Abstract
Objective
Tenofovir disoproxil fumarate is a widely used antiretroviral for HIV infection that has been associated with an increased risk of chronic kidney disease (CKD). Our objective was to derive a scoring system to predict 5-year risk of developing CKD in HIV-infected individuals and to estimate difference in risk associated with tenofovir use.
Design
We evaluated time to first occurrence of CKD (estimated glomerular filtration rate <60 ml/min per 1.73 m2) in 21 590 HIV-infected men from the Veterans Health Administration initiating antiretroviral therapy from 1997 to 2010.
Methods
We developed a point-based score using multivariable Cox regression models. Median follow-up was 6.3 years, during which 2059 CKD events occurred.
Results
Dominant contributors to the CKD risk score were traditional kidney risk factors (age, glucose, SBP, hypertension, triglycerides, proteinuria); CD4+ cell count was also a component, but not HIV RNA. The overall 5-year event rate was 7.7% in tenofovir users and 3.8% in nonusers [overall adjusted hazard ratio 2.0, 95% confidence interval (CI) 1.8–2.2]. There was a progressive increase in 5-year CKD risk, ranging from less than 1% (zero points) to 16% (≥9 points) in nonusers of tenofovir, and from 1.4 to 21.4% among tenofovir users. The estimated number-needed-to-harm (NNH) for tenofovir use ranged from 108 for those with zero points to 20 for persons with at least nine points. Among tenofovir users with at least 1 year exposure, NNH ranged from 68 (zero points) to five (≥9 points).
Conclusion
The CKD risk score can be used to predict an HIV-infected individual’s absolute risk of developing CKD over 5 years and may facilitate clinical decision-making around tenofovir use.
Keywords: chronic kidney disease, HIV, risk score, tenofovir
Introduction
Use of HAARTs in the setting of HIV infection has dramatically reduced morbidity and mortality in HIV-infected persons. Tenofovir disoproxil fumarate is a widely used and highly effective antiretroviral medication that is recommended as a component in all four listed preferred regimens for antiretroviral-naive patients in the current Department of Health and Human Services Panel on Antiretroviral Guidelines for Adults and Adolescents [1]. The 2013 treatment guidelines advanced by the WHO recommend tenofovir in combination with emtrictabine and efavirenz as the preferred regimen for all HIV-infected patients with CD4+ cell counts less than 500 cells/μl [2].
HIV-infected persons are at an increased risk for chronic kidney disease (CKD) and end-stage renal disease (ESRD) relative to persons without HIV infection [3]. Renal insufficiency is of major public health importance in persons both with and without HIV infection, as it increases the risk of cardiovascular disease, heart failure and mortality [4,5]. Certain antiretroviral drugs – particularly tenofovir – have also been associated with kidney disease. In randomized clinical studies of generally healthy adults with limited other risk factors for kidney disease, tenovir use was generally associated with minimal nephrotoxicity [6,7]. In clinical practice, however, wherein individuals might have multiple other risk factors, the risk of tenovovir-associated CKD is likely higher [8]. We previously evaluated cumulative and ever use of tenofovir in over 10 000 HIV-infected veterans, and found that exposure to tenofovir was associated with a higher relative risk of CKD, proteinuria and rapid decline in kidney function [9]. However, relative risks are only partially informative for clinicians adjudicating treatment decisions for patients, who will have varying risk factors for renal disease prior to initiating tenofovir or alternative antiretroviral drugs.
In response to this need for tools to optimize individualized decision making, we developed a risk score for CKD in HIV-infected patients utilizing readily available clinical variables. We studied a national sample of HIV-infected male veterans who initiated antiretroviral therapy between 1997 and 2010. We used data from 21 590 HIV-infected men within the Veterans Health Administration system over 193 771 years of follow-up for this analysis. We then employed this score to determine the additional risk of kidney disease associated with the use of tenofovir.
Materials and methods
We utilized a national sample of HIV-infected U.S. veterans to create a risk score for CKD. Data sources used to assemble the analytic cohort have been described in detail [10]. In brief, the Department of Veterans Affairs (VA) HIV Clinical Case Registry (CCR) actively monitors all HIV-infected persons receiving care in the VA nationally and automatically extracts demographic, clinical, laboratory, pharmacy, utilization and death information from the VA electronic medical record into a centralized database [11].
Patients
The target population for this analysis was HIV-infected male veterans who were treatment-naive (i.e. no prior exposure to any antiretroviral) at the time they entered clinical care in the Veterans Health Administration (VHA) system, and who subsequently received antiretroviral therapy followed by regular visits and laboratory monitoring. We focused on patients who initiated therapy during or after 1997, as this was when combination antiretroviral therapy became standard of care. The baseline visit was defined as the date of starting antiretroviral therapy. Patients were followed until 1 January 2011. We excluded patients with prevalent kidney failure (receipt of chronic dialysis treatment or kidney transplant) and those with estimated glomerular filtration rate (eGFR) at baseline of less than 60 ml/min per 1.73 m2. We also excluded those who did not have at least one plasma HIV RNA value, CD4+ cell count, record of an outpatient visit and kidney function assessment. A total of 21 590 patients were included in the final analytic cohort.
Outcome
The primary study outcome was time to first occurrence of eGFR less than 60 ml/min per 1.73 m2. eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula based on age, sex, race and serum creatinine [12]. CKD was defined by two consecutive estimates of GFR less than 60 ml/min per 1.73 m2, wherein consecutive estimates were required to be at least 3 months apart and not obtained during inpatient admissions.
Independent variables
We ascertained drug utilization in the CCR medication files on the basis of pharmacy fill and refill information. Previous work demonstrated that VA pharmacy data are comprehensive and reliable for assessing medication use [13–17]. A strict definition of HAART utilization was defined as in previous reports [13,18].
Demographic information (age and race) from the CCR was supplemented with Medicare database information. We defined comorbid conditions as described previously [18]. Hypertension was defined on the basis of two outpatient ICD-9 codes or a combination of diagnostic codes and use of antihypertensive treatment [16,19]. Proteinuria was defined as urine dipstick measurements greater than 30 mg/dl. Diabetes was defined by any prescription for insulin or an oral hypoglycemic agent, or two or more diagnoses from inpatient or outpatient visits [20]. Hepatitis B virus (HBV) infection was defined by positive surface antigens or a detectable hepatitis viral load. Hepatitis C virus (HCV) infection was defined using ICD-9 codes and HCV antibody positive status. Blood pressure, BMI, CD4+ cell counts, HIV RNA level, low-density lipoprotein (LDL) and high-density lipoprotein (HDL) levels, triglycerides, total cholesterol and serum glucose were included in statistical models or used to define clinical characteristics. At any given time, the most recent previous measurement was used to define time-dependent covariates.
Statistical analysis
For the development of the CKD risk score, we considered the following baseline clinical variables: age, race, diabetes, glucose, hypertension, SBP and DBP, dyslipidemia, LDL, HDL, triglyceride, total cholesterol, cardiovascular disease, smoking, illicit drug use, proteinuria, HBV and HCV infection status, BMI, CD4+ cell count and HIV RNA level. Multivariable Cox proportional hazards regression models were used to assess risk factors for CKD. Patients were censored at time of death or the last day of follow-up for this analysis, 1 January 2011. Cox regression model assumptions were checked by comparing plots of log [−log(survival)] versus log of survival time and the Schoenfeld test. We first used stepwise backward selection with a significance level of α equal to 0.05 to remove candidate covariates. As an alternative model building approach, we used Bayesian model averaging to identify candidate models with high posterior probabilities [21]. Models constructed using the two approaches were similar. We then used 10-fold cross-validation to compare candidate models and identify the best fitting model.
Using the final model, we developed a risk scoring system using previously established methods developed by the Framingham Heart Study [22] to calculate points associated with each risk factor and determine the 5-year probability of developing CKD. The total number of points was calculated for each participant using this risk score and associated with the risk of developing CKD. We used c-statistics to assess model discrimination, and goodness-of-fit testing to assess calibration. Bootstrap simulation was used to assess overoptimism. We assessed model performance by comparing observed event rates across the range of points to expected rates, which represent the predicted rate based on the proportion at risk within each subgroup. We also calculated the number-needed-to-harm (NNH) for the analyses involving tenofovir use, which represents the number of patients who would need to be treated with tenofovir for one CKD event to occur.
Analyses were conducted using Stata version 11 and SAS version 9.3. Bayesian model averaging was performed using the BMA package for the R statistical computing language. This study was approved by the Committee on Human Research of the San Francisco VA Medical Center and the VA Public Health Strategic Healthcare Group.
Results
After excluding persons with CKD at baseline, 21 590 HIV-infected male veterans were at a risk for CKD and included in this study. Overall, there were 2059 CKD events during 193 771 person-years of follow-up. Baseline demographic and clinical characteristics are presented in Table 1, stratified by end-of-study tenofovir exposure status. Mean ± standard deviation (SD) age at baseline was 47.2 ± 9.8 years. Individuals ever exposed to tenofovir were similar at baseline in terms of race, diabetes, hypertension, proteinuria, HIV RNA level and other risk factors compared with those never exposed to tenofovir. Median follow-up was 6.3 years [interquartile range (IQR) 2.8–10.3].
Table 1.
Summary of demographic and baseline characteristics, stratified by tenofovir exposure status at end of study.
| Risk factor | TDF exposure (n = 14 687) | No TDF exposure (n = 6903) |
|---|---|---|
| Age (years)a | 47 (40, 53) | 48 (42, 54) |
| Black | 52% | 56% |
| Glucose (mg/dl) | 95 (87, 106) | 96 (87, 109) |
| Diabetic | 5.4% | 7.0% |
| SBP (mmHg) | 127 (116, 138) | 128 (115, 140) |
| DBP (mmHg) | 77 (70, 85) | 77 (70, 85) |
| Hypertension | 27% | 28% |
| ACE-I or ARB use | 5.8% | 7.4% |
| Statin use | 3.8% | 3.4% |
| Proteinuria | 23% | 23% |
| HDL cholesterol (mg/dl) | 38 (30, 47) | 39 (31, 49) |
| LDL cholesterol (mg/dl) | 100 (78, 125) | 101 (76, 129) |
| Triglycerides (mg/dl) | 138 (94, 216) | 144 (98, 223) |
| CVD | 3.9% | 4.9% |
| Current smoking | 21% | 17% |
| BMI (kg/m2) | 25 (22, 28) | 24 (22, 27) |
| Illicit drug use | 26% | 28% |
| HIV viral load (/1000) | 14 (0, 92) | 10 (0, 93) |
| CD4+ cell count (cells/μl) | 314 (160, 492) | 310 (143, 526) |
| Hepatitis C virus infection | 23% | 28% |
| Hepatitic B virus infection | 7.5% | 7.0% |
ACE-I, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; CVD, cardiovascular disease; HDL, high-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein; TDF, tenofovir disoproxil fumarate.
Continuous variables reported as median (IQR). Proteinuria defined by urinalysis protein 30 mg/dl or greater.
Table 2 lists all variables included in the risk score, along with the adjusted hazard ratio and points calculated for each risk factor. In Cox multivariable regression analysis, we found that older age, elevated glucose (>140 mg/dl), elevated SBP (>140 mmHg), hypertension, elevated triglyceride (>200 mg/dl), proteinuria and low CD4+ cell count were independently associated with a higher risk of developing CKD. Higher levels of HIV RNA did not appear to be associated with a greater risk of developing CKD [hazard ratio 0.97 per 10-fold increase, 95% confidence interval (CI) 0.94–1.01]. The oldest age group (age >60 years) had the greatest points assigned of any risk factor category (+6). The possible risk scores ranged from zero (lowest comorbidity score) to +15 (highest comorbidity score).
Table 2.
Points assigned to chronic kidney disease risk factor categories.
| Risk factor | Category | Hazard ratio (95% CI) | Raw regression coefficient | Points |
|---|---|---|---|---|
| Age (per year) | 1.06 (1.05–1.07) | 0.0619 = β1 | ||
| Age (categories) | 19–39 | Reference | 0 | |
| 40–49 | 2.07 (1.75–2.44) | 0.7268 | 2 | |
| 50–59 | 3.40 (2.88–4.00) | 1.2227 | 4 | |
| 60–90 | 7.24 (6.08–8.63) | 1.9799 | 6 | |
| Glucose >140 mg/dl | No | 1.60 (1.41–1.82) | 0.4719 = β2 | 0 |
| Yes | 2 | |||
| SBP >140 mmHg | No | 1.23 (1.11–1.35) | 0.2049 = β3 | 0 |
| Yes | 1 | |||
| Hypertension | No | 1.76 (1.61–1.92) | 0.5632 = β4 | 0 |
| Yes | 2 | |||
| TG >200 mg/dl | No | 1.31 (1.19–1.44) | 0.2696 = β5 | 0 |
| Yes | 1 | |||
| Proteinuria | No | 1.99 (1.77–2.23) | 0.6871 = β6 | 0 |
| Yes | 2 | |||
| CD4+ cell count <200 cells/μl | No | 1.18 (1.07–1.31) | 0.1692 = β7 | 0 |
| Yes | 1 |
CI, confidence interval; SBP, systolic blood pressure; TG, triglycerides.
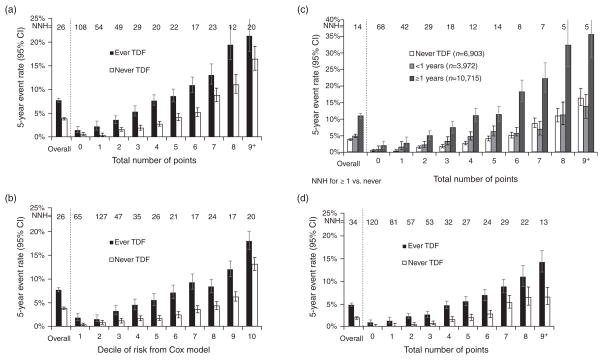
The overall 5-year CKD event rate was 7.7% in tenofovir users and 3.8% in nonusers (Fig. 1a), corresponding to an NNH of 26. We observed a progressive increase in the risk of CKD with increasing number of points from the risk score, with 5-year risks in nonusers of tenofovir ranging from less than 1% in those with zero points to 16.4% in those with 9 or more points, and from 1.4 to 21.4% among tenofovir users. As a result, the estimated NNH for tenofovir use ranged from 108 for those with zero points to 20 for persons with 9 or more points. After controlling for the risk score, the overall relative risk of CKD was two-fold higher in tenofovir users than in nonusers (95% CI 1.83–2.18, P < 0.0001). A similar pattern of increasing risk was seen when we examined risk of CKD by increasing decile of risk from the Cox model (Fig. 1b).
Fig. 1. Five-year risk of chronic kidney disease in tenofovir disoproxil fumarate ever and never users.
(a) Five-year risk of CKD associated with number of points in TDF ever and never users. *If 26 patients are exposed to TDF for 5 years, one would develop CKD that would not have otherwise. (b) Five-year risk of CKD associated with decile of risk from Cox model in TDF ever and never users. (c) Five-year risk of CKD associated with number of points, stratified by duration of TDF exposure. *NNH for ≥1 years versus never TDF. (d) Five-year risk of CKD associated with number of points, restricting analysis to most recent 5 years. CKD, chronic kidney disease; NNH, number-needed-to-harm; TDF, tenofovir disoproxil fumarate.
We observed a nonlinear association of duration of tenofovir use with risk of CKD, with an inflection point near 1 year of use. Increasing duration of tenofovir exposure (≥1 year versus <1 year) was associated with a higher overall 5-year CKD event rate (10.9% in those with ≥1 year of tenofovir versus 4.9% in those with <1 year of exposure, Fig. 1c). This increased risk associated with longer tenofovir duration was observed across the range of risk scores in our model (overall hazard ratio 2.1 controlling for risk score, P < 0.0001). The NNH among those with at least 1 year of tenofovir ranged from 5 for those with 8 or more points to 68 for those with 0 points. In an analysis limited to the most recent 5 years (from 2006 to 2010) (Fig. 1d), tenofovir exposure was associated with an increased risk of CKD (hazard ratio 2.47, 95% CI 2.17–2.81) after controlling for the risk score, and discrimination was similar (c = 0.72).
As an illustration of the use of the risk score, we present the example of a hypothetical 55-year-old man with normal glucose, hypertension, hypertriglyceridemia, no proteinuria and a normal CD4+ cell count (Table 3). In this example, the risk score calculation would assign 4 points for age, 1 point for having elevated SBP, 2 points for having hypertension and 1 point for having elevated triglycerides, yielding a total score of 8. As shown in Fig. 1a, a patient with a total score of 8 would have an 11% chance of developing CKD over 5 years as a nonuser and a 19% chance as a user of tenofovir.
Table 3.
Example of risk calculation for a 55-year-old patient with normal glucose, hypertension, hypertriglyceridemia, no proteinuria and normal CD4+ cell count.
| Risk factor | Pointsa |
|---|---|
| Age 55 years | 4 |
| Glucose >140 mg/dl | 0 |
| SBP >140 mmHg | 1 |
| Hypertension | 2 |
| TG >200 mg/dl | 1 |
| No proteinuria | 0 |
| CD4+ cell count >200 cells/μl | 0 |
| Total score | 8 |
| Estimated 5-year risk of CKDa | |
| If patient is put on tenofovir | 19% chance of developing CKD |
| If patient is not put on tenofovir | 11% chance of developing CKD |
CKD, chronic kidney disease; TG, triglycerides.
Using points from Table 2 and estimates from supplemental Table 2, http://links.lww.com/QAD/A503.
We assessed the performance of our risk prediction model and found that it had good discrimination (overall c statistic 0.73) and calibration (goodness of fit P = 0.99). Bootstrap simulation indicated a very low degree of over optimism (bias 0.0003). Results were similar in a sensitivity analysis in which we applied our risk score model to those with at least two kidney assessments, excluding the less than 5% of participants with only one assessment (Supplemental Table 1, http://links.lww.com/QAD/A503). A comparison of the observed and expected event rates (Supplemental Fig. 1, http://links.lww.com/QAD/A503) showed a progressive increase in both observed and expected risk with increasing number of points (test for trend: P < 0.0001) with similar calibration and discrimination (c = 0.70 versus c = 0.74) in tenofovir users and nonusers. Event rates and person years of follow-up in users and nonusers of tenofovir are summarized in Supplemental Table 2, http://links.lww.com/QAD/A503 (by total number of points) and Supplemental Table 3, http://links.lww.com/QAD/A503 (by decile of risk from the Cox model).
Discussion
In this large, national sample of over 20 000 male HIV-infected veterans, we found that tenofovir users had a higher 5-year risk of developing CKD than never users of tenofovir. This finding held in those with both a lower and higher burden of comorbidities typically associated with renal dysfunction. We developed a 5-year risk score for CKD in HIV-infected men using risk factors that are commonly measured in clinical practice, including age, glucose, SBP, hypertension, triglyceride level, proteinuria and CD4+ cell count. This risk score had good discrimination, with a c-statistic of 0.73. Although the relative risk associated with tenofovir was statistically significant across the range of predicted CKD risk, both the absolute risks and the NNH differed substantially, with higher risk patients being the most adversely affected. In particular, those with higher predicted risk were especially susceptible to more than 1 year of tenofovir use.
Our results found a higher risk of CKD with increasing duration of tenofovir exposure, consistent with our previous findings [9] but in contrast to observations from some clinical trials [6,7]. We also found that tenofovir exposure was associated with an increased risk of CKD even when restricting our analysis to the most recent 5 years of data. Although tenofovir was typically prescribed in treatment-experienced patients upon its approval in 2001, current guidelines recommend the inclusion of tenofovir in first-line therapy.
A recent study [23] in 4350 HIV-infected patients suggested that tenofovir use should be contraindicated in persons with one or more traditional risk factors for kidney disease. Recommendations have also been published advising kidney function monitoring in tenofovir-treated patients [24]. Our scoring system allows risk assessment to be quantified, providing physicians and patients with an estimate of the absolute risk of developing CKD and providing a more nuanced algorithm for HIV treatment. Our results find that among those with more than 1 year of tenofovir exposure, the NNH is as low as 18 for persons with a risk score of 3 or more. This risk scoring system will inform clinicians of the additional risk incurred in an individual patient when prescribed tenofovir. Given that the typical nucleoside reverse transcriptase inhibitor (NRTI) combination alternative to tenofovir/emtricitabine (i.e. abacavir/lamivudine) is less likely to worsen renal function, our CKD risk score could aid clinicians in designing first-line HIV treatment regimens optimized for safety as well as efficacy.
It is noteworthy that although low CD4+ cell count was associated with a higher risk of CKD in our cohort, we did not find an association of higher HIV RNA levels or other HIV-related risk factors with increased CKD risk. By contrast, our group has previously reported that higher HIV RNA levels are associated with an increased risk of ESRD in HIV-infected veterans [3], and it has also been reported that increasing HIV RNA levels are associated with proteinuria in HIV-infected women [25]. It is possible that this lack of an association between HIV RNA levels and CKD is explained in part by the competing risk of death, for which HIV RNA is a strong risk factor. It has been posited that the kidney may act as a reservoir of HIV-1 in HIV-infected individuals [26], and the circulating viral load level may therefore be a less sensitive indicator of CKD risk. Prior HIV viremia even when subsequently suppressed may also lead to long-lasting kidney damage, accelerating the occurrence of CKD.
We also found that elevated triglyceride level was an important risk factor for CKD. Although not considered a traditional risk factor, the most common form of dyslipidemia in persons with CKD is hypertriglyceridemia [27], and its prevalence appears to increase in later stages of CKD. Mechanisms posited to explain hypertriglyceridemia in CKD include diminished clearance and increased synthesis [28].
A major strength of our study is the inclusion of a large number of participants, which resulted in a large number of events in both tenofovir users and nonusers. Study limitations include our inability to measure GFR directly, similar to all large studies of HIV-infected persons. We used creatinine-based estimates of GFR, which are known to be biased in the setting of HIV infection [29], yet remain the clinical standard. This is an observational study, and not a randomized study. Although users and nonusers of tenofovir had a similar baseline risk profile, there may have been incomplete or inadequate control of factors that may confound the association between tenofovir use and kidney disease. We did not have HCV RNA data to confirm chronic HCV infection, and it is likely that hepatitis C resolved in some patients. However, the NA-ACCORD study recently reported that individuals who were HCV Ab positive but HCV RNA negative also had an increased risk for CKD similar to individuals who were HCVAb and RNA positive [30]. In addition, our results may not generalize to nonveterans, women, children or patients not receiving regular clinical care. However, our population does include patients who might have been excluded from clinical trials and who might not qualify or volunteer for epidemiological cohort studies. Finally, our risk score algorithm has not been validated in an independent cohort.
In conclusion, the CKD risk score can be used to predict the absolute 5-year risk of developing CKD for individual HIV-infected men. The availability of this information should guide clinicians and individual patients who are weighing the benefits of using tenofovir against the long-term risks of kidney damage. Future studies of HIV-infected persons (including women) and persons utilizing tenofovir for hepatitis B and for preexposure prophylaxis (PrEP) are needed to validate this risk equation.
Acknowledgments
R.S. and M.G.S. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
This work was supported by the National Institutes of Health [K23DK080645-01A1 (A.I.C.), 1R03AG034871-01 (A.I.C./M.G.S.), K24AI069994, R01 DK066488-01 (M.G.S./A.I.C.), RO1 AI098472 (M.G.)], the National Center for Research Resources [KL2 RR024130], the American Heart Association Established Investigator Award (M.G.S.) and the VA Public Health Strategic Healthcare Group, which were administered by the Northern California Institute for Research and Education, and with resources of the Veterans Affairs Medical Center, San Francisco, California. These funding sources had no involvement in the design or execution of this study. S.G.D. received grant support from Gilead and Merck, and honorarium for ad hoc consulting from Gilead and GlaxoSmithKline. C.G. has received prior research funding and/or honorarium from Merck, Bristol-Myers Squibb, Abbott, Gilead Sciences, EMD Serono and Theratechnologies.
Footnotes
Conflicts of interest
There are no conflicts of interest.
Conception and design of the research was done by R.S., C.A.P., M.G.S. The acquisition of data was done by M.G.S. and R.S. Analysis and interpretation of the data was carried out by R.S., C.A.P., M.G.S., P.T., M.M.E. R.S. performed the statistical analysis. M.G.S., R.S., C.G. obtained funding for this study. R.S., M.G., M.M.E., P.C.T., S.G.D., C.G., C.A.P., M.G.S. did the drafting of the manuscript. R.S., M.G., M.M.E., P.C.T., S.G.D., C.G., C.A.P., M.G.S. did the critical revision of manuscript for important intellectual content. M.G.S. did the supervision.
References
- 1.Panel on Antiretroviral Guidelines for Adults and Adolescents. [Accessed 24 February 2014];Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. [Updated 12 February 2013]
- 2.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach [Revised] Geneva: WHO; 2010. [Accessed 24 February 2014]. http://www.who.int/hiv/pub/arv/adult2010/en/index.html. [PubMed] [Google Scholar]
- 3.Jotwani V, Li Y, Grunfeld C, Choi AI, Shlipak MG. Risk factors for ESRD in HIV-infected individuals: traditional and HIV-related factors. Am J Kidney Dis. 2012;59:628–635. doi: 10.1053/j.ajkd.2011.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McAlister FA, Ezekowitz J, Tonelli M, Armstrong PW. Renal insufficiency and heart failure: prognostic and therapeutic implications from a prospective cohort study. Circulation. 2004;109:1004–1009. doi: 10.1161/01.CIR.0000116764.53225.A9. [DOI] [PubMed] [Google Scholar]
- 5.Choi A, Scherzer R, Bacchetti P, Tien PC, Saag MS, Gibert CL, et al. Cystatin C, albuminuria, and 5-year all-cause mortality in HIV-infected persons. Am J Kidney Dis. 2010;56:872–882. doi: 10.1053/j.ajkd.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallant JE, Staszewski S, Pozniak AL, DeJesus E, Suleiman JM, Miller MD, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292:191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 7.Squires K, Pozniak AL, Pierone G, Jr, Steinhart CR, Berger D, Bellos NC, et al. Tenofovir disoproxil fumarate in nucleoside-resistant HIV-1 infection: a randomized trial. Ann Intern Med. 2003;139:313–320. doi: 10.7326/0003-4819-139-5_part_1-200309020-00006. [DOI] [PubMed] [Google Scholar]
- 8.Del Palacio M, Romero S, Casado JL. Proximal tubular renal dysfunction or damage in HIV-infected patients. AIDS Rev. 2012;14:179–187. [PubMed] [Google Scholar]
- 9.Scherzer R, Estrella M, Li Y, Choi AI, Deeks SG, Grunfeld C, et al. Association of tenofovir exposure with kidney disease risk in HIV infection. AIDS. 2012;26:867–875. doi: 10.1097/QAD.0b013e328351f68f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi AI, Li Y, Deeks SG, Grunfeld C, Volberding PA, Shlipak MG. Association between kidney function and albuminuria with cardiovascular events in HIV-infected persons. Circulation. 2010;121:651–658. doi: 10.1161/CIRCULATIONAHA.109.898585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Backus LI, Gavrilov S, Loomis TP, Halloran JP, Phillips BR, Belperio PS, et al. Clinical case registries: simultaneous local and national disease registries for population quality management. J Am Med Inform Assoc. 2009;16:775–783. doi: 10.1197/jamia.M3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi AI, Rodriguez RA, Bacchetti P, Volberding PA, Havlir D, Bertenthal D, et al. Low rates of antiretroviral therapy among HIV-infected patients with chronic kidney disease. Clin Infect Dis. 2007;45:1633–1639. doi: 10.1086/523729. [DOI] [PubMed] [Google Scholar]
- 14.Justice AC, Dombrowski E, Conigliaro J, Fultz SL, Gibson D, Madenwald T, et al. Veterans Aging Cohort Study (VACS): overview and description. Med Care. 2006;44:S13–S24. doi: 10.1097/01.mlr.0000223741.02074.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Office USGA. Veterans healthcare: use of VA services by Medicare-eligible veterans. GAO Report. Washington, DC: United States General Accounting Office; 1994. [Google Scholar]
- 16.Shen Y, Hendricks A, Zhang S, Kazis LE. VHA enrollees’ healthcare coverage and use of care. Med Care Res Rev. 2003;60:253–267. doi: 10.1177/1077558703060002007. [DOI] [PubMed] [Google Scholar]
- 17.Wagner JH, Justice AC, Chesney M, Sinclair G, Weissman S, Rodriguez-Barradas M, et al. Patient- and provider-reported adherence: toward a clinically useful approach to measuring antiretroviral adherence. J Clin Epidemiol. 2001;54 (Suppl 1):S91–S98. doi: 10.1016/s0895-4356(01)00450-4. [DOI] [PubMed] [Google Scholar]
- 18.Choi AI, Vittinghoff E, Deeks SG, Weekley CC, Li Y, Shlipak MG. Cardiovascular risks associated with abacavir and tenofovir exposure in HIV-infected persons. AIDS. 2011;25:1289–1298. doi: 10.1097/QAD.0b013e328347fa16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karras A, Lafaurie M, Furco A, Bourgarit A, Droz D, Sereni D, et al. Tenofovir-related nephrotoxicity in human immunodeficiency virus-infected patients: three cases of renal failure, Fanconi syndrome, and nephrogenic diabetes insipidus. Clin Infect Dis. 2003;36:1070–1073. doi: 10.1086/368314. [DOI] [PubMed] [Google Scholar]
- 20.Peyriere H, Reynes J, Rouanet I, Daniel N, de Boever CM, Mauboussin JM, et al. Renal tubular dysfunction associated with tenofovir therapy: report of 7 cases. J Acquir Immune Defic Syndr. 2004;35:269–273. doi: 10.1097/00126334-200403010-00007. [DOI] [PubMed] [Google Scholar]
- 21.Hoeting JM, Raftery A, Volinsky C. Bayesian model averaging: a tutorial. Stat Sci. 1999;14:382–401. [Google Scholar]
- 22.Sullivan LM, Massaro JM, D’Agostino RB., Sr Presentation of multivariate data for clinical use: the Framingham Study risk score functions. Stat Med. 2004;23:1631–1660. doi: 10.1002/sim.1742. [DOI] [PubMed] [Google Scholar]
- 23.Morlat P, Vivot A, Vandenhende MA, Dauchy FA, Asselineau J, Deti E, et al. Role of traditional risk factors and antiretroviral drugs in the incidence of chronic kidney disease, ANRS CO3 Aquitaine Cohort, France, 2004–2012. PLoS One. 2013;8:e66223. doi: 10.1371/journal.pone.0066223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tourret J, Deray G, Isnard-Bagnis C. Tenofovir effect on the kidneys of HIV-infected patients: a double-edged sword? J Am Soc Nephrol. 2013;24:1519–1527. doi: 10.1681/ASN.2012080857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szczech LA, Gange SJ, van der Horst C, Bartlett JA, Young M, Cohen MH, et al. Predictors of proteinuria and renal failure among women with HIV infection. Kidney Int. 2002;61:195–202. doi: 10.1046/j.1523-1755.2002.00094.x. [DOI] [PubMed] [Google Scholar]
- 26.Saksena NK, Wang B, Zhou L, Soedjono M, Ho YS, Conceicao V. HIV reservoirs in vivo and new strategies for possible eradication of HIV from the reservoir sites. HIV AIDS (Auckl) 2010;2:103–122. doi: 10.2147/hiv.s6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiner DE, Sarnak MJ. Managing dyslipidemia in chronic kidney disease. J Gen Intern Med. 2004;19:1045–1052. doi: 10.1111/j.1525-1497.2004.40049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chmielewski M, Carrero JJ, Nordfors L, Lindholm B, Stenvinkel P. Lipid disorders in chronic kidney disease: reverse epidemiology and therapeutic approach. J Nephrol. 2008;21:635–644. [PubMed] [Google Scholar]
- 29.Odden MC, Scherzer R, Bacchetti P, Szczech LA, Sidney S, Grunfeld C, et al. Cystatin C level as a marker of kidney function in human immunodeficiency virus infection: the FRAM study. Arch Intern Med. 2007;167:2213–2219. doi: 10.1001/archinte.167.20.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lucas GM, Jing Y, Sulkowski M, Abraham AG, Estrella MM, Atta MG, et al. Hepatitis C viremia and the risk of chronic kidney disease in HIV-infected individuals. J Infect Dis. 2013;208:1240–1249. doi: 10.1093/infdis/jit373. [DOI] [PMC free article] [PubMed] [Google Scholar]