Abstract
Background
In Huntington’s disease (HD) increased variability is seen in performance of motor tasks that require implicit control of timing. We examined if timing variability was also evident in an explicit interval-timing task.
Methods
Sixty subjects (21 controls, 19 manifest HD and 20 pre-manifest HD) performed a single interval production task with three target intervals (1.1s, 2.2s, 3.3s). We analyzed accuracy (proportional error) and precision (standard deviation) across groups and intervals.
Results
No differences were seen in accuracy across groups or intervals. Precision was significantly lower in manifest (p=0.0001) and pre-manifest HD (p=0.04) compared with controls. This was particularly true for pre-manifest subjects close to diagnosis (based on probability of diagnosis in five years). Precision was correlated with proximity to diagnosis (r2=0.3, p<0.01). To examine the source of reduced precision, we conducted linear regression of standard deviation with interval duration. Slope of the regression was significantly higher in manifest HD (p=0.02) and in pre-manifest HD close to diagnosis (p=0.04) compared with controls and pre-manifest participants far from diagnosis.
Conclusions
Timing precision is impaired before clinical diagnosis in Huntington’s disease. Slope analysis suggests that timing variability (decreased precision) was due to deficits in timing dependent processes. Our results provide additional support for the proposal that the basal ganglia are implicated in central timekeeping functions. Since the single interval production task was sensitive to deficits in pre-manifest HD, temporal precision may be a useful outcome measure in future clinical trials.
Keywords: Huntington’s disease, timing, interval production, variability, pre-manifest
Introduction
The diagnosis of Huntington’s disease (HD) is based on clinical observation of motor impairments [1]. Motor impairments in HD include inaccurate saccadic eye movements with increased latency and variability [2], slow and variable arm movements [3, 4], slow, asymmetric gait with increased variability [5, 6], and increased force and timing variability during precision grip [7, 8]. An impairment common to all motor tasks mentioned above is increased timing variability. In motor tasks, timing is thought to be an emergent property rather than being controlled explicitly [9, 10]. Increased timing variability in HD can be explained by the fact that the basal ganglia are implicated in timing control, as seen from lesion studies in animals and humans [11–13], as well as imaging studies in healthy humans [14, 15]. However, it is unclear if deficits in explicit timing accompany those in implicit timing control seen in motor tasks.
Sensitivity to time as a stimulus dimension (i.e. duration) is typically evaluated using perception or production tasks. There is a lack of clarity on time perception impairments in HD: while pre-manifest HD (pHD) subjects demonstrate impairments on a single alternative discrimination task [16], they do not demonstrate impairments on a two-alternative forced choice variation of the task [17]. Time production (interval timing) is tested through repetitive tapping (in the range of hundreds of milliseconds) [18] or single interval production (SIP), ranging from the hundreds of milliseconds to minutes [19, 20]. Repetitive tapping at paced and self-selected intervals is impaired in manifest HD [21] and pre-manifest HD [22–24]. However, results from speeded repetitive tapping are inconsistent: one study [17] reported that performance of pre-manifest HD subjects was similar to controls, whereas another study [25] reported impaired performance.
SIP may be a better test of explicit timing than repetitive tapping. The cyclic pattern of responses in repetitive tapping may confer a performance advantage by reducing variability and may engage neural mechanisms implicated in motor control [10, 26]. Moreover, SIP is sensitive in differentiating between controls and individuals with Parkinson’s disease (PD) [11] and elderly [27–29]. Only one study has examined SIP in HD, which reported similar error rate for manifest, pre-manifest HD, PD and elderly [30]. Since this study only tested a single interval (1.2 sec) and did not measure the magnitude of error or variability, we examined SIP in pre-manifest and manifest HD at three intervals using measures of accuracy and precision. The purpose of this study was to examine (a) if single interval production task could distinguish between pre-manifest, manifest HD and matched controls, (b) if performance on single interval production is associated with proximity to diagnosis, (c) the source of timing variability [10].
Methods
Participants
Sixty participants (20 healthy controls, 21 pre-manifest HD (pHD) and 19 early manifest HD (mHD)) enrolled in this study. pHD participants were recruited from ongoing multi-site observational studies if they 1) had a family history of HD, 2) had a confirmed genetic test (expended CAG allele >36), 3) were rated by a movement disorder specialist as not meeting clinical criteria for diagnosis of HD based on clinical motor examination and diagnostic confidence rating from the UHDRS, and 4) were free of orthopedic or neurological disorders.
mHD participants were recruited from the Huntington’s disease Center of Excellence at Columbia University and were included if they 1) had a confirmed diagnosis of HD, 2) had total functional capacity (TFC) scale score of at least 9 out of 13 indicating mild functional limitations, 3) had negative history of neurological disorders other than HD; and 4) could follow directions in English. Healthy control participants were recruited from among family members of mHD or pHD without the expanded CAG allele. Additional control participants were included through public advertisement if they had a negative family history of HD. Control subjects were matched with pHD and mHD for age and were not administered the modified MMSE or the UHDRS because they were not at risk of HD. The Institutional Review Board at Columbia University approved study procedures and all subjects provided written informed consent before participation. Subjects were compensated for participation.
Interval Timing Assessment
Testing was done in a quiet, well-lit room. Participants sat on a comfortable chair at a table in front of a laptop computer (Macbook Pro 15″, Apple, Inc.). The single interval production (SIP) task consisted of two phases: synchronization and continuation, as seen in Figure 1A [31]. During synchronization (training) trials, participants were presented with two tones. The time period between the tones corresponded to one of the target intervals (1.1 s ‘short’, 2.2 s ‘medium’, or 3.3 s ‘long’). Participants were instructed to respond by pressing a key (spacebar) in synchrony with the second tone at the end of the interval. The trial ended after a response or was terminated if participants failed to respond. Two blocks of 20 trials were presented for each of three target intervals (1.1s, 2.2s, 3.3s). The order of presentation of the blocks was randomized. At the beginning of each block, target interval was identified on the screen and the tester provided instructions.
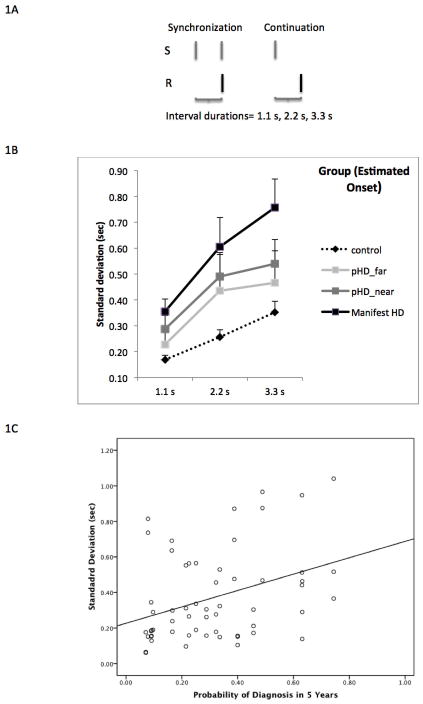
Figure 1.
A) Description of synchronization and continuation phases of the single interval production task. During the synchronization phase subjects were presented stimuli (S) at the beginning and end of the interval (1.1s, 2.2s or 3.3s). Subjects were asked to respond (R) by pressing a key (spacebar) at the end of the interval. During the continuing phase, stimulus was provided only at the beginning of the interval and subjects were asked to respond by indicating the end of the interval. B) Mean ± standard error for precision (standard deviation) at each interval (1.1s, 2.2s, 3.3s) for each group. Pre-manifest subjects were divided into two groups based on median probability of diagnosis. C) Relationship between precision (standard deviation) and probability of diagnosis in 5 years for pre-manifest HD subjects at all three target intervals. Linear regression line is presented (r2= 0.3, p<0.01)
The continuation phase began immediately after the synchronization phase. During continuation trials participants were presented with a single tone at the beginning of the target interval, and were instructed to respond by pressing the spacebar at the end of the interval. No tone was presented at the end of the target interval. The cue turned red following a response (pressing the spacebar) or a time out. Two blocks of 20 trials were presented for the three intervals in a randomized order. At the beginning of each block, target interval was identified on the screen and the tester provided instructions. The inter-trial interval was 0.5 s. Performance feedback was not provided during either phase. The total duration of testing was 20 minutes. In this report, we present results for the continuation phase because it explicitly evaluates time estimation (whereas the purpose of the synchronization phase was training).
Clinical Assessment
1) Motor Assessment
pHD and mHD participants were administered the motor section of the Unified HD Rating Scale (UHDRS), a standardized assessment of involuntary and voluntary motor function [32]. We computed the total motor score (TMS), as the sum of scores of 31 individual items, each of which was scored on a scale of 0–4 (total of 124 points). Raters were blinded to genetic status of pHD participants. A movement disorder specialist rated the motor items of the UHDRS and assigned a rating of diagnostic confidence ranging from 0–4, defined as follows: (0) no motor abnormalities; (1) non-specific motor abnormality (<50% confidence), (2) motor abnormalities that may be signs of HD (50–89% confidence), (3) motor abnormalities that are likely signs of HD (90–98% confidence), (4) motor abnormalities that are unequivocal signs of HD (>99% confidence) [33]. Participants with diagnosis confidence rating ≤ 2 were included in the analysis. While some recent studies (Track-HD[24]) have used a criterion of TMS < 5 to recruit pre-manifest subjects, we included subjects with higher TMS, as long as their diagnostic confidence was ≤ 2, because there are no well-defined criteria for diagnosis purely based on TMS, and because none of the individual item scores were greater than 2/4 (mild-moderate impairment).
2) Cognitive Assessment
pHD and mHD participants completed the Symbol digit modality test (SDMT) [34]. The SDMT assesses psychomotor speed and working memory and is sensitive to performance differences between controls, pHD and mHD [35]. Participants were provided with a reference key that paired digits with symbols on top of a page. Symbols were presented in rows below the key. Participants were required to write the corresponding digit for each symbol. The number of correct matches completed in 90 s was recorded.
Proximity to Diagnosis
Estimated onset and probability of diagnosis in 5 years were computed from CAG repeats on the expanded allele and the participants’ age by an investigator blinded to subject assignment [36]. We used probability of diagnosis for regression analysis because regression relationships are approximately linear [22]. For between group analyses, we used estimated onset.
Data Analysis
Normally distributed data were analyzed with parametric statistics. Data that were not normally distributed were analyzed with non-parametric statistics. We analyzed the continuation phase of the SIP task using analysis of variance and linear regression. Dependent variables included accuracy (proportional error defined as (response latency–target)/target) and precision (standard deviation) [22, 23]. The first two responses, the fastest and slowest response from each block of trials were excluded from analysis to eliminate spurious variability.
1. Single interval production across groups
We compared accuracy and precision across groups by conducting repeated measures analysis of variance (ANOVA) with group (control, pHD_far, pHD_near and mHD) and interval (1.1s, 2.2s, 3.3s) as factors. We divided the pHD group based on estimated onset (near onset= <9 years and far from onset= ≥ 9 years), based on prior work[22]. We included age, sex, education, medication status and music training as covariates, defined a priori, in the analysis. Post-hoc analyses, defined a-priori, were conducted using Tukey’s honestly significant difference.
2. Association with proximity to diagnosis
We performed linear regression analysis to examine if timing performance (precision) was predictive of probability of diagnosis in five years.
3. Effect of Motor and Cognitive Function on interval timing
In separate analyses, we divided pHD subjects into pHD_near and pHD_far based on median total motor score (7.0) and median score on symbol digit modality test-SDMT (53.0) and conducted group × interval ANOVA with repeated measures.
4. Source of timing variability
We performed slope analysis [10], which assumes that total variability in a timing task can be decomposed into variability associated with timing dependent and timing independent processes. The method involves linear regression of standard deviation and interval duration, where the slope represents the timing dependent processes and the intercept represents timing independent processes (attention, memory, motor implementation) [10]. The slope and intercept were separately analyzed using a non-parametric test (Jonckheere-Terpstra Test for Ordered Alternatives) with group as the single factor (control, pHD_far, pHD_near, and mHD). pHD groups were divided based on estimated onset. We used a non-parametric test because slope and intercept were not normally distributed.
Demographic variables were analyzed with one-way ANOVA (for continuous variables) and Chi-square (for discrete variables). All analyses used SPSS 18.0 and we used 0.05 as the level of significance.
Results
Table 1 presents demographic and clinical data for each group. There were no differences in age (p= 0.22) or music training (p= 0.46) across groups. Control participants were, on average, more educated than pHD (p=0.001) and mHD (p=0.001) participants, likely because a number of them were recruited from an academic institution. pHD participants had a five-year probability of diagnosis ranging from 0.07–0.74, and had an estimated onset ranging from 3.2–15.5 years.
Table 1.
Demographic and Clinical data by group (control, pHD and mHD). pHD group were divided into Far from onset and Near onset based on estimated onset
| Control | Pre-manifest HD (pHD) | Pre-manifest HD Far | Pre-manifest HD Near | Manifest HD (mHD) | |
|---|---|---|---|---|---|
| Number of subjects | 21 | 20 | 10 | 10 | 19 |
| Gender, F/M | 16/5 | 11/9 | 6/4 | 5/5 | 7/12 |
| Age, years (range) | 42.45 (31–54) | 41.75 (30–52) | 41.56 (30–52) | 42.3 (32–51) | 44 (30–58) |
| Years of Education | 19.19** | 16.32 | 15.44 | 17.1 | 15.78 |
| Music training (Number of subjects) | 4 | 3 | 2 | 1 | 2 |
| Probability of Diagnosis (range) | N/A | 0.31*** (0.07 – 0.74) | 0.15 (0.07 – 0.27) | 0.47 (0.28 – 0.74) | 1.00 |
| Estimated Onset (range) | N/A | 9.13 (3.2–15.5) | 12.7 (9.8–15.5) | 6.12 (3.2–8.7) | N/A |
| UHDRS motor score (SD) | N/A | 9.35 (7.4)* | 5.6 (4.1) | 13.1 (8.2) | 24.89 (10.7) |
| SDMT (SD) | N/A | 53.11 (10.5) | 57.63 (7.4) | 49.5 (11.58) | 29.63 (7.7) |
| UHDRS TFC (SD) | N/A | 13.0 (0) | 11.0 (2.52) | ||
| UHDRS IS (SD) | N/A | 95 (7.07) | 91.5 (8.8) |
UHDRS= Unified Huntington’s disease rating scale; SDMT= Symbol digit modality test; TFC= Total functional capacity scale; IS= Independence scale;
= p<0.05;
= p<0.01;
p<0.0001
1. Single Interval Production across Groups
We divided pHD participants into two groups: far from onset and near onset based on estimated onset. Group (control, pHD_far, pHD_near, mHD) × interval ANOVA for accuracy did not reveal any significant effects for either factor, as seen in Table 2. Precision showed a main effect of interval (F= 15.96, p= 0.0001), with all groups demonstrating a significant linear trend (F= 31.17, p<0.0001) but not a significant quadratic trend (F= 1.49, p=0.23). This result indicates that precision demonstrated the scalar property of interval timing [10]. A main effect of group (p= 0.001) was also seen, after controlling for age, sex, education and music training as covariates (Figure 1A). None of the covariates demonstrated significant effects. Post-hoc analysis indicated that controls had significantly higher precision than mHD (p=0.0001) and pHD_near (p=0.04). Differences between controls and pHD_far were not significant (p=0.17). Cohen’s d for differences between controls and mutation positive groups were 1.2 (mHD) and 0.8 (pHD_near). There were no significant group × condition interaction effects (F=1.01, p= 0.42).
Table 2.
Mean (SEM) for proportional error and standard deviation for group (control, pHD_far, pHD_near, mHD) × interval. pHD groups divided into two based on estimated onset. Results of statistical analysis are presented (significant results in bold).
| 1.1 s | 2.2 s | 3.3 s | F (Interval) | Sig. | F (Group) | Sig. | Post-hoc (Group) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Contorl vs pHD_near | Control vs pHD_far | Control vs mHD | |||||||||
| Proportional error, s (SD) | Control | 0.089 (0.08) | 0.245 (0.52) | 0.425 (0.17) | 1.82 | 0.17 | 0.73 | 0.54 | NS | NS | NS |
| pHD_far | −0.206 (0.15) | 0.176 (0.31) | −0.148 (0.42) | ||||||||
| pHD_near | 0.189 (0.18) | 0.339 (0.23) | 0.543 (0.39) | ||||||||
| mHD | 0.236 (0.17) | 0.231 (0.22) | 0.733 (0.46) | ||||||||
| Standard deviation, s (SD) | Control | 0.17 (0.02) | 0.26 (0.03) | 0.35 (0.04) | 15.51 | 0.0001 | 6.83 | 0.001 | 0.17 | 0.04 | 0.0001 |
| pHD_far | 0.23 (0.06) | 0.44 (0.14) | 0.47 (0.12) | ||||||||
| pHD_near | 0.29 (0.05) | 0.49 (0.09) | 0.54 (0.09) | ||||||||
| mHD | 0.36 (0.05) | 0.61 (0.11) | 0.76 (0.11) | ||||||||
S= second; pHD= pre-manifest HD; mHD= Manifest HD; Sig.= significance
2. Association with Proximity to Diagnosis
Linear regression revealed that precision was a significant predictor of probability of diagnosis (r2=0.3, p<0.01) after controlling for age, sex, education and music training, as shown in Figure 1B.
3. Effect of Motor and Cognitive Performance
Motor function, assessed by the TMS, was significantly worse for mHD compared with pHD_near (p=0.005) and pHD_far (p=0.00001) participants. The two pHD groups were not different from each other (p= 0.15). To examine the effect of motor function on interval timing, we divided pHD participants into two groups (higher and lower motor function) based on the median UHDRS total motor score (7.0) and performed a group (control, pHD_higher motor, pHD_lower motor, mHD) × interval ANOVA for precision. There was a significant main effect of interval (F= 16.18, p= 0.0001) and a significant main effect of group (F= 9.63, p=0. 0001) but no interaction effect (p= 0.38). Control subjects demonstrated significantly higher precision compared with pHD_lower motor function (p= 0.001) and mHD (p= 0.0001) participants, but not compared with pHD_higher motor function (p= 0.84).
Cognitive function, assessed by the Symbol Digit Modality Test (SDMT) was worse for mHD participants compared with pHD_near (p= 0.0001) and pHD_far (p= 0.0001). There were no differences between pHD_near and pHD_far groups (P= 0.18). We examined the effect of cognitive function on interval timing by dividing the pHD participants into higher and lower cognitive function groups based on the median SDMT score (53.0) and conducted a group (control, pHD_higher cog, pHD_lower cog, mHD) × interval ANOVA for precision. There was a significant effect of interval (F= 15.34, p= 0.0001) and group (F= 7.36, p= 0.0001). Control participants demonstrated higher precision compared with mHD (p= 0.0001), pHD_lower cognitive function (p= 0.01), but not when compared with pHD_higher cognitive function (p= 0.3). Thus both motor and cognitive performance influenced precision on single interval production.
4. Source of Timing Variability
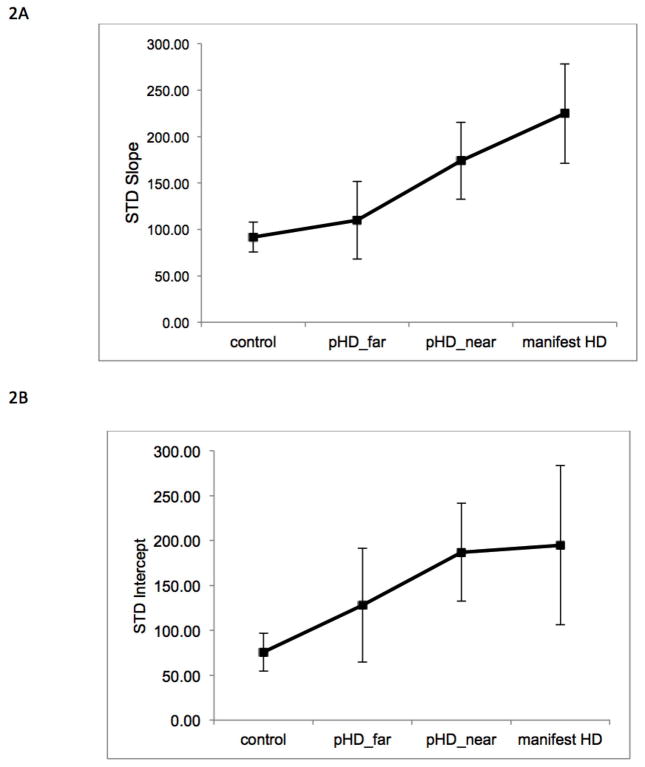
We conducted linear regression of precision (standard deviation) with interval duration, and compared the slope and intercept of the regression across groups (control, pHD_far, pHD_near, mHD). Non-parametric Test of Ordered Alternatives (Jonckheere-Terpstra) for slope demonstrated a significant effect (Test Statistic= 2.14, p=0.03). As seen in Figure 2, control subjects had a significantly lower slope compared with mHD (p=0.02, Cohen’s d= 0.6), pHD_near (p −0.04, Cohen’s d=0.5) but not pHD_far (p=0.58). pHD_far had a significantly lower slope compared with pHD_near (p=0.02, Cohen’s d= 0.4). The results indicate that mHD and pHD_near participants had a deficit in timing dependent processes. Non-parametric Test for intercept was not significant (p= 0.07). The results indicate that deficits in timing independent processes did not account for between-group differences in timing variability in our sample.
Figure 2.
Results of regression of standard deviation with interval duration across groups. A) Presents the regression slope for each group (control, pHD_far, pHD_near, and mHD), and B) Presents the regression intercept for each group.
Discussion
Individuals with manifest Huntington’s disease (mHD) and pre-manifest mutation carriers (pHD) demonstrate greater variability in motor skills that require implicit control of timing. Here we investigated whether individuals with the expanded CAG mutation demonstrated increased variability on a task that requires explicit control of timing. Our results demonstrate that mHD and pHD participants have intact accuracy but impaired precision in a single interval production task compared with matched control subjects. Group differences were maintained after accounting for sex, education, and music training. Precision was associated with proximity to diagnosis, indicating that the single interval production task is sensitive to physiological changes in the pre-manifest stage of HD. A precise estimate of time intervals is necessary for functional motor skills such as stepping on to a moving escalator, estimating time available for crossing a busy street, and intercepting a moving object, to name a few. Since the SIP task is easy to administer without special equipment, and is a sensitive measure of timing control, precision in SIP may be a useful outcome measure in clinical trials in pre-manifest HD aimed at improving motor function.
Slope analysis (regression of precision with interval duration) allowed us to examine the source of impaired precision. While there were no differences across groups in the regression intercept, the slope was significantly higher in mHD and pHD participants near diagnosis. In both these groups the relationship between precision and interval duration violated the scalar property of interval timing [9, 10]. Since deviations in slope represent timing dependent processes, our results indicate that increased timing variability in HD may be due to deficits in timing dependent processes [10, 19].
Our results are comparable to recent studies on repetitive tapping in pre-manifest and manifest HD [22–25]. Precision, rather than accuracy, was impaired and was correlated with predicted disease onset, clinical motor function and basal ganglia volume (but see [17]). However, tapping may engage motor control networks rather than timing specific mechanisms because it involves repetitive movement at very short intervals [26, 37]. Our results establish that late pre-manifest and early manifest HD subjects have lower precision in single interval production that is due to deficits in timing dependent processes. Models of timing control are proposed to have a time dependent mechanism (clock) and a time independent mechanism, which may include attention and motor implementation [10, 19, 38]. The basal ganglia are thought to be important for regulating the clock mechanism, particularly for time intervals above 1s [15]. This suggestion was partly based on observation of explicit timing impairment in PD [11, 38]. In Huntington’s disease, impairments have been observed in motor skills that require implicit control of timing. Our result of impairments in explicit timing before clinical diagnosis and functional limitations underscores the role of the basal ganglia in timing control.
Our study has limitations. First, while our data did not confirm deficits in timing independent processes (based on the regression intercept), it is possible that our small sample size obscured differences between groups. However, differences in slope emerged despite the relatively small sample, indicating that timing dependent deficits are readily evident in pre-manifest and manifest HD. Second, total motor score for some of the pHD participants were well above what has been reported in recent studies [24]. Despite having higher motor scores, precision was not different for pHD_far compared to pHD_near participants (see Figure 1A). Notwithstanding the inclusion of pHD participants who may be classified as “peri-manifest” rather than pre-manifest, single interval production was sensitive to proximity to diagnosis. Finally, we were unable to directly compare the sensitivity of single interval production and repetitive tapping in the same subjects. Future work will attempt to compare performance across single interval production and other timing tasks. Despite the limitations, single interval production was sensitive in detecting deficits in temporal processing in individuals with the HD gene mutation years before clinical diagnosis, and may be a useful measure of function in future clinical trials.
Acknowledgments
We thank pre-manifest, manifest HD subjects and controls for participating in the study. We also thank Drs. Jane Paulsen and Jeffrey Long for providing access to clinical data for subjects enrolled from Predict-HD, and Paula Leber-Wasserman for help with subject recruitment.
Footnotes
AKR was involved with study conception and design, analysis and interpretation of data, drafting the article, revising it critically for important intellectual content and final approval of the version to be published.
KSM was involved with study conception, interpretation of data, revising the article critically for important intellectual content and final approval of the version to be published.
JU was involved with data collection, analysis and interpretation of data, revising the article critically for important intellectual content and final approval of the version to be published.
BCR was involved with study conception and design, analysis and interpretation of data, and revising the article critically for important intellectual content and final approval of the version to be published.
Financial Disclosures/Conflict of interest: None of the authors report a conflict of interest with respect to financial or personal relationships with organizations that may have an influence on the work. This work was funded by National Institute of Health grant (Ashwini K. Rao K01-HD060912), partially funded by National Institute of Health grant (Paulsen 5R01NS040068), Huntington Disease Family Fund (Marder), and Huntington Disease Society of America Center of Excellence grant (Marder). Ashwini K. Rao also received support from NIH grant (Co-Investigator 5R01NS042859). Karen S. Marder receives support from NIH #NS036630 (PI), 1UL1 RR024156-01 (Director PCIR), PO412196-G (Co-I), and PO412196-G (Co-I)]. Brian C. Rakitin receives support from the Parkinson’s Disease Foundation. This work was also supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1 TR000040, formerly the National Center for Research Resources, Grant Number UL1 RR024156. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Contributor Information
Ashwini K. Rao, Department of Rehabilitation & Regenerative Medicine (Program in Physical Therapy), and G.H. Sergievsky Center, College of Physicians & Surgeons, Columbia University. 710 West 168th Street, 8th Floor, New York, NY. 10032. USA. New York, NY. USA
Karen S. Marder, Department of Neurology, Psychiatry, G.H. Sergievsky Center, Taub Institute of Alzheimer’s Disease and the Aging Brain. College of Physicians & Surgeons, Columbia University. P&S Box 16, 630 West 168th Street, New York, NY. USA
Jasim Uddin, Program in Physical Therapy, College of Physicians & Surgeons, Columbia University. 710 West 168th Street, 8th Floor, New York, NY. 10032. USA. New York, NY. USA.
Brian C. Rakitin, Cognitive Neuroscience Division-Taub Institute of Alzheimer’s Disease and the Aging Brain, Department of Neurology. College of Physicians & Surgeons, Columbia University. P&S Box 16, 630 West 168th Street,New York, NY. USA
References
- 1.Paulsen JS, et al. Detection of Huntington’s disease decades before diagnosis: the Predict-HD study. J Neurol Neurosurg Psychiatry. 2008;79(8):874–80. doi: 10.1136/jnnp.2007.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blekher T, et al. Saccades in presymptomatic and early stages of Huntington disease. Neurology. 2006;67(3):394–9. doi: 10.1212/01.wnl.0000227890.87398.c1. [DOI] [PubMed] [Google Scholar]
- 3.Carella F, et al. A study of arm movements in Huntington’s disease under visually controlled and blindfolded conditions. Neurol Sci. 2003;23(6):287–93. doi: 10.1007/s100720300003. [DOI] [PubMed] [Google Scholar]
- 4.Quinn L, et al. Control of Multijoint Arm Movements in Huntington’s Disease. J Neuro Rehab. 1997;11:47–60. [Google Scholar]
- 5.Rao AK, et al. Longitudinal Change in Gait and Motor Function in Pre-manifest Huntington’s Disease. PLoS Curr. 2011;3:RRN1268. doi: 10.1371/currents.RRN1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rao AK, et al. Spectrum of gait impairments in presymptomatic and symptomatic Huntington’s disease. Mov Disord. 2008;23(8):1100–1107. doi: 10.1002/mds.21987. [DOI] [PubMed] [Google Scholar]
- 7.Rao AK, Gordon AM, Marder KS. Coordination of fingertip forces during precision grip in premanifest Huntington’s disease. Mov Disord. 2011;26(5):862–9. doi: 10.1002/mds.23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reilmann R, et al. Grasping premanifest Huntington’s disease - shaping new endpoints for new trials. Mov Disord. 2010;25(16):2858–62. doi: 10.1002/mds.23300. [DOI] [PubMed] [Google Scholar]
- 9.Spencer RM, Zelaznik HN. Weber (slope) analyses of timing variability in tapping and drawing tasks. J Mot Behav. 2003;35(4):371–81. doi: 10.1080/00222890309603157. [DOI] [PubMed] [Google Scholar]
- 10.Ivry RB, Hazeltine RE. Perception and production of temporal intervals across a range of durations: evidence for a common timing mechanism. J Exp Psychol Hum Percept Perform. 1995;21(1):3–18. doi: 10.1037//0096-1523.21.1.3. [DOI] [PubMed] [Google Scholar]
- 11.Malapani C, et al. Coupled temporal memories in Parkinson’s disease: a dopamine-related dysfunction. J Cogn Neurosci. 1998;10(3):316–31. doi: 10.1162/089892998562762. [DOI] [PubMed] [Google Scholar]
- 12.Malapani C, Rakitin BC. Interval timing in the dopamine-depleted basal ganglia: From empirical data to timing theory. In: Meck WH, editor. Functional and neural mechanisms of interval timing. CRC Press; Boca Raton, FL: 2003. pp. 485–514. [Google Scholar]
- 13.Harrington DL, Haaland KY. Neural underpinnings of temporal processing: a review of focal lesion, pharmacological, and functional imaging research. Rev Neurosci. 1999;10(2):91–116. doi: 10.1515/revneuro.1999.10.2.91. [DOI] [PubMed] [Google Scholar]
- 14.Rao SM, Mayer AR, Harrington DL. The evolution of brain activation during temporal processing. Nat Neurosci. 2001;4(3):317–23. doi: 10.1038/85191. [DOI] [PubMed] [Google Scholar]
- 15.Wiener M, Turkeltaub P, Coslett HB. The image of time: a voxel-wise meta-analysis. Neuroimage. 2010;49(2):1728–40. doi: 10.1016/j.neuroimage.2009.09.064. [DOI] [PubMed] [Google Scholar]
- 16.Paulsen JS, et al. fMRI biomarker of early neuronal dysfunction in presymptomatic Huntington’s Disease. AJNR Am J Neuroradiol. 2004;25(10):1715–21. [PMC free article] [PubMed] [Google Scholar]
- 17.Beste C, et al. Time processing in Huntington’s disease: a group-control study. PLoS One. 2007;2(12):e1263. doi: 10.1371/journal.pone.0001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wing AM, Kristofferson AB. Response delays and the timing of discrete motor responses. Perception and Psychophysics. 1973;14(1):5–12. [Google Scholar]
- 19.Rakitin BC. The effects of spatial stimulus-response compatibility on choice time production accuracy and variability. J Exp Psychol Hum Percept Perform. 2005;31(4):685–702. doi: 10.1037/0096-1523.31.4.685. [DOI] [PubMed] [Google Scholar]
- 20.Rakitin BC, et al. Scalar expectancy theory and peak-interval timing in humans. J Exp Psychol Anim Behav Process. 1998;24(1):15–33. doi: 10.1037//0097-7403.24.1.15. [DOI] [PubMed] [Google Scholar]
- 21.Freeman JS, CF, O’Boyle DJ, Crauford D, Neary D, Snowden JS. Abnormalities of motor timing in Huntington’s disease. Parkinsonism and Related Disorders. 1996;2(2):81–93. doi: 10.1016/1353-8020(96)00009-0. [DOI] [PubMed] [Google Scholar]
- 22.Rowe KC, et al. Self-paced timing detects and tracks change in prodromal Huntington disease. Neuropsychology. 2010;24(4):435–42. doi: 10.1037/a0018905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hinton SC, et al. Motor timing variability increases in preclinical Huntington’s disease patients as estimated onset of motor symptoms approaches. J Int Neuropsychol Soc. 2007;13(3):539–43. doi: 10.1017/S1355617707070671. [DOI] [PubMed] [Google Scholar]
- 24.Tabrizi SJ, et al. Biological and clinical manifestations of Huntington’s disease in the longitudinal TRACK-HD study: cross-sectional analysis of baseline data. Lancet Neurol. 2009;8(9):791–801. doi: 10.1016/S1474-4422(09)70170-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bechtel N, et al. Tapping linked to function and structure in premanifest and symptomatic Huntington disease. Neurology. 2010;75(24):2150–60. doi: 10.1212/WNL.0b013e3182020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merchant H, et al. The context of temporal processing is represented in the multidimensional relationships between timing tasks. PLoS One. 2008;3(9):e3169. doi: 10.1371/journal.pone.0003169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rakitin BC, Stern Y, Malapani C. The effects of aging on time reproduction in delayed free-recall. Brain Cogn. 2005;58(1):17–34. doi: 10.1016/j.bandc.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Rakitin BC, Malapani C. Effects of feedback on time production errors in aging participants. Brain Res Bull. 2008;75(1):23–33. doi: 10.1016/j.brainresbull.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 29.Rakitin BC, et al. Single-dose levodopa administration and aging independently disrupt time production. J Cogn Neurosci. 2006;18(3):376–87. doi: 10.1162/089892906775990615. [DOI] [PubMed] [Google Scholar]
- 30.Wild-Wall N, et al. Time estimation in healthy ageing and neurodegenerative basal ganglia disorders. Neurosci Lett. 2008;442(1):34–8. doi: 10.1016/j.neulet.2008.06.069. [DOI] [PubMed] [Google Scholar]
- 31.Gooch CM, et al. Oxycodone lengthens reproductions of suprasecond time intervals in human research volunteers. Behav Pharmacol. 2011;22(4):354–61. doi: 10.1097/FBP.0b013e328348d8b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huntington Study Group. Unified Huntington’s Disease Rating Scale: reliability and consistency. Huntington Study Group. Mov Disord. 1996;11(2):136–42. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]
- 33.Paulsen JS, et al. Preparing for preventive clinical trials: the Predict-HD study. Arch Neurol. 2006;63(6):883–90. doi: 10.1001/archneur.63.6.883. [DOI] [PubMed] [Google Scholar]
- 34.Stout JC, et al. Neurocognitive signs in prodromal Huntington disease. Neuropsychology. 2011;25(1):1–14. doi: 10.1037/a0020937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stout JC, et al. Evaluation of longitudinal 12 and 24 month cognitive outcomes in premanifest and early Huntington’s disease. J Neurol Neurosurg Psychiatry. 2012;83(7):687–94. doi: 10.1136/jnnp-2011-301940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langbehn DR, et al. A new model for prediction of the age of onset and penetrance for Huntington’s disease based on CAG length. Clin Genet. 2004;65(4):267–77. doi: 10.1111/j.1399-0004.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- 37.Merchant H, Zarco W, Prado L. Do we have a common mechanism for measuring time in the hundreds of millisecond range? Evidence from multiple-interval timing tasks. J Neurophysiol. 2008;99(2):939–49. doi: 10.1152/jn.01225.2007. [DOI] [PubMed] [Google Scholar]
- 38.Shea-Brown E, et al. A firing rate model of Parkinsonian deficits in interval timing. Brain Res. 2006;1070(1):189–201. doi: 10.1016/j.brainres.2005.10.070. [DOI] [PubMed] [Google Scholar]