Abstract
The spider mite sub-family Tetranychinae includes many agricultural pests. The internal transcribed spacer (ITS) region of nuclear ribosomal RNA genes and the cytochrome c oxidase subunit I (COI) gene of mitochondrial DNA have been used for species identification and phylogenetic reconstruction within the sub-family Tetranychinae, although they have not always been successful. The 18S and 28S rRNA genes should be more suitable for resolving higher levels of phylogeny, such as tribes or genera of Tetranychinae because these genes evolve more slowly and are made up of conserved regions and divergent domains. Therefore, we used both the 18S (1,825–1,901 bp) and 28S (the 5′ end of 646–743 bp) rRNA genes to infer phylogenetic relationships within the sub-family Tetranychinae with a focus on the tribe Tetranychini. Then, we compared the phylogenetic tree of the 18S and 28S genes with that of the mitochondrial COI gene (618 bp). As observed in previous studies, our phylogeny based on the COI gene was not resolved because of the low bootstrap values for most nodes of the tree. On the other hand, our phylogenetic tree of the 18S and 28S genes revealed several well-supported clades within the sub-family Tetranychinae. The 18S and 28S phylogenetic trees suggest that the tribes Bryobiini, Petrobiini and Eurytetranychini are monophyletic and that the tribe Tetranychini is polyphyletic. At the genus level, six genera for which more than two species were sampled appear to be monophyletic, while four genera (Oligonychus, Tetranychus, Schizotetranychus and Eotetranychus) appear to be polyphyletic. The topology presented here does not fully agree with the current morphology-based taxonomy, so that the diagnostic morphological characters of Tetranychinae need to be reconsidered.
Introduction
The spider mite sub-family Tetranychinae includes some pests that cause serious economic losses throughout the world [1], [2], [3]. The family consists of more than 1,200 species, some of which have a wide host range, whereas others are highly host-specific [4], [5]. For example, Tetranychus urticae Koch, Panonychus citri (McGregor) and Oligonychus coffeae (Nietner), have an especially strong effect on agricultural and horticultural crops, and they are polyphagous. However, these genera also include mono-, oligophagous species, such as Tetranychus bambusae Wang & Ma, Panonychus bambusicola Ehara & Gotoh, Oligonychus orthius Rimando, Oligonychus modestus (Banks) and Oligonychus rubicundus Ehara which inhabit only gramineous plants.
Although exact species identification is the first step in any biological study, spider mites are difficult to distinguish by morphological characters alone because of their small size (<0.5 mm) and limited number of diagnostic characters [6], [7], [8]. Therefore, the use of DNA-based methods to identify species has increasingly been used for some genera of the Tetranychinae. For example, Navajas and Boursot [9] showed that T. urticae and Tetranychus turkestani Ugarov & Nikolskii, which are very closely related species, can be identified by using the internal transcribed spacer 2 (ITS2) region of nuclear ribosomal RNA (rRNA) genes. More recently, Matsuda et al. [10], [11] revealed that almost all species of Japanese Oligonychus (17 of 18 species) and all species of Tetranychus (13 species) can be identified by using the cytochrome c oxidase subunit I (COI) gene of mitochondrial DNA.
Despite recent advances in DNA-based methods for identifying spider mites, most phylogenetic relationships of sub-families, tribes and genera of the Tetranychinae remain poorly understood, as is reflected by the low support values for most nodes of the phylogenetic trees. However, phylogenetic trees clearly show that the genus Oligonychus is polyphyletic. Navajas et al. [12] and Ros and Breeuwer [13] analyzed the phylogeny of Tetranychinae including three Oligonychus species (Oligonychus ununguis (Jacobi), Oligonychus platani (McGregor) and Oligonychus gossypii (Zacher)) using the COI gene. Although these three species have the same empodium shape, O. gossypii, whose aedeagus curves dorsally, can be easily distinguished from O. ununguis and O. platani whose aedeagi curve ventrally. In the phylogenetic trees of these two studies, O. gossypii clustered more closely with Tetranychus species whose aedeagi also curve dorsally, while O. ununguis and O. platani formed a separate group. Polyphyly in the genus Oligonychus was also reported in the ITS2 region [14].
The unresolved phylogeny among the taxa of the sub-family Tetranychinae based on the COI sequences is probably due to the strongly biased nucleotide composition and the saturation at the third codon positions [13]. Because both the 18S and 28S rRNA genes evolve more slowly and are made up of conserved regions and divergent domains [15], these genes have been used for phylogenetic analyses of higher taxonomic relationships (from “phyla” to “classes” within Ecdysozoa) [16], [17]. In resolving tick genera (Acari: Ixodida), combining the 18S and 28S rRNA genes provided more detailed relationships than did the 18S gene alone [18], [19]. Therefore, we used both the 18S (1,825–1,901 bp) and 28S (the 5′ end of 646–743 bp) rRNA genes to infer phylogenetic relationships within the sub-family Tetranychinae. Then, we compared the trees based on the 18S and 28S genes with the tree based on the mitochondrial COI gene (618 bp). Another problem in previous studies [12], [13], [14] was that only 16 to 25 species were used for the phylogenetic analyses. Limited taxon sampling can seriously influence the resulting phylogenetic inferences (for reviews, see [20], [21], [22]). Therefore, to assess the phylogenetic relationships among tribes and genera of the sub-family Tetranychinae, we examined a total of 88 strains (15 genera and 4 tribes) most of which were from Japan.
Results
Mitochondrial COI gene
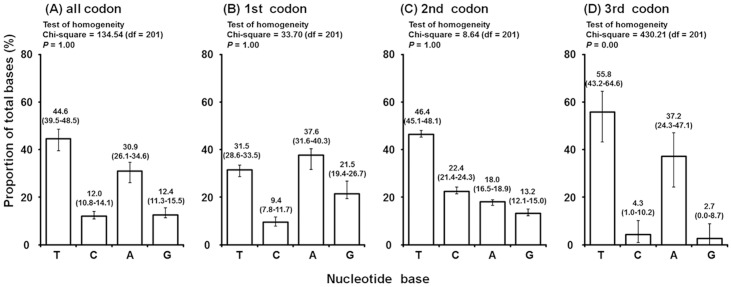
We obtained the COI sequences of 38 strains determined in this study (Table 1) and 30 strains from previously published data [10], [11]. The COI sequences contained no insertions or deletions. After alignment, the COI fragment had 618 nucleotides, of which 282 were parsimony-informative sites (File S1). The AT contents of the COI sequences of the tetranychid mites were very high (75.5%), especially at the 3rd codon position (93.0%). Chi-square tests revealed no significant heterogeneity in the first and second codon positions of the COI sequences, but significant heterogeneity at third codon positions (Figure 1). Similar high AT contents have been observed in previous studies of tetranychid mites [10], [11], [12], [13].
Table 1. Classification and sources of Tetranychid mites used in this study.
| Sub-family | Tribe | Genus | Species | Date | Locality | Host plant | Voucher specimen no.a | Accession no. | ||
| COI | 18S | 28S | ||||||||
| Bryobiinae | Bryobiini | Bryobia | B. eharai Pritchard & Keifer | Sept. 11, 2012 | Ibaraki, Japan | Chrysanthemum morifolium | 0612 | – | AB926227 | AB926318 |
| B. praetiosa Koch | July 27, 2008 | Hokkaido, Japan | Trifolium repens | 0609 | AB981203 | AB926228 | AB926319 | |||
| Petrobiini | Petrobia | P. latens (Müller) | Mar. 30, 2012 | Tokushima, Japan | Daucus carota | 0482 | AB981204 | AB926229 | AB926320 | |
| Tetranychina | T. harti (Ewing) | June 11, 2012 | Ibaraki, Japan | Oxalis corniculata | 0602 | – | AB926230 | AB926321 | ||
| Tetranychinae | Eurytetranychini | Eurytetranychoides | E. japonicus (Ehara) | Sept. 22, 2010 | Tokyo, Japan | Lithocarpus edulis | 0493 | AB981205 | AB926231 | AB926322 |
| Eutetranychus | E. africanus (Tucker) | June 30, 2008 | Taichung, Taiwan | Pueraria montana | 0377 | – | AB926232 | AB926323 | ||
| Aponychus | A. corpuzae Rimando | Apr. 10, 2001 | Ibaraki, Japan | Sasa senanensis | 0607 | AB981206 | AB926233 | AB926324 | ||
| A. firmianae (Ma & Yuan) | Aug. 7, 2010 | Ibaraki, Japan | Firmiana simplex | 0405 | – | AB926234 | AB926325 | |||
| Tetranychini | Panonychus | P. bambusicola Ehara & Gotoh | June 4, 1989 | Hokkaido, Japan | Sasa senanensis | 0606 | AB981207 | AB926235 | AB926326 | |
| P. caglei Mellot | Aug. 19, 2009 | Okinawa, Japan | Trichosanthes pilosa | 0611 | – | AB926236 | AB926327 | |||
| P. citri (McGregor) | May 6, 1993 | Ibaraki, Japan | Ilex crenata | 0226 | AB981208 | AB926237 | AB926328 | |||
| P. elongatus Manson | July 27, 2010 | Hangzhou, China | Broussonetia papyrifera | 0398 | – | AB926238 | AB926329 | |||
| P. mori Yokoyama | Apr. 22, 2007 | Hokkaido, Japan | Morus australis | 0239 | AB981209 | AB926239 | AB926330 | |||
| P. osmanthi Ehara & Gotoh | Nov. 16, 2001 | Guilin, China | Osmanthus fragrans | 0229 | AB981210 | AB926240 | AB926331 | |||
| P. thelytokus Ehara & Gotoh | Aug. 4, 2010 | Hokkaido, Japan | Ulmus davidiana | 0407 | AB981211 | AB926241 | AB926332 | |||
| P. ulmi (Koch) | Aug. 2, 2012 | Nagano, Japan | Malus pumila | 0603 | AB981212 | AB926242 | AB926333 | |||
| Sasanychus | S. akitanus (Ehara) | June 23, 1986 | Hokkaido, Japan | Sasa senanensis | 0605 | AB981213 | AB926243 | AB926334 | ||
| S. pusillus Ehara & Gotoh | July 31, 2012 | Hokkaido, Japan | Sasa chartacea | 0575 | AB981214 | AB926244 | AB926335 | |||
| Schizotetranychus | S. bambusae Reck | Aug. 27, 2011 | Chiba, Japan | Phyllostachys edulis | 0503 | AB981215 | AB926245 | AB926336 | ||
| S. brevisetosus Ehara | Oct. 13, 2011 | Kochi, Japan | Quercus glauca | 0527 | AB981216 | AB926246 | AB926337 | |||
| S. cercidiphylli Ehara | Aug. 3, 2010 | Hokkaido, Japan | Cercidiphyllum japonicum | 0411 | AB981217 | AB926247 | AB926338 | |||
| S. gilvus Ehara & Ohashi | May 22, 2012 | Nara, Japan | Quercus gilva | 0549 | AB981218 | AB926248 | AB926339 | |||
| S. lespedezae Begljarov & Mitrofanov | Aug. 26, 2011 | Ibaraki, Japan | Pueraria montana | 0515 | AB981219 | AB926249 | AB926340 | |||
| S. recki Ehara | Aug. 4, 2010 | Hokkaido, Japan | Sasa senanensis | 0408 | AB981220 | AB926250 | AB926341 | |||
| S. schizopus (Zacher) | June 14, 2010 | Tokyo, Japan | Salix integra | 0532 | AB981221 | AB926251 | AB926342 | |||
| S. shii (Ehara) | June 14, 2010 | Tokyo, Japan | Castanopsis sieboldii | 0533 | AB981222 | AB926252 | AB926343 | |||
| Stigmaeopsis | S. celarius Banks | Aug. 7, 2011 | Ibaraki, Japan | Pleioblastus chino | 0506 | AB981223 | AB926253 | AB926344 | ||
| S. longus (Saito) | June 4, 1989 | Hokkaido, Japan | Sasa senanensis | 0542 | AB981224 | AB926254 | AB926345 | |||
| S. miscanthi (Saito) | Feb. 16, 2009 | Nagasaki, Japan | Miscanthus sinensis | 0404 | AB981225 | AB926255 | AB926346 | |||
| S. saharai Saito & Mori | Aug. 5, 2011 | Chiba, Japan | Pleioblastus chino | 0501 | AB981226 | AB926256 | AB926347 | |||
| S. takahashii Saito & Mori | Oct. 27, 1997 | Hokkaido, Japan | Sasa senanensis | 0541 | AB981227 | AB926257 | AB926348 | |||
| Yezonychus | Y. sapporensis Ehara | Aug. 4, 2010 | Hokkaido, Japan | Sasa senanensis | 0406 | AB981228 | AB926258 | AB926349 | ||
| Eotetranychus | E. asiaticus Ehara | Mar. 19, 2007 | Nagasaki, Japan | Citrus reticulata | 0546 | AB981229 | AB926259 | AB926350 | ||
| E. boreus Ehara | June 3, 2010 | Wakayama, Japan | Armeniaca mume | 0415 | – | AB926260 | AB926351 | |||
| E. celtis Ehara | Aug. 27, 2011 | Chiba, Japan | Aphananthe aspera | 0502 | AB981230 | AB926261 | AB926352 | |||
| E. cornicola Ehara | Aug. 5, 2011 | Chiba, Japan | Cornus controversa | 0498 | AB981231 | AB926262 | AB926353 | |||
| E. dissectus Ehara | Aug. 3, 2010 | Hokkaido, Japan | Acer pictum | 0412 | AB981232 | AB926263 | AB926354 | |||
| E. nomurai Ehara | Aug. 20, 2011 | Ibaraki, Japan | Celtis sinensis | 0514 | AB981233 | AB926264 | AB926355 | |||
| E. pruni (Oudemans) | Sept. 1, 2012 | Ibaraki, Japan | Castanea crenata | 0562 | – | AB926265 | AB926356 | |||
| E. querci Reeves | Aug. 3, 2010 | Hokkaido, Japan | Tilia japonica | 0403 | – | AB926266 | AB926357 | |||
| E. quercifoliae Ehara & Gotoh | July 6, 2011 | Ibaraki, Japan | Quercus serrata | 0507 | AB981234 | AB926267 | AB926358 | |||
| E. rubricans Ehara | Sept. 1, 2012 | Ibaraki, Japan | Carpinus tschonoskii | 0559 | – | AB926268 | AB926359 | |||
| E. smithi Pritchard & Baker | Aug. 14, 2007 | Nagasaki, Japan | Rosa multiflora | 0545 | AB981235 | AB926269 | AB926360 | |||
| E. spectabilis Ehara | Sept. 7, 2011 | Hokkaido, Japan | Acer pictum | 0524 | – | AB926270 | AB926361 | |||
| E. suginamensis (Yokoyama) | Aug. 26, 2011 | Ibaraki, Japan | Morus australis | 0517 | AB981236 | AB926271 | AB926362 | |||
| E. tiliarium (Hermann) | Aug. 3, 2010 | Hokkaido, Japan | Alnus hirsuta | 0409 | – | AB926272 | AB926363 | |||
| E. toyoshimai Ehara & Gotoh | Aug. 29, 2011 | Iwate, Japan | Magnolia obovata | 0519 | – | AB926273 | AB926364 | |||
| E. uchidai Ehara | Aug. 15, 2011 | Hokkaido, Japan | Ulmus davidiana | 0528 | AB981237 | AB926274 | AB926365 | |||
| E. uncatus Garman | Aug. 3, 2010 | Hokkaido, Japan | Betula platyphylla | 0413 | – | AB926275 | AB926366 | |||
| Oligonychus | O. amiensis Ehara & Gotoh | July 13, 2005 | Ibaraki, Japan | Lithocarpus edulis | 0116 | AB683672 | AB926276 | AB926367 | ||
| O. biharensis (Hirst) | Dec. 21, 2007 | Okinawa, Japan | Mangifera indica | 0012 | AB683678 | AB926277 | AB926368 | |||
| O. camelliae Ehara & Gotoh | May 13, 2000 | Fukushima, Japan | Camellia japonica | 0082 | AB683662 | AB926278 | AB926369 | |||
| O. castaneae Ehara & Gotoh | May 5, 2009 | Ibaraki, Japan | Castanea crenata | 0297 | AB683667 | AB926279 | AB926370 | |||
| O. clavatus (Ehara) | July 28, 2009 | Kanagawa, Japan | Pinus thunbergii | 0360 | AB683654 | AB926280 | AB926371 | |||
| O. coffeae (Nietner) | May 30, 2005 | Okinawa, Japan | Mangifera indica | 0078 | AB683670 | AB926281 | AB926372 | |||
| O. gotohi Ehara | July 1, 2007 | Ibaraki, Japan | Lithocarpus edulis | 0076 | AB683668 | AB926282 | AB926373 | |||
| O. hondoensis (Ehara) | Aug. 22, 2009 | Aomori, Japan | Cryptomeria japonica | 0376 | AB683658 | AB926283 | AB926374 | |||
| O. ilicis (McGregor) | Oct. 30, 2000 | Kagoshima, Japan | Camellia sinensis | 0081 | AB683660 | AB926284 | AB926375 | |||
| O. karamatus (Ehara) | Aug. 27, 2009 | Hokkaido, Japan | Larix kaempferi | 0358 | AB683656 | AB926285 | AB926376 | |||
| O. modestus (Banks) | Sept. 9, 2008 | Okinawa, Japan | Digitaria ciliaris | 0092 | AB683677 | AB926286 | AB926377 | |||
| O. orthius Rimando | July 9, 2009 | Okinawa, Japan | Saccharum officinarum | 0378 | AB683675 | AB926287 | AB926378 | |||
| O. perditus Pritchard & Baker | Sept. 17, 2009 | Kanagawa, Japan | Juniperus sp. | 0364 | AB683665 | AB926288 | AB926379 | |||
| O. pustulosus Ehara | Aug. 22, 2009 | Aomori, Japan | Cryptomeria japonica | 0363 | AB683655 | AB926289 | AB926380 | |||
| O. rubicundus Ehara | Oct. 17, 2008 | Kochi, Japan | Miscanthus sinensis | 0290 | AB683681 | AB926290 | AB926381 | |||
| O. ununguis (Jacobi) | July 27, 2008 | Hokkaido, Japan | Cryptomeria japonica | 0088 | AB683664 | AB926291 | AB926382 | |||
| Amphitetranychus | A. quercivorus (Ehara & Gotoh) | July 9, 2003 | Ibaraki, Japan | Quercus crispula | 0610 | AB981238 | AB926292 | AB926383 | ||
| A. viennensis (Zacher) | Sept. 21, 2010 | Tokyo, Japan | Armeniaca vulgaris | 0613 | AB981239 | AB926293 | AB926384 | |||
| Tetranychus | T. bambusae Wang & Ma | July 5, 2009 | Okinawa, Japan | Phyllostachys edulis | 0343 | AB926294 | AB926385 | |||
| T. evansi Baker & Pritchard | Nov. 3, 2006 | Tokyo, Japan | Solanum nigrum | 0210 | AB736039 | AB926295 | AB926386 | |||
| T. ezoensis Ehara | Sept. 3, 2008 | Ibaraki, Japan | Taxus cuspidata | 0281 | AB736042 | AB926296 | AB926387 | |||
| T. huhhotensis Ehara, Gotoh & Hong | July 26, 2007 | Inner Mongolia Autonomous Region, Mongolia | Zea mays | 0201 | – | AB926297 | AB926388 | |||
| T. kanzawai Kishida | May 19, 1993 | Shizuoka, Japan | Thea sinensis | 0158 | AB736043 | AB926298 | AB926389 | |||
| T. lombardinii Baker & Pritchard | July 10, 2008 | Durban, South Africa | Erythrina variegata | 0381 | – | AB926299 | AB926390 | |||
| T. ludeni Zacher | Oct.17, 1995 | Ibaraki, Japan | Solidago virgaurea | 0189 | AB736051 | AB926300 | AB926391 | |||
| T. macfarlanei Baker & Pritchard | Sept. 30, 2008 | Mymensingh, Bangladesh | Dolichos lablab | 0389 | – | AB926301 | AB926392 | |||
| T. merganser Boudreaux | Apr. 6, 2007 | El Talo, Sonora, Mexico | Cucurbita maxima | 0225 | – | AB926302 | AB926393 | |||
| T. misumaiensis Ehara & Gotoh | Aug. 23, 2005 | Hokkaido, Japan | Apios sp. | 0218 | AB736054 | AB926303 | AB926394 | |||
| T. neocaledonicus Andre | May 27, 1998 | Tokyo, Japan | Morus australis | 0192 | AB736055 | AB926304 | AB926395 | |||
| T. okinawanus Ehara | June 19, 2003 | Okinawa, Japan | Pueraria montana | 0208 | AB736058 | AB926305 | AB926396 | |||
| T. parakanzawai Ehara | June 5, 1993 | Ibaraki, Japan | Pueraria montana | 0155 | AB736060 | AB926306 | AB926397 | |||
| T. phaselus Ehara | June 29, 2000 | Ibaraki, Japan | Glycine max | 0191 | AB736066 | AB926307 | AB926398 | |||
| T. piercei McGregor | Dec. 20, 2007 | Okinawa, Japan | Cucumis melo | 0014 | AB736068 | AB926308 | AB926399 | |||
| T. pueraricola Ehara & Gotoh | Oct. 23, 1993 | Ibaraki, Japan | Pueraria montana | 0203 | AB736071 | AB926309 | AB926400 | |||
| T. truncatus Ehara | May 8, 2004 | Kyoto, Japan | Solanum nigrum | 0195 | AB736075 | AB926310 | AB926401 | |||
| T. turkestani Ugarov & Nikolski | Sept. 15, 2007 | Hamedan, Iran | Phaseolus vulgaris | 0219 | AB981240 | AB926311 | AB926402 | |||
| T. urticae Koch (green form) | July 16, 2001 | Hokkaido, Japan | Citrullus lanatus | 0181 | AB736076 | AB926312 | AB926403 | |||
| T. urticae Koch (red form) | Aug. 27, 2001 | Nagano, Japan | Dianthus sp. | 0171 | AB736079 | AB926313 | AB926404 | |||
| T. zeae Ehara, Gotoh & Hong | July 26, 2007 | Inner Mongolia Autonomous Region, Mongolia | Zea mays | 0202 | – | AB926314 | AB926405 | |||
aVoucher specimens are preserved at the Laboratory of Applied Entomology and Zoology, Faculty of Agriculture, Ibaraki University under the serial voucher specimen number.
Figure 1. Base compositions of the codons of the mitochondrial COI gene.
(A) All codon positions, (B) 1st codon position, (C) 2nd codon position, (D) 3rd codon position, averaged over all 68 mite strains used in this study. Error bars depict range. Results of the homogeneity test are given for each codon position.
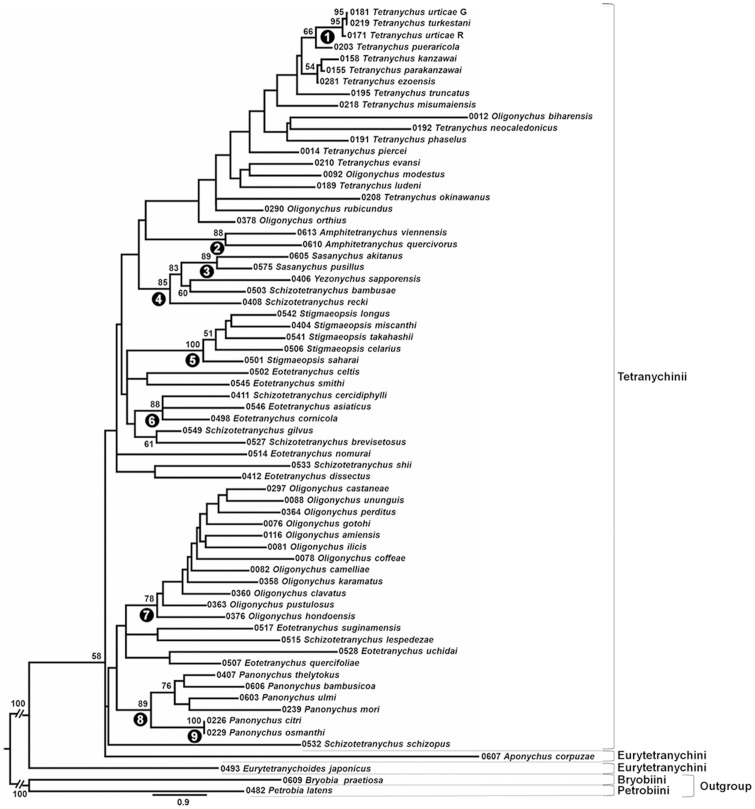
A phylogenetic tree of the sub-family Tetranychinae based on the COI gene is shown in Figure 2. Among the eight genera for which more than two strains were sampled, four genera (Panonychus, Sasanychus, Stigmaeopsis and Amphitetranychus) appear to be monophyletic with >80 bootstrap values, while the other four (Oligonychus, Tetranychus, Schizotetranychus and Eotetranychus) are polyphyletic. The four monophyletic genera are in clades 8, 3, 5 and 2, respectively (Figure 2). As was observed in previous studies, Oligonychus species whose aedeagus curves ventrally (clade 7) can be easily distinguished from Oligonychus biharensis (Hirst), O. modestus, O. orthius and O. rubicundus whose aedeagi curve dorsally. Although Schizotetranychus and Eotetranychus are scattered across the tree, some species formed well-supported clades. Schizotetranychus bambusae Reck & Schizotetranychus recki Ehara clustered with Sasanychus and Yezonychus species (clade 4). The clade including Schizotetranychus cercidiphylli Ehara, Eotetranychus asiaticus Ehara and Eotetranychus cornicola Ehara are supported with high bootstrap value (clade 6: bootstrap value (BP) = 88). The COI tree also shows monophyly of closely related species that morphologically and molecularly resemble each other, such as P. citri and Panonychus osmanthi Ehara & Gotoh [23], [24] (clade 9) and T. urticae and T. turkestani [9] (clade 1). These results are consistent with the 18S and 28S topologies described below. However, the COI phylogeny was not resolved and the deep-level relationships were especially unresolved, as shown by the low bootstrap values (Figure 2), as was observed in previous studies [12], [13]. The deep-level phylogeny of the sub-family Tetranychinae was also not resolved in the Bayesian tree (data not shown).
Figure 2. Maximum likelihood (ML) phylogenetic tree of the sub-family Tetranychinae based on the mitochondrial COI gene using the GTR Gamma model.
Bootstrap values (>50%) based on 1,000 replications are indicated at nodes. Each operational taxonomic unit is indicated by the voucher specimen no. and scientific name. Black circles with numbers indicate the clade no. which corresponds with the article.
18S and 28S rRNA genes
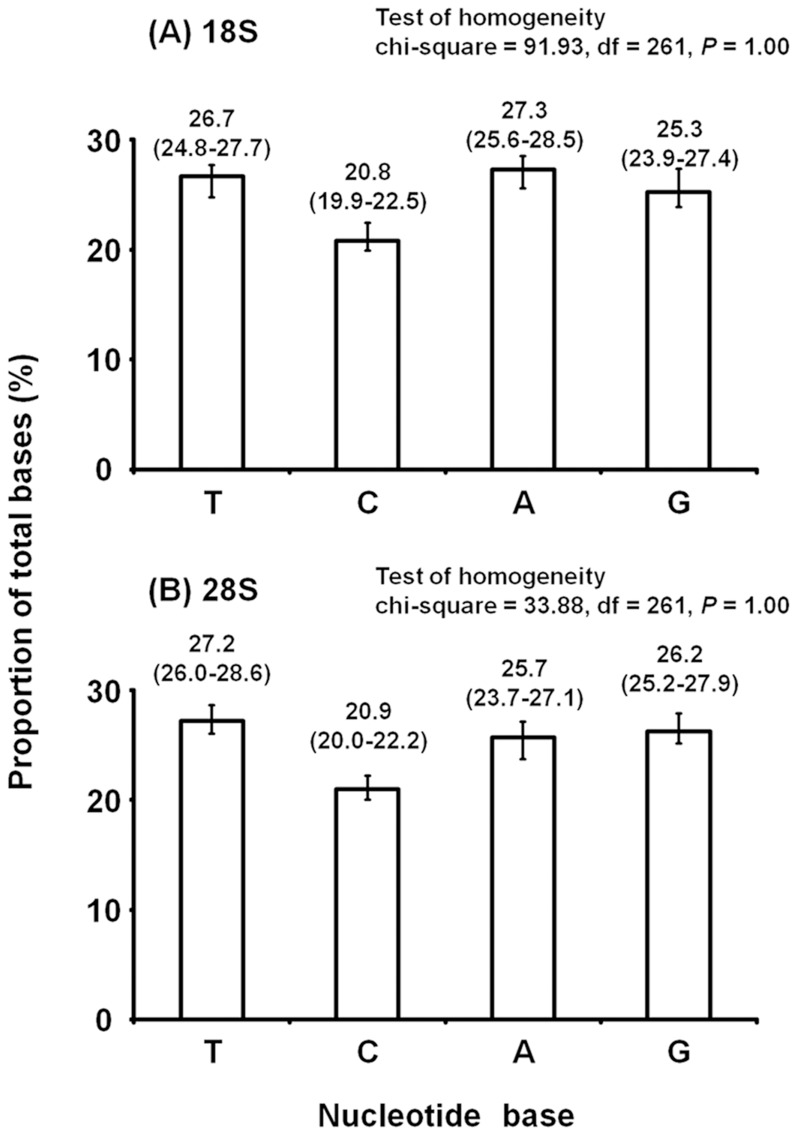
We determined the 18S and the 5′ end of the 28S rRNA sequences of all 88 strains used in this study (Table 1). The lengths of the 18S sequences obtained were 1,825–1,901 bp. The 18S and 28S sequences contained a number of gaps (insertions and deletions). After alignment and deletion of the ambiguous part of the aligned data, the final length was 1,863 bp, containing 495 parsimony-informative sites. The lengths of the 28S sequences were 646–743 bp, with a final length of 671 bp, containing 201 parsimony-informative sites. The aligned sequences before and after deleting the ambiguous parts are shown in Supporting Information (Files S2–S4). Chi-square tests revealed no significant heterogeneity in the nucleotide composition of the 18S and 28S sequences (Figure 3).
Figure 3. Base compositions of the (A) 18S and (B) 28S rRNA genes, averaged over all 88 mite strains used in this study.
Error bars depict range. Results of the homogeneity test are given for each gene.
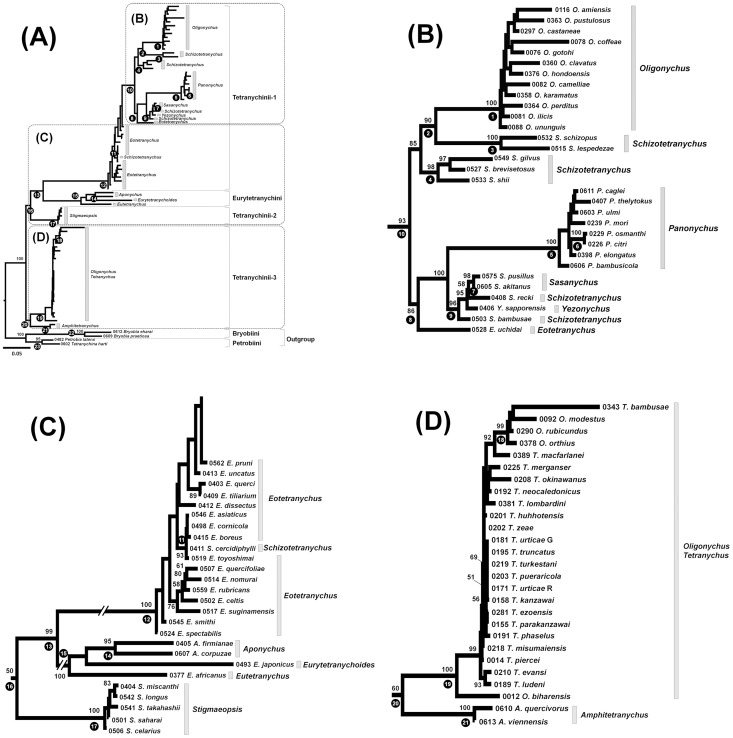
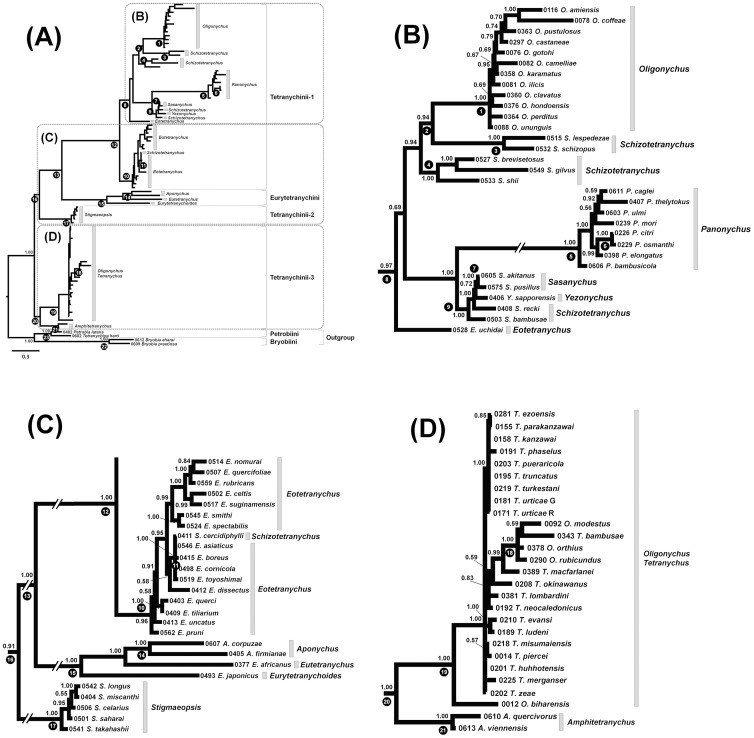
Phylogenetic trees based on a single gene were not as well resolved as phylogenetic trees based on the combined 18S and 28S data sets. Therefore, only the combined data set was used for the ML and Bayesian analyses. The 18S and 28S trees suggest that the tribes Bryobiini and Petrobiini of the sub-family Bryobiinae, which were used as outgroups, are both monophyletic (Figures 4A and 5A, clades 22 and 23). Within the Tetranychinae, Clade 15 is composed of species of Eurytetranychini, and clades 12,17 and 20 are composed of species of Tetranychini (Figures 4A and 5A). Among the 10 genera for which more than two strains were sampled, six genera (Bryobia, Aponychus, Panonychus, Sasanychus, Stigmaeopsis and Amphitetranychus), appear to be monophyletic with >95 bootstrap values and 1.00 posterior probabilities, while four genera (Oligonychus, Tetranychus, Schizotetranychus and Eotetranychus) are polyphyletic. The monophyletic genera are in clades 22, 14, 5, 7, 17 and 21, respectively (Figures 4A–4D and 5A–5D). Species of the genus Oligonychus are separated into 2 clades (clades 1 and 19), with the Tetranychus species included in clade 19 (Figures 4B, 4D, 5B and 5D). Schizotetranychus species, with the exception of S. cercidiphylli, are separated into 3 clades (clades 3, 4 and 9), with the Sasanychus and Yezonychus species included in clade 9 (Figures 4B and 5B). In the ML tree (Figures 4B–4C), S. cercidiphylli and Eotetranychus species, with the exception of Eotetranychus uchidai Ehara, are paraphyletic with respect to clade 10. E. uchidai forms a sister group with Panonychus, Sasanychus, Schizotetranychus and Yezonychus species (Figure 4B, clade 8). In the Bayesian tree (Figures 5B–5C), a well-supported clade consisting of S. cercidiphylli and Eotetranychus species, with the exception of E. uchidai (clade 10: Bayesian posterior probabilities (BPP) = 0.96) clustered with clade 8.
Figure 4. Maximum likelihood (ML) phylogenetic tree of the sub-family Tetranychinae based on the 18S and 28S rRNA genes using the GTR Gamma model.
Bootstrap values (>50%) based on 1,000 replications are indicated at nodes. Each operational taxonomic unit is indicated by the voucher specimen no. and scientific name. Black circles with numbers indicate the clade no. which corresponds with the article. The tree is divided into three sections: (A) The entire tree, (B) Tetranychini-1, (C) Tetranychini-1, Eurytetranychini and Tetranychini-2 and (D) Tetranychini-3.
Figure 5. Bayesian phylogenetic tree of the sub-family Tetranychinae based on the 18S and 28S rRNA genes using the GTR Gamma model.
Bayesian posterior probabilities (>0.50) are indicated at nodes. Each operational taxonomic unit is indicated by the voucher specimen no. and scientific name. Black circles with numbers indicate the clade no. which corresponds with the article. The tree is divided into three sections: (A) The entire tree, (B) Tetranychini-1, (C) Tetranychini-1, Eurytetranychini and Tetranychini-2 and (D) Tetranychini-3.
As was observed in the COI tree, the 18S and 28S trees also show the monophyly of P. citri and P. osmanthi which are closely related species (Figures 4B and 5B, clade 6). S. cercidiphylli forms a well-supported clade with four Eotetranychus species (E. asiaticus, Eotetranychus boreus Ehara, E. cornicola and Eotetranychus toyoshimai Ehara & Gotoh) in both ML and Bayesian trees (Figures 4C and 5C, clade 11: BP/BPP = 93/1.00). On the other hand, closely related Eotetranychus species (Eotetranychus pruni (Oudemans), Eotetranychus querci Reeves and Eotetranychus uncatus Garman), which have long, flagellate and undulate aedeagi [25], did not cluster together in either tree (Figures 4C and 5C).
Discussion
Only a few studies have examined the molecular phylogeny of the sub-family Tetranychinae, and they often used genes or regions that had limited discriminating ability. As observed in previous studies, our tree based on the COI gene did not resolve deep-level phylogeny because of the low bootstrap values for deep nodes of tree (Figure 2). Therefore, we used the 18S and 28S rRNA genes for phylogenetic analyses because of their better discriminating ability. Indeed, our phylogenetic tree of the 18S and 28S sequences revealed several well-supported clades, allowing us to consider the phylogenetic relationships among the sub-family Tetranychinae.
Our phylogenetic trees based on the 18S and 28S rRNA genes suggest that the tribes Bryobiini and Petrobiini of the sub-family Bryobiinae are both monophyletic, but the tribe Tetranychini is polyphyletic because the monophyletic clade of Eurytetranychini is placed inside Tetranychini (Figures 4A and 5A). At the generic level, 4 genera (Oligonychus, Tetranychus, Schizotetranychus and Eotetranychus) are polyphyletic. The phylogenetic tree separates the Oligonychus species into two clades (Figures 4B, 4D, 5B and 5D, clades 1 and 19). That is, the two clades comprising the genus Oligonychus coincide with their morphology based on the direction of curvature of the aedeagus. These results are in agreement with our COI phylogeny (Figure 2) and previous phylogenies based on the COI gene and ITS2 region [10], [12], [13], [14]. Although phylogenies based on the COI gene and ITS2 region could not establish the exact phylogenetic positions of the two clades of Oligonychus, our tree suggests that species whose aedeagi curve ventrally form a sister group with some of the Schizotetranychus species (Figures 4B and 5B, clade 2) and species whose aedeagi curve dorsally are more closely related to Tetranychus species whose aedeagi also curve dorsally (Figures 4D and 5D, clade 19). Though Oligonychus and Tetranychus can be distinguished by their empodium shape, our phylogenetic trees reveal that the shape of the aedeagi can help to discriminate these two genera.
Species of the genus Schizotetranychus and Eotetranychus appear to be polyphyletic within clade 12 (Figures 4B–4C and 5B–5C). Puzzlingly, S. cercidiphylli and E. uchidai are separated from other congeneric species in the tree. The placement of Eotetranychus species is different between the ML and Bayesian trees. In the ML tree (Figures 4B–4C), we could not establish the exact phylogenetic position of the species of Eotetranychus which are paraphyletic respect to clade 10 because bootstrap values are relatively low. On the other hand, in the Bayesian tree (Figure 5C), S. cercidiphylli and the Eotetranychus species, with the exception of E. uchidai, clustered into a well-supported clade (clade 10: BPP = 0.96). Similarly, the phylogenetic position of the genus Stigmaeopsis is resolved in the Bayesian analysis but not in the ML analysis. In the ML tree (Figure 4C), Stigmaeopsis species (clade 17) clustered with clade 13, which includes the Eurytetranychini species and some of the Tetranychini species, but the topology is not well supported (clade 16: BP = 50). In the Bayesian tree (Figure 5C), Stigmaeopsis species (clade 17) clustered with clade 13 with high Bayesian posterior probabilities (clade 16: BPP = 0.91). Although our data suggests that the Bayesian tree (Figures 5A–5D) is better supported than the ML tree (Figures 4A–4D), it is common knowledge that posterior probabilities are generally higher than bootstrap values [26].
Phylogenetic trees can be used to assess associations between spider mites and their host plants [13]. In the ML and Bayesian trees (Figures 4D and 5D), Oligonychus and Tetranychus species inhabiting gramineous plants (O. orthius, O. modestus, O. rubicundus and T. bambusae) clustered separately from other species and formed a monophyletic clade (Figures 4D and 5D, clade 18). Clade 4 includes Schizotetranychus brevisetosus Ehara, Schizotetranychus gilvus Ehara & Ohashi and Schizotetranychus shii (Ehara) which inhabit fagaceous plants (Figures 4B and 5B). Clade 9 include species irrespective of genus, which inhabit bamboo plants, Sasanychus akitanus (Ehara), Sasanychus pusillus Ehara & Gotoh, S. bambusae, S. recki and Yezonychus sapporoensis Ehara (Figures 4B and 5B). All Stigmaeopsis species inhabiting gramineous plants are separated from other Tetranychini species and appear to be monophyletic (Figures 4C and 5C, clade 17). These results indicate that the phylogenetic relationships of some species of spider mites are closely linked with their host plant, as reported in other phytophagous arthropods [27], [28], [29].
We consider the phylogenies of the Tetranychinae based on the 18S and 28S rRNA genes to be a major improvement over previous phylogenies because they reveal several well-supported clades that were not distinguished by phylogenetic relationships based on the COI gene and ITS2 region. Our finding that the tribe Tetranychini and four genera (Oligonychus, Tetranychus, Schizotetranychus and Eotetranychus) are polyphyletic indicates that the diagnostic morphological characters of tribes and genera of Tetranychinae need to be reconsidered. Although we examined a large number of species in this study, most of them were collected in Japan. Analyzing a number of undescribed genera remaining throughout the world may help achieve a deeper understanding of the phylogenetic relationships among the family Tetranychinae. In addition, a large number of nuclear genes need to be examined to resolve poorly understood relationships in the ML tree (Figures 4A–4D), such as the phylogenetic positions of the genera Eotetranychus and Stigmaeopsis.
Materials and Methods
Mites
Eighty-four strains representing 12 genera and two tribes in Tetranychinae, were used in this study and four strains of the tribes Bryobiini and Petrobiini of the sub-family Bryobiinae (Acari: Tetranychidae) were used as outgroups (Table 1). Mite samples that could be reared in the laboratory were maintained on leaf discs of common bean leaves (Phaseolus vulgaris L.), mulberry leaves (Morus bombycis Koidz.) or the original host plants placed on a water-saturated polyurethane mat in a plastic dish (90 mm diameter, 20 mm depth) at 25°C under a 16L-8D photoperiod until analysis. Samples that could not be maintained in the laboratory and samples that were imported from abroad were preserved in 99.5% ethanol for molecular analyses and 70% ethanol for morphological identification. Specimens were mounted in Hoyer’s medium and identified under phase-contrast and differential interference-contrast microscopes. Voucher specimens are preserved at the Laboratory of Applied Entomology and Zoology, Faculty of Agriculture, Ibaraki University under the serial voucher specimen numbers (Table 1).
DNA extraction, amplification, cloning and sequencing
Total DNA was extracted from the whole body of each female individual by using a Wizard Genomic DNA Purification Kit (Promega). Live female individuals for DNA samples and female individuals for voucher specimen were obtained from the same leaf discs. A few of the strains could not be maintained in the laboratory. For these strains, DNA samples were obtained from ethanol-preserved female individuals. The PCR primers are given in Table 2. The mitochondrial COI fragments were amplified using primer sets C1-J-1718 [30] and COI REVA [8] for species of 12 genera (Bryobia, Petrobia, Eurytetranychoides, Aponychus, Panonychus, Sasanychus, Schizotetranychus, Yezonychus, Eotetranychus, Oligonychus, Amphitetranychus and Tetranychus) and primer sets C1-J-1718-stig and COI REVA-stig for species of the genus Stigmaeopsis. COI sequences for Oligonychus and Tetranychus species were obtained from previously published data [10], [11]. PCR amplification was performed with the following profile: 3 min at 94°C, followed by 35 cycles of 1 min at 94°C, 1 min at 45°C for COI, 60°C for 28S and 65°C for 18S and 1.5 min at 72°C. An additional 10 min at 72°C was allowed for last strand elongation. The resultant DNA solutions were purified by using MinElute PCR Purification Kit (Qiagen) and sequenced directly. Sequencing was carried out using the sequencing primers (Table 2) with a BigDye Terminator Cycle Sequencing Kit v.3.1 (Applied Biosystems) and on an ABI 3130×l automated sequencer.
Table 2. Primers used in polymerase chain reaction amplification and sequencing of the mitochondrial COI gene and the 18S and 28S rRNA genes.
| Primer name | Sequence | Application | References | ||
| COI | |||||
| C1-J-1718 | Forward primer | 5′-GGAGGATTTGGAAATTGATTAGTTCC-3′ | PCR amplification & sequencing | Simon et al. [30] | |
| COI REVA | Reverse primer | 5′-GATAAAACGTAATGAAAATGAGCTAC-3′ | PCR amplification & sequencing | Gotoh et al. [8] | |
| C1-J-1718-stig | Forward primer | 5′-GGAGGTTTTGGTAATTGGTTAATCCC-3′ | PCR amplification & sequencing | This study | |
| COI REVA-stig | Reverse primer | 5′-GAAAGAACATAATGAAAATGAGCAAC-3′ | PCR amplification & sequencing | This study | |
| 18S | |||||
| 18S-1F | Forward primer | 5′-ACCGCGAATGGCTCATTAAATCAGTT-3′ | PCR amplification & sequencing | This study | |
| 18S-2F | Forward primer | 5′-TGGCCTCTGAGCCGACGATGTAT-3′ | Sequencing | This study | |
| 18S-2R | Reverse primer | 5′-ACCCCATAGGTTCGACTGAAATC-3′ | Sequencing | This study | |
| 18S-5R | Reverse primer | 5′-TCCAATAGATCCTCGTTAAAGGAT-3′ | Sequencing | This study | |
| 18S-8R | Reverse primer | 5′-TCTCGTTCGTTATCGGAATTAAC-3′ | Sequencing | This study | |
| 18S-9F | Forward primer | 5′-AGCTTCCGGGAAACCAAAGTTT-3′ | Sequencing | This study | |
| 18S-9R | Reverse primer | 5′-AGGGCATCACAGACCTGTTATT-3′ | Sequencing | This study | |
| 18S-10F | Forward primer | 5′-AGTTGGTGGAGTGATTTGTCTGGT-3′ | Sequencing | This study | |
| 18S-10R | Reverse primer | 5′-ACAAAGGGCAGGGACGTAATCAA-3′ | PCR amplification & sequencing | This study | |
| 28S | |||||
| 28v-5′ | Forward primer | 5′-AAGGTAGCCAAATGCCTCATC-3′ | PCR amplification & sequencing | Hillis and Dixon [31], Palumbi [32] | |
| 28jj-3′ | Reverse primer | 5′-AGTAGGGTAAAACTAACCT-3′ | PCR amplification & sequencing | Hillis and Dixon [31], Palumbi [32] | |
Data analysis
All sequences obtained were deposited in DDBJ/EMBL/GenBank International Nucleotide Sequence Databases under the accession numbers AB981203 to AB981240, AB926227 to AB926314 and AB926318 to AB926405 (Table 1). Sequences were aligned using the 'auto' option of the MAFFT software [33]. Gaps (insertions and deletions) included in the 18S and 28S rRNA sequences were treated using the 'automated1' option of the trimAl software [34], which trimmed ambiguous sites by using a heuristic selection of the automatic method based on similarity statistics. The homogeneity of nucleotide composition was checked using chi-square tests implemented in PAUP* version 4.0b10 software [35].
Maximum likelihood (ML) and Bayesian phylogenetic trees were constructed with RAxML [36] and MrBayes5D [37], respectively. We used the tribes Bryobiini and Petrobiini of the sub-family Bryobiinae as outgroups to root the tree. For all analyses, we used the GTR Gamma model selected by the Akaike Information Criterion (AIC) using the program Kakusan4 [38]. The RAxML search was executed for the best-scoring ML tree in one single program run (the ‘-f a' option) instead of the default maximum parsimony-starting tree. Statistical support was evaluated with 1,000 rapid bootstrap inferences. The MrBayes5D analyses were implemented with two parallel runs of 10 million generations each and using one cold and two incrementally heated Markov chains and sampling every 100 steps. Tracer v.1.6 [39] was used to assess if the search had reached stationarity and to check whether the sample sizes for each parameter (ESS>100) were adequate. The first 10% of the trees were discarded as burn-in and the consensus tree with Bayesian posterior probabilities was constructed based on the trees sampled after the burn-in.
Supporting Information
Aligned COI sequences in FASTA format.
(ZIP)
Aligned 18S sequences in FASTA format.
(ZIP)
Aligned 28S sequences in FASTA format.
(ZIP)
Aligned 18S sequences after deleting the ambiguous parts in FASTA format.
(ZIP)
Aligned 28S sequences after deleting the ambiguous parts in FASTA format.
(ZIP)
Acknowledgments
We are specifically thankful to Drs. K. Ishii and T. Kozaki for their help in data analyses. We are very grateful to Drs. Y. Kitashima, K. Ito, H. Kishimoto, S. Ohno and Y. Sato and M. Arimoto, M. Kakizaki, T. Kamata, M. Minamishima and A. Okada for collecting spider mites. We also thank to A. Miyagi and Y. Shimizu for assistance with rearing the spider mites.
Data Availability
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. All sequences obtained were deposited in DDBJ/EMBL/GenBank International Nucleotide Sequence Databases under the accession numbers AB981203 to AB981240, AB926227 to AB926314 and AB926318 to AB926405.
Funding Statement
Funding provided by Grant number 25292033, Japan Society for the Promotion of Science, http://www.jsps.go.jp/english/index.html. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Helle W, Sabelis MW (1985) Spider Mites: Their Biology, Natural Enemies and Control Vol. 1A (World Crop Pests). Amsterdam: Elsevier. 405p.
- 2.Helle W, Sabelis MW (1985) Spider Mites: Their Biology, Natural Enemies and Control Vol. 1B (World Crop Pests). Amsterdam: Elsevier. 458 p. [Google Scholar]
- 3.Zhang ZQ (2003) Mites of greenhouses, identification, biology and control. Wallington: CABI Publishing. 244 p. [Google Scholar]
- 4.Bolland HR, Gutierrez J, Flechtmann CHW (1998) World catalogue of the spider mite family (Acari: Tetranychidae). Leiden: Brill Academic Publishers. 392 p. [Google Scholar]
- 5.Migeon A, Dorkeled F (2006–2013) Spider Mites Web: a comprehensive database for the Tetranychidae. Available: http://www.montpellier.inra.fr/CBGP/spmweb. Accessed 11 July 2014.
- 6. Wauthy G, Noti MI, Leponce M, Bauchau V (1998) Taxy and variations of leg setae and solenidia in Tetranychus urticae (Acari, Prostigmata). Acarologia 39: 233–255. [Google Scholar]
- 7. Zhang ZQ, Jacobson RJ (2000) Using adult female morphological characters for differentiating Tetranychus urticae complex (Acari: Tetranychidae) from greenhouse tomato crops in UK. Syst Appl Acarol 5: 69–76. [Google Scholar]
- 8. Gotoh T, Araki R, Boubou A, Migeon A, Ferragut F, et al. (2009) Evidence of co-specificity between Tetranychus evansi and Tetranychus takafujii (Acari: Prostigmata, Tetranychidae): comments on taxonomic and agricultural aspects. Int J Acarol 35: 485–501. [Google Scholar]
- 10. Matsuda T, Hinomoto N, Singh RN, Gotoh T (2012) Molecular-based identification and phylogeny of Oligonychus species (Acari: Tetranychidae). J Econ Entomol 105: 1043–1050. [DOI] [PubMed] [Google Scholar]
- 11. Matsuda T, Fukumoto C, Hinomoto N, Gotoh T (2013) DNA-based identification of spider mites: molecular evidence for cryptic species of the genus Tetranychus (Acari: Tetranychidae). J Econ Entomol 106: 463–472. [DOI] [PubMed] [Google Scholar]
- 13. Ros VID, Breeuwer JAJ (2007) Spider mite (Acari: Tetranychidae) mitochondrial COI phylogeny reviewed: host plant relationships, phylogeography, reproductive parasites and barcoding. Exp Appl Acarol 42: 239–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ben-David T, Melamed S, Gerson U, Morin S (2007) ITS2 sequences as barcodes for identifying and analyzing spider mites (Acari: Tetranychidae). Exp Appl Acarol 41: 169–181. [DOI] [PubMed] [Google Scholar]
- 15. Zhao YE, Wu LP, Hu L, Xu Y, Wang ZH, et al. (2012) Sequencing for complete rDNA sequences (18S, ITS1, 5.8S, ITS2, and 28S rDNA) of Demodex and phylogenetic analysis of Acari based on 18S and 28S rDNA. Parasitol Res 111: 2109–2114. [DOI] [PubMed] [Google Scholar]
- 16. Mallatt JM, Garey JR, Shultz JW (2004) Ecdysozoan phylogeny and Bayesian inference: first use of nearly complete 28S and 18S rRNA gene sequences to classify the arthropods and their kin. Mol Phylogenet Evol 31: 178–191. [DOI] [PubMed] [Google Scholar]
- 17. Mallatt J, Giribet G (2006) Further use of nearly complete 28S and 18S rRNA genes to classify Ecdysozoa: 37 more arthropods and a kinorhynch. Mol Phylogenet Evol 40: 772–794. [DOI] [PubMed] [Google Scholar]
- 18. Burger TD, Shao R, Beati L, Miller H, Barker SC (2012) Phylogenetic analysis of ticks (Acari: Ixodida) using mitochondrial genomes and nuclear rRNA genes indicates that the genus Amblyomma is polyphyletic. Mol Phylogenet Evol 64: 45–55. [DOI] [PubMed] [Google Scholar]
- 19. Burger TD, Shao R, Barker SC (2013) Phylogenetic analysis of the mitochondrial genomes and nuclear rRNA genes of ticks reveals a deep phylogenetic structure within the genus Haemaphysalis, and further elucidates the polyphyly of the genus Amblyomma with respect to Amblyomma sphenodonti and Amblyomma elaphense . Ticks Tick Borne Dis 4: 265–274. [DOI] [PubMed] [Google Scholar]
- 20. Hillis DM (1998) Taxonomic sampling, phylogenetic accuracy, and investigator bias. Syst Biol 47: 3–8. [DOI] [PubMed] [Google Scholar]
- 21. Pollock DD, Zwickl DJ, McGuire JA, Hillis DM (2002) Increased taxon sampling is advantageous for phylogenetic inference. Syst Biol 51: 664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hedtke SM, Townsend TM, Hillis DM (2006) Resolution of phylogenetic conflict in large data sets by increased taxon sampling. Syst Biol 55: 522–529. [DOI] [PubMed] [Google Scholar]
- 23. Ehara S, Gotoh T (1996) Two New Species of Spider Mites Occurring in Japan (Acari, Tetranychidae). J Acarol Soc Jpn 5: 17–25. [Google Scholar]
- 24. Toda S, Osakabe MH, Komazaki S (2000) Interspecific diversity of mitochondrial COI sequences in Japanese Panonychus species (Acari: Tetranychidae). Exp Appl Acarol 24: 821–829. [DOI] [PubMed] [Google Scholar]
- 25. Ehara S (1999) Revision of the spider mite family Tetranychidae of Japan (Acari, Prostigmata). Species Divers 4: 63–141. [Google Scholar]
- 26. Klicka J, Voelker G, Spellman GM (2005) A molecular phylogenetic analysis of the “true thrushes” (Aves: Turdinae). Mol Phylogenet Evol 34: 486–500. [DOI] [PubMed] [Google Scholar]
- 27. Futuyma DJ, Agrawal AA (2009) Macroevolution and the biological diversity of plants and herbivores. Proc Natl Acad Sci USA 106: 18054–18061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nyman T, Vikberg V, Smith DR, Boevé JL (2010) How common is ecological speciation in plant-feeding insects? A 'higher' Nematinae perspective. BMC Evol Biol 10: 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bennett GM, O’Grady PM (2012) Host-plants shape insect diversity: Phylogeny, origin, and species diversity of native Hawaiian leafhoppers (Cicadellidae: Nesophrosyne). Mol Phylogenet Evol 65: 705–717. [DOI] [PubMed] [Google Scholar]
- 30. Simon C, Frati F, Backenbach A, Crespi B, Liu H, et al. (1994) Evolution, weighting and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann Entomol Soc Am 87: 651–701. [Google Scholar]
- 31. Hillis DM, Dixon MT (1991) Ribosomal DNA: molecular evolution and phylogenetic inference. Q Rev Biol 66: 411–453. [DOI] [PubMed] [Google Scholar]
- 32.Palumbi SR (1996) Nucleic acids II: the polymerase chain reaction. In: Hillis DM, Moritz C, Mable BK, editors. Molecular Systematics 2nd ed. Sunderland, MA: Sinauer Associates, Inc. pp.205–247.
- 33. Katoh K, Toh H (2008) Recent developments in the MAFFT multiple sequence alignment program. Brief Bioinform 9: 286–298. [DOI] [PubMed] [Google Scholar]
- 34. Capella-Gutiérrez S, Silla-Martínez JM, Gabaldón T (2009) trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinfomatics 25: 1972–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swofford DL (2002) PAUP*. Phylogenetic analysis using parsimony (*and other methods), version 4.0b10 [computer program]. Sunderland, MA: Sinauer Associates, Inc.
- 36. Stamatakis A (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. [DOI] [PubMed] [Google Scholar]
- 37.Tanabe AS (2008) MrBayes5D. Available: http://fifthdimension.jp/products/mrbayes5d. Accessed 3 July 2014.
- 38. Tanabe AS (2011) Kakusan4 and Aminosan: two programs for comparing nonpartitioned, proportional and separate models for combined molecular phylogenetic analyses of multilocus sequence data. Mol Ecol Resour 11: 914–921. [DOI] [PubMed] [Google Scholar]
- 39.Rambaut A, Drummond A (2013) Tracer v1.6. Available: http://tree.bio.ed.ac.uk/software/tracer. Accessed 3 July 2014.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Aligned COI sequences in FASTA format.
(ZIP)
Aligned 18S sequences in FASTA format.
(ZIP)
Aligned 28S sequences in FASTA format.
(ZIP)
Aligned 18S sequences after deleting the ambiguous parts in FASTA format.
(ZIP)
Aligned 28S sequences after deleting the ambiguous parts in FASTA format.
(ZIP)
Data Availability Statement
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. All sequences obtained were deposited in DDBJ/EMBL/GenBank International Nucleotide Sequence Databases under the accession numbers AB981203 to AB981240, AB926227 to AB926314 and AB926318 to AB926405.