Abstract
Pituitary adenomas are neoplasms of the anterior pituitary lobe and account for 15–20% of all intracranial tumors. Although most pituitary tumors are benign they can cause severe symptoms related to tumor size as well as hypopituitarism and/or hypersecretion of one or more pituitary hormones. Most pituitary adenomas are sporadic, but it has been estimated that 5% of patients have a familial background. Germline mutations of the tumor suppressor gene aryl hydrocarbon receptor-interacting protein (AIP) predispose to hereditary pituitary neoplasia. Recently, it has been demonstrated that AIP mutations predispose to pituitary tumorigenesis through defective inhibitory GTP binding protein (Gαi) signaling. This finding prompted us to examine whether germline loss-of-function mutations in inhibitory guanine nucleotide (GTP) binding protein alpha (GNAI) loci are involved in genetic predisposition of pituitary tumors. To our knowledge, this is the first time GNAI genes are sequenced in order to examine the occurrence of inactivating germline mutations. Thus far, only somatic gain-of-function hot-spot mutations have been studied in these loci. Here, we have analyzed the coding regions of GNAI1 , GNAI2, and GNAI3 in a set of young sporadic somatotropinoma patients (n = 32; mean age of diagnosis 32 years) and familial index cases (n = 14), thus in patients with a disease phenotype similar to that observed in AIP mutation carriers. In addition, expression of Gαi proteins was studied in human growth hormone (GH), prolactin (PRL), adrenocorticotropic hormone (ACTH)-secreting and non-functional pituitary tumors. No pathogenic germline mutations affecting the Gαi proteins were detected. The result suggests that loss-of-function mutations of GNAI loci are rare or nonexistent in familial pituitary adenomas.
Introduction
Pituitary adenomas are neoplasms of the anterior pituitary lobe. They account for 15–20% of all the intracranial tumors [1] and approximately 16% of all the primary brain and central nervous system tumors [2]. The hallmarks of pituitary tumors are hormonal dysfunction, i.e hormonal hypersecretion or hypopituitarism and local symptoms related to the tumor mass. Compression of neighboring structures may cause headaches and visual impairment [3]. Pituitary adenomas are classified based on the pituitary cell of origin and the type of hormone secreted. The most common functional pituitary tumors hypersecrete prolactin (PRL) (40–45%). Patients with prolactinomas present with amenorrhea, infertility and galactorrhea in females, and infertility in males. Somatotropinomas hypersecrete growth hormone (GH) (20–25%), causing acromegaly with clinical features of enlarged extremities, coarse facial structures and comorbidities such as hypertension, cardiovascular disease and diabetes mellitus [4]. The rate of mortality associated to untreated acromegaly has been reported to be two to four times higher than that seen in the healthy population [5], [6]. In many cases, slow progression of the symptoms delays the diagnosis [7]. Somatotropinoma during childhood or adolescence, before the growth of the long bones is complete, leads to gigantism. Tumors secreting adrenocorticotropic hormone (ACTH) (10–12%) cause Cushing’s disease, which is characterized by hypercortisolism. The majority of the other adenomas are non-functioning (non-secreting) pituitary adenomas (NFPA) [4]. All in all, pituitary adenomas cause a heavy clinical burden due to increased morbidity and the treatment modalities involved, i.e neurosurgery, chronic medical therapies and radiotherapy.
Most pituitary adenomas are sporadic but it has been estimated that 5% of affected patients have a familial background [8]. Pituitary adenomas occur as components of familial tumor syndromes such as multiple endocrine neoplasia type 1 (MEN1) [9], [10], Carney’s complex (CNC) [11], [12] and MEN4 [13]. Furthermore, in 2006, Vierimaa et al. found that germline mutations in the aryl hydrocarbon receptor interacting protein (AIP) gene cause pituitary adenoma predisposition (PAP) [14]. AIP mutations are mostly associated with somatotropinomas (78%), although cases with prolactinomas, NFPAs and Cushing’s syndrome have also been reported [15], [16]. The patients with AIP mutations are typically young (mean age at diagnosis 25 years) and do not necessary have a strong family history of the disease. AIP associated pituitary tumors are often large and invasive and resistant to the effects of available treatments, such as somatostatin analogues, which are used in acromegaly [17]–[19]. Familial occurrence of pituitary tumors is also the main feature in familial isolated pituitary adenoma (FIPA) [8], [20]. Subsequently, it was found that AIP germline mutations explain 15–20% of FIPA families and 50% of families with isolated familial somatotropinomas (IFS) [15]. Thus, the majority of FIPA families appear to be influenced by some other, as yet unidentified genes responsible for familiar clustering of pituitary tumors. Identification of new predisposing genes would enable earlier detection of pituitary adenomas and contribute to clinical management of patients.
The stimulatory guanine nucleotide (GTP) binding protein alpha (GNAS; encoding Gαs subunit) has been found to be mutated in 30–40% of sporadic somatotropinomas. These somatic gain-of-function mutations lead to constitutive activation of cyclic adenosine monophosphate (cAMP) synthesis and increased proliferation through cAMP mediated mitogenic signaling [21]–[24]. Activating mutations on GNAS are also associated to McCune-Albright syndrome [25], [26]. Along with well-established GNAS mutations, somatic mutations in other Gα family members, namely GNAQ and GNA11, have been linked to tumorigenesis in melanocytic neoplasms [27], [28].
We have recently demonstrated that AIP loss-of-function mutations predispose to pituitary tumorigenesis through defective inhibitory GTP binding protein (Gαi) signaling and consequent elevated intracellular cAMP concentrations [29]. We found that Gαi-2 and Gαi-3 proteins are not capable of inhibiting cAMP synthesis during AIP deficiency and that Gαi-2 protein levels are significantly reduced in AIP-mutated somatotropinomas. As the AIP protein seems to be an essential regulator of Gαi signaling, the possibility that inactivating germline mutations in GNAI loci (encoding Gαi subunits) would predispose to pituitary adenomas prompted us to investigate the role of these genes in pituitary tumorigenesis. Here we sequenced all the coding exons of GNAI1 , GNAI2 and GNAI3 in a set of young sporadic somatotropinoma patients and familial index cases, thus in patients with a disease phenotype similar to that observed in AIP mutation carriers.
Materials and Methods
Gαi immunohistochemistry
To investigate the expression of Gαi proteins in human pituitary tumors, Gαi-1, Gαi-2 and Gαi-3 immunostainings were performed in four prolocatinomas, six somatotropinomas, three ACTH and four NFPA tumors. All tumors were AIP mutation negative. Antibodies used were mouse monoclonal antibody against Gαi-1 (SPM397, sc-56536, Santa Cruz, 1: 40), rabbit polyclonal antibody against Gαi-2 (T19, sc-7276, Santa Cruz, 1: 60) and mouse polyclonal antibody against Gαi-3 (H00002773-B01P, Abnova Corp. Taipei city, Taiwan, 1: 50). Anti-mouse/rabbit/rat secondary antibody, Poly-HRP-GAM/R/R (DPVB55HRP, Immunologic, Duiven, Netherlands) and DAB chromogen (Lab Vision Corporation, Fremont, CA, USA, Thermo Fisher Scientific, Watham, MA, USA) were used for detection. Immunostaining protocol was applied as described [30]. The staining intensities of Gαi proteins were scaled as negative (0), weak (1), moderate (2), or strong (3). The images were taken and edited by Leica DM LB microscope (Meyer Instruments, Houston, TX, USA), Olympus DP50 camera (Olympus Corporation, Tokyo, Japan) and Studio Lite software (Licor, Lincoln, NE, USA).
Patients
This study included a set of 32 young sporadic GH-secreting pituitary adenoma cases in which three of the tumors were secreting both GH and PRL. Age at diagnosis for sporadic cases ranged from 14 to 56 years with a mean of 32 years (Table 1). A majority of the tumors were macroadenomas. The second set of samples included 14 index cases with a familial history of pituitary adenomas (Table 1). The hormones secreted by the tumors were GH (n = 11), PRL (n = 1), ACTH (n = 1) and NFPA (n = 1). All the patients had previously been sequenced negative for AIP. From familial cases 9/14 were earlier screened negative for large germline deletions of AIP [31]. The study and the consent procedures were approved by the Ethics Committee of the Hospital district of Helsinki and Uusimaa (HUS) (approval number: 408/13/03/03/2009) and the Institutional Review Board of the Department of Internal Medicine, General Hospital, Montebelluna (Treviso). Signed informed consent was obtained from all the study participants. In case of the minor/children, the consent was obtained from parent/guardian. Consents are stored and managed together with patient information in the central office/ambulatories where the access is restricted.
Table 1. Patient information and variants detected in the coding regions of GNAI loci.
| Patient | Sex | Age at Dg | Age at Op | Origin | Clinical Dg | TumorSize | Affectedfamilymember(s) | GNAI1 | GNAI2 | GNAI3 |
| S1 | M | 37 | – | Spain | GH | Macro | – | – | – | – |
| S2 | M | 40 | – | Tunisia | GH | Macro | – | – | – | – |
| S3 | F | 38 | – | Finland | GH | Macro | – | – | – | – |
| S4 | M | 14 | – | Finland | GH | Macro | – | – | – | – |
| S5 | F | 24 | – | Italy | GH/PRL | NA | – | – | – | – |
| S6 | F | 24 | – | Italy | GH/PRL | Macro | – | – | – | – |
| S7 | F | 23 | – | Italy | GH | Macro | – | – | – | – |
| S8 | M | 22 | – | Italy | GH | NA | – | – | – | – |
| S9 | F | 19 | – | Italy | GH | Macro | – | – | – | – |
| S10 | F | 17 | – | Italy | GH | Macro | – | – | – | – |
| S11 | M | 33 | – | Italy | GH | Macro | – | c.468G>GA(rs12721456) | – | c.105G>GA(rs2230350) c.987G>GA(rs61758987) |
| S12 | M | 30 | – | Italy | GH | Macro | – | c.468G>GA(rs12721456) | c.138C>CT(rs762707) | – |
| S13 | F | 37 | – | Italy | GH | Macro | – | c.846T>TC(rs10241877) | – | – |
| S14 | F | 36 | – | Italy | GH | Micro | – | – | – | – |
| S15 | F | 33 | – | Italy | GH | Macro | – | – | – | – |
| S16 | M | 36 | – | Italy | GH | Macro | – | – | – | – |
| S17 | M | 23 | – | Italy | GH | Macro | – | – | – | – |
| S18 | M | 35 | – | Italy | GH | Macro | – | c.846T>TC(rs10241877) | – | – |
| S19 | F | 32 | – | Italy | GH | Macro | – | – | – | – |
| S20 | F | 36 | – | Italy | GH | Macro | – | c.468G>GA(rs12721456) | c.138C>CT(rs762707) | – |
| S21 | M | 39 | – | Italy | GH | Macro | – | – | – | (c.105G>GA)rs2230350 |
| S22 | M | 38 | – | Italy | GH | Micro | – | c.468G>GA(rs12721456) | – | – |
| S23 | M | 26 | – | Finland | GH | NA | – | c.846T>TC (rs10241877) | – | – |
| S24 | F | 40 | – | Italy | GH | NA | – | – | – | – |
| S25 | M | 23 | – | Finland | GH/PRL | Macro | – | c.846T>TC(rs10241877) | – | – |
| S26 | F | 43 | – | Finland | GH | NA | – | c.846T>TC(rs10241877) | – | – |
| S27 | F | 24 | – | Finland | GH | Macro | – | – | – | – |
| S28 | F | 39 | – | Estonia | GH | Macro | – | – | – | – |
| S29 | M | 40 | – | Finland | GH | NA | – | – | – | – |
| S30 | F | 25 | – | Italy | GH | NA | – | – | – | – |
| S31 | M | 56 | – | Finland | GH | NA | – | c.846T>TC(rs10241877) | – | – |
| S32 | F | 55 | – | Finland | GH | NA | – | – | – | – |
| F1 | F | 40 | – | Italy | GH | Micro | NFPP (father) | – | – | – |
| *F2 | F | 56 | NA | Italy | GH | NA | GH (aunt) | – | – | – |
| *F3 | M | 56 | NA | Italy | NFPA | NA | GH (mother) | – | – | – |
| *F4 | F | NA | 67 | Italy | ACTH | NA | GH (son) | – | – | – |
| *F5 | F | NA | 36 | Italy | PRL | NA | GH (aunt) | – | c.138C>CT(rs762707) | – |
| *F6 | F | NA | 49 | Italy | GH | NA | PRL (daughter) | – | – | – |
| *F7 | M | 42 | NA | Italy | GH | NA | GH (cousin) | – | – | – |
| *F8 | M | 36 | – | Finland | GH | NA | GH (uncle) | c.846T>TC(rs10241877) | – | – |
| F9 | F | NA | 59 | Finland | GH | NA | PRL (niece) | – | – | (c.105G>GA)rs2230350 |
| *F10 | M | NA | 44 | Italy | GH | NA | NFPA (niece) | c.468G>GA(rs12721456) | – | – |
| F11 | M | 24 | NA | Italy | GH | NA | GH/PRL (sister) | – | – | – |
| F12 | F | 36 | NA | Italy | GH | NA | GH (brother) | – | – | – |
| F13 | F | 63 | NA | Finland | GH | NA | ACTH (cousin) | c.846T>TC(rs10241877) | – | – |
| *F14 | M | 40 | NA | Finland | GH | Macro | PRL (cousin) | – | – | – |
Dg: diagnosis, Op: operation, S: sporadic, F: familial, M: male, F: female, NA: not available, Micro: <10 mm, Macro: >10 mm. * Screened negative for AIP germline deletions by MLPA.
Mutation Analysis on GNAI loci
The coding regions of GNAI1 (ENST00000442586 and ENST00000351004; Ensemble release 75), GNAI2 (ENST00000422163, ENST00000451956 and ENST00000266027), and GNAI3 (ENST00000369851) were amplified and sequenced from blood-derived DNA. Also intronic regions flanking the exons were included in the analyses. PCR was carried out by mixing 0.25 µl 20 mM of each primers (Table 2), 5 ng/ul of DNA, 0.4 µl 40 mM of dNTP, 2.5 µl 10xPCR Buffer, and 0.1 µl AmpliTaq Gold DNA Polymerase (Invitrogen Life Science Technologies, Foster City, CA) in a final volume of 25 µl. PCR products were purified by using ExoSAP-IT PCR product cleanup reaction (Affymetrix, USB Products, CA, USA). DNA was sequenced by using BigDye v.3.1 sequencing chemistry and ABI3830x DNA sequencer (Applied Biosystems, Foster City, CA, USA). Sequences were analyzed with Mutation Surveyor software V4.0.8 (Soft-Genetics, State College, PA, USA).
Table 2. Primer sequences, annealing temperatures and Ensembl transcripts for 23 amplicons of GNAI loci.
| Primer | Sequence (5′ –3′) | Tm (°C) | Trancript |
| Gαi1_ex1_F | GGATTCCCCTGTGCTTGGA | 60 | ENST00000442586 |
| Gαi1_ex1_R | GTTTCCAAACGCCGAGGG | ||
| Gαi1_ex2&3_F | CACACAGAGAGAGACTGGGTG | 60 | ENST00000351004 |
| Gαi1_ex2&3_R | GGTCCTGATAGTTGACAAGCC | ||
| Gαi1_ex4_F | AAGGAAGTTCGCTATTGCC | 60 | ENST00000351004 |
| Gαi1_ex4_R | AATGTGTCAGCCAATTCTGC | ||
| Gαi1_ex5_F | GTTTTGGATGATCTTTATTGGC | 60 | ENST00000351004 |
| Gαi1_ex5_R | TCTCCCAAACATTCTTTTGTCC | ||
| Gαi1_ex6_F | CCCATAAAGTCCTTCTCTCCTTC | 62×1, 61×1, 60×2, 59×2,58×2 | ENST00000351004 |
| Gαi1_ex6_R | CTTGGCAACACCTTCAGCTC | ||
| Gαi1_ex7_F | TGTTCTGAAATGGCAGAAATG | 60 | ENST00000351004 |
| Gαi1_ex7_R | CTGAATTCTTGCCTTAGGGG | ||
| Gαi1_ex8_F | GGAGTCCATGAATGAAACTGTATG | 60 | ENST00000351004 |
| Gαi1_ex8_R | TTTGGTCAAGTCCCAGATGC | ||
| Gαi2_ex1c_F | TCACCCACATCACCGTCTAA | 59 | ENST00000422163 |
| Gαi2_ex1c_R | ACGCGTCCTCTTGCAACTA | ||
| Gαi2_ex1d_F | CGCTGTCCATTGCTCTTCAT | 60 | ENST00000451956 |
| Gαi2_ex1d_R | GCACATGTGAGCATTCAGGT | ||
| Gαi2_ex2_F | AGCTGAAGTGTGACGCTGTG | 58 | ENST00000266027 |
| Gαi2_ex2_R | CTTGGCCAGCCATGAAGG | ||
| Gαi2_ex3&4_F | ATGTGAGAACAGGGTGGCTC | 58 | ENST00000266027 |
| Gαi2_ex3&4_R | GGATTCCCTAGGATGAGACTTG | ||
| Gαi2_ex5_F | CCAAGAATACCCTAGCCTGG | 60 | ENST00000266027 |
| Gαi2_ex5_R | GCAAAGACCAGCAGTGTCC | ||
| Gαi2_ex6_F | CTACCTGAACGACCTGGAGCGTA | 58 | ENST00000266027 |
| Gαi2_ex6_R | CTCTGCTACCCCAGAGGCTG | ||
| Gαi2_ex7&8_F | AAATGGGGTAGAAAGCCTCC | 58 | ENST00000266027 |
| Gαi2_ex7&8_R | TGGTCACCATAGGCTACTTGG | ||
| Gαi2_ex9_F | CTTGCTGCACACGTAGGATG | 58 | ENST00000266027 |
| Gαi2_ex9_R | CGCTTAGTTCTTCCCCAGC | ||
| Gαi2_ex9b_F | GTCCACCTGCTCATTCTCGT | 60 | ENST00000266027 |
| Gαi2_ex9b_R | TGGAACCCAATTCTGTGGAG | ||
| Gαi3_ex1_F | GCAGTTTCCGTGGTGTGAG | 58 | ENST00000369851 |
| Gαi3_ex1_R | GTTCAGGCCTTCCAAGCG | ||
| Gαi3_ex2&3_F | TAGGACCCGTGGTTTTCATC | 60 | ENST00000369851 |
| Gαi3_ex2&3_R | TTGTTGCTTAAATTCATTTCCC | ||
| Gαi3_ex4_F | CTGGCCTGTCAGAAAAGGTC | 60 | ENST00000369851 |
| Gαi3_ex4_R | AAACATTTCCTTAAGTGGGGAC | ||
| Gαi3_ex5_F | TTTGCTATGCACATGGTTGG | 60 | ENST00000369851 |
| Gαi3_ex5_R | AAATTTTACCCTGATTAAGAGATGG | ||
| Gαi3_ex6_F | CATTTCAGTTTAGGGGAAGGTG | 60 | ENST00000369851 |
| Gαi3_ex6_R | TTATTTTCCATTTCCTGGCTAC | ||
| Gαi3_ex7_F | TGAATGCCATTTAGTGCTGC | 60 | ENST00000369851 |
| Gαi3_ex7_R | GCCACTACCACTGAATACTCTCC | ||
| Gαi3_ex8_F | TTGGGTTATGTTCCCTCTCC | 60 | ENST00000369851 |
| Gαi3_ex8_R | CAAGAGACATCACTGTAGCACTATAAC |
Tm: annealing temperature.
Results
Gαi immunohistochemistry
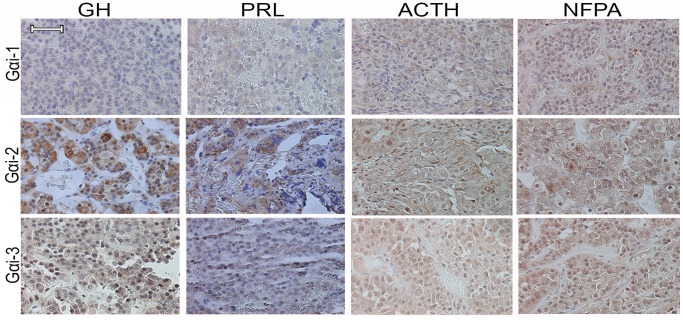
To examine the Gαi protein expressions in human pituitary adenomas, Gαi-1, Gαi-2, Gαi-3 expressions were immunohistochemically (IHC) analyzed in AIP mutation negative somatotropinomas, prolactinomas, NFPA and ACTH tumors. Weak and speckled cytoplasmic expression of Gαi-1 was detected in GH- (mean±SD; 0.8±0.4) and PRL- (1±0.8) secreting tumors, whereas NFPA (1.8±0.5) and ACTH (1.7±0.6) tumors showed weak to moderate cytoplasmic expression (Figure 1). Consistent with the earlier observation in human GH-secreting tumors [29], Gαi-2 was prominently expressed in the cytoplasm of the somatotropinomas (2.8±0.4). Prolactinomas displayed moderate to strong expression of Gαi-2 (2.5±0.6). NFPA (1.8±0.5) and ACTH (1.7±0.6) adenomas showed moderate cytoplasmic and occasional nuclear Gαi-2 staining. All tumor types displayed moderate cytoplasmic expression of Gαi-3 (GH: 2.3±0.5, PRL: 2±0.8, ACTH: 1.6±0.6, NFPA: 1.8±0.5). Weak to moderate nuclear Gαi-3 staining was also observed in all tumor types (GH: 1.3±0.8, PRL: 0.8±0.5, ACTH: 1.3±0.6, NFPA: 1.5±0.6).
Figure 1. Gαi-1, Gαi-2, and Gαi-3 protein expressions in GH, PRL, ACTH and non-functioning (NFPA) pituitary adenomas.
Scale bar = 20 µm.
GNAI loci mutation analysis
All the GNAI coding exons (23 amplicons per sample) were successfully sequenced and analyzed in 32 young sporadic somatotropinoma and 14 index familial cases (Table 1). In GNAI1, earlier reported synonymous heterozygous variations were detected in exon 6 (rs12721456/5 samples) and in exon 7 (rs10241877/8 samples). In GNAI2, one reported heterozygous and synonymous variation was found in exon 4 (rs762707/3 samples). Also in GNAI3, only previously observed heterozygous synonymous variations were detected in exon 1 (rs2230350/3 samples) and exon 8 (rs61758987/1 sample). None of these variants modified amino acid sequence, indicating the polymorphic nature of these changes. Additionally, several reported and unreported variations were observed in intronic regions (Table S1). All the intronic variants located outside of the splice site consensus sequences and are thus not assumed to affect splicing events.
Discussion
Many G proteins have been linked to tumor development, starting with the discovery that somatic gain-of-function mutations of codons 201 and 227 in the GNAS gene are responsible in one third of the sporadic somatotropinomas with elevated cAMP levels [23], [32]. Activating GNAS hot-spot mutations have been detected in many other tumor types. For instance, biliary tract, thyroid, pancreatic, colon, and testis tumors are common targets of somatic GNAS mutations. Additionally, activating somatic hotspot mutations have been reported in GNAQ (Gαq) and GNA11 (Gα11) genes in melanomas and meningeal tumors [33]. Somatic mutations in other Gα subunit genes have been detected, albeit in a low frequency.
Proteins of the inhibitory Gα subfamily, Gαi/Gαo, mediate several cellular and metabolic functions [34]–[37]. Unlike the Gαo, Gαi-1, Gαi-2 and Gαi-3 subunits are involved in the hormonal inhibition of adenylate cyclase (AC) activity with subsequent decrease of intracellular cAMP levels [38], [39]. Previous studies have been focusing on screening GNAI2 somatic hot-spot mutations (termed gip2 oncogene) in codons 179 and 205. Somatic gip2 mutations have been found in ovarian, adrenal, ACTH and NFPA tumors [32], [40], [41] . However, other studies have failed to confirm these initial findings [42]–[48]. Although isolated somatic mutations of GNAI genes have also been observed in next-generation sequencing efforts, further experiments are needed to validate the existence and relevance of these findings [49], [50].
In our original study, we found that AIP deficiency is associated in pituitary tumorigenesis via reduced Gαi signaling followed by elevated cAMP concentrations [29]. In the current study, we searched for germline mutations in GNAI loci in pituitary adenoma patients compatible with the AIP phenotype; young patients with somatotropinoma and familial index cases (Table 1). Also protein expressions of Gαi-1, Gαi-2 and Gαi-3 were examined in human GH-, PRL-, ACTH- and non-secreting (NFPA) pituitary adenomas. We have earlier shown that Gαi-2 and Gαi-3 proteins are expressed in human somatotropinomas [29]. Here we observed that also the Gαi-1 protein, although at low levels, is present in GH-secreting pituitary adenomas. Moreover, immunoreactions against all three Gαi proteins were detected in human prolactinomas, ACTH and NFPA tumors (Figure 1), suggesting a biological role of all these proteins in these tumor types as well.
We screened for germline mutations in the GNAI loci in sporadic somatotropinoma patients (n = 32) and familial index cases (n = 14) characterized by the AIP phenotype (Table 1). No pathogenic mutations were observed in any of the patients studied. All the detected variants were either known polymorphisms or located in intronic regions. Although certain intronic variants may cause impaired splicing, the observed variants were not proximal to known splice sites. We acknowledge that the sample size in the present study is insufficient to draw a definite conclusion of the involvement of GNAI germline mutations in genetic predisposition of pituitary tumors. Moreover, due to the small sample size there is no adequate power to detect possible associations between the observed variant alleles and a pituitary tumor phenotype.
To our knowledge, this is the first time that all the coding exons of GNAI1, GNAI2 and GNAI3 have been sequenced to detect germline loss-of-function mutations in a set of selected pituitary adenoma patients. All in all, our sequencing results suggest that germline mutations of the GNAI loci seem not to be associated to, or are rare in familial pituitary tumorigenesis. However, a larger set of samples, somatic mutation screenings, copy number profiling and additional cellular works would provide a more comprehensive result of the role of GNAI genes in pituitary tumorigenesis.
Supporting Information
Intronic variations in GNAI loci.
(DOCX)
Acknowledgments
We thank Inga-Lill Svedberg, Iina Vuoristo and Alison Ollikainen for technical assistance. Institute for Molecular Medicine Finland (FIMM) for the sequencing service and the Biomedicum Imaging Unit for the microscopy service are acknowledged.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the Worldwide Cancer Research (Grant no: 13–1075, http://www.worldwidecancerresearch.org/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Heaney AP, Melmed S (2004) Molecular targets in pituitary tumours. Nat Rev Cancer 4: 285–295. [DOI] [PubMed] [Google Scholar]
- 2. Dolecek TA, Propp JM, Stroup NE, Kruchko C (2012) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol 14: v1–v49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Asa SL, Ezzat S (2009) The pathogenesis of pituitary tumors. Annu Rev Pathol Mech Dis 4: 97–126. [DOI] [PubMed] [Google Scholar]
- 4. Arafah B, Nasrallah M (2001) Pituitary tumors: pathophysiology, clinical manifestations and management. Endocr Relat Cancer 8: 287–305. [DOI] [PubMed] [Google Scholar]
- 5. Dekkers O, Biermasz N, Pereira A, Romijn J, Vandenbroucke J (2008) Mortality in acromegaly: a metaanalysis. J Clin Endocrinol Metab 93: 61–67. [DOI] [PubMed] [Google Scholar]
- 6. Swearingen B, Barker FG, Katznelson L, Biller BM, Grinspoon S, et al. (1998) Long-Term Mortality after Transsphenoidal Surgery and Adjunctive Therapy for Acromegaly 1. J Clin Endocrinol Metab 83: 3419–3426. [DOI] [PubMed] [Google Scholar]
- 7. Reid TJ, Post KD, Bruce JN, Nabi Kanibir M, Reyes-Vidal CM, et al. (2010) Features at diagnosis of 324 patients with acromegaly did not change from 1981 to 2006: acromegaly remains under-recognized and under-diagnosed. Clin Endocrinol (Oxf) 72: 203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tichomirowa M, Daly A, Beckers A (2009) Familial pituitary adenomas. J Intern Med 266: 5–18. [DOI] [PubMed] [Google Scholar]
- 9. Larsson C, Skogseid B, Öberg K, Nakamura Y, Nordenskjöld M (1988) Multiple endocrine neoplasia type 1 gene maps to chromosome 11 and is lost in insulinoma. Nature 332: 85–87. [DOI] [PubMed] [Google Scholar]
- 10. Chandrasekharappa SC, Guru SC, Manickam P, Olufemi S-E, Collins FS, et al. (1997) Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science 276: 404–407. [DOI] [PubMed] [Google Scholar]
- 11. Casey M, Vaughan CJ, He J, Hatcher CJ, Winter JM, et al. (2000) Mutations in the protein kinase A R1α regulatory subunit cause familial cardiac myxomas and Carney complex. J Biol Chem 106: R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kirschner LS, Carney JA, Pack SD, Taymans SE, Giatzakis C, et al. (2000) Mutations of the gene encoding the protein kinase A type I-α regulatory subunit in patients with the Carney complex. Nat Genet 26: 89–92. [DOI] [PubMed] [Google Scholar]
- 13. Pellegata NS, Quintanilla-Martinez L, Siggelkow H, Samson E, Bink K, et al. (2006) Germ-line mutations in p27Kip1 cause a multiple endocrine neoplasia syndrome in rats and humans. Proc Natl Acad Sci U S A 103: 15558–15563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vierimaa O, Georgitsi M, Lehtonen R, Vahteristo P, Kokko A, et al. (2006) Pituitary adenoma predisposition caused by germline mutations in the AIP gene. Science 312: 1228–1230. [DOI] [PubMed] [Google Scholar]
- 15. Daly AF, Vanbellinghen J-F, Khoo SK, Jaffrain-Rea M-L, Naves LA, et al. (2007) Aryl hydrocarbon receptor-interacting protein gene mutations in familial isolated pituitary adenomas: analysis in 73 families. J Clin Endocrinol Metab 92: 1891–1896. [DOI] [PubMed] [Google Scholar]
- 16. Cazabat L, Bouligand J, Salenave S, Bernier M, Gaillard S, et al. (2012) Germline AIP mutations in apparently sporadic pituitary adenomas: prevalence in a prospective single-center cohort of 443 patients. J Clin Endocrinol Metab 97: E663–E670. [DOI] [PubMed] [Google Scholar]
- 17. Leontiou CA, Gueorguiev M, van der Spuy J, Quinton R, Lolli F, et al. (2008) The role of the aryl hydrocarbon receptor-interacting protein gene in familial and sporadic pituitary adenomas. J Clin Endocrinol Metab 93: 2390–2401. [DOI] [PubMed] [Google Scholar]
- 18. Daly AF, Tichomirowa MA, Petrossians P, Heliövaara E, Jaffrain-Rea M-L, et al. (2010) Clinical characteristics and therapeutic responses in patients with germ-line AIP mutations and pituitary adenomas: an international collaborative study. J Clin Endocrinol Metab 95: E373–E383. [DOI] [PubMed] [Google Scholar]
- 19. Beckers A, Aaltonen LA, Daly AF, Karhu A (2013) Familial isolated pituitary adenomas (FIPA) and the pituitary adenoma predisposition due to mutations in the aryl hydrocarbon receptor interacting protein (AIP) gene. Endocr Rev 34: 239–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Daly A, Jaffrain-Rea M-L, Ciccarelli A, Valdes-Socin H, Rohmer V, et al. (2006) Clinical characterization of familial isolated pituitary adenomas. J Clin Endocrinol Metab 91: 3316–3323. [DOI] [PubMed] [Google Scholar]
- 21. Vallar L, Spada A, Giannattasio G (1987) Altered Gs and adenylate cyclase activity in human GH-secreting pituitary adenomas. Nature 330: 566–568. [DOI] [PubMed] [Google Scholar]
- 22. Boikos SA, Stratakis CA (2007) Molecular genetics of the cAMP-dependent protein kinase pathway and of sporadic pituitary tumorigenesis. Hum Mol Genet 16: R80–R87. [DOI] [PubMed] [Google Scholar]
- 23. Landis CA, Masters SB, Spada A, Pace AM, Bourne HR, et al. (1989) GTPase inhibiting mutations activate the α chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature 340: 692–696. [DOI] [PubMed] [Google Scholar]
- 24. Gupta S, Gallego C, Johnson G (1992) Mitogenic pathways regulated by G protein oncogenes. Mol Biol Cell 3: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, et al. (1991) Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med 325: 1688–1695. [DOI] [PubMed] [Google Scholar]
- 26. Schwindinger WF, Francomano CA, Levine MA (1992) Identification of a mutation in the gene encoding the alpha subunit of the stimulatory G protein of adenylyl cyclase in McCune-Albright syndrome. Proc Natl Acad Sci U S A 89: 5152–5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Van Raamsdonk CD, Bezrookove V, Green G, Bauer J, Gaugler L, et al. (2009) Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature 457: 599–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Van Raamsdonk CD, Griewank KG, Crosby MB, Garrido MC, Vemula S, et al. (2010) Mutations in GNA11 in uveal melanoma. N Engl J Med 363: 2191–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tuominen I, Heliövaara E, Raitila A, Rautiainen M, Mehine M, et al.. (2014) AIP inactivation leads to pituitary tumorigenesis through defective Gαi-cAMP signaling. Oncogene. doi: 10.1038/onc.2014.50. [DOI] [PubMed]
- 30. Raitila A, Lehtonen HJ, Arola J, Heliövaara E, Ahlsten M, et al. (2010) Mice with Inactivation of Aryl Hydrocarbon Receptor-Interacting Protein (Aip) Display Complete Penetrance of Pituitary Adenomas with Aberrant ARNT Expression. Am J Pathol 177: 1969–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Georgitsi M, Heliovaara E, Paschke R, Kumar AV, Tischkowitz M, et al. (2008) Large genomic deletions in AIP in pituitary adenoma predisposition. J Clin Endocrinol Metab 93: 4146–4151. [DOI] [PubMed] [Google Scholar]
- 32. Lyons J, Landis CA, Harsh G, Vallar L, Grunewald K, et al. (1990) Two G protein oncogenes in human endocrine tumors. Science 249: 655–659. [DOI] [PubMed] [Google Scholar]
- 33. O'Hayre M, Vázquez-Prado J, Kufareva I, Stawiski EW, Handel TM, et al. (2013) The emerging mutational landscape of G proteins and G-protein-coupled receptors in cancer. Nat Rev Cancer 13: 412–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wettschureck N, Moers A, Offermanns S (2004) Mouse models to study G-protein-mediated signaling. Pharmacol Ther 101: 75–89. [DOI] [PubMed] [Google Scholar]
- 35. Spiegel AM (1996) Mutations in G proteins and G protein-coupled receptors in endocrine disease. J Clin Endocrinol Metab 81: 2434–2442. [DOI] [PubMed] [Google Scholar]
- 36. Dhanasekaran N, Heasley LE, Johnson GL (1995) G protein-coupled receptor systems involved in cell growth and oncogenesis. Endocr Rev 16: 259–270. [DOI] [PubMed] [Google Scholar]
- 37. Epstein FH, Farfel Z, Bourne HR, Iiri T (1999) The expanding spectrum of G protein diseases. N Engl J Med 340: 1012–1020. [DOI] [PubMed] [Google Scholar]
- 38. Kobayashi I, Shibasaki H, Takahashi K, Tohyama K, Kurachi Y, et al. (1990) Purification and characterization of five different α subunits of guanine-nucleotide-binding proteins in bovine brain membranes. Eur J Biochem 191: 499–506. [DOI] [PubMed] [Google Scholar]
- 39. Peverelli E, Busnelli M, Vitali E, Giardino E, Galés C, et al. (2013) Specific roles of Gi protein family members revealed by dissecting SST5 coupling in human pituitary cells. J Cell Sci 126: 638–644. [DOI] [PubMed] [Google Scholar]
- 40. Williamson EA, Daniels M, Foster S, Kelly WF, Kendall-Taylor P, et al. (1994) Gsα and Gi2α mutations in clinically non-functioning pituitary tumours. Clin Endocrinol (Oxf) 41: 815–820. [DOI] [PubMed] [Google Scholar]
- 41. Williamson E, Ince P, Harrison D, Kendall-Taylor P, Harris P (1995) G-protein mutations in human pituitary adrenocorticotrophic hormone-secreting adenomas. Eur J Clin Invest 25: 128–131. [DOI] [PubMed] [Google Scholar]
- 42. Tordjman K, Stern N, Ouaknine G, Yossiphov Y, Razon N, et al. (1993) Activating mutations of the Gs alpha-gene in nonfunctioning pituitary tumors. J Clin Endocrinol Metab 77: 765–769. [DOI] [PubMed] [Google Scholar]
- 43. Ruggeri R, Santarpia L, Curtò L, Torre M, Galatioto M, et al. (2008) Non-functioning pituitary adenomas infrequently harbor G-protein gene mutations. J Endocrinol Invest 31: 946–949. [DOI] [PubMed] [Google Scholar]
- 44. Petersenn S, Heyens M, Lüdecke DK, Beil FU, Schulte HM (2000) Absence of somatostatin receptor type 2 A mutations and gip oncogene in pituitary somatotroph adenomas. Clin Endocrinol (Oxf) 52: 35–42. [DOI] [PubMed] [Google Scholar]
- 45. Kan B, Esapa C, Sipahi T, Nacar C, Özer F, et al. (2003) G protein mutations in pituitary tumors: a study on Turkish patients. Pituitary 6: 75–80. [DOI] [PubMed] [Google Scholar]
- 46. Reincke M, Karl M, Travis W, Chrousos GP (1993) No evidence for oncogenic mutations in guanine nucleotide-binding proteins of human adrenocortical neoplasms. J Clin Endocrinol Metab 77: 1419–1422. [DOI] [PubMed] [Google Scholar]
- 47. Villares Fragoso MCB, Latronico AC, Carvalho FM, Zerbini MCN, Marcondes JAM, et al. (1998) Activating Mutation of the Stimulatory G Protein (gsp) as a Putative Cause of Ovarian and Testicular Human Stromal Leydig Cell Tumors 1. J Clin Endocrinol Metab 83: 2074–2078. [DOI] [PubMed] [Google Scholar]
- 48. Shen Y, Mamers P, Jobling T, Burger HG, Fuller P (1996) Absence of the previously reported G protein oncogene (gip2) in ovarian granulosa cell tumors. J Clin Endocrinol Metab 81: 4159–4161. [DOI] [PubMed] [Google Scholar]
- 49. Forbes SA, Bindal N, Bamford S, Cole C, Kok CY, et al. (2011) COSMIC: mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res 39: D945–D950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fu W, O’Connor TD, Jun G, Kang HM, Abecasis G, et al. (2013) Analysis of 6,515 exomes reveals the recent origin of most human protein-coding variants. Nature 493: 216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Intronic variations in GNAI loci.
(DOCX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.