoxynaphthalen-1-yl)piperazin-1-yl]butyl-4-methyl-2H-[1,2,4]triazine-3,5-dione: A novel 5-HT1A receptor agonist positron emission tomography ligand. J Med Chem. 2006;49:125–134. doi: 10.1021/jm050725j. [DOI] [PubMed] [Google Scholar]
- 121.Prabhakaran J, Parsey RV, Majo VJ, Hsiung SC, Milak MS, Tamir H, Simpson NR, Van Heertum RL, Mann JJ, Dileep Kumar JS. Synthesis, in vitro and in vivo evaluation of [O-methyl-11C] 2-4-[4-(3-methoxyphenyl)piperazin-1-yl]-butyl-4-methyl-2H-[1,2,4]-triazi ne-3,5-dione: A novel agonist 5-HT1A receptor PET ligand. Bioorg Med Chem Lett. 2006;16:2101–2104. doi: 10.1016/j.bmcl.2006.01.052. [DOI] [PubMed] [Google Scholar]
- 122.Kumar JS, Prabhakaran J, Majo VJ, Milak MS, Hsiung SC, Tamir H, Simpson NR, Van Heertum RL, Mann JJ, Parsey RV. Synthesis and in vivo evaluation of a novel 5-HT1A receptor agonist radioligand [O-methyl-11C]2-(4-(4-(2-methoxyphenyl)piperazin-1-yl)butyl)-4-methyl-1,2,4-triazine -3,5(2H,4H)dione in nonhuman primates. Eur J Nucl Med Mol Imaging. 2007;34:1050–1060. doi: 10.1007/s00259-006-0324-y. [DOI] [PubMed] [Google Scholar]
- 123.Milak MS, Severance AJ, Prabhakaran J, Kumar JS, Majo VJ, Ogden RT, Mann JJ, Parsey RV. In vivo serotonin-sensitive binding of [(11)C]CUMI-101: A serotonin 1A receptor agonist positron emission tomography radiotracer. J Cereb Blood Flow Metab. 2011;31:243–249. doi: 10.1038/jcbfm.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Palner M, Underwood MD, Kumar DJ, Arango V, Knudsen GM, Mann JJ, Parsey RV. Ex vivo evaluation of the serotonin 1A receptor partial agonist [(3)H]CUMI-101 in awake rats. Synapse. 2010 doi: 10.1002/syn.20888. [DOI] [PubMed] [Google Scholar]
- 125.Milak MS, Delorenzo C, Zanderigo F, Prabhakaran J, Kumar JS, Majo VJ, Mann JJ, Parsey RV. In vivo quantification of human serotonin 1A receptor using 11C-CUMI-101, an agonist PET radiotracer. J Nucl Med. 2010;51:1892–1900. doi: 10.2967/jnumed.110.076257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Maurel JL, Autin JM, Funes P, Newman-Tancredi A, Colpaert F, Vacher B. High-efficacy 5-HT1A agonists for antidepressant treatment: A renewed opportunity. J Med Chem. 2007;50:5024–5033. doi: 10.1021/jm070714l. [DOI] [PubMed] [Google Scholar]
- 127.Newman-Tancredi A, Martel JC, Assie MB, Buritova J, Lauressergues E, Cosi C, Heusler P, Bruins Slot L, Colpaert FC, Vacher B, Cussac D. Signal transduction and functional selectivity of F15599, a preferential post-synaptic 5-HT1A receptor agonist. Br J Pharmacol. 2009;156:338–353. doi: 10.1111/j.1476-5381.2008.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lu S, Liow JS, Zoghbi SS, Hong J, Innis RB, Pike VW. Evaluation of [C]S14506 and [F]S14506 in rat and monkey as agonist PET radioligands for brain 5-HT(1A) receptors. Curr Radiopharm. 2010;3:9–18. doi: 10.2174/1874471011003010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Domenech T, Beleta J, Palacios JM. Characterization of human serotonin 1D and 1B receptors using [3H]-GR-125743, a novel radiolabelled serotonin 5HT1D/1B receptor antagonist. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:328–334. doi: 10.1007/pl00005058. [DOI] [PubMed] [Google Scholar]
- 130.Bonaventure P, Schotte A, Cras P, Leysen JE. Autoradiographic mapping of 5-HT1B- and 5-HT1D receptors in human brain using [3H]alniditan, a new radioligand. Recept Channels. 1997;5:225–230. [PubMed] [Google Scholar]
- 131.Doenicke A, Brand J, Perrin VL. Possible benefit of GR43175, a novel 5-HT1-like receptor agonist, for the acute treatment of severe migraine. Lancet. 1988;1:1309–1311. doi: 10.1016/s0140-6736(88)92122-8. [DOI] [PubMed] [Google Scholar]
- 132.Ruf BM, Bhagwagar Z. The 5-HT1B receptor: A novel target for the pathophysiology of depression. Curr Drug Targets. 2009;10:1118–1138. doi: 10.2174/138945009789735192. [DOI] [PubMed] [Google Scholar]
- 133.Olivier B, van Oorschot R. 5-HT1B receptors and aggression: A review. Eur J Pharmacol. 2005;526:207–217. doi: 10.1016/j.ejphar.2005.09.066. [DOI] [PubMed] [Google Scholar]
- 134.Sari Y. Serotonin1B receptors: From protein to physiological function and behavior. Neurosci Biobehav Rev. 2004;28:565–582. doi: 10.1016/j.neubiorev.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 135.Finnema SJ, Varrone A, Hwang TJ, Gulyas B, Halldin C, Farde L. Fenfluramine induced serotonin release decreases [11C]AZ10419369 binding to 5-HT1B receptors in the primate brain. Synapse. 2010;64:573–577. doi: 10.1002/syn.20780. [DOI] [PubMed] [Google Scholar]
- 136.Pierson ME, Andersson J, Nyberg S, McCarthy DJ, Finnema SJ, Varnas K, Takano A, Karlsson P, Gulyas B, Medd AM, Lee CM, Powell ME, Heys JR, Potts W, Seneca N, Mrzljak L, Farde L, Halldin C. [11C]AZ10419369: A selective 5-HT1B receptor radioligand suitable for positron emission tomography (PET). Characterization in the primate brain. Neuroimage. 2008;41:1075–1085. doi: 10.1016/j.neuroimage.2008.02.063. [DOI] [PubMed] [Google Scholar]
- 137.Varnas K, Nyberg S, Halldin C, Varrone A, Takano A, Karlsson P, Andersson J, McCarthy D, Smith M, Pierson ME, Soderstrom J, Farde L. Quantitative analysis of [(11)C]AZ10419369 binding to 5-HT(1B) receptors in human brain. J Cereb Blood Flow Metab. 2011;31:113–123. doi: 10.1038/jcbfm.2010.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Maier DL, Sobotka-Briner C, Ding M, Powell ME, Jiang Q, Hill G, Heys JR, Elmore CS, Pierson ME, Mrzljak L. [N-methyl-3H3]AZ10419369 binding to the 5-HT1B receptor: In vitro characterization and in vivo receptor occupancy. J Pharmacol Exp Ther. 2009;330:342–351. doi: 10.1124/jpet.109.150722. [DOI] [PubMed] [Google Scholar]
- 139.Gallezot JD, Nabulsi N, Neumeister A, Planeta-Wilson B, Williams WA, Singhal T, Kim S, Maguire RP, McCarthy T, Frost JJ, Huang Y, Ding YS, Carson RE. Kinetic modeling of the serotonin 5-HT(1B) receptor radioligand [(11)C]P943 in humans. J Cereb Blood Flow Metab. 2010;30:196–210. doi: 10.1038/jcbfm.2009.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nabulsi N, Huang Y, Weinzimmer D, Ropchan J, Frost JJ, McCarthy T, Carson RE, Ding YS. High-resolution imaging of brain 5-HT 1B receptors in the rhesus monkey using [11C]P943. Nucl Med Biol. 2010;37:205–214. doi: 10.1016/j.nucmedbio.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Varnas K, Hall H, Bonaventure P, Sedvall G. Autoradiographic mapping of 5-HT(1B) and 5-HT(1D) receptors in the post mortem human brain using [(3)H]GR 125743. Brain Res. 2001;915:47–57. doi: 10.1016/s0006-8993(01)02823-2. [DOI] [PubMed] [Google Scholar]
- 142.Hu J, Henry S, Gallezot JD, Ropchan J, Neumaier JF, Potenza MN, Sinha R, Krystal JH, Huang Y, Ding YS, Carson RE, Neumeister A. Serotonin 1B receptor imaging in alcohol dependence. Biol Psychiatry. 2010;67:800–803. doi: 10.1016/j.biopsych.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Murrough JW, Henry S, Hu J, Gallezot JD, Planeta-Wilson B, Neumaier JF, Neumeister A. Reduced ventral striatal/ventral pallidal serotonin(1B) receptor binding potential in major depressive disorder. Psychopharmacology. 2011;213:547–553. doi: 10.1007/s00213-010-1881-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Miller KJ, Teitler M. Quantitative autoradiography of 5-CT-sensitive (5-HT1D) and 5-CT-insensitive (5-HT1E) serotonin receptors in human brain. Neurosci Lett. 1992;136:223–226. doi: 10.1016/0304-3940(92)90054-b. [DOI] [PubMed] [Google Scholar]
- 145.Wainscott DB, Krushinski JH, Jr, Audia JE, Schaus JM, Zgombick JM, Lucaites VL, Nelson DL. [3H]LY334370, a novel radioligand for the 5-HT1F receptor. I. In vitro characterization of binding properties. Naunyn Schmiedebergs Arch Pharmacol. 2005;371:169–177. doi: 10.1007/s00210-005-1035-9. [DOI] [PubMed] [Google Scholar]
- 146.Ferrari MD, Farkkila M, Reuter U, Pilgrim A, Davis C, Krauss M, Diener HC. Acute treatment of migraine with the selective 5-HT1F receptor agonist lasmiditan—A randomised proof-of-concept trial. Cephalalgia. 2010;30:1170–1178. doi: 10.1177/0333102410375512. [DOI] [PubMed] [Google Scholar]
- 147.Ward SE, Watson JM. Recent advances in the discovery of selective and non-selective 5-HT(1D) receptor ligands. Curr Topics Med Chem. 2010;10:479–492. doi: 10.2174/156802610791111533. [DOI] [PubMed] [Google Scholar]
- 148.Lucaites VL, Krushinski JH, Schaus JM, Audia JE, Nelson DL. [3H]LY334370, a novel radioligand for the 5-HT1F receptor. II. Autoradiographic localization in rat, guinea pig, monkey and human brain. Naunyn Schmiedebergs Arch Pharmacol. 2005;371:178–184. doi: 10.1007/s00210-005-1036-8. [DOI] [PubMed] [Google Scholar]
- 149.Rosel P, Arranz B, San L, Vallejo J, Crespo JM, Urretavizcaya M, Navarro MA. Altered 5-HT(2A) binding sites and second messenger inositol trisphosphate (IP(3)) levels in hippocampus but not in frontal cortex from depressed suicide victims. Psychiatry Res. 2000;99:173–181. doi: 10.1016/s0925-4927(00)00076-7. [DOI] [PubMed] [Google Scholar]
- 150.Pazos A, Probst A, Palacios JM. Serotonin receptors in the human brain–IV. Autoradiographic mapping of serotonin-2 receptors. Neuroscience. 1987;21:123–139. doi: 10.1016/0306-4522(87)90327-7. [DOI] [PubMed] [Google Scholar]
- 151.Cornea-Hebert V, Riad M, Wu C, Singh SK, Descarries L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol. 1999;409:187–209. doi: 10.1002/(sici)1096-9861(19990628)409:2<187::aid-cne2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 152.Hall H, Farde L, Halldin C, Lundkvist C, Sedvall G. Autoradiographic localization of 5-HT(2A) receptors in the human brain using [(3)H]M100907 and [(11)C]M100907. Synapse. 2000;38:421–431. doi: 10.1002/1098-2396(20001215)38:4<421::AID-SYN7>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 153.Leysen JE. 5-HT2 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3:11–26. doi: 10.2174/1568007043482598. [DOI] [PubMed] [Google Scholar]
- 154.Gonzalez-Maeso J, Sealfon SC. Psychedelics and schizophrenia. Trends Neurosci. 2009;32:225–232. doi: 10.1016/j.tins.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 155.Sargent T, 3rd, Budinger TF, Braun G, Shulgin AT, Braun U. An iodinated catecholamine congener for brain imaging and metabolic studies. J Nucl Med. 1978;19:71–76. [PubMed] [Google Scholar]
- 156.Sargent T, Kalbhen DA, Shulgin AT, Braun G, Stauffer H, Kusubov N. In vivo human pharmacodynamics of the psychodysleptic 4-BR-2,5-dimethoxyphenylisopropylamine labelled with ’BRor ’BR. Neuropharmacology. 1975;14:165–174. doi: 10.1016/0028-3908(75)90001-5. [DOI] [PubMed] [Google Scholar]
- 157.Glennon RA, Seggel MR, Soine WH, Herrick-Davis K, Lyon RA, Titeler M. [125I]-1-(2,5-dimethoxy-4-iodophenyl)-2-amino-propane: An iodinated radioligand that specifically labels the agonist high-affinity state of 5-HT2 serotonin receptors. J Med Chem. 1988;31:5–7. doi: 10.1021/jm00396a003. [DOI] [PubMed] [Google Scholar]
- 158.Appel NM, Mitchell WM, Garlick RK, Glennon RA, Teitler M, De Souza EB. Autoradiographic characterization of (+-)-1-(2,5-dimethoxy-4-[125I] iodophenyl)-2-aminopropane ([125I]DOI) binding to 5-HT2 and 5-HT1c receptors in rat brain. J Pharmacol Exp Ther. 1990;255:843–857. [PubMed] [Google Scholar]
- 159.Nazarali AJ, McKenna DJ, Saavedra JM. Autoradiographic localization of 5HT2 receptors in rat brain using [125I]-DOI, a selective psychotomimetic radioligand. Prog Neuropsychopharmacol Biol Psychiatry. 1989;13:573–581. doi: 10.1016/0278-5846(89)90149-8. [DOI] [PubMed] [Google Scholar]
- 160.Zea-Ponce Y, Kegeles LS, Guo N, Raskin L, Bakthavachalam V, Laruelle M. Pharmacokinetics and brain distribution in non human primate of R(−)[123I]DOI, A 5HT(2A/2C) serotonin agonist. Nucl Med Biol. 2002;29:575–583. doi: 10.1016/s0969-8051(02)00306-2. [DOI] [PubMed] [Google Scholar]
- 161.Samnick S, Remy N, Ametamey S, Bader JB, Brandau W, Kirsch CM. 123I-MSP and F[11C]MSP: New selective 5-HT2A receptor radiopharmaceuticals for in vivo studies of neuronal 5-HT2 serotonin receptors. Synthesis, in vitro binding study with unlabelled analogues and preliminary in vivo evaluation in mice. Life Sci. 1998;63:2001–2013. doi: 10.1016/s0024-3205(98)00478-0. [DOI] [PubMed] [Google Scholar]
- 162.Busatto GF, Pilowsky LS, Costa DC, Mertens J, Terriere D, Ell PJ, Mulligan R, Travis MJ, Leysen JE, Lui D, Gacinovic S, Waddington W, Lingford-Hughes A, Kerwin RW. Initial evaluation of 123I-5-I-R91150, a selective 5-HT2A ligand for single-photon emission tomography, in healthy human subjects. Eur J Nucl Med. 1997;24:119–124. doi: 10.1007/BF02439542. [DOI] [PubMed] [Google Scholar]
- 163.Abi-Dargham A, Zea-Ponce Y, Terriere D, al-Tikriti M, Baldwin RM, Hoffer P, Charney D, Leysen JE, Laruelle M, Mertens J, Innis RB. Preclinical evaluation of [123I]R93274 as a SPECT radiotracer for imaging 5-HT2A receptors. Eur J Pharmacol. 1997;321:285–293. doi: 10.1016/s0014-2999(96)00906-5. [DOI] [PubMed] [Google Scholar]
- 164.Mertens J, Terriere D, Sipido V, Gommeren W, Janssen PMF, Leysen JE. Radiosynthesis of a new radioiodinated ligand for serotonin-5HT2-receptors, a promising tracer for gamma-emission tomography. J Labl Compd Radiopharm. 1994;34:795–806. [Google Scholar]
- 165.Terriere D, Janssen PM, Gommeren W, Gysemans M, Mertens JJ, Leysen JE. Evaluation of radioiodo-4-amino-N-[1-[3-(4-fluorophenoxy)-propyl]-4-methyl-4-piperidinyl]-5-iodo-2-methoxy-benzamide as a potential 5HT2 receptor tracer for SPE(C)T. Nucl Med Biol. 1995;22:1005. doi: 10.1016/0969-8051(95)02023-3. [DOI] [PubMed] [Google Scholar]
- 166.Catafau AM, Danus M, Bullich S, Llop J, Perich J, Cunningham VJ, Plaza P, Penengo MM, Eersels JL, Squassante L, Ros D, Barbanoj M. Characterization of the SPECT 5-HT2A receptor ligand 123I-R91150 in healthy volunteers: Part 1—Pseudoequilibrium interval and quantification methods. J Nucl Med. 2006;47:919–928. [PubMed] [Google Scholar]
- 167.Catafau AM, Danus M, Bullich S, Nucci G, Llop J, Abanades S, Cunningham VJ, Eersels JL, Pavia J, Farre M. Characterization of the SPECT 5-HT2A receptor ligand 123I-R91150 in healthy volunteers: Part 2—Ketanserin displacement. J Nucl Med. 2006;47:929–937. [PubMed] [Google Scholar]
- 168.Travis MJ, Busatto GF, Pilowsky LS, Mulligan R, Acton PD, Gacinovic S, Mertens J, Terriere D, Costa DC, Ell PJ, Kerwin RW. 5-HT2A receptor blockade in patients with schizophrenia treated with risperidone or clozapine. A SPET study using the novel 5-HT2A ligand 123I-5-I-R-91150. Br J Psychiatry. 1998;173:236–241. doi: 10.1192/bjp.173.3.236. [DOI] [PubMed] [Google Scholar]
- 169.Jones HM, Travis MJ, Mulligan R, Visvikis D, Gacinovic S, Ell PJ, Kerwin RW, Pilowsky LS. In vivo serotonin 5-HT(2A) receptor occupancy and quetiapine. Am J Psychiatry. 2000;157:148. doi: 10.1176/ajp.157.1.148. [DOI] [PubMed] [Google Scholar]
- 170.Jones HM, Travis MJ, Mulligan R, Bressan RA, Visvikis D, Gacinovic S, Ell PJ, Pilowsky LS. In vivo 5-HT2A receptor blockade by quetiapine: An R91150 single photon emission tomography study. Psychopharmacology. 2001;157:60–66. doi: 10.1007/s002130100761. [DOI] [PubMed] [Google Scholar]
- 171.Versijpt J, Van Laere KJ, Dumont F, Decoo D, Vandecapelle M, Santens P, Goethals I, Audenaert K, Slegers G, Dierckx RA, Korf J. Imaging of the 5-HT2A system: Age-, gender-, and Alzheimer’s disease-related findings. Neurobiol Aging. 2003;24:553–561. doi: 10.1016/s0197-4580(02)00137-9. [DOI] [PubMed] [Google Scholar]
- 172.Audenaert K, Van Laere K, Dumont F, Slegers G, Mertens J, van Heeringen C, Dierckx RA. Decreased frontal serotonin 5-HT 2a receptor binding index in deliberate self-harm patients. Eur J Nucl Med. 2001;28:175–182. doi: 10.1007/s002590000392. [DOI] [PubMed] [Google Scholar]
- 173.van Heeringen C, Audenaert K, Van Laere K, Dumont F, Slegers G, Mertens J, Dierckx RA. Prefrontal 5-HT2a receptor binding index, hopelessness and personality characteristics in attempted suicide. J Affect Disord. 2003;74:149–158. doi: 10.1016/s0165-0327(01)00482-7. [DOI] [PubMed] [Google Scholar]
- 174.Audenaert K, Van Laere K, Dumont F, Vervaet M, Goethals I, Slegers G, Mertens J, van Heeringen C, Dierckx RA. Decreased 5-HT2a receptor binding in patients with anorexia nervosa. J Nucl Med. 2003;44:163–169. [PubMed] [Google Scholar]
- 175.Goethals I, Vervaet M, Audenaert K, Jacobs F, Ham H, Van de Wiele C, Vandecapelle M, Slegers G, Dierckx R, van Heeringen C. Differences of cortical 5-HT2A receptor binding index with SPECT in subtypes of anorexia nervosa: Relationship with personality traits? J Psychiatr Res. 2007;41:455–458. doi: 10.1016/j.jpsychires.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 176.Baeken C, D’Haenen H, Flamen P, Mertens J, Terriere D, Chavatte K, Boumon R, Bossuyt A. 123I-5-I-R91150, a new single-photon emission tomography ligand for 5-HT2A receptors: Influence of age and gender in healthy subjects. Eur J Nucl Med. 1998;25:1617–1622. doi: 10.1007/s002590050339. [DOI] [PubMed] [Google Scholar]
- 177.Muhlhausen U, Ermert J, Coenen HH. Synthesis, labelling and first evaluation of [F-18]R91150 as a serotonin 5-HT2A receptor antagonist for PET. J Labl Compd Radiopharm. 2009;52:13–22. [Google Scholar]
- 178.Fu X, Tan PZ, Kula NS, Baldessarini R, Tamagnan G, Innis RB, Baldwin RM. Synthesis, receptor potency, and selectivity of halogenated diphenylpiperidines as serotonin 5-HT2A ligands for PET or SPECT brain imaging. J Med Chem. 2002;45:2319–2324. doi: 10.1021/jm0200411. [DOI] [PubMed] [Google Scholar]
- 179.Blanckaert P, Burvenich I, Staelens L, Dierckx RA, Slegers G. Synthesis, radiosynthesis and in vivo evaluation in mice of [123I]-(4-fluorophenyl) 1-[2-(4-iodophenyl)ethyl]piperidin-4-ylmetha-none for visualization of the 5-HT2A receptor with SPECT. Appl Radiat Isot. 2005;62:737–743. doi: 10.1016/j.apradiso.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 180.Blanckaert P, Vandecapelle M, Staelens L, Burvenich I, Dierckx RA, Slegers G. Synthesis, radiosynthesis and preliminary in vivo evaluation of [I-123](4-fluorophenyl) 1-[2-(2(2-iodophenyl)-ethyl]piperidin-4-ylmethanone, a potential 5-HT2A-antagonist for SPECT brain imaging. J Labl Compd Radiopharm. 2004;47:591–598. [Google Scholar]
- 181.Blanckaert P, Burvenich I, Devos F, Slegers G. Synthesis and in vivo evaluation in mice of (I-123)-(4-fluorophenyl)(1-(3-iodophenethyl)piperidin-4-yl) methanone as a potential SPECT-tracer for the serotonin 5-HT2A receptor. J Labl Compd Radiopharm. 2007;50:183–188. [Google Scholar]
- 182.Blanckaert PB, Burvenich I, Wyffels L, De Bruyne S, Moerman L, De Vos F. In vivo evaluation in rodents of [(123)I]-3-I-CO as a potential SPECT tracer for the serotonin 5-HT(2A) receptor. Nucl Med Biol. 2008;35:861–867. doi: 10.1016/j.nucmedbio.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 183.Baron JC, Samson Y, Comar D, Crouzel C, Deniker P, Agid Y. In vivo study of central serotoninergic receptors in man using positron tomography. Rev Neurol. 1985;141:537–545. [PubMed] [Google Scholar]
- 184.Moerlein SM, Perlmutter JS. Central serotonergic S2 binding in Papio anubis measured in vivo with N-omega-[18F]fluoroethylketanserin and PET. Neurosci Lett. 1991;123:23–26. doi: 10.1016/0304-3940(91)90149-n. [DOI] [PubMed] [Google Scholar]
- 185.Lyon RA, Titeler M, Frost JJ, Whitehouse PJ, Wong DF, Wagner HN, Jr, Dannals RF, Links JM, Kuhar MJ. 3H-3-N-methylspiperone labels D2 dopamine receptors in basal ganglia and S2 serotonin receptors in cerebral cortex. J Neurosci. 1986;6:2941–2949. doi: 10.1523/JNEUROSCI.06-10-02941.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Frost JJ, Smith AC, Kuhar MJ, Dannals RF, Wagner HN., Jr In vivo binding of 3H-N-methylspiperone to dopamine and serotonin receptors. Life Sci. 1987;40:987–995. doi: 10.1016/0024-3205(87)90321-3. [DOI] [PubMed] [Google Scholar]
- 187.Wong DF, Wagner HN, Jr, Dannals RF, Links JM, Frost JJ, Ravert HT, Wilson AA, Rosenbaum AE, Gjedde A, Douglass KH, et al. Effects of age on dopamine and serotonin receptors measured by positron tomography in the living human brain. Science. 1984;226:1393–1396. doi: 10.1126/science.6334363. [DOI] [PubMed] [Google Scholar]
- 188.Okubo Y, Suhara T, Suzuki K, Kobayashi K, Inoue O, Terasaki O, Someya Y, Sassa T, Sudo Y, Matsushima E, Iyo M, Tateno Y, Toru M. Serotonin 5-HT2 receptors in schizophrenic patients studied by positron emission tomography. Life Sci. 2000;66:2455–2464. doi: 10.1016/s0024-3205(00)80005-3. [DOI] [PubMed] [Google Scholar]
- 189.Nyberg S, Farde L, Eriksson L, Halldin C, Eriksson B. 5-HT2 and D2 dopamine receptor occupancy in the living human brain. A PET study with risperidone. Psychopharmacology. 1993;110:265–272. doi: 10.1007/BF02251280. [DOI] [PubMed] [Google Scholar]
- 190.Nordstrom AL, Farde L, Halldin C. High 5-HT2 receptor occupancy in clozapine treated patients demonstrated by PET. Psychopharmacology. 1993;110:365–367. doi: 10.1007/BF02251294. [DOI] [PubMed] [Google Scholar]
- 191.Nordstrom AL, Farde L, Nyberg S, Karlsson P, Halldin C, Sedvall G. D1, D2, and 5-HT2 receptor occupancy in relation to clozapine serum concentration: A PET study of schizophrenic patients. Am J Psychiatry. 1995;152:1444–1449. doi: 10.1176/ajp.152.10.1444. [DOI] [PubMed] [Google Scholar]
- 192.Grunder G, Yokoi F, Offord SJ, Ravert HT, Dannals RF, Salzmann JK, Szymanski S, Wilson PD, Howard DR, Wong DF. Time course of 5-HT2A receptor occupancy in the human brain after a single oral dose of the putative antipsychotic drug MDL 100,907 measured by positron emission tomography. Neuropsychopharmacology. 1997;17:175–185. doi: 10.1016/S0893-133X(97)00044-4. [DOI] [PubMed] [Google Scholar]
- 193.Reimold M, Solbach C, Noda S, Schaefer JE, Bartels M, Beneke M, Machulla HJ, Bares R, Glaser T, Wormstall H. Occupancy of dopamine D(1), D (2) and serotonin (2A) receptors in schizophrenic patients treated with flupentixol in comparison with risperidone and haloperidol. Psychopharmacology. 2007;190:241–249. doi: 10.1007/s00213-006-0611-0. [DOI] [PubMed] [Google Scholar]
- 194.Lever JR, Dannals RF, Wilson AA, Ravert HT, Scheffel U, Hoffman BJ, Hartig PR, Wong DF, Wagner HN., Jr Synthesis and in vivo characterization of D-(+)-(N1-[11C]methyl)-2-Br-LSD: A radioligand for positron emission tomographic studies of serotonin 5-HT2 receptors. Int J Rad Appl Instrum B. 1989;16:697–704. doi: 10.1016/0883-2897(89)90141-4. [DOI] [PubMed] [Google Scholar]
- 195.Wong DF, Lever JR, Hartig PR, Dannals RF, Villemagne V, Hoffman BJ, Wilson AA, Ravert HT, Links JM, Scheffel U, Wagner HN., Jr Localization of serotonin 5-HT2 receptors in living human brain by positron emission tomography using N1-([11C]-methyl)-2-Br-LSD. Synapse. 1987;1:393–398. doi: 10.1002/syn.890010502. [DOI] [PubMed] [Google Scholar]
- 196.Burris KD, Breeding M, Sanders-Bush E. (+)Lysergic acid diethylamide, but not its nonhallucinogenic congeners, is a potent serotonin 5HT1C receptor agonist. J Pharmacol Exp Ther. 1991;258:891–896. [PubMed] [Google Scholar]
- 197.Blin J, Pappata S, Kiyosawa M, Crouzel C, Baron JC. [18F]setoperone: A new high-affinity ligand for positron emission tomography study of the serotonin-2 receptors in baboon brain in vivo. Eur J Pharmacol. 1988;147:73–82. doi: 10.1016/0014-2999(88)90635-8. [DOI] [PubMed] [Google Scholar]
- 198.Blin J, Sette G, Fiorelli M, Bletry O, Elghozi JL, Crouzel C, Baron JC. A method for the in vivo investigation of the serotonergic 5-HT2 receptors in the human cerebral cortex using positron emission tomography and 18F-labeled setoperone. J Neurochem. 1990;54:1744–1754. doi: 10.1111/j.1471-4159.1990.tb01229.x. [DOI] [PubMed] [Google Scholar]
- 199.Kapur S, Zipursky R, Remington G, Jones C, McKay G, Houle S. PET evidence that loxapine is an equipotent blocker of 5-HT2 and D2 receptors: Implications for the therapeutics of schizophrenia. Am J Psychiatry. 1997;154:1525–1529. doi: 10.1176/ajp.154.11.1525. [DOI] [PubMed] [Google Scholar]
- 200.Trichard C, Paillere-Martinot ML, Attar-Levy D, Recassens C, Monnet F, Martinot JL. Binding of antipsychotic drugs to cortical 5-HT2A receptors: A PET study of chlorpromazine, clozapine, and amisulpride in schizophrenic patients. Am J Psychiatry. 1998;155:505–508. doi: 10.1176/ajp.155.4.505. [DOI] [PubMed] [Google Scholar]
- 201.Mamo D, Kapur S, Shammi CM, Papatheodorou G, Mann S, Therrien F, Remington G. A PET study of dopamine D2 and serotonin 5-HT2 receptor occupancy in patients with schizophrenia treated with therapeutic doses of ziprasidone. Am J Psychiatry. 2004;161:818–825. doi: 10.1176/appi.ajp.161.5.818. [DOI] [PubMed] [Google Scholar]
- 202.Blin J, Baron JC, Dubois B, Crouzel C, Fiorelli M, Attar-Levy D, Pillon B, Fournier D, Vidailhet M, Agid Y. Loss of brain 5-HT2 receptors in Alzheimer’s disease. In vivo assessment with positron emission tomography and [18F]setoperone. Brain. 1993;116 :497–510. doi: 10.1093/brain/116.3.497. [DOI] [PubMed] [Google Scholar]
- 203.Chabriat H, Tehindrazanarivelo A, Vera P, Samson Y, Pappata S, Boullais N, Bousser MG. 5HT2 receptors in cerebral cortex of migraineurs studied using PET and 18F-fluorosetoperone. Cephalalgia. 1995;15:104–108. doi: 10.1046/j.1468-2982.1995.015002104.x. discussion 177. [DOI] [PubMed] [Google Scholar]
- 204.Vera P, Zilbovicius M, Chabriat H, Amarenco P, Kerdraon J, Menard JF, Amarenco G, Bousser MG, Rancurel G, Samson Y. Post-stroke changes in cortical 5-HT2 serotonergic receptors. J Nucl Med. 1996;37:1976–1981. [PubMed] [Google Scholar]
- 205.Massou JM, Trichard C, Attar-Levy D, Feline A, Corruble E, Beaufils B, Martinot JL. Frontal 5-HT2A receptors studied in depressive patients during chronic treatment by selective serotonin reuptake inhibitors. Psychopharmacology. 1997;133:99–101. doi: 10.1007/s002130050377. [DOI] [PubMed] [Google Scholar]
- 206.Meyer JH, Kapur S, Houle S, DaSilva J, Owczarek B, Brown GM, Wilson AA, Kennedy SH. Prefrontal cortex 5-HT2 receptors in depression: An [18F]setoperone PET imaging study. Am J Psychiatry. 1999;156:1029–1034. doi: 10.1176/ajp.156.7.1029. [DOI] [PubMed] [Google Scholar]
- 207.Strome EM, Clark CM, Zis AP, Doudet DJ. Electroconvulsive shock decreases binding to 5-HT2 receptors in nonhuman primates: An in vivo positron emission tomography study with [18F]setoperone. Biol Psychiatry. 2005;57:1004–1010. doi: 10.1016/j.biopsych.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 208.Lemaire C, Cantineau R, Guillaume M, Plenevaux A, Christiaens L. Fluorine-18-altanserin: A radioligand for the study of serotonin receptors with PET: Radiolabeling and in vivo biologic behavior in rats. J Nucl Med. 1991;32:2266–2272. [PubMed] [Google Scholar]
- 209.Biver F, Goldman S, Luxen A, Monclus M, Forestini M, Mendlewicz J, Lotstra F. Multicompartmental study of fluorine-18 altanserin binding to brain 5HT2 receptors in humans using positron emission tomography. Eur J Nucl Med. 1994;21:937–946. doi: 10.1007/BF00238117. [DOI] [PubMed] [Google Scholar]
- 210.Sadzot B, Lemaire C, Maquet P, Salmon E, Plenevaux A, Degueldre C, Hermanne JP, Guillaume M, Cantineau R, Comar D, et al. Serotonin 5HT2 receptor imaging in the human brain using positron emission tomography and a new radioligand, [18F]altanserin: Results in young normal controls. J Cereb Blood Flow Metab. 1995;15:787–797. doi: 10.1038/jcbfm.1995.99. [DOI] [PubMed] [Google Scholar]
- 211.Tan PZ, Baldwin RM, Van Dyck CH, Al-Tikriti M, Roth B, Khan N, Charney DS, Innis RB. Characterization of radioactive metabolites of 5-HT2A receptor PET ligand [18F]altanserin in human and rodent. Nucl Med Biol. 1999;26:601–608. doi: 10.1016/s0969-8051(99)00022-0. [DOI] [PubMed] [Google Scholar]
- 212.Price JC, Lopresti BJ, Mason NS, Holt DP, Huang Y, Mathis CA. Analyses of [(18)F] altanserin bolus injection PET data. I: Consideration of radiolabeled metabolites in baboons. Synapse. 2001;41:1–10. doi: 10.1002/syn.1054. [DOI] [PubMed] [Google Scholar]
- 213.Price JC, Lopresti BJ, Meltzer CC, Smith GS, Mason NS, Huang Y, Holt DP, Gunn RN, Mathis CA. Analyses of [(18)F]altanserin bolus injection PET data. II: Consideration of radiolabeled metabolites in humans. Synapse. 2001;41:11–21. doi: 10.1002/syn.1055. [DOI] [PubMed] [Google Scholar]
- 214.van Dyck CH, Tan PZ, Baldwin RM, Amici LA, Garg PK, Ng CK, Soufer R, Charney DS, Innis RB. PET quantification of 5-HT2A receptors in the human brain: A constant infusion paradigm with [18F]altanserin. J Nucl Med. 2000;41:234–241. [PubMed] [Google Scholar]
- 215.Haugbol S, Pinborg LH, Arfan HM, Frokjaer VM, Madsen J, Dyrby TB, Svarer C, Knudsen GM. Reproducibility of 5-HT2A receptor measurements and sample size estimations with [18F]altanserin PET using a bolus/infusion approach. Eur J Nucl Med Mol Imaging. 2007;34:910–915. doi: 10.1007/s00259-006-0296-y. [DOI] [PubMed] [Google Scholar]
- 216.Meltzer CC, Smith G, Price JC, Reynolds CF, 3rd, Mathis CA, Greer P, Lopresti B, Mintun MA, Pollock BG, Ben-Eliezer D, Cantwell MN, Kaye W, DeKosky ST. Reduced binding of [18F]altanserin to serotonin type 2A receptors in aging: Persistence of effect after partial volume correction. Brain Res. 1998;813:167–171. doi: 10.1016/s0006-8993(98)00909-3. [DOI] [PubMed] [Google Scholar]
- 217.Sheline YI, Mintun MA, Moerlein SM, Snyder AZ. Greater loss of 5-HT(2A) receptors in midlife than in late life. Am J Psychiatry. 2002;159:430–435. doi: 10.1176/appi.ajp.159.3.430. [DOI] [PubMed] [Google Scholar]
- 218.Adams KH, Pinborg LH, Svarer C, Hasselbalch SG, Holm S, Haugbol S, Madsen K, Frokjaer V, Martiny L, Paulson OB, Knudsen GM. A database of [(18)F]-altanserin binding to 5-HT(2A) receptors in normal volunteers: Normative data and relationship to physiological and demographic variables. Neuroimage. 2004;21:1105–1113. doi: 10.1016/j.neuroimage.2003.10.046. [DOI] [PubMed] [Google Scholar]
- 219.Marner L, Knudsen GM, Haugbol S, Holm S, Baare W, Hasselbalch SG. Longitudinal assessment of cerebral 5-HT2A receptors in healthy elderly volunteers: An [18F]-altanserin PET study. Eur J Nucl Med Mol Imaging. 2009;36:287–293. doi: 10.1007/s00259-008-0945-4. [DOI] [PubMed] [Google Scholar]
- 220.Sheline YI, Mintun MA, Barch DM, Wilkins C, Snyder AZ, Moerlein SM. Decreased hippocampal 5-HT(2A) receptor binding in older depressed patients using [18F]altanserin positron emission tomography. Neuropsychopharmacology. 2004;29:2235–2241. doi: 10.1038/sj.npp.1300555. [DOI] [PubMed] [Google Scholar]
- 221.Mintun MA, Sheline YI, Moerlein SM, Vlassenko AG, Huang Y, Snyder AZ. Decreased hippocampal 5-HT2A receptor binding in major depressive disorder: In vivo measurement with [18F]altanserin positron emission tomography. Biol Psychiatry. 2004;55:217–224. doi: 10.1016/j.biopsych.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 222.Frank GK, Kaye WH, Meltzer CC, Price JC, Greer P, McConaha C, Skovira K. Reduced 5-HT2A receptor binding after recovery from anorexia nervosa. Biol Psychiatry. 2002;52:896–906. doi: 10.1016/s0006-3223(02)01378-1. [DOI] [PubMed] [Google Scholar]
- 223.Bailer UF, Price JC, Meltzer CC, Mathis CA, Frank GK, Weissfeld L, McConaha CW, Henry SE, Brooks-Achenbach S, Barbarich NC, Kaye WH. Altered 5-HT(2A) receptor binding after recovery from bulimia-type anorexia nervosa: Relationships to harm avoidance and drive for thinness. Neuropsychopharmacology. 2004;29:1143–1155. doi: 10.1038/sj.npp.1300430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Adams KH, Hansen ES, Pinborg LH, Hasselbalch SG, Svarer C, Holm S, Bolwig TG, Knudsen GM. Patients with obsessive-compulsive disorder have increased 5-HT2A receptor binding in the caudate nuclei. Int J Neuropsychopharmacol. 2005;8:391–401. doi: 10.1017/S1461145705005055. [DOI] [PubMed] [Google Scholar]
- 225.Meltzer CC, Price JC, Mathis CA, Greer PJ, Cantwell MN, Houck PR, Mulsant BH, Ben-Eliezer D, Lopresti B, DeKosky ST, Reynolds CF., 3rd PET imaging of serotonin type 2A receptors in late-life neuropsychiatric disorders. Am J Psychiatry. 1999;156:1871–1878. doi: 10.1176/ajp.156.12.1871. [DOI] [PubMed] [Google Scholar]
- 226.Hasselbalch SG, Madsen K, Svarer C, Pinborg LH, Holm S, Paulson OB, Waldemar G, Knudsen GM. Reduced 5-HT2A receptor binding in patients with mild cognitive impairment. Neurobiol Aging. 2008;29:1830–1838. doi: 10.1016/j.neurobiolaging.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 227.Haugbol S, Pinborg LH, Regeur L, Hansen ES, Bolwig TG, Nielsen FA, Svarer C, Skovgaard LT, Knudsen GM. Cerebral 5-HT2A receptor binding is increased in patients with Tourette’s syndrome. Int J Neuropsychopharmacol. 2007;10:245–252. doi: 10.1017/S1461145706006559. [DOI] [PubMed] [Google Scholar]
- 228.Hurlemann R, Boy C, Meyer PT, Scherk H, Wagner M, Herzog H, Coenen HH, Vogeley K, Falkai P, Zilles K, Maier W, Bauer A. Decreased prefrontal 5-HT2A receptor binding in subjects at enhanced risk for schizophrenia. Anat Embryol. 2005;210:519–523. doi: 10.1007/s00429-005-0036-2. [DOI] [PubMed] [Google Scholar]
- 229.Hurlemann R, Matusch A, Kuhn KU, Berning J, Elmenhorst D, Winz O, Kolsch H, Zilles K, Wagner M, Maier W, Bauer A. 5-HT2A receptor density is decreased in the at-risk mental state. Psychopharmacology. 2008;195:579–590. doi: 10.1007/s00213-007-0921-x. [DOI] [PubMed] [Google Scholar]
- 230.Erritzoe D, Rasmussen H, Kristiansen KT, Frokjaer VG, Haugbol S, Pinborg L, Baare W, Svarer C, Madsen J, Lublin H, Knudsen GM, Glenthoj BY. Cortical and subcortical 5-HT(2A) receptor binding in neuroleptic-naive first-episode schizophrenic patients. Neuropsychopharmacology. 2008;33:2435–2441. doi: 10.1038/sj.npp.1301656. [DOI] [PubMed] [Google Scholar]
- 231.Rasmussen H, Ebdrup BH, Erritzoe D, Aggernaes B, Oranje B, Kalbitzer J, Pinborg LH, Baare WF, Svarer C, Lublin H, Knudsen GM, Glenthoj B. Serotonin2A receptor blockade and clinical effect in first-episode schizophrenia patients treated with quetiapine. Psychopharmacology. 2011;213:583–592. doi: 10.1007/s00213-010-1941-5. [DOI] [PubMed] [Google Scholar]
- 232.Frokjaer VG, Mortensen EL, Nielsen FA, Haugbol S, Pinborg LH, Adams KH, Svarer C, Hasselbalch SG, Holm S, Paulson OB, Knudsen GM. Frontolimbic serotonin 2A receptor binding in healthy subjects is associated with personality risk factors for affective disorder. Biol Psychiatry. 2008;63:569–576. doi: 10.1016/j.biopsych.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 233.Frokjaer VG, Vinberg M, Erritzoe D, Baare W, Holst KK, Mortensen EL, Arfan H, Madsen J, Jernigan TL, Kessing LV, Knudsen GM. Familial risk for mood disorder and the personality risk factor, neuroticism, interact in their association with frontolimbic serotonin 2A receptor binding. Neuropsychopharmacology. 2010;35:1129–1137. doi: 10.1038/npp.2009.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Erritzoe D, Frokjaer VG, Haugbol S, Marner L, Svarer C, Holst K, Baare WF, Rasmussen PM, Madsen J, Paulson OB, Knudsen GM. Brain serotonin 2A receptor binding: Relations to body mass index, tobacco and alcohol use. Neuroimage. 2009;46:23–30. doi: 10.1016/j.neuroimage.2009.01.050. [DOI] [PubMed] [Google Scholar]
- 235.Frokjaer VG, Erritzoe D, Madsen J, Paulson OB, Knudsen GM. Gender and the use of hormonal contraception in women are not associated with cerebral cortical 5-HT 2A receptor binding. Neuroscience. 2009;163:640–645. doi: 10.1016/j.neuroscience.2009.06.052. [DOI] [PubMed] [Google Scholar]
- 236.Pinborg LH, Arfan H, Haugbol S, Kyvik KO, Hjelmborg JV, Svarer C, Frokjaer VG, Paulson OB, Holm S, Knudsen GM. The 5-HT2A receptor binding pattern in the human brain is strongly genetically determined. Neuroimage. 2008;40:1175–1180. doi: 10.1016/j.neuroimage.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 237.van Dyck CH, Soares JC, Tan PZ, Staley JK, Baldwin RM, Amici LA, Fu X, Garg PK, Seibyl JP, Charney DS, Innis RB. Equilibrium modeling of 5-HT(2A) receptors with [18F]deuteroaltanserin and PET: Feasibility of a constant infusion paradigm. Nucl Med Biol. 2000;27:715–722. doi: 10.1016/s0969-8051(00)00160-8. [DOI] [PubMed] [Google Scholar]
- 238.Soares JC, van Dyck CH, Tan P, Zoghbi SS, Garg P, Soufer R, Baldwin RM, Fujita M, Staley JK, Fu X, Amici L, Seibyl J, Innis RB. Reproducibility of in vivo brain measures of 5-HT2A receptors with PET and [18F]deuteroaltanserin. Psychiatry Res. 2001;106:81–93. doi: 10.1016/s0925-4927(01)00071-3. [DOI] [PubMed] [Google Scholar]
- 239.Staley JK, Van Dyck CH, Tan PZ, Al Tikriti M, Ramsby Q, Klump H, Ng C, Garg P, Soufer R, Baldwin RM, Innis RB. Comparison of [(18)F]altanserin and [(18)F]deuteroaltanserin for PET imaging of serotonin(2A) receptors in baboon brain: Pharmacological studies. Nucl Med Biol. 2001;28:271–279. doi: 10.1016/s0969-8051(00)00212-2. [DOI] [PubMed] [Google Scholar]
- 240.Kugaya A, Epperson CN, Zoghbi S, van Dyck CH, Hou Y, Fujita M, Staley JK, Garg PK, Seibyl JP, Innis RB. Increase in prefrontal cortex serotonin 2A receptors following estrogen treatment in postmenopausal women. Am J Psychiatry. 2003;160:1522–1524. doi: 10.1176/appi.ajp.160.8.1522. [DOI] [PubMed] [Google Scholar]
- 241.Santhosh L, Estok KM, Vogel RS, Tamagnan GD, Baldwin RM, Mitsis EM, Macavoy MG, Staley JK, van Dyck CH. Regional distribution and behavioral correlates of 5-HT(2A) receptors in Alzheimer’s disease with [(18)F]deuteroaltanserin and PET. Psychiatry Res. 2009;173:212–217. doi: 10.1016/j.pscychresns.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 242.Doble A, Girdlestone D, Piot O, Allam D, Betschart J, Boireau A, Dupuy A, Gueremy C, Menager J, Zundel JL, et al. Pharmacological characterization of RP 62203, a novel 5-hydroxytryptamine 5-HT2 receptor antagonist. Br J Pharmacol. 1992;105:27–36. doi: 10.1111/j.1476-5381.1992.tb14206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Besret L, Dauphin F, Huard C, Lasne MC, Vivet R, Mickala P, Barbelivien A, Baron JC. Specific in vivo binding in the rat brain of [18F]RP 62203: A selective 5-HT2A receptor radioligand for positron emission tomography. Nucl Med Biol. 1996;23:169–171. doi: 10.1016/0969-8051(95)02008-x. [DOI] [PubMed] [Google Scholar]
- 244.Ashworth S, Hume SP, Lammertsma AA, Opacka-Juffry J, Shah F, Pike VW. Development of central 5-HT2A receptor radioligands for PET: Comparison of [3H]RP 62203 and [3H]SR 46349B kinetics in rat brain. Nucl Med Biol. 1996;23:245–250. doi: 10.1016/0969-8051(95)02053-5. [DOI] [PubMed] [Google Scholar]
- 245.Lopez-Gimenez JF, Vilaro MT, Palacios JM, Mengod G. [3H]MDL 100,907 labels 5-HT2A serotonin receptors selectively in primate brain. Neuropharmacology. 1998;37:1147–1158. doi: 10.1016/s0028-3908(98)00102-6. [DOI] [PubMed] [Google Scholar]
- 246.Kristiansen H, Elfving B, Plenge P, Pinborg LH, Gillings N, Knudsen GM. Binding characteristics of the 5-HT2A receptor antagonists altanserin and MDL 100907. Synapse. 2005;58:249–257. doi: 10.1002/syn.20205. [DOI] [PubMed] [Google Scholar]
- 247.Lundkvist C, Halldin C, Ginovart N, Nyberg S, Swahn CG, Carr AA, Brunner F, Farde L. [11C]MDL 100907, a radioligland for selective imaging of 5-HT(2A) receptors with positron emission tomography. Life Sci. 1996;58:187–192. doi: 10.1016/0024-3205(96)00013-6. [DOI] [PubMed] [Google Scholar]
- 248.Johnson MP, Siegel BW, Carr AA. [3H]MDL 100,907: A novel selective 5-HT2A receptor ligand. Naunyn Schmiedebergs Arch Pharmacol. 1996;354:205–209. doi: 10.1007/BF00178722. [DOI] [PubMed] [Google Scholar]
- 249.Lopez-Gimenez JF, Mengod G, Palacios JM, Vilaro MT. Selective visualization of rat brain 5-HT2A receptors by autoradiography with [3H]MDL 100,907. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:446–454. doi: 10.1007/pl00005075. [DOI] [PubMed] [Google Scholar]
- 250.Ito H, Nyberg S, Halldin C, Lundkvist C, Farde L. PET imaging of central 5-HT2A receptors with carbon-11-MDL 100,907. J Nucl Med. 1998;39:208–214. [PubMed] [Google Scholar]
- 251.Watabe H, Channing MA, Der MG, Adams HR, Jagoda E, Herscovitch P, Eckelman WC, Carson RE. Kinetic analysis of the 5-HT2A ligand [11C]MDL 100,907. J Cereb Blood Flow Metab. 2000;20:899–909. doi: 10.1097/00004647-200006000-00002. [DOI] [PubMed] [Google Scholar]
- 252.Hinz R, Bhagwagar Z, Cowen PJ, Cunningham VJ, Grasby PM. Validation of a tracer kinetic model for the quantification of 5-HT(2A) receptors in human brain with [(11)C]MDL 100,907. J Cereb Blood Flow Metab. 2007;27:161–172. doi: 10.1038/sj.jcbfm.9600323. [DOI] [PubMed] [Google Scholar]
- 253.Meyer PT, Bhagwagar Z, Cowen PJ, Cunningham VJ, Grasby PM, Hinz R. Simplified quantification of 5-HT2A receptors in the human brain with [11C]MDL 100,907 PET and non-invasive kinetic analyses. Neuroimage. 2010;50:984–993. doi: 10.1016/j.neuroimage.2010.01.037. [DOI] [PubMed] [Google Scholar]
- 254.Perani D, Garibotto V, Gorini A, Moresco RM, Henin M, Panzacchi A, Matarrese M, Carpinelli A, Bellodi L, Fazio F. In vivo PET study of 5HT(2A) serotonin and D(2) dopamine dysfunction in drug-naive obsessive-compulsive disorder. Neuroimage. 2008;42:306–314. doi: 10.1016/j.neuroimage.2008.04.233. [DOI] [PubMed] [Google Scholar]
- 255.Herth MM, Debus F, Piel M, Palner M, Knudsen GM, Luddens H, Rosch F. Total synthesis and evaluation of [18F]MHMZ. Bioorg Med Chem Lett. 2008;18:1515–1519. doi: 10.1016/j.bmcl.2007.12.054. [DOI] [PubMed] [Google Scholar]
- 256.Herth MM, Piel M, Debus F, Schmitt U, Luddens H, Rosch F. Preliminary in vivo and ex vivo evaluation of the 5-HT2A imaging probe [(18)F]MH. MZ Nucl Med Biol. 2009;36:447–454. doi: 10.1016/j.nucmedbio.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 257.Herth MM, Kramer V, Piel M, Palner M, Riss PJ, Knudsen GM, Rosch F. Synthesis and in vitro affinities of various MDL 100907 derivatives as potential 18F-radioligands for 5-HT2A receptor imaging with PET. Bioorg Med Chem. 2009;17:2989–3002. doi: 10.1016/j.bmc.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 258.Debus F, Herth MM, Piel M, Buchholz HG, Bausbacher N, Kramer V, Luddens H, Rosch F. 18F-labeling and evaluation of novel MDL 100907 derivatives as potential 5-HT2A antagonists for molecular imaging. Nucl Med Biol. 2010;37:487–495. doi: 10.1016/j.nucmedbio.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 259.Ettrup A, Palner M, Gillings N, Santini MA, Hansen M, Kornum BR, Rasmussen LK, Någren K, Madsen J, Begtrup M, Knudsen GM. Radiosynthesis and evaluation of [11C]Cimbi-5 as a 5-HT2A receptor agonist radioligand for positron emission tomography. J Nucl Med. 2010;51:1763–1770. doi: 10.2967/jnumed.109.074021. [DOI] [PubMed] [Google Scholar]
- 260.Ettrup A, Hansen M, Santini MA, Paine J, Gillings N, Palner M, Lehel S, Herth MM, Madsen J, Kristensen J, Begtrup M, Knudsen GM. Radiosynthesis and in vivo evaluation of a series of substituted 11C-phenethylamines as 5-HT2A agonist PET tracers. Eur J Nucl Med Mol Imaging. 2011;38:681–693. doi: 10.1007/s00259-010-1686-8. [DOI] [PubMed] [Google Scholar]
- 261.Bonhaus DW, Bach C, DeSouza A, Salazar FH, Matsuoka BD, Zuppan P, Chan HW, Eglen RM. The pharmacology and distribution of human 5-hydroxytryptamine2B (5-HT2B) receptor gene products: Comparison with 5-HT2A and 5-HT2C receptors. Br J Pharmacol. 1995;115:622–628. doi: 10.1111/j.1476-5381.1995.tb14977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Kursar JD, Nelson DL, Wainscott DB, Baez M. Molecular cloning, functional expression, and mRNA tissue distribution of the human 5-hydroxytryptamine2B receptor. Mol Pharmacol. 1994;46:227–234. [PubMed] [Google Scholar]
- 263.Poissonnet G, Parmentier JG, Boutin JA, Goldstein S. The emergence of selective 5-HT 2B antagonists structures, activities and potential therapeutic applications. Mini Rev Med Chem. 2004;4:325–330. doi: 10.2174/1389557043487312. [DOI] [PubMed] [Google Scholar]
- 264.Leonhardt S, Gorospe E, Hoffman BJ, Teitler M. Molecular pharmacological differences in the interaction of serotonin with 5-hydroxytryptamine1C and 5-hydroxytryptamine2 receptors. Mol Pharmacol. 1992;42:328–335. [PubMed] [Google Scholar]
- 265.Hoyer D, Pazos A, Probst A, Palacios JM. Serotonin receptors in the human brain. II. Characterization and autoradiographic localization of 5-HT1C and 5-HT2 recognition sites. Brain Res. 1986;376:97–107. doi: 10.1016/0006-8993(86)90903-0. [DOI] [PubMed] [Google Scholar]
- 266.Molineaux SM, Jessell TM, Axel R, Julius D. 5-HT1c receptor is a prominent serotonin receptor subtype in the central nervous system. Proc Natl Acad Sci USA. 1989;86:6793–6797. doi: 10.1073/pnas.86.17.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Hemedah M, Coupar IM, Mitchelson FJ. [3H]-Mesulergine labels 5-HT7 sites in rat brain and guinea-pig ileum but not rat jejunum. Br J Pharmacol. 1999;126:179–188. doi: 10.1038/sj.bjp.0702293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Reynolds GP, Kirk SL. Metabolic side effects of antipsychotic drug treatment—Pharmacological mechanisms. Pharmacol Ther. 2010;125:169–179. doi: 10.1016/j.pharmthera.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 269.Millan MJ, Gobert A, Lejeune F, Dekeyne A, Newman-Tancredi A, Pasteau V, Rivet JM, Cussac D. The novel melatonin agonist agomelatine (S20098) is an antagonist at 5-hydroxy-tryptamine2C receptors, blockade of which enhances the activity of frontocortical dopaminergic and adrenergic pathways. J Pharmacol Exp Ther. 2003;306:954–964. doi: 10.1124/jpet.103.051797. [DOI] [PubMed] [Google Scholar]
- 270.Costall B, Naylor RJ. 5-HT3 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3:27–37. doi: 10.2174/1568007043482624. [DOI] [PubMed] [Google Scholar]
- 271.Fletcher S, Barnes NM. Distribution and characterisation of the [3H](S)-zacopride labelled 5-HT3 receptor in pig forebrain. Brain Res. 1996;729:281–284. [PubMed] [Google Scholar]
- 272.Abi-Dargham A, Laruelle M, Lipska B, Jaskiw GE, Wong DT, Robertson DW, Weinberger DR, Kleinman JE. Serotonin 5-HT3 receptors in schizophrenia: A postmortem study of the amygdala. Brain Res. 1993;616:53–57. doi: 10.1016/0006-8993(93)90191-o. [DOI] [PubMed] [Google Scholar]
- 273.Bufton KE, Steward LJ, Barber PC, Barnes NM. Distribution and characterization of the [3H]granisetron-labelled 5-HT3 receptor in the human forebrain. Neuropharmacology. 1993;32:1325–1331. doi: 10.1016/0028-3908(93)90027-z. [DOI] [PubMed] [Google Scholar]
- 274.Parker RM, Barnes JM, Ge J, Barber PC, Barnes NM. Autoradiographic distribution of [3H]-(S)-zacopride-labelled 5-HT3 receptors in human brain. J Neurol Sci. 1996;144:119–127. doi: 10.1016/s0022-510x(96)00211-0. [DOI] [PubMed] [Google Scholar]
- 275.Engleman EA, Rodd ZA, Bell RL, Murphy JM. The role of 5-HT3 receptors in drug abuse and as a target for pharmacotherapy. CNS Neurol Disord Drug Targets. 2008;7:454–467. doi: 10.2174/187152708786927886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Ford AC, Brandt LJ, Young C, Chey WD, Foxx-Orenstein AE, Moayyedi P. Efficacy of 5-HT3 antagonists and 5-HT4 agonists in irritable bowel syndrome: Systematic review and meta-analysis. Am J Gastroenterol. 2009;104:1831–1843. doi: 10.1038/ajg.2009.223. quiz 1844. [DOI] [PubMed] [Google Scholar]
- 277.Hewlett WA, Trivedi BL, Zhang ZJ, de Paulis T, Schmidt DE, Lovinger DM, Ansari MS, Ebert MH. Characterization of (S)-des-4-amino-3-[125I]iodozacopride ([125I]DAIZAC), a selective high-affinity radioligand for 5-hydroxytryptamine3 receptors. J Pharmacol Exp Ther. 1999;288:221–231. [PubMed] [Google Scholar]
- 278.Camsonne R, Barre L, Petit-Taboue MC, Travere JM, Jones R, Debruyne D, Moulin MA, MacKenzie ET, Baron JC. Positron emission tomographic studies of [11C]MDL 72222, a potential 5-HT3 receptor radioligand: Distribution, kinetics and binding in the brain of the baboon. Neuropharmacology. 1993;32:65–71. doi: 10.1016/0028-3908(93)90131-l. [DOI] [PubMed] [Google Scholar]
- 279.Ishiwata K, Ishii K, Ishii S, Senda M. Synthesis of 5-HT3 receptor antagonists, [11C]Y-25130 and [11C]YM060. Appl Radiat Isot. 1995;46:907–910. doi: 10.1016/0969-8043(95)00147-6. [DOI] [PubMed] [Google Scholar]
- 280.Ishiwata K, Saito N, Yanagawa K, Furuta R, Ishii S, Kiyosawa M, Homma Y, Ishii K, Suzuki F, Senda M. Synthesis and evaluation of 5-HT3 receptor antagonist [11C]KF17643. Nucl Med Biol. 1996;23:285–290. doi: 10.1016/0969-8051(95)02079-9. [DOI] [PubMed] [Google Scholar]
- 281.Guillouet S, Barre L, Gourand F, Lasne MC, Rault S. Synthesis of [11C]-S21007 a novel 5HT3 partial agonist as a potential tracer for PET studies. J Labl Compd Radiopharm. 1996;38:367–371. [Google Scholar]
- 282.Besret L, Dauphin F, Guillouet S, Dhilly M, Gourand F, Blaizot X, Young AR, Petit-Taboue MC, Mickala P, Barbelivien A, Rault S, Barre L, Baron JC. [11C]S21007, a putative partial agonist for 5-HT3 receptors PET studies. Rat and primate in vivo biological evaluation. Life Sci. 1998;62:115–129. doi: 10.1016/s0024-3205(97)01058-8. [DOI] [PubMed] [Google Scholar]
- 283.Katounina T, Besret L, Dhilly M, Petit-Taboue MC, Barbelivien A, Baron JC, Dauphin F, Barre L. Synthesis and biological investigations of [18F]MR18445, a 5-HT3 receptor partial agonist. Bioorg Med Chem. 1998;6:789–795. doi: 10.1016/s0968-0896(98)00035-2. [DOI] [PubMed] [Google Scholar]
- 284.Thorell JO, Stone-Elander S, Eriksson L, Ingvar M. N-Methylquipazine: Carbon-11 labelling of the 5-HT3 agonist and in vivo evaluation of its biodistribution using PET. Nucl Med Biol. 1997;24:405–412. doi: 10.1016/s0969-8051(97)00003-6. [DOI] [PubMed] [Google Scholar]
- 285.Gao M, Wang M, Hutchins GD, Zheng QH. Synthesis of new carbon-11 labeled benzoxazole derivatives for PET imaging of 5-HT(3) receptor. Eur J Med Chem. 2008;43:1570–1574. doi: 10.1016/j.ejmech.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 286.Bockaert J, Claeysen S, Compan V, Dumuis A. 5-HT4 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3:39–51. doi: 10.2174/1568007043482615. [DOI] [PubMed] [Google Scholar]
- 287.Bonaventure P, Hall H, Gommeren W, Cras P, Langlois X, Jurzak M, Leysen JE. Mapping of serotonin 5-HT(4) receptor mRNA and ligand binding sites in the post-mortem human brain. Synapse. 2000;36:35–46. doi: 10.1002/(SICI)1098-2396(200004)36:1<35::AID-SYN4>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 288.Jakeman LB, To ZP, Eglen RM, Wong EH, Bonhaus DW. Quantitative autoradiography of 5-HT4 receptors in brains of three species using two structurally distinct radioligands, [3H]GR113808 and [3H]BIMU-1. Neuropharmacology. 1994;33:1027–1038. doi: 10.1016/0028-3908(94)90162-7. [DOI] [PubMed] [Google Scholar]
- 289.Waeber C, Sebben M, Grossman C, Javoy-Agid F, Bockaert J, Dumuis A. [3H]-GR113808 labels 5-HT4 receptors in the human and guinea-pig brain. Neuroreport. 1993;4:1239–1242. doi: 10.1097/00001756-199309000-00007. [DOI] [PubMed] [Google Scholar]
- 290.Marner L, Gillings N, Comley RA, Baare WF, Rabiner EA, Wilson AA, Houle S, Hasselbalch SG, Svarer C, Gunn RN, Laruelle M, Knudsen GM. Kinetic modeling of 11C-SB207145 binding to 5-HT4 receptors in the human brain in vivo. J Nucl Med. 2009;50:900–908. doi: 10.2967/jnumed.108.058552. [DOI] [PubMed] [Google Scholar]
- 291.Marner L, Gillings N, Madsen K, Erritzoe D, Baare WF, Svarer C, Hasselbalch SG, Knudsen GM. Brain imaging of serotonin 4 receptors in humans with [11C]SB207145-PET. Neuroimage. 2010;50:855–861. doi: 10.1016/j.neuroimage.2010.01.054. [DOI] [PubMed] [Google Scholar]
- 292.Brown AM, Young TJ, Patch TL, Cheung CW, Kaumannm AJ, Gaster L, King FD. [125I]SB207710, a potent, selective radioligand for 5-HT4 receptors. Br J Pharmacol. 1993;110:10P. [Google Scholar]
- 293.Patel S, Roberts J, Moorman J, Reavill C. Localization of serotonin-4 receptors in the striatonigral pathway in rat brain. Neuroscience. 1995;69:1159–1167. doi: 10.1016/0306-4522(95)00314-9. [DOI] [PubMed] [Google Scholar]
- 294.Pike VW, Halldin C, Nobuhara K, Hiltunen J, Mulligan RS, Swahn CG, Karlsson P, Olsson H, Hume SP, Hirani E, Whalley J, Pilowsky LS, Larsson S, Schnell PO, Ell PJ, Farde L. Radioiodinated SB 207710 as a radioligand in vivo: Imaging of brain 5-HT4 receptors with SPET. Eur J Nucl Med Mol Imaging. 2003;30:1520–1528. doi: 10.1007/s00259-003-1307-x. [DOI] [PubMed] [Google Scholar]
- 295.Gee AD, Martarello L, Passchier J, Wishart M, Parker C, Matthews J, Comley R, Hopper R, Gunn R. Synthesis and evaluation of [11C]SB207145 as the first in vivo serotonin 5-HT4 receptor radioligand for PET imaging in man. Curr Radiopharm. 2008;1:110–114. [Google Scholar]
- 296.Kornum BR, Lind NM, Gillings N, Marner L, Andersen F, Knudsen GM. Evaluation of the novel 5-HT4 receptor PET ligand [11C]SB207145 in the Gottingen minipig. J Cereb Blood Flow Metab. 2009;29:186–196. doi: 10.1038/jcbfm.2008.110. [DOI] [PubMed] [Google Scholar]
- 297.Volk B, Nagy BJ, Vas S, Kostyalik D, Simig G, Bagdy G. Medicinal chemistry of 5-HT5A receptor ligands: A receptor subtype with unique therapeutical potential. Curr Topics Med Chem. 2010;10:554–578. doi: 10.2174/156802610791111588. [DOI] [PubMed] [Google Scholar]
- 298.Gannon RL, Peglion JL, Millan MJ. Differential influence of selective 5-HT5A vs 5-HT1A, 5-HT1B, or 5-HT2C receptor blockade upon light-induced phase shifts in circadian activity rhythms: Interaction studies with citalopram. Eur Neuropsychopharmacol. 2009;19:887–897. doi: 10.1016/j.euroneuro.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 299.Grailhe R, Grabtree GW, Hen R. Human 5-HT(5) receptors: The 5-HT(5A) receptor is functional but the 5-HT(5B) receptor was lost during mammalian evolution. Eur J Pharmacol. 2001;418:157–167. doi: 10.1016/s0014-2999(01)00933-5. [DOI] [PubMed] [Google Scholar]
- 300.Nelson DL. 5-HT5 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3:53–58. doi: 10.2174/1568007043482606. [DOI] [PubMed] [Google Scholar]
- 301.Woolley ML, Marsden CA, Fone KC. 5-ht6 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3:59–79. doi: 10.2174/1568007043482561. [DOI] [PubMed] [Google Scholar]
- 302.East SZ, Burnet PW, Leslie RA, Roberts JC, Harrison PJ. 5-HT6 receptor binding sites in schizophrenia and following antipsychotic drug administration: Autoradiographic studies with [125I]SB-258585. Synapse. 2002;45:191–199. doi: 10.1002/syn.10097. [DOI] [PubMed] [Google Scholar]
- 303.Hirst WD, Minton JA, Bromidge SM, Moss SF, Latter AJ, Riley G, Routledge C, Middlemiss DN, Price GW. Characterization of [(125)I]-SB-258585 binding to human recombinant and native 5-HT(6) receptors in rat, pig and human brain tissue. Br J Pharmacol. 2000;130:1597–1605. doi: 10.1038/sj.bjp.0703458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Zhou P, Yan Y, Bernotas R, Harrison BL, Huryn D, Robichaud AJ, Zhang GM, Smith DL, Schechter LE. 4-(2-Aminoethoxy)-N-(phenylsulfonyl)indoles as novel 5-HT6 receptor ligands. Bioorg Med Chem Lett. 2005;15:1393–1396. doi: 10.1016/j.bmcl.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 305.Tang S, Verdurand M, Joseph B, Lemoine L, Daoust A, Billard T, Fournet G, Le Bars D, Zimmer L. Synthesis and biological evaluation in rat and cat of [18F]12ST05 as a potential 5-HT6 PET radioligand. Nucl Med Biol. 2007;34:995–1002. doi: 10.1016/j.nucmedbio.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 306.Martarello L, Cunningham VJ, Matthews JC, Rabiner EA, Jakobsen S, Gee AD. Radiolabelling and in vivo evaluation of [11C]GSK215083 as potential PET radioligand for the 5-HT6 receptor in porcine brain. J Cereb Blood Flow Metab. 2005;25:S598. [Google Scholar]
- 307.Huiban M, Passchier J, Martarello L, Cunningham VJ, Jakobsen S, Gee AD. [11C]GSK224558 as a potential PET ligand for the delineation of 5-HT6 receptors. Neuroimage. 2006;31:T12–T43. [Google Scholar]
- 308.Parker CA, Cunningham VJ, Martarello L, Rabiner EA, Searle GE, Gee AD, Davy M, Johnson CN, Ahmed M, Gunn RN, Laruelle M. Evaluation of the novel 5-HT6 receptor radioligand, [11C]GSK215083 in human. Neuroimage. 2008;41:T20. [Google Scholar]
- 309.Parker CA, Martarello L, Rabiner EA, Gunn RN, Laruelle M, Slifstein M, Cunningham VJ. Evaluation of the in vivo ED50 for the PET radioligand, [11C]GSK215083, in papio anubis. Neuroimage. 2010;52:S37. [Google Scholar]
- 310.Thomas DR, Atkinson PJ, Hastie PG, Roberts JC, Middlemiss DN, Price GW. [3H]-SB-269970 radiolabels 5-HT7 receptors in rodent, pig and primate brain tissues. Neuropharmacology. 2002;42:74–81. doi: 10.1016/s0028-3908(01)00151-4. [DOI] [PubMed] [Google Scholar]
- 311.Hedlund PB, Sutcliffe JG. Functional, molecular and pharmacological advances in 5-HT7 receptor research. Trends Pharmacol Sci. 2004;25:481–486. doi: 10.1016/j.tips.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 312.Thomas DR, Hagan JJ. 5-HT7 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3:81–90. doi: 10.2174/1568007043482633. [DOI] [PubMed] [Google Scholar]
- 313.Krobert KA, Bach T, Syversveen T, Kvingedal AM, Levy FO. The cloned human 5-HT7 receptor splice variants: A comparative characterization of their pharmacology, function and distribution. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:620–632. doi: 10.1007/s002100000369. [DOI] [PubMed] [Google Scholar]
- 314.Andries J, Lemoine L, Mouchel-Blaisot A, Tang S, Verdurand M, Le Bars D, Zimmer L, Billard T. Looking for a 5-HT7 radiotracer for positron emission tomography. Bioorg Med Chem Lett. 2010;20:3730–3733. doi: 10.1016/j.bmcl.2010.04.076. [DOI] [PubMed] [Google Scholar]
- 315.Kikuchi C, Hiranuma T, Koyama M. Tetrahydrothienopyridylbutyl-tetrahydrobenzindoles: New selective ligands of the 5-HT7 receptor. Bioorg Med Chem Lett. 2002;12:2549–2552. doi: 10.1016/s0960-894x(02)00485-7. [DOI] [PubMed] [Google Scholar]
- 316.Zhang MR, Haradahira T, Maeda J, Okauchi T, Kida T, Obayashi S, Suzuki K, Suhara T. Synthesis and preliminary PET study of the 5-HT7 receptor antagonist [11C]DR4446. J Labl Compd Radiopharm. 2002;45:857–866. [Google Scholar]
- 317.Palacin M, Estevez R, Bertran J, Zorzano A. Molecular biology of mammalian plasma membrane amino acid transporters. Physiol Rev. 1998;78:969–1054. doi: 10.1152/physrev.1998.78.4.969. [DOI] [PubMed] [Google Scholar]
- 318.Murphy DL, Lesch KP. Targeting the murine serotonin transporter: Insights into human neurobiology. Nat Rev Neurosci. 2008;9:85–96. doi: 10.1038/nrn2284. [DOI] [PubMed] [Google Scholar]
- 319.Huang Y, Zheng MQ, Gerdes JM. Development of effective PET and SPECT imaging agents for the serotonin transporter: has a twenty-year journey reached its destination? Curr Topics Med Chem. 2010;10:1499–1526. doi: 10.2174/156802610793176792. [DOI] [PubMed] [Google Scholar]
- 320.Rosel P, Arranz B, Vallejo J, Oros M, Menchon JM, Alvarez P, Navarro MA. High affinity [3H]imipramine and [3H]paroxetine binding sites in suicide brains. J Neural Transm. 1997;104:921–929. doi: 10.1007/BF01285560. [DOI] [PubMed] [Google Scholar]
- 321.Smith DF, Jensen PN, Gee AD, Hansen SB, Danielsen E, Andersen F, Saiz PA, Gjedde A. PET neuroimaging with [11C]venlafaxine: Serotonin uptake inhibition, biodistribution and binding in living pig brain. Eur Neuropsychopharmacol. 1997;7:195–200. doi: 10.1016/s0924-977x(97)00403-3. [DOI] [PubMed] [Google Scholar]
- 322.Stanley M, Virgilio J, Gershon S. Tritiated imipramine binding sites are decreased in the frontal cortex of suicides. Science. 1982;216:1337–1339. doi: 10.1126/science.7079769. [DOI] [PubMed] [Google Scholar]
- 323.Hashimoto K, Inoue O, Suzuki K, Yamasaki T, Kojima M. Synthesis and evaluation of [11C]cyanoimipramine. Int J Rad Appl Instrum B. 1987;14:587–592. doi: 10.1016/0883-2897(87)90030-4. [DOI] [PubMed] [Google Scholar]
- 324.Laruelle M, Baldwin RM, Malison RT, Zea-Ponce Y, Zoghbi SS, al-Tikriti MS, Sybirska EH, Zimmermann RC, Wisniewski G, Neumeyer JL, Milius RA, Wang S, Smith EO, Roth RH, Charney DS, Hoffer PB, Innis RB. SPECT imaging of dopamine and serotonin transporters with [123I]beta-CIT: Pharmacological characterization of brain uptake in nonhuman primates. Synapse. 1993;13:295–309. doi: 10.1002/syn.890130402. [DOI] [PubMed] [Google Scholar]
- 325.Brucke T, Kornhuber J, Angelberger P, Asenbaum S, Frassine H, Podreka I. SPECT imaging of dopamine and serotonin transporters with [123I]beta-CIT. Binding kinetics in the human brain. J Neural Transm Gen Sect. 1993;94:137–146. doi: 10.1007/BF01245007. [DOI] [PubMed] [Google Scholar]
- 326.Lundkvist C, Halldin C, Swahn CG, Ginovart N, Farde L. Different brain radioactivity curves in a PET study with [11C]beta-CIT labelled in two different positions. Nucl Med Biol. 1999;26:343–350. doi: 10.1016/s0969-8051(98)00111-5. [DOI] [PubMed] [Google Scholar]
- 327.Bergstrom KA, Halldin C, Hall H, Lundkvist C, Ginovart N, Swahn CG, Farde L. In vitro and in vivo characterisation of nor-beta-CIT: A potential radioligand for visualisation of the serotonin transporter in the brain. Eur J Nucl Med. 1997;24:596–601. doi: 10.1007/BF00841395. [DOI] [PubMed] [Google Scholar]
- 328.Hiltunen J, Akerman KK, Kuikka JT, Bergstrom KA, Halldin C, Nikula T, Rasanen P, Tiihonen J, Vauhkonen M, Karhu J, Kupila J, Lansimies E, Farde L. Iodine-123 labeled nor-beta-CIT as a potential tracer for serotonin transporter imaging in the human brain with single-photon emission tomography. Eur J Nucl Med. 1998;25:19–23. doi: 10.1007/s002590050189. [DOI] [PubMed] [Google Scholar]
- 329.Reneman L, Booij J, Lavalaye J, De Bruin K, De Wolff FA, Koopmans RP, Stoof JC, Den Heeten GJ. Comparative in vivo study of iodine-123-labeled beta-CIT and nor-beta-CIT binding to serotonin transporters in rat brain. Synapse. 1999;34:77–80. doi: 10.1002/(SICI)1098-2396(199910)34:1<77::AID-SYN9>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 330.Lehto S, Tolmunen T, Joensuu M, Saarinen PI, Vanninen R, Ahola P, Tiihonen J, Kuikka J, Lehtonen J. Midbrain binding of [123I]nor-beta-CIT in atypical depression. Prog Neuropsycho-pharmacol Biol Psychiatry. 2006;30:1251–1255. doi: 10.1016/j.pnpbp.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 331.Joensuu M, Tolmunen T, Saarinen PI, Tiihonen J, Kuikka J, Ahola P, Vanninen R, Lehtonen J. Reduced midbrain serotonin transporter availability in drug-naive patients with depression measured by SERT-specific [(123)I] nor-beta-CIT SPECT imaging. Psychiatry Res. 2007;154:125–131. doi: 10.1016/j.pscychresns.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 332.Maron E, Kuikka JT, Ulst K, Tiihonen J, Vasar V, Shlik J. SPECT imaging of serotonin transporter binding in patients with generalized anxiety disorder. Eur Arch Psychiatry Clin Neurosci. 2004;254:392–396. doi: 10.1007/s00406-004-0520-3. [DOI] [PubMed] [Google Scholar]
- 333.Zitterl W, Aigner M, Stompe T, Zitterl-Eglseer K, Gutierrez-Lobos K, Wenzel T, Zettinig G, Hornik K, Pirker W, Thau K. Changes in thalamus-hypothalamus serotonin transporter availability during clomipramine administration in patients with obsessive-compulsive disorder. Neuropsychopharmacology. 2008;33:3126–3134. doi: 10.1038/npp.2008.35. [DOI] [PubMed] [Google Scholar]
- 334.Maron E, Kuikka JT, Shlik J, Vasar V, Vanninen E, Tiihonen J. Reduced brain serotonin transporter binding in patients with panic disorder. Psychiatry Res. 2004;132:173–181. doi: 10.1016/j.pscychresns.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 335.Caretti V, Stoffers D, Winogrodzka A, Isaias IU, Costantino G, Pezzoli G, Ferrarese C, Antonini A, Wolters EC, Booij J. Loss of thalamic serotonin transporters in early drug-naive Parkinson’s disease patients is associated with tremor: An [(123)I]beta-CIT SPECT study. J Neural Transm. 2008;115:721–729. doi: 10.1007/s00702-007-0015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Kung MP, Hou C, Oya S, Mu M, Acton PD, Kung HF. Characterization of [(123)I]IDAM as a novel single-photon emission tomography tracer for serotonin transporters. Eur J Nucl Med. 1999;26:844–853. doi: 10.1007/s002590050458. [DOI] [PubMed] [Google Scholar]
- 337.Oya S, Kung MP, Acton PD, Mu M, Hou C, Kung HF. A new single-photon emission computed tomography imaging agent for serotonin transporters: [123I]IDAM, 5-iodo-2-((2-((dimethylamino)-methyl)phenyl)thio)benzyl alcohol. J Med Chem. 1999;42:333–335. doi: 10.1021/jm9806751. [DOI] [PubMed] [Google Scholar]
- 338.Acton PD, Kung MP, Mu M, Plossl K, Hou C, Siciliano M, Oya S, Kung HF. Single-photon emission tomography imaging of serotonin transporters in the non-human primate brain with the selective radioligand [(123)I]IDAM. Eur J Nucl Med. 1999;26:854–861. doi: 10.1007/s002590050459. [DOI] [PubMed] [Google Scholar]
- 339.Acton PD, Mu M, Plossl K, Hou C, Siciliano M, Zhuang ZP, Oya S, Choi SR, Kung HF. Single-photon emission tomography imaging of serotonin transporters in the nonhuman primate brain with [(123)I]ODAM. Eur J Nucl Med. 1999;26:1359–1362. doi: 10.1007/s002590050596. [DOI] [PubMed] [Google Scholar]
- 340.Newberg AB, Plossl K, Mozley PD, Stubbs JB, Wintering N, Udeshi M, Alavi A, Kauppinen T, Kung HF. Biodistribution and imaging with (123)I-ADAM: A serotonin transporter imaging agent. J Nucl Med. 2004;45:834–841. [PubMed] [Google Scholar]
- 341.Huang WS, Ma KH, Cheng CY, Chen CY, Fu YK, Chou YH, Wey SP, Liu JC. Imaging serotonin transporters with 123I-ADAM brain SPECT in healthy non-human primates. Nucl Med Commun. 2004;25:515–519. doi: 10.1097/00006231-200405000-00014. [DOI] [PubMed] [Google Scholar]
- 342.Catafau AM, Perez V, Penengo MM, Bullich S, Danus M, Puigdemont D, Pascual JC, Corripio I, Llop J, Perich J, Alvarez E. SPECT of serotonin transporters using 123I-ADAM: Optimal imaging time after bolus injection and long-term test-retest in healthy volunteers. J Nucl Med. 2005;46:1301–1309. [PubMed] [Google Scholar]
- 343.Frokjaer VG, Pinborg LH, Madsen J, de Nijs R, Svarer C, Wagner A, Knudsen GM. Evaluation of the serotonin transporter ligand 123I-ADAM for SPECT studies on humans. J Nucl Med. 2008;49:247–254. doi: 10.2967/jnumed.107.046102. [DOI] [PubMed] [Google Scholar]
- 344.Erlandsson K, Sivananthan T, Lui D, Spezzi A, Townsend CE, Mu S, Lucas R, Warrington S, Ell PJ. Measuring SSRI occupancy of SERT using the novel tracer [123I]ADAM: A SPECT validation study. Eur J Nucl Med Mol Imaging. 2005;32:1329–1336. doi: 10.1007/s00259-005-1912-y. [DOI] [PubMed] [Google Scholar]
- 345.Klein N, Sacher J, Geiss-Granadia T, Attarbaschi T, Mossaheb N, Lanzenberger R, Potzi C, Holik A, Spindelegger C, Asenbaum S, Dudczak R, Tauscher J, Kasper S. In vivo imaging of serotonin transporter occupancy by means of SPECT and [123I]ADAM in healthy subjects administered different doses of escitalopram or citalopram. Psychopharmacology. 2006;188:263–272. doi: 10.1007/s00213-006-0486-0. [DOI] [PubMed] [Google Scholar]
- 346.Herold N, Uebelhack K, Franke L, Amthauer H, Luedemann L, Bruhn H, Felix R, Uebelhack R, Plotkin M. Imaging of serotonin transporters and its blockade by citalopram in patients with major depression using a novel SPECT ligand [123I]-ADAM. J Neural Transm. 2006;113:659–670. doi: 10.1007/s00702-005-0429-7. [DOI] [PubMed] [Google Scholar]
- 347.Catafau AM, Perez V, Plaza P, Pascual JC, Bullich S, Suarez M, Penengo MM, Corripio I, Puigdemont D, Danus M, Perich J, Alvarez E. Serotonin transporter occupancy induced by paroxetine in patients with major depression disorder: A 123I-ADAM SPECT study. Psycho-pharmacology. 2006;189:145–153. doi: 10.1007/s00213-006-0540-y. [DOI] [PubMed] [Google Scholar]
- 348.Klein N, Sacher J, Geiss-Granadia T, Mossaheb N, Attarbaschi T, Lanzenberger R, Spindelegger C, Holik A, Asenbaum S, Dudczak R, Tauscher J, Kasper S. Higher serotonin transporter occupancy after multiple dose administration of escitalopram compared to citalopram: An [123I]ADAM SPECT study. Psychopharmacology. 2007;191:333–339. doi: 10.1007/s00213-006-0666-y. [DOI] [PubMed] [Google Scholar]
- 349.Newberg AB, Amsterdam JD, Wintering N, Ploessl K, Swanson RL, Shults J, Alavi A. 123I-ADAM binding to serotonin transporters in patients with major depression and healthy controls: A preliminary study. J Nucl Med. 2005;46:973–977. [PubMed] [Google Scholar]
- 350.Schuh-Hofer S, Richter M, Geworski L, Villringer A, Israel H, Wenzel R, Munz DL, Arnold G. Increased serotonin transporter availability in the brainstem of migraineurs. J Neurol. 2007;254:789–796. doi: 10.1007/s00415-006-0444-0. [DOI] [PubMed] [Google Scholar]
- 351.Koch W, Schaaff N, Popperl G, Mulert C, Juckel G, Reicherzer M, Ehmer-von Geiso C, Moller HJ, Hegerl U, Tatsch K, Pogarell O. [I-123] ADAM and SPECT in patients with borderline personality disorder and healthy control subjects. J Psychiatry Neurosci. 2007;32:234–240. [PMC free article] [PubMed] [Google Scholar]
- 352.Lundgren JD, Newberg AB, Allison KC, Wintering NA, Ploessl K, Stunkard AJ. (123)I-ADAM SPECT imaging of serotonin transporter binding in patients with Night Eating Syndrome: A preliminary report. Psychiatry Res. 2008;162:214–220. doi: 10.1016/j.pscychresns.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Szabo Z, Kao PF, Scheffel U, Suehiro M, Mathews WB, Ravert HT, Musachio JL, Marenco S, Kim SE, Ricaurte GA, Wong DF, Wagner HA, Jr, Dannals RF. Positron emission tomography imaging of serotonin transporters in the human brain using [11C](+)McN5652. Synapse. 1995;20:37–43. doi: 10.1002/syn.890200107. [DOI] [PubMed] [Google Scholar]
- 354.Frankle WG, Huang Y, Hwang DR, Talbot PS, Slifstein M, Van Heertum R, Abi-Dargham A, Laruelle M. Comparative evaluation of serotonin transporter radioligands 11C-DASB and 11C-McN 5652 in healthy humans. J Nucl Med. 2004;45:682–694. [PubMed] [Google Scholar]
- 355.Buchert R, Thiele F, Thomasius R, Wilke F, Petersen K, Brenner W, Mester J, Spies L, Clausen M. Ecstasy-induced reduction of the availability of the brain serotonin transporter as revealed by [11C](+)McN5652-PET and the multi-linear reference tissue model: Loss of transporters or artifact of tracer kinetic modelling? J Psychopharmacol. 2007;21:628–634. doi: 10.1177/0269881106071975. [DOI] [PubMed] [Google Scholar]
- 356.McCann UD, Szabo Z, Seckin E, Rosenblatt P, Mathews WB, Ravert HT, Dannals RF, Ricaurte GA. Quantitative PET studies of the serotonin transporter in MDMA users and controls using [11C]McN5652 and [11C]DASB. Neuropsychopharmacology. 2005;30:1741–1750. doi: 10.1038/sj.npp.1300736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Frankle WG, Lombardo I, New AS, Goodman M, Talbot PS, Huang Y, Hwang DR, Slifstein M, Curry S, Abi-Dargham A, Laruelle M, Siever LJ. Brain serotonin transporter distribution in subjects with impulsive aggressivity: A positron emission study with [11C]McN 5652. Am J Psychiatry. 2005;162:915–923. doi: 10.1176/appi.ajp.162.5.915. [DOI] [PubMed] [Google Scholar]
- 358.Miller JM, Kinnally EL, Ogden RT, Oquendo MA, Mann JJ, Parsey RV. Reported childhood abuse is associated with low serotonin transporter binding in vivo in major depressive disorder. Synapse. 2009;63:565–573. doi: 10.1002/syn.20637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Brust P, Hinz R, Kuwabara H, Hesse S, Zessin J, Pawelke B, Stephan H, Bergmann R, Steinbach J, Sabri O. In vivo measurement of the serotonin transporter with (S)-([18F]fluoro-methyl)-(+)-McN5652. Neuropsychopharmacology. 2003;28:2010–2019. doi: 10.1038/sj.npp.1300281. [DOI] [PubMed] [Google Scholar]
- 360.Huang Y, Hwang DR, Narendran R, Sudo Y, Chatterjee R, Bae SA, Mawlawi O, Kegeles LS, Wilson AA, Kung HF, Laruelle M. Comparative evaluation in nonhuman primates of five PET radiotracers for imaging the serotonin transporters: [11C]McN 5652, [11C]ADAM, [11C]DASB, [11C]DAPA, and [11C]AFM. J Cereb Blood Flow Metab. 2002;22:1377–1398. doi: 10.1097/01.WCB.0000040948.67415.05. [DOI] [PubMed] [Google Scholar]
- 361.Shiue GG, Choi SR, Fang P, Hou C, Acton PD, Cardi C, Saffer JR, Greenberg JH, Karp JS, Kung HF, Shiue CY. N,N-Dimethyl-2-(2-amino-4-(18)F-fluorophenylthio)-benzylamine (4-(18)F-ADAM): An improved PET radioligand for serotonin transporters. J Nucl Med. 2003;44:1890–1897. [PubMed] [Google Scholar]
- 362.Fang P, Shiue GG, Shimazu T, Greenberg JH, Shiue CY. Synthesis and evaluation of N,N-dimethyl-2-(2-amino-5-[18F]fluorophenylthio)benzylamine (5-[18F]-ADAM) as a serotonin transporter imaging agent. Appl Radiat Isot. 2004;61:1247–1254. doi: 10.1016/j.apradiso.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 363.Zessin J, Deuther-Conrad W, Kretzschmar M, Wust F, Pawelke B, Brust P, Steinbach J, Bergmann R. [11C]SMe-ADAM, an imaging agent for the brain serotonin transporter: Synthesis, pharmacological characterization and microPET studies in rats. Nucl Med Biol. 2006;33:53–63. doi: 10.1016/j.nucmedbio.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 364.Huang Y, Hwang DR, Zhu Z, Bae SA, Guo N, Sudo Y, Kegeles LS, Laruelle M. Synthesis and pharmacological characterization of a new PET ligand for the serotonin transporter: [11C]5-bromo-2-[2-(dimethylaminomethylphenylsulfanyl)]phenylamine ([11C]DAPA) Nucl Med Biol. 2002;29:741–751. doi: 10.1016/s0969-8051(02)00337-2. [DOI] [PubMed] [Google Scholar]
- 365.Huang Y, Narendran R, Bae SA, Erritzoe D, Guo N, Zhu Z, Hwang DR, Laruelle M. A PET imaging agent with fast kinetics: Synthesis and in vivo evaluation of the serotonin transporter ligand [11C]2-[2-dimethylaminomethylphenylthio)]-5-fluorophenylamine ([11C]AFA) Nucl Med Biol. 2004;31:727–738. doi: 10.1016/j.nucmedbio.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 366.Jarkas N, McConathy J, Votaw JR, Voll RJ, Malveaux E, Camp VM, Williams L, Goodman RR, Kilts CD, Goodman MM. Synthesis and characterization of EADAM: A selective radioligand for mapping the brain serotonin transporters by positron emission tomography. Nucl Med Biol. 2005;32:75–86. doi: 10.1016/j.nucmedbio.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 367.Houle S, Ginovart N, Hussey D, Meyer JH, Wilson AA. Imaging the serotonin transporter with positron emission tomography: Initial human studies with [11C]DAPP and [11C]DASB. Eur J Nucl Med. 2000;27:1719–1722. doi: 10.1007/s002590000365. [DOI] [PubMed] [Google Scholar]
- 368.Zhu Z, Guo N, Narendran R, Erritzoe D, Ekelund J, Hwang DR, Bae SA, Laruelle M, Huang Y. The new PET imaging agent [11C]AFE is a selective serotonin transporter ligand with fast brain uptake kinetics. Nucl Med Biol. 2004;31:983–994. doi: 10.1016/j.nucmedbio.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 369.Halldin C, Lundberg J, Sovago J, Gulyas B, Guilloteau D, Vercouillie J, Emond P, Chalon S, Tarkiainen J, Hiltunen J, Farde L. [(11)C]MADAM, a new serotonin transporter radioligand characterized in the monkey brain by PET. Synapse. 2005;58:173–183. doi: 10.1002/syn.20189. [DOI] [PubMed] [Google Scholar]
- 370.Wilson AA, Ginovart N, Hussey D, Meyer J, Houle S. In vitro and in vivo characterisation of [11C]-DASB: A probe for in vivo measurements of the serotonin transporter by positron emission tomography. Nucl Med Biol. 2002;29:509–515. doi: 10.1016/s0969-8051(02)00316-5. [DOI] [PubMed] [Google Scholar]
- 371.Wilson AA, Houle S. Radiosynthesis of carbon-11 labelled N-methyl-2-(arylthio)benzylamines: Potential radiotracers for the serotonin reuptake receptor. J Labl Compd Radiopharm. 1999;42:1277–1288. [Google Scholar]
- 372.Ginovart N, Wilson AA, Meyer JH, Hussey D, Houle S. Positron emission tomography quantification of [(11)C]-DASB binding to the human serotonin transporter: Modeling strategies. J Cereb Blood Flow Metab. 2001;21:1342–1353. doi: 10.1097/00004647-200111000-00010. [DOI] [PubMed] [Google Scholar]
- 373.Szabo Z, McCann UD, Wilson AA, Scheffel U, Owonikoko T, Mathews WB, Ravert HT, Hilton J, Dannals RF, Ricaurte GA. Comparison of (+)-(11)C-McN5652 and (11)C-DASB as serotonin transporter radioligands under various experimental conditions. J Nucl Med. 2002;43:678–692. [PMC free article] [PubMed] [Google Scholar]
- 374.Kim JS, Ichise M, Sangare J, Innis RB. PET imaging of serotonin transporters with [11C]DASB: Test-retest reproducibility using a multilinear reference tissue parametric imaging method. J Nucl Med. 2006;47:208–214. [PubMed] [Google Scholar]
- 375.Parsey RV, Kent JM, Oquendo MA, Richards MC, Pratap M, Cooper TB, Arango V, Mann JJ. Acute occupancy of brain serotonin transporter by sertraline as measured by [11C]DASB and positron emission tomography. Biol Psychiatry. 2006;59:821–828. doi: 10.1016/j.biopsych.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 376.Takano A, Suzuki K, Kosaka J, Ota M, Nozaki S, Ikoma Y, Tanada S, Suhara T. A dose-finding study of duloxetine based on serotonin transporter occupancy. Psychopharmacology. 2006;185:395–399. doi: 10.1007/s00213-005-0304-0. [DOI] [PubMed] [Google Scholar]
- 377.Voineskos AN, Wilson AA, Boovariwala A, Sagrati S, Houle S, Rusjan P, Sokolov S, Spencer EP, Ginovart N, Meyer JH. Serotonin transporter occupancy of high-dose selective serotonin reuptake inhibitors during major depressive disorder measured with [11C]DASB positron emission tomography. Psychopharmacology. 2007;193:539–545. doi: 10.1007/s00213-007-0806-z. [DOI] [PubMed] [Google Scholar]
- 378.Meyer JH, Houle S, Sagrati S, Carella A, Hussey DF, Ginovart N, Goulding V, Kennedy J, Wilson AA. Brain serotonin transporter binding potential measured with carbon 11-labeled DASB positron emission tomography: Effects of major depressive episodes and severity of dysfunctional attitudes. Arch Gen Psychiatry. 2004;61:1271–1279. doi: 10.1001/archpsyc.61.12.1271. [DOI] [PubMed] [Google Scholar]
- 379.Bhagwagar Z, Murthy N, Selvaraj S, Hinz R, Taylor M, Fancy S, Grasby P, Cowen P. 5-HTT binding in recovered depressed patients and healthy volunteers: A positron emission tomography study with [11C]DASB. Am J Psychiatry. 2007;164:1858–1865. doi: 10.1176/appi.ajp.2007.06111933. [DOI] [PubMed] [Google Scholar]
- 380.Reimold M, Batra A, Knobel A, Smolka MN, Zimmer A, Mann K, Solbach C, Reischl G, Schwarzler F, Grunder G, Machulla HJ, Bares R, Heinz A. Anxiety is associated with reduced central serotonin transporter availability in unmedicated patients with unipolar major depression: A [(11)C]DASB PET study. Mol Psychiatry. 2008;13:606–613. doi: 10.1038/sj.mp.4002149. [DOI] [PubMed] [Google Scholar]
- 381.Frankle WG, Narendran R, Huang Y, Hwang DR, Lombardo I, Cangiano C, Gil R, Laruelle M, Abi-Dargham A. Serotonin transporter availability in patients with schizophrenia: A positron emission tomography imaging study with [11C]DASB. Biol Psychiatry. 2005;57:1510–1516. doi: 10.1016/j.biopsych.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 382.Reimold M, Smolka MN, Zimmer A, Batra A, Knobel A, Solbach C, Mundt A, Smoltczyk HU, Goldman D, Mann K, Reischl G, Machulla HJ, Bares R, Heinz A. Reduced availability of serotonin transporters in obsessive-compulsive disorder correlates with symptom severity—A [11C]DASB PET study. J Neural Transm. 2007;114:1603–1609. doi: 10.1007/s00702-007-0785-6. [DOI] [PubMed] [Google Scholar]
- 383.Brown AK, George DT, Fujita M, Liow JS, Ichise M, Hibbeln J, Ghose S, Sangare J, Hommer D, Innis RB. PET [11C]DASB imaging of serotonin transporters in patients with alcoholism. Alcohol Clin Exp Res. 2007;31:28–32. doi: 10.1111/j.1530-0277.2006.00261.x. [DOI] [PubMed] [Google Scholar]
- 384.Cannon DM, Ichise M, Fromm SJ, Nugent AC, Rollis D, Gandhi SK, Klaver JM, Charney DS, Manji HK, Drevets WC. Serotonin transporter binding in bipolar disorder assessed using [11C]DASB and positron emission tomography. Biol Psychiatry. 2006;60:207–217. doi: 10.1016/j.biopsych.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 385.Kalbitzer J, Frokjaer VG, Erritzoe D, Svarer C, Cumming P, Nielsen FA, Hashemi SH, Baare WF, Madsen J, Hasselbalch SG, Kringelbach ML, Mortensen EL, Knudsen GM. The personality trait openness is related to cerebral 5-HTT levels. Neuroimage. 2009;45:280–285. doi: 10.1016/j.neuroimage.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 386.Kalbitzer J, Erritzoe D, Holst KK, Nielsen FA, Marner L, Lehel S, Arentzen T, Jernigan TL, Knudsen GM. Seasonal changes in brain serotonin transporter binding in short serotonin transporter linked polymorphic region-allele carriers but not in long-allele homozygotes. Biol Psychiatry. 2010;67:1033–1039. doi: 10.1016/j.biopsych.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 387.Frokjaer VG, Vinberg M, Erritzoe D, Svarer C, Baare W, Budtz-Joergensen E, Madsen K, Madsen J, Kessing LV, Knudsen GM. High familial risk for mood disorder is associated with low dorsolateral prefrontal cortex serotonin transporter binding. Neuroimage. 2009;46:360–366. doi: 10.1016/j.neuroimage.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 388.Lundberg J, Odano I, Olsson H, Halldin C, Farde L. Quantification of 11C-MADAM binding to the serotonin transporter in the human brain. J Nucl Med. 2005;46:1505–1515. [PubMed] [Google Scholar]
- 389.Chalon S, Tarkiainen J, Garreau L, Hall H, Emond P, Vercouillie J, Farde L, Dasse P, Varnas K, Besnard JC, Halldin C, Guilloteau D. Pharmacological characterization of N,N-dimethyl-2-(2-amino-4-methylphenyl thio)benzylamine as a ligand of the serotonin transporter with high affinity and selectivity. J Pharmacol Exp Ther. 2003;304:81–87. doi: 10.1124/jpet.102.042226. [DOI] [PubMed] [Google Scholar]
- 390.Cortes R, Soriano E, Pazos A, Probst A, Palacios JM. Autoradiography of antidepressant binding sites in the human brain: Localization using [3H]imipramine and [3H]paroxetine. Neuroscience. 1988;27:473–496. doi: 10.1016/0306-4522(88)90282-5. [DOI] [PubMed] [Google Scholar]
- 391.Plenge P, Mellerup ET, Laursen H. Regional distribution of the serotonin transport complex in human brain, identified with 3H-paroxetine, 3H-citalopram and 3H-imipramine. Prog Neuro-psychopharmacol Biol Psychiatry. 1990;14:61–72. doi: 10.1016/0278-5846(90)90064-n. [DOI] [PubMed] [Google Scholar]
- 392.Lundberg J, Halldin C, Farde L. Measurement of serotonin transporter binding with PET and [11C]MADAM: A test-retest reproducibility study. Synapse. 2006;60:256–263. doi: 10.1002/syn.20297. [DOI] [PubMed] [Google Scholar]
- 393.Lundberg J, Christophersen JS, Petersen KB, Loft H, Halldin C, Farde L. PET measurement of serotonin transporter occupancy: A comparison of escitalopram and citalopram. Int J Neuropsychopharmacol. 2007;10:777–785. doi: 10.1017/S1461145706007486. [DOI] [PubMed] [Google Scholar]
- 394.Henningsson S, Borg J, Lundberg J, Bah J, Lindstrom M, Ryding E, Jovanovic H, Saijo T, Inoue M, Rosen I, Traskman-Bendz L, Farde L, Eriksson E. Genetic variation in brain-derived neurotrophic factor is associated with serotonin transporter but not serotonin-1A receptor availability in men. Biol Psychiatry. 2009;66:477–485. doi: 10.1016/j.biopsych.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 395.Jovanovic H, Lundberg J, Karlsson P, Cerin A, Saijo T, Varrone A, Halldin C, Nordstrom AL. Sex differences in the serotonin 1A receptor and serotonin transporter binding in the human brain measured by PET. Neuroimage. 2008;39:1408–1419. doi: 10.1016/j.neuroimage.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 396.Jovanovic H, Karlsson P, Cerin A, Halldin C, Nordstrom AL. 5-HT(1A) receptor and 5-HTT binding during the menstrual cycle in healthy women examined with [(11)C] WAY100635 and [(11)C] MADAM PET. Psychiatry Res. 2009;172:31–37. doi: 10.1016/j.pscychresns.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 397.Lundberg J, Borg J, Halldin C, Farde L. A PET study on regional coexpression of 5-HT1A receptors and 5-HTT in the human brain. Psychopharmacology. 2007;195:425–433. doi: 10.1007/s00213-007-0928-3. [DOI] [PubMed] [Google Scholar]
- 398.Peng CJ, Huang YY, Huang WS, Shiue CY. An automated synthesis of N,N-dimethyl-2-(2-amino-4-[(18)F]fluorophenylthio)benzylamine (4-[(18)F]-ADAM) for imaging serotonin transporters. Appl Radiat Isot. 2008;66:625–631. doi: 10.1016/j.apradiso.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 399.Huang YY, Huang WS, Chu TC, Shiue CY. An improved synthesis of 4-[F-18]-ADAM, a potent serotonin transporter imaging agent. Appl Radiat Isot. 2009;67:1063–1067. doi: 10.1016/j.apradiso.2009.02.090. [DOI] [PubMed] [Google Scholar]
- 400.Ma KH, Huang WS, Kuo YY, Peng CJ, Liou NH, Liu RS, Hwang JJ, Liu JC, Chen HJ, Shiue CY. Validation of 4-[18F]-ADAM as a SERT imaging agent using micro-PET and autoradiography. Neuroimage. 2009;45:687–693. doi: 10.1016/j.neuroimage.2008.12.060. [DOI] [PubMed] [Google Scholar]
- 401.Huang YY, Ma KH, Tseng TW, Chou TK, Ng H, Mirsalis JC, Fu YK, Chu TC, Huang WS, Shiue CY. Biodistribution, toxicity and radiation dosimetry studies of the serotonin transporter radioligand 4-[18F]-ADAM in rats and monkeys. Eur J Nucl Med Mol Imaging. 2010;37:545–555. doi: 10.1007/s00259-009-1281-z. [DOI] [PubMed] [Google Scholar]
- 402.Diksic M, Young SN. Study of the brain serotonergic system with labeled alpha-methyl-L-tryptophan. J Neurochem. 2001;78:1185–1200. doi: 10.1046/j.1471-4159.2001.00536.x. [DOI] [PubMed] [Google Scholar]
- 403.Cohen Z, Tsuiki K, Takada A, Beaudet A, Diksic M, Hamel E. In-vivo synthesized radioactively labeled alpha-methyl serotonin as a selective tracer for visualization of brain-serotonin neurons. Synapse. 1995;21:21–28. doi: 10.1002/syn.890210104. [DOI] [PubMed] [Google Scholar]
- 404.Diksic M, Nagahiro S, Sourkes TL, Yamamoto YL. A new method to measure brain-serotonin synthesis in vivo 1. Theory and basic data for a biological model. J Cereb Blood Flow Metab. 1990;10:1–12. doi: 10.1038/jcbfm.1990.1. [DOI] [PubMed] [Google Scholar]
- 405.Shoaf SE, Carson R, Hommer D, Williams W, Higley JD, Schmall B, Herscovitch P, Eckelman W, Linnoila M. Brain serotonin synthesis rates in rhesus monkeys determined by [C-11]alpha-methyl-L-tryptophan and positron emission tomography compared to CSF 5-hydroxyindole-3-acetic acid concentrations. Neuropsychopharmacology. 1998;19:345–353. doi: 10.1016/S0893-133X(98)00032-3. [DOI] [PubMed] [Google Scholar]
- 406.Muzik O, Chugani DC, Chakraborty P, Mangner T, Chugani HT. Analysis of [(C-11)]alpha-methyl-tryptophan kinetics for the estimation of serotonin synthesis rate in vivo. J Cereb Blood Flow Metab. 1997;17:659–669. doi: 10.1097/00004647-199706000-00007. [DOI] [PubMed] [Google Scholar]
- 407.Chugani DC, Muzik O, Chakraborty P, Mangner T, Chugani HT. Human brain serotonin synthesis capacity measured in vivo with alpha-[C-11]methyl-L-tryptophan. Synapse. 1998;28:33–43. doi: 10.1002/(SICI)1098-2396(199801)28:1<33::AID-SYN5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 408.Gharib A, Balende C, Sarda N, Weissmann D, Plenevaux A, Luxen A, Bobillier P, Pujol JF. Biochemical and autoradiographic measurements of brain serotonin synthesis rate in the freely moving rat: A reexamination of the alpha-methyl-L-tryptophan method. J Neurochem. 1999;72:2593–2600. doi: 10.1046/j.1471-4159.1999.0722593.x. [DOI] [PubMed] [Google Scholar]
- 409.Shoaf SE, Carson RE, Hommer D, Williams WA, Higley JD, Schmall B, Herscovitch P, Eckelman WC, Linnoila M. The suitability of [C-11]-alpha-methyl-L-tryptophan as a tracer for serotonin synthesis: Studies with dual administration of [C-11] and [C-14] labeled tracer. J Cereb Blood Flow Metab. 2000;20:244–252. doi: 10.1097/00004647-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 410.Chugani DC, Muzik O. Alpha[C-11]methyl-L-tryptophan PET maps brain serotonin synthesis and kynurenine pathway metabolism. J Cereb Blood Flow Metab. 2000;20:2–9. doi: 10.1097/00004647-200001000-00002. [DOI] [PubMed] [Google Scholar]
- 411.Perreau-Linck E, Beauregard M, Gravel P, Paquette V, Soucy JP, Diksic M, Benkelfat C. In vivo measurements of brain trapping of C-11-labelled alpha-methyl-L-tryptophan during acute changes in mood states. J Psychiatr Neurosci. 2007;32:430–434. [PMC free article] [PubMed] [Google Scholar]
- 412.Berney A, Nishikawa M, Benkelfat C, Debonnel G, Gobbi G, Diksic M. An index of 5-HT synthesis changes during early antidepressant treatment: Alpha-[C-11]methyl-L-tryptophan PET study. Neurochem Int. 2008;52:701–708. doi: 10.1016/j.neuint.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 413.Bjurling P, Watanabe Y, Tokushige M, Oda T, Langstrom B. Syntheses of beta-C-11-labeled L-tryptophan and 5-hydroxy-L-tryptophan using a multi-enzymatic reaction route. J Chem Soc Perkin Trans. 1989;1:1331–1334. [Google Scholar]
- 414.Hartvig P, Tedroff J, Lindner KJ, Bjurling P, Chang CW, Tsukada H, Watanabe Y, Langstrom B. Positron emission tomographic studies on aromatic L-amino-acid decarboxylase activity in-vivo for L-dopa and 5-hydroxy-L-tryptophan in the monkey brain. J Neural Transm. 1993;94:127–135. doi: 10.1007/BF01245006. [DOI] [PubMed] [Google Scholar]
- 415.Hartvig P, Lindner KJ, Bjurling P, Langstrom B, Tedroff J. Pyridoxine effect on synthesis rate of serotonin in the monkey brain measured with positron emission tomography. J Neural Transm. 1995;102:91–97. doi: 10.1007/BF01276505. [DOI] [PubMed] [Google Scholar]
- 416.Reibring L, Agren H, Hartvig P, Tedroff J, Lundqvist H, Bjurling P, Kihlberg T, Langstrom B. Uptake and utilization of [Beta-C-11]5-hydroxytryptophan in human brain studied by positron emission tomography. Psychiatry Res Neuroimaging. 1992;45:215–225. doi: 10.1016/0925-4927(92)90017-x. [DOI] [PubMed] [Google Scholar]
- 417.Hagberg GE, Torstenson R, Marteinsdottir I, Fredrikson M, Langstrom B, Blomqvist G. Kinetic compartment modeling of [11C]-5-hydroxy-L-tryptophan for positron emission tomography assessment of serotonin synthesis in human brain. J Cereb Blood Flow Metab. 2002;22:1352–1366. doi: 10.1097/01.WCB.0000040946.89393.9d. [DOI] [PubMed] [Google Scholar]
- 418.Leyton M, Diksic M, Benkelfat C. Brain regional alpha-[C-11]methyl-L-tryptophan trapping), correlates with post-mortem tissue serotonin content and [C-11]5-hydroxytryptophan accumulation. Int J Neuropsychopharmacol. 2005;8:633–634. doi: 10.1017/S1461145705005420. [DOI] [PubMed] [Google Scholar]
- 419.Lundquist P, Hartvig P, Blomquist G, Hammarlund-Udenaes M, Langstrom B. 5-Hydroxy-L-[beta-C-11]tryptophan versus alpha-[C-11]methyl-L-tryptophan for positron emission tomography imaging of serotonin synthesis capacity in the rhesus monkey brain. J Cereb Blood Flow Metab. 2007;27:821–830. doi: 10.1038/sj.jcbfm.9600381. [DOI] [PubMed] [Google Scholar]