Abstract
Background
Hypertension is a major global public health problem that affects both pediatric and adult populations. ACE I/D, AGT M235T, and ADD Gly460Trp polymorphisms are thought to be associated with primary hypertension. In the present study, we examined the frequency of these polymorphisms in a pediatric population with secondary hypertension.
Material/Methods
Included in the study were 58 hypertensive and 58 normotensive pediatric patients. ACE I/D and AGT M235T polymorphisms are determined by conventional PCR; ADD Gly460Trp polymorphism was investigated using PCR amplification of genomic DNA.
Results
There were significant differences between the control group and pediatric hypertensive group in terms of ACE I/D (P<0.05) and AGT M235T (P<0.05) polymorphisms, but there were no differences in ADD Gly460Trp (P>0.05) polymorphism.
Conclusions
We suggest that RAS gene polymorphisms (ACE-I/D, AGT M235T) are significantly associated with susceptibility to diseases that lead to secondary hypertension.
MeSH Keywords: Genes, ras; Hypertension; Polymorphism, Genetic
Background
Hypertension is a major public health problem affecting many people of all ages. The emergence of anatomic changes at a young age is important in establishing some of the negative effects of risk factors of hypertension. This situation is not limited to hypertension; even cardiovascular and renal diseases begin in childhood [1,2].
Pediatric hypertension often occurs secondary to renal, endocrine, or vascular pathologies, but primary hypertension has become more prevalent in recent years [3]. The Renin-angiotensin system (RAS) plays an important role in sodium homeostasis, body fluid balance, and blood pressure. Angiotensin-converting enzyme (ACE) is the main component of RAS. ACE gene encoding is subjected to an insertion/deletion (I/D) polymorphism that is a chief determinant of plasma and tissue ACE levels [4].
ACE and the angiotensinogen (AGT) genes sequences have identified a variety of polymorphisms which may contribute to primary hypertension. ACE I/D polymorphism is the insertion or deletion (I/D) of a 287 base pair in intron 16 of the ACE gene. AGT is also a major component of RAS. The M235T polymorphism of the AGT gene is associated with increased plasma levels of AGT [5].
Adducin is a heterodimer cytoskeleton protein containing the α subunit and either β or γ subunit. Mutation of the adducin α subunit causes the stimulation of sodium-potassium adenosine triphosphatase (Na-K ATPase) activity in renal tubular cells, which increases renal sodium reabsorption and subsequently leads to hypertension [6].
α adducin Gly460Trp (ADD Gly460T) polymorphism of α adducin affects the capacity of the kidney to reabsorb Na. Individuals who have 1 or more of these polymorphisms are thought to be predisposed to primary hypertension. However, this polymorphism in patients with secondary hypertension has not been studied in detail. Many studies have shown a relationship between renal disease and genetic variants of the RAS genes as well as the rate of progression of renal damage [7].
We investigated the frequency of ACE I/D, AGT M235T, and ADD Gly460Trp polymorphisms in pediatric patients with hypertension secondary to chronic kidney disease (CKD).
Material and Methods
Subjects
Included in this study were 58 children (31 boys and 27 girls) with a clinical diagnosis of advanced CKD according to the National Kidney Foundation classification. Thirty-three of the patients were undergoing maintenance hemodialysis; the others were undergoing conservative treatments. We enrolled 31 patients with CKD, 18 with nephrotic syndrome, 9 with Henoch-Schönlein purpura (HSP), and a control group of 58 healthy children. Descriptive properties of groups are listed in Table 1.
Table 1.
Main descriptive features of study groups.
| CKD | Control group | p value | |
|---|---|---|---|
| Mean age (years) | 10.5±3.7 | 9.7±3.1 | N.S. |
| Male/Female | 31/27 | 30/28 | N.S. |
| BMI (kg/m2) | 16.8±1.1 | 19.9±0.9 | N.S. |
| SBP (mmHg) | 99.1±7.4 | 94.7±8.5 | N.S. |
| DBP (mmHg) | 77.8±7.1 | 60.8±9.4 | N.S. |
| Age at disease onset, (years) | 6.4±2.5 | – | <0.001 |
| Proteinuria (%) (>300 mg/24 h) | 33 | – | <0.001 |
| Hematuria (%) (≥10 red blood cell/high power field) | 5 | – | N.S. |
| Renal squeal (%) (persistent renal involvement; follow-up >6 months) | 100 | – | <0.001 |
CKD – chronic kidney disease.
None of the patients with CKD had cardiovascular events on the basis of examination and detailed clinical history. All control subjects were healthy with no clinical signs of vascular or renal disease and no family history of renal disease as assessed using medical history and clinical examination. Healthy control subjects were matched for age, sex, and BMI range to the patient groups. The study was performed with local ethics committee approval, and informed consent was obtained from all the participants. All subjects were from the cities of Diyarbakır and Elazığ in Turkey.
Assessment of genotypes
Whole blood samples were collected into EDTA tubes for DNA analysis. Blood samples were stored at −20°C for 10 to 90 days. Genomic DNA was extracted from peripheral blood leukocytes according to standard protocols [8].
Polymorphism of ACE I/D
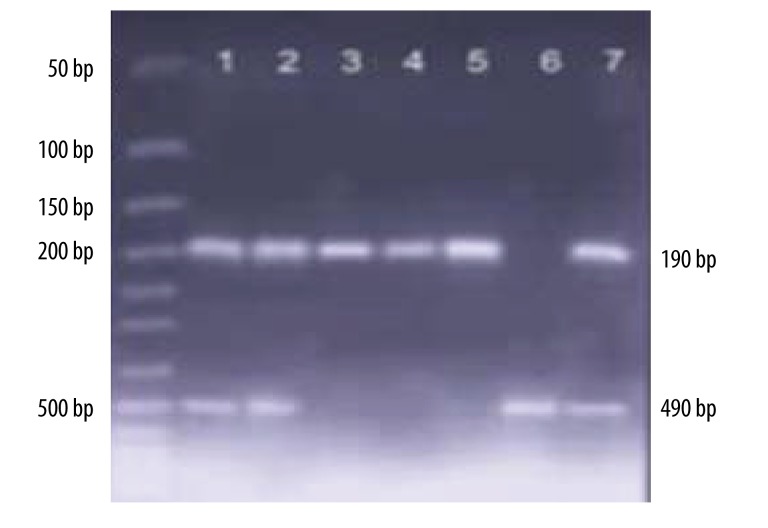
Forward 5′CTGGAGACC ACTCCCATCCTTTCT′3 and reverse 5′GATGTGGCCATCAC ATTCGTCAGAT′3 primer pairs were used for the ACE I/D polymorphism. Annealing temperatures of 59ºC were used to amplify the intron 16 region where the I/D fragment is located. The amplified products were analyzed by electrophoresis on 1.5% agarose gel. ACE, I, and D alleles resulted in 490- and 190-bp amplicons (Figure 1) [9].
Figure 1.
Genotypes of ACE I/D polymorphism; numbers 1, 2 and 7 show I/D genotype; numbers 3,4 and 5 show D/D genotype; number 6 shows I/I genotype.
Polymorphism of AGT M235T
The single-nucleotide polymorphisms (SNPs) in the AGT gene were examined using a polymerase chain reaction/restriction fragment length polymorphism (PCR-RFLP). Primer pairs used for amplification for the AGT M235T polymorphism were forward 5′CAGGGTGCTGTCCACACTGGACCCC′3′ and reverse 5′CCGTTTGTGCAGGGCCTGGCTCTCT′3. Annealing temperature was 60ºC. PCR products were subjected to enzymatic digestion with Psy I enzyme (Lot: 00022395, Fermentas UAB, Vilnius, Lt). Alleles resulted in 165-bp amplicons for M/M, 142-bp, and 24-bp amplicons for TT (Figure 2) [10].
Figure 2.
Genotypes of AGT M235T polymorphism; numbers 1and 2 show T/T genotype; numbers 3 and 4 show M/T genotype; numbers 5, 6 and 7 show M/M genotype.
Polymorphism of ADD Gly460T
The SNPs in the ADD gene were examined using PCR-RFLP. The primer pairs used for the ADD Gly460T polymorphism were forward 5′GAC TTG GGA CTG CTT CCA TTC GGC C′3 and reverse 5′CTC CTT TGC TAG TGA CGG TGA TTC′3. Annealing temperature was 62ºC. PCR products were subjected to enzymatic digestion with Sau96I enzyme (Lot: 00041101, Fermentas UAB, Vilnius, Lt). Alleles resulted in 147-bp amplicons for W/W, 122-bp and 25-bp amplicons for GG (Figure 3) [11].
Figure 3.
Genotypes of ADD G460W polymorphism; numbers 1, 2 and 5 show G/G genotype; numbers 3 and 6 show W/W genotype; numbers 4 and 7 show G/W genotype.
Statistical analysis
Findings obtained in our study were evaluated with SPSS (Statistical Package for Social Sciences) 17.0 program for Windows. Allele and genotypic frequencies for alleles were calculated with the gene counting method. Pearson chi-square (χ2) test and A 2×2 contingency table were used to test the differences of allele frequencies between cases and controls. Odds ratios (OR) with 95% confidence intervals (CI) were estimated for the effects of high-risk factors.
Results
Genetic association analyses with Pearson chi-square test were performed and data are summarized in Table 2. Polymorphisms of related genes are depicted in Figures 1–3. In examining ACE I/D polymorphism there was a significant difference between the CKD group and the controls with regards to DD genotype (p<0.0001). This may suggest that patients with DD genotype are at a high risk of developing renal disease (OR=6.69, 95% CI=2.70–16.6). The genotypic level was also visible at the allelic level as D allele was found in a higher frequency in CHD patients than in the controls (p<0.0001, OR=5.14, 95% CI=2.25–11.7). The CKD group showed an increased frequency of the T allele (p = 0.0001, OR=5.83, 95% CI=1.92–17.7) and the homozygous genotype TT of the AGT M235T polymorphism compared to the controls (p<0.0001, OR=1.38, 95% CI=1.18–1.62). No significant differences were observed between CKD patients and the controls with regards to ADD Gly460Trp genotypes or alleles.
Table 2.
Distribution of alleles and gene polymorphisms in CKD patients and in controls.
| Gene | CKD patients n | (%) | Controls n | (%) | p | OR (95% CI) | |
|---|---|---|---|---|---|---|---|
| ACE Alleles | I | 108 | 87 | 60 | 42 | <0.001 | 0.15 (0.08–0.28) |
| D | 16 | 13 | 84 | 58 | <0.001 | 5.14 (2.25–11.7) | |
| ACE genotypes | II | 28 | 48 | 50 | 86 | 0.006 | 0.19 (0.06–0.63) |
| ID | 14 | 24 | 4 | 6 | 0.019 | 0.23 (0.07–0.76) | |
| DD | 16 | 28 | 4 | 6 | <0.001 | 6.69 (2.70–16.6) | |
| AGT Alleles | M | 84 | 43 | 116 | 55 | 0.001 | 1.38 (1.23–1.45) |
| T | 112 | 57 | 96 | 45 | 0.001 | 5.83 (1.92–17.7) | |
| AGT genotypes | MM | 10 | 17 | 2 | 3 | 0.029 | 0.17 (0.04–0.82) |
| MT | 48 | 83 | 40 | 69 | 0.128 | 0.46 (0.19–1.12) | |
| TT | 0 | 0 | 16 | 28 | <0.001 | 1.38 (1.18–1.62) | |
| Adducin Alleles | G | 104 | 76 | 88 | 71 | 0.009 | 2.76 (1.32–5.74) |
| W | 32 | 24 | 36 | 29 | 0.665 | 0.87 (0.48–1.49) | |
| Adducin genotypes | GG | 40 | 69 | 42 | 72 | 0.839 | 1.12 (0.53–2.63) |
| GW | 4 | 7 | 10 | 18 | 0.152 | 2.81 (0.83–9.56) | |
| WW | 14 | 24 | 6 | 10 | 0.084 | 0.36 (0.13–1.02) |
Discussion
Primary hypertension is a complex genetic disorder affected by environmental factors of a variety of different physiological pathways that act on the homeostasis of blood pressure. RAS is known to play a critical role as a key regulator of blood pressure as well as renal function and may play a role in their interaction. The role of RAS in the pathogenesis of HT is well known. However, whether RAS contributes to chronic renal failure and progression of nephropathy is still controversial [12].
ACE is a key component of RAS. I/D polymorphism of the RAS gene has been shown to cause HT. Several studies have shown the importance of ACE I / D polymorphism in the pathogenesis of hypertension [13]. There are many studies showing that the DD genotype is more frequent in hypertensive individuals [14,15].
A Chinese cohort study supports the idea that RAS gene polymorphism plays a role in susceptibility to diseases resulting in hypertension. In this study, the ACE I/D polymorphism in the secondary hypertensives show that susceptibility to Henoch-Schönlein purpura (HSP) [16]. Similar polymorphisms are associated with posterior urethral valves (PUV) disease and chronic renal disease [17].
In our study, we found significant differences between the control group and pediatric secondary hypertensive group in terms of the ACE I/D polymorphism (p<0.0001).
ACE gene polymorphism appears to be an important genetic predisposition in causation and progression of renal disease. DD genotype was found to be significantly associated with advanced CKD in children [18].
Ferenc et al. concluded that the DD genotype of ACE was more frequent in pediatric end-stage renal disease (ESRD). This genotype, associated with higher circulating and tissue ACE levels, could be a genetic risk factor for renal parenchyma destruction, renal scar formation, and the development of ESRD in children, except those with original renal disease [19,20].
There are many studies on the AGT gene, which is another important component of RAS, indicating that TT genotype of AGT M235T polymorphism is associated with primary hypertension [21,22].
There are no studies in the literature reporting a relationship between secondary hypertension and AGT M235T polymorphism. In our study, we found significant differences between the control group and patient group in terms of the AGT M235T polymorphism (p<0.0001).
Similar studies have associated ADD Gly460Trp polymorphism with primary hypertension [23,24]. We found no significant difference between those groups.
The meta-analysis by Kuo et al. failed to provide evidence for the genetic association of ADD Gly460Trp polymorphism with hypertension [25]. This was also true for secondary hypertension in our study.
Determining the relationship between a gene and a complex genetic disease is difficult due to the necessity of including a large number of genes in the etiology of essential hypertension.
Additionally, these genes may interact with each other in different formations to ensure a similar disease phenotype. The size of this problem makes the frequency of any polymorphism contributing to a disease phenotype marginally higher in the disease group compared to the control group [26].
These data suggest that RAS polymorphisms may participate in the regulation of the system, causing vascular endothelia damage. RAS genotypes may have a synergistic effect on hypertension. However, given the multi-factorial pathogenesis of hypertension, a very large sample would certainly be needed to detect a role for a given pattern of RAS genes.
Polymorphisms in the ACE and AGT genes act as markers for individual variations in the plasma and, possibly, cellular levels of their respective protein products. This suggests a mechanism by which genotype might play a role in the physiology of the renin-angiotensin axis, even though the connection between these RAS gene polymorphisms and secondary hypertension to diseases remains vague [27]. Together with other risk factors, RAS polymorphisms could determine the development of organ complications and disease susceptibility.
Conclusions
According the results of our study, we suggest that RAS gene polymorphisms (ACE-I/D, AGT M235T) are significantly associated with susceptibility to diseases that lead to secondary hypertension.
Acknowledgement
We are grateful to Dicle University DUBAP for their assistance in the English editing of this manuscript.
Footnotes
Source of support: Departmental sources
References
- 1.Berenson G, Srinivasan SR. Cardiovascular Risk Factors in Youth with Implications for Aging: The Bogalusa Heart Study. Neurobiol Aging. 2005;26:303–7. doi: 10.1016/j.neurobiolaging.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Freedman DS, Wattigney WA. The Relation Of Atherosclerotic Lesions To Antemortem And Postmortem Lipid Levels: The Bogalusa Heart Study. Atherosclerosis. 1993;104:37–46. doi: 10.1016/0021-9150(93)90174-s. [DOI] [PubMed] [Google Scholar]
- 3.Falkner B. Pediatric Hypertension. 2nd Edition. 2004. Development of Blood Pressure Norms in Children. [Google Scholar]
- 4.Van Der Kleij FGH, De Jong PE, Henning RH, et al. Enhanced response of blood pressure, renal function and aldosterone to angiotensin I in DD genotype are blunted by low sodium intake. J Am Soc Nephrol. 2002;13:1025–33. doi: 10.1681/ASN.V1341025. [DOI] [PubMed] [Google Scholar]
- 5.Campbell CY. Associations Between Genetic Variants In The ACE, AGT, AGTR1 and AGTR2 Genes And Renal Functionin The Multi-Ethnic Study Of Atherosclerosis. Am J Nephrol. 2010;32(2):156–62. doi: 10.1159/000315866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Staessen JA, Bianchi G. Adducin and hypertension. Pharmacogenomics. 2005;6(7):665–69. doi: 10.2217/14622416.6.7.665. [DOI] [PubMed] [Google Scholar]
- 7.Nakayama Y, Nonoguchi H, Kohda Y, et al. Different Mechanisms for the Progression of CKD with ACE Gene Polymorphisms. Nephron Clin Pract. 2009;111:c240–46. doi: 10.1159/000209150. [DOI] [PubMed] [Google Scholar]
- 8.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 9.Rigat B, Hubert C, Corvol P, Soubrier F. PCR detection of the insertion/deletion polymorphism of the human angiotensin converting enzyme gene (DCP1) (dipeptidyl carboxypeptidase 1) Nucleic Acids Res. 1992;20:1433. doi: 10.1093/nar/20.6.1433-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russ AP, Maerz W, Ruzicka V, et al. Rapid detection of the hypertension-associated Met235-->The allele of the human angiotensinogen gene. Hum Mol Genet. 1993;2:609–10. doi: 10.1093/hmg/2.5.609. [DOI] [PubMed] [Google Scholar]
- 11.Morrison AC, Doris PA, Folsom AR, et al. G-protein beta3 subunit and alpha-adducin polymorphisms and risk of subclinical and clinical stroke. Stroke. 2001;32:822–29. doi: 10.1161/01.str.32.4.822. [DOI] [PubMed] [Google Scholar]
- 12.Mondry A, Loh M, Liu P, et al. Polymorphism of the insertion/deletion ACE and M235T AGT genes and hypertension: surprising new finding and meta-analysis of data. BMC Nephrol. 2005;6:1. doi: 10.1186/1471-2369-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang YL, Zhou SX. Association of angiotensin I-converting enzyme gene polymorphism with ACE and PAI-1 levels in Guangdong Chinese Han patients with essential hypertension. Nan Fang Yi Ke Da Xue Xue Bao. 2007;27(11):1681–84. [PubMed] [Google Scholar]
- 14.Bawazier LA, Sja’bani M. Relationship Of Angiotensin Converting Enzyme Gene Polymorphism And Hypertension In Yogyakarta, Indonesia. Acta Med Indones. 2010;42(4):192–98. [PubMed] [Google Scholar]
- 15.Safar ME, Lajemi M. Angiotensin-Converting Enzyme D/I Gene Polymorphism and Age-Related Changes In Pulse Pressure In Subjects With Hypertension. Arterioscler Thromb Vasc Biol. 2004;24(4):782–86. doi: 10.1161/01.ATV.0000119354.41615.33. [DOI] [PubMed] [Google Scholar]
- 16.Liu D, Lu F, Zhai S, et al. Renin-angiotensin system gene polymorphisms in children with Henoch-Schönlein purpura in West China. J Renin Angiotensin Aldosterone Syst. 2010;11(4):248–55. doi: 10.1177/1470320310374214. [DOI] [PubMed] [Google Scholar]
- 17.Laksmi NK, Khullar M, Kaur B, et al. Association of angiotensin converting enzyme and angiotensin type 2 receptor gene polymorphisms with renal damage in posterior urethral valves. J Pediatr Urol. 2010;6(6):560–66. doi: 10.1016/j.jpurol.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Elshamaa MF, Sabry SM, Bazaraa HM, et al. Genetic polymorphism of ACE and the angiotensin II type1 receptor genes in children with chronic kidney disease. J Inflamm. 2011;8(1):20. doi: 10.1186/1476-9255-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papp F, Friedman AL, Bereczki C, et al. Renin-angiotensin gene polymorphism in children with uremia and essential hypertension. Pediatr Nephrol. 2003;18(2):150–54. doi: 10.1007/s00467-002-1032-x. [DOI] [PubMed] [Google Scholar]
- 20.Filler G, Yang F, Martin A, et al. Renin angiotensin system gene polymorphisms in pediatric renal transplant recipients. Pediatr Transplant. 2001;5(3):166–73. doi: 10.1034/j.1399-3046.2001.00053.x. [DOI] [PubMed] [Google Scholar]
- 21.Ji LD, Zhang LN. Association of Angiotensinogen Gene M235T and Angiotensin-Converting Enzyme Gene I/D Polymorphisms with Essential Hypertension In Han Chinese Population: A Meta-Analysis. J Hypertens. 2010;28(3):419–28. doi: 10.1097/HJH.0b013e32833456b9. [DOI] [PubMed] [Google Scholar]
- 22.Zafarmand MH, Franx A. The M235T Variant Of The Angiotensinogen Gene Is Related To Development Of Self-Reported Hypertension During Pregnancy: The Prospect-EPIC Cohort Study. Hypertens Res. 2008;31(7):1299–305. doi: 10.1291/hypres.31.1299. [DOI] [PubMed] [Google Scholar]
- 23.Glorioso N, Filigheddu F. Adducin 460Trp Allele Is Associated With Erythrocyte Na Transport Rate in North Sardinian Primary Hypertensives. Hypertension. 2002;39(2):357–62. doi: 10.1161/hy0202.103065. [DOI] [PubMed] [Google Scholar]
- 24.Glorioso N, Manunta P. The Role of a-Adducin Polymorphism in Blood Pressure and Sodium Handling Regulation May Not Be Excluded by a Negative Association Study. Hypertension. 1999;34:649–54. doi: 10.1161/01.hyp.34.4.649. [DOI] [PubMed] [Google Scholar]
- 25.Liu K, Liu J. Alpha-Adducin Gly460Trp Polymorphism and Hypertension Risk: A Meta-Analysis of 22 Studies Including 14303 Cases and 15961 Controls. PLoS One. 2010;5(9):13057. doi: 10.1371/journal.pone.0013057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta S, Agrawal BK. Angiotensin-converting enzyme gene polymorphism in hypertensive rural population of Haryana, India. J Emerg Trauma Shock. 2009;2(3):150–54. doi: 10.4103/0974-2700.55323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duprez DA. Role of the renin-angiotensin-aldosterone system in vascular remodeling and inflammation: a clinical review. J Hypertens. 2006;24:983–84. doi: 10.1097/01.hjh.0000226182.60321.69. [DOI] [PubMed] [Google Scholar]