Abstract
Objective
To determine if asthma control improves in patients who receive physician-pharmacist collaborative management (PPCM) during visits to primary care medical offices.
Design
Prospective pre-post study of patients who received the intervention in primary care offices for 9 months. The primary outcome was the sum of asthma-related emergency department (ED) visits and hospitalizations at 9 months before, 9 months during, and 9 months following the intervention. Events were analyzed using linear mixed effects regression. Secondary analysis was conducted for patients with uncontrolled asthma (Asthma Control Test [ACT]<20). Additional secondary outcomes included the ACT, the Asthma Quality of Life Questionnaire by Marks (AQLQ-M) scores, and medication changes.
Intervention
Pharmacists provided patients with an asthma self-management plan and education and made pharmacotherapy recommendations to physicians when appropriate.
Results
Of 126 patients, the number of emergency department (ED) visits and/or hospitalizations decreased 30% during the intervention (p=0.052) and then returned to pre-enrollment levels after the intervention was discontinued (p=0.83). Secondary analysis of patients with uncontrolled asthma at baseline (ACT<20), showed 37 ED visits and hospitalizations prior to the intervention, 21 during the intervention, and 33 after the intervention was discontinued (p=0.019). ACT and AQLQ-M scores improved during the intervention (ACT mean absolute increase of 2.11, AQLQ-M mean absolute decrease of 4.86, p<0.0001 respectively) and sustained a stable effect after discontinuation of the intervention. Inhaled corticosteroid use increased during the intervention (p=0.024).
Conclusions
The PPCM care model reduced asthma-related ED visits and hospitalizations and improved asthma control and quality of life. However, the primary outcome was not statistically significant for all patients. There was a significant reduction in ED visits and hospitalizations during the intervention for patients with uncontrolled asthma at baseline. Our findings support the need for further studies to investigate asthma outcomes achievable with the PPCM model.
Keywords: Asthma, Pharmacist, Collaboration, Team-based care
Introduction
Over 25 million Americans have asthma, and the number of diagnoses increased nearly 15% in the last decade.1 While deaths related to asthma have declined in recent years, it remains a significant cause of morbidity with 479,300 hospitalizations and almost 2 million emergency department (ED) visits in 2009.2 African Americans are at the highest risk for developing asthma and are two to three times more likely to die from asthma than any other race or ethnic group.2 Asthma exacerbations can be prevented by proper pharmacotherapy and guideline adherence3, yet missing asthma action plans, suboptimal use of prescribed medications, poor adherence, and insufficient patient education contribute to significant gaps in care.4–6
Pharmacists can assist with asthma management.7 Most studies that evaluated a pharmacist intervention for asthma were conducted in community pharmacies.4,6,7–21 In a large randomized controlled trial evaluating pharmacist-assisted care for patients with asthma or COPD in chain community pharmacies in Indiana, peak expiratory flow rates (PEFRs) were greater in the intervention arm compared to usual care after one year.22 However, there was no benefit when compared to patients who simply received a peak flow meter and instructions on use. Additionally, there were more ED and hospital visits when compared to usual care.22
Physician-pharmacist collaborative management (PPCM) is a process in which pharmacists work directly with primary care physicians in the medical office to optimize therapy. PPCM has been especially beneficial for specific cardiovascular risks and is a key component of the patient-centered medical home.23–25 However, few studies have examined PPCM for asthma in a primary care medical office, and results have been mixed.26,27 In one study of physician-pharmacist collaboration for asthma, significant reductions in ED visits were found compared to two similar time periods prior to study initiation.27 The National Asthma Education and Prevention Program’s (NAEPP) most recent Expert Panel Report 3 (EPR-3) specifically mentioned pharmacists and recommended that they should “be considered; [as] such programs are feasible, and they require further studies of effectiveness.3,7” In addition, it is not known if the effects of a pharmacist intervention can be sustained after it is discontinued.
The true benefit derived from PPCM for asthma is unknown, as many gaps in research remain. The purpose of this study was to determine if patients who received PPCM within primary care medical offices achieved improved asthma control.
Methods
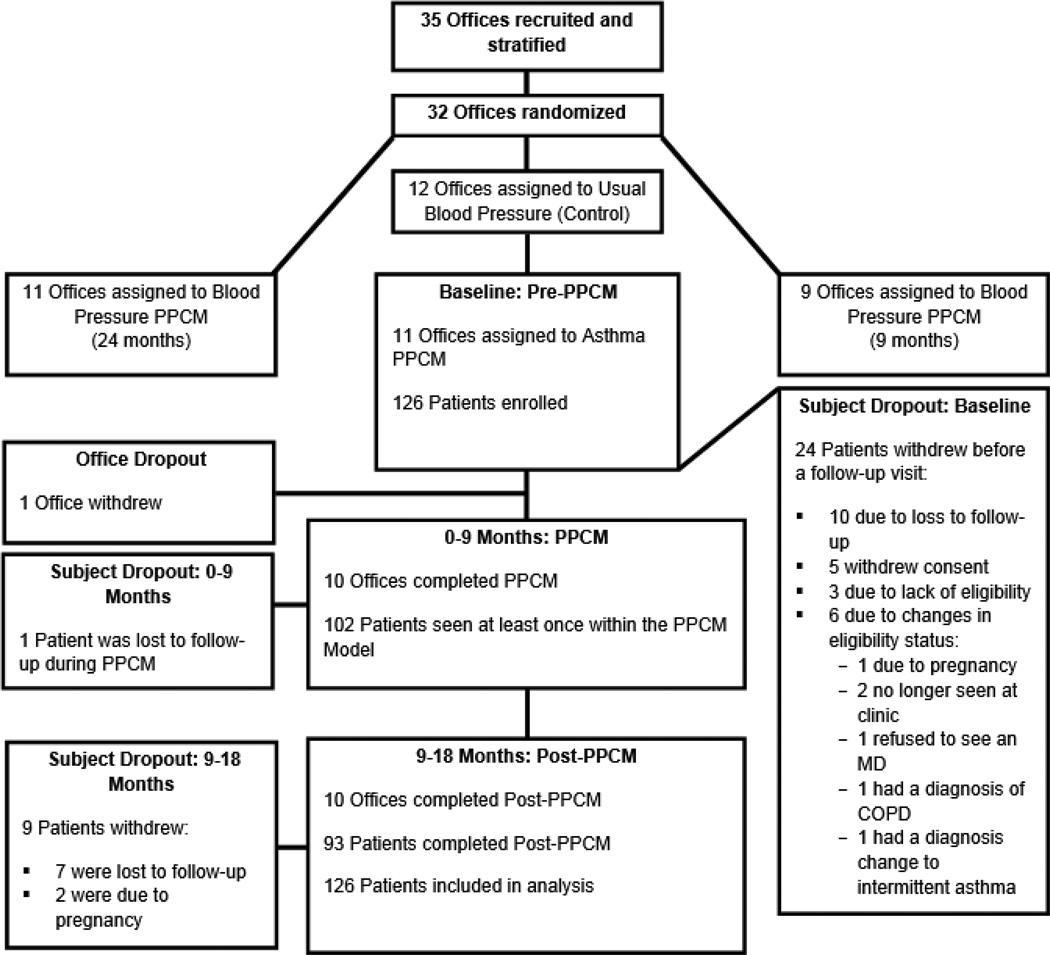
This study was conducted in one arm of a three-arm randomized control trial, the “Collaboration Among Pharmacist and Physicians to Improve Blood Pressure Now” (CAPTION). CAPTION was an effectiveness trial with specific aims to examine blood pressure (BP) control following implementation of PPCM. The background study design and baseline data for hypertensive patients have previously been reported.8,28 CAPTION was an effectiveness trial to determine if the intervention would be implemented in a large number of diverse offices. CAPTION was a 5-year, prospective, cluster-randomized multi-center clinical trial in 32 medical offices from 13 states. All offices employed pharmacists with a Doctor of Pharmacy degree, 95% of whom had also completed post-doctoral residencies or fellowships.29 Offices were stratified based on the structure of pharmacy services and percent minority patients.8,30 Clinics were then randomized to one of three groups: usual BP care, a 9-month BP intervention, or a 24-month BP intervention (Figure 1). Clinics randomized to the usual BP care arm also enrolled an unrelated second group of patients with a diagnosis of asthma and implemented a distracter PPCM asthma intervention. The PPCM asthma intervention is the subject of the present study.
Figure 1.
Design of the CAPTION trial: Asthma Arm
The primary outcome was the sum of asthma-related ED visits and hospitalizations in three time periods: 9 months before (Pre-PPCM), 9 months during (PPCM), and 9 months following the intervention (Post-PPCM). Secondary outcomes included the sum of asthma-related ED visits and hospitalizations in patients with uncontrolled asthma at baseline, Asthma Control Test (ACT) scores31, Asthma Quality of Life Questionnaire by Marks (AQLQ-M) scores32, and asthma medication changes.
The ACT is a 5-question test that identifies asthma exacerbation and asthma-related symptom frequency in the past month. Patients answer in a multiple choice fashion, with five selections available for each question, leaving a total score of 0 to 25. A high score (≥20) indicates “well-controlled” asthma.31 The AQLQ-M is a 20-item questionnaire that measures the impact of asthma on the lives of patients in the past month. AQLQ-M measures physical, emotional, and social impact and health concerns related to asthma.32 Scores range from 0 to 60, and higher scores indicate a lower quality of life due to asthma. Both instruments have been validated.31,32
Study Coordinator and Pharmacist Training
Two investigators (GM & BLC) designed the asthma training program for site personnel at all CAPTION asthma sites. One trainer (GM) was a faculty member at the University of Iowa Hospitals and Clinics Pediatric Allergy and Pulmonary Clinic. He had extensive experience in implementing national asthma guidelines and strategies to promote proper medication adherence, especially in pediatric patients. One-day asthma training sessions were conducted at 4 regional locations (California, Texas, Florida, and Wisconsin). An educational presentation based on the 2007 NAEPP EPR-33 was provided which focused on making the appropriate diagnosis, choosing drug therapy, therapy implementation, counseling, and monitoring of outcomes. The program focused on distinguishing intermittent from persistent asthma, described the various therapeutic options based on the pattern of disease, and illustrated the stepwise approach to care. Training included methods of intensifying therapy, step-down therapy, or stopping therapy if indicated and were completed by lead physicians, nurses, and pharmacists from each asthma intervention site. Following the training sessions, attendees provided the same educational sessions for other personnel in their offices in a “train-the-trainer” model.
Patient Recruitment
The study was approved by the Institutional Review Board (IRB) for each office. One nurse or medical assistant employed in each office was trained to specifically recruit patients and collect data in their offices (hereafter referred to as research nurses). A research nurse at each of the 11 medical offices generated electronic lists of patients seen in the previous 24 months with a diagnosis of asthma (ICD9 code 493.x). The goal was to recruit 10–15 patients per office. Each patient list was de-identified and then randomized by the CAPTION biostatistician (CC). To prevent selection bias, the biostatistician sent the randomized patient list to research nurses to begin enrollment. Research Nurses screened patient’s medical records, in order from the randomized list, for available inclusion and exclusion criteria and mailed invitations to patients who appeared to qualify. The invitation letter described the study and requested that patients return an enclosed response card by a specified date. The letter also informed the patient that they would be called if the response card was not returned by the specified date and included staff contact information should they wish to call. In order to ensure adequate recruitment, the research nurse continued this process until 10–15 patients per office had been enrolled.
Inclusion & Exclusion Criteria
Eligible patients were males or females 12 years of age or older with a diagnosis of persistent asthma who were being managed by primary care physicians. Patients who had a history of severe, life-threatening asthma evidenced by a history of loss of consciousness, intensive care unit admissions or mechanical ventilation due to asthma were excluded. Patients with severe asthma were excluded for safety reasons and also because they would be more likely to be managed by pulmonologists and not strictly by primary care providers. Patients with a diagnosis of chronic obstructive pulmonary disease (COPD), previous involvement in a pulmonologist or multidisciplinary asthma management clinic, self-reported pregnancy, poor prognosis with a life expectancy estimated less than 2 years, residence in a nursing home, or a diagnosis of dementia were also excluded from the study.
Asthma PPCM Model
The on-site pharmacist conducted a baseline interview upon receipt of the signed, written informed consent form. The pharmacist and physician decided upon short-term and long-term treatment goals. Drug therapy goals included proper maintenance and rescue asthma medications, correct administration, high adherence, and strategies to reduce adverse event rates. Pharmacists assessed asthma severity and control (via ACT scores), provided asthma education (e.g. importance of “controller” medication adherence) and training in asthma management skills such as self-monitoring education (e.g. symptoms and peak flow measurement). Pharmacists wrote and supplied patients with an asthma action plan based on the NAEPP EPR-33 guidelines for daily treatment and self-management of exacerbations or symptomatic episodes. The pharmacist observed the patient’s inhaler technique and provided instructions on proper technique if necessary. Next, the pharmacist assessed the medication regimen and made recommendations to physicians regarding potential improvement of the current regimen in accordance with the NAEPP EPR 3.3 This often included initiation and/or titration of inhaled corticosteroids, modifications to how β-agonists were used (e.g., pre-exercise for exercise induced asthma, rate and frequency of use, etc.), and prescribing a spacer. The PPCM model did not require the use of a collaborative drug therapy management (CDTM) protocol.
The 9-month asthma PPCM intervention suggested a visit frequency and activities including structured face-to-face visits with the pharmacist at baseline and 1, 2, 4, 6, and 9 months. Optional visits at 3, 5, 7, and 8 months and a telephone call at 2 weeks were available for patients with continued poor asthma control. This visit frequency was designed to parallel a similar frequency used in the hypertension intervention in the other arms of the CAPTION trial. Because this was an effectiveness study, pharmacists were asked to use discretion for patient management and were free to modify or reduce this frequency, especially if they felt patients were well-controlled. We expected to see diversity in the visit frequency between offices. The pharmacist assessed medication delivery technique, AQLQ-M scores, and ACT scores at each visit. Medication therapy was reviewed, with special attention given to frequency of β-agonist use and need for asthma maintenance medications (i.e. inhaled corticosteroids). The pharmacist then reviewed goals, adjusted the asthma action plan as needed, and presented recommendations to the physician. Physicians were free to accept or reject any recommendation.
Data Collection
Research nurses collected the following data by patient-report at baseline, 9-month, and 18-month visits (with the option of conducting the 18-month visit by telephone): demographics, list of asthma medications, number of clinic visits, number of hospitalizations and/or ED visits for asthma in the past 9 months, number of non-ED visits for asthma in past 9 months, number of courses of oral corticosteroids in past 9 months, and days of missed work or school in past 9 months. For patients with chronic asthma medications, adherence was assessed using self-report via a validated 6 question instrument.33 Patients were considered “nonadherent” if they endorsed ≥ 2 of the 6 items, as previously validated. Research nurses administered the ACT and the AQLQ-M at the same time points. All research data elements were confirmed by medical record review (e.g., documentation of any hospitalizations, ED visits, medication changes, adverse events, etc.). Because all CAPTION offices admitted patients to their own local hospital and had access to these records, all data were complete unless a patient was seen at another hospital that failed to provide discharge notes. Formative evaluations with the study personnel indicated that such events were rare.
Analysis
The primary outcome was the combined number of asthma-related ED visits and hospitalizations. Because the main CAPTION trial was powered based on hypertension endpoints, a formal sample size and power calculation were not conducted. All analyses were performed on the intention-to-treat data set, with the last observation carried forward (LOCF). In order to assess the sensitivity of our conclusions on the chosen approach for handing missing data, we also conducted a sensitivity analysis using only those patients with observed data. The results and overall conclusions from both approaches were identical, thus only the primary intention-to-treat results are reported. These events were analyzed using a linear mixed effects regression model to compare total number of visits across the three time periods: 9-months before (Pre-PPCM), 9-months during (PPCM), and 9-months after the intervention (Post-PPCM). We used Restricted Maximum Likelihood (REML) methods to account for the repeated measures across the three time periods.
Secondary analyses were performed on patients with uncontrolled asthma at baseline (ACT<20) and on asthma medication regimens for all three time periods. Secondary outcomes also included ACT scores and AQLQ-M results. Categorical outcomes, including dichotomous ACT and medication adherence results, were compared across the three time periods using a generalized linear mixed effect model with a logit link function and continuous outcomes were compared using a linear mixed effect model.
Results
Research nurses enrolled 126 patients from the 11 medical offices in the asthma arm of CAPTION. The recruitment and enrollment period spanned from March 2010 to October 2011. There were 33 patients (26%) who were prematurely terminated because of loss of follow-up (n=17), withdrawal of consent (n=5), ineligibility (n=3), and changes in eligibility status (n=8) (Figure 1). One study site was discontinued early due to poor patient enrollments. Demographic characteristics are displayed in Table 1. Age ranged from 12 to 83 years (mean 39.7 years), and African Americans (n=50, 39.7%) and Hispanics (n=18, 14.3%) were well represented. A large number of patients had household income below $25,000 per year (n=59, 46.8%) or had either Medicaid or self-pay as their pay source (n=59, 46.8%). Patients saw the pharmacist an average of 2.52 times over the course of the 9-month intervention, with visit frequency ranging from 0 to 7 visits per patient. The most common encounter frequency was 2 (n=40; 31.8%) and ≥ 4 encounters (n=33 patients; 26.2%). Patients with uncontrolled asthma at baseline met with pharmacists an average of 2.81 times, versus controlled patients averaging 1.84 encounters. Baseline visits lasted 15–90 minutes, with an average of approximately 60 minutes. Follow-up visits required approximately 15 minutes based on formative evaluations with the pharmacists. At baseline, 100 patients were taking chronic asthma medications. Data were available for 76 patients at 9 months and 66 patients at 18 months. When using the LOCF for missing data, there was a significant increase in the number of patients with high adherence when comparing baseline (64%) to 9 months (75%, p=0.007) but not at 18 months (73%,p=0.067). When considering only those patients with actual observed data at each visit, 64% had high adherence at baseline, 80.3% had high adherence at 9 months (n=76, p=0.004) and 81.8% at 18 months (n=66, p=0.03).
Table 1.
Demographic Characteristics
| Variable | Asthma group (N=126) N (%) |
|---|---|
| Gender | |
| Male | 32 (25.4) |
| Female | 94 (74.6) |
| Race/Ethnicity | |
| Non-Hispanic Caucasian | 53 (42.1) |
| Minority | 70 (55.6) |
| Declined to answer/Missing | 3 (2.4) |
| Education | |
| <= 12 Years | 76 (60.3) |
| > 12 Years | 50 (39.7) |
| Marital Status | |
| Married | 35 (27.8) |
| Not married | 91 (72.2) |
| Insurance Status | |
| Medicare/Medicaid | 66 (52.4) |
| Other | 60 (47.6) |
| Annual Income | |
| < $25,000 | 59 (46.8) |
| >= $25,000 | 66 (52.4) |
| Missing | 1 (0.8) |
| Smoking Status | |
| Current smoker | 25 (19.8) |
| Former smoker | 30 (23.8) |
| Never smoker | 70 (55.6) |
| Missing | 1 (0.8) |
| Alcohol Intake | |
| No alcohol intake | 81 (64.3) |
| Any alcohol intake | 43 (34.1) |
| Missing | 2 (1.6) |
| Duration of Asthma | |
| <= 3 years | 13 (10.3) |
| > 3 – 10 years | 25 (19.8) |
| > 10 years | 88 (69.8) |
| Missing | 0 (0.0) |
| Asthma Diagnosis | |
| Persistent | 42 (33.3) |
| Mild | 50 (39.7) |
| Moderate/severe | 34 (27.0) |
| Age | |
| Mean (SD) | 39.7 (17.8) |
| Min.-Max. | (12, 83) |
Primary Outcome
The number of ED visits and/or hospitalizations decreased 30% during the intervention (p=0.052), but returned to pre-enrollment levels after the intervention was discontinued (p=0.83) (Table 2).
Table 2.
Summary of Asthma Emergency Department Visits, Hospitalizations, ACT and Quality of Life Scores for All Patients
| Pre-PPCM: Baseline (N=126) |
PPCM Intervention: 0–9 months (N=126) |
Post-PPCM Intervention: 9–18 months (N=126) |
||||
|---|---|---|---|---|---|---|
| Patients N (%) |
Events N |
Patients N (%) |
Events N |
Patients N (%) |
Events N |
|
| ED Visits & Hospitalizations1 | 21 (16.7%) | 47 | 16 (12.7%) | 33 | 23 (18.3%) | 45 |
| ED Visits | 21 (16.7%) | 43 | 16 (12.7%) | 30 | 22 (17.5%) | 39 |
| Hospitalizations | 3 (2.4%) | 4 | 2 (1.6%) | 3 | 5 (4%) | 6 |
| Mean Number of ED visits and/or Hospitalizations (SD) | 0.37 (1.02) | 0.26 (0.81)3 | 0.36 (0.91)4 | |||
| ACT Mean Scores (SD)1 | 16.76 (4.54) | 18.87 (4.29)5 | 19.02 (4.34)5,6 | |||
| Asthma Control Test2 (Categorical) | ||||||
| Uncontrolled (ACT < 20) | 88 (69.8%) | 62 (49.2%)5 | 58 (46%)5,7 | |||
| Controlled (ACT ≥ 20) | 38 (30.2%) | 64 (50.8%)5 | 68 (54%)5 | |||
| AQLQ-M Mean Scores (SD)1 | 20.65 (13.83)1 | 15.79 (12.39)5 | 15.43 (13.55)5,8 | |||
Linear mixed effects models were used to determine the p-values. Models incorporate centers as random effects and assume that random error terms are nested within subject.
A generalized mixed effect model with a logit link function were used to determine the p-values.
p=0.052 comparison to baseline
p=0.833 comparison to baseline
p<0.0001 comparison to baseline
p = 0.6766 comparison to 0–9 months
p = 0.4556 comparison to 0–9 months
p = 0.6592 comparison to 0–9 months
Secondary Outcomes
Patients with Uncontrolled Asthma at Baseline (ACT<20)
Almost one-third (n=38) of patients had controlled asthma at baseline (ACT ≥20), therefore an additional intention-to-treat analysis was performed to examine only those patients who had uncontrolled asthma at baseline (ACT<20). The number ED visits and/or hospitalizations decreased 43.2% during the intervention (p=0.016) and then returned to near pre-enrollment levels after the intervention was discontinued (p=0.64) (Table 3).
Table 3.
Asthma Emergency Department Visits, Hospitalizations, ACT and Quality of Life Scores for Patients Uncontrolled at Baseline (ACT < 20)1
| Pre-PPCM: Baseline (N=88) |
PPCM Intervention: 0–9 months (N=88) |
Post-PPCM Intervention: 9–18 months (N=88) |
||||
|---|---|---|---|---|---|---|
| Patients N (%) |
Events N |
Patients N (%) |
Events N |
Patients N (%) |
Events N |
|
| ED Visits & Hospitalizations | 18 (20.5%) | 37 | 12 (13.6%) | 21 | 17 (19.3%) | 33 |
| ED Visits | 18 (20.5%) | 36 | 12 (13.6%) | 20 | 17 (19.3%) | 30 |
| Hospitalizations | 1 (1.1%) | 1 | 1 (1.1%) | 1 | 3 (3.4%) | 3 |
| Mean Number of ED visits and/or Hospitalizations (SD) | 0.42 (1.05) | 0.24 (0.73)2 | 0.38 (0.90)3,4 | |||
| ACT Mean Scores (SD) | 14.48 (3.28) | 17.65 (4.30)5 | 18.11 (4.55)5,6 | |||
| AQLQ-M Mean Scores (SD) | 25.49 (13.45) | 19.10 (12.94)5 | 18.08 (14.67)5,7 | |||
Linear mixed effects models were used to determine the p-values. Models incorporate centers as random effects and assume that random error terms are nested within subject.
p=0.016 comparison to baseline
p=0.64 comparison to baseline
p=0.0698 comparison to 0–9 months
p<0.0001 comparison to baseline
p=0.2768 comparison to 0–9 months
p=0.3454 comparison to 0–9 months
Asthma Control Test (ACT)
There was a significant increase (improvement) in mean ACT scores after implementing the PPCM intervention (p<0.0001) (Table 2). When compared to the 9 months prior to the intervention, ACT scores showed significant improvement during both the 9-month period of the intervention (0–9 months mean absolute increase of 2.11 [95% CI: 1.44, 2.78] p<0.001 compared to baseline) and the 9-month period following discontinuation of the intervention (9–18 months mean absolute increase of 2.26 [95% CI: 1.42, 3.09] p<0.001 compared to baseline). No significant difference was found between the intervention period compared to the post-intervention period (p=0.67) suggesting there was a sustained effect of the intervention on asthma control.
The percentage of patients with controlled asthma, as measured by the ACT, also showed a significant increase over time (p<0.0001) (Table 2). Asthma control rates increased from 30.2% at baseline to 50.8% at 9 months (p<0.0001), and 54% at 18 months (p<0.0001).
Asthma Quality of Life (AQLQ-M)
There was a significant improvement in the mean asthma quality of life score after implementing the intervention (p<0.001) (Table 2). Compared to the 9 months prior to initiation of the intervention, AQLQ-M scores significantly improved during both the 9-month period of the intervention (0–9 months mean absolute decrease [ie, improvement] of 4.86 [95% CI: 3.23, 6.48, p<0.0001 compared to baseline]) and the 9-month period following the intervention (9–18 months mean absolute decrease of 5.22 [95% CI: 3.11, 7.33, p<0.0001 compared to baseline]).
Asthma Medication Regimens
Asthma medication regimens were evaluated during all three time periods. Regimens that included inhaled corticosteroids prescriptions increased from 90 to 105 during the intervention (p=0.0239) and were sustained after the intervention (n=103). Use of leukotriene modifiers and short-acting beta agonists remained fairly constant (Table 4).
Table 4.
Asthma Medications at each Time Period1
| Medication Class | Pre-PPCM Intervention: Baseline (N=126) |
PPCM Intervention: 0–9 months (N=126) |
Post-PPCM Intervention: 9– 18 months (N=126) |
|---|---|---|---|
| Patients N (%) |
Patients N (%) |
Patients N (%) |
|
| Inhaled Corticosteroids | 90 (71.43%) | 105 (83.33%)2 | 103 (81.75%) |
| Long Acting Beta 2 Bronchodilators | 0 (0.00%) | 0 (0.00%) | 1 (0.79%) |
| Leukotriene Modifiers | 34 (26.98%) | 39 (30.95%)3 | 33 (26.19%) |
| Short Acting Beta Agonists | 117 (92.86%) | 116 (92.06%) | 120 (95.24%) |
| Short-Acting Anticholinergics | 4 (3.17%) | 2 (1.59%) | 3 (2.38%) |
| Long-Acting Anticholinergics | 1 (0.79%) | 1 (0.79%) | 1 (0.79%) |
| Oral Corticosteroid | 4 (3.17%) | 1 (0.79%) | 1 (0.79%) |
Chi-squared tests were used to determine the p-values.
p=0.024
p=0.488
Discussion
We observed a non-significant reduction in asthma-related ED visits and hospitalizations during the 9-month pharmacist intervention. However, when only patients with uncontrolled asthma at baseline was evaluated, there was a significant reduction in events (p=0.016). This reduction in ED visits and hospitalizations shows the importance of targeting the intervention for those with uncontrolled asthma. We also found significant improvements in ACT and AQLQ-M scores in patients who received the PPCM intervention. Furthermore, ACT and AQLQ-M scores sustained improvement following the discontinuation of the intervention. Asthma control rates (ACT ≥ 20) increased to 54% by 18 months. To our knowledge, this is the first study to show a sustained effect on ACT and AQLQ-M following discontinuation of a pharmacist-led intervention. These findings are important because the population consisted of a large number of patients from racial minority groups and those with lower socioeconomic status who have been largely under-represented in previous studies. This population increases our external validity.
Another study assessing a physician-pharmacist care model in asthma demonstrated reductions in ED visits for patients who had been seen in the ED at least three times in the past year before study enrollment.27 These findings could indicate that intensifying the intervention for those who chronically seek additional care might minimize ED visits. Our study randomly enrolled patients with asthma irrespective of the degree of asthma control at baseline, and 30.2% were controlled (ACT ≥ 20) at baseline. These patients may be less likely to benefit from the intervention (i.e., asthma education and pharmacotherapy adjustments), and thus could explain the lack of statistical significance for ED visits and hospitalizations. However, there was a significant reduction in these events when patients with controlled asthma at baseline were removed from analysis. This finding suggests future research should selectively target patients who suffer from uncontrolled asthma.
Patients met with their pharmacist an average 2.52 times over the course of the intervention versus the 5 visits that were recommended. However, there were more visits for patients with uncontrolled asthma. There was also a wide range of visit frequency (0–7 visits/patient) at the medical offices. These differences were expected because this study was an effectiveness trial in diverse medical offices with variable degrees of asthma control at baseline. The study was designed to specifically allow pharmacists to use total discretion in their visit frequency, especially if asthma became controlled early.
ACT and AQLQ-M scores displayed sustained improvement even though ED visits and hospitalizations increased in the post-intervention period. One explanation for this finding may be that once the intervention was stopped, patients were alerted and increased their care-seeking behavior. This theory is supported by a previous study of patients with asthma and COPD, where an increase in breathing-related ED visits were noted in patients who received a peak flow meter, and in those who received a peak flow meter and pharmaceutical care, compared to usual care.25 The authors speculated that the patients associated their PEFR values and symptoms, which led to more care-seeking. Another possible explanation for the increase in ED visits when the intervention was discontinued may be due to the patients’ inability to easily access the primary care provider. Pharmacists served to increase access and time for patient care, with the ability to provide quick primary care access and answer critical questions when sudden asthma exacerbations arose. Without this access, patients may have sought care from the ED rather than thorough primary care.” Self-reported adherence data demonstrated a significant improvement at 9 months and some reduction in high adherence at 18 months (using LOCF), which supports the primary outcome findings.
The present study also found a significant increase in inhaled corticosteroids during the intervention (p=0.024) showing that more asthma regimens included chronic “controller” medications. These changes may have positively impacted the reduction in ED visits and hospitalizations. However, many other variables impact drug use and ED visits.
There were limitations to the present study. First, the study used a pre-post design and lacked a control group. However, this study was unique because we evaluated patients after the intervention was discontinued. Second, asthma-related ED visits and hospitalizations were self-reported which could have influenced the findings. We confirmed self-reported ED visits and hospitalizations by medical record audit, however some self-reported events were unable to be verified. ACT questionnaires were also self-reported and completed repeatedly by patients, which may increase bias. Third, the intervention was conducted for 9 months to be consistent with the BP intervention in the CAPTION trial. Asthma control may have varied based on seasonal changes, which would be hard to identify without 12 month data. Fourth, 26% of patients dropped out of the study which may be due to high asthma control rates at baseline.
Conclusion
The PPCM care model reduced asthma-related ED visits and hospitalizations and improved asthma control and quality of life. The primary outcome was not statistically significant. However, there was a significant reduction in ED visits and hospitalizations during the intervention for patients with uncontrolled asthma at baseline. Out findings support the use of the PPCM models in treating asthma. Additional research is needed to investigate both the effects on asthma outcomes achievable through the PPCM model and the cost-effectiveness of the intervention when implemented in medical offices.
Acknowledgements
The authors would like to acknowledge the Executive Committee of the National Interdisciplinary Primary Care Practice-Based Research Network who assisted with the development of this network and the study design: John Gums, Lori M. Dickerson, Oralia V. Bazaldua, Timothy Ives, Connie Kraus, Grace Kuo, and the Data and Safety Monitoring Board, Barry R. Davis, Keith C. Ferdinand, Michael Murray, and Nakela Cook.
Funding:
Supported by the National Heart, Lung, and Blood Institute, RO1HL091841 and RO1HL091843.
Abbreviation List
- ACT
Asthma Control Test
- AQLQ-M
Asthma Quality of Life Questionnaire by Marks
- BP
Blood Pressure
- COPD
Chronic Obstructive Pulmonary Disease
- ED
Emergency Department
- GEE
Generalized Estimating Equation
- PPCM
Physician-Pharmacist Collaborative Management
- PEFR
Peak Expiratory Flow Rate
Site investigators for the CAPTION study
Carlos Rojas, Fourth & Lewis Medical Office Family Medicine, San Diego, CA, Kevin B. Sneed, H. James Brownlee, Jr., Kymia Love Jackson, University of South Florida Department of Family Medicine, Tampa, FL, Jeanette Figueroa, Meredith Snyder, Jefferson Family Medicine Clinic, Buffalo, NY, Rebecca Edwards, Geraldine Zurek, David Townsend, Wake Forest University Baptist Medical Center, Northwest Area Health Education Center, Winston Salem, NC, Lynda Lowe, Kris Madala, I.S. Simon, Spartanburg Family Medicine Residency Program, Spartanburg, SC, Debbie Hermes, Texas Tech Center for Community and Family Medicine, Amarillo, TX, Jeri J. Sias,; Margie E. Perez-Padilla, Clara Castrellon, Jose Luna, Texas Tech Community Partnership Clinics, El Paso, TX, Carrie Stoltenberg, Jody Pankow, Louis Sanner, Northeast Family Practice Pharmacy, Madison, WI.
Footnotes
Previous Presentations:
Poster at the American Society of Health-System Pharmacists (ASHP) Midyear Conference. Orlando, Florida. Poster Number: 5-228. December 10, 2013.
Poster at the University of Iowa Health Science Research Week. Iowa City, Iowa. Poster Number 123. April 23, 2014.
References
- 1.Centers for Disease Control and Prevention. Asthma facts—CDC’s national asthma control program grantees. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2013. [Google Scholar]
- 2.Centers for Disease Control and Prevention. Asthma's impact on the nation. [Accessed August 5, 2013]; Retrieved from: http://www.cdc.gov/asthma/impacts_nation.
- 3.National Asthma, Education and Prevention Program (2007) Expert Panel Report 3 (EPR-3): Guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;120:S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 4.Mehuys E, Van Bortel L, De Bolle L, Van Tongelen I, Annemans L, Remon JP, Brusselle G. Effectiveness of pharmacist intervention for asthma control improvement. Eur Respir. 2008;31:790–799. doi: 10.1183/09031936.00112007. [DOI] [PubMed] [Google Scholar]
- 5.Gillisen A. Patient's adherence in asthma. J Physiol Pharmacol. 2007;58:205–222. [PubMed] [Google Scholar]
- 6.Young HN, Havican SN, Griesbach S, Thorpe JM, Chewning BA, Sorkness CA. Patient and phaRmacist Telephonic Encounters (PARTE) in an underserved rural patient population with asthma: results of a pilot study. Telemed J E Health. 2012;18:427–433. doi: 10.1089/tmj.2011.0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benavides S, Rodriguez JC, Maniscalco-Feichtl M. Pharmacist involvement in improving asthma outcomes in various healthcare settings: 1997 to present. Ann Pharmacother. 2009;43:85–97. doi: 10.1345/aph.1K612. [DOI] [PubMed] [Google Scholar]
- 8.Smith L, Bosnic-Anticevich SZ, Mitchell B, Saini B, Krass I, Armour C. Treating asthma with a self-management model of illness behaviour in an Australian community pharmacy setting. Soc Sci Med. 2007;64:1501–1511. doi: 10.1016/j.socscimed.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Saini B, Smith L, Armour C, Krass I. An educational intervention to train community pharmacists in providing specialized asthma care. Am J Pharm Educ. 2006;70:118. doi: 10.5688/aj7005118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stergachis A, Gardner JS, Anderson MT, Sullivan SD. Improving pediatric asthma outcomes in the community setting: does pharmaceutical care make a difference? J Am Pharm Assoc. 2002;42:743–752. doi: 10.1331/108658002764653522. [DOI] [PubMed] [Google Scholar]
- 11.García-Cárdenas V, Sabater-Hernández D, Kenny P, Martínez-Martínez F, Faus MJ, Benrimoj SI. Effect of a pharmacist intervention on asthma control. A cluster randomised trial. Respir Med. 2013;107:1346–1355. doi: 10.1016/j.rmed.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 12.Bereznicki BJ, Peterson G, Jackson S, Walters EH, George J, Stewart K, March GJ. Uptake and effectiveness of a community pharmacy intervention programme to improve asthmamanagement. J Clin Pharm Ther. 2013;38:212–218. doi: 10.1111/jcpt.12017. [DOI] [PubMed] [Google Scholar]
- 13.Armour CL, Reddel HK, LeMay KS, et al. Feasibility and effectiveness of an evidence-based asthma service in Australian community pharmacies: a pragmatic cluster randomized trial. J Asthma. 2013;50:302–309. doi: 10.3109/02770903.2012.754463. [DOI] [PubMed] [Google Scholar]
- 14.Saini B, LeMay K, Emmerton L, et al. Asthma disease management-Australian pharmacists' interventions improve patients' asthmaknowledge and this is sustained. Patient Educ Couns. 2011;83:295–302. doi: 10.1016/j.pec.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Bereznicki B, Peterson G, Jackson S, Walters EH, DeBoos I, Hintz P. Perceived feasibility of a community pharmacy-based asthma intervention: a qualitative follow-up study. J Clin Pharm Ther. 2011;36:348–355. doi: 10.1111/j.1365-2710.2010.01187.x. [DOI] [PubMed] [Google Scholar]
- 16.Bereznicki B, Peterson G, Jackson S, Walters EH, Gee P. The sustainability of a community pharmacy intervention to improve the quality use of asthma medication. J Clin Pharm Ther. 2011;36:144–151. doi: 10.1111/j.1365-2710.2010.01165.x. [DOI] [PubMed] [Google Scholar]
- 17.Hämmerlein A, Müller U, Schulz M. Pharmacist-led intervention study to improve inhalation technique in asthma and COPD patients. J Eval Clin Pract. 2011;17:61–70. doi: 10.1111/j.1365-2753.2010.01369.x. [DOI] [PubMed] [Google Scholar]
- 18.René-Henri N, Khamla Y, Nadaira N, et al. Community pharmacists' interventions in asthma care: a descriptive study. Ann Pharmacother. 2009;43:104–111. doi: 10.1345/aph.1L308. [DOI] [PubMed] [Google Scholar]
- 19.Bereznicki BJ, Peterson GM, Jackson SL, Walters EH, Fitzmaurice K, Gee P. Pharmacist-initiated general practitioner referral of patients with suboptimal asthma management. Pharm World Sci. 2008;30:869–875. doi: 10.1007/s11096-008-9242-3. [DOI] [PubMed] [Google Scholar]
- 20.Pradel FG, Obeidat NA, Tsoukleris MG. Factors affecting pharmacists' pediatric asthma counseling. J Am Pharm Assoc. 2007;47:737–746. doi: 10.1331/JAPhA.2007.06138. [DOI] [PubMed] [Google Scholar]
- 21.Stergachis A, Gardner JS, Anderson MT, Sullivan SD. Improving pediatric asthma outcomes in the community setting: does pharmaceutical care make a difference? J Am Pharm Assoc. 2002;42:743–752. doi: 10.1331/108658002764653522. [DOI] [PubMed] [Google Scholar]
- 22.Weinberger M, Murray MD, Marrero DG, et al. Effectiveness of pharmacist care for patients with reactive airways disease: a randomized controlled trial. JAMA. 2002;288:1594–1602. doi: 10.1001/jama.288.13.1594. [DOI] [PubMed] [Google Scholar]
- 23.Carter BL, Clarke W, Ardery G, et al. A cluster-randomized effectiveness trial of a physician-pharmacist collaborative model to improve blood pressure control. Circ Cardiovasc Qual Outcomes. 2010;3:418–423. doi: 10.1161/CIRCOUTCOMES.109.908038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carter BL, Ardery G, Dawson JD, et al. Physician and pharmacist collaboration to improve blood pressure control. Arch Intern Med. 2009;169:1996–2002. doi: 10.1001/archinternmed.2009.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carter BL, Rogers M, Daly J, Zheng S, James PA. The potency of team-based care interventions for hypertension. Arch Intern Med. 2009;169:1748–1755. doi: 10.1001/archinternmed.2009.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knoell DL, Pierson JF, Marsh CB, Allen JN, Pathak DS. Measurement of outcomes in adults receiving pharmaceutical care in a comprehensive asthma outpatient Clinic. Pharmacotherapy. 1998;18:1365–1374. [PubMed] [Google Scholar]
- 27.Pauley TR, Magee MJ, Cury JD. Pharmacist-managed, physician-directed asthma management program reduces emergency department visits. Ann Pharmacother. 1995;29:5–9. doi: 10.1177/106002809502900101. [DOI] [PubMed] [Google Scholar]
- 28.Carter BL, Coffey CS, Uribe L, et al. Similar blood pressure values across racial and economic groups: baseline data from a group randomized clinical trial. J Clin Hypertens. 2013;15:404–412. doi: 10.1111/jch.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dickerson LM, Kraus CK, Kuo GM, et al. Formation of a primary care pharmacist practice-based research network. Am J Health-Syst Pharm. 2007;64:2044–2049. doi: 10.2146/ajhp060650. [DOI] [PubMed] [Google Scholar]
- 30.Billups SJ, Okano G, Malone D, et al. Assessing the structure and process for providing pharmaceutical care in Veterans Affairs medical centers. Am J Health-Syst Pharm. 2000;57:29–39. doi: 10.1093/ajhp/57.1.29. [DOI] [PubMed] [Google Scholar]
- 31.Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113:59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Marks GB, Dunn SM, Woolcock AJ. A scale for the measurement of quality of life in adults with asthma. J Clin Epidemiol. 1992;45:461–472. doi: 10.1016/0895-4356(92)90095-5. [DOI] [PubMed] [Google Scholar]
- 33.Rose AJ, Berlowitz DR, Orner MB, Kressin NR. Understanding uncontrolled hypertension: is it the patient or the provider? J Clin Hypertens. 2007;9:937–943. doi: 10.1111/j.1524-6175.2007.07332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]