Abstract
Objective
Among rheumatoid arthritis (RA) patients, pain may be due to peripheral inflammation or other causes, such as central pain mechanisms. The objective was to use self-report measures and physical examination to identify clusters of RA patients who may have different causes of pain and different prognoses and treatment options.
Methods
Data were analyzed from 169 RA patients in the Brigham Rheumatoid Arthritis Sequential Study who had pain > 0/10 and completed questionnaires on pain, fatigue and psychosocial factors. A hierarchical agglomerative clustering procedure with Ward’s method was used to obtain subgroups. Multivariate analysis of variance was used to determine the contribution of each variable in a cluster. General linear regression models were used to examine differences in clinical characteristics across subgroups. Discriminant analyses were performed to determine coefficients for linear combinations of variables that assigned cluster membership to individual cases.
Results
Three clusters best fit these data. Cluster 1 consisted of 89 individuals with low inflammation, pain, fatigue and psychosocial distress. Cluster 2 consisted of 57 individuals with minimal inflammation but high pain, fatigue and psychosocial distress. Cluster 3 consisted of 23 individuals with active inflammatory disease, manifested by high joint counts, high C-reactive protein and high pain and fatigue.
Conclusion
Although most patients had low levels of inflammation, pain and fatigue, 47.3% continued to report moderate to high pain and fatigue. Most of these patients had minimal signs of inflammation but high levels of fatigue, pain catastrophizing and sleep disturbance, indicative of a chronic widespread pain syndrome.
Rheumatoid arthritis (RA) is the most common systemic rheumatic disease, affecting approximately 1.5 million adults in the United States (1). Historically, physicians have focused on the inflammatory components of the disease (e.g. synovitis), whereas RA patients have cited pain, fatigue, sleep problems and other quality of life outcomes as their main priorities (2). Both patients and physicians frequently assume that these symptoms are correlated with heightened systemic inflammation. However, many studies indicate significant discordance between inflammation, pain and fatigue among RA patients (3, 4). In a prospective observational cohort of established RA patients experiencing sustained inflammatory disease remission, 12% continued to report clinically significant pain (≥ 4 on a 10-point numeric rating scale) (5). A study including 2,096 RA patients in a clinical setting and 14,607 RA patients from a survey-based cohort reported weak correlations of 0.07–0.11 between fatigue and measures of inflammation (6). Similarly, van Hoogmoed found no association between fatigue and either the erythrocyte sedimentation rate or C-reactive protein (CRP) among 228 RA outpatients in the Netherlands (7). These observations suggest that patient-reported outcomes, such as pain, fatigue and sleep problems, should not automatically be attributed to inflammation in an inflammatory disease population, such as RA.
Central, non-inflammatory pain conditions, such as fibromyalgia, tend to be associated with high levels of pain, fatigue and sleep problems (8, 9). Compared to the general population (10, 11), the prevalence of non-inflammatory pain conditions is significantly higher in RA, with 15–25% of RA patients also meeting criteria for fibromyalgia and an additional 7–15% meeting criteria for chronic widespread pain (12, 13). Thus, while it is not debated that RA is an inflammatory condition, many patients with RA also have features characteristic of non-inflammatory centralized pain conditions (e.g., fatigue, sleep disturbances).
The objective of this study was to determine whether subgroups of RA patients could be characterized by a latent construct, representing the involvement of central, non-inflammatory pain processes. The latent construct was defined by grouping individuals based on the presence or absence of symptoms indicative of aberrant central nervous system involvement (e.g., fatigue, sleep problems, negative mood, catastrophic attributions and perception of overall illness burden) using cluster analysis. Whereas factor analysis aggregates variables into patterns based on correlations, cluster analysis categorizes individuals into non-overlapping subgroups (14). Cluster analysis is particularly valuable in identifying subgroups which differ in underlying pathogenic mechanisms and clinical outcomes (15). The identification of clinically distinct phenotypes within a heterogeneous population of RA patients would be the first step in understanding the pathogenic mechanisms underlying each symptom cluster and, ultimately, identifying potential targets for treatment. The variables used in this cluster analysis were markers of central pain processes, similar to those included in a previous cluster analysis examining the role of central, non-inflammatory pain in osteoarthritis (16).
We hypothesized the presence of at least three subgroups: one group comprised of RA patients with well-controlled disease, minimal fatigue and low psychosocial distress; a second group of RA patients with active inflammatory joint pain and moderate levels of psychosocial distress, and a third group of RA patients with low swollen joint counts and high levels of pain, fatigue and other symptoms characteristic of a chronic, non-inflammatory, pain syndrome. This hypothesis was based on clinical observations, as well as studies in other painful chronic conditions. These studies have reported similar subgroups consisting of: a) participants predominantly affected by peripheral pain generators (e.g., joint inflammation and/or mechanical/structural markers), and b) participants in whom inflammation and/or mechanical factors appear to have minimal impact compared to the influence of central, non-inflammatory pain processes(16, 17). Additional subgroups may also exist. For example, other studies have described a group with high levels of depression but low levels of other symptoms (16, 17). However, given the low levels of depression in our patient population (18), we did not expect to identify this group in this cluster analysis.
Patients and Methods
Study population
The study population was derived from a subgroup of the Brigham Rheumatoid Arthritis Sequential Study (BRASS). BRASS is a prospective observational cohort of over 1,300 participants over the age of 18, with a confirmed diagnosis of RA by a board-certified rheumatologist (19). Two hundred and eight of these participants participated in a sub-study to examine the effects of widespread pain on functional status. Exclusion criteria for the current study included: 1) absence of pain (average BPI pain severity score = 0) and 2) incomplete data for any of the clustering variables. Written informed consent was obtained for both the BRASS study and for the sub-study on widespread pain and functional status. This study was approved by the Partners Institutional Review Board.
Measures
Inflammation
Board-certified rheumatologists performed 28-joint counts to assess tenderness and swelling. Both physicians and participants also provided global assessments of disease activity. Samples of blood were collected for a standard laboratory panel, including CRP. Based on these measures, the Disease Activity Score in 28 joints (DAS28) was calculated.(20)
Pain
To quantify pain intensity, we used the average pain severity score on the Brief Pain Inventory – short form (BPI-sf). The BPI-sf is a validated, nine-question survey that assesses the sensory and reactive aspects of clinical pain. The sensory portion includes questions regarding the severity of pain on average (the average BPI-sf pain severity score), “at its worst, at its least and right now”(21). We also measured the distribution of non-joint pain using the Widespread Pain Index (WPI), which assesses pain in 19 areas (22). The distribution of joint pain was quantified using the joint score from the Rheumatoid Arthritis Disease Activity Index (RADAI) (23, 24).
Mood
Depression and anxiety were measured using the Hospital Anxiety and Depression Scale (HADS), a 14-item questionnaire validated in physically ill patients (25).
Fatigue
The fatigue numeric rating scale on the Multidimensional Health Assessment Questionnaire (MDHAQ) was used to assess fatigue. Specifically, the MDHAQ asks, “How much of a problem has fatigue or tiredness been for you in the past week?” (26).
Illness burden
We quantified illness burden using a count of patient-reported symptoms of headaches, migraines, poor concentration, poor memory and poor word-finding. This measure was based on the concept of illness burden described by Murphy et al. in a similar cluster analysis of symptoms in osteoarthritis patients (16).
Sleep problems
Sleep problems were measured using the Medical Outcomes Study (MOS) sleep problems index II, a validated, 12-item questionnaire that assesses sleep problems in chronically ill populations (27).
Catastrophizing
The Pain Catastrophizing Scale (PCS), a validated 13-item scale that assesses negative emotional and cognitive processes (e.g., helplessness, rumination, pessimism and magnification of symptoms) was used to evaluate catastrophizing (28).
Fibromyalgianess
“Fibromyalgianess,” first coined by Wolfe in 2009 to describe polysymptomatic features of chronic widespread pain (e.g., fatigue, somatic symptoms, cognitive and sleep problems, decreased pain threshold, etc.) (29), was measured by summing the WPI and the Symptom Severity Score, which also assesses the presence of headaches, abdominal pain and depression. Both the WPI and the Symptom Severity Score are components of the 2010 ACR Diagnostic Criteria for fibromyalgia (22).
Statistical analysis
Descriptive measures, including medians, interquartile ranges (IQRs) and frequencies were determined. Based on Formann’s methodology (30), which states that the maximum number of clustering variables should be m where sample size = 2m, the number of clustering variables was limited to seven (31, 32). Clustering variables were chosen based on previous studies, indicating the presence of depression-pain-fatigue clusters and fatigue-insomnia-pain clusters in chronic disease populations, such as cancer and osteoarthritis (16). In addition, catastrophizing was included as a measure of negative cognitive and emotional processes, in accordance with a study examining psychological subgrouping of low back pain patients (33). Swollen joint count was included as a measure of joint inflammation. Swollen joint count was chosen to represent RA-related inflammation because: 1) swollen joint count is considered an “objective” measure of inflammation, whereas the tender joint count and physician global assessment may be influenced by other factors, such as widespread pain sensitivity, and 2) swollen joint count directly reflects active inflammation at joint sites whereas CRP is a marker of overall systemic inflammation and Sharp erosion scores reflect cumulative damage but not acute inflammation.
A hierarchical agglomerative clustering procedure with Ward’s method with Squared Euclidian Distances was used to obtain the clusters. To determine the optimal number of clusters, we used the Cubic Clustering Criterion and constructed a dendogram to visually inspect the distances between clusters (34).
To determine the relative contribution of each clustering variable, all variables were included in a multivariate analysis of variance (MANOVA) model with the cluster assignment as the independent variable. The clustering variables were standardized by subtracting each data point from the mean and dividing by the standard deviation. This standardization process minimized deviations from normality, but some variables, particularly swollen joint count, illness burden and pain catastrophizing, continued to have a skewed distribution. In general, the F-test, which is the basis of the MANOVA, is robust to non-normal distributions, particularly when the sample size is large, because the sampling distribution of the mean approximates the normal distribution (central limit theorem) (35, 36). We used the Wilks’ λ as an evaluation of the dissimilarity measure between clusters. Unadjusted general linear regression models were used to identify differences in clustering variables and demographic and clinical characteristics across clusters.
A discriminant analysis was performed to examine cluster groupings. To define the discriminant functions, a constant and seven coefficients (one for each clustering variable) were calculated for each cluster. To determine the relative contribution of each clustering variable to the discriminant functions, the total canonical structure was analyzed. The proportion of misclassified observations in each group was assessed. All statistical analyses were performed using the SAS 9.2 software package (SAS Institute, Cary, NC, USA).
Results
Participant characteristics
Among the over 1,300 RA participants in BRASS, 208 completed baseline questionnaires for the sub-study to examine the effects of widespread pain on functional status. Of these 208 participants, 13 participants (6.3%) were excluded because they did not report any pain, and 26 participants (12.5%) were excluded due to incomplete data for at least one clustering variable. The majority of missing data were from participants who did not complete the PCS, the MDHAQ fatigue scale, or questions used to calculate illness burden. These 26 participants did not differ from participants without missing data in any of the non-missing clustering variables (swollen joint count, BPI average pain intensity, HADS depression score, MDHAQ fatigue score, illness burden, MOS sleep problems index II score, PCS score).
Of the 169 participants in this analysis, 136 (80.5%) were female (Table 1). One hundred and fifty-seven out of 166 (94.6%, three participants did not provide information on race) were Caucasian. Median disease duration was 13.0 years (IQR 7.0–23.0 years). One-hundred and four of 166 participants (62.7%, three participants were missing data regarding rheumatoid factor status) were rheumatoid factor positive. Median DAS28 was 2.6 (IQR 2.0–3.8), and the median MDHAQ fatigue score was 35 (IQR 15.0–65.0). The median BPI pain score was 3.0 (IQR 2.0–5.0).
Table 1.
Clinical characteristics of the study population (N = 169).
| Variable | Median (interquartile range)/Number (%) |
|---|---|
| Age (years) | 58.0 (50.0–65.0) |
| Female (N) | 136 (80.5%) |
| Caucasian (N) | 157 (94.6%)a |
| Body mass index (kg/m2) | 26.1 (23.4–29.8) |
| Disease duration (years) | 13.0 (7.0–23.0) |
| Rheumatoid factor positive (N) | 104 (62.7%)† |
| DAS28-CRP4 | 2.6 (2.0–3.8) |
| BPI pain intensity | 3.0 (2.0–5.0) |
| Fatigue | 35.0 (15.0–65.0) |
| Current DMARD use (N) | 149 (88.2%) |
| Current biologic DMARD use (N) | 98 (58.0%) |
| Current synthetic DMARD use (N) | 108 (63.9%) |
Percentages of Caucasian and rheumatoid factor positive participants are derived from a denominator of 166, due to missing data in these two categories.
DAS28-CRP = Disease Activity Score in 28 Joints – C-Reactive Protein, BPI = Brief Pain Inventory, DMARD = disease-modifying antirheumatic drug.
Cluster analysis
Cluster analyses identified three groups of RA patients (Figure 1). Cluster 1 consisted of the largest number of patients (N = 89, 52.7%) and was characterized by the lowest swollen joint counts (median 0.0, IQR 0.0–1.0; P < 0.0001 compared to both Clusters 2 and 3) (Table 2). Participants in Cluster 1 also had the lowest levels of fatigue (P < 0.0001 compared to both Clusters 2 and 3) and depression (P = 0.01 compared to Cluster 2 and P = 0.004 compared to Cluster 3). Cluster 2 consisted of 57 participants (33.7%), characterized by low swollen joint counts (median 2.0, IQR 0.0–4.0) and high levels of fatigue, catastrophizing and sleep problems. Cluster 3 consisted of 23 participants (13.6%), characterized by high swollen joint counts and moderate levels of fatigue, catastrophizing and sleep problems. MANOVA confirmed that clustering variables were significantly different between groups (Wilks’ λ =0.08, F(14, 320) = 60.0, P <0.0001). These differences were confirmed in unadjusted general linear regression models, which showed statistically significant differences in all clustering variables (P < 0.03) except for illness burden (P = 0.06) between groups.
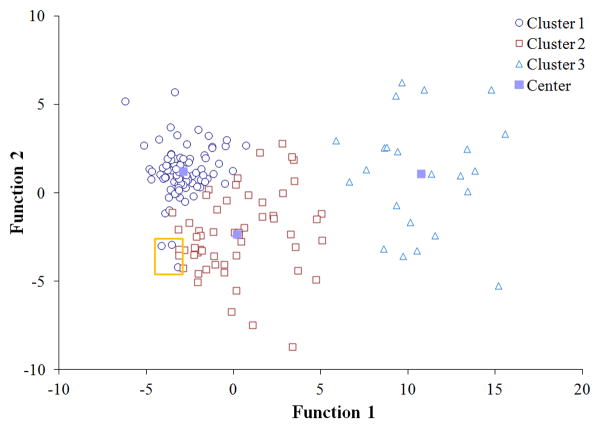
Figure 1. Canonical discriminant functions used to differentiate clusters.
Two discriminant functions significantly distinguished the clusters, accounting for 88.3% and 11.7% of the variance (P <0.0001). Three potential outliers (orange square) were noted.
Table 2.
Clinical characteristics used to define the clusters. Values are expressed as medians and interquartile ranges.a
| Characteristic | Cluster 1 (N = 89) | Cluster 2 (N = 57) | Cluster 3 (N = 23) | P-valueb |
|---|---|---|---|---|
| Swollen joint count | 0.0 (0.0–1.0) | 2.0 (0.0–4.0) c | 12.0 (10.0–14.0) d,e | <0.0001 |
| BPI pain intensity | 3.0 (2.0–4.0) | 3.0 (2.0–5.0) c | 3.0 (2.0–5.0) | 0.03 |
| Fatigue | 20.0 (10.0–30.0) | 70.0 (50.0–80.0) c | 60.0 (25.0–80.0) d | <0.0001 |
| Sleep problems | 27.2 (16.1–41.1) | 38.3 (27.2–46.7) c | 35.6 (17.5–47.8) | 0.009 |
| HADS Depression | 3.0 (1.0–5.0) | 4.0 (1.0–7.0) c | 5.0 (2.0–8.0) d | 0.004 |
| Illness burden | 1.0 (0.0–3.0) | 2.0 (1.0–3.0) c | 1.0 (0.0–2.0) e | 0.06 |
| Catastrophizing | 6.0 (1.0–12.0) | 12.0 (5.0–21.0) c | 9.0 (3.0–18.0) | <0.0001 |
BPI = Brief Pain Inventory, HADS = Hospital Anxiety and Depression Scale.
P-value that any one cluster is different from the others.
Cluster 2 is significantly different from cluster 1 at P ≤ 0.05.
Cluster 3 is significantly different from cluster 1 at P ≤ 0.05.
Clusters 3 is significantly different from cluster 2 at P ≤ 0.05.
Clinical variables among subgroups
Cluster 1
Compared to Clusters 2 and 3, Cluster 1 had the lowest WPI scores (median 2 vs. 5 in both Clusters 2 and 3; P = 0.0006 compared to Cluster 2 and P = 0.005 compared to Cluster 3), consistent with localized distribution of pain. Measures of inflammatory disease activity were also significantly lower in Cluster 1 than in Clusters 2 and 3. The median DAS28-CRP was 2.4 compared to 2.9 (Cluster 2) (P = 0.0005) and 5.1 (Cluster 3) (P ≤ 0.0001), and the median physician global assessment score was 10, compared to 20 (Cluster 2) (P = 0.02) and 40 (Cluster 3) (P ≤ 0.0001). Similarly, the median patient global assessment score was 15 compared to 30 in both Clusters 2 (P =< 0.0001) and 3 (P = 0.001). Subjects in Cluster 1 also had low CRP levels (median 1.7), Sharp erosion scores (median 1) and joint narrowing scores (median 2), though these values were not significantly different from cluster 2.
Cluster 2
Similar to Cluster 1, Cluster 2 had low CRP levels, low tender joint counts, low Sharp erosion scores and low Sharp joint narrowing scores. However, subjects in Cluster 2 had significantly higher DAS28-CRP levels than subjects in Cluster 1 (P = 0.0005), likely due to higher patient global assessment scores (P < 0.0001). These subjects also had significantly higher physician global assessment scores (P = 0.02) and more widespread distribution of pain, assessed by WPI scores (P = 0.0006).
Cluster 3
Compared to Clusters 1 and 2, Cluster 3 had significantly higher measures of inflammatory disease activity, assessed by the DAS28-CRP (P < 0.0001), CRP (P = 0.0001), tender joint count (P < 0.0001) and physician global assessment (P < 0.0001). Although patient global assessment was significantly higher in Cluster 3 compared to Cluster 1 (P = 0.001), patient global assessment was not higher in Cluster 3 compared to Cluster 2. Measures of joint destruction were consistently higher in Cluster 3 compared to Clusters 1 and 2 (P = 0.009 for erosions and P = 0.0005 for joint space narrowing). Subjects in cluster 3 also had significantly higher disease duration (P =0.002) and significantly lower current DMARD use (P = 0.04) than subjects in clusters 1 and 2. Past DMARD use did not differ across groups.
Discriminant functions
The clusters were distinguished by two discriminant functions, corresponding to 88.3% and 11.7% of the variance (P <0.0001). The coefficients for the discriminant functions for each cluster are reported in Table 4. Function 1 was mostly influenced by swollen joint count and fatigue, whereas Function 2 was mostly influenced by fatigue and catastrophizing. Function 1 was primarily responsible for distinguishing Clusters 1 and 2 from Cluster 3, whereas Function 2 was required to distinguish between Clusters 1 and 2. Using discriminant function analysis, we were able to correctly categorize 95.9% of the participants into the groups determined by cluster analysis.
Table 4.
| Variable | Cluster 1 | Cluster 2 | Cluster 3 |
|---|---|---|---|
| Constant | −2.31 | −1.95 | −18.80 |
| Swollen joint count | −3.74 | 0.09 | 14.26 |
| BPI pain intensity | 0.59 | −0.47 | −1.11 |
| Fatigue | −2.57 | 2.35 | 4.13 |
| Sleep problems | 1.18 | −0.92 | −2.28 |
| HADS Depression | 0.12 | −0.12 | −0.16 |
| Illness burden | −0.03 | 0.14 | −0.24 |
| Catastrophizing | −0.69 | 0.62 | 1.14 |
Values are the coefficients for discriminant functions for each cluster. Values in boldface indicate the highest variable loads for each cluster.
BPI = Brief Pain Inventory, HADS = Hospital Anxiety and Depression Scale.
Outliers
Three potential outliers were noted. Each were grouped into Cluster 1 according to cluster analysis, although based on graphical presentation, they appeared more similar to observations in Cluster 2 (Figure 1). These individuals had no swollen joints but high levels of pain catastrophizing, sleep problems and fatigue. Discriminant analyses ultimately re-categorized two of these three patients into Cluster 2.
Discussion
The theory underlying this study is that symptoms, such as pain, fatigue and sleep problems, may be predominantly associated with either: 1) active inflammatory disease, or 2) a chronic, non-inflammatory pain syndrome. Our results characterize three distinct groups of RA patients, based on inflammatory disease activity (swollen joints), co-occurring symptoms (e.g., pain, fatigue, sleep problems) cognitive and emotional factors (e.g., catastrophizing and depression) and functional illness burden. Although the majority of RA patients in this cohort were doing well (Cluster 1), a significant number continued to have multiple areas of pain and difficulties with fatigue, sleep and catastrophizing. Of the 80 patients who had significant problems in these areas, 23 (28.8%) had active inflammatory disease, manifested by elevated swollen joint counts, tender joint counts and serum CRP (Cluster 3). In contrast, the remaining 71.2% (Cluster 2: N = 57) had low levels of inflammatory disease activity, despite the worst patient global scores and the highest levels of fatigue and pain catastrophizing of all three groups. These patients also had low Sharp erosion and joint narrowing scores, indicating that joint damage was not a major cause for pain and mood disturbances. Taken together, these results suggest that Cluster 2 represents a subgroup of RA patients who likely have a more centralized chronic widespread pain syndrome, such as fibromyalgia in addition to RA.
Several other studies have used cluster analysis to identify subgroups of RA patients, but, within each study, the clustering variables were limited to one dimension (e.g., pain behaviors or psychosocial measures) (37, 38). To our knowledge, none have included a wide assessment of clinical factors, such as inflammatory disease activity, cognitive and emotional factors, and functional illness burden, as we did in our study. The study most similar to ours was a cluster analysis of 104 RA patients based on observed pain behaviors (guarding, bracing, active rubbing, grimacing, sighing and rigidity) (37). This study yielded five subgroups with varying behavior patterns, ranging from those with very few pain behaviors to those with multiple distressing behaviors. Although the subgroups differed in behavior patterns, all five subgroups reported similar pain intensity, highlighting a disconnect between physical responses to pain and verbal reports of pain. In a large longitudinal study, cluster analysis was used to classify 561 RA patients based on five psychosocial measures (ability to cope, RA impact, health satisfaction, adequacy of social support, psychological mastery) (38). The authors identified three subgroups of psychosocial risk, which predicted the development of depression, poor functional status and global pain over an 8-year period. These results showcased the ability of cluster analysis to identify clinically meaningful subgroups. Notably, no previous studies have used cluster analysis to identify subgroups with similar sources and patterns of pain in an inflammatory arthritis population, such as RA.
In chronic non-inflammatory pain populations, however, researchers have frequently identified subgroups of patients with widespread pain and psychosocial impairment, in the absence of inflammation and other obvious causes of pain. A cluster analysis of 104 older women identified three distinct groups, which were strikingly similar to the clusters defined in this study: 1) a “healthy” group, 2) a group with high levels of psychosocial distress and illness burden and 3) a group with poor physical health and moderate levels of psychosocial distress (39). Similarly, in a sample of 121 patients with chronic neck pain, three distinct subgroups were noted, which varied in the severity of psychosocial distress, sleep disorder and disability (40). In a longitudinal study of 843 pediatric functional abdominal pain patients, Walker et al. used a variety of clustering variables, including pain intensity, gastrointestinal symptoms, coping abilities, catastrophizing, negative affection and physical activity, to categorize patients into three subgroups, including a “high pain dysfunctional” group, which was associated with a high risk for functional abdominal pain disorder in adolescence/adulthood (41).
Compared to the cluster analyses done in non-inflammatory chronic pain conditions, our results are most similar to a study using cluster analysis to characterize symptomatic subjects with osteoarthritis of the knee and hip (16). Both studies identified a group of patients with high pain, fatigue, sleep problems and mood disturbances who comprised approximately one-third of the study population. In the osteoarthritis study, the chronic widespread pain group also had significantly higher levels of illness burden, assessed by MANOVA. In our study, illness burden was not significantly different between groups based on MANOVA (P = 0.06). The lack of a difference may partially be attributed to our measure of illness burden, which was assessed based on five common somatic symptoms, as opposed to the 41 symptoms used in the previous study. Thus, we may not have captured the full spectrum of illness burden. We do note, however, that when the clusters were separately compared with each other using a general linear model, the differences in illness burden between Cluster 2 and Clusters 1 and 3 were both statistically significant at P = 0.04 (Table 2).
Post hoc analyses highlighted key differences between pain measures. Although BPI average pain severity scores did not differ between groups, clinical pain measures which incorporate pain distribution (e.g., the RADAI joint count and WPI) significantly distinguished Cluster 1 from Clusters 2 and 3, with subjects in Cluster 1 scoring significantly lower than subjects in the other two clusters. However, neither the RADAI joint count nor the WPI significantly differentiated Clusters 2 and 3, even though the RADAI and WPI are designed to focus on joint pain and non-joint pain, respectively. Interestingly, the tender joint count was significantly lower among patients in Cluster 2, compared to those in Cluster 3, suggesting that, despite previously reported flaws in its specificity for assessing arthritis pain (42), the tender joint count may offer greater discriminating capacity compared to either the RADAI or the WPI.
Our results have important theoretical and clinical implications. Specifically, our results indicate that, in a population of established RA patients, many continue to have widespread pain, despite relatively low levels of inflammation. Thus, physicians should carefully assess symptoms, such as fatigue, sleep problems, depression and catastrophizing, when evaluating RA patients, particularly if these patients have high assessments of disease activity, in the face of low objective measures of inflammation. In theory, this group may be more likely to respond to psychological interventions (e.g., cognitive behavioral therapy, pain coping skills training) (43) or medications aimed at treating chronic widespread pain syndromes, such as serotonin norepinephrine reuptake inhibitors and neuroleptic pain medications. Studies are currently underway to examine whether similar medications may be effective for RA patients with stable inflammatory disease activity who continue to have widespread pain and fatigue.
This study does have limitations. Specifically, the generalizability of these analyses is limited by the study population, a cohort of established RA patients (median disease duration 13 years), treated at a single academic medical institution. Eighty-eight percent of this population was treated with a disease-modifying antirheumatic drug (DMARD), including 58.0% who were on biologic DMARDs. The proportion of patients with active inflammatory disease would likely be much higher in a population that was not as intensively treated. Although subjects in Cluster 3 were significantly less likely, than subjects in Clusters 1 and 2, to be on a DMARD at the time of the study, all of the subjects had been treated with DMARDs in the past. Past biologic DMARD use was not significantly different between clusters, with 87% of subjects in Cluster 3 reporting past biologic DMARD use. These results suggest that Cluster 3 may consist of patients with particularly treatment resistant disease.
Other limitations include the use of a hierarchical agglomerative clustering technique, which may be influenced by outliers, and the lack of quantitative information about pain mechanisms. Future studies are needed to replicate these findings and further characterize these patients using quantitative sensory testing techniques. Quantitative sensory testing may help determine whether subjects in Cluster 2 have measureable differences in central pain regulatory mechanisms, such as loss of conditioned pain modulation, which are associated with chronic widespread pain conditions. The identification of different subgroups of RA patients may lead to a better understanding of the mechanisms causing pain and fatigue in RA, which can facilitate the identification of appropriate treatment targets.
In conclusion, a clustering algorithm based on swollen joint count, co-occurring symptoms (e.g., pain, fatigue, sleep problems), psychological distress (catastrophizing and depression) and functional illness burden identified three groups of established RA patients. Although most patients were doing well, 47.3% continued to have moderate to high levels of pain, fatigue and sleep problems. The majority of these patients had low markers of inflammation but high measures of catastrophizing, consistent with a chronic widespread pain syndrome. These results are poignant because they indicate that: 1) chronic widespread pain syndromes are common among patients with established RA; 2) active inflammatory disease may account for only a minority of patients with problems with pain, fatigue and mood disturbance; and 3) chronic widespread pain syndromes are associated with significantly diminished quality of life, even compared to patients with active inflammatory disease.
Table 3.
Sociodemographic and RA-disease related clinical characteristics between clusters. Values are expressed as medians and interquartile ranges or numbers and percentages.a
| Characteristic | Cluster 1 (N = 89) | Cluster 2 (N = 57) | Cluster 3 (N = 23) | P-valueb |
|---|---|---|---|---|
| Sociodemographic | ||||
|
| ||||
| Age (years) | 58.0 (50.0–65.0) | 56.0 (49.0–64.0) | 60.0 (52.0–70.0) | 0.23 |
| Female (N) | 70 (78.7%) | 47 (82.5%) | 19 (82.6%) | 0.82 |
| Caucasian (N)c | 84 (95.5%)† | 51 (91.1%)† | 22 (100%)† | 0.25 |
| Body mass index (kg/m2) | 26.2 (22.9–29.6) | 25.7 (23.3–29.1) | 28.3 (24.8–31.1) | 0.14 |
|
| ||||
| Disease-related | ||||
|
| ||||
| Disease duration (years) | 12.0 (7.0–21.0) | 13.0 (8.0–22.0) | 22.0 f,g (13.0–27.0) | 0.006 |
| Rheumatoid factor > 15 (N)d | 51 (58.0%)‡ | 34 (61.8%)‡ | 19 (82.6%)‡ | 0.09 |
| DAS28-CRP | 2.4 (1.7–3.0) | 2.9 (2.2–3.8) e | 5.1 (4.5–5.9) f,g | <0.0001 |
| C-reactive protein (mg/L) | 1.7 (0.6–2.9) | 1.7 (0.6–4.8) | 2.6 (1.5–22.2) f,g | 0.0003 |
| Tender joint count (0–28) | 1.0 (0.0–3.0) | 2.0 (0.0–6.0) | 14.0 (12.0–16.0) f,g | <0.0001 |
| Patient global assessment (0–100) | 15.0 (5.0–25.0) | 30.0 (15.0–60.0) e | 30.0 (20.0–50.0) f | <0.0001 |
| Physician global assessment (0–100) | 10.0 (10.0–30.0) | 20.0 (10.0–30.0) e | 40.0 (30.0–50.0) f,g | <0.0001 |
| Sharp erosion (0–160) | 1.0 (0.0–10.0) | 1.0 (0.0–4.0) | 12.0 f,g (1.0–31.0) | 0.03 |
| Sharp joint space narrowing (0–120) | 2.0 (0.0–23.0) | 2.0 (0.0–18.5) | 26.0 (2.0–53.0) f,g | 0.002 |
| RADAI joint score (0–10) | 1.0 (0.4–1.9) | 1.7 (0.8–2.9) e | 2.7 (1.0–3.3) f | 0.0007 |
| Widespread pain index (0–19) | 2.0 (1.0–5.0) | 5.0 (2.0–7.0) e | 5.0 (2.0–8.0) f | 0.0004 |
| Fibromyalgianess (0–31) | 7.0 (4.0–9.0) | 10.0 (7.0–14.0) e | 11.0 (6.0–15.0) f | <0.0001 |
|
| ||||
| Medications | ||||
|
| ||||
| Current DMARD use (N) | 85 (95.5%) | 47 (82.5%) e | 17 (73.9%) f | 0.004 |
| Current biologic DMARD use (N) | 54 (60.7%) | 33 (57.9%) | 11 (47.8%) | 0.54 |
| Current synthetic DMARD use (N) | 64 (71.9%) | 32 (56.1%) e | 12 (52.2%) f | 0.07 |
| Current corticosteroid use (N) | 14 (15.7%) | 8 (14.0%) | 5 (21.7%) | 0.69 |
| Past DMARD use (N) | 89 (100%) | 57 (100%) | 23 (100%) | - |
| Past biologic DMARD use (N) | 61 (68.5%) | 44 (77.2%) | 20 (87.0%) | 0.16 |
| Past synthetic DMARD use (N) | 89 (100%) | 57 (100%) | 23 (100%) | - |
RA = rheumatoid arthritis, DAS28-CRP = Disease Activity Score in 28 Joints – C-Reactive Protein, RADAI = Rheumatoid Arthritis Disease Activity Index, DMARD = disease-modifying antirheumatic drug
P-value that any one cluster is different from the others.
Due to missing data, percentages of Caucasian participants are derived from denominators of 88, 56 and 22 for clusters 1, 2 and 3, respectively.
Due to missing data, percentages of rheumatoid factor positive participants are derived from denominators of 88, 55 and 23 for clusters 1, 2 and 3, respectively.
Cluster 2 is significantly different from cluster 1 at P ≤ 0.05.
Cluster 3 is significantly different from cluster 1 at P ≤ 0.05.
Clusters 3 is significantly different from cluster 2 at P ≤ 0.05.
Acknowledgments
Grant Support: Dr. Lee’s work was supported by the NIH (K23 AR057578) and the Katherine Swan Ginsburg Fund.
Footnotes
Disclosures: The Brigham Rheumatoid Arthritis Sequential Study (BRASS) receives financial support from MedImmune, Crescendo Biosciences and Bristol Myers Squibb. Dr. Yvonne Lee receives research support from Forest. She also has stock in Merck, Novartis, Cubist and Elan Corporation. Dr. Shadick receives research grant support from MedImmune, Crescendo Biosciences, Amgen, ABBVIE, and Genentech. Dr. Weinblatt receives grant support and is a consultant to MedImmune and Crescendo Biosciences and Bristol Myers Squibb. Dr. Williams is a consultant for Pfizer, Lilly, Forest and Bristol Myers Squibb.
References
- 1.Myasoedova E, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. Is the incidence of rheumatoid arthritis rising?: results from Olmsted County, Minnesota, 1955–2007. Arthritis Rheum. 2010;62(6):1576–82. doi: 10.1002/art.27425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanderson T, Morris M, Calnan M, Richards P, Hewlett S. Patient perspective of measuring treatment efficacy: the rheumatoid arthritis patient priorities for pharmacologic interventions outcomes. Arthritis care & research. 2010;62(5):647–56. doi: 10.1002/acr.20151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pollard LC, Choy EH, Gonzalez J, Khoshaba B, Scott DL. Fatigue in rheumatoid arthritis reflects pain, not disease activity. Rheumatology (Oxford) 2006;45(7):885–9. doi: 10.1093/rheumatology/kel021. [DOI] [PubMed] [Google Scholar]
- 4.Stebbings S, Herbison P, Doyle TC, Treharne GJ, Highton J. A comparison of fatigue correlates in rheumatoid arthritis and osteoarthritis: disparity in associations with disability, anxiety and sleep disturbance. Rheumatology (Oxford) 2010;49(2):361–7. doi: 10.1093/rheumatology/kep367. [DOI] [PubMed] [Google Scholar]
- 5.Lee YC, Cui J, Lu B, Frits ML, Iannaccone CK, Shadick NA, et al. Pain persists in DAS28 rheumatoid arthritis remission but not in ACR/EULAR remission: a longitudinal observational study. Arthritis Res Ther. 2011;13(3):R83. doi: 10.1186/ar3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergman MJ, Shahouri SS, Shaver TS, Anderson JD, Weidensaul DN, Busch RE, et al. Is fatigue an inflammatory variable in rheumatoid arthritis (RA)? Analyses of fatigue in RA, osteoarthritis, and fibromyalgia. J Rheumatol. 2009;36(12):2788–94. doi: 10.3899/jrheum.090561. [DOI] [PubMed] [Google Scholar]
- 7.van Hoogmoed D, Fransen J, Bleijenberg G, van Riel P. Physical and psychosocial correlates of severe fatigue in rheumatoid arthritis. Rheumatology (Oxford) 2010 doi: 10.1093/rheumatology/keq043. [DOI] [PubMed] [Google Scholar]
- 8.Ulus Y, Akyol Y, Tander B, Durmus D, Bilgici A, Kuru O. Sleep quality in fibromyalgia and rheumatoid arthritis: associations with pain, fatigue, depression, and disease activity. Clin Exp Rheumatol. 2011;29(6 Suppl 69):S92–6. [PubMed] [Google Scholar]
- 9.Roehrs T, Diederichs C, Gillis M, Burger AJ, Stout RA, Lumley MA, et al. Nocturnal sleep, daytime sleepiness and fatigue in fibromyalgia patients compared to rheumatoid arthritis patients and healthy controls: A preliminary study. Sleep medicine. 2012 doi: 10.1016/j.sleep.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 10.Bannwarth B, Blotman F, Roue-Le Lay K, Caubere JP, Andre E, Taieb C. Fibromyalgia syndrome in the general population of France: a prevalence study. Joint Bone Spine. 2009;76(2):184–7. doi: 10.1016/j.jbspin.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Branco JC, Bannwarth B, Failde I, Abello Carbonell J, Blotman F, Spaeth M, et al. Prevalence of fibromyalgia: a survey in five European countries. Semin Arthritis Rheum. 2010;39(6):448–53. doi: 10.1016/j.semarthrit.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Wolfe F, Hauser W, Hassett AL, Katz RS, Walitt BT. The development of fibromyalgia - I: examination of rates and predictors in patients with rheumatoid arthritis (RA) Pain. 2011;152(2):291–9. doi: 10.1016/j.pain.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 13.Dhir V, Lawrence A, Aggarwal A, Misra R. Fibromyalgia is common and adversely affects pain and fatigue perception in North Indian patients with rheumatoid arthritis. J Rheumatol. 2009;36(11):2443–8. doi: 10.3899/jrheum.090157. [DOI] [PubMed] [Google Scholar]
- 14.Newby PK, Tucker KL. Empirically derived eating patterns using factor or cluster analysis: a review. Nutr Rev. 2004;62(5):177–203. doi: 10.1301/nr.2004.may.177-203. [DOI] [PubMed] [Google Scholar]
- 15.Aktas A, Walsh D, Rybicki L. Symptom clusters: myth or reality? Palliat Med. 2010;24(4):373–85. doi: 10.1177/0269216310367842. [DOI] [PubMed] [Google Scholar]
- 16.Murphy SL, Lyden AK, Phillips K, Clauw DJ, Williams DA. Subgroups of older adults with osteoarthritis based upon differing comorbid symptom presentations and potential underlying pain mechanisms. Arthritis Res Ther. 2011;13(4):R135. doi: 10.1186/ar3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Illi J, Miaskowski C, Cooper B, Levine JD, Dunn L, West C, et al. Association between pro- and anti-inflammatory cytokine genes and a symptom cluster of pain, fatigue, sleep disturbance, and depression. Cytokine. 2012;58(3):437–47. doi: 10.1016/j.cyto.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee YC, Chibnik LB, Lu B, Wasan AD, Edwards RR, Fossel AH, et al. The relationship between disease activity, sleep, psychiatric distress and pain sensitivity in rheumatoid arthritis: a cross-sectional study. Arthritis Res Ther. 2009;11(5):R160. doi: 10.1186/ar2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iannaccone CK, Lee YC, Cui J, Frits ML, Glass RJ, Plenge RM, et al. Using genetic and clinical data to understand response to disease-modifying anti-rheumatic drug therapy: data from the Brigham and Women’s Hospital Rheumatoid Arthritis Sequential Study. Rheumatology (Oxford) 2011;50(1):40–6. doi: 10.1093/rheumatology/keq263. [DOI] [PubMed] [Google Scholar]
- 20.van Riel PL, Fransen J. DAS28: a useful instrument to monitor infliximab treatment in patients with rheumatoid arthritis. Arthritis Res Ther. 2005;7(5):189–90. doi: 10.1186/ar1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–38. [PubMed] [Google Scholar]
- 22.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis care & research. 2010;62(5):600–10. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- 23.Stucki G, Liang MH, Stucki S, Bruhlmann P, Michel BA. A self-administered rheumatoid arthritis disease activity index (RADAI) for epidemiologic research. Psychometric properties and correlation with parameters of disease activity. Arthritis Rheum. 1995;38(6):795–8. doi: 10.1002/art.1780380612. [DOI] [PubMed] [Google Scholar]
- 24.Houssien DA, Stucki G, Scott DL. A patient-derived disease activity score can substitute for a physician-derived disease activity score in clinical research. Rheumatology (Oxford) 1999;38(1):48–52. doi: 10.1093/rheumatology/38.1.48. [DOI] [PubMed] [Google Scholar]
- 25.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52(2):69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 26.Pincus T, Swearingen C, Wolfe F. Toward a multidimensional Health Assessment Questionnaire (MDHAQ): assessment of advanced activities of daily living and psychological status in the patient-friendly health assessment questionnaire format. Arthritis Rheum. 1999;42(10):2220–30. doi: 10.1002/1529-0131(199910)42:10<2220::AID-ANR26>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 27.Hays RD, Stewart AL. Sleep Measures. In: Ware ALSaJE., editor. Measuring functioning and well-being: The Medical Outcomes Study approach. Durham, NC: Durham University Press; 1992. pp. 235–59. [Google Scholar]
- 28.Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess. 1995;7:524–32. [Google Scholar]
- 29.Wolfe F. Fibromyalgianess. Arthritis Rheum. 2009;61(6):715–6. doi: 10.1002/art.24553. [DOI] [PubMed] [Google Scholar]
- 30.Formann AK. Die Latent-Class-Analyse: Einfuhrung in die Theorie und Anwendung. Weinham, Germany: Beltz; 1984. [Google Scholar]
- 31.Dolnicar S. A Review of Unquestioned Standards in Using Cluster Analysis for Data-Driven Market Segmentation. Australian and New Zealand Marketing Academy Conference 2002; December 2–4, 2002; Melbourne: Deakin University; 2002. [Google Scholar]
- 32.Mooi E, Sarstedt A. A Concise Guide to Market Research. Berlin Heidelberg: Springer-Verlag; 2011. Cluster Analysis; pp. 237–84. [Google Scholar]
- 33.Beneciuk JM, Robinson ME, George SZ. Low back pain subgroups using fear-avoidance model measures: results of a cluster analysis. Clin J Pain. 2012;28(8):658–66. doi: 10.1097/AJP.0b013e31824306ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inc. SI. SAS Technical Report A-108, Cubic Clustering Criterion. Cary, NC: Sas Insitute INc; 1983. [Google Scholar]
- 35.Box GEP, Anderson SL. Permutation theory in the derivation of robust criteria and the study of departures from assumptions. Journal of the Royal Statistical Society. 1955;17:1–34. [Google Scholar]
- 36.Lindman HR. Analysis of variance in complex experimental designs. San Francisco: W.H. Freeman and Company; 1974. [Google Scholar]
- 37.Waters SJ, Riordan PA, Keefe FJ, Lefebvre JC. Pain behavior in rheumatoid arthritis patients: identification of pain behavior subgroups. Journal of pain and symptom management. 2008;36(1):69–78. doi: 10.1016/j.jpainsymman.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morris A, Yelin EH, Wong B, Katz PP. Patterns of psychosocial risk and long-term outcomes in rheumatoid arthritis. Psychology, health & medicine. 2008;13(5):529–44. doi: 10.1080/13548500801927113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hart-Johnson TA, Green CR. Physical and psychosocial health in older women with chronic pain: comparing clusters of clinical and nonclinical samples. Pain medicine. 2010;11(4):564–74. doi: 10.1111/j.1526-4637.2010.00803.x. [DOI] [PubMed] [Google Scholar]
- 40.Kang JH, Chen HS, Chen SC, Jaw FS. Disability in patients with chronic neck pain: heart rate variability analysis and cluster analysis. The Clinical journal of pain. 2012;28(9):797–803. doi: 10.1097/AJP.0b013e3182442afd. [DOI] [PubMed] [Google Scholar]
- 41.Walker LS, Sherman AL, Bruehl S, Garber J, Smith CA. Functional abdominal pain patient subtypes in childhood predict functional gastrointestinal disorders with chronic pain and psychiatric comorbidities in adolescence and adulthood. Pain. 2012;153(9):1798–806. doi: 10.1016/j.pain.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ton E, Bakker MF, Verstappen SM, Ter Borg EJ, van Albada-Kuipers IA, Schenk Y, et al. Look Beyond the Disease Activity Score of 28 Joints (DAS28): Tender Points Influence the DAS28 in Patients with Rheumatoid Arthritis. J Rheumatol. 2012;39(1):22–7. doi: 10.3899/jrheum.110072. [DOI] [PubMed] [Google Scholar]
- 43.Hoffman BM, Papas RK, Chatkoff DK, Kerns RD. Meta-analysis of psychological interventions for chronic low back pain. Health Psychol. 2007;26(1):1–9. doi: 10.1037/0278-6133.26.1.1. [DOI] [PubMed] [Google Scholar]
- 44.Lee YC, Lu B, Edwards RR, Wasan AD, Nassikas NJ, Clauw DJ, et al. The role of sleep problems in central pain processing in rheumatoid arthritis. Arthritis and rheumatism. 2013;65(1):59–68. doi: 10.1002/art.37733. [DOI] [PMC free article] [PubMed] [Google Scholar]